Abstract
Purpose:
Lower urinary tract symptoms (LUTS) are common in men and women. The Lower Urinary Tract Dysfunction Research Network (LURN) sought to create a brief, clinically relevant tool to improve upon existing measurements of LUTS in both men and women.
Materials and Methods:
Using a modified Delphi methodology during an expert consensus meeting, we reduced the LURN Comprehensive Assessment of Self-Reported Urinary Symptoms (CASUS) questionnaire to a very brief set of clinically-relevant items measuring LUTS. The sum score of these items was evaluated by comparing to the American Urological Association Symptom Index (AUA-SI), the Urinary Distress Inventory Short Form (UDI-6; in women only), and LUTS screening questions from CASUS, using Pearson correlations, regression analysis, and receiver operating characteristic (ROC) curves.
Results:
The 10-item LURN-Symptom Index (LURN SI-10) assesses urinary frequency, nocturia, urgency, incontinence, bladder pain, voiding, and post-micturition symptoms (score range: 0–38). The correlation between the LURN SI-10 score and the AUA-SI was 0.77 in men and 0.70 in women. The UDI-6 and LURN SI-10 were highly correlated in women (r=0.76). The LURN SI-10 showed good accuracy in predicting both moderate and severe LUTS as defined by the AUA-SI (area under the ROC curve [AUC] range 0.82–0.90). Similar accuracy was shown in predicting different levels of symptom status using the UDI (AUC range 0.84–0.86).
Conclusions:
The LURN SI-10 correlates well with the AUA-SI and UDI-6. It includes items related to a broader spectrum of LUTS, particularly incontinence, bladder pain, and post-micturition symptoms, and applies to both men and women.
Keywords: LUTS, urinary symptoms, LURN SI-10, patient reported outcomes
Introduction
Lower urinary tract symptoms (LUTS) are common in men and women, and patients often present with multiple symptoms.1,2 Although there are several available questionnaires to assess LUTS,3–5 there remains a need for a brief-yet-comprehensive clinical questionnaire that can be used in both men and women to capture a broad spectrum of symptoms. For example, the American Urological Association Symptom Index (AUA-SI) is widely-used, but because it was developed initially for men, it does not include a question on urinary incontinence.5 Similarly, the Pelvic Floor Distress Inventory6 is widely used, but is condition-specific and only applies to women.
A brief clinical assessment that can be used informatively with both men and women would therefore be an advance. An objective of the Symptoms of Lower Urinary Tract Dysfunction Research Network (LURN)7 was to create a brief, comprehensive, and clinically relevant tool to improve upon existing measurements of LUTS in men and women. LURN provides such an opportunity with its Comprehensive Assessment of Self-Reported Urinary Symptoms (CASUS) questionnaire, developed to support phenotyping of patients with LUTS by characterizing patients through detailed symptom reporting, including the nature, severity, and bother associated with the full range of LUTS.8 The 93-item CASUS phenotyping questionnaire was reduced to a 29-item outcome questionnaire (LURN SI-29) that includes assessment of incontinence, urgency, voiding difficulty, bladder pain, nocturia, and other symptoms.9 The current paper describes a procedure to reduce the LURN SI-29 to a brief form that covers the major LUTS and is practical to use in a clinical setting.
Methods
Data were obtained from the LURN Observational Cohort Study.10 Treatment-seeking men and women were recruited between June 2015 and January 2017 and completed in-person clinic visits at baseline, 3 months, and 12 months. At baseline, participants completed a physical exam and questionnaires related to LUTS and other symptoms. Questionnaires were repeated at 3 and 12 months. Because CASUS was developed while the observational cohort study was underway, only a subset of participants were administered the CASUS (and therefore the LURN SI-29) at baseline. Most participants, however, completed CASUS at 12 months, so the analyses for this report used responses from the LURN SI-29 at the 12-month visit. We included all participants who had completed 85% or more of the CASUS items at the 12-month visit.
At the 12-month assessment in the LURN Observational Study, participants completed the CASUS questionnaire,8 the AUA-SI,5 and the Urinary Distress Inventory Short Form (UDI-6; women only).11 The CASUS is a 93-item phenotyping questionnaire developed for use in the LURN Observational Study and elsewhere, intended to help advance the search for well-characterized subgroups of patients who present for treatment of LUTS.8 Included within CASUS were four general questions about bladder and urinary function that were used to sort participants into severity groups for receiver operating characteristic (ROC) analysis: Satisfaction with bladder function (Very/Extremely Satisfied vs. Not at all/Somewhat Satisfied); Urinary symptom bother (Very/Extremely Bothered vs. Not at all/Somewhat Bothered); Urinary/bladder problem frequency (About half the time or more vs. Never/A few times); and Rating urinary/bladder function (Good/Very good vs. Poor/Very Poor).
