Abstract
Foxp3+T-regulatory (Treg) cells control autoimmune response by suppressing proliferation and effector functions of self-reactive Foxp3-CD4+/CD8+ T (Teff) cells and thereby maintain the critical balance between self-tolerance and autoimmunity. Earlier, we have shown that OX40L-JAG1 co-signaling mediated through their cognate receptors OX40 and Notch3 preferentially expressed on murine Tregs can selectively induce their proliferation in the absence of TCR stimulation. However, the differential molecular mechanisms regulating TCR-independent vs TCR-dependent Treg proliferation and lineage stability of the expanded Tregs remained unknown. Herein, we show that OX40L-JAG1 treatment induced TCR-independent proliferation of Tregs in the thymus and periphery. The use of Src kinase inhibitor permitted us to demonstrate selective inhibition of TCR-dependent T-cell proliferation with little to no effect on OX40L-JAG1 induced TCR-independent Treg expansion in vitro, which was critically dependent on non-canonical NF-kB signaling. OX40L-JAG1 expanded Tregs showed sustained lineage stability as indicated by stable demethylation marks in Treg signature genes such as Foxp3, Il2ra, Ctla4, Ikzf2, and Ikzf4. Furthermore, OX40L-JAG1 treatment significantly increased CTLA4+ and TIGIT+ Tregs and ameliorated experimental autoimmune thyroiditis in mice. Relevance of our findings to humans became apparent when human OX40L and JAG1 induced TCR-independent selective expansion of human Tregs in thymocyte cultures, and increased human Tregs in the liver tissue of humanized NSG mice. Our findings suggest that OX40L-JAG1-induced TCR-independent Treg proliferation is a conserved mechanism that can be used to expand lineage stable Tregs to treat autoimmune diseases.
Keywords: OX40L, JAG1, Treg, Foxp3, NF-κB2, thyroiditis
Introduction
CD4+Foxp3+T-regulatory (Treg) cells control autoimmune response by suppressing proliferation and/or effector functions of self-reactive CD4+/CD8+ T cells, B cells, NK cells, and APCs, and thereby maintain self-tolerance (1, 2). Reduced Treg numbers and functions have been noted in many autoimmune diseases including type-1 diabetes (T1D) and autoimmune thyroiditis and enhancing functional Foxp3+ Tregs has been shown to ameliorate these autoimmune diseases (3, 4). Despite conferring protection against autoimmune diseases (3, 5, 6), the tendency of Tregs to lose lineage stability and morph into pathogenic Teff cells (“ex-Foxp3” cells) remains poorly understood and thus impedes clinical translation (7, 8). Treg-specific DNA demethylation (TSDR) at Foxp3 gene locus allows for the constitutive expression of Foxp3, which is essential for the repression of TCR activation-induced expression of inflammatory genes like Ifn-g and Il-2 in Tregs (9). Additionally, Foxp3 expression alone is insufficient for optimal Treg function. CpG hypomethylation of Il2ra (Cd25), Ctla4, Tnfrsf18 (Gitr) and Ikzf4 (Eos) gene loci in nTregs represent a Foxp3-independent nTreg signature(8). Constitutive expression of these genes along with Foxp3 determines the lineage stability and optimum function of Tregs, and loss/reduced expression of these genes in Tregs can lead to impaired suppressive function (9).
Treg proliferation can occur through two different mechanisms: 1) Antigen/TCR-dependent proliferation, 2) Antigen/TCR-independent proliferation. Of these, TCR-dependent Treg proliferation is the most widely studied mechanism which requires two signals for proliferation. Recognition of MHC-bound antigenic peptides presented on APCs by the cognate TCRs expressed on the surface of Tregs acts as the primary activation signal. The secondary signal is provided by the interaction between co-stimulatory ligands such as CD80/CD86 expressed on APCs with their cognate receptors such as CD28 on Tregs (10). On the contrary, we and others have shown that Treg proliferation can be induced through an antigen/TCR-independent, but IL-2 dependent, mechanism by co-culturing T-cells with GM-CSF derived bone-marrow derived dendritic cells (G-BMDCs) (11, 12). Further, we identified that co-signaling through two membrane-bound ligands namely, OX40L, which belongs to the TNFRSF, and Jagged (JAG)-1, which belongs to Notch family ligands, expressed on G-BMDCs is required and sufficient to cause TCR-independent Treg proliferation (13).
The most commonly used ex vivo Treg expansion protocols rely on TCR-dependent mechanism and use αCD3 and αCD28 monoclonal antibodies (mAbs) to provide antigen receptor crosslinking and co-stimulatory signal. Although this is an effective approach for expanding Tregs, it can also cause concomitant proliferation of Teff cells due to ubiquitous expression of CD3 and CD28 receptors on both Foxp3+ Tregs and Foxp3- conventional T (Tconv)-cells, limiting it’s in vivo application (14, 15). In contrast, we found that OX40L-JAG1 induced TCR-independent Treg proliferation to be selective due to the preferential/constitutive expression of their cognate receptors OX40 and Notch3 on Tregs over Tconv cells. More importantly, soluble OX40L and JAG1 co-treatment induced selective proliferation Tregs from NOD mice ex vivo, increased Tregs in vivo and delayed the onset of diabetes suggesting the potential utility of this approach to expand Tregs for treating T1D (16). However, the signaling involved in TCR-dependent vs TCR-independent Treg proliferation remained poorly understood and a better understanding of differential signaling, if it exists, might aid in selectively inhibiting TCR-dependent cell proliferation while permitting TCR-independent Treg proliferation. During TCR-dependent Treg expansion in vitro, repeated TCR-stimulation can induce cell-cycle dependent recruitment of DNA Methyl Transferase (DNMT)-1 which facilitates methylation of the TSDR leading to loss of Foxp3 expression in a subset of Tregs and give rise to pathogenic “exFoxp3” cells producing inflammatory cytokines (17). We speculated that the signaling required for Treg expansion and the epigenetic changes induced by OX40L-JAG1 might be different from that noted in TCR-dependent Treg proliferation. Additionally, the suitability of this approach to treat other autoimmune diseases and expand human Tregs remained to be determined.
In the present study, we showed that OX40L-JAG1 can induce proliferation of lineage stable Tregs in vivo; the TCR-dependent vs TCR-independent Treg proliferation were driven by distinct signaling pathways; OX40L-JAG1 treatment could prevent experimental autoimmune thyroiditis (EAT) in mice; and OX40L-JAG1 induced Treg expansion is conserved in humans.
Materials and methods
Animals, human tissues and antibodies
C57BL/6J (Stock # 000664), NOD/ShiLtJ (Stock # 001976), OX40−/− (Stock # 012838), Foxp3.eGFP mice (Stock # 018628), CBA/J (Stock # 000656) and NSG (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ, stock # 005557) mice were purchased from Jackson Laboratories. Breeding colonies were established and maintained in a pathogen-free facility of the biological resources laboratory (BRL) of the University of Illinois at Chicago (Chicago, IL). All animal experiments were approved and performed in accordance with the guidelines set forth by the Animal Care and Use Committee at the University of Illinois at Chicago.
Normal pediatric thymus tissues (n=16) were obtained through the Cooperative Human Tissue Network (CHTN), Ohio State University in accordance with the policies stated by the University of Illinois at Chicago Institutional Review Board. CHTN is funded by the National Cancer Institute and other investigators may have received specimens from the same subjects.
Antibodies and Reagents
Anti-mouse CD4 (Clone # GK1.5), anti-mouse CD8a (53.6.7), Anti-mouse CD25 (PC61.5), Anti mouse/Rat Foxp3 (FJK16S), anti- mouse Nur77 (12.14), Anti-mouse CD134/OX40 (OX86), Anti-human CD4 (RTA-T4), Anti-human CD8a (SK1), Anti-human FOXP3 (236A/E7 and PCH101), Anti-human CD25 (BC96), Anti-human CD134/OX40 (ACT35), anti-human CTLA4 (14D3), anti-human GITR (eBioAITR), anti-human TIGIT (MBSA43), anti-mouse/human Helios (22F6), Anti-mouse CD11c (N418), Anti-mouse CD39 (24DMS1), anti-mouse F4/80 (BM8), anti-mouse B220 (RA3–6B2), Anti-mouse TIGIT (GIGD7), Anti-mouse IFN-γ (XMG1.2), Anti-mouse OX40 (OX86), Anti-mouse GITR (DTA1), Anti-mouse/RAT IL17A (EBIO17B7), Anti-mouse IL4 (11B11), Anti-mouse CTLA-4 (UC10–4B9), Anti-mouse CCR4 (2G12), anti-human CD45 (HI30), and appropriate isotype control antibodies were purchased from eBioscience, Thermo Fisher Scientific. Anti-Bcl2 (BCL/10C4), anti-human/mouse Ki67 (11F6), anti-mouse CD69 (H1.2F3), anti-mouse PD1 (29F.1A12), anti-mouse LAG-3 (C9B7W), anti-mouse Tim-3 (B8.2C12), anti-human CD45RA (HI100) were purchased from Biolegend Inc. CD4+/CD4+CD25+ EasySep T-cell isolation kits were from StemCell Technologies. Mouse recombinant OX40L-Fc was provided by Dr. Alan L Epstein (Keck School of Medicine, LA). Mouse recombinant Jagged-1-Fc was produced from stable CHO(r) cells expressing soluble JAG1 as described previously (18). Human recombinant OX40L-Fc and Jagged-1-Fc were from Sino Biologicals. T-cells were cultured in PRIME-XV® T Cell Expansion XSFM medium (Irvine Scientific). Purified porcine thyroglobulin was from Sigma. Mouse anti-CD3 (Clone # 2C11) and anti-CD28 (Clone # PV10) were purchased from Bioxcell. Human and mouse recombinant IL-2 from eBioscience -Thermo Fisher. Porcine thyroglobulin was from Sigma-Aldrich. Enzo screewell kinase assay library was provided by High-Throughput Screening Core, Research resource center, UIC. Compounds PP1, R406, AG-126 were from Selleckchem. Inhibitor of NF-kB inducible kinase (NIK) was generously gifted by Dr. Nico Ghilradi, Genentech.
