Abstract
Inflammatory bowel disease (IBD) is a complex autoimmune disease with dysfunction in pattern recognition responses, including within the NLR family. Nucleotide-binding oligomerization domain, leucine rich repeat containing X1 (NLRX1) is a unique NLR with regulatory and anti-inflammatory functions resulting in protection from IBD in mouse models. NX-13 is an orally active, gut-restricted novel drug candidate that selectively targets and activates the NLRX1 pathway locally in the gut. In vitro and in vivo efficacy of NLRX1 activation by NX-13 was examined. Oral treatment with NX-13 ameliorates disease severity, colonic leukocytic infiltration and cytokine markers of inflammation in three mouse models of IBD (DSS, Mdr1a−/− and CD45RBhi adoptive transfer). Treatment of naïve CD4+ T cells with NX-13 in vitro decreases differentiation into Th1 and Th17 subsets with increased oxidative phosphorylation and decreased NF-κB activation and ROS. With stimulation by PMA/ionomycin, TNFα, or H2O2, PBMCs from ulcerative colitis (UC) patients treated with NX-13 had decreased NF-κB activity, TNFα+ and IFNγ+ CD4+ T cells and overall production of IL-6, MCP1 and IL-8. NX-13 activates NLRX1 to mediate a resistance to both inflammatory signaling and oxidative stress in mouse models and human primary cells from UC patients with effects on NF-κB activity and oxidative phosphorylation. NX-13 is a promising oral, gut-restricted NLRX1 agonist for treating IBD.
Introduction
Over 3 million people in America and 5 million worldwide suffer from inflammatory bowel disease (IBD). This widespread and debilitating illness results in decreased quality of life and significant healthcare related costs (1). The underlying immune dysfunction in Crohn’s disease (CD) (2, 3) and ulcerative colitis (UC) (4, 5) is with involvement of both innate and adaptive immunity culminating in a perturbed immune tolerance to the gut microbiome and other environmental factors. Despite this complexity, current therapeutics are unrefined steroids, immunosuppressants and neutralizing antibodies to single cytokines such as TNFα. Although current treatments for IBD have improved (6, 7), these therapies remain modestly successful with adverse side effects including immunosuppression, increased risk for infection, malignancies and death. Thus, an unmet clinical need exists for novel targets capable of restoring immune tolerance and controlling the wide array of immune responses.
NLRs are a family of cytosolic pattern recognition receptors that function in surveillance of metabolic stress, discrimination between pathogenic bacteria versus beneficial symbionts, and recognition of inflammatory signaling (8–12). Genetic polymorphisms in several NLRs have been described in IBD such as NLRC1 (NOD1) and NLRC2 (NOD2) (13–15). While NOD1 and NOD2 in addition to inflammasome associated NLRs are well-characterized to contribute to human disease, a smaller class of negative regulatory NLRs remain relatively unknown. Indeed, genetic abnormalities in these well-characterized NLR genes are linked to IBD pathology (16–19). This smaller family of NLRs is comprised of NLRX1, NLRP12, and NLRC3 and has shown potent effects in the attenuation of inflammation in nonclinical models of autoimmune and infectious disease. NLRX1 regulates inflammation and interferon production in response to pathogens (20–23), and antagonizes NF-κB (20, 23, 24). However, many of these preclinical studies focus solely on the worsening of disease in loss of function designs. The ability to modulate function by pharmacologic activation of the receptor, the prevalence of function-disrupting mutations and native expression patterns in response to inflammation is unknown in human disease.
NLRX1 is a mitochondria-associated receptor involved in down-regulating inflammation during bacterial and viral exposure, acute non-infectious colitis, colorectal cancer and chronic pulmonary disease (20, 22, 23). NLRX1−/− mice with DSS colitis have significantly worse disease activity associated with more severe gut mucosal inflammation and fibrosis when compared to their wild-type counterparts (25). Colonic NLRX1 expression is significantly suppressed in colons of mice with colitis (26). RNA-seq data from colons of WT and NLRX1−/− mice unveil that loss of NLRX1 during colitis causes overzealous inflammatory chemokine and cytokine signaling (>10-fold increase) in myeloid and epithelial cell response elements such as Il6, Tnf, Ifng, Il21 and Mcp1. Previously, we demonstrated that the deficiency of NLRX1 worsens inflammatory responses in vitro in both CD4+ T cells and epithelial cells of mice and shifts the metabolic preferences of cells. When the metabolic differences are prevented, the immune effects resulting from the loss of NLRX1 are abrogated (25) both in vitro and in vivo that are integrated with changes in inflammation-associated bacterial taxa within the colonic microenvironment (27).
In line with these beneficial preclinical responses in IBD, we have developed 1, 3, 5-tris (6-methylpyridin-2-yloxy) benzene (NX-13), as an orally active, gut-restricted NLRX1-activating therapeutic with limited systemic exposure for the treatment of IBD. NX-13 was generated by medicinal chemistry-based optimization of novel scaffolds designed to target the previously categorized binding pocket of NLRX1. In preliminary safety and pharmacokinetic studies, NX-13 is gut-restricted with no effects on systemic hematology parameters and a NOAEL ≥ 1,000 mg/kg (28). Following through in silico testing of affinity, safety and pharmacokinetics, NX-13 was synthesized to characterize its efficacy in the treatment of IBD. This manuscript provides the first evidence for NX-13 as a promising immunometabolic therapeutic in three models of IBD and primary cells from human UC patients.
Materials and Methods
Mice
Wild-type (WT) and Rag2−/− mice were used on a C57BL/6 background. Mdr1a−/− were used on an FVB background. All procedures and experiments were approved by an Institutional Animal Care and Use Committee (IACUC). Experimental groupings were age-, gender- and litter-matched with equal distribution to groups in co-housed conditions. For dextran sulfate sodium (DSS) experiments, 8-wk-old WT mice were administered dextran sulfate sodium in drinking water for 7 days. Mice were scored daily for disease activity. For adoptive transfer experiments, 7-wk-old Rag2−/− mice were injected with 4×105 naïve CD4+ T cells (CD45RBhi) per mouse by intraperitoneal injection. Mice were scored weekly post-transfer for disease activity for 8 weeks. For Mdr1a−/− experiments, Mdr1a−/− mice were started on treatment at 4 wk of age and continued treatment through 10 wk of age. Mice were scored weekly. Disease activity was scored on a range from 0 to 4 encompassing parameters pertaining to rectal inflammation, stool consistency, presence of blood in stool and overall appearance/behavior. General assignment of score corresponded to 0, no symptoms; 1, soft/loose/watery stool; 2, blood or moderate rectal inflammation; 3, blood and moderate or greater inflammation; 4, severe gastrointestinal symptoms with reduced activity or weight loss. NX-13 treatments, or vehicle control, were administered by orogastric gavage daily for the duration of each experiment.