The AUA-SI5 was originally designed for use in patients with benign prostatic hyperplasia (BPH) and assesses the frequency of various LUTS; it also includes one question about the degree of bother associated with LUTS. The UDI-6 has six items about the degree of bother associated with LUTS that occur with female pelvic floor disorders.11
The previously-scaled LURN SI-299 was the starting point for the development of the brief clinical form. We chose this starting point because prior work had distilled the 93-item CASUS into this set of representative items targeted for use in outcomes research, such as clinical trials.9 To evaluate validity of the new brief clinical form, we compared responses and summary scores to those on the AUA-SI and UDI-6, using these existing tools as anchors to which the newly-developed LURN clinical form could be compared. We also used the four general CASUS questions described above as severity anchors to help evaluate validity of the clinical form.
Procedure.
On October 23, 2017, the LURN Steering Committee convened a panel of experts from its membership. The expert review panel was comprised of 24 participating investigators in the LURN Network. These experts included 10 urologists, 5 urogynecologists, 4 patient reported outcome measurement specialists, 3 quantitative specialists, a nephrologist, and a transplant surgeon. Of the 24 participants, 10 were women. Only the LUTS clinicians on the panel (7 women; 8 men) participated in the discussion and decision-making regarding the composition of the brief clinical form.
We employed a modified Delphi approach to facilitating group discussion and consensus-based decision-making. This four-hour meeting had two objectives: 1) To develop an outcome tool with subscales derived from the CASUS questionnaire; and 2) To develop a brief clinical form that improves upon existing measures of clinical symptom reporting, derived from the set of questions selected under the first objective. The first objective included reviewing results of factor analyses and making final decisions on the naming and item content of subscales (Incontinence, Urgency, Voiding Difficulty, Bladder Pain, Nocturia), and selecting additional clinically-relevant questions, resulting in the LURN SI-29 as reported elsewhere.9 The second objective – the brief clinical form – is the subject of this manuscript.
As reported elsewhere, the first half of this meeting, with discussion and input from the entire panel of LURN investigators, resulted in the formation of the LURN SI-29, comprised of five multi-item subscales and nine additional questions not included in any of those subscales. Optional item sets were proffered, discussed, and decided upon through a consensus-building discussion. The resulting composition was unanimously endorsed. Upon completion of this 29-item set, the clinicians on the panel continued discussion in the second half of the meeting, with the instruction of selecting one to two items from each subscale and a finite number of items from among the miscellaneous nine items not included in one of the multi-item subscales. Discussion ensued, scale-by-scale, until consensus was reached on which item (or items) from each set should be included on the clinical form. The focus of discussion was on selecting those items that were regarded as actionable or at least indicative of the need for further clinical interrogation. All item selection decisions were made without member dissent and were thereby assumed to be unanimous.
The 10 items (including frequency, nocturia, urgency, incontinence, bladder pain, voiding, and post-micturition symptoms) were summed to form a 10-item LURN-Symptom Index (LURN SI-10), with score range from 0 to 38. We used all available response data from the 12-month assessment to evaluate the validity of the brief clinical form. Specifically, we compared the LURN SI-10 total score to participant responses on the general screening questions regarding bladder and urinary function described above. Medians and interquartile ranges (IQR) for LURN SI-10 scores were reported by response level on the general screening questions, and relationships between LURN SI-10 scores and responses to the screening questions were examined using proportional odds models. The LURN SI-10 score was then compared to the AUA-SI and the UDI-6 using Pearson correlations and ROC curves. ROC curves were plotted to illustrate the diagnostic ability of various LURN-SI discrimination thresholds as binary classifiers of case definitions defined by the AUA-SI, UDI-6, and the four global screening items from CASUS.
Results
The longitudinal LURN Observational Study enrolled 1064 LUTS treatment-seeking men and women whose characteristics have been described elsewhere.10 The subsample used for this study includes 717 participants with complete response data on the LURN SI-10 at the 12-month assessment. As with the overall LURN Observational cohort, the participants were, on average, 60 years old; they were mostly non-Hispanic white and well educated (Table 1). We have treatment information on 90% of the enrolled patients: During the year on study prior to the assessment used for this report, 79% of participants received at least one of the following three treatments: 43% were taking medication for LUTS; 50% reported some sort of non-traditional or non-medicinal treatment; 17% underwent a surgical procedure.
