Abstract
In an observational longitudinal study of a sub-sample of the Aberdeen 1936 birth cohort, from age 62 to 77 years, we investigated childhood intelligence, social class, education, life-course social mobility, memory test performance and memory decline in late life. We examined 388 local residents who had attended school in Aberdeen in 1947 and measured Auditory-Verbal Learning Test (AVLT) at recruitment age about 64 years and up to five times until age about 77 years. Better performance at age about 64 on AVLT was predicted by early socioeconomic status (SES), social mobility and childhood intelligence. The trajectory of AVLT decline was steeper in those who had received less education. This relationship was independent of childhood ability, sex, SES in childhood and social mobility. The protection of memory by education suggests that education supports resilience to age-related cognitive impairment. Upward social mobility does not enhance this effect, suggesting that resilience to age-related decline may be established in early life.
Keywords: memory decline, social class, education, social mobility, childhood intelligence, older people
Introduction
The maintenance of cognitive function in late life is desirable for individuals and society. Factors known to influence cognitive performance in late life include education, childhood mental ability, material wealth, job complexity and social mobility [1]. These and other factors may interact in complex ways throughout the life course, and a greater understanding of these interactions influence on cognitive ageing is needed. Suo et al. [2] have shown that managerial experience, high socioeconomic status, is associated with the structure and function of the hippocampus. Similarly, Bennett et al. [3] have suggested that higher levels of mental activity associated with higher occupational achievement has a beneficial effect on life-course cognitive outcomes.
Research has highlighted benefits of social mobility, and its associated mental endeavours, on life-course cognitive functioning [4]. However, a limitation of these studies is the frequent absence of early life cognitive ability measures, a key driver for social mobility. This limitation opens the possibility that differences observed in late life could be brought about by social selection with superior early cognition driving upward social mobility via education and occupational attainment. In addition, these studies also focus on cross-sectional estimates of cognition in late life and implied protection from the individual differences in social status and mobility. What remains unanswered is if these differences in cognition and cognitive decline are a result of life-course gains, differential development or the accumulation of resilience over the adult life course resulting in differential decline.
Focusing broadly on population well-being and developmental dynamics, research highlights that both social inequality and lower social status are associated with negative effects on health and other outcomes [5]. Social mobility is defined as ‘the movement of an individual between social classes’ [6]. Although it is unclear if upward social mobility can improve well-being and support positive outcomes, there is optimism that it might [7]. This optimism should be set against an understanding of the effects of early life on late life diseases [8] and related effects on neurodevelopment [9, 10], and the accumulation of ageing neuropathology. In this study, we extend understanding of these dynamics by examining both early life influences and upward social mobility on trajectories of cognitive ageing.
Specifically, in a well-characterised birth cohort [11], we investigate how social mobility, early life circumstances, education and childhood intelligence are related to memory performance and its trajectory in late life. In 1997, the Scottish Council for Research in Education allowed us access to their archives of intelligence tests obtained in the Scottish Mental Survey of 1947 when Scottish Children born in 1936 sat a group administered test. We recruited volunteers from among local residents who had participated in this survey. From 1998, we obtained volunteers accounts of their childhood and current social circumstances and repeated scores on cognitive tests from 1999 to 2014. Drawing upon this data, we pose two research questions:
[1] Do early life social class, education and social mobility influence memory function in late life and is this influence independent of childhood ability and sex?
[2] Do early life social class, education and social mobility influence the rate of decline in memory function in late life and is this influence independent of childhood ability and sex?
Methods
Study population
All data were provided by the Aberdeen Birth Cohort of 1936. This is a sub-sample of the 1947 Scottish Mental Survey (N = 75,211) which was a national survey of childhood general mental ability (intelligence). An extended description of recruitment and data acquisition is available [11]. Following guidance by the Local Ethics of Research Committee (University of Aberdeen and NHS Grampian) who approved study procedures of the study, volunteers gave written informed consent to a longitudinal observational study of brain ageing and health. We invited individuals who were matched exactly with the Scottish Mental Survey (1947) archive; 506 (75%) of 676 invited to volunteer agreed to participate in some form. An audit trail of the dropout at each stage of the data acquisition is detailed in the cohort description publication [11] and in Supplementary text (ST1 available in Age and Ageing online). Three hundred and eighty-eight gave data required for this analysis at baseline. In general, those who were approached and did not take part had lower childhood intelligence scores. Similarly, those who continued in the study across multiple waves of longitudinal assessment displayed higher intelligence test scores at the first occasion of testing and in childhood [12].