CD4+ T cell culture
Naïve CD4+ T cells were obtained from spleens of WT mice by magnetic sorting of a cellular suspension of splenocytes. Naïve CD4+ T cells were cultured in Iscove’s Modified Dulbecco’s Medium (IMDM) supplemented with fetal bovine serum within anti-CD3/anti-CD28 coated 96 well plates. Cells were differentiated into Th1 or Th17 cells by cytokine mixture: [IL-12 (10 ng/mL), anti-IL-4 (500 ng/mL)] or [IL-6 (10 ng/mL), TGFβ (5 ng/mL), anti-IL-4 (500 ng/mL)], respectively. During differentiation, cells were treated with NX-13. After 48 h, cells were stimulated with phorbol 12-myristate-13-acetate (5 ng/mL) and ionomycin (500 ng/mL) (PMA/I) for 6 hours. After stimulation, cells were assayed for cellular phenotype by flow cytometry, gene expression by qRT-PCR, proliferation by carboxyfluorescein succinimidyl ester (CFSE) staining, or NF-κB, reactive oxygen species (ROS), lactate dehydrogenase (LDH) activity, and glucose uptake according to manufacturer’s instructions.
Flow cytometry
Lamina propria lymphocytes were obtained from colons by collagenase/DNase digestion followed by purification by Percoll gradient. Cultured cells were obtained directly from culture. Cells were washed and stained for extracellular antigens (CD45, CD3, CD4, CD8, CD25, Gr1). Cells were then fixed and permeabilized for staining of intracellular antigens (IFNγ, Tbet, IL10, FOXP3, IL17, RORγT, IL4). Data was acquired on a BD FACSCelesta II and analyzed using BD FACSDiva version 9.0.
Gene expression
Colon or cells were homogenized within RLT buffer. RNA was isolated from homogenate by RNAEasy Mini kit, according to manufacturer’s instructions. From RNA, cDNA was produced by iScript reverse transcriptase. qRT-PCR was conducted with SybrGreen mastermix. Starting quantity was calculated according to standard curve and normalized to beta-actin as previously described.
Histopathology
Colons were fixed within 10% buffered formalin, embedded in paraffin, sectioned and stained with hematoxylin and eosin for examination. Colons were scored (0–4) for leukocytic infiltration, mucosal thickening, and epithelial erosion by blinded pathologist.
PBMC culture
Peripheral blood mononuclear cells (PBMCs) were isolated from fresh whole blood of male and female ulcerative colitis patients. PBMCs were separated by LeucoSep tube and remaining red blood cells were lysed by hypotonic lysis. Isolated cells were treated with NX-13 (0, 50, 100, 500 nM). Cells were stimulated by TNFα (0.5 ng/mL) for 24 h, hydrogen peroxide (1 mM) for 24 h, or PMA/I for 6 h. Cytokine responses were measured by Luminex and NF-κB activity was measured by TransAM NF-κB p65 kit (Active Motif).
Statistical analysis
Data is presented as mean ± SEM. Quantitative data were analyzed using ANOVA, followed by the Scheffe multiple-comparisons test. ANOVA was performed using the general linear model procedure of SAS (SAS Institute, Cary, NC). Statistical significance was determined at p < 0.05.
Results
NX-13 modulates CD4+ T cell differentiation and metabolism
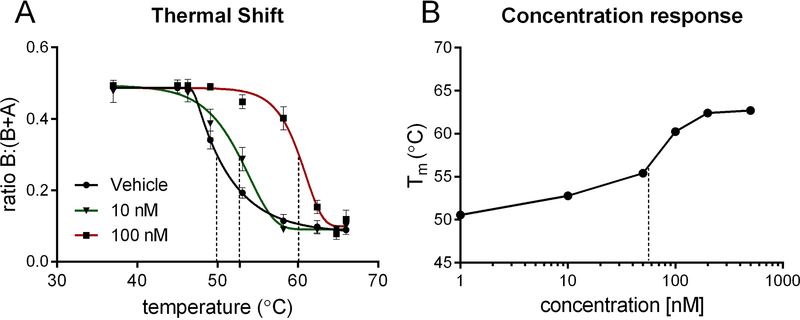
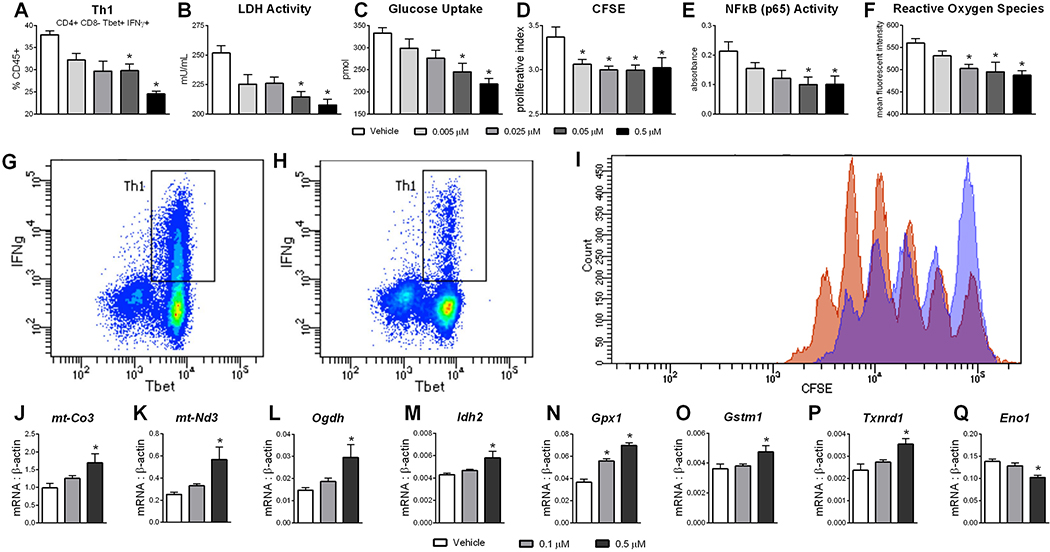
NX-13 is a symmetric tri-phenyl compound designed to activate NLRX1 in the GI tract. By CETSA, NX-13 has in situ binding effects on the stability of NLRX1 at concentrations below 100 nM, with an estimated EC50 of 58 nM (Figure 1). As NLRX1 has previously been associated with immunometabolic effects in CD4+ T cells in IBD (25), the differentiation of naïve CD4+ T cells was assessed in the presence of NX-13. Treatment with NX-13 significantly decreased the differentiation of Th1 (Figure 2A) and Th17 (Figure S1) at 0.05 μM with a dose response and suppressive effect observed down to 0.005 μM. The effect on CD4+ T cell differentiation was observed to be dependent on the presence of NLRX1, whereby CD4+ T cell deficient in NLRX1 did not respond to NX-13 treatment (Figure S1). Reduction of Th1 phenotyped cells corresponded with a marked decrease in IFNγ production (Figure 2G–H). In line with the previously described metabolic functions of NLRX1 activation, NX-13 decreased LDH activity (Figure 2B) and glucose uptake (Figure 2C) in in vitro differentiated Th1 cells paired with a decrease in proliferation (Figure 2D, I). No effect on cell death or apoptosis was observed (Figure S1). NX-13 also decreased NF-κB activity (Figure 2E) and intracellular reactive oxygen species (ROS) levels (Figure 2F), two inflammatory signaling elements inhibited by NLRX1 activation. Additionally, after the observation of effects on metabolism and ROS levels, we further characterized the effects of NX-13 treatment by qRT-PCR of in vitro differentiated Th1 cells. While NX-13 is associated with increased oxidative phosphorylation (Figure 2J–M), it is also associated with increased expression of antioxidant enzymes including Gpx1, Gstm1, and Txnrd1 (Figure 2N–P). Meanwhile, Eno1, a marker of anaerobic glycolysis activity and a moonlighting enzyme associated with inflammatory signaling, is decreased by NX-13 (Figure 2Q).