T-cell proliferation assays and thymic organ culture
Spleen from 6–8-week old mice was excised, single cell suspensions were prepared and CD4+/CD4+CD25-/CD4+CD25+ T-cells were isolated according to the Manufacturer’s (StemCell Technologies) protocol. Isolated cells were stained with Cell-Trace Violet (Life technologies) and treated with IL-2 (25U/ml), recombinant OX40L-Fc (5μg/ml), JAG1-Fc (1 μg/ml), αCD3/CD28 monoclonal antibodies (mAbs) (2 μg/ml each) for indicated duration. For kinase inhibition assays, cells were pre-treated with inhibitors for 2h and then stimulated with either OX40L-JAG1 or anti-CD3/CD28 for 4 days. Human thymuses were dissected into ~2mm3 fragments and cultured over Matrigel (Corning) in 24 well transwell plates with PRIME-XV® T Cell Expansion XSFM medium in the presence of IL-2, human OX40L-Fc and JAG1-Fc were prepared and used as described previously (19).
Flow cytometry and FACS analysis
Cells were washed with PBS containing 0.5% BSA, surface stained followed by fixation and permeabilization using Foxp3/Transcription Factor Staining Buffer Kit (Tonbo Biosciences), and stained with appropriate isotype controls and test antibodies in the dark at 4°C. Samples were analyzed using CyAn ADP Analyzer and CytoFLEX (Beckman and Coulter) and data were analyzed using Summit v4.3 and Kaluza v2.1 software (Beckman and Coulter). In some experiments, cell proliferation was measured by division index calculated using Flowjo_v10.6.1 (BD Biosciences). FACS sorting was performed using MoFlo Astrios cell sorter (Beckman and Coulter). Sort purity was more than 95% as confirmed by post-sort analysis.
Micro-array and whole genome bisulfite sequencing (WGBS) analyses
For microarray analysis, Foxp3.GFP+ Tregs were sorted from fresh, OX40L-JAG1 and anti-CD3/DC28 treated cultures. Total RNA was isolated from these cells using RNAeasy columns (Qiagen) converted into cDNA and microarray analysis was performed in duplicate using the Affymetrix GeneChip Mouse Genome 430 2.0 microarray at the Center for Genomics core facility at the University of Illinois at Chicago. Briefly, biotinylated cDNA was synthesized from total RNA using biotinylated dNTPs and allowed to hybridize with microarrays and scanned. Arrays passing quality control tests were further subjected to gene expression analysis after normalization with housekeeping gene controls. Data were analyzed using the R-package software.
For whole genome bi-sulfite sequencing, genomic DNA was isolated from sorted CD4+CD25- and CD4+CD25+ T-cells. Shotgun genomic libraries were prepared with the Hyper Library construction kit from Kapa Biosystems (Roche) and treated with the Lightning EZ kit from Zymo Research. The libraries were quantitated by qPCR and sequenced on one lane for 151 cycles from each end of the fragments on a NovaSeq 6000. Fastq files were generated and demultiplexed with the bcl2fastq v2.20 Conversion Software (Illumina). Reads were mapped to the reference genome in a bisulfite-conversion-aware manner using Bismark. Apparent PCR duplicates were removed using deduplicate_bismark. Methylation status per read obtained with bismark_methylation_extractor. Percent methylation levels, plus counts of methylated and unmethylated bases were then summarized per CpG. CpGs were further filtered to the subset with measurable methylation levels (minimum 10x coverage) in all the samples of differential analysis. The lineage stability of Tregs was analyzed in terms of percent methylation in Treg signature genes such as Foxp3, Il-2ra, Ctla4, Ikzf2 and Ikzf4. (Refer to NCBI-GEO accession ID GSE136582 https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE136582 and GSE130617 https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE130617 for more information).
Western blot
CD4+CD25+ T- cells (2 × 106 cells/ml) were treated with soluble OX40L, JAG1 and IL-2 or anti-CD3/D28 as described above. Cells were washed with PBS and lysed in Laemmli buffer (Biorad). Proteins were resolved in 10% SDS-PAGE gels and transferred to PVDF membranes (Biorad), blocked with 5% skimmed milk and incubated with primary anti-mouse TRAF1 (1:1000, Santacruz Biotechnologies), anti-mouse phospho p65 (Ser536) (1:500) and anti-mouse NF-κB2 p100/p52 (1:500, Cell Signaling Technology) antibodies. Blots were then washed, incubated with secondary anti-rabbit IgG-HRP and developed using ECL detection kit (Pierce Scientific). Blots were stripped and re-probed with the anti-mouse β-actin-HRP antibody (1:5000; Santacruz Biotechnology), anti-mouse p65 (1:1000, Cell Signaling Technology), and developed.
Animal experiments
8 week old female NOD mice (n=6 per group) were injected (i.p.) with recombinant OX40L (100μg) and JAG1 (100μg) once a week for three consecutive weeks. Age and sex-matched control mice received PBS. On week 12, mice were sacrificed and analyzed for Treg cell numbers and suppressive phenotype. CD4+CD25- Tconv cells and CD4+CD25+ Tregs were sorted and subjected to whole genome bisulfite sequencing as described above.
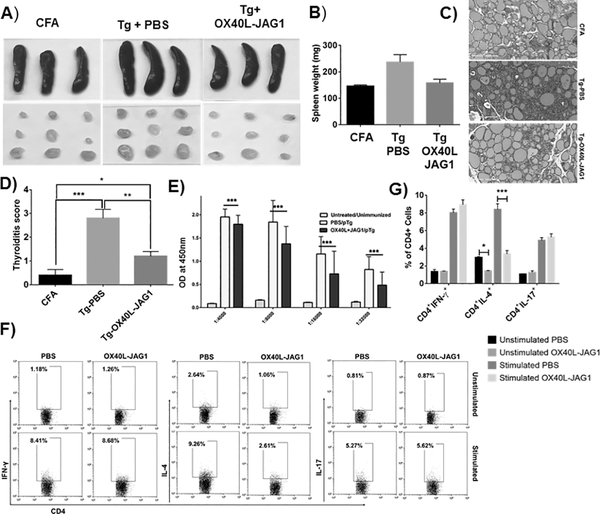
For EAT induction, 6–8-week old female CBA/j mice were divided into three groups (n=6 mice/group) namely; 1) Complete Freund’s adjuvant (CFA) control, 2) PBS vehicle control, 3) OX40L-JAG1 (100μg each). Mice were treated with PBS or OX40L-JAG1 on day 1, 7 and 14. On day 12 mice were immunized with CFA alone or CFA + porcine thyroglobulin (Tg-100μg) emulsion subcutaneously to induce EAT (20, 21). A booster dose was given on day 26. On day 40, mice were euthanized, and spleen, draining lymph nodes of thyroid and neck region, and thyroid tissue were harvested. Serum samples were analyzed for anti-porcine thyroglobulin IgG antibodies by ELISA. Draining lymph node cells were stimulated with thyroglobulin antigen (20μg/ml) in the presence of PMA-Ionomycin for 6 h and protein transport inhibitor cocktail (eBioscience) for final 2h and analyzed for CD4+Foxp3-IFN-γ+, IL-4+ and IL-17+ Teff cells by flow cytometry.
Thyroid tissues from CFA, PBS, and OX40L-JAG1 treated mice were excised and fixed in 10% formalin and processed for hematoxylin and eosin staining. Thyroiditis scoring was done as described previously (22). Scoring was done on a scale of 1 to 5 with reference to the extent of lymphocyte infiltration in blinded fashion by two individuals. 1: Infiltration of at least 125 cells in one or several foci. 2: 10–20 foci of cellular infiltration with up to 25% of the gland. 3: Infiltration of up to 25–50%. 4: >50% destruction of the gland. 5: Near-complete destruction of the gland.
22-week-old female NSG mice engrafted with human CD34 precursors at 4-weeks of age at Jackson labs were used to study the effect of OX40L-JAG1 treatment on human Tregs in vivo. Engraftment efficiency was checked by quantifying human CD45+ cells in the peripheral blood before treatment. Distribution frequency of human CD45+ cells ranged from 38.6 to 65.0%. Mice were divided randomly (n=4) and treated with PBS or human OX40L and JAG1 (100μg/each) three times at 5 day interval. Mice were sacrificed a week after the final treatment. Spleen and liver were collected, single cell suspensions were prepared and analyzed for increased human Tregs by flow cytometry as described above.
In vitro suppression assays
Suppression assays were performed by co-culturing anti-CD3 (0.5 μg/ml) stimulated CD4+CD25- (Teff) and CD4+CD25+ (Treg) cells in the presence of Tg (20μg/ml) loaded APCs at 1:0, 1:1, 1:2, 1:4 and 1:8 Teff: Treg ratios for 3 days. The extent of suppression was measured using the division index of Teff cells in no Treg control vs Treg co-cultures.