Childhood socioeconomic status and education
Demographic and social conditions data were obtained at a structured interview at age 64 (±1) years by a research nurse. These data were previously used by ourselves when examining the influence of personality on social mobility [13]. Participants were asked to recall home conditions and paternal occupation at age 11 years. Questions included the number of rooms in the family home, the number of occupants (giving the number of residents/room to quantify overcrowding (OCR)); how many shared their sanitation facility (SAN). Participants’ best-ever occupation provided their Occupational Social Class in adulthood (OSCA). Similarly, paternal occupation was used to categorise Occupational Social Class (OSCP). For both paternal and participant’s own occupation, OSC was coded using the UK Office of Population Statistics Classification of Occupations HMSO, 1971. We coded occupations so that high values represented occupations of higher status. The participant’s current UK post-code was recorded and then used to estimate the relative level of deprivation at that address using the Scottish Index of Multiple Deprivation (SIMD) (http://simd.scotland.gov.uk).
Childhood socioeconomic status (SESC) is derived as a latent, unobserved variable, composed of OCD, sanitation share (SAN) and paternal occupational social class (OSCP). Adult socioeconomic status (SESA) is derived from participants’ OSCA and residential deprivation (SIMD). We recorded the number of year’s education an individual had before the age of 25 (EDU). Female homemakers were placed in their husband’s SES category. Social mobility (SESM) was calculated as the relative standardized difference between childhood and late life social position (SESC–SESA).
Cognitive tests
Childhood intelligence data were provided from the Scottish Mental Survey (1947) archive. All children born in 1936 and at school in Scotland on the 4 June 1947 sat a group administered intelligence test (The Moray House Test (MHT)) [14].
Psychological tests were administered by a psychologist following standard procedures. The Rey Auditory-Verbal Learning Test (AVLT) was used to measure age-related memory decline [15]. Cognitive longitudinal modelling can be confounded by practice effects [16]. The pattern of improvement presented by our data suggests that AVLT practice improvement is largest between the first and second occasion and there is little improvement after that. Such practice effects were accounted for using an initial practice model [17]. AVLT performance is also affected by age, education, childhood intelligence and sex [18]. Declines in performance with age are well-documented [19]. Reports of the effects of education, ability and sex on AVLT performance are mixed, but it is generally accepted that education and sex affects performance [18]. In the current study, the AVLT was administered on the first occasion and repeated on up to five occasions over the next 14 years.
Statistical analysis
We used MLwin software from the University of Bristol [20] and SPSSv24 (www.ibm.com) for all statistical analyses. To create a summary variable for socioeconomic status in both childhood and later life, we used a principal component analysis technique using father’s occupation (OSCP), SAN and OCR to calculate the childhood estimate; and we used participants occupation and SIMD decile to calculate the adult estimate. Raw AVLT scores and MHT scores were standardized to a mean of 100 and a standard deviation of 15. Age at testing was measured as time since their 60th birthday. Practice was modelled using an initial practice model approach [17].
To examine the association between late life memory and its trajectory with age, we used multilevel linear modelling which included age, sex, practice, education, SESc, mobility and childhood ability (MHT). In Model 1, we examined the main effects (fixed) of each variable on late life memory (AVLT): age, sex, practice, childhood ability (MHT), SESc and social mobility (SESm). This is described by equation (1): here, we also modelled the intercept and slope with age as both a fixed and random effect. In equation (1), i represents the occasion of testing and j the individual being tested; represents the fixed part of the intercept and the random departure from ; is constructed so that it has a mean of zero and a variation of ; similarly, represents the fixed part of the slope and the random departure form ; is constructed so that it has a mean of zero and a variation ; the covariance between the intercept is also modelled ; provides an estimate of the residual.
| (1) |
where and
In Model 2, we hypothesised that there would be additional contributions to memory from a variety of interaction effects: Age × Sex (), Age × SESc () and Age × SESm (). As such, these interactions were added to the components of Model 1. Finally in Model 3, we removed childhood ability from Model 2 to examine if the association remain if this rarely available variable is not present.
