Abstract
Background
Adolescence is a critical period for neural development and alcohol exposure during adolescence can lead to an elevated risk for health consequences as well as alcohol use disorders. Clinical and experimental data suggest that chronic alcohol exposure may produce immunomodulatory effects that can lead to the activation of proinflammatory cytokine pathways as well as microglial markers. The present study evaluated, in brain and blood, the effects of adolescent alcohol exposure and withdrawal on microglia and on the most representative pro and anti-inflammatory cytokines and major chemokines that can contribute to the establishing of a neuroinflammatory environment.
Methods
Wistar rats (males, n=96) were exposed to ethanol vapors, or air control, for 5 weeks over adolescence (PD 22–58). Brains and blood samples were collected at three time points: 1) after 35 days of vapor/air exposure (PD58); 2) after 1 day of withdrawal (PD59), and 3) 28 days after withdrawal (PD86). The ionized calcium binding adapter molecule 1 (Iba-1) was used to index microglia activation, and cytokine/chemokine responses were analyzed using Magnetic Bead Panels.
Results
After 35 days of adolescent vapor exposure, a significant increase in Iba-1 immunoreactivity was seen in: amygdala, frontal cortex, hippocampus and substantia nigra. However, Iba-1 density returned to control levels at both 1 day and 28 days of withdrawal except in the hippocampus where Iba-1 density was significantly lower than controls. In serum, adolescent ethanol exposure induced a reduction of IL-13 and an increase in fractalkine at day 35. After 1 day of withdrawal IL-18 was reduced, and IP-10 was elevated, whereas both IP-10 and IL-10 were elevated at 28 days following withdrawal. In the frontal cortex adolescent ethanol exposure induced an increase of IL-1β at day 35, and 28 days of withdrawal, and IL-10 was increased after 28 days of withdrawal.
Conclusion
These data demonstrate that ethanol exposure during adolescence produces significant microglial activation, however, inflammatory markers seen in the blood appear to differ from those observed in the brain.
Keywords: adolescent, cytokines, Wistar rats, microglial activation, alcohol
Introduction
Alcohol use and binge drinking among adolescents is associated with increased health risks (Miller et al., 2007), and enhanced propensity for the development of alcohol use disorders (AUD) in later life (Dawson et al., 2008; Ehlers et al., 2006; Hingson et al., 2006; Johnston et al., 2009). Adolescence is a critical period for the onset of binge drinking, and alcohol exposure during this time has been demonstrated to produce persistent alterations in neural development, behavior and drinking levels in adulthood in several rodent models (see (Alaux-Cantin et al., 2013; Crews et al., 2016; Crews et al., 2019; Maldonado-Devincci et al., 2010; Spear, 2016; Spear, 2018; Spear and Swartzwelder, 2014)).
Adolescence is also a period of significant cortical modification (Gogtay et al., 2004) that coincides with increases in vulnerability to the effects of excessive alcohol exposure on behavior and memory (Hermens and Lagopoulos, 2018). We have shown, using an alcohol vapor exposure model, that alcohol exposure during adolescence can lead to deficits in several physiological and behavioral responses in rats that persist into adulthood (Amodeo et al., 2018; Ehlers et al., 2011; Ehlers et al., 2013a). We have also shown, in young adult humans and in rats, that alcohol exposure during adolescence can cause a delay in the development of age associated increases in cortical connectivity (Ehlers et al., 2019). These data are also supportive of findings that show changes in neural connectivity as indexed by fMRI in cortical and subcortical structures following adolescent alcohol exposure in rats (Broadwater et al., 2018), and deficits in connectivity and neuropsychological performance in human adolescents with early age of onset drinking (Nguyen-Louie et al., 2018).
The brain mechanisms that underlie neural developmental changes associated with adolescent alcohol exposure remains under studied. However, clinical and experimental data suggest that alcohol may be an immunomodulatory agent (Afshar et al., 2015; Szabo and Mandrekar, 2009). Alcohol exposure can impact systemic and brain innate immune signaling leading to activation of proinflammatory cytokine pathways (Szabo and Saha, 2015), variation in serum cytokine levels (Gonzalez-Quintela et al., 2000; Neupane et al., 2016), and induction of neuroinflammation (Crews et al., 2017; Crews et al., 2015; de Timary et al., 2017; Qin et al., 2008).
Preclinical studies have demonstrated that in some cases, following alcohol exposure, that immune proteins act as important signaling molecules in the brain in addition to their role in the periphery (Crews et al., 2006; Szabo and Lippai, 2014). For example, repeated cycles of exposure and/or larger doses of alcohol (Crews, 2008; Peng et al., 2017; Zahr et al., 2010; Marshall et al., 2016; Zhao et al., 2013) are known to produce a long lasting induction of neuroimmune genes as well as increases in levels of serum cytokines (Crews and Vetreno, 2016; Doremus-Fitzwater et al., 2014; Doremus-Fitzwater et al., 2018; Zou and Crews, 2010). Several preclinical studies have additionally demonstrated that adolescent alcohol exposure can also induce neuroinflammatory changes (see (Crews and Vetreno, 2011; Crews et al., 2016; Guerri and Pascual, 2018; Montesinos et al., 2016; Pascual et al., 2018)).
More recently, it has been posited that microglia may act as important modulators of alcohol neurotoxicity (Chastain and Sarkar, 2014; Henriques et al., 2018). Microglial activation has been demonstrated to occur in adult rodents after chronic alcohol exposure and also following withdrawal (Marshall et al., 2013; McClain et al., 2011; Sanchez-Alavez et al., 2018). In clinical studies, that evaluated the post-mortem brains of persons with a lifetime diagnosis of alcoholism, the microglia activation marker, Iba-1, was also shown to be increased (Crews and Vetreno, 2016; He and Crews, 2008). However, the extent to which microglia and cytokine markers are impacted during adolescent exposure to alcohol is less known. Also, it is not clear how more moderate alcohol doses may affect both blood-based markers of inflammation as well as brain signaling measures following adolescent alcohol exposure. Additionally, the relationship between microglial activation, as indexed by Iba-1, and other immune signaling molecules following exposure and protracted withdrawal has been little studied.
The specific aims of the current study were to investigate the time course of microglial activation, as well as chemokine and cytokine signaling in blood and in frontal cortex, following chronic adolescent alcohol exposure and withdrawal in young adult Wistar rats. In this study we measured the levels of IL-1α, IL-1β, IL-12 IL-17, IL-18 and TNF which are some of the most critical pro-inflammatory cytokines known to initiate and sustain inflammation. The levels of IL-4, IL-5, IL-10 and IL-13, that can reduce inflammation by reducing the amount of pro-inflammatory molecules, were also measured. Finally, the chemokines eotaxin, fractalkine, IP-10, LIX and RANTES were measured because of their role as a chemoattractant in the recruitment of leukocytes that can contribute to local inflammatory responses. Notably, some of these modulators of inflammation can also affect the function of neuronal receptors. For instance, IL-1β can increase phosphorylation of the NR2B subunit of the NMDA receptor resulting in an increase in neuronal excitability (Balosso et al., 2008). Similarly, the anti-inflammatory cytokine IL-10 is capable of regulating GABAergic transmission in dentate gyrus neurons by lowering excitability and changing LTP responses (Kelly et al., 2001).
Experimental Procedures
Animal subjects
Ninety-six adolescent male Wistar rats, from Charles River (USA), arrived with their dams and were weaned on postnatal day (PD) 21. Rats were housed in groups of 3 in standard plastic cages in a temperature and light controlled room with a 12h light/dark cycle. Ad libitum food and water were available. These procedures were approved by The Scripps Research Institute’s Animal Care and Use Committee and additionally adheres to the guidelines outlined in the NIH Guide for the Care and Use of Laboratory Animals (NIH publication No. 80–23, revised 1996).
Alcohol vapor exposure
Alcohol was administered by infusing sealed chambers with alcohol vapor. The alcohol vapor inhalation chambers and the experimental procedures used in this study have been described previously (Ehlers et al., 2011; Slawecki, 2002). For this study the alcohol vapor chambers were adjusted to sustain blood alcohol levels between 150–200 mg/dL. These blood levels represent levels that would be achieved in binge drinking adolescents (Ehlers et al., 2019). Rats exposed to this intermittent alcohol vapor exposure protocol were monitored to determine that blood ethanol concentrations (BEC) were within the target range. Following each BEC measurement, adjustments were made as appropriate. The day after arrival, rats were divided into four groups of 24 rats each. Adolescent rats were exposed to alcohol vapor in two separate groups of 24 rats each with their own air exposed control group of 24 rats. Tissue from one vapor/control pair was used for microglia staining and the other for cytokine assays (n=8 for each time point). Alcohol chambers were infused with vaporized 95% alcohol for 14 hours from 20:00 to 10:00. Alcohol vapor was not infused into the chambers for the rest of the 24-hour cycle. Rats were exposed to vapor for 35 days over the peri-adolescent period, (PD 22–58). Although this does not simulate all the patterns of binge drinking typically seen in human adolescents it does allow for coverage of the entire adolescent period in the rat that in the human would span over 10 years.