Statistical analysis
Statistical analyses were performed using Prism GraphPad (V6.0). Data were expressed as Mean ± SEM of multiple experiments. Student’s t-test was used to compare two groups, whereas ANOVA with Tukey’s multiple comparisons was used to compare more than two groups. A p-value < 0.05 was considered significant.
Results
OX40L-JAG1 induced selective Treg proliferation is not blocked by the inhibition of proximal TCR signal initiating Src family kinase
Previous studies from our laboratory have shown that OX40L-JAG1 co-signaling can induce selective expansion of Foxp3+Tregs, but not, Foxp3-Teff cells independent of TCR stimulation (13, 16). Therefore, we investigated as to why Tregs proliferated independent of TCR-stimulation while Tconv cells did not. We performed differential gene expression profiling of Foxp3+ Tregs and Foxp3- Tconv cells sorted from Foxp3.GFP reporter mice and found that Tregs had higher expression levels of T-cell activation related genes like CD25, OX40, GITR, 4–1BB, TNFR2, CD69, Nur77, IL-2Ra and IL-2Rb compared to Tconv cells (Fig-S1A). Moreover, Tregs had increased CD44hi CD62low activated population and decreased CD44low CD62Lhi naïve population compared to Tconv cells under resting conditions (Fig-S1B–C). These results suggested a lower activation threshold required for Treg proliferation compared to Tconv cells which can be achieved without TCR stimulation.
Total CD4+ T-cells were pre-treated with a library of kinase (e.g. CDK, CKI & II, EGFR, GSK, IKK, Insulin receptor, JAK, JNK, MAPK, MEK, PI3-Kinase, AKT, mTOR, PDGFR, PKA, PKC, and Src-family, etc.) inhibitors and stimulated with α-CD3/CD28 mAbs or soluble OX40L-JAG1. Treg and Teff cell proliferation was assayed by cell trace violet dilution assay. Inhibitors causing more than 50% cell death were eliminated from analysis. Proliferation index was calculated as the ratio between proliferating/resting Tregs. Inhibitors causing 50% reduction in the proliferation index with respect to the proliferation index of no inhibitors control in both the treatments are shown in Fig-S1D. Inhibitors of MEK (U-0126), p38 MAPK (SB-203580 and SB-202190), PKA-PKG-MLCK-PKC (H7–2HCl), PDGFRK (TYRPHOSTIN 9), GSK-3-beta (Indirubin-3’-monooxime), IKK2 (sc-514), PI3K (LY 294002 and Wortmannin), Akt (BML-257), mTOR (Rapamycin) and CDK (Roscovitine) effectively inhibited both αCD3/CD28 mAbs and OX40L-JAG1 induced Treg proliferation. Inhibitors of Src family kinase (PP1 and PP2), JAK-2 (AG-490), EGFRK (Erbstatin analog), cRAF (GW 5074) specifically inhibited αCD3/CD28 induced Treg and Teff cell proliferation, but not, OX40L-JAG1 induced Treg proliferation. Thus, OX40L-JAG1-induced TCR-independent Treg proliferation was not blocked by the inhibitors of proximal TCR signaling kinase such as Src-family kinase, but both αCD3/CD28 and OX40L-JAG1 induced Treg proliferation was inhibited by inhibitors of downstream signaling pathways like PKC, PI3-K-AKT-mTOR and MEK pathways (Fig-S1D).
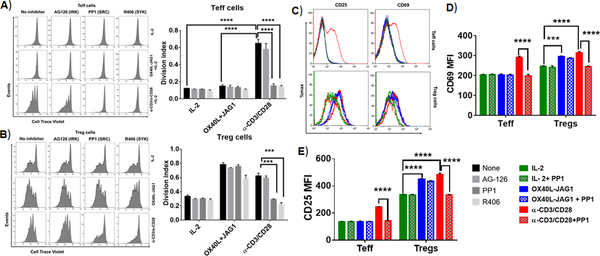
During TCR-mediated T-cell proliferation, recognition of peptide-MHC complex by TCRs leads to activation of Lck (a member of Src family kinases), that phosphorylates immune receptor tyrosine-based activation motifs (ITAMs) of CD3 and ζ-chain leading to the recruitment and phosphorylation of Syk-family kinase (ZAP70). Activated ZAP70 then propagates signaling downstream of TCR (23). Though SRC and Syk-family kinases predominantly mediate TCR dependent T-cell proliferation, their plausible role in TCR-independent T-cell proliferation had not been ruled out. Furthermore, to avoid interference from IL-2 produced by Teff cells in total CD4 T-cell culture that might boost Treg proliferation/differentiation (24), we sorted CD4+CD25- Tconv cells and CD4+CD25+ Treg cells and pre-treated them with the inhibitors of Src kinase (PP1), SYK (R406) and IRAK (AG-126) and stimulated them with IL-2 alone or αCD3/CD28 or OX40L-JAG1 with IL-2, and measured Teff/Treg proliferation using cell division index. IL-2 and OX40L-JAG1 did not induce Teff cell proliferation while αCD3/CD28 treatment induced significant proliferation of both Teff and Treg cells as shown by higher cell division index. α-CD3/CD28 induced Teff proliferation was significantly inhibited by Src and Syk kinase inhibitors as evidenced by a significant reduction in cell division index. IL-2 induced basal proliferation of Tregs which was further enhanced by the addition of OX40L-JAG1. While αCD3/CD28 induced proliferation of Tregs was suppressed by the inhibitors of Src and Syk kinases (Fig-1A–B), they had little or no effect on either IL-2 alone or OX40L-JAG1 induced Treg proliferation.
Figure-1:
A-B) CD4+CD25- Tconv cells (A) and CD4+CD25+ Treg (B) cells were pre-treated with indicated kinase inhibitors (10μM/ml) for 2h and co-treated with IL-2 (25U/ml) and OX40L (5 μg/ml) + JAG1 (1 μg/ml) and αCD3+ αCD28 monoclonal antibodies in the presence of IL-2 for 4 days. Cell proliferation was measured by cell trace violet dilution assay. Bar graphs show division index calculated from cell trace violet dilution in each culture conditions (Values represent Mean ± SEM, n=3, ****p<0.001, ***p<0.05). C) Overlay histograms show CD69 and CD25 expression in gated CD4+Foxp3- Teff cells (top panel) and CD4+Foxp3+ Tregs (bottom panel) from the above experiments. Bar graphs show MFI values of CD69 (D) and CD25 expression (E) in Teff and Treg cells summarized from C. Values are expressed as Mean ± SEM (n=3 independent experiments; ***p<0.005, ****p<0.001).
To further ascertain the differential role of Src-kinase signaling in TCR-dependent vs independent T-cell activation and proliferation, we stimulated CD4+ T-cells with α-CD3/CD28 and OX40L-JAG1 in the presence/absence of Src-kinase inhibitor PP1 and analyzed the expression of T-cell activation markers such as CD69 and CD25 in Foxp3- Teff cells and Foxp3+ Treg cells (Fig-1C–E). Increases in the expression of CD69 and CD25 after α-CD3/CD28 stimulation were significantly suppressed upon PP1 treatment in both Teff and Treg cells. In contrast, we observed a significant increase in CD69 and CD25 expression in Tregs upon OX40L-JAG1 treatment both in the presence or absence of PP1. However, there was no increase in the levels of expression of CD69 and CD25 in Teff cells upon IL-2 and OX40L-JAG1 treatment and remained unaltered in the presence of PP1 (Fig-1C–E).
TCR-independent Treg proliferation is induced via OX40-TRAF1 mediated non-canonical NF-kB signaling
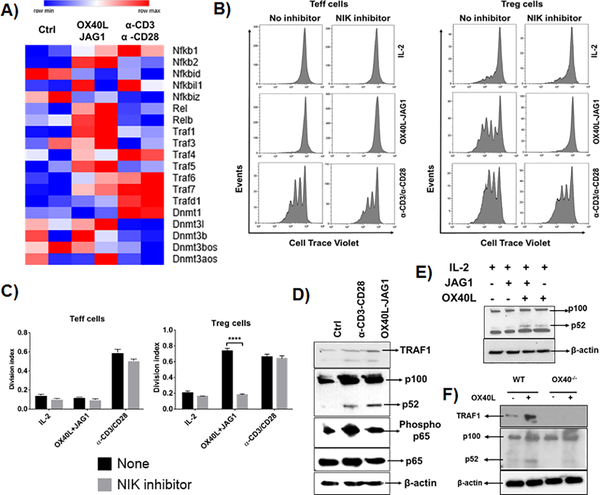
Unlike the molecular mechanisms of TCR-dependent Treg proliferation which are well documented, those of TCR-independent Treg proliferation are not completely known. Earlier, we have shown the involvement of canonical NF-kB signaling and AKT-mTOR signaling in this Treg proliferation (16, 25). However, these pathways are not specific to OX40L-JAG1 induced proliferation as they can also mediate TCR-dependent Treg proliferation (Fig-S1D). To determine the specific signaling pathway mediating OX40L-JAG1 induced TCR-independent Treg proliferation, but not TCR-dependent Treg proliferation, we analyzed the gene expression profile of fresh, α-CD3/CD28 and OX40L-JAG1 expanded Foxp3.GFP Tregs. In silico pathway analysis using Metacore software (Thomson Reuters) identified differential expression of a gene cluster related to non-canonical NF-kB activation involving TRAF1, TRAF5, RelB and NF-kB2 genes in OX40L-JAG1 expanded Tregs relative to αCD3/CD28 expanded and control Tregs (Fig-2A). Genes involved in canonical NF-kB signaling such as RelA and NF-kb1 were upregulated in Tregs expanded by both OX40L-JAG1 and α-CD3/CD28 stimulation compared to control Tregs. We also found specific upregulation of Dnmt1 in α-CD3/CD28 expanded Tregs compared to control and OX40L-JAG1 expanded Tregs (Fig-2A).