Results
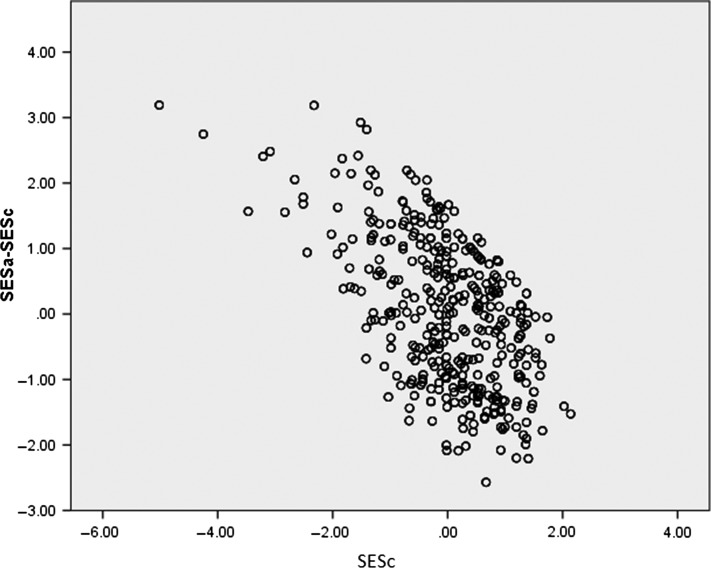
The sample is described in Table 1. The principal component analysis of the early life data reveals a single component that explains 52.9% of the variance (Component loadings Table 1). Similarly, the later life data using the participant’s occupation and SIMD reveals a single component that explained 69.1% of the variance (Component loadings Table 1). Table 1 also shows correlations between each variable and memory performance on recruitment. With the exception of age at entry, all variables were associated with memory. Change in social status is plotted against childhood SES (Figure 1). The figure indicates relative social mobility in both directions, both upward and downward. A Spaghetti plot for the standardized AVLT score over time is shown in Supplementary Figure S1 available in Age and Ageing online.
Table 1.
Sociodemographic, educational, childhood intelligence scores for 388 participants recruited in 1998-2000 from local survivors of the 1947 Scottish Mental Survey. Auditory-Verbal Learning Test (AVLT); Moray House Test (MHT); socioeconomic status (SES); socioeconomic status adult (SESa); socioeconomic status childhood (SESc) and socioeconomic status mobility (SESm). Pearson correlation with AVLT at entry and comparison between genders for each measure. *P < 0.001.
| N = 388 Male 192 (49.5%) | Mean (standard deviation) Min–Max | Component loading on SES | Correlation with AVLT score at entry | Male (standard deviation) | Female (standard deviation) |
|---|---|---|---|---|---|
| Age when first tested as an adult | 64.8 (1.40) 62.6–77.3 | 0.04 | 64.7 (1.2) | 64.9 (1.5) | |
| Father occupational status | 6.22 (2.33) 1.00–9.00 | 0.50 | 0.11* | 6.33 (2.36) | 6.13 (2.31) |
| Overcrowding | 1.75 (0.75) 0.43–5.00 | 0.83 | –0.17* | 1.78 (0.78) | 1.72 (0.07) |
| Sanitation share | 7.88 (4.87) 2.00–18.00 | 0.80 | –0.16* | 8.37 (5.69) | 7.40 (3.54) |
| SESc | 0.21* | –0.09 (1.13) | 0.09 (0.84) | ||
| Education year | 11.11 (1.99) 9.00–20.00 | 0.28* | 11.0 (2.1) | 11.2 (1.9) | |
| Participants occupation status | 4.47 (2.19) 1.00–9.00 | 0.83 | 0.25* | 4.79 (2.35) | 4.66 (2.03) |
| SIMD | 6.66 (3.03) 1.00–10.00 | 0.83 | 0.22* | 6.73 (3.05) | 6.60 (3.01) |
| SESa | 0.28* | –0.01 (1.04) | 0.01 (0.96) | ||
| SESm | 0.28* | 0.09 (1.21) | –0.08 (1.04) | ||
| MHT | 42.61 (12.52) 1.00–72.00 | 0.38* | 41.8 (12.7) | 43.4 (12.3) | |
| AVLT Score at entry | 44.97 (7.80) 12.00–72.0 | 41.8 (9.7)* | 48.1 (8.9) |
Figure 1.
A scatter plot showing the relationship between childhood socioeconomic status SESc and socioeconomic change between age 11 years and late life (SESa–SESc).