Over the course of the 5-week vapor exposure, blood samples were collected every 3–4 days, at 0:800, from the tip of the tail in both alcohol and the control rats, in order to determine BECs (5-week average, microglia group: 177.1 ± 7.9 mg/dL; cytokine group: 183.3 ± 8.0 mg/dL). BECs were quantitated using the Analox micro-statAM1 (Analox Instr. Ltd., Lunenberg, MA). After the alcohol exposure period all rats were housed in standard caging for the rest of the experiment.
Tissue collection and preparation for assay
Blood serum and brain samples were collected from the rats at 3 time-points during and after the 5-week adolescent alcohol vapor exposure or control conditions. The time points used were: after 35 days of vapor/air exposure (PD58), 24 hours after the withdrawal (PD59) and 28 days after withdrawal (PD86). Withdrawal is the period after termination of ethanol vapor exposure at which the rats were returned to the vivarium, where they remained until sacrifice at 24 hours and 28 days after alcohol cessation. For each time point, rats were terminally anesthetized with Fatal-Plus and then a blood sample was obtained using cardiac puncture. The animals were then promptly perfused with phosphate buffered saline (PBS) to remove excess blood from the brain.
To prepare the tissue for Multiplex assay, as described previously (Sanchez-Alavez et al., 2018), one hemisphere, chosen randomly, of flash frozen brain tissue from 48 of the rats were homogenized in RIPA buffer (Thermo Fisher Scientific, cat#89900) and Halt Protease Inhibitor Cocktail (Thermo Fisher Scientific, 1X-cat#87786). Resulting homogenized samples were then cooled on ice and gently agitated before being centrifuged at 14,000 xg for 15 minutes at 4° C, with the supernatant separated and stored for the assays.
Immunohistochemical analysis of microglial activation
One half of the brain from the 48 rats was used for the evaluation of microglial activation using Iba-1 DAB immunohistochemical analysis. Using a Leica cryostat 35-μm brain slices were obtained. Endogenous peroxidases were neutralized using a 3% hydrogen peroxide with 0.1 M phosphate buffer (PB) solution for 15 min. Sections were then washed with 0.1 M PB twice and blocked in a 0.1 PB solution with 5% normal goat serum, 1% BSA, and 0.1% Tween. Sections were then incubated with primary antibody rabbit anti-Iba-1 (1:500, Wako, cat# 019–19741) overnight at 4 °C in blocking buffer. After incubation, brain sections were then washed three times with BSA (0.5% in 0.1 M PB) and incubated for 1 hour at room temperature using a secondary antibody (biotinylated goat-anti rabbit, 1:400, Vector labs, cat# BA-1000) in BSA (0.5% in 0.1 M PB). Sections were washed twice more with BSA (0.5% in 0.1 M PB) prior to 1-hour incubation in ABC elite vectastatin (Vector Labs, catalog #PK-6100) followed by two more washes with 0.1 M PB. Finally, brain sections were stained for five minutes (Impact DAB, Vector Labs, cat#SK4105) and placed in 0.1 M PB before being mounted in VectaMount™.
Microscopic quantification and image analysis of microglial activation
Five brain regions were selected for image analysis using coordinates from the Paxinos and Watson rat brain atlas (Paxinos and Watson, 1986): central nucleus of amygdala (CeA), frontal cortex, region CA1 of the hippocampus, substantia nigra pars reticulata (SNpr), and white matter fiber tracts of the cerebellum. For every 6th section, three slices were quantified using digital scans taken with a Leica Aperio AT2. Each slice was scanned under 20× magnification four times. Activated microglia pixel density (cells with an area >200 mm2) was quantified using ImageJ software (National Institute of Health, version 1.45) by utilizing a stitching algorithm (Preibisch et al., 2009). Methods for preparation and quantification of microglial activation have been described previously (Sanchez-Alavez et al., 2018). We sought to determine how widespread microglial activation might be in brain following alcohol exposure. The specific brain regions were selected based on our previous studies demonstrating that these brain areas are important in neuroimmune functions as well as sensitive to the effects of alcohol (Alboni et al., 2009; Amodeo et al., 2017; Ehlers et al., 1992; Ehlers et al., 2013a; Ehlers et al., 2013b; Hwang et al., 1999; Mori et al., 2016; Morrison et al., 2012; Sugama et al., 2007; Sugama et al., 2013; Mori et al., 2017).
Determination of cytokine /chemokine concentrations using multiplex assays
Rat cytokine/chemokine magnetic bead panels (RECYTMAG-65K, EMD Millipore, Billerica, MA) were used to quantitate values for serum samples and frontal cortex brain tissue lysate using methods described previously (Sanchez-Alavez et al., 2018). The main goal of this measurement was to determine whether there was a correlation between the circulating and the central levels of cytokines, as well as to determine if peripheral measurements can be used as an index of central cytokine levels during ethanol exposure or withdrawal. The frontal cortex was selected as representative for the following reasons: 1) clinical studies in human alcoholic brain had found that neuroimmune signaling was especially affected in that area (Crews et al., 2013; Vetreno et al., 2013), 2) it showed robust microglia activation in our experimental paradigm; and 3) this region is relatively large providing enough tissue for the analysis without the need to pool samples from more than one animal. In brief, the blood and brain samples were mixed with antibody beads and were incubated in wells on a plate shaker at room temperature (RT) for 2 hours. Well contents were then washed, and detection antibodies were added for a 1-hour incubation at room temperature. Samples were treated with streptavidin-phycoerythrin and incubated for a further 30 mins. Following incubation the supernatant was removed, plates washed, and beads re-suspended using sheath fluid/drive fluid/assay buffer. Plates were stored at 4 °C prior to quantification using MAGPIX plate reader.
Levels of the most important pro-inflammatory cytokines known to initiate and sustain inflammation were measured (e.g. interleukin-1 alpha (IL-1α), interleukin-1 beta (IL-1β), interleukin-12p70 (IL-12p70), interleukin-17A (IL-17A) interleukin-18 (IL-18) and tumor necrosis factor (TNF)). Levels of interleukin-4 (IL-4), g interleukin-5 (IL-5), interleukin-10 (IL-10) and interleukin-13 (IL-13), cytokines that can lower inflammation through a reduction in the amount of pro-inflammatory molecules, were also measured. Finally, the chemokines eotaxin, fractalkine, interferon gamma-induced protein-10 (IP-10), lipopolysaccharide-inducible CXC chemokine (LIX), normal T cell expressed and secreted (RANTES) were measured as they have the ability to act as a chemoattractant in the recruitment of leukocytes and can contribute to local inflammatory responses.
Statistical analyses
Data obtained from all measures were analyzed using SPSS (IBM Corp, Armonk, NY) statistical software. The effects of adolescent alcohol vapor exposure (EtOH-exposed vs. control) on chemokine/cytokine expression were analyzed using a nonparametric method (Mann-Whitney), in order to account for potentially non-normal distribution of the data. Data for chemokine/cytokine expression are shown as a percentage of the control animals’ values, with controls set to 100%. Iba-1 immunoreactivity was analyzed using two-factor ANOVA followed by post hoc analyses using ANOVAs. Significance level was set at p<0.05.
Results
Effects of adolescent ethanol exposure on the time course of microglial activation
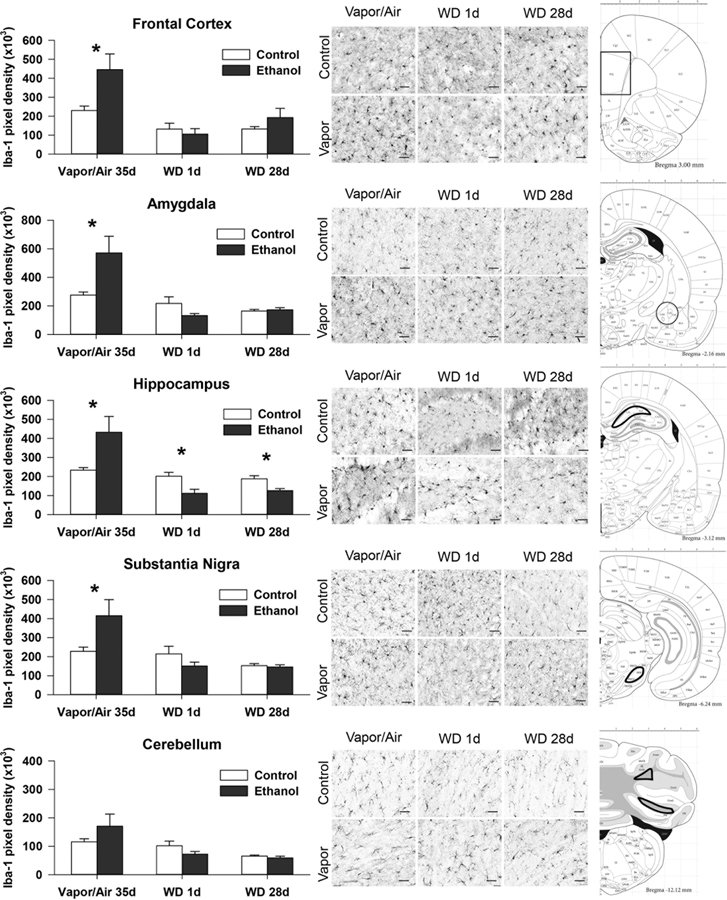
Iba-1 immunoreactivity analysis was accomplished at 3 time points after alcohol exposure: 1) after 35 days of vapor/air exposure (PD58); 2) after 1 day of withdrawal (PD59), and 3) after 28 days of withdrawal (PD86). Tissue from five brain regions were analyzed: amygdala (AMYG), frontal cortex (FCTX), hippocampus (HC), substantia nigra (SN), and cerebellum (CB) (Fig. 1). A significant main effect of adolescent alcohol exposure was only found in the frontal cortex (FCTX: F = 6.2; p = 0.018). A significant main effect of time, however, was seen in all brain regions: (AMYG: F = 19.1; p < 0.00; FCTX: F = 14.8; p < 0.001; HC: F = 18.2, p < 0.001; SN: F = 12.4; p < 0.001; CB: F = 12.9; p < 0.001). Ethanol exposure X time interactions were significant for all of the brain regions investigated (FCTX: F = 4.2; p = 0.024; AMYG: F = 8.7; p = 0.001; HC: F = 11.1, p < 0.001; SN: F = 6.3; p = 0.004; CB: F = 3.6; p = 0.039). Post hoc ANOVAs of Iba-1 density identified significantly higher values in the control vs. the ethanol-exposed group (Table 1). Significant increases in values in FCTX, AMYG, HC, and SN were seen after 35 days of adolescent vapor exposure. Following 1 day and 28 days of withdrawal, Iba-1 density was returned to control levels in all regions except for the hippocampus where Iba-1 intensity in the ethanol group was lower than in the controls at both 1 and 28 days of withdrawal. There were no significant correlations between microglial activation and BEC at time of sacrifice.