Figure-2:
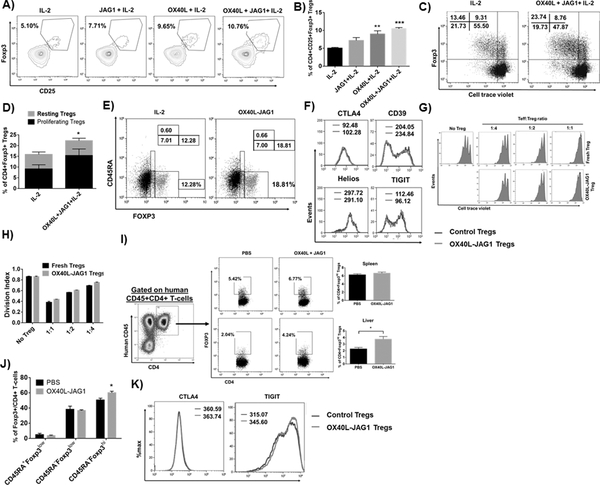
A) CD4+ T-cells from Foxp3.GFP mice were treated with OX40L-JAG1 or α-CD3/CD28 for 3 days. CD4+Foxp3-GFP- Teff cells and CD4+Foxp3+GFP+ Tregs were sorted and subjected to microarray analysis. Unstimulated fresh Teff cells and Tregs used as a control. Heat map shows differential mRNA expression of TRAFs, NF-kB and DNMT signaling related genes between control, OX40L-JAG1 and α-CD3/CD28 treated Tregs. B) CD4+CD25- Tconv cells and CD4+CD25+ Treg cells were pre-treated with indicated NIK inhibitor (10μM/ml) for 2h and co-treated with IL-2 (25U/ml) and OX40L (5 μg/ml) + JAG1 (1 μg/ml) and αCD3+ αCD28 monoclonal antibodies in the presence of IL-2 for 4 days. Cell proliferation was measured by cell trace violet dilution assay. C) Bar graphs show division index calculated from cell trace violet dilution in each culture conditions (Values represent Mean ± SEM, n=3, ****p<0.001). D) Western blots show TRAF1 expression, p100 to p52 processing and total and phospho p65 levels in OX40L-JAG1 treated Tregs compared to fresh and α-CD3/CD28 treated Tregs after 24hr. E) Western blots show p100 to p52 processing in Tregs treated with IL-2 alone, JAG1 alone, OX40L-alone and OX40L+JAG1+IL-2. F) Western blots show TRAF1 and p100 to p52 processing in WT and OX40−/− Tregs treated with or without OX40L.
Interestingly, NIK (NF-kB inducing kinase of non-canonical NF-kB signaling) inhibitor selectively blocked OX40L-JAG1 induced, but not IL-2 or αCD3/CD28-induced Treg proliferation (Fig-2B–C). Further, we found increased TRAF1 and p100 to p52 processing in OX40L-JAG1 treated Tregs compared to αCD3/CD28 treated Tregs while p65 phosphorylation was increased in both OX40L-JAG1 and αCD3/CD28 treated Tregs which is in line with our gene expression profiling results (Fig-2D). Next, we analyzed the effects of treatment with either OX40L or JAG1 on non-canonical NF-kB activation. While OX40L activated p100 to p52 processing, JAG1 failed to show synergistic effect on OX40L induced non-canonical NF-kB signaling (Fig-2E). Furthermore, TRAF1 and p100 to p52 processing were decreased in OX40−/− Tregs compared to WT Tregs in the presence/absence of OX40L stimulation (Fig-2F). Taken together, these results showed OX40L-induced non-canonical NF-kB signaling as a key signaling mechanism differentially driving TCR-independent Treg proliferation.
OX40L-JAG1 treatment induces Treg proliferation in vivo and expands lineage stable Tregs in autoimmune-prone NOD mice
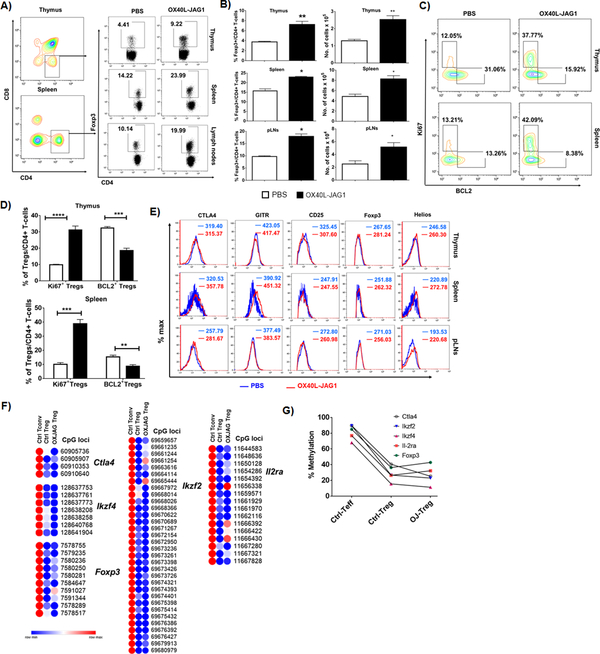
Earlier, we had shown that OX40L-JAG1 treatment of pre-diabetic NOD (10–12-week old) mice increased Tregs in the periphery and delayed the onset of autoimmune diabetes(16). However, we did not determine if it involved increased thymic output or increased Treg survival/proliferation. Therefore, we treated pre-diabetic female NOD mice with OX40L-JAG1 and found significantly increased Treg frequency and numbers in the thymus (**p<0.01), spleen (*p<0.05) and peripheral lymph nodes (*p<0.05) (Fig-3A–B). We also analyzed the expression of proliferation marker Ki67 and pro-survival factor BCL2, which arrests cell cycle progression (26, 27). There was a significant increase in Ki67+BCL2-Foxp3+ proliferating Tregs (****p<0.001 in thymus and ***p<0.005 in spleen) and a significant reduction in Ki67-BCL2+Foxp3+ resting Tregs (***p<0.01 in thymus and **p<0.005 in spleen) in OX40L-JAG1 treated mice compared to PBS treated mice (Fig-3C–D). These results indicated that it is the increased Treg proliferation, and not increased survival, that contributed to the increase in Tregs. To further confirm that OX40L-JAG1 treatment significantly increased matured thymic Tregs and Treg precursors, we analyzed CD4+CD25+Foxp3-/CD4+CD25-Foxp3low Treg precursors and CD4+CD25+Foxp3+ Tregs in the thymus and found a significant increase in CD4+CD25-Foxp3low Treg precursors and CD4+CD25+Foxp3+ matured Tregs (****p<0.001) with no significant increase in CD4+CD25+Foxp3- Treg precursors (Fig-S2A). Further, the increased thymic Tregs could contribute to increased peripheral Treg numbers. Next, we analyzed peripheral Treg expansion in the absence of continuous thymic Treg output in thymectomized NOD mice and observed increased Tregs in the spleen of thymectomized mice upon OX40L-JAG1 treatment compared to control mice (*p<0.05) indicating Treg expansion in the periphery (Fig-S2B). These data indicated that increased thymic and peripheral Treg proliferation might contributed to increased Treg numbers.
Figure-3:
A) 6–8week-old female NOD mice were injected with PBS or soluble OX40L and JAG1 (100μg each) once a week for 3 consecutive weeks and percentages of CD4+Foxp3+ Tregs were analyzed in the thymus, spleen and peripheral lymph nodes. B) Bar graph show frequency of and number of Tregs (Values represent Mean ± SEM, n=6, *p<0.05, **p<0.01). C) CD4+Foxp3+ from the thymus and spleen were gated and contour plots show Ki67+BCL2- (proliferating) and Ki67-BCL2+ (resting) cells. D) Bar graphs summarize frequencies of Ki67+BCL2- (proliferating) and Ki67-BCL2+ (resting) Tregs in the thymus and spleen calculated from Fig-3C. (Values represent Mean ± SEM, n=6, **p<0.01, ***p<0.005, ****p<0.001). E) Overlay histograms show the expression levels of Treg signature markers CTLA4, GITR, CD25, Foxp3 and Helios expression in CD4+Foxp3+ cells in the thymus, spleen and pancreatic lymph nodes of PBS (Blue) Vs OX40L (Red) treated mice. F) Heat maps show methylation index of individual CpG islands between control and OX40l-JAG1 treated Tregs compared to control Teff cells. G) Percentage methylation levels in the promoter and intronic regions of Treg signature genes Ctla4, Ikzf4, Ikzf2, Il-2ra and Foxp3 in sorted splenic CD4+CD25+ Tregs and CD4+CD25- Teff cells from PBS and OX40L-JAG1 treated NOD mice as analyzed by WGBS.