The results of the multilevel modelling are shown in Table 2. The results show a significant decline in memory performance with age. In addition, significant practice, sex, childhood ability and SESc effects on memory performance are observed (Model 1). The influence of SESm on memory performance is not significant (Model 1).
Table 2.
Three longitudinal linear mixed models (see equation (1)) of data from 388 participants in the Aberdeen 1936 Birth Cohort Study. Socioeconomic status childhood (SESc) and socioeconomic status mobility (SESm). All associations are significant unless indicated (ns).
| Model 1 | Model 2 | Model 3 | ||
|---|---|---|---|---|
| Effect (standard error) | Effect (standard error) | Effect (standard error) | ||
| Intercept | 63.4 (5.6) | 70.7 (6.3) | 89.7 (5.0) | |
| Age | –0.54 (0.11) | –1.73 (0.54) | –1.74 (0.54) | |
| Practice | 4.33 (0.66) | 4.42 (0.66) | 4.51 (0.66) | |
| Sex | 8.56 (1.27) | 8.57 (1.12) | 8.94 (1.15) | |
| Childhood ability | 0.24 (0.05) | 0.24 (0.05) | ||
| Education | 0.84 (0.33) | 0.20 (0.44) ns | 0.60 (0.44) ns | |
| SESc | 1.84 (0.84) | 2.47 (1.16) | 4.00 (1.14) | |
| SESm | 1.17 (0.70) ns | 1.88 (0.96) | 3.00 (0.95) | |
| Age × education | 0.11 (0.05) | 0.11 (0.05) | ||
| Age × SESc | –0.10 (0.13) ns | –0.10 (0.13) ns | ||
| Age × SESm | –0.12 (0.11) ns | –0.12 (0.11) ns | ||
| Intercept variance | 86.5 (16.3) | 87.1 (16.3) | 95.2 (16.9) | |
| Age variance | 0.62 (0.17) | 0.61 (0.16) | 0.61 (0.16) | |
| Intercept/age covariance | –1.56 (1.44) ns | –1.56 (1.43) ns | –1.63 (1.45) ns | |
| Residual | 59.6 (3.49) | 59.3 (3.5) | 59.3 (3.5) | |
| −2Loglikihood | 8841.5 | 8836.2 | 8859.5 | |
In Model 2, the effects of education (EDU), SESc and SESm, and the interaction terms Age x SESc and Age x SESm are not significant. However, Model 2 reveals a significant Age x EDU interaction effect. Since childhood ability is rarely available, Model 2 was repeated without childhood ability to examine the predictive value of the other variables in its absence (Model 3). All models indicate a difference between the sexes with women performing better than men.
Discussion
Main findings
Focusing on memory performance the current study found that SESc and social mobility predicted memory; however, effects of SESc and social mobility on trajectories of change over time were non-significant. The data also indicate the trajectory of memory decline was steeper in those with less education. This relationship was independent of other variables modelled. Although we did not measure the accumulation of age-related brain pathology, the apparent protection of memory by education suggests that education contributes to resilience against the ageing processes as shown by higher memory scores by those participants with more education. This is indicated by the significant Age x EDU which shows that on average those with more education had a less steep rate of decline.
The correlation between SESa–SESc and SESc is –0.58 ‘moderate’ and perhaps greater than expected. As demonstrated by the scatter plot (Figure 1), those who were disadvantaged in childhood achieved greater upward social mobility relative to those who were advantaged. When these observations and their inter-relationships are placed in the context of profound socioeconomic changes brought about post war: the welfare state, improved housing, better health care, and improved access to higher and further education, these correlations appear understandable. In addition, marked increased opportunities in Aberdeen were also brought about by the North Sea oil boom.
Strengths and limitations
A particular study strength lies in the generalisability of our results from a population-based volunteer sample with good rates of recruitment (75%) [11]. Recruitment to cognitive ageing studies is affected by self-selection to better educated, healthy volunteers. Our findings may be biased towards those of higher cognitive performance.
We compared OSC between fathers and participants and observed considerable occupational gains between generations (1.5 points, P < 0.001 paired t-test). This observation is typical of the extent of gains expected during the twentieth century in the UK [21]. Although we evaluated relative SES in father and participant, we are aware that both classifications of socioeconomic status were not exactly comparable. A simple approach would have been to use participant occupation and father’s occupation as estimates of SES. We have repeated our analysis replacing SES with occupation finding similar results.