Figure 1:
Iba-1 measures of microglia activation and staining are shown for all brain regions collected from adolescent rats exposed to ethanol vapor or air for 35 days (PD58) and two time points of withdrawal (1 and 28 days), PD59 and PD86 respectively. On the left, pixel density of Iba staining is shown. Means and S.E.M. shown for post hoc ANOVA by treatment group. * indicates p<0.05. On the right, representative brain sections are shown for each brain region that were stained with Impact DAB staining, the scale line at the bottom right of each representative section is 50 μm.
Table 1: Iba-1 staining in 5 brain regions.
lba-1 measures (pixel density, x103) of microglia activation across 5 brain sites. Means and S.E.M. shown for post hoc ANOVA by treatment group.
| Control | Ethanol | df | F Stat | p value | |
|---|---|---|---|---|---|
| Vapor/Air 35 Days | Mean ± SEM | Mean ± SEM | |||
| Frontal Cortex | 230.6 ±23.5 | 445.8 ±82.7 | 1,10 | 7.4 | 0.024 |
| Amygdala | 275.9 ±21.6 | 571.5 ±116.0 | 1,12 | 7.3 | 0.02 |
| Hippocampus | 233.7 ±12.6 | 432.4 ±83.1 | 1,12 | 6.5 | 0.026 |
| Substantia Nigra | 228.0 ±22.3 | 415.4 ±84.0 | 1,12 | 5.3 | 0.041 |
| Cerebellum | 115.9 ±10.7 | 170.9 ±42.1 | 1,12 | 2.1 | 0.17 |
| Withdrawal 1 Day | |||||
| Frontal Cortex | 132.3 ±30.7 | 105.6 ±28.8 | 1,13 | 0.3 | 0.54 |
| Amygdala | 217.1 ±46.4 | 132.5 ±13.9 | 1,12 | 3.5 | 0.08 |
| Hippocampus | 201.4 ±20.1 | 111.3 ±21.3 | 1,13 | 9.4 | 0.01 |
| Substantia Nigra | 214.6 ±40.2 | 150.9 ±20.4 | 1,14 | 1.8 | 0.2 |
| Cerebellum | 101.8 ±16.3 | 72.3 ±9.7 | 1,14 | 2.2 | 0.15 |
| Withdrawal 28 days | |||||
| Frontal Cortex | 132.7 ±12.2 | 192.9 ±48.7 | 1,14 | 1.2 | 0.28 |
| Amygdala | 163.6 ±11.6 | 172.5 ±15.3 | 1,15 | 0.2 | 0.65 |
| Hippocampus | 187.8 ±16.4 | 126.2 ±10.4 | 1,13 | 10.9 | 0.006 |
| Substantia Nigra | 153.0 ±10.2 | 145.9 ±11.4 | 1,15 | 0.2 | 0.64 |
| Cerebellum | 65.6 ±3.7 | 59.2 ±6.0 | 1,14 | 0.8 | 0.36 |
Effects of adolescent ethanol exposure on frontal cortex cytokine/chemokines.
Using a multiplex system (Millipore) we measured the relative amounts of the anti-inflammatory cytokines (IL-4, IL-5, IL-10, IL-13), pro- inflammatory cytokines (IL-1α, IL-1β, IL-12, IL-17, IL-18 and TNF-α), and chemokines (fractalkine, eotaxin/CCL11, IP-10, LIX and RANTES) in the frontal cortex.
The results of the adolescent alcohol exposure are presented as a relative percentage of the control group’s mean (control set at 100%) and are shown in table 2. Mann-Whitney nonparametric analysis revealed that the pro-inflammatory cytokine IL-1β (p=0.033) showed a statistically significant increase after 35 days of vapor exposure in the frontal cortex. Other pro- and anti-inflammatory cytokines, and chemokines, did not show significant variation (see table 2). No significant changes were observed for the pro- and anti-inflammatory cytokines or the chemokines 1 day following withdrawal (see table 2). The pro-inflammatory cytokine IL-1β (p=0.019), and the anti-inflammatory cytokine IL-10 (p=0.028) were significantly higher at 28 days of withdrawal in alcohol vapor exposed animals as compared to controls. There were no correlations between cytokine levels in FC and BEC at time of sacrifice.
Table 2: Frontal cortex cytokine/chemokine profile.
Mean levels for vapor-exposed animals presented as a percent of the control values (% ctrl) (reference set at 100%) for each cytokine/chemokine at three time points in the frontal cortex. Non-parametric statistical results from Mann Whitney U (MWU), Z-score (Z), and p value (p) shown. p values less than 0.05 are shown in bold.
| Vapor (35 days) | Withdrawal (1 day) | Withdrawal (28 days) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| % Ctrl | MWU | Z | P | % Ctrl | MWU | Z | P | % Ctrl | MWU | Z | P |
| 86.88 | 13 | −1.039 | 0.299 | 114.05 | 11 | −0.852 | 0.394 | 124.07 | 9 | −1.615 | 0.106 |
| 110.1 | 5.5 | −2.137 | 0.033 | 97.07 | 12 | −0.679 | 0.497 | 131.58 | 4 | −2.342 | 0.019 |
| 97.99 | 4 | −0.258 | 0.796 | 115.09 | 7.5 | −0.707 | 0.48 | 91.08 | 6 | −0.313 | 0.754 |
| 104.01 | 18.5 | −0.22 | 0.826 | 102.59 | 11 | −0.852 | 0.394 | 112.14 | 14 | −0.88 | 0.379 |
| 86.54 | 10 | −1.464 | 0.143 | 100.81 | 14 | −0.34 | 0.734 | 115.09 | 12 | −1.171 | 0.242 |
| ND | ND | ND | |||||||||
| % Ctrl | MWU | Z | P | % Ctrl | MWU | Z | P | % Ctrl | MWU | Z | P |
| 92.79 | 15.5 | −0.663 | 0.507 | 117.71 | 11 | −0.857 | 0.392 | 117.59 | 10 | −1.472 | 0.141 |
| 154.09 | 8 | −1.776 | 0.076 | 96.49 | 15 | −0.174 | 0.862 | 128.72 | 12.5 | −1.115 | 0.265 |
| 114.9 | 7.5 | −1.832 | 0.067 | 104.05 | 12 | −0.679 | 0.497 | 135.53 | 5 | −2.196 | 0.028 |
| 84.31 | 14.5 | −0.851 | 0.395 | 126.31 | 11 | −0.871 | 0.384 | 98.91 | 11 | −0.206 | 0.837 |
| % Ctrl | MWU | Z | P | % Ctrl | MWU | Z | P | % Ctrl | MWU | Z | P |
| ND | ND | ND | |||||||||
| 85.27 | 7 | −1.903 | 0.057 | 115.3 | 14 | −0.34 | 0.734 | 124.27 | 8 | −1.757 | 0.079 |
| 107.67 | 18 | −0.293 | 0.77 | 140.87 | 5 | −1.868 | 0.062 | 131.98 | 10 | −1.464 | 0.143 |
| 99.29 | 14 | −0.183 | 0.855 | 98 | 11.5 | −0.103 | 0.918 | 117.63 | 8.5 | −0.463 | 0.643 |
| 109.12 | 14 | −0.878 | 0.38 | 118.44 | 11 | −0.849 | 0.396 | 108.66 | 13 | −1.025 | 0.306 |
Effects of chronic adolescent alcohol exposure and withdrawal on serum cytokine/chemokine profiles
A pattern of changes was seen in serum cytokine levels over the course of alcohol exposure and withdrawal that differed from the changes seen in frontal cortex. As seen in Table 3, the anti-inflammatory cytokine IL-13 (p=0.046) showed a statistically significant reduction after 35 days of vapor, and the chemokine Fractalkine (p=0.028) showed a significant increase. Other cytokines and the chemokines did not show significant effects (see table 3). Following 24 hours of withdrawal a significant reduction in IL-18 (p=0.042) along with a significant increase in IP-10 (p= 0.027) was found, but no significant changes were observed for other cytokines or chemokines (see table 3). The anti-inflammatory cytokine IL-10 (p=0.042) and the chemokine IP-10 (p=0.019) were found to be elevated in the ethanol vapor exposed animals following 28 days of withdrawal. A correlation between IL-5 (Pearson’s rho: −0.84 p<0.01) Il-17A (Pearson’s rho: −0.7,<0.05) and LIX (Pearson’s rho −0.76, p<0.03).