Next, we investigated the lineage stability of OX40L-JAG1 expanded Tregs and found sustained expression of Treg lineage stability markers CD25, CTLA4, Helios and GITR in CD4+Foxp3+ cells from the thymus, spleen and peripheral LNs of control vs OX40L-JAG1 treated mice (Fig-3E). Furthermore, the epigenetic stability of Treg lineage was evaluated by analyzing the methylation levels of the nTreg signature genes in sorted CD4+CD25+ Tregs and CD4+CD25- Tconv cells from PBS and OX40L-JAG1 treated mice by WGBS. Treg specific de/hypomethylated regions were selected by comparing methylation levels between CD4+CD25+ Tregs (20–40%) and CD4+CD25- Tconv cells (70–90%) from control mice. As shown in Fig-3F and 3G, we observed minimal differences in the degree of methylation in the relevant CpG islands between control and OX40L-JAG1 expanded Tregs indicating that OX40L-JAG1 stimulation did not lead to hypermethylation of the relevant CPG islands and thus might have contributed to their lineage stability.
OX40L-JAG1 treatment expands functional Tregs and ameliorates EAT
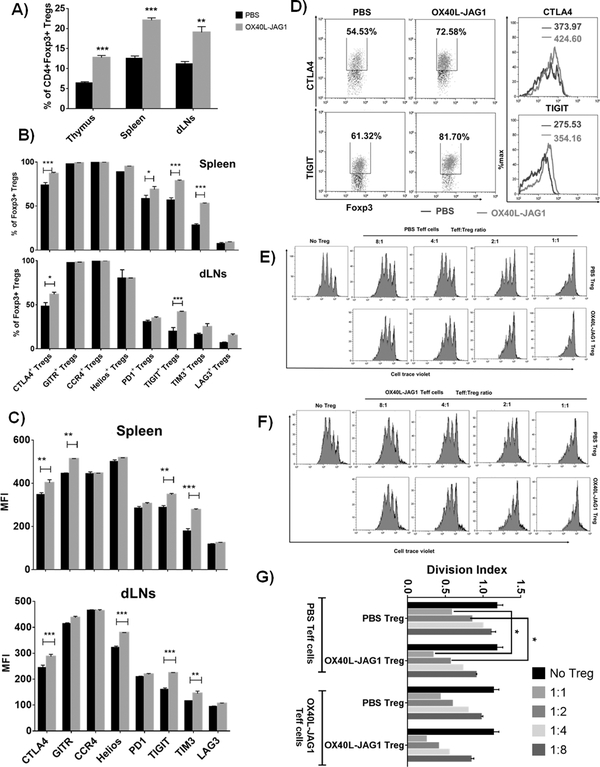
We examined whether OX40L-JAG1 co-treatment can expand functionally suppressive Tregs and prevent EAT in CBA/j mice which is a highly susceptible strain for EAT induction (28). Firstly, we treated 6-week-old CBA/j mice with soluble OX40L and JAG1 once a week for two weeks and immunized them with porcine thyroglobulin emulsified in CFA. An additional (3rd) OX40L-JAG1 treatment was given two days after immunization. Mice received booster immunization 14 days after the first immunization. Mice were monitored for thyroiditis development 14 days after the booster dose. A subset of mice was sacrificed after final OX40L-JAG1 treatment to determine the suppressive phenotype and functionality of Tregs. We observed significantly increased Tregs in the thymus (***p<0.001), spleen (***p <0.001) and dLNs (**p<0.01) of OX40L-JAG1 treated mice (Fig-4A) with significantly increased frequencies of CTLA4+Foxp3+ and TIGIT+ Foxp3+ Tregs in the spleen (***p<0.005) and dLNs (*p<0.05 (CTLA4) and ***p<0.005 (TIGIT) (Fig-4B). In addition, increased TIM3+Foxp3+ and PD1+Foxp3+Tregs were observed in the spleen, but not dLNs (Fig-4B). Additionally, expression levels of CTLA4, TIGIT, and TIM3 were also significantly increased in OX40L-JAG1 expanded Tregs in the spleen and lymph nodes (Fig-4C). Increased CTLA4+/TIGIT+ Foxp3+ Tregs and CTLA4/TIGIT expression per se were observed in OX40L-JAG1 treated mice compared to control mice (Fig-4D). We did not find any difference in the total CD4+ T-cells, CD8+ T-cells, B220+ B-cells, F4/80+CD11c- macrophages and F4/80-CD11c+ dendritic cells in the spleen and lymph nodes of PBS vs OX40L-JAG1 treated mice (Fig-S3A) indicating the major effect of the treatment was on the Treg compartment. Furthermore, we observed higher levels of suppression of Teff cell proliferation when co-cultured with Tregs from OX40L-JAG1 treated mice compared to PBS treated mice (Fig-4E–G) which is in line with enhanced expression of suppressive markers in these Tregs. Treg suppressive functions were generally higher against OX40L-JAG1 treated Teff cells indicating that these Teff cells were more sensitive to Treg suppression.
Figure-4:
A) 6–8week-old female CBA/j mice were injected with PBS or soluble OX40L and JAG1 (100μg each) and immunized with porcine thyroglobulin antigen emulsified with CFA. A subset of mice from PBS/OX40L-JAG1 treated mice were sacrificed immediately after 1st immunization and last OX40L-JAG1 treatment as described in materials and methods. Percentages of CD4+Foxp3+ Tregs were analyzed in the thymus, spleen and peripheral lymph nodes. (Values represent Mean ± SEM, n=6, **p<0.01, ***p<0.005 vs PBS). B) Frequencies of CTLA4+, GITR+, CCR4+, Helios+, PD1+, TIGIT+, TIM3+ and LAG3+ Tregs in the spleen (top panel) and draining lymph nodes (bottom panel) were analyzed by flow cytometry. C) MFI values of the above mentioned suppressive markers are summarized. (Values represent Mean ± SEM, n=6, **p<0.01, ***p<0.005 vs PBS). D) Representative dot plots and overlay histograms show increased CTLA4+/TIGIT+ Foxp3+ Tregs and CTLA4/TIGIT expression per se in OX40L-JAG1 treated mice. E-F) PBS and OX40L-JAG1 expanded CD4+CD25+ Treg cells from CBA/j mice were co-cultured with cell trace violet labeled CD4+CD25- Teff cells from PBS and OX40L-JAG1 treated mice in depicted combinations at indicated ratios and stimulated with anti-CD3 in the presence of thyroglobulin antigen (20μg/ml) loaded APCs for 3 days. The extent of Teff cell proliferation was measured by cell trace violet dilution assay. G) Bar graphs show division index of Teff cells calculated from each culture conditions (Values represent Mean ± SEM, n=3, *p<0.05).
As shown in Fig-5A–B, we noticed splenomegaly and lymphadenopathy in thyroglobulin immunized control mice compared to CFA control and OX40L-JAG1 treated mice. Histopathology and thyroiditis scores showed significantly increased lymphocyte infiltration in thyroglobulin immunized and PBS (***p< 0.005) treated mice compared to CFA alone treated mice. In contrast, significantly reduced (**p<0.01) lymphocytic infiltration with more intact thyroid follicles were seen in thyroglobulin immunized OX40L-JAG1 treated mice compared to PBS treated mice (Fig-5C–D). Reduced anti-thyroglobulin antibody titers was also seen in OX40L-JAG1 treated mice (Fig-5E). Furthermore, intracellular cytokine staining showed increased IL-4+ Th2 cells in the dLNs in PBS treated mice compared to OX40L-JAG1 treated mice. Upon in vitro stimulation, pro-inflammatory IFN-γ, IL-4 and IL-17 cytokine expressions were significantly increased in PBS treated mice, whereas IL-4, but not IFN-γ and IL-17, expression was significantly reduced in OX40L-JAG1 treated mice (Fig-5F–G).
Figure-5:
A) All mice from thyroiditis experiments were sacrificed at the end of the experiment 14 days after the second immunization. Images of the spleen and draining lymph nodes are shown. B) Bar graph summarizing spleen weights. C) H & E staining analysis of thyroid sections from CFA control, Tg immunized and treated with PBS, or OX40L-JAG1 (n=6). D) Thyroiditis scoring was done as described in materials and methods. (Values represent Mean ± SEM, n=6, *p<0.05, **p<0.01, ***p<0.005). E) Serum anti-thyroglobulin levels were measured by ELISA and bar graph shows absorbance at 450 nm measured at different dilutions (Values represent Mean ± SEM, n=6). F) Representative dot plots showing frequency of CD4+Foxp3-IFN-γ+ Th1, CD4+Foxp3-IL-4+ Th2 and CD4+Foxp3-IL-17+ Th17 cells in the draining lymph node cells before and after stimulation with PMA/ionomycin. G) Bar graph summarizing results of Fig-5F (Values represent Mean ± SEM, n=6, *p<0.05, **p<0.01 vs PBS).
Human OX40L-JAG1 treatment induces TCR-independent proliferation of human Tregs
Next, we examined whether human OX40L-JAG1 can expand human Tregs. First, we analyzed the expression levels of OX40 in CD4+Foxp3+ Tregs and CD4+Foxp3- Tconv cells from human and murine PBMCs, spleen and thymus. Interestingly, we observed very low levels of OX40 expression in Tregs from the PBMCs compared to those from spleen and thymus. Similarly, Tconv cells had very low levels of OX40 expression (Fig-S4). Furthermore, we observed a significant increase (**p<0.01) in human CD4+CD25+FOXP3+ human Tregs in OX40L treated human thymic organ cultures compared to IL-2 alone treated cultures. Although there was considerable increase in Tregs in JAG1+IL-2 treated cultures, it was not significantly different from IL-2 alone. OX40L+JAG1 treated cultures showed the highest increase in Tregs (***p<0.005) (Fig-6A–B). Next, we treated thymocytes devoid of APCs with IL-2 alone or OX40L+JAG1+IL-2 without TCR stimulation for 5 days and measured Treg proliferation. While we found a significant increase in proliferating Tregs upon OX40L-JAG1 treatment compared to IL-2 control, we failed to find a significant increase in the proliferation of Foxp3- Tconv cells (Fig-6C–D).