Table 1:
Sample Characteristics of Subsample Completing the LURN SI-10
| Overall (n=717) |
Women (n=320) |
Men (n=397) |
|
|---|---|---|---|
| Age | 59.6 (13.4) | 56.7 (14.0) | 62.0 (12.5) |
| Race | |||
| African-American | 82 (12%) | 47 (15%) | 35 (9%) |
| Other | 62 (9%) | 20 (6%) | 42 (11%) |
| White | 569 (80%) | 251 (79%) | 318 (81%) |
| Ethnicity | |||
| Hispanic/Latino | 31 (4%) | 14 (4%) | 17 (4%) |
| Non-Hispanic/Non-Latino | 672 (94%) | 298 (93%) | 374 (94%) |
| Ethnicity unknown | 14 (2%) | 8 (3%) | 6 (2%) |
| Education | |||
| < High School/GED | 14 (2%) | 4 (1%) | 10 (3%) |
| HS Diploma/GED | 59 (8%) | 21 (7%) | 38 (10%) |
| Some college | 155 (22%) | 84 (26%) | 71 (19%) |
| Associate’s Degree | 54 (8%) | 29 (9%) | 25 (7%) |
| Bachelor’s Degree | 196 (28%) | 99 (31%) | 97 (25%) |
| Graduate Degree | 222 (32%) | 82 (26%) | 140 (37%) |
| Employment Status | |||
| Employed part-time | 83 (12%) | 48 (15%) | 35 (9%) |
| Employed full-time | 275 (39%) | 127 (40%) | 148 (38%) |
| Unemployed | 22 (3%) | 9 (3%) | 13 (3%) |
| Not employed | 328 (46%) | 135 (42%) | 193 (50%) |
| Marital Status | |||
| Married/civil union | 480 (67%) | 190 (60%) | 290 (74%) |
| Living with partner | 19 (3%) | 9 (3%) | 10 (3%) |
| Separated/Divorced | 89 (13%) | 50 (16%) | 39 (10%) |
| Widowed | 30 (4%) | 23 (7%) | 7 (2%) |
| Single | 94 (13%) | 47 (15%) | 47 (12%) |
| Body mass index | 28.8 (25.4–32.9) | 29.1 (24.9–34.2) | 28.6 (25.8–32.4) |
| Current Smoker | 44 (6%) | 19 (6%) | 25 (6%) |
| Diabetes | 113 (16%) | 46 (14%) | 67 (17%) |
| Functional Comorbidity Index | 2.0 (1.0–3.0) | 2.0 (1.0–3.5) | 2.0 (1.0–3.0) |
| Postvoid residual (ml) | 25.0 (0.0–70.0) | 21.0 (5.0–60.0) | 28.0 (0.0–79.0) |
| AUA-SI at 12 months | 9.0 (5.0–14.0) | 8.0 (5.0–12.0) | 10.0 (6.0–16.0) |
| UDI-6 at 12 months | - | 25.0 (8.3–41.7) | - |
Most participants had AUA-SI scores in the moderate range (median score=9, IQR=5–14). Among women, median UDI-6 score was 25 (IQR 8.3–41.7). The median LURN SI-10 score was 7 in both men and women, with IQR from 5 to 10 and 4 to 10, respectively. The maximum score was 26 in women and 25 in men.
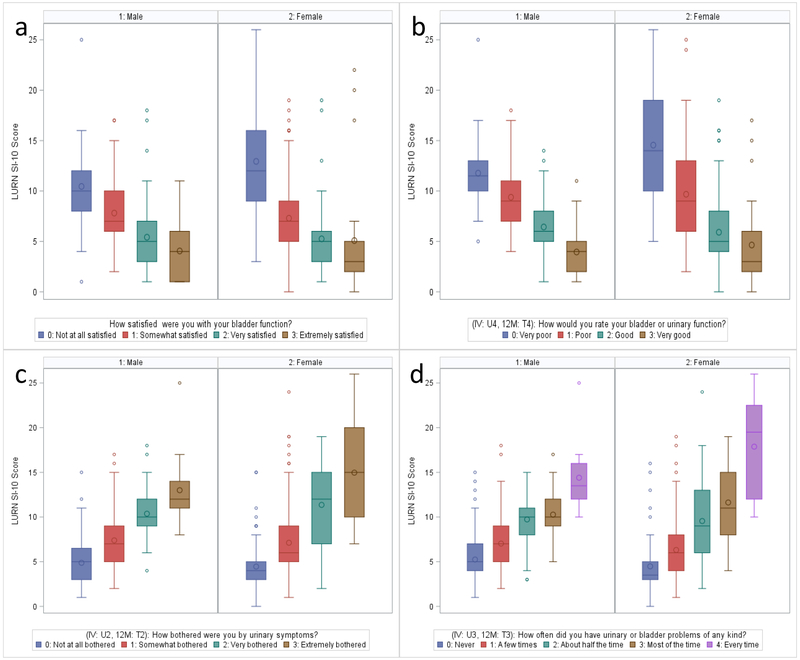
Among both men and women, LURN SI-10 scores were consistent with responses to general screening questions relating bladder and urinary function (Table 2, Figures 1a–d). For positive questions such as satisfaction and rating of bladder function where higher ratings indicated more satisfaction and better assessment of function, LURN SI-10 scores decreased (improved) with increased ratings (Figures 1a and 1b). For both men and women, median LURN SI-10 scores were five or lower for participants indicating they were very or extremely satisfied with their bladder function and six or lower for participants rating their bladder or urinary function as good or very good. Proportional odds models indicated odds of higher ratings for these items to decrease by 78–92% per 5 unit increase in LURN SI-10 score (Table 3).