Table 3: Serum cytokine/chemokine profile.
Mean levels for vapor-exposed animals presented as a percent of the control values (% ctrl) (reference set at 100%) for each cytokine/chemokine at three time points in serum. Non-parametric statistical results from Mann Whitney U (MWU), Z-score (Z), and p value (p) shown. p values less than 0.05 are shown in bold.
| Vapor (35 days) | Withdrawal (1 day) | Withdrawal (28 days) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| % Ctrl | MWU | Z | P | % Ctrl | MWU | Z | P | % Ctrl | MWU | Z | P |
| 68.65 | 10 | −1.472 | 0.141 | 101.22 | 15.5 | −0.087 | 0.931 | 93.88 | 17 | −0.44 | 0.66 |
| 124.73 | 18 | −0.293 | 0.77 | 419.65 | 7 | −1.531 | 0.126 | 145.23 | 7 | −1.903 | 0.057 |
| 91.54 | 14 | −0.88 | 0.379 | 107.1 | 10.5 | −0.939 | 0.348 | 93.5 | 7.5 | −1.835 | 0.067 |
| 72.73 | 13.5 | −0.953 | 0.341 | 101.37 | 15.5 | −0.086 | 0.932 | 109.68 | 14 | −0.878 | 0.38 |
| 138.69 | 16 | −0.586 | 0.558 | 60.56 | 4 | −2.038 | 0.042 | 87.35 | 14 | −0.878 | 0.38 |
| 81.78 | 5.44 | −1.835 | 0.067 | 95.42 | 6.31 | −0.256 | 0.798 | 96.31 | 6.38 | −0.734 | 0.463 |
| % Ctrl | MWU | Z | P | % Ctrl | MWU | Z | P | % Ctrl | MWU | Z | P |
| 87.22 | 9 | −1.642 | 0.101 | 98.04 | 15 | −0.174 | 0.862 | 97.76 | 18.5 | −0.22 | 0.826 |
| 90.13 | 10.5 | −1.393 | 0.164 | 97.53 | 15.5 | −0.085 | 0.932 | 99.98 | 18.5 | −0.22 | 0.826 |
| 116.54 | 15 | 0 | 1 | 201.5 | 4 | −1.89 | 0.073 | 210.69 | 5 | −2.03 | 0.042 |
| 70.51 | 6.5 | −1.993 | 0.046 | 123.56 | 9.5 | −1.124 | 0.261 | 105.45 | 16.5 | −0.519 | 0.604 |
| % Ctrl | MWU | Z | P | % Ctrl | MWU | Z | P | % Ctrl | MWU | Z | P |
| 89.7 | 12 | −1.179 | 0.238 | 102.28 | 14 | −0.346 | 0.729 | 95.7 | 14 | −0.88 | 0.379 |
| 169.09 | 5 | −2.196 | 0.028 | 104.11 | 14 | −0.34 | 0.734 | 101.11 | 18 | −0.293 | 0.77 |
| 139.06 | 19 | −0.146 | 0.884 | 155.85 | 3 | −2.208 | 0.027 | 123.5 | 4 | −2.342 | 0.019 |
| 119.5 | 15 | −0.732 | 0.464 | 100.07 | 15 | −0.17 | 0.865 | 113.54 | 12 | −1.171 | 0.242 |
| 110.39 | 16 | −0.586 | 0.558 | 84.18 | 12 | −0.679 | 0.497 | 122.76 | 13 | −1.025 | 0.306 |
Discussion
Alcohol-mediated dysregulation of the immune system is proposed to be among the potential mechanisms that can affect neuronal maturation or survival and possibly increase the risk for alcohol use disorders (reviewed by (Crews and Vetreno, 2011; Crews et al., 2016; Szabo and Mandrekar, 2009)). Several studies have reported that alcohol can have effects on the level of blood and/or brain cytokines, however, findings appear to also depend on the duration of ethanol exposure, the dosage given, the developmental epoch of exposure, as well as the species and tissue studied (see (Asquith et al., 2014; Crews and Vetreno, 2014)). In the current study we evaluated the effects of adolescent ethanol exposure and withdrawal on microglia activation across the brain. Levels of several chemokines and cytokines in the serum and the frontal cortex were also evaluated. These data provide an index of the systemic effects of alcohol on immune function, as well as an evaluation of the “inflammatory environment” in cerebral cortex, a brain region previously shown to be impacted by alcohol and proposed to contribute to the long-lasting effects of alcohol exposure (He and Crews, 2008; Pascual et al., 2014).
Like most groups, we used the cytoplasmic calcium-binding protein Iba-1 as a marker of microglia activation. Iba1 has actin-crosslinking activity and its expression is associated with motility rearrangement of the actin cytoskeleton that occurs during microglia activation (Ito et al., 1998). Thus, strictly speaking Iba-1 is an index of morphological rather than functional activation. Information on functional activation may be extrapolated by the level of anti- and pro-inflammatory cytokines but only to some extent. In fact, although these molecules are too large to cross an intact blood brain barrier they can be produced by fully activated microglia.
Like for cytokines, the effects of ethanol on microglia activation varies with experimental conditions, animal species, dose and age. In general, the longer the duration of alcohol administration and the higher the dose, the more the brain regions appear to be activated (Chastain and Sarkar, 2014). For example, in adolescent exposure studies, intermittent ethanol administration (EtOH dose: 2 or 3 g/kg, for 2 days a week, three times/day, for a month) has been shown to induce microglia activation in the hippocampal dentate gyrus but not in other brain regions (Ward et al., 2009). In other studies, when ethanol (3 g/kg i.p.) was given for 14 days, for 2 days on and 2 days off, the resulting inflammatory changes were found in both hippocampus and prefrontal cortex (Pascual et al., 2007). Whereas in experiments where even higher ethanol doses were administered (4 days of 5 g/kg i.g., 3× day) microglia infiltration was found in many more brain areas, including the hippocampal dentate gyrus (McClain et al., 2011). Additionally, in a model of intermittent binge alcohol in adolescent female rats, MHC-II expression was also found to be increased (Ward et al., 2009). This suggests that multiple withdrawal episodes may have cumulative effects that cause partially activated microglia to express a more inflammatory phenotype (Crews and Vetreno, 2014; McClain et al., 2011; Montesinos et al., 2016). In the current study we found that chronic exposure of adolescent rats to moderate levels of ethanol vapors, produced a transient increase of microglia activation that returned to basal level within 24 hours with the exception of the hippocampus where it further decreased over time.
Microglia have different states of activation that have distinct morphologies that are triggered by regional inflammation and can also lead to their proliferation, and migration to the inflamed tissue (for review see (Chastain and Sarkar, 2014)). Human postmortem brains obtained from alcoholics and moderately drinking controls have suggested that ethanol causes sensitization through a process where resting microglia become hyper-ramified, bushy, and then amoeboid (Crews and Vetreno, 2016; He and Crews, 2008). Two distinct activated macrophage-like phenotypes have been characterized: the M1 phenotype (associated with secretion of proinflammatory factors and neurotoxic activity) and the M2 phenotype (associated with secretion of anti-inflammatory factors (Marshall et al., 2013). The mechanism underlying microglia activation by alcohol exposure remains unknown but could be caused by a direct effect of alcohol on glia cells or neurons or an indirect action through cytokine signaling (Fernandez-Lizarbe et al., 2009; Qin et al., 2008; Ward et al., 2014). Alcohol induces (HMGB1)/toll-like receptor (TLR) −4 signaling and play an important role in the induction of proinflammatory mediators(Montesinos et al., 2015; Pascual et al., 2015; Vetreno and Crews, 2015). High-mobility group box 1 (HMGB1) is an endogenous signaling molecule that contributes to the induction of NF-kB transcription of IL-1β as well as multiple interleukikn-1/Toll-like receptors (IL-1/TLRs) that ultimately lead to the neuroimmune activation of proinflammatory cytokines (Crews et al., 2013).