Figure-6:
A) Human thymic fragments were cultured in 3D thymic organ cultures with human IL-2 (50U/ml) or human JAG1-Fc (1μg/ml), human OX40L-Fc (5μg/ml) and JAG1+OX40L in the presence of IL-2 for 5 days and frequencies of CD4+CD25+FOXP3+ Tregs were analyzed. B) Bar graph summarizes the frequencies of CD25+FOXP3+ Tregs. (Values represent Mean ± SEM, n=8 independent experiments, **p<0.01, ***p<0.005 vs IL-2). C) Human thymocytes were cultured with either IL-2 alone or OX40L+JAG1+IL-2 for 5 days and the proliferation of CD4+Foxp3+ and CD4+Foxp3- cells was assayed by cell trace violet dilution. D) The bar graph shows percentages of resting and proliferating CD4+Foxp3+ Tregs (Values represent Mean ± SEM, n=5, *p<0.05 Vs IL-2). E) Representative dot plot from the above experiments show the frequencies of CD45RA+Foxp3low (naïve Tregs), CD45RA-Foxp3hi (effector Tregs) and CD45RA-Foxp3low (effector T-cells) in the culture. F) Overlay histograms show the expression levels of Treg functional markers CTLA4, CD39, Helios and TIGIT in CD4+Foxp3hi Tregs expanded from IL-2 (Blue) Vs OX40L+JAG1 (Red) treated cultures. G) In vitro Treg suppression assay was performed using autologous CD4+CD25-CD127high Teff cells and fresh/OX40L-JAG1 expanded CD4+CD25hiCD127low Tregs in the presence of anti-CD3 stimulation for 4 days. The extent of Teff cell proliferation was measured by cell trace violet dilution assay. H) Bar graphs show division index of Teff cells calculated from each culture conditions I) NSG mice engrafted with human CD34 precursor cells were treated with soluble OX40L and JAG1. Representative dot plots show the development of human CD45+CD4+ T-cells in humanized NSG mice. Representative dot plots and bar graphs show the frequencies of Foxp3hi human Tregs within gated human CD45+CD4+ T-cells in spleen (top panel) and liver of PBS vs OX40L-JAG1 treated mice. (Values represent Mean ± SEM, n=4, *p<0.05).J) Bar graph shows the frequencies of CD45RA+Foxp3low (naïve Tregs), CD45RA-Foxp3hi (effector Tregs) and CD45RA-Foxp3low (effector T-cells) within CD45+CD4+Foxp3+ cells in PBS vs OX40L-JAG1 treated NSG mice. K) Overlay histogram analysis show CTLA4 and TIGIT expression in CD45RA-Foxp3hi effector Tregs from PBS vs OX40L-JAG1 treated NSG mice.
Unlike murine Tregs in which Foxp3 expression is strictly confined to Tregs and accepted as a lineage-specific marker, transient expression of FOXP3 has been noted in non-regulatory activated Teff cells lacking suppressive functions in humans (29, 30). Therefore, we analyzed the proportions of CD45RA-Foxp3hi (effector Tregs), CD45RA+Foxp3low (naïve Tregs), and CD45RA-Foxp3low (effector like T-cells) and noted a significant increase in CD45RA-Foxp3hi effector Tregs and not in CD45RA-Foxp3low effector like T-cells upon OX40L-JAG1 treatment compared to IL-2 control (Fig-6E). Furthermore, OX40L-JAG1 expanded Tregs had comparable levels of Treg markers such as CTLA4, CD39, Helios, and TIGIT compared to IL-2 control Tregs (Fig-6F), indicative of their ability to maintain suppressive phenotype. We tested the functional competency of OX40L-JAG1 expanded CD4+CD25hi Tregs by co-culturing them with autologous CD4+CD25- Tconv cells and observed comparable suppressive function between fresh vs OX40L-JAG1 expanded Tregs (Fig-6G–H).
Finally, we tested the ability of OX40L-JAG1 treatment to expand human Tregs in vivo using the humanized NSG mice engrafted with CD34 precursors. We did not observe any signs of graft-vs-host disease such as weight loss/lethargy in control or OX40L-JAG1 treated mice. While the frequency of Foxp3hi Tregs in human CD45+CD4+ T-cells in the spleen was not significantly different from that in control mice, there was a significant increase in Foxp3hi Tregs in the liver of treated mice (Fig-6I). We found a significant, increase within CD45RA-Foxp3hi effector Treg subset in OX40L-JAG1 expanded Tregs (Fig-6J) without loss of expression suppressive markers CTLA4 and TIGIT (Fig-6K).
Discussion
Positive selection of Tregs in the thymus requires higher TCR signal strength, which enables those cells to preferentially overexpress T-cell activation and co-stimulatory/co-inhibitory receptors such as CD25, ICOS, GITR, OX40, 4–1BB, CTLA4, PD-1 and TIGIT relative to Tconv cells and they require TCR-activation to gain expression of these receptors (31). This allows co-signaling receptors to selectively modulate Treg proliferation and function, and thereby contribute significantly to Treg homeostasis and immune tolerance. Here, we show that stimulation of OX40 and Notch3 receptors that are preferentially over-expressed on Tregs can lead to selective activation and proliferation of Tregs without concomitant expansion of Tconv cells in T-cell cultures in the absence of TCR stimulation. Earlier, studies have shown that a sharp TCR signaling threshold is obligatory for T-cell proliferation (32). Although TCR signaling is activated shortly after TCR engagement, T-cell proliferation is observed days later due to the time required for the propagation of progressive events to reach the threshold needed for proliferation. After reaching the threshold T-cells can proliferate even in the absence of TCR signaling as evidenced by ZAP70 kinase-independent proliferation of T-cells (32). It is possible that Tregs might have attained higher activation threshold than Tconv cells during their thymic selection which renders them susceptible to TCR-independent proliferation when stimulated through co-activation receptors such as OX40. Furthermore, inhibitors of proximal TCR signaling kinase such as Src family kinase (PP1) blocked αCD3/CD28 induced TCR-dependent Tconv cell activation as evidenced by reduced CD69 and CD25 expression, and proliferation as well. Tregs, due to their selection against higher TCR signal strength, expressed higher levels of CD69 and CD25 relative to Tconv cells under resting state which was further increased upon TCR/OX40L-JAG1 stimulation. More importantly, TCR-dependent, but not TCR-independent, upregulation of CD69 and CD25 expression in Tregs was inhibited by PP1. These results suggest two key interpretations. i) Even TCR-independent stimulation through co-activation receptors like OX40/Notch3 can upregulate CD69 and CD25 expression in Tregs. ii) PP1 is a selective inhibitor of TCR mediated T-cell activation and proliferation which does not inhibit TCR-independent activation/proliferation of Tregs induced OX40L-JAG1 treatment. These results suggested that Tregs differ from Tconv cells in terms of their activation state and signaling requirement for proliferation, and can be induced to proliferate with OX40L-JAG1 treatment in the absence of TCR stimulation.
We found a significant increase in Ki67+BCl2- proliferating Tregs with a concomitant decrease in Ki67-BCL2+ resting Tregs in thymus and spleen of OX40L-JAG1 co-treated mice compared to PBS treated mice. These findings demonstrated the ability of OX40L-JAG1 treatment to induce Treg proliferation in vivo even under autoimmune condition in NOD mice. In addition, we found a significant increase in CD4+CD25-Foxp3low thymic Treg precursors and CD4+CD25+Foxp3+ matured Tregs in OX40L-JAG1 treated mice indicating the possible role of these ligands in thymic Treg differentiation as well. Furthermore, since TCR-independent phase is an integral part of thymic Treg development, it is likely that OX40L and JAG1 contributed to increased thymic output of Tregs by inducing both Treg differentiation and proliferation during that phase (16, 25).These findings are consistent with earlier reports of OX40 signaling enhancing IL-2 dependent thymic Treg maturation in a TCR-independent manner (25, 33).