Conclusions
Social and neurobiological pathways underlie the association between life-course social mobility and cognition. Socioeconomic status effects are likely exerted over the life course via complex pathways of influence involving SESc, education and cognitive ability, but their exact nature remains uncertain. Nevertheless, several levels of explanation seem plausible. (1) Those from lower of socioeconomic origins may receive less education and enter the labour market with higher risks of being exposed to environmental hazards [22]. (2) Low social status is associated with less disposable income and fewer opportunities for leisure pursuits fostering greater social engagement, cognitively effortful activities and more physical exercise, each of which may support resilience [23]. (3) Lower incomes and less education could influence diet negatively [24]. The overall picture might be confounded by complex associations between the demands of coping with the material privations and paucity of opportunities to improve social status, coupled with the consequences for neural health of poor dietary habits and exposure to neurotoxins. Among the most socially disadvantaged, exposure to hazards for brain health are not only more frequent but the resources available to help cope are also reduced [25].
Previous studies have highlighted that lower levels of education and low status occupations are risk factors for most common late-onset diseases. [26] and Alzheimer’s disease [27]. Investigations into the relationship between demographic measures and cognitive function in later life have highlighted similar effects [28]. Previous studies have also suggested that education and occupational status contribute independently to reserve [29]. Results here suggest that education and SES are not independent. Childhood ability measures are rarely available in studies of this type, and removing childhood ability from the model used in the current study revealed that both SESc and SESm contribute to late life memory but not the trajectory of decline.
As the number of older people increases, these results suggest that the increase in educational exposure that recent generations have experienced may impact on their late life cognition in two ways. First, by increasing the amount of life course gains and secondly by reducing the rate of decline in late life.
Supplementary Material
Funding
Support for the Aberdeen 1936 Birth Cohort follow-up studies was provided by grants from BBSRC (1999–2002), Wellcome Trust (2001–2006) and the Alzheimer UK Research Trust (2002–2005).
Conflicts of interest
None.
Key points.
Better memory is predicted by Social Class and mobility across the life course.
Education predicts the trajectory of decline in late life.
Resilience to age-related decline may be established in early life through education.
Early life social class provides no protection against the trajectory of decline in old age
Social mobility provides no protection against the trajectory of decline in old age.
References
- 1. Lee Y, Back JH, Kim J et al. Systematic review of health behavioral risks and cognitive health in older adults. Int Psychogeriatr 2010; 22: 174–87. [DOI] [PubMed] [Google Scholar]
- 2. Suo C, Gates N, Singh MF et al. Midlife managerial experience is linked to late life hippocampal morphology and function. Brain Imaging Behav 2017; 11: 333–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bennett DA, Arnold SE, Valenzuela MJ, Brayne C, Schneider JA. Cognitive and social lifestyle: links with neuropathology and cognition in late life. Acta Neuropathol 2014; 127: 137–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Turrell G, Lynch J, Kaplan G et al. Socioeconomic position across the lifecourse and cognitive function in late middle age. Journals of Gerontology Series B-Psychological Sciences and Social Sciences 2002; 57: S43–51. [DOI] [PubMed] [Google Scholar]
- 5. Lorgelly PK, Lindley J. What is the relationship between income inequality and health? Evidence from the BHPS. Health Econ 2008; 17: 249–65. [DOI] [PubMed] [Google Scholar]
- 6. Forrest LF, Hodgson S, Parker L, Pearce MS. The influence of childhood IQ and education on social mobility in the Newcastle thousand families birth cohort. BMC Public Health 2011; 11: 895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hart CL, Smith GD, Blane D. Social mobility and 21 year mortality in a cohort of Scottish men. Soc Sci Med 1998; 47: 1121–30. [DOI] [PubMed] [Google Scholar]