In the current study, ethanol vapor exposure was found to induce microglia activation in most brain regions studied (amygdala, frontal cortex, hippocampus, substantia nigra) except in the cerebellum. This indicates that although ethanol can generally effect microglia activation, the mechanisms by which this occur are likely to be subjected to some regional specificity. This may be more pronounced in pre-adolescent rats (P3–P5) (Topper et al., 2015), or in adult rats, although in adults, cerebellar activation occurred to a lower extent than in the other regions investigated (Sanchez-Alavez et al., 2018). Microglia activation appeared to be dependent on the presence of ethanol as levels were comparable to the controls within 24 hours of withdrawal. In the hippocampus, the intensity of Iba-1 staining at both withdrawal time points was lower than the controls. This differs from what seen in adult rats where microglia activation 24 hr after withdrawal Iba-1 staining was still significantly higher than in the controls (Sanchez-Alavez et al., 2018). Thus, the extent of microglia activation in adolescents was lower than in the adults (Sanchez-Alavez et al., 2018), an indication that the microglia of younger animals are more resilient to the effects of alcohol and rapidly return to a non-activated state. It is unlikely that this is due to differences in ethanol metabolism since the blood concentration of alcohol was similar in adolescent and in adults.
Levels of serum cytokines have previously been demonstrated to be elevated especially after the administration of large “binge” doses of alcohol, possibly in concert with the liver, while lower doses can actually reduce cytokine levels (Mandrekar et al., 2006; Szabo and Iracheta-Vellve, 2015). In the current study we found that ethanol significantly altered the serum concentration of two cytokine/chemokines, producing a reduction in the level of IL-13 and an increase in fractalkine. The levels of both cytokine/chemokines returned to baseline within 24 hours of withdrawal. This differs from what was found in adult rats where the same experimental conditions decreased the serum level of all cytokines (pro /anti-inflammatory), as well as those of the chemokines eotaxin, IP-10 and LIX (Sanchez-Alavez et al., 2018). In adolescent rats, withdrawal was found to reduce only IL-18 while increasing IL-10 and IP10. These changes, observed at 24 hours following withdrawal, were still significant 28 days later for IL-10 and IP10. These profiles indicate ethanol-induced changes in cytokines are not identical in adolescent and adult rats and that, like what was observed for Iba-1, ethanol responses seemed to be more modest in the adolescent than in adult animals. Previous comparisons of adolescent and adult neuroimmune responses to ethanol exposure and endotoxin-LPS have also found that adolescents have a blunted neuroimmune response compared to similarly treated adults (Doremus-Fitzwater et al., 2015). These findings may also explain why in alcohol-associated inflammatory liver disease is rarely seen in human adolescents as compared to adult drinkers (Novick et al., 1985; Stone et al., 1968).
In the frontal cortex, the only brain region for which the cytokine profile was investigated, changes were only observed for the anti-inflammatory cytokine IL-10 and the pro-inflammatory cytokine IL-1β. Only IL-1β was significantly increased following adolescent vapor exposure. Whereas the levels of both cytokines were comparable to the controls upon acute withdrawal but was found to be significantly higher after protracted withdrawal at day 28. In adults, alcohol vapors did not elevate IL-10 and elevated the IL-1α member of the IL-1 family whereas withdrawal increased the chemokine fractalkine (Sanchez-Alavez et al., 2018). Fractalkine plays a key role in interactions between glia and neurons and has been demonstrated to be both neuroprotective or neurotoxic depending on the specific immune condition (see (Lauro et al., 2015; Luo et al., 2019))
Blood ethanol concentrations (BEC) at sacrifice after 5 weeks of alcohol exposure were found to correlate with three of the cytokines measured, however, no correlations were found between any frontal cortex cytokine concentrations and BEC. These results indicate that the effects of adolescent alcohol exposure and its withdrawal on cytokine/chemokine levels are not necessarily similar in the periphery and in frontal cortex. This further suggests that evaluating the role of cytokines in mediating the central effects of alcohol cannot be done solely by relying on the changes induced peripherally and measurable in the blood. Cytokines are too large to cross an intact blood brain barrier but can be produced in the CNS. Thus, while it was reasonable to assume that ethanol could affect cytokines similarly in the periphery and in the brain, our data suggest that this may not be the case. Alcohol was reported to affect cytokine levels in brain and in the plasma in different ways depending on the ethanol amount, the mode of administration, the duration as well as on the age of the animals used. We found that chronic exposure to moderate amount of alcohol vapors during adolescence affected IL-1β in the frontal cortex and the plasma primarily during the recovery phase.
Studies using adolescent rats and mouse models (Montesinos et al., 2015; Pascual et al., 2007), have shown that intermittent “binge-like” alcohol treatment may trigger pro-inflammatory cytokines and other mediators in the PFC (Montesinos et al., 2015), as well as reduction and/or loss of neurogenesis in the hippocampus (Vetreno and Crews, 2015). One of the pathways activated by alcohol is the (TLRs)—innate immune receptors that result in the release of inflammatory mediators and neuroinflammation (Alfonso-Loeches et al., 2010). Later it was determined that TLR4- deficient mice (TLR4-KO) (Montesinos et al., 2016) or TLR2-deficient mice (Pascual et al., 2015; Pascual et al., 2018) do not demonstrate these effects in response to ethanol. TLR4-deficient mice are also protected against ethanol-induced synaptic and myelin deficits as well as associated cognitive dysfunction, suggesting a critical role of TLR4 in ethanol- induced neuroinflammation (see (Pascual et al., 2018)). Adolescent alcohol exposure has also been demonstrated to cause persistent increases in multiple pro-inflammatory TLR mRNAs in the adult hippocampus that is accompanied by enhanced expression of the nuclear transcription factor pNF-κB p65 as well as several neuroimmune NF-κB target genes later in adulthood, that can lead to the induction of neuroimmune signaling cascades (e.g., TNFα, MCP-1). It has been additionally suggested that this activation may result in “positive feedback loops” in neuroimmune signaling following adolescent intermittent exposure that could potentially persist well into adulthood (Crews and Vetreno, 2011; Vetreno et al., 2018).
In contrast, studies in adult rodent models show that heavy session drinking (5 g/kg, e.g., BEC: 310 mg/dl ± 8.9) for 1 day vs 10 day regimen induces different patterns of microglia and cytokine pathway induction. A single dose of ethanol was not found to result in elevated blood, liver and brain cytokines, whereas 10 daily doses induced significant increases in TNFα and MCP-1 protein in both brain and liver (Qin et al., 2008). In contrast, (Zahr et al., 2010) found that a single 4-day binge EtOH exposure did not induce the expression of cytokines in either blood or brain. In contrast, adult WT C57BL6 mice who were exposed to ethanol in their drinking water for over 5 months have been shown to have significantly increased levels of chemokines (MCP-1, MIP-1a and CX3CL1 or Fractalkine) and cytokines (IL-1b, IL-17, TNF-a) in the striatum, and increased MCP-1, MIP-1a, CX3CL1 levels in the blood. Additionally, after 24 h of alcohol withdrawal an increase in IFN-g levels were observed in addition to elevated levels of IL-1b, IL-17 and MIP-1a, CX3CL1 in striatum. These changes are not induced in ethanol-treated TLR4-KO and TLR2-KO mice (Pascual et al., 2015).
When the effect of acute high-dose alcohol consumption on blood levels of cytokines was evaluated in alcohol-experienced healthy male adult volunteers (vodka, 4.28 mL/kg), serum levels of IL-1Ra were found to be elevated and the levels of the chemokine MCP-1 decreased acutely followed by a sustained elevation levels of MCP-1, even blood alcohol level had returned to non-detectable levels (Neupane et al., 2016). Taken together these findings indicate that, in the adult, multiple cycles of alcohol exposure (Marshall et al., 2016; Zhao et al., 2013) and/or prior ethanol exposure can produce long-lasting increases in the expression of neuroimmune genes, microglial activation and elevated levels of serum cytokines (Crews and Vetreno, 2016; Zou and Crews, 2010). However, it has also been demonstrated, in rats, that abstinence from alcohol can result in a “rebound” of hippocampal neurogenesis during recovery. It appears that this process is anteceded by microglial proliferation, suggesting the possibility that microglia may facilitate some aspects of brain recovery following alcohol exposure (McClain et al., 2011; Nixon et al., 2008).
The present data, as well as our previous findings in adult rats, also provide clear evidence that while alcohol can alter cytokines levels, such changes are not consistent with a clear pro- or anti-inflammatory phenotype in the periphery or the CNS, instead, individual cytokines may be functioning as independent and distinct neuromodulators. For instance, IL-1β is not only a prototypic inflammatory cytokine but it can increase the phosphorylation of the NMDA receptor NR2B subunit resulting in increased neuronal excitability (Balosso et al., 2008). Similarly, the anti-inflammatory cytokine IL-10 is capable of rapidly regulating GABAergic transmission in dentate gyrus neurons by lowering excitability and changing LTP responses (Almolda et al., 2015; Suryanarayanan et al., 2016). Several interleukins can also be produced by astrocytes, by neurons or by leukocytes that can be driven in the brain by specific chemokines. Thus, alteration of cytokines is an index of inflammatory processes but cannot be attributed exclusively to microglia. In the present study we measured immune molecules in the serum and the brain to determine whether peripheral levels could be used as an index of central inflammation. Experimental evidence indicates that microglia and astrocytes are activated by ethanol and may alter neuronal signaling through interactive pathways (Crews and Vetreno, 2016; Erickson et al., 2018; Kane and Drew, 2016; Wilhelm et al., 2016). It is likely that both astrocytes and microglia release factors that modulate each other’s activation status. Astrocytes may then modulate neuronal signaling through an effect on glutamate and ATP levels. Neurons also release factors such as chemokines and cytokines, that can in turn affect the level of microglial and astrocyte activation (Coleman et al., 2017; Crews et al., 2013; Lawrimore and Crews, 2017; Liddelow et al., 2017). Ethanol can also effect microglia activation by altering the production and the activity of the inflammasome through the JAK/STAT signaling pathway. IL-1β, IL-18, HGMB1 and STAT have all been shown to be affected by ethanol and to alter neuronal excitability (Krueger et al., 2011; Kubota et al., 2001; Zielinski et al., 2017).