Table 2.
Distribution of LURN SI-10 Scores by Sex and Global Screening Questions
| Question | Response | N | Median (IQR) | Mean (SD) | Range |
|---|---|---|---|---|---|
| Women | |||||
| Overall | 320 | 7 (4, 10) | 7.77 (4.90) | 0–26 | |
| How satisfied were you with your bladder function? | 0: Not at all satisfied | 61 | 12 (9, 16) | 12.93 (5.34) | 3–26 |
| 1: Somewhat satisfied | 164 | 7 (5, 9) | 7.30 (3.63) | 0–19 | |
| 2: Very satisfied | 68 | 5 (3, 6) | 5.25 (3.27) | 1–19 | |
| 3: Extremely satisfied | 25 | 3 (2, 5) | 5.08 (5.80) | 0–22 | |
| How bothered were you by urinary symptoms? | 0: Not at all bothered | 68 | 4 (3, 5) | 4.46 (2.94) | 0–15 |
| 1: Somewhat bothered | 185 | 6 (5, 9) | 7.12 (3.73) | 1–24 | |
| 2: Very bothered | 36 | 12 (7, 15) | 11.36 (4.64) | 2–19 | |
| 3: Extremely bothered | 30 | 15 (10, 20) | 14.97 (5.64) | 7–26 | |
| How often did you have urinary or bladder problems of any kind? | 0: Never | 54 | 4 (3, 5) | 4.48 (3.37) | 0–16 |
| 1: A few times | 159 | 6 (4, 8) | 6.31 (3.05) | 1–19 | |
| 2: About half the time | 40 | 9 (6, 13) | 9.55 (5.01) | 2–24 | |
| 3: Most of the time | 46 | 11 (8, 15) | 11.63 (4.22) | 4–19 | |
| 4: Every time | 16 | 20 (12, 23) | 17.88 (5.33) | 10–26 | |
| How would you rate your bladder or urinary function? | 0: Very poor | 29 | 14 (10, 19) | 14.55 (5.45) | 5–26 |
| 1: Poor | 102 | 9 (6, 13) | 9.68 (4.72) | 2–25 | |
| 2: Good | 151 | 5 (4, 8) | 5.91 (3.14) | 0–19 | |
| 3: Very good | 36 | 3 (2, 6) | 4.64 (3.81) | 0–17 | |
| Men | |||||
| Overall | 397 | 7 (5, 10) | 7.41 (3.40) | 1–25 | |
| How satisfied were you with your bladder function? | 0: Not at all satisfied | 55 | 10 (8, 12) | 10.45 (3.49) | 1–25 |
| 1: Somewhat satisfied | 223 | 7 (6, 10) | 7.82 (2.83) | 2–17 | |
| 2: Very satisfied | 99 | 5 (3, 7) | 5.41 (2.85) | 1–18 | |
| 3: Extremely satisfied | 19 | 4 (1, 6) | 4.05 (3.21) | 1–11 | |
| How bothered were you by urinary symptoms? | 0: Not at all bothered | 88 | 5 (3, 7) | 4.88 (2.61) | 1–15 |
| 1: Somewhat bothered | 246 | 7 (5, 9) | 7.39 (2.77) | 2–17 | |
| 2: Very bothered | 46 | 10 (9, 12) | 10.37 (3.01) | 4–18 | |
| 3: Extremely bothered | 15 | 12 (11, 14) | 13.00 (4.05) | 8–25 | |
| How often did you have urinary or bladder problems of any kind? | 0: Never | 113 | 5 (4, 7) | 5.25 (2.72) | 1–15 |
| 1: A few times | 178 | 7 (5, 9) | 7.03 (2.59) | 2–18 | |
| 2: About half the time | 53 | 10 (8, 11) | 9.74 (2.89) | 3–15 | |
| 3: Most of the time | 41 | 10 (9, 12) | 10.27 (2.64) | 5–17 | |
| 4: Every time | 10 | 14 (12, 16) | 14.40 (4.40) | 10–25 | |
| How would you rate your bladder or urinary function? | 0: Very poor | 22 | 12 (10, 13) | 11.77 (4.12) | 5–25 |
| 1: Poor | 123 | 9 (7, 11) | 9.38 (3.12) | 4–18 | |
| 2: Good | 209 | 6 (5, 8) | 6.43 (2.44) | 1–14 | |
| 3: Very good | 39 | 4 (2, 5) | 3.95 (2.28) | 1–11 | |
Figure 1:
Distribution of LURN SI-10 score by responses to global screening questions rating (a) satisfaction with bladder function, (b) rating of bladder or urinary function, (c) bother associated with urinary symptoms, and (d) frequency of urinary or bladder problems.