- 8. Boekelheide K, Blumberg B, Chapin RE et al. Predicting later-life outcomes of early-life exposures. Environ Health Perspect 2012; 120: 1353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Staff RT, Murray AD, Ahearn TS, Mustafa N, Fox HC, Whalley LJ. Childhood socioeconomic status and adult brain size: childhood socioeconomic status influences adult hippocampal size. Ann Neurol 2012; 71: 653–60. [DOI] [PubMed] [Google Scholar]
- 10. Noble KG, Houston SM, Brito NH et al. Family income, parental education and brain structure in children and adolescents. Nat Neurosci 2015; 18: 773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Whalley LJ, Murray AD, Staff RT et al. How the 1932 and 1947 mental surveys of Aberdeen schoolchildren provide a framework to explore the childhood origins of late onset disease and disability. Maturitas 2011; 69: 365–72. [DOI] [PubMed] [Google Scholar]
- 12. Staff RT, Chapko D, Hogan MJ, Whalley LJ. Life course socioeconomic status and the decline in information processing speed in late life. Soc Sci Med 2016; 151: 130–8. [DOI] [PubMed] [Google Scholar]
- 13. Staff RT, Hogan MJ, Whalley LJ. Childhood intelligence and personality traits neuroticism and openness contributes to social mobility: a study in the Aberdeen 1936 Birth Cohort. Pers Individ Dif 2017; 114: 206–12. [Google Scholar]
- 14. Whalley LJ, Deary IJ. Longitudinal cohort study of childhood IQ and survival up to age 76. Br Med J 2001; 322: 819–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Andersson C, Lindau M, Almkvist O, Engfeldt P, Johansson S, Jonhagen M. Identifying patients at high and low risk of cognitive decline using Rey auditory verbal learning test among middle-aged memory clinic outpatients. Dement Geriatr Cogn Disord 2006; 21: 251–9. [DOI] [PubMed] [Google Scholar]
- 16. Goldberg TE, Harvey PD, Wesnes KA, Snyder PJ, Schneider LS. Practice effects due to serial cognitive assessment: implications for preclinical Alzheimer’s disease randomized controlled trials. Alzheimer’s & dementia (Amsterdam, Netherlands) 2015; 1: 103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Staff RT, Hogan MJ, Whalley LJ. Aging trajectories of fluid intelligence in late life: the influence of age, practice and childhood IQ on Raven’s progressive matrices. Intelligence 2014; 47: 194–201. [Google Scholar]
- 18. Schmidt M. Rey auditory verbal learning test (RAVLT): A handbook. Los Angeles: Western Psychological Services, 1996. [Google Scholar]
- 19. Crossen J, Wiens A. Comparison of the auditory-verbal learning test (AVLT) and the California verbal-learning test (CVLT) in a sample of normal subjects, (vol 16, pg 190, 1994). J Clin Exp Neuropsychol 1994; 16: 649. [DOI] [PubMed] [Google Scholar]
- 20. Centre for Multilevel Modelling, University of Bristol MLwiN version 2.1 2009.
- 21. Matheson J, Summerfield C, eds. Social trends 30 2000 edition, 2000th London: The office of National Statistics, 2000. [Google Scholar]
- 22. WHO Regional Office for Europe Environment and health risks: A review of the influence and effects of social inequalities. Copenhagen: WHO, 2010. [Google Scholar]
- 23. Hogan M. Physical and cognitive activity and exercise for older adults: a review. Int J Aging Hum Dev 2005; 60: 95–126. [DOI] [PubMed] [Google Scholar]
- 24. Gray L, Leyland AH. A multilevel analysis of diet and socio-economic status in Scotland: investigating the ‘Glasgow effect’. Public Health Nutr 2009; 12: 1351–8. [DOI] [PubMed] [Google Scholar]
- 25. Meyer IH, Schwartz S, Frost DM. Social patterning of stress and coping: does disadvantaged social statuses confer more stress and fewer coping resources? Soc Sci Med 2008; 67: 368–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hemingway H, Marmot M. Evidence based cardiology—psychosocial factors in the aetiology and prognosis of coronary heart disease: systematic review of prospective cohort studies. Br Med J 1999; 318: 1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R. Influence of education and occupation on the incidence of Alzheimers-disease. Jama-Journal of the American Medical Association 1994; 271: 1004–10. [PubMed] [Google Scholar]
- 28. Coffey C, Saxton J, Ratcliff G, Bryan R, Lucke J. Relation of education to brain size in normal aging—implications for the reserve hypothesis. Neurology 1999; 53: 189–96. [DOI] [PubMed] [Google Scholar]
- 29. Mortel K, Meyer J, Herod B, Thornby J. Education and occupation as risk-factors for dementias of the Ālzheimer and ischemic vascular types. Dementia 1995; 6: 55–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.