Thus, the observed elevation of the level the pro-inflammatory cytokine IL-1β and the anti-inflammatory cytokine IL-10 in the frontal cortex during ethanol exposure and/or withdrawal should perhaps not be interpreted for their opposite action on inflammation but rather for their ability to affect neuronal activity. The reduction or induction of both anti- and pro-inflammatory mediators may result in a disruption in the delicate balance necessary for the optimal neurophysiological actions of immune processes. Such changes may also need to be investigated time and brain region specific manner. There are a number of limitations of the present study that should be considered. The model of alcohol exposure used allowed us to carefully titrate blood levels to simulate the amounts typically used by adolescent binge drinkers (see (Ehlers et al., 2019; Hingson and Zha, 2018; Hingson et al., 2017)), and to cover the entire adolescent development period in the rat. However, it does not represent the range of drinking amounts and frequencies seen in human adolescents. In our study cytokine levels were only measured in frontal cortex limiting our ability to describe effects from other brain areas. Our study also only investigated male rats, sex specific differences have been reported in some neuro-immune mediators following adolescent drinking in mice (Pascual et al., 2017) and in neuro-immune responses to stress (Hudson et al., 2014), response to microglia depletion (Nelson et al., 2017), and alcohol consumption and withdrawal (Silva and Madeira, 2012). However, the data presented here confirm the notion that chronic moderate ethanol exposure during adolescence activates microglia cells and produces neuro-immune activation during exposure and following acute and prolonged withdrawal. Our data also provide evidence to suggest that moderate alcohol exposure can increase or reduce some cytokine levels and increase some chemokines in serum while producing a different profile of cytokine elevations in brain. Investigating this specificity may be important towards understanding how ethanol consumption during adolescence can affect the brain differently than in adults.
Acknowledgments
This work was supported by grants: U01 AA019969 and R01 AA006059 to Cindy L. Ehlers from the National Institute on Alcohol Abuse and Alcoholism (NIAAA). The authors thank Jessica Benedict for her help with manuscript editing and Phil Lau for his statistical expertise.
Footnotes
Conflicts of Interest
None
REFERENCES
- Afshar M, Richards S, Mann D, Cross A, Smith GB, Netzer G, Kovacs E & Hasday J (2015) Acute immunomodulatory effects of binge alcohol ingestion. Alcohol, 49, 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaux-Cantin S, Warnault V, Legastelois R, Botia B, Pierrefiche O, Vilpoux C & Naassila M (2013) Alcohol intoxications during adolescence increase motivation for alcohol in adult rats and induce neuroadaptations in the nucleus accumbens. Neuropharmacology, 67, 521–31. [DOI] [PubMed] [Google Scholar]
- Alboni S, Cervia D, Ross B, Montanari C, Gonzalez AS, Sanchez-Alavez M, Marcondes MC, De Vries D, Sugama S, Brunello N, Blom J, Tascedda F & Conti B (2009) Mapping of the full length and the truncated interleukin-18 receptor alpha in the mouse brain. J Neuroimmunol, 214, 43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfonso-Loeches S, Pascual-Lucas M, Blanco AM, Sanchez-Vera I & Guerri C (2010) Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. J Neurosci, 30, 8285–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almolda B, De Labra C, Barrera I, Gruart A, Delgado-Garcia JM, Villacampa N, Vilella A, Hofer MJ, Hidalgo J, Campbell IL, Gonzalez B & Castellano B (2015) Alterations in microglial phenotype and hippocampal neuronal function in transgenic mice with astrocyte-targeted production of interleukin-10. Brain Behav Immun, 45, 80–97. [DOI] [PubMed] [Google Scholar]
- Amodeo LR, Kneiber D, Wills DN & Ehlers CL (2017) Alcohol drinking during adolescence increases consumptive responses to alcohol in adulthood in Wistar rats. Alcohol, 59, 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodeo LR, Wills DN, Sanchez-Alavez M, Nguyen W, Conti B & Ehlers CL (2018) Intermittent voluntary ethanol consumption combined with ethanol vapor exposure during adolescence increases drinking and alters other behaviors in adulthood in female and male rats. Alcohol, 73, 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asquith M, Pasala S, Engelmann F, Haberthur K, Meyer C, Park B, Grant KA & Messaoudi I (2014) Chronic ethanol consumption modulates growth factor release, mucosal cytokine production, and microRNA expression in nonhuman primates. Alcohol Clin Exp Res, 38, 980–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balosso S, Maroso M, Sanchez-Alavez M, Ravizza T, Frasca A, Bartfai T & Vezzani A (2008) A novel non-transcriptional pathway mediates the proconvulsive effects of interleukin-1beta. Brain, 131, 3256–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadwater MA, Lee SH, Yu Y, Zhu H, Crews FT, Robinson DL & Shih YI (2018) Adolescent alcohol exposure decreases frontostriatal resting-state functional connectivity in adulthood. Addict Biol, 23, 810–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chastain LG & Sarkar DK (2014) Role of microglia in regulation of ethanol neurotoxic action. Int Rev Neurobiol, 118, 81–103. [DOI] [PubMed] [Google Scholar]
- Coleman LG Jr., Zou J& Crews FT (2017) Microglial-derived miRNA let-7 and HMGB1 contribute to ethanol-induced neurotoxicity via TLR7. J Neuroinflammation, 14, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT (2008) Alcohol-related neurodegeneration and recovery: mechanisms from animal models. Alcohol Res Health, 31, 377–88. [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Bechara R, Brown LA, Guidot DM, Mandrekar P, Oak S, Qin L, Szabo G, Wheeler M & Zou J (2006) Cytokines and alcohol. Alcohol Clin Exp Res, 30, 720–30. [DOI] [PubMed] [Google Scholar]
- Crews FT, Lawrimore CJ, Walter TJ & Coleman LG Jr. (2017) The role of neuroimmune signaling in alcoholism. Neuropharmacology, 122, 56–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Qin L, Sheedy D, Vetreno RP & Zou J (2013) High mobility group box 1/Toll-like receptor danger signaling increases brain neuroimmune activation in alcohol dependence. Biol Psychiatry, 73, 602–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Robinson DL, Chandler LJ, Ehlers CL, Mulholland PJ, Pandey SC, Rodd ZA, Spear LP, Swartzwelder HS & Vetreno RP (2019) Mechanisms of persistent neurobiological changes following adolescent alcohol exposure. Alcoholism: Clinical and Experimental Research, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Sarkar DK, Qin L, Zou J, Boyadjieva N & Vetreno RP (2015) Neuroimmune Function and the Consequences of Alcohol Exposure. Alcohol Res, 37, 331–41, 344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT & Vetreno RP (2011) Addiction, adolescence, and innate immune gene induction. Front Psychiatry, 2, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT & Vetreno RP (2014) Neuroimmune basis of alcoholic brain damage. Int Rev Neurobiol, 118, 315–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT & Vetreno RP (2016) Mechanisms of neuroimmune gene induction in alcoholism. Psychopharmacology (Berl), 233, 1543–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Vetreno RP, Broadwater MA & Robinson DL (2016) Adolescent Alcohol Exposure Persistently Impacts Adult Neurobiology and Behavior. Pharmacol Rev, 68, 1074–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DA, Goldstein RB, Chou SP, Ruan WJ & Grant BF (2008) Age at first drink and the first incidence of adult-onset DSM-IV alcohol use disorders. Alcohol Clin Exp Res, 32, 2149–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Timary P, Starkel P, Delzenne NM & Leclercq S (2017) A role for the peripheral immune system in the development of alcohol use disorders? Neuropharmacology, 122, 148–160. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Buck HM, Bordner K, Richey L, Jones ME & Deak T (2014) Intoxication- and withdrawal-dependent expression of central and peripheral cytokines following initial ethanol exposure. Alcohol Clin Exp Res, 38, 2186–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Gano A, Paniccia JE & Deak T (2015) Male adolescent rats display blunted cytokine responses in the CNS after acute ethanol or lipopolysaccharide exposure. Physiol Behav, 148, 131–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Paniccia JE, Gano A, Vore AS & Deak T (2018) Differential effects of acute versus chronic stress on ethanol sensitivity: Evidence for interactions on both behavioral and neuroimmune outcomes. Brain Behav Immun, 70, 141–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Chaplin RI, Wall TL, Lumeng L, Li TK, Owens MJ & Nemeroff CB (1992) Corticotropin releasing factor (CRF): studies in alcohol preferring and non-preferring rats. Psychopharmacology (Berl), 106, 359–364. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Criado JR, Wills DN, Liu W & Crews FT (2011) Periadolescent ethanol exposure reduces adult forebrain ChAT+IR neurons: correlation with behavioral pathology. Neuroscience, 199, 333–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Liu W, Wills DN & Crews FT (2013a) Periadolescent ethanol vapor exposure persistently reduces measures of hippocampal neurogenesis that are associated with behavioral outcomes in adulthood. Neuroscience, 244, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Oguz I, Budin F, Wills DN & Crews FT (2013b) Peri-adolescent ethanol vapor exposure produces reductions in hippocampal volume that are correlated with deficits in prepulse inhibition of the startle. Alcohol Clin Exp Res, 37, 1466–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Phillips E, Wills D, Benedict J & Sanchez-Alavez M (2019) Phase locking of event-related oscillations is decreased in both young adult humans and rats with a history of adolescent alcohol exposure. Addict Biol, In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Slutske WS, Gilder DA, Lau P & Wilhelmsen KC (2006) Age at first intoxication and alcohol use disorders in Southwest California Indians. Alcohol Clin Exp Res, 30, 1856–65. [DOI] [PubMed] [Google Scholar]