Figure 1.
NX-13 binding to NLRX1 in situ. Splenocytes were cultured with NX-13 (1, 10, 50, 100, 200, 500 nM) for 3 h following which cells were exposure to acute thermal stress. Protein was isolated, NLRX1 content was assessed by Western blot and compared at a ratio of soluble to total (A). Melting temperature (B) was calculated by 5-parameter logistic fit of ratio of soluble to insoluble/denatured NLRX1. EC50 estimated to be 58 nM in situ by protection from denaturation.
Figure 2.
In vitro responses to NX-13. Naïve CD4+ T cells were obtained from wild-type spleens and differentiated into Th1 cells in vitro in presence of NX-13 (0 – 0.5 μM) (A). After differentiation LDH activity (B), glucose uptake (C), proliferation (D), NF-κB activity (E) and reactive oxygen species (F) were measured. Representative plots of Th1 populations in untreated (G) and 0.5 μM NX-13 (H). Representative histogram (I) of untreated (orange) and 0.05 μM NX-13 (blue). Expression of mt-Co3 (J), mt-Nd3 (K), Odgh (L), Idh2 (M), Gpx1 (N), Gstm1 (O), Txnrd1 (P), and Eno1 (Q) were measured by qRT-PCR. Data presented as mean ± SEM (n = 6). Asterisks mark significance (p ≤ 0.05).
NX-13 reduces inflammation in a model of DSS colitis
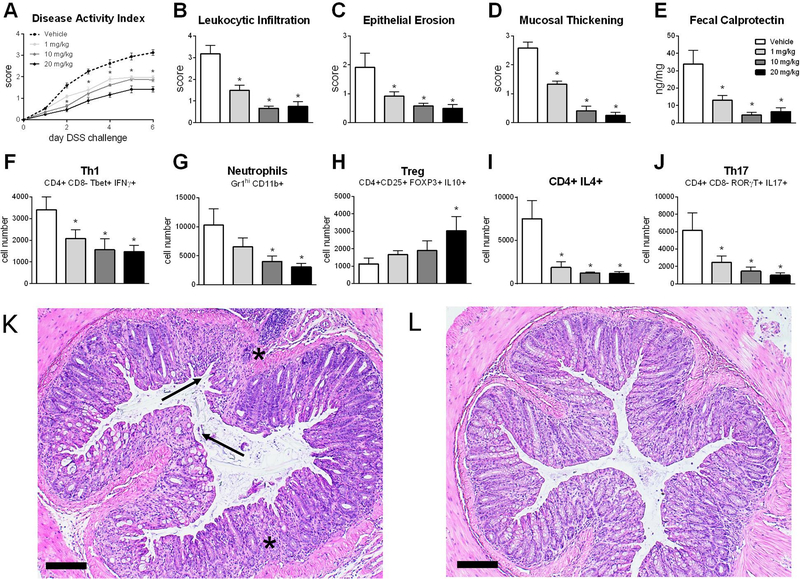
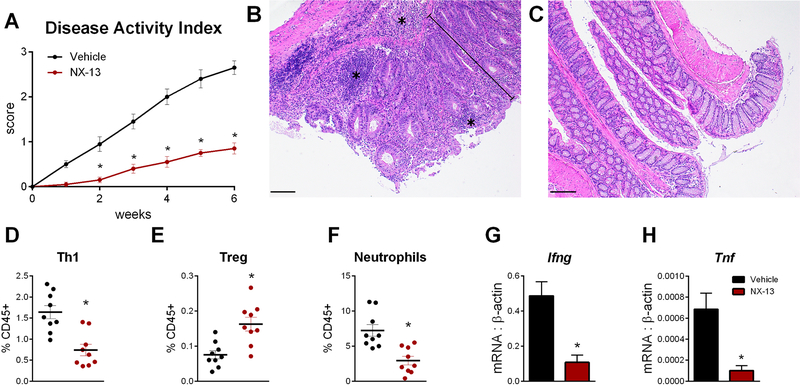
Upon validation of NLRX1-mediated activity in vitro, NX-13 was tested in a DSS model of colitis to characterize its therapeutic efficacy and underlying immunological changes induced by NX-13 in the gut. Oral treatment with NX-13 reduced disease activity index (Figure 3A), a summarized score encompassing diarrhea, rectal bleeding, rectal inflammation and overall activity, with significant reduction retained throughout the 7-day DSS period, at doses as low as 1 mg/kg. Similar trends were observed microscopically with reductions in leukocytic infiltration, epithelial erosion and mucosal thickening recorded at 1 mg/kg and higher upon examination of colon (Figure 3B–D). Reduction in leukocytic infiltration was observed at both the mucosal and submucosal levels, while NX-13 also protected against ulceration and loss of crypt architecture (Figure 3K, L). Treatment with NX-13 resulted in an increased colonic expression of Nlrx1 (Figure S2) with significant increases observed at 10 and 20 mg/kg. Fecal calprotectin, a clinical biomarker for intestinal inflammation and treatment response and remission, was decreased by NX-13 treatment on d 7 (Figure 3E). Notably, NX-13 treatment resulted in a greater reduction of fecal calprotectin in the DSS model when compared to current UC therapeutics (Figure S3). By flow cytometry immunophenotyping, 1 mg/kg oral NX-13 significantly decreased Th1, Th2 and Th17 (Figure 3F–H) locally within the colon. A significant decrease in neutrophils was observed at 10 mg/kg and higher with a noted suppressive trend at lower doses (Figure 3I). In contrast, CD25+ Tregs were significantly increased at the highest test dose, 20 mg/kg, and slightly increased at lower doses (Figure 3J). Similar trends in cellular populations were also observed based on percentage of CD45+ cells (Figure S2). When NX-13 was dosed to unchallenged mice, splenic immune cell populations were unchanged (Figure S2), suggestive of local action.