One of the major hurdles associated with Treg therapy is the lineage stability of expanded Tregs. Treg-specific DNA demethylation at Foxp3 gene locus allows for the constitutive expression of Foxp3, which is essential for the repression of TCR activation-induced inflammatory genes like Ifn-g and Il-2 expression in Tregs (9). Thus, loss/reduced expression of Foxp3 in Tregs renders them unstable resulting in the expression of pro-inflammatory genes, which can exacerbate autoimmune response (29, 34). It has been shown that repeated TCR-stimulation during ex vivo expansion of Tregs can lead to increased methylation of the CpG islands of TSDR at Foxp3 gene locus in a subset of Tregs resulting in loss of Foxp3 expression/lineage stability (7, 17). Accordingly, TGF-β has been shown to antagonize TCR-induced cell-cycle dependent recruitment of DNA Methyl Transferase (DNMT1)-1 to Foxp3 locus and thereby helps maintain Foxp3 expression (35). In our differential gene expression microarray analysis, we observed a specific increase in the expression of DNMT1 in Tregs expanded by α-CD3/CD28, but not in OX40L/JAG1 expanded or fresh Tregs reiterating the TCR signaling requirement for DNMT1 upregulation (Fig-2A). Moreover, we found that OX40L-JAG1 induced Treg proliferation occurred independent of canonical TCR signaling and was mediated specifically by non-canonical NF-kB activation. Our findings are consistent with recent studies which showed massive inflammation and impaired suppressive function in Tregs upon deletion of NF-kB2 in Tregs indicating a key role for non-canonical NF-kB signaling in Treg homeostasis (36). Therefore, it is likely that the TCR-independent nature of OX40L-JAG1 induced Treg expansion might favor the epigenetic lineage stability of the expanded Tregs. In support of this notion, we found a stable expression of lineage stability markers CD25, Foxp3, Helios, CTLA4 and GITR in Tregs expanded in the thymus, spleen and lymph nodes of NOD mice. Moreover, WGBS analysis showed stable demethylation marks in nTreg signatures genes such as Foxp3, Il-2ra, Ctla4, Ikzf2 and Ikzf4 in OX40L-JAG1 expanded Tregs compared to control Tregs in the periphery implying that OX40L-JAG1 expanded Tregs are lineage stable.
In the EAT model, we found OX40L-JAG1 treatment to increase functional Tregs in the thymus and periphery of CBA/j mice and ameliorate EAT. This protective effect was accompanied by reduced thyroid lymphocytic infiltration, Th2 response and autoantibody production. Among the expanded Tregs, we found a specific increase in subsets expressing elevated levels of co-inhibitory receptors such as CTLA4 and TIGIT in the dLNs. These co-inhibitory receptors are known to modulate APC functions through two independent co-inhibitory signaling axes: CTLA4-CD28-CD80/CD86 and TIGIT-CD226-CD155/CD112 axes (37, 38). There was a modest increase in TIM3 expressing Tregs as well in the dLNs. Unlike CTLA4 which is constitutively expressed by Tregs and is critical for their function, TIGIT and TIM3 are expressed on a subset of Tregs and TIGIT+/TIM3+ Tregs were functionally superior to TIGIT-/TIM3- Tregs under autoimmune conditions (31, 37). In line with these findings, we noted enhanced Treg suppressive functions in OX40L-JAG1 expanded Tregs compared to control Tregs and more interestingly, Teff cells from OX40L-JAG1 treated mice were more susceptible to Treg mediated suppression (Fig-4E–G).
Signaling through TNFSF receptors including OX40 has been shown to impair Treg functions and render Teff cells resistant to Treg mediated suppression (39, 40). In contrast, we observed enhanced suppression by Tregs from OX40L-JAG1 treated mice. Earlier, we have seen OX40L treatment alone to expand “non-functional” Tregs in older NOD mice when the inflammatory responses had already unfolded (41) and co-treatment with JAG1 was necessary for the expansion of functional Tregs in older NOD mice (16). These observations indicated that although OX40 signaling is sufficient to induce Treg expansion, JAG1 co-signaling is essential to maintain suppressive function of Tregs. This is in accordance with the findings from other groups which showed Notch3 signaling to regulate Foxp3 transcription (42) and in vivo functions of nTregs (43).
Many encouraging immune interventions discovered in mice have failed in human trials likely due to inherent differences between human Vs murine system. Majority of the human Treg studies have been carried out using PBMCs which are strikingly different in their phenotype from Tregs present in the lymphoid organs and very little is known about the expansion of Tregs in the human lymphoid organs (44). We observed preferential expression of OX40 on Tregs over Tconv cells in human thymus and spleen, but not in PBMCs indicating a role for this receptor in human Treg expansion in lymphoid organs. Moreover, unlike murine Tregs, human Tregs are highly heterogeneous and have substantial differences in their phenotype (45, 46). In spite of these differences, we found selective expansion of human Tregs, but not Tconv cells, in thymocyte cultures treated with OX40L-JAG1 and a major proportion of expanded Tregs were of CD45-Foxp3hi effector Tregs and not CD45-Foxp3low effector memory like cells. Moreover, the expanded Tregs expressed functional markers CTLA4 and TIGIT and showed comparable suppressive function to that of freshly isolated Tregs.
Furthermore, we observed increased Foxp3hi Tregs in the liver, but not in the spleen, of humanized NSG mice treated with OX40L-JAG1. Lack of Treg increase in the spleen upon OX40L-JAG1 treatment could be in part explained by the lack of thymic selection in humanized NSG mice. Increase in human Tregs observed in the liver of OX40L-JAG1 treated mice indicates a potential hitherto unidentified site for Treg expansion. Given that the humanized NSG mice are not ideal to study human tTreg expansion in vivo, our findings are significant. Evolving humanized mouse models such as NSG.BLT (bone marrow-liver-thymus) mice might be more suitable to study the effect of OX40L-JAG1 treatment on human tTreg expansion (47). Collectively, our results show a novel and conserved mechanism of Treg homeostasis and have important implications for treating autoimmunity.
Supplementary Material
Key points.
OX40L-JAG1 induced Treg proliferation mediated via non-canonical NF-kB signaling.
OX40L-JAG1 expanded epigenetically stable and suppressive Tregs to ameliorate EAT.
OX40L-JAG1 induced TCR-independent selective proliferation of human thymic Tregs.
Acknowledgments
We thank UIC-Research Resource center’s high throughput core, flow cytometry core, genomics core, and research informatics core for their technical help. We thank Dr. Nico Ghilradi, Genentech for providing NIK inhibitor.
This study was supported by the grants from NIH R01 AI107516-01A1 and #1R41AI125039-01, Juvenile Diabetes Research Foundation (JDRF) # 2-SRA-2016-245-S-B and Sirazi Foundation to Dr. Prabhakar.
References
- 1.Sakaguchi S, Yamaguchi T, Nomura T, and Ono M. 2008. Regulatory T cells and immune tolerance. Cell 133: 775–787. [DOI] [PubMed] [Google Scholar]
- 2.Josefowicz SZ, Lu LF, and Rudensky AY. 2012. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol 30: 531–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, Ye J, Masteller EL, McDevitt H, Bonyhadi M, and Bluestone JA. 2004. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med 199: 1455–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glick AB, Wodzinski A, Fu P, Levine AD, and Wald DN. 2013. Impairment of regulatory T-cell function in autoimmune thyroid disease. Thyroid 23: 871–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bluestone JA, Buckner JH, Fitch M, Gitelman SE, Gupta S, Hellerstein MK, Herold KC, Lares A, Lee MR, Li K, Liu W, Long SA, Masiello LM, Nguyen V, Putnam AL, Rieck M, Sayre PH, and Tang Q. 2015. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci Transl Med 7: 315ra189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grant CR, Liberal R, Mieli-Vergani G, Vergani D, and Longhi MS. 2015. Regulatory T-cells in autoimmune diseases: challenges, controversies and--yet--unanswered questions. Autoimmun Rev 14: 105–116. [DOI] [PubMed] [Google Scholar]
- 7.Komatsu N, Okamoto K, Sawa S, Nakashima T, Oh-hora M, Kodama T, Tanaka S, Bluestone JA, and Takayanagi H. 2014. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat Med 20: 62–68. [DOI] [PubMed] [Google Scholar]
- 8.Sawant DV, and Vignali DAA. 2014. Once a Treg, always a Treg? Immunol Rev 259: 173–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohkura N, Hamaguchi M, Morikawa H, Sugimura K, Tanaka A, Ito Y, Osaki M, Tanaka Y, Yamashita R, Nakano N, Huehn J, Fehling HJ, Sparwasser T, Nakai K, and Sakaguchi S. 2012. T cell receptor stimulation-induced epigenetic changes and Foxp3 expression are independent and complementary events required for Treg cell development. Immunity 37: 785–799. [DOI] [PubMed] [Google Scholar]
- 10.Chen L, and Flies DB. 2013. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol 13: 227–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhattacharya P, Gopisetty A, Ganesh BB, Sheng JR, and Prabhakar BS. 2011. GM-CSF-induced, bone-marrow-derived dendritic cells can expand natural Tregs and induce adaptive Tregs by different mechanisms. J Leukoc Biol 89: 235–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zou T, Caton AJ, Koretzky GA, and Kambayashi T. 2010. Dendritic Cells Induce Regulatory T Cell Proliferation through Antigen-Dependent and -Independent Interactions. J Immunol 185: 2790–2799. [DOI] [PubMed] [Google Scholar]