- Erickson EK, Farris SP, Blednov YA, Mayfield RD & Harris RA (2018) Astrocyte-specific transcriptome responses to chronic ethanol consumption. Pharmacogenomics J, 18, 578–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Lizarbe S, Pascual M & Guerri C (2009) Critical role of TLR4 response in the activation of microglia induced by ethanol. J Immunol, 183, 4733–44. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL & Thompson PM (2004) Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A, 101, 8174–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Quintela A, Dominguez-Santalla MJ, Perez LF, Vidal C, Lojo S & Barrio E (2000) Influence of acute alcohol intake and alcohol withdrawal on circulating levels of IL-6, IL-8, IL-10 and IL-12. Cytokine, 12, 1437–40. [DOI] [PubMed] [Google Scholar]
- Guerri C & Pascual M (2018) Impact of neuroimmune activation induced by alcohol or drug abuse on adolescent brain development. Int J Dev Neurosci. [DOI] [PubMed] [Google Scholar]
- He J & Crews FT (2008) Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp Neurol, 210, 349–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriques JF, Portugal CC, Canedo T, Relvas JB, Summavielle T & Socodato R (2018) Microglia and alcohol meet at the crossroads: Microglia as critical modulators of alcohol neurotoxicity. Toxicol Lett, 283, 21–31. [DOI] [PubMed] [Google Scholar]
- Hermens DF & Lagopoulos J (2018) Binge Drinking and the Young Brain: A Mini Review of the Neurobiological Underpinnings of Alcohol-Induced Blackout. Front Psychol, 9, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingson RW, Heeren T & Winter MR (2006) Age at drinking onset and alcohol dependence: age at onset, duration, and severity. Arch. Pediatr. Adolesc. Med, 160, 739–746. [DOI] [PubMed] [Google Scholar]
- Hingson RW & Zha W (2018) Binge Drinking Above and Below Twice the Adolescent Thresholds and Health-Risk Behaviors. Alcohol Clin Exp Res, 42, 904–913. [DOI] [PubMed] [Google Scholar]
- Hingson RW, Zha W & White AM (2017) Drinking Beyond the Binge Threshold: Predictors, Consequences, and Changes in the U.S. Am J Prev Med, 52, 717–727. [DOI] [PubMed] [Google Scholar]
- Hudson SP, Jacobson-Pick S & Anisman H (2014) Sex differences in behavior and pro-inflammatory cytokine mRNA expression following stressor exposure and re-exposure. Neuroscience, 277, 239–49. [DOI] [PubMed] [Google Scholar]
- Hwang BH, Zhang JK, Ehlers CL, Lumeng L & Li TK (1999) Innate differences of neuropeptide Y (NPY) in hypothalamic nuclei and central nucleus of the amygdala between selectively bred rats with high and low alcohol preference. Alcohol Clin. Exp. Res, 23, 1023–1030. [PubMed] [Google Scholar]
- Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y & Kohsaka S (1998) Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Res Mol Brain Res, 57, 1–9. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’malley PM, Bachman JG & Schulenberg JE (2009) Monitoring the Future national results on adolescent drug use: Overview of key findings, 2008, Bethesda, Md., National Institute on Drug Abuse, U.S. Dept. of Health and Human Services, National Institutes of Health. [Google Scholar]
- Kane CJ & Drew PD (2016) Inflammatory responses to alcohol in the CNS: nuclear receptors as potential therapeutics for alcohol-induced neuropathologies. J Leukoc Biol, 100, 951–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly A, Lynch A, Vereker E, Nolan Y, Queenan P, Whittaker E, O’neill LA & Lynch MA (2001) The anti-inflammatory cytokine, interleukin (IL)-10, blocks the inhibitory effect of IL-1 beta on long term potentiation. A role for JNK. J Biol Chem, 276, 45564–72. [DOI] [PubMed] [Google Scholar]
- Krueger JM, Clinton JM, Winters BD, Zielinski MR, Taishi P, Jewett KA & Davis CJ (2011) Involvement of cytokines in slow wave sleep. Prog Brain Res, 193, 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota T, Fang J, Brown RA & Krueger JM (2001) Interleukin-18 promotes sleep in rabbits and rats. Am J Physiol Regul Integr Comp Physiol, 281, R828–38. [DOI] [PubMed] [Google Scholar]
- Lauro C, Catalano M, Trettel F & Limatola C (2015) Fractalkine in the nervous system: neuroprotective or neurotoxic molecule? Ann N Y Acad Sci, 1351, 141–8. [DOI] [PubMed] [Google Scholar]
- Lawrimore CJ & Crews FT (2017) Ethanol, TLR3, and TLR4 Agonists Have Unique Innate Immune Responses in Neuron-Like SH-SY5Y and Microglia-Like BV2. Alcohol Clin Exp Res, 41, 939–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Munch AE, Chung WS, Peterson TC, Wilton DK, Frouin A, Napier BA, Panicker N, Kumar M, Buckwalter MS, Rowitch DH, Dawson VL, Dawson TM, Stevens B & Barres BA (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature, 541, 481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo P, Chu SF, Zhang Z, Xia CY & Chen NH (2019) Fractalkine/CX3CR1 is involved in the cross-talk between neuron and glia in neurological diseases. Brain Res Bull, 146, 12–21. [DOI] [PubMed] [Google Scholar]
- Maldonado-Devincci AM, Badanich KA & Kirstein CL (2010) Alcohol during adolescence selectively alters immediate and long-term behavior and neurochemistry. Alcohol, 44, 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrekar P, Catalano D, White B & Szabo G (2006) Moderate alcohol intake in humans attenuates monocyte inflammatory responses: inhibition of nuclear regulatory factor kappa B and induction of interleukin 10. Alcohol Clin Exp Res, 30, 135–9. [DOI] [PubMed] [Google Scholar]
- Marshall SA, Geil CR & Nixon K (2016) Prior Binge Ethanol Exposure Potentiates the Microglial Response in a Model of Alcohol-Induced Neurodegeneration. Brain Sci, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall SA, Mcclain JA, Kelso ML, Hopkins DM, Pauly JR & Nixon K (2013) Microglial activation is not equivalent to neuroinflammation in alcohol-induced neurodegeneration: The importance of microglia phenotype. Neurobiol Dis, 54, 239–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcclain JA, Morris SA, Deeny MA, Marshall SA, Hayes DM, Kiser ZM & Nixon K (2011) Adolescent binge alcohol exposure induces long-lasting partial activation of microglia. Brain Behav Immun, 25 Suppl 1, S120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JW, Naimi TS, Brewer RD & Jones SE (2007) Binge drinking and associated health risk behaviors among high school students. Pediatrics, 119, 76–85. [DOI] [PubMed] [Google Scholar]
- Montesinos J, Alfonso-Loeches S & Guerri C (2016) Impact of the Innate Immune Response in the Actions of Ethanol on the Central Nervous System. Alcohol Clin Exp Res, 40, 2260–2270. [DOI] [PubMed] [Google Scholar]
- Montesinos J, Pascual M, Pla A, Maldonado C, Rodriguez-Arias M, Minarro J & Guerri C (2015) TLR4 elimination prevents synaptic and myelin alterations and long-term cognitive dysfunctions in adolescent mice with intermittent ethanol treatment. Brain Behav Immun, 45, 233–44. [DOI] [PubMed] [Google Scholar]