Table 3:
Proportional Odds Models by Sex
| Odds Ratio | Lower 95% CI | Upper 95% CI | p-value | |
|---|---|---|---|---|
| Women | ||||
| Probability of increasing bother per 5 unit increase in LURN SI-10 Score | 7.26 | 5.15 | 10.22 | <.0001 |
| Probability of increasing frequency of urinary/bladder problems per 5 unit increase in LURN SI-10 Score | 9.07 | 6.52 | 12.62 | <.0001 |
| Probability of increasing positive rating of urinary/bladder function per 5 unit increase in LURN SI-10 Score | 0.13 | 0.10 | 0.19 | <.0001 |
| Probability of increasing satisfaction per 5 unit increase in LURN SI-10 Score | 0.22 | 0.17 | 0.29 | <.0001 |
| Men | ||||
| Probability of increasing bother per 5 unit increase in LURN SI-10 Score | 10.22 | 5.22 | 19.98 | <.0001 |
| Probability of increasing frequency of urinary/bladder problems per 5 unit increase in LURN SI-10 Score | 17.11 | 8.88 | 32.97 | <.0001 |
| Probability of increasing positive rating of urinary/bladder function per 5 unit increase in LURN SI-10 Score | 0.08 | 0.04 | 0.16 | <.0001 |
| Probability of increasing satisfaction per 5 unit increase in LURN SI-10 Score | 0.17 | 0.12 | 0.24 | <.0001 |
Similarly, for questions regarding bother and frequency of symptoms, participants with higher ratings (indicating more bother / higher frequency) reported higher (worse) LURN SI-10 scores (Table 2, Figures 1c and 1d). Participants reporting to be “very” or “extremely” bothered by their urinary symptoms or having urinary or bladder problems of any kind “most of the time” or “every time” had median LURN SI-10 scores of 10 or higher. Among women, estimated odds of higher bother and frequency of symptoms was 7–9 times higher per 5 unit increase in LURN SI-10 score. Among men, odds were 10–17 times higher (Table 3). Overall, higher symptom severity as defined by the LURN SI-10 was strongly positively associated with reporting higher levels of bother and more frequent symptoms.
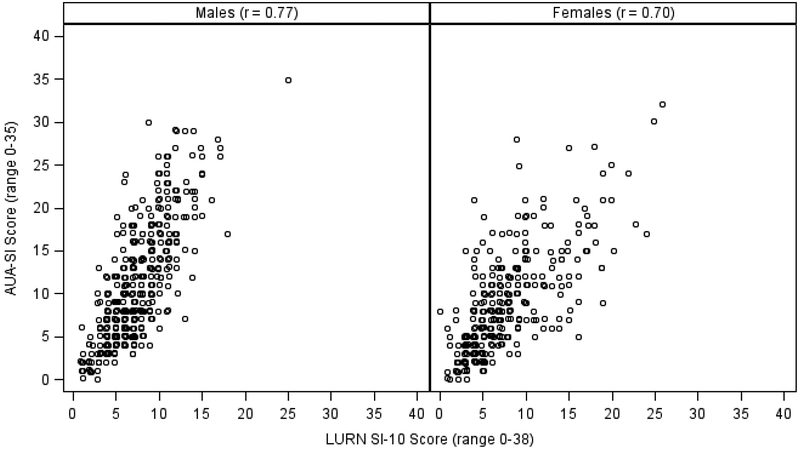
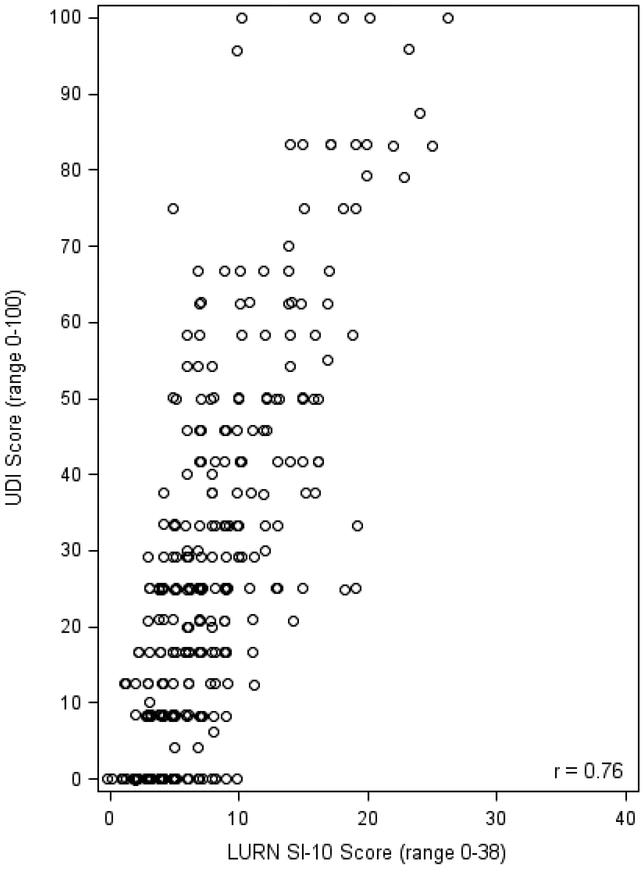
The correlation between the LURN SI-10 score and the AUA-SI was 0.77 in men and 0.70 in women (Figure 2). The UDI-6 and LURN SI-10 were also highly correlated in women (r = 0.76, Figure 3). The LURN SI-10 showed good accuracy in predicting both moderate (AUA SI>7) and severe (AUA SI>19) LUTS as defined by the AUA-SI (AUC range 0.82–0.90, Table 4, Supplementary Figure 1). Using cutoffs of 0 (no urinary distress) and 16.7 (90th percentile among continent participants) resulted in good to excellent accuracy (AUC range 0.84–0.86, Table 4, Supplementary Figure 2). In addition, 67 (21%) and 5 (1.6%) participants scored the minimum and maximum score on the UDI-6, respectively, while only 2 (0.6%) participants scored the minimum on the LURN SI-10 and none scored the maximum score.