Figure 3.
Efficacy of NX-13 in DSS colitis. Wild-type mice were challenged with DSS and treated daily with oral NX-13 (0 – 20 mg/kg). Disease activity was assessed daily (A). Colon histology was scored at day 7 of DSS challenge for leukocytic infiltration (B), epithelial erosion (C), and mucosal thickening (D). Fecal calprotectin was assessed in colonic contents on day 7 (E). Th1 (F, CD4+ CD8- Tbet+ IFNγ+), neutrophils (G, Gr1hi CD11b+), Treg (H, CD4+ CD25+ FOXP3+ IL10+), IL4+ CD4+ (I), and Th17 (J, CD4+ CD8- RORγT+ IL17+) were quantified by flow cytometry on day 7. Representative photomicrographs of H&E stained colon from vehicle (K) and 10 mg/kg NX-13 (L) groups; asterisks mark leukocytic infiltration, arrows mark ulceration (scale bar, 100 μm). Data presented as mean ± SEM (n = 9). Asterisks mark significance (p ≤ 0.05).
NX-13 maintains immune homeostasis after CD45RBhi adoptive transfer
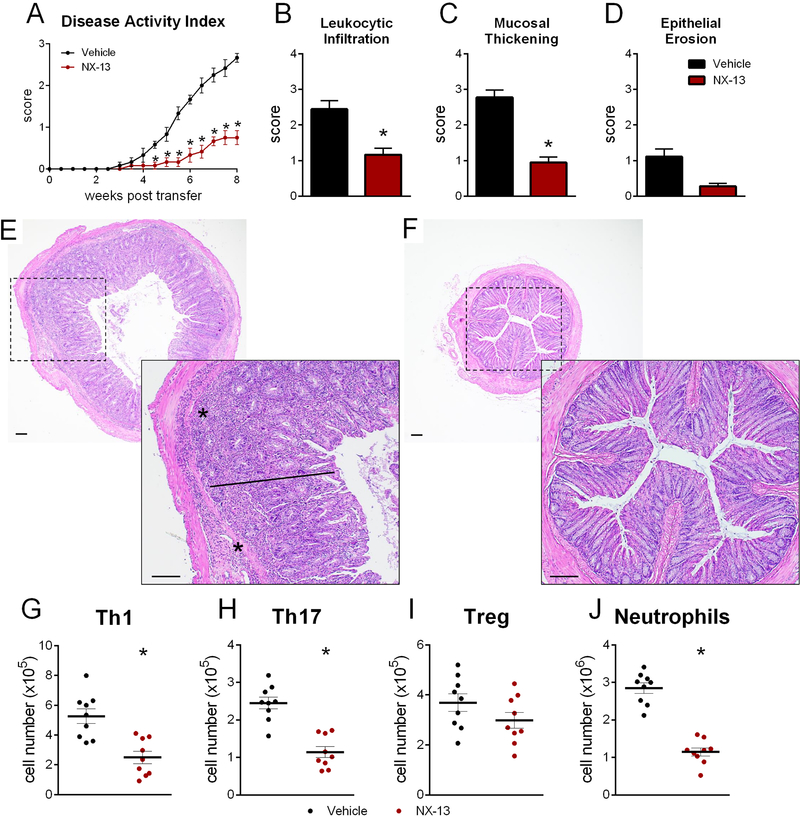
Based on the in vitro response to NX-13 in CD4+ T cells, its therapeutic efficacy was also assessed in the CD45RBhi adoptive transfer model of IBD. Oral NX-13 treatment significantly reduced signs of disease post-transfer with reduction of overall disease activity (Figure 4A). Significant reductions in colonic histopathology parameters (Figure 4B–D) were similarly observed with NX-13 with notable decreases in leukocytic infiltration and mucosal thickening (Figure 4E, F). In an environment of altered immune tolerance, NX-13 decreased Th1 and Th17 cells while the number of CD25+ Tregs was unchanged (Figure 4G–I). Reduced neutrophils were also observed in the colonic lamina propria of NX-13-treated mice (Figure 4J). In regards to the percentage of CD45+ cells, similar trends were observed in the proportion of Th1 and neutrophil cells while the proportion of Tregs was increased (Figure S2).
Figure 4.
Efficacy of NX-13 in CD45RBhi adoptive transfer. Rag2−/− were adoptively transferred 4×105 naïve CD4+ T cells and treated daily with oral NX-13 (10 mg/kg). Mice were scored twice weekly for disease activity until eight weeks post-transfer (A). Colon histology was scored at 8 weeks post-transfer for leukocytic infiltration (B), mucosal thickening (C), and epithelial erosion (D). Representative photomicrographs of H&E stained colon from vehicle (E) and 10 mg/kg NX-13 (L) groups; asterisks mark leukocytic infiltration, bars mark mucosal thickening (scale bar, 100 μm). Th1 (G, CD4+ CD8- Tbet+ IFNγ+), Th17 (H, CD4+ CD8- RORγT+ IL17+), Treg (I, CD4+ CD25+ FOXP3+ IL10+), and neutrophils (J, Gr1hi CD11b+) were quantified by flow cytometry in colon at eight weeks post-transfer. Data presented as mean ± SEM (n = 10). Asterisks mark significance (p ≤ 0.05).
Therapeutic efficacy of NX-13 in an Mdr1a−/− model of colitis
The knockout of Mdr1a was used as a spontaneous model of severe colitis. Similar to other models of colitis, oral NX-13 treatment results in an early reduction of inflammation that is maintained throughout the course of the experiment in Mdr1a−/− (Figure 5A). By histopathology, NX-13 reduces the occurrence of crypt destruction and structural abnormalities with reduced infiltration of neutrophils and eosinophils (Figure 5B–C). Cellular reductions in inflammation were confirmed by flow cytometry by observation of reduced Th1 cells and neutrophils and increased proportion of IL-10-producing Tregs in the colonic lamina propria (Figure 5D–F). Due to a decrease in total immune cell infiltration in the colon, Th1 and neutrophil numbers were also significantly reduced, while the total number of Tregs were unchanged (Figure S2). At the transcriptional level, colonic expression of Ifng and Tnf was reduced with oral NX-13 treatment (Figure 5G–H).
Figure 5.
Efficacy of NX-13 in Mdr1a−/− mice. Mdr1a−/− mice were administered oral NX-13 (20 mg/kg) daily for six weeks during which disease activity was assessed weekly (A). Representative photomicrographs of vehicle (B) and NX-13-treated (C) mice after six weeks of treatment; asterisks mark sites of leukocytic infiltration, brackets mark mucosal thickening (scale bar, 100 μm). Th1 (D, CD4+ CD8- Tbet+ IFNγ+), Treg (E, CD4+ CD25+ FOXP3+ IL10+), and neutrophils (F, Gr1hi CD11b+) were quantified by flow cytometry in colon after six weeks of treatment. Expression of colonic Ifng (G) and Tnf (H) were measured by qRT-PCR. Data presented as mean ± SEM (n = 9). Asterisks mark significance (p ≤ 0.05).
In vivo validation of NX-13-induced metabolic changes in Mdr1a−/− colitis
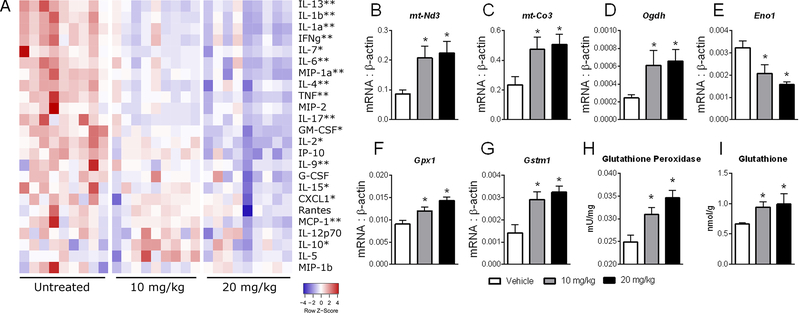
Upon demonstration of therapeutic efficacy, we examined local biomarkers of NX-13 activity. A panel of cytokines was used to determine changes in inflammatory response representative of the effects of NX-13 (Figure 6A). Notable cytokines prominent in IBD, such as TNF, IL-6 and MCP1 were decreased by NX-13, while an increase in IL-10 was observed in NX-13-treated mice. Additional cytokines with a lower degree of characterization in IBD, such as CXCL1, MIP1α, IL-9 and IL-1α were also decreased, indicating an ability of NX-13 to extend anti-inflammatory properties beyond a single cytokine. Next, expression of metabolic genes was measured in Mdr1a−/− mice after treatment with 10 or 20 mg/kg NX-13 to determine local metabolic biomarkers of NX-13 activity. In a similar manner to that observed in vitro, NX-13 resulted in an increase in markers of oxidative phosphorylation including the mitochondrially encoded Nd3 and Co3, and rate-limiting enzyme, Ogdh (Figure 6B–D). In contrast, Eno1 was decreased by NX-13 (Figure 6E). The change in oxidative phosphorylation was paired with increased expression of antioxidant enzymes, Gpx1 and Gstm1 (Figure 6F, G). In an activity-based assay, colonic glutathione peroxidase was also observed to be increased with NX-13 (Figure 6H), with similar increases observed in colonic glutathione levels (Figure 6I). Similar to the DSS model, these cytokines and metabolic changes correlated to increased colonic expression of Nlrx1 at 10 and 20 mg/kg (Figure S2).
Figure 6.
In vivo biomarker and metabolic effects of NX-13 in Mdr1a−/− mice. Mdr1a−/− mice were administered oral NX-13 (0, 10, or 20 mg/kg) daily for six weeks. Protein levels (A) of cytokines and chemokines in colon after six weeks were measured by Luminex; single asterisks mark significance relative to vehicle in the 20 mg/kg group and double asterisks mark significance at both 10 and 20 mg/kg. Expression of mt-Nd3 (B), mt-Co3 (C), Odgh (D), Eno1 (E), Gpx1 (F), and Gstm1 (G) were measured by qRT-PCR. Glutathione peroxidase (H) and glutathione (I) were measured in colon after six weeks of treatment. Data presented as mean ± SEM (n = 8). Asterisks mark significance (p ≤ 0.05).
NX-13 reduces inflammation and alters NLRX1 signaling in PBMCs from UC patients
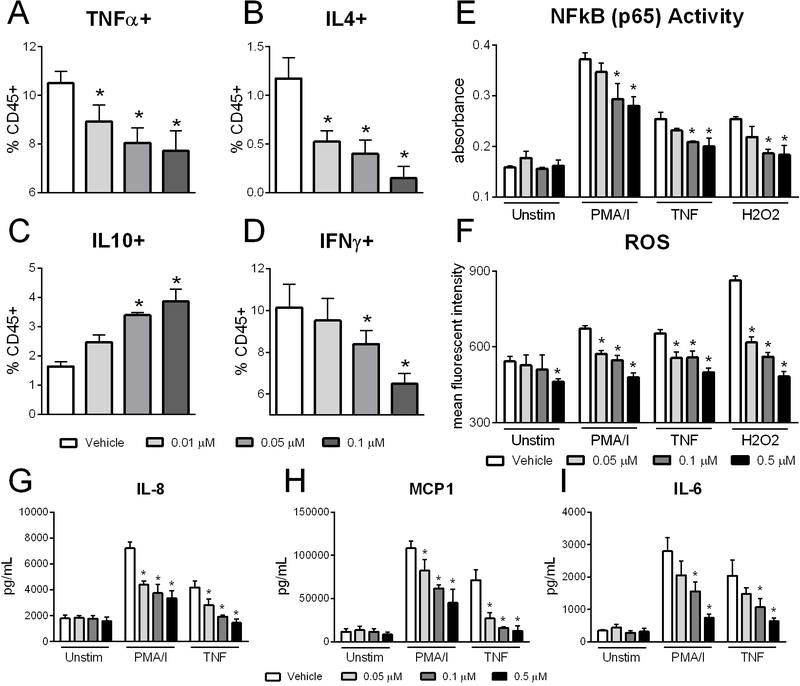
Based on the efficacy of NX-13 in mouse models of IBD, the translational value and potency of response to NX-13 treatment was tested within PBMCs from moderate to severe UC patients. At concentrations of 0.01 μM and higher, NX-13 significantly reduced TNFα and IL-4-producing cells after 24 h of culture (Figure 7A, B). Meanwhile at 0.05 μM and above, IL-10-producing cells were increased and IFNγ producing cells were decreased (Figure 7C, D). NX-13 reduces both NF-κB and ROS production upon stimulation with PMA/I, TNF, or hydrogen peroxide (Figure 7E, F). Importantly, NX-13 greatly reduced ROS production with oxidative stress caused by hydrogen peroxide. Additionally, NX-13 decreased inflammatory cytokines implicated in IBD, including IL-8, MCP1, and IL-6 upon stimulation with PMA/I or TNF (Figure 7 G–I). These results suggest a translatable potency of NX-13 between mice and humans, with clear therapeutic benefit ex vivo for the reduction of inflammation in primary cells from UC.