- Mori S, Maher P & Conti B (2016) Neuroimmunology of the Interleukins 13 and 4. Brain Sci, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Sugama S, Nguyen W, Michel T, Sanna MG, Sanchez-Alavez M, Cintron-Colon R, Moroncini G, Kakinuma Y, Maher P & Conti B (2017) Lack of interleukin-13 receptor alpha1 delays the loss of dopaminergic neurons during chronic stress. J Neuroinflammation, 14, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison BE, Marcondes MC, Nomura DK, Sanchez-Alavez M, Sanchez-Gonzalez A, Saar I, Kim KS, Bartfai T, Maher P, Sugama S & Conti B (2012) Cutting edge: IL-13Ralpha1 expression in dopaminergic neurons contributes to their oxidative stress-mediated loss following chronic peripheral treatment with lipopolysaccharide. J Immunol, 189, 5498–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson LH, Warden S & Lenz KM (2017) Sex differences in microglial phagocytosis in the neonatal hippocampus. Brain Behav Immun, 64, 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neupane SP, Skulberg A, Skulberg KR, Aass HC & Bramness JG (2016) Cytokine Changes following Acute Ethanol Intoxication in Healthy Men: A Crossover Study. Mediators Inflamm, 2016, 3758590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen-Louie TT, Simmons AN, Squeglia LM, Alejandra Infante M, Schacht JP & Tapert SF (2018) Earlier alcohol use onset prospectively predicts changes in functional connectivity. Psychopharmacology (Berl), 235, 1041–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon K, Kim DH, Potts EN, He J & Crews FT (2008) Distinct cell proliferation events during abstinence after alcohol dependence: microglia proliferation precedes neurogenesis. Neurobiol Dis, 31, 218–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick DM, Enlow RW, Gelb AM, Stenger RJ, Fotino M, Winter JW, Yancovitz SR, Schoenberg MD & Kreek MJ (1985) Hepatic cirrhosis in young adults: association with adolescent onset of alcohol and parenteral heroin abuse. Gut, 26, 8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Balino P, Aragon CM & Guerri C (2015) Cytokines and chemokines as biomarkers of ethanol-induced neuroinflammation and anxiety-related behavior: role of TLR4 and TLR2. Neuropharmacology, 89, 352–9. [DOI] [PubMed] [Google Scholar]
- Pascual M, Blanco AM, Cauli O, Minarro J & Guerri C (2007) Intermittent ethanol exposure induces inflammatory brain damage and causes long-term behavioural alterations in adolescent rats. Eur J Neurosci, 25, 541–50. [DOI] [PubMed] [Google Scholar]
- Pascual M, Montesinos J & Guerri C (2018) Role of the innate immune system in the neuropathological consequences induced by adolescent binge drinking. J Neurosci Res, 96, 765–780. [DOI] [PubMed] [Google Scholar]
- Pascual M, Montesinos J, Marcos M, Torres JL, Costa-Alba P, Garcia-Garcia F, Laso FJ & Guerri C (2017) Gender differences in the inflammatory cytokine and chemokine profiles induced by binge ethanol drinking in adolescence. Addict Biol, 22, 1829–1841. [DOI] [PubMed] [Google Scholar]
- Pascual M, Pla A, Minarro J & Guerri C (2014) Neuroimmune activation and myelin changes in adolescent rats exposed to high-dose alcohol and associated cognitive dysfunction: a review with reference to human adolescent drinking. Alcohol Alcohol, 49, 187–92. [DOI] [PubMed] [Google Scholar]
- Paxinos G & Watson C (1986) The rat brain in stereotaxic coordinates, Sydney, Australia, Academic Press. [Google Scholar]
- Peng H, Geil Nickell CR, Chen KY, Mcclain JA & Nixon K (2017) Increased expression of M1 and M2 phenotypic markers in isolated microglia after four-day binge alcohol exposure in male rats. Alcohol, 62, 29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preibisch S, Saalfeld S & Tomancak P (2009) Globally optimal stitching of tiled 3D microscopic image acquisitions. Bioinformatics, 25, 1463–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, He J, Hanes RN, Pluzarev O, Hong JS & Crews FT (2008) Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. J Neuroinflammation, 5, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Alavez M, Nguyen W, Mori S, Wills DN, Otero D, Ehlers CL & Conti B (2018) Time course of microglia activation and brain and blood cytokine/chemokine levels following chronic ethanol exposure and protracted withdrawal in rats. Alcohol, 76, 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva SM & Madeira MD (2012) Effects of chronic alcohol consumption and withdrawal on the response of the male and female hypothalamic-pituitary-adrenal axis to acute immune stress. Brain Res, 1444, 27–37. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ (2002) Altered EEG responses to ethanol in adult rats exposed to ethanol during adolescence. Alcohol Clin Exp Res, 26, 246–54. [PubMed] [Google Scholar]
- Spear LP (2016) Consequences of adolescent use of alcohol and other drugs: Studies using rodent models. Neurosci Biobehav Rev, 70, 228–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP (2018) Effects of adolescent alcohol consumption on the brain and behaviour. Nat Rev Neurosci, 19, 197–214. [DOI] [PubMed] [Google Scholar]
- Spear LP & Swartzwelder HS (2014) Adolescent alcohol exposure and persistence of adolescent-typical phenotypes into adulthood: a mini-review. Neurosci Biobehav Rev, 45, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone WD, Islam NR & Paton A (1968) The natural history of cirrhosis. Experience with an unselected group of patients. Q J Med, 37, 119–32. [PubMed] [Google Scholar]
- Sugama S, Fujita M, Hashimoto M & Conti B (2007) Stress induced morphological microglial activation in the rodent brain: involvement of interleukin-18. Neuroscience, 146, 1388–99. [DOI] [PubMed] [Google Scholar]
- Sugama S, Takenouchi T, Fujita M, Kitani H, Conti B & Hashimoto M (2013) Corticosteroids limit microglial activation occurring during acute stress. Neuroscience, 232, 13–20. [DOI] [PubMed] [Google Scholar]
- Suryanarayanan A, Carter JM, Landin JD, Morrow AL, Werner DF & Spigelman I (2016) Role of interleukin-10 (IL-10) in regulation of GABAergic transmission and acute response to ethanol. Neuropharmacology, 107, 181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo G & Iracheta-Vellve A (2015) Inflammasome activation in the liver: Focus on alcoholic and non-alcoholic steatohepatitis. Clin Res Hepatol Gastroenterol, 39 Suppl 1, S18–23. [DOI] [PubMed] [Google Scholar]
- Szabo G & Lippai D (2014) Converging actions of alcohol on liver and brain immune signaling. Int Rev Neurobiol, 118, 359–80. [DOI] [PubMed] [Google Scholar]
- Szabo G & Mandrekar P (2009) A recent perspective on alcohol, immunity, and host defense. Alcohol Clin Exp Res, 33, 220–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo G & Saha B (2015) Alcohol’s Effect on Host Defense. Alcohol Res, 37, 159–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topper LA, Baculis BC & Valenzuela CF (2015) Exposure of neonatal rats to alcohol has differential effects on neuroinflammation and neuronal survival in the cerebellum and hippocampus. J Neuroinflammation, 12, 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetreno RP & Crews FT (2015) Binge ethanol exposure during adolescence leads to a persistent loss of neurogenesis in the dorsal and ventral hippocampus that is associated with impaired adult cognitive functioning. Front Neurosci, 9, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetreno RP, Lawrimore CJ, Rowsey PJ & Crews FT (2018) Persistent Adult Neuroimmune Activation and Loss of Hippocampal Neurogenesis Following Adolescent Ethanol Exposure: Blockade by Exercise and the Anti-inflammatory Drug Indomethacin. Front Neurosci, 12, 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetreno RP, Qin L & Crews FT (2013) Increased receptor for advanced glycation end product expression in the human alcoholic prefrontal cortex is linked to adolescent drinking. Neurobiol Dis, 59, 52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward RJ, Colivicchi MA, Allen R, Schol F, Lallemand F, De Witte P, Ballini C, Corte LD & Dexter D (2009) Neuro-inflammation induced in the hippocampus of ‘binge drinking’ rats may be mediated by elevated extracellular glutamate content. J Neurochem, 111, 1119–28. [DOI] [PubMed] [Google Scholar]
- Ward RJ, Lallemand F & De Witte P (2014) Influence of adolescent heavy session drinking on the systemic and brain innate immune system. Alcohol Alcohol, 49, 193–7. [DOI] [PubMed] [Google Scholar]
- Wilhelm CJ, Hashimoto JG, Roberts ML, Bloom SH, Andrew MR & Wiren KM (2016) Astrocyte Dysfunction Induced by Alcohol in Females but Not Males. Brain Pathol, 26, 433–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, Luong R, Sullivan EV & Pfefferbaum A (2010) Measurement of serum, liver, and brain cytokine induction, thiamine levels, and hepatopathology in rats exposed to a 4-day alcohol binge protocol. Alcohol Clin Exp Res, 34, 1858–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YN, Wang F, Fan YX, Ping GF, Yang JY & Wu CF (2013) Activated microglia are implicated in cognitive deficits, neuronal death, and successful recovery following intermittent ethanol exposure. Behav Brain Res, 236, 270–82. [DOI] [PubMed] [Google Scholar]
- Zielinski MR, Gerashchenko D, Karpova SA, Konanki V, Mccarley RW, Sutterwala FS, Strecker RE & Basheer R (2017) The NLRP3 inflammasome modulates sleep and NREM sleep delta power induced by spontaneous wakefulness, sleep deprivation and lipopolysaccharide. Brain Behav Immun, 62, 137–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J & Crews F (2010) Induction of innate immune gene expression cascades in brain slice cultures by ethanol: key role of NF-kappaB and proinflammatory cytokines. Alcohol Clin Exp Res, 34, 777–89. [DOI] [PubMed] [Google Scholar]