Figure 2:
Correlation between LURN SI-10 and AUA-SI by sex.
Figure 3:
Correlation between LURN SI-10 and UDI-6 (women only)
Table 4:
ROC analysis (AUC with 95% CI)
| Outcome | Men (n=397) | Women (n=320) | Overall (n=717) |
|---|---|---|---|
| AUA-SI>7 (Moderate/Severe vs. Mild)* | 0.86 (0.83–0.90) | 0.82 (0.78–0.87) | 0.84 (0.81–0.87) |
| AUA-SI>19 (Severe vs. Mild/Moderate)* | 0.90 (0.86–0.94) | 0.88 (0.79–0.97) | 0.87 (0.84–0.91) |
| Satisfaction with bladder function (Very/Extremely Satisfied vs. Not at all/Somewhat Satisfied) | 0.79 (0.74–0.84) | 0.76 (0.71–0.83) | 0.78 (0.74–0.82) |
| Urinary symptom bother (Very/Extremely Bothered vs. Not at all/Somewhat Bothered) | 0.84 (0.79–0.89) | 0.85 (0.80–0.90) | 0.84 (0.81–0.88) |
| Urinary/bladder problem frequency (About half the time or more vs. Never/A few times) | 0.84 (0.80–0.89) | 0.83 (0.78–0.88) | 0.83 (0.80–0.87) |
| Rating urinary/bladder function (Good/Very good vs. Poor/Very Poor) | 0.81 (0.76–0.85) | 0.81 (0.76–0.85) | 0.80 (0.77–0.84) |
| UDI-6>0 | - | 0.84 (0.80–0.89) | - |
| UDI-6>16.7 | - | 0.86 (0.83–0.90) | - |
Excludes 17 males and 15 females due to missing AUA-SI (4.5% of overall sample). All other analyses exclude ≤1% of sample due to missing data.
Discussion
The LURN SI-10, a brief clinical assessment derived from the longer LURN CASUS, shows strong and expected associations with the AUA-SI and UDI-6. It improves upon these questionnaires by including items related to a broader spectrum of LUTS, particularly incontinence, bladder pain, and post-micturition symptoms, that apply to both men and women. An advantage of the LURN SI-10 is that both men and women were included in the development, from the initial qualitative interviews, to the item cognitive debriefing, to formal psychometric testing.8,9 In addition, the group of experts that reviewed and selected items for inclusion represented both men and women from urology and urogynecology. Thus, unlike the AUA-SI and the UDI-6, male and female perspectives were equally represented in the development of these items. Our approach to development provides evidence for the content validity of this measure.