- 13.Gopisetty A, Bhattacharya P, Haddad C, Bruno JC Jr., Vasu C, Miele L, and Prabhakar BS. 2013. OX40L/Jagged1 cosignaling by GM-CSF-induced bone marrow-derived dendritic cells is required for the expansion of functional regulatory T cells. J Immunol 190: 5516–5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Earle KE, Tang Q, Zhou X, Liu W, Zhu S, Bonyhadi ML, and Bluestone JA. 2005. In vitro expanded human CD4+CD25+ regulatory T cells suppress effector T cell proliferation. Clin Immunol 115: 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masteller EL, Warner MR, Tang Q, Tarbell KV, McDevitt H, and Bluestone JA. 2005. Expansion of functional endogenous antigen-specific CD4+CD25+ regulatory T cells from nonobese diabetic mice. J Immunol 175: 3053–3059. [DOI] [PubMed] [Google Scholar]
- 16.Kumar P, Alharshawi K, Bhattacharya P, Marinelarena A, Haddad C, Sun Z, Chiba S, Epstein AL, and Prabhakar BS. 2017. Soluble OX40L and JAG1 Induce Selective Proliferation of Functional Regulatory T-Cells Independent of canonical TCR signaling. Sci Rep 7: 39751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffmann P, Boeld TJ, Eder R, Huehn J, Floess S, Wieczorek G, Olek S, Dietmaier W, Andreesen R, and Edinger M. 2009. Loss of FOXP3 expression in natural human CD4+CD25+ regulatory T cells upon repetitive in vitro stimulation. Eur J Immunol 39: 1088–1097. [DOI] [PubMed] [Google Scholar]
- 18.Shimizu K, Chiba S, Kumano K, Hosoya N, Takahashi T, Kanda Y, Hamada Y, Yazaki Y, and Hirai H. 1999. Mouse jagged1 physically interacts with notch2 and other notch receptors. Assessment by quantitative methods. J Biol Chem 274: 32961–32969. [DOI] [PubMed] [Google Scholar]
- 19.Okamoto Y, Douek DC, McFarland RD, and Koup RA. 2002. Effects of exogenous interleukin-7 on human thymus function. Blood 99: 2851–2858. [DOI] [PubMed] [Google Scholar]
- 20.Gangi E, Vasu C, Cheatem D, and Prabhakar BS. 2005. IL-10-producing CD4+CD25+ regulatory T cells play a critical role in granulocyte-macrophage colony-stimulating factor-induced suppression of experimental autoimmune thyroiditis. J Immunol 174: 7006–7013. [DOI] [PubMed] [Google Scholar]
- 21.Vasu C, Dogan RN, Holterman MJ, and Prabhakar BS. 2003. Selective induction of dendritic cells using granulocyte macrophage-colony stimulating factor, but not fms-like tyrosine kinase receptor 3-ligand, activates thyroglobulin-specific CD4+/CD25+ T cells and suppresses experimental autoimmune thyroiditis. J Immunol 170: 5511–5522. [DOI] [PubMed] [Google Scholar]
- 22.Ganesh BB, Cheatem DM, Sheng JR, Vasu C, and Prabhakar BS. 2009. GM-CSF-induced CD11c+CD8a--dendritic cells facilitate Foxp3+ and IL-10+ regulatory T cell expansion resulting in suppression of autoimmune thyroiditis. Int Immunol 21: 269–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Courtney AH, Lo WL, and Weiss A. 2018. TCR Signaling: Mechanisms of Initiation and Propagation. Trends Biochem Sci 43: 108–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baeyens A, Saadoun D, Billiard F, Rouers A, Gregoire S, Zaragoza B, Grinberg-Bleyer Y, Marodon G, Piaggio E, and Salomon BL. 2015. Effector T cells boost regulatory T cell expansion by IL-2, TNF, OX40, and plasmacytoid dendritic cells depending on the immune context. J Immunol 194: 999–1010. [DOI] [PubMed] [Google Scholar]
- 25.Kumar P, Marinelarena A, Raghunathan D, Ragothaman VK, Saini S, Bhattacharya P, Fan J, Epstein AL, Maker AV, and Prabhakar BS. 2019. Critical role of OX40 signaling in the TCR-independent phase of human and murine thymic Treg generation. Cell Mol Immunol 16: 138–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vail ME, Chaisson ML, Thompson J, and Fausto N. 2002. Bcl-2 expression delays hepatocyte cell cycle progression during liver regeneration. Oncogene 21: 1548–1555. [DOI] [PubMed] [Google Scholar]
- 27.Zinkel S, Gross A, and Yang E. 2006. BCL2 family in DNA damage and cell cycle control. Cell Death Differ 13: 1351–1359. [DOI] [PubMed] [Google Scholar]
- 28.Chen KM, Wei YZ, Sharp GC, and Braley-Mullen H. 2001. Induction of experimental autoimmune thyroiditis in IL-12(−/−) mice. J Immunol 167: 1720–1727. [DOI] [PubMed] [Google Scholar]
- 29.Wang J, Ioan-Facsinay A, van der Voort EI, Huizinga TW, and Toes RE. 2007. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur J Immunol 37: 129–138. [DOI] [PubMed] [Google Scholar]
- 30.Kmieciak M, Gowda M, Graham L, Godder K, Bear HD, Marincola FM, and Manjili MH. 2009. Human T cells express CD25 and Foxp3 upon activation and exhibit effector/memory phenotypes without any regulatory/suppressor function. Journal of Translational Medicine 7:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar P, Bhattacharya P, and Prabhakar BS. 2018. A comprehensive review on the role of co-signaling receptors and Treg homeostasis in autoimmunity and tumor immunity. J Autoimmun. 95:77–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Au-Yeung BB, Zikherman J, Mueller JL, Ashouri JF, Matloubian M, Cheng DA, Chen Y, Shokat KM, and Weiss A. 2014. A sharp T-cell antigen receptor signaling threshold for T-cell proliferation. Proc Natl Acad Sci U S A 111: E3679–3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahmud SA, Manlove LS, Schmitz HM, Xing Y, Wang Y, Owen DL, Schenkel JM, Boomer JS, Green JM, Yagita H, Chi H, Hogquist KA, and Farrar MA. 2014. Costimulation via the tumor-necrosis factor receptor superfamily couples TCR signal strength to the thymic differentiation of regulatory T cells. Nat Immunol 15: 473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, Parizot C, Taflin C, Heike T, Valeyre D, Mathian A, Nakahata T, Yamaguchi T, Nomura T, Ono M, Amoura Z, Gorochov G, and Sakaguchi S. 2009. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 30: 899–911. [DOI] [PubMed] [Google Scholar]
- 35.Josefowicz SZ, Wilson CB, and Rudensky AY. 2009. Cutting Edge: TCR Stimulation Is Sufficient for Induction of Foxp3 Expression in the Absence of DNA Methyltransferase 1. J Immunol 182: 6648–6652. [DOI] [PubMed] [Google Scholar]
- 36.Grinberg-Bleyer Y, Caron R, Seeley JJ, De Silva NS, Schindler CW, Hayden MS, Klein U, and Ghosh S. 2018. The Alternative NF-kappa B Pathway in Regulatory T Cell Homeostasis and Suppressive Function. J Immunol 200: 2362–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anderson AC, Joller N, and Kuchroo VK. 2016. Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity 44: 989–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walker LS 2013. Treg and CTLA-4: two intertwining pathways to immune tolerance. J Autoimmun 45: 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takeda I, Ine S, Killeen N, Ndhlovu LC, Murata K, Satomi S, Sugamura K, and Ishii N. 2004. Distinct roles for the OX40-OX40 ligand interaction in regulatory and nonregulatory T cells. J Immunol 172: 3580–3589. [DOI] [PubMed] [Google Scholar]
- 40.Barsoumian HB, Yolcu ES, and Shirwan H. 2016. 4–1BB Signaling in Conventional T Cells Drives IL-2 Production That Overcomes CD4+CD25+FoxP3+ T Regulatory Cell Suppression. PLoS One 11: e0153088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haddad CS, Bhattacharya P, Alharshawi K, Marinelarena A, Kumar P, El-Sayed O, Elshabrawy HA, Epstein AL, and Prabhakar BS. 2016. Age-dependent divergent effects of OX40L treatment on the development of diabetes in NOD mice. Autoimmunity 49: 298–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barbarulo A, Grazioli P, Campese AF, Bellavia D, Di Mario G, Pelullo M, Ciuffetta A, Colantoni S, Vacca A, Frati L, Gulino A, Felli MP, and Screpanti I. 2011. Notch3 and canonical NF-kappaB signaling pathways cooperatively regulate Foxp3 transcription. J Immunol 186: 6199–6206. [DOI] [PubMed] [Google Scholar]
- 43.Campese AF, Grazioli P, Colantoni S, Anastasi E, Mecarozzi M, Checquolo S, De Luca G, Bellavia D, Frati L, Gulino A, and Screpanti I. 2009. Notch3 and pTalpha/pre-TCR sustain the in vivo function of naturally occurring regulatory T cells. Int Immunol 21: 727–743. [DOI] [PubMed] [Google Scholar]
- 44.Nagar M, Jacob-Hirsch J, Vernitsky H, Berkun Y, Ben-Horin S, Amariglio N, Bank I, Kloog Y, Rechavi G, and Goldstein I. 2010. TNF Activates a NF-kappa B-Regulated Cellular Program in Human CD45RA(−) Regulatory T Cells that Modulates Their Suppressive Function. J Immunol 184: 3570–3581. [DOI] [PubMed] [Google Scholar]
- 45.Rudensky AY 2011. Regulatory T cells and Foxp3. Immunol Rev 241: 260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sakaguchi S, Miyara M, Costantino CM, and Hafler DA. 2010. FOXP3(+) regulatory T cells in the human immune system. Nature Reviews Immunology 10: 490–500. [DOI] [PubMed] [Google Scholar]
- 47.Smith DJ, Lin LJ, Moon H, Pham AT, Wang X, Liu S, Ji S, Rezek V, Shimizu S, Ruiz M, Lam J, Janzen DM, Memarzadeh S, Kohn DB, Zack JA, Kitchen SG, An DS, and Yang L. 2016. Propagating Humanized BLT Mice for the Study of Human Immunology and Immunotherapy. Stem Cells Dev 25: 1863–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.