Figure 7.
Efficacy of NX-13 in ulcerative colitis PBMCs. PBMCs were isolated from blood samples of UC donors and cultured ex vivo with NX-13 (0, 0.01, 0.05, 0.1, 0.5 μM) for 24 h. Cells were stimulated with PMA (5 ng/mL) / ionomycin (500 ng/mL), TNF (0.5 ng/mL), or hydrogen peroxide (1 mM) as indicated. TNFα+ (A), IL4+ (B), IL10+ (C), and IFNγ+ (D) CD4+ T cells by flow cytometry. NF-κB activity (E) and reactive oxygen species levels (F) after 24 h treatment. Secreted IL-8 (G), MCP1 (H) and IL-6 (I) protein levels by Luminex. Data presented as mean ± SEM (n = 5). Asterisks mark significance (p ≤ 0.05).
Discussion
NX-13 is a promising immunometabolic oral, gut-restricted therapeutic for CD and UC that is effective at reducing inflammation in DSS, adoptive transfer and Mdr1a−/− mouse models of IBD plus in in vitro studies using primary murine and human cells. Through the activation of NLRX1, NX-13 increases oxidative phosphorylation in immune cells resulting in a decrease in the inflammation-associated anaerobic glycolysis. However, despite the increase in oxidative metabolism, NX-13 treatment decreases cellular ROS through activation and increased expression of antioxidant enzymes. Downstream of NLRX1, these immunometabolic changes result in decreased NF-κB activity and lower production of inflammatory cytokines such as TNFα and IFNγ. Both in vitro and in vivo, these signaling changes result in lower levels of Th1 cells. Oral NX-13 treatment reduces colonic lesions and overall disease severity at a dose as low as 1 mg/kg indicating a high potential for further development as an investigational new drug for CD and UC.
The concept that immune and metabolic pathways have important co-dependencies has clear relevance to the understanding of disease and therapeutic development, particularly IBD with diminished activity of the electron transport chain and lower ATP levels observed in the inflamed GI (29, 30). Effector CD4+ T cells (Th1 and Th17) drive glycolytic responses to produce energy quickly (31). Immune cells unable to establish fatty acid metabolism can show insensitivity to regulatory and suppressive signals (32). Our data suggests that NLRX1 acts as a key regulator of metabolic re-programming within immune cells with greater oxidative phosphorylation by NX-13 treatment in line with the previously described augmentation of glycolytic flux and decreased substrate-switching capability displayed by NLRX1 deficient cells (25). Indeed, NLRX1 directly influences the maturation of mitochondrial RNA for important oxidative phosphorylation enzymes (33). Further, Eno1, which is decreased by NX-13, is connected to TNFα, IL-1β, and IFNγ, is increased in colonic tissues and may serve as an important antigen in IBD with 50% of UC and CD patients presenting with serum anti-ENO1 antibodies (34, 35). The remaining question was whether the increased oxidative phosphorylation would lead to an increase in intracellular ROS and activation of inflammatory pathways. However, activation of NLRX1 by NX-13 leads to a decrease in ROS that is paired with increased antioxidant enzyme activity. Due to its previous connection to the regulation of MAVS, NLRX1 could be acting as an important switch through c-Abl that would serve to activate GPX1 (36, 37). The role in this regulation merits further exploration. With previous connections to the prevention of oxidative stress (25, 38) and the described data herein including increases to Gpx1, Gstm1, and Txnrd1, NLRX1 is a potent controller of the cellular oxidative state. Meanwhile, impaired function of these enzymes has been linked to enhanced susceptibility to IBD and leukocytic infiltration in colons of patients (39–41).
The implication of ROS in the NLRX1 pathway extends beyond the metabolic effects but the downstream signaling. Among other molecules, ROS are proposed to be a key mediator in the downstream effects of NLRX1 on NF-κB and potentially other inflammatory pathways including the phosphorylation of ERK1 and ERK2 leading to upregulation of MAPK activity (42). While cytoplasmic NLRX1 has direct effects on NF-κB activity, the control of Gpx1 and other antioxidant enzymes may provide further inhibitory support of the pathway as IKKα can be inhibited by the decrease in hydrogen peroxide levels (43). Conversely, NF-κB activity has also been linked to antioxidant enzyme transcription suggesting a potential co-regulation and feedback in this linkage in parallel to NF-κB-mediated upregulation of ROS producers (44). Dysfunction of this feedback may be important in the pathogenesis of IBD and a cause of the increased intestinal oxidative stress observed in UC and CD (45). Additionally, GPX1 activity has been connected to a decreased ability of TNF to activate expression of VCAM and ICAM molecules (46), which would lead to further support of in vivo anti-inflammatory effects locally in the GI tract, and serve as a mechanistic link for the observed decreased leukocytic infiltration observed in the three models of IBD. While current data supports a lack of effect on systemic immune profiles (28), risk for infection remains a concern in the development of immune-targeted therapeutics. The immunometabolic effects induced by NX-13 may support specific anti-viral responses through a depletion of lactate and modulation of the overall cellular energy phenotype. Many of the viral responses connected to NLRX1 have been through its interaction with MAVS; however, MAVS is also highly responsive to metabolic change with recent publications linking high lactate to impaired MAVS and Type I IFN production (47) as well as the necessity of O-GlcNAcylation in MAVS signaling (48). Therefore, the metabolic change combined with NLRX1 activation provide a balance in antiviral signaling. However, additional studies on the effect of NX-13 on both the susceptibility and severity of bacterial and viral infections is needed.
Via control of NF-κB, it is proposed that NLRX1 activation influences a wide array of cytokine responses. Based on the presented findings, oral NX-13 treatment provides anti-inflammatory control over a number of cytokines within the colonic mucosa. These cytokines cover a broad range of functions including potent inflammatory mediators of activation and differentiation (TNF, IL-6), neutrophil and monocyte chemokines (MCP1, CXCL1) as well as those with newly emergent functions in IBD (MIP1α, IL-9, IL-1α). Recently, CXCL1 has been identified as a differentiator between responders and non-responders clinically (49) and IL-9 positively correlated to clinical scores, including mucosal healing and endoscopic appearance in UC (50) while changes in MIP1α and IL-1α, indicate a potential involvement of myeloid cells in the response to NX-13 treatment (51).
As regulators of many of these molecular changes, this manuscript largely focuses on the effect of NX-13 in CD4+ T cells due to their role in the pathogenesis and chronicity of IBD and the previous implication of NLRX1 in this cell type. NX-13 shows a direct ability to decrease effector CD4+ T cells, including Th1 and Th17 cells. Recent evidence on the dual regulation of these cell types (52), along with the development of anti-IL-23 antibodies (53) which serve to suppress both Th1 and Th17 differentiation, support that the potential down-modulation of these cell types independently can reduce gastrointestinal inflammation in IBD. Based on prior knowledge of NLRX1 and therapeutic efficacy across mouse models of IBD, NX-13 is likely to also induce direct effects on epithelial and myeloid cells, although currently unexplored. Given the shared immunometabolic pathways in each of these cell types, these direct effects would be supportive of the overall anti-inflammatory nature of NX-13 treatment.
Combined with the described therapeutic efficacy in this manuscript, NX-13 is safe up to oral doses of 1,000 mg/kg in preliminary safety studies in rats (28). This benign safety profile is mediated in part by gut-restricted pharmacokinetics with local colonic concentrations over 1000-fold higher than those in plasma (28). NX-13 is effective in vivo at oral doses as low as 1 mg/kg with in vitro responses below 100 nM. With a wide therapeutic window permitted by this large safety margin, NX-13 is a prime candidate for advancement into investigational new drug (IND)-enabling studies and clinical testing in UC and CD patients. By immunometabolic mechanisms targeting enhanced oxidative phosphorylation and controlled ROS levels, NLRX1 activation is an innovative first-in-class strategy for the treatment of autoimmune disease. Further, the categorization of NLRX1 as a regulatory NLR offers the potential to intrinsically restore immune tolerance to the gut.
Supplementary Material
Key Points.
NLRX1 activation induces immunometabolic mechanisms to reduce effector CD4+ T cells.
NX-13 is a novel NLRX1-targeting therapeutic for inflammatory bowel disease.
NX-13 is effective in three mouse models of IBD and primary human cells from UC.
Acknowledgments
This work was supported by National Institutes of Health Public Service Grant 1R43DK121561.
Footnotes
Disclosures: All authors are employees of Landos Biopharma. JBR is a shareholder of Landos Biopharma.
References
- 1.Kappelman MD, Rifas-Shiman SL, Porter CQ, Ollendorf DA, Sandler RS, Galanko JA, and Finkelstein JA. 2008. Direct Health Care Costs of Crohn’s Disease and Ulcerative Colitis in US Children and Adults. Gastroenterology 135: 1907–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baumgart DC, and Sandborn WJ. 2012. Crohn’s disease. Lancet 380: 1590–1605. [DOI] [PubMed] [Google Scholar]
- 3.Torres J, Mehandru S, Colombel JF, and Peyrin-Biroulet L. 2017. Crohn’s disease. Lancet 389: 1741–1755. [DOI] [PubMed] [Google Scholar]
- 4.Ordas I, Eckmann L, Talamini M, Baumgart DC, and Sandborn WJ. 2012. Ulcerative colitis. Lancet 380: 1606–1619. [DOI] [PubMed] [Google Scholar]
- 5.Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, and Colombel JF. 2017. Ulcerative colitis. Lancet 389: 1756–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Camilleri M 2003. GI clinical research 2002–2003: the year in review. Clin Gastroenterol Hepatol 1: 415–420. [DOI] [PubMed] [Google Scholar]
- 7.Kozuch PL, and Hanauer SB. 2008. Treatment of inflammatory bowel disease: a review of medical therapy. World J Gastroenterol 14: 354–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ting JP, Lovering RC, Alnemri ES, Bertin J, Boss JM, Davis BK, Flavell RA, Girardin SE, Godzik A, Harton JA, Hoffman HM, Hugot JP, Inohara N, Mackenzie A, Maltais LJ, Nunez G, Ogura Y, Otten LA, Philpott D, Reed JC, Reith W, Schreiber S, Steimle V, and Ward PA. 2008. The NLR gene family: a standard nomenclature. Immunity 28: 285–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franchi L, McDonald C, Kanneganti TD, Amer A, and Nunez G. 2006. Nucleotide-binding oligomerization domain-like receptors: intracellular pattern recognition molecules for pathogen detection and host defense. J Immunol 177: 3507–3513. [DOI] [PubMed] [Google Scholar]
- 10.Harton JA, Linhoff MW, Zhang J, and Ting JP. 2002. Cutting edge: CATERPILLER: a large family of mammalian genes containing CARD, pyrin, nucleotide-binding, and leucine-rich repeat domains. J Immunol 169: 4088–4093. [DOI] [PubMed] [Google Scholar]
- 11.Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, and Tschopp J. 2004. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity 20: 319–325. [DOI] [PubMed] [Google Scholar]
- 12.Lara-Tejero M, Sutterwala FS, Ogura Y, Grant EP, Bertin J, Coyle AJ, Flavell RA, and Galan JE. 2006. Role of the caspase-1 inflammasome in Salmonella typhimurium pathogenesis. The Journal of experimental medicine 203: 1407–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hugot JP, Chamaillard M, Zouali H, Lesage S, Cezard JP, Belaiche J, Almer S, Tysk C, O’Morain CA, Gassull M, Binder V, Finkel Y, Cortot A, Modigliani R, Laurent-Puig P, Gower-Rousseau C, Macry J, Colombel JF, Sahbatou M, and Thomas G. 2001. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature 411: 599–603. [DOI] [PubMed] [Google Scholar]
- 14.Lesage S, Zouali H, Cezard JP, Colombel JF, Belaiche J, Almer S, Tysk C, O’Morain C, Gassull M, Binder V, Finkel Y, Modigliani R, Gower-Rousseau C, Macry J, Merlin F, Chamaillard M, Jannot AS, Thomas G, and Hugot JP. 2002. CARD15/NOD2 mutational analysis and genotype-phenotype correlation in 612 patients with inflammatory bowel disease. Am J Hum Genet 70: 845–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Limbergen J, Geddes K, Henderson P, Russell RK, Drummond HE, Satsangi J, Griffiths AM, Philpott DJ, and Wilson DC. 2015. Paneth cell marker CD24 in NOD2 knockout organoids and in inflammatory bowel disease (IBD). Gut 64: 353–354. [DOI] [PubMed] [Google Scholar]
- 16.Feldmann J, Prieur AM, Quartier P, Berquin P, Certain S, Cortis E, Teillac-Hamel D, Fischer A, and de Saint Basile G. 2002. Chronic infantile neurological cutaneous and articular syndrome is caused by mutations in CIAS1, a gene highly expressed in polymorphonuclear cells and chondrocytes. Am J Hum Genet 71: 198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffman HM, Mueller JL, Broide DH, Wanderer AA, and Kolodner RD. 2001. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nature genetics 29: 301–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Villani AC, Lemire M, Fortin G, Louis E, Silverberg MS, Collette C, Baba N, Libioulle C, Belaiche J, Bitton A, Gaudet D, Cohen A, Langelier D, Fortin PR, Wither JE, Sarfati M, Rutgeerts P, Rioux JD, Vermeire S, Hudson TJ, and Franchimont D. 2009. Common variants in the NLRP3 region contribute to Crohn’s disease susceptibility. Nature genetics 41: 71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henckaerts L, Pierik M, Joossens M, Ferrante M, Rutgeerts P, and Vermeire S. 2007. Mutations in pattern recognition receptor genes modulate seroreactivity to microbial antigens in patients with inflammatory bowel disease. Gut 56: 1536–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allen IC, Moore CB, Schneider M, Lei Y, Davis BK, Scull MA, Gris D, Roney KE, Zimmermann AG, Bowzard JB, Ranjan P, Monroe KM, Pickles RJ, Sambhara S, and Ting JP. 2011. NLRX1 protein attenuates inflammatory responses to infection by interfering with the RIG-I-MAVS and TRAF6-NF-kappaB signaling pathways. Immunity 34: 854–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lei Y, Wen HT, Yu YB, Taxman DJ, Zhang L, Widman DG, Swanson KV, Wen KW, Damania B, Moore CB, Giguere PM, Siderovski DP, Hiscott J, Razani B, Semenkovich CF, Chen X, and Ting JPY. 2012. The Mitochondrial Proteins NLRX1 and TUFM Form a Complex that Regulates Type I Interferon and Autophagy. Immunity 36: 933–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore CB, Bergstralh DT, Duncan JA, Lei Y, Morrison TE, Zimmermann AG, Accavitti-Loper MA, Madden VJ, Sun L, Ye Z, Lich JD, Heise MT, Chen Z, and Ting JP. 2008. NLRX1 is a regulator of mitochondrial antiviral immunity. Nature 451: 573–577. [DOI] [PubMed] [Google Scholar]
- 23.Philipson CW, Bassaganya-Riera J, Viladomiu M, Kronsteiner B, Abedi V, Hoops S, Michalak P, Kang L, Girardin SE, and Hontecillas R. 2015. Modeling the Regulatory Mechanisms by Which NLRX1 Modulates Innate Immune Responses to Helicobacter pylori Infection. PLoS One 10: e0137839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coutermarsh-Ott S, Simmons A, Capria V, LeRoith T, Wilson JE, Heid B, Philipson CW, Qin Q, Hontecillas-Magarzo R, Bassaganya-Riera J, Ting JP, Dervisis N, and Allen IC. 2016. NLRX1 suppresses tumorigenesis and attenuates histiocytic sarcoma through the negative regulation of NF-kappaB signaling. Oncotarget 7: 33096–33110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leber A, Hontecillas R, Tubau-Juni N, Zoccoli-Rodriguez V, Hulver M, McMillan R, Eden K, Allen IC, and Bassaganya-Riera J. 2017. NLRX1 Regulates Effector and Metabolic Functions of CD4+ T Cells. J Immunol 198: 2260–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu P, Hontecillas R, Abedi V, Kale S, Leber A, Heltzel C, Langowski M, Godfrey V, Philipson C, Tubau-Juni N, Carbo A, Girardin S, Uren A, and Bassaganya-Riera J. 2015. Modeling-Enabled Characterization of Novel NLRX1 Ligands. PLoS One 10: e0145420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leber A, Hontecillas R, Tubau-Juni N, Zoccoli-Rodriguez V, Abedi V, and Bassaganya-Riera J. 2018. NLRX1 Modulates Immunometabolic Mechanisms Controlling the Host-Gut Microbiota Interactions during Inflammatory Bowel Disease. Front Immunol 9: 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leber A, Hontecillas R, Zoccoli-Rodriguez V, Ehrich M, Chauhan J, and Bassaganya-Riera J. 2019. Exploratory Studies with NX-13: Oral toxicity and pharmacokinetics in rodents of an orally active, gut-restricted first-in-class therapeutic for IBD that targets NLRX1. Drug Chem Toxicol In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Novak EA, and Mollen KP. 2015. Mitochondrial dysfunction in inflammatory bowel disease. Front Cell Dev Biol 3: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santhanam S, Rajamanickam S, Motamarry A, Ramakrishna BS, Amirtharaj JG, Ramachandran A, Pulimood A, and Venkatraman A. 2012. Mitochondrial electron transport chain complex dysfunction in the colonic mucosa in ulcerative colitis. Inflamm Bowel Dis 18: 2158–2168. [DOI] [PubMed] [Google Scholar]
- 31.Fox CJ, Hammerman PS, and Thompson CB. 2005. Fuel feeds function: energy metabolism and the T-cell response. Nat Rev Immunol 5: 844–852. [DOI] [PubMed] [Google Scholar]
- 32.Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF, Sullivan SA, Nichols AG, and Rathmell JC. 2011. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol 186: 3299–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh K, Sripada L, Lipatova A, Roy M, Prajapati P, Gohel D, Bhatelia K, Chumakov PM, and Singh R. 2018. NLRX1 resides in mitochondrial RNA granules and regulates mitochondrial RNA processing and bioenergetic adaptation. Biochim Biophys Acta Mol Cell Res 1865: 1260–1276. [DOI] [PubMed] [Google Scholar]
- 34.Bae S, Kim H, Lee N, Won C, Kim HR, Hwang YI, Song YW, Kang JS, and Lee WJ. 2012. alpha-Enolase expressed on the surfaces of monocytes and macrophages induces robust synovial inflammation in rheumatoid arthritis. J Immunol 189: 365–372. [DOI] [PubMed] [Google Scholar]
- 35.Ji H, Wang J, Guo J, Li Y, Lian S, Guo W, Yang H, Kong F, Zhen L, Guo L, and Liu Y. 2016. Progress in the biological function of alpha-enolase. Anim Nutr 2: 12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cao C, Leng Y, Huang W, Liu X, and Kufe D. 2003. Glutathione peroxidase 1 is regulated by the c-Abl and Arg tyrosine kinases. J Biol Chem 278: 39609–39614. [DOI] [PubMed] [Google Scholar]
- 37.Jacobs JL, and Coyne CB. 2013. Mechanisms of MAVS regulation at the mitochondrial membrane. J Mol Biol 425: 5009–5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stokman G, Kors L, Bakker PJ, Rampanelli E, Claessen N, Teske GJD, Butter L, van Andel H, van den Bergh Weerman MA, Larsen PWB, Dessing MC, Zuurbier CJ, Girardin SE, Florquin S, and Leemans JC. 2017. NLRX1 dampens oxidative stress and apoptosis in tissue injury via control of mitochondrial activity. The Journal of experimental medicine 214: 2405–2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karban A, Krivoy N, Elkin H, Adler L, Chowers Y, Eliakim R, and Efrati E. 2011. Non-Jewish Israeli IBD patients have significantly higher glutathione S-transferase GSTT1-null frequency. Dig Dis Sci 56: 2081–2087. [DOI] [PubMed] [Google Scholar]
- 40.Mrowicki J, Mrowicka