Regarding concurrent validity, high correlations of LURN SI-10 with AUA-SI and UDI-6 were very reassuring. Similarly, the LURN SI-10 scores of participants satisfied with their bladder function were better than those of dissatisfied participants, and the LURN SI-10 scores of those bothered by their symptoms were worse than those not bothered. The ROC analyses of all these “anchors” produced highly significant C statistics, with AUC ranging from 76% to 94%.
There are some limitations that help point to further research needed. First, although patients were included extensively during item development and testing, the LURN SI-10 selection meeting did not include patient representatives. While these items were selected by a clinical panel to optimize their value in practice settings, it will be important to confirm their clinical utility from the patient perspective as well. Second, this was a cross sectional data set used to test performance of the instrument. Therefore, we do not know about the stability (test-retest reliability) or responsiveness of the questions to meaningful change. Finally, the level of symptom severity in this sample was, on average, in the mild range, perhaps because this cohort had been in treatment for at least a year. More data on people with moderate to severe pathology will be of value.
Future studies evaluating the correlation of LURN SI-10 scores with symptom improvements, and defining meaningful changes in LURN SI-10 scores are planned. We anticipate that future work with the LURN SI-10 will determine its responsiveness to change over time with known effective interventions, which will help clinicians gauge treatment effectiveness. It is also possible that these items – and the overall summary score – will help to subtype and diagnose patients as well as to guide treatment planning. Although there is a summary score option, it may be that the clinical utility of the LURN SI-10 will be enhanced by attention to individual item responses. Any individual item response could spark discussion with the patient about symptom severity, frequency, life impact, bother, and need for clinical action, beyond its contribution to a single total score.
Future work should examine how the LURN SI-10 items differ across diagnostic groups, and how it performs across varying levels of symptom severity. It will also be important to assess how well the SI-10 guides treatment, and how accurately it reflects the outcomes of those treatments. Because the questionnaire is brief, it also may be a good option for epidemiology studies or other large protocols where many concepts are being assessed, necessitating a small number of items dedicated to LUTS.
Supplementary Material
Acknowledgements
This is publication number 22 of the Symptoms of Lower Urinary Tract Dysfunction Research Network (LURN).
The following individuals were instrumental in the planning and conduct of this study at each of the participating institutions:
Duke University, Durham, North Carolina (DK097780): PI: Cindy Amundsen, MD, Kevin Weinfurt, PhD; Co-Is: Kathryn Flynn, PhD, Matthew O. Fraser, PhD, Todd Harshbarger, PhD, Eric Jelovsek, MD, Aaron Lentz, MD, Drew Peterson, MD, Nazema Siddiqui, MD, Alison Weidner, MD; Study Coordinators: Carrie Dombeck, MA, Robin Gilliam, MSW, Akira Hayes, Shantae McLean, MPH
University of Iowa, Iowa City, IA (DK097772): PI: Karl Kreder, MD, MBA, Catherine S Bradley, MD, MSCE, Co-Is: Bradley A. Erickson, MD, MS, Susan K. Lutgendorf, PhD, Vince Magnotta, PhD, Michael A. O’Donnell, MD, Vivian Sung, MD; Study Coordinator: Ahmad Alzubaidi
Northwestern University, Chicago, IL (DK097779): PIs: David Cella, Brian Helfand, MD, PhD; Co-Is: James W Griffith, PhD, Kimberly Kenton, MD, MS, Christina Lewicky-Gaupp, MD, Todd Parrish, PhD, Jennie Yufen Chen, PhD, Margaret Mueller, MD; Study Coordinators: Sarah Buono, Maria Corona, Beatriz Menendez, Alexis Siurek, Meera Tavathia, Veronica Venezuela, Azra Muftic, Pooja Talaty, Jasmine Nero. Dr. Helfand, Ms. Talaty, and Ms. Nero are at NorthShore University HealthSystem.
University of Michigan Health System, Ann Arbor, MI (DK099932): PI: J Quentin Clemens, MD, FACS, MSCI; Co-Is: Mitch Berger, MD, PhD, John DeLancey, MD, Dee Fenner, MD, Rick Harris, MD, Steve Harte, PhD, Anne P. Cameron, MD, John Wei, MD; Study Coordinators: Morgen Barroso, Linda Drnek, Greg Mowatt, Julie Tumbarello
University of Washington, Seattle Washington (DK100011): PI: Claire Yang, MD; Co-I: John L. Gore, MD, MS; Study Coordinators: Alice Liu, MPH, Brenda Vicars, RN
Washington University in St. Louis, St. Louis Missouri (DK100017): PI: Gerald L. Andriole, MD, H. Henry Lai; Co-I: Joshua Shimony, MD, PhD; Study Coordinators: Susan Mueller, RN, BSN, Heather Wilson, LPN, Deborah Ksiazek, BS, Aleksandra Klim, RN, MHS, CCRC
National Institute of Diabetes and Digestive and Kidney Diseases, Division of Kidney, Urology, and Hematology, Bethesda, MD: Project Scientist: Ziya Kirkali MD; Project Officer: Christopher Mullins PhD; NIH Personnel: Tamara Bavendam, MD, Robert Star, MD, Jenna Norton
Arbor Research Collaborative for Health, Data Coordinating Center (DK097776 and DK099879): PI: Robert Merion, MD, FACS; Co-Is: Victor Andreev, PhD, DSc, Brenda Gillespie, PhD, Gang Liu, PhD, Abigail Smith, PhD; Project Manager: Melissa Fava, MPA, PMP; Clinical Study Process Manager: Peg Hill-Callahan, BS, LSW; Clinical Monitor: Timothy Buck, BS, CCRP; Research Analysts: Margaret Helmuth, MA, Jon Wiseman, MS; Project Associate: Julieanne Lock, MLitt
Funding/Support
This study is supported by the National Institute of Diabetes & Digestive & Kidney Diseases through cooperative agreements (grants DK097780, DK097772, DK097779, DK099932, DK100011, DK100017, DK097776, DK099879).
Research reported in this publication was supported at Northwestern University, in part, by the National Institutes of Health’s National Center for Advancing Translational Sciences, Grant Number UL1TR001422. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviation Key
- AUC
Area under the curve
- AUA-SI
American Urological Association Symptom Index
- BPH
Benign prostatic hyperplasia
- CASUS
Comprehensive Assessment of Self-Reported Urinary Symptoms
- IQR
interquartile ranges
- LURN
The Symptoms of Lower Urinary Tract Dysfunction Research Network
- LURN SI-10
10-item LURN-Symptom Index
- LUTS
Lower urinary tract symptoms
- ROC
Receiver operating characteristic
- UDI
Urinary Distress Inventory Short Form
References
- 1.Coyne KS, Sexton CC, Thompson CL, et al. The prevalence of lower urinary tract symptoms (LUTS) in the USA, the UK and Sweden: results from the Epidemiology of LUTS (EpiLUTS) study. BJU international. 104: 352–360, 2009. [DOI] [PubMed] [Google Scholar]
- 2.Sexton CC, Coyne KS, Kopp ZS, et al. The overlap of storage, voiding and postmicturition symptoms and implications for treatment seeking in the USA, UK and Sweden: EpiLUTS. BJU international. 103 Suppl 3: 12–23, 2009. [DOI] [PubMed] [Google Scholar]
- 3.Griffith JW. Self-Report Measurement of Lower Urinary Tract Symptoms: A Commentary on the Literature Since 2011. Curr Urol Rep.;13: 420–426, 2012. [DOI] [PubMed] [Google Scholar]
- 4.Abrams P, Avery K, Gardener N, Donovan J, Board IA. The International Consultation on Incontinence Modular Questionnaire: www.iciq.net. J Urol 175 :1063–1066; 2006d. iscussion 1066. [DOI] [PubMed] [Google Scholar]
- 5.Barry MJ, Fowler FJ Jr., O’Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol 148: 1549–1557; 1992. discussion 1564. [DOI] [PubMed] [Google Scholar]
- 6.Barber MD, Kuchibhatla MN, Pieper CF, Bump RC. Psychometric evaluation of 2 comprehensive condition-specific quality of life instruments for women with pelvic floor disorders. Am J Obstet Gynecol.185: 1388–1395, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Yang CC, Weinfurt KP, Merion RM, et al. Symptoms of Lower Urinary Tract Dysfunction Research Network. J Urol. 196: 146–152, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weinfurt KP, Griffith JW, Flynn KE, et al. The Comprehensive Assessment of Self-Reported Urinary Symptoms (CASUS): A New Tool for Research on Subtypes of Patients with Lower Urinary Tract Symptoms. J Urol. 2019: Epub ahead of print] DOI 10.1097/JU.0000000000000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cella D, Smith AR, Griffith JW, et al. A New Outcome Measure for LUTS: Symptoms of Lower Urinary Tract Dysfunction Research Network Symptom Index-29 (LURN SI-29) Questionnaire. Neurourol Urodynam. 2019, under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cameron AP, Lewicky-Gaupp C, Smith AR, et al. Baseline lower urinary tract symptoms in patients enrolled in the Symptoms of Lower Urinary Tract Dysfunction Research Network (LURN): a prospective, observational cohort study. J Urol. 199: 1023, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shumaker SA, Wyman JF, Uebersax J, McClish D, Fantl JA. Health-related quality of life measures for women with urinary incontinence: the Incontinence Impact Questionnaire and the Urogenital Distress Inventory. Qual Life Res.. 3: 291–306, 1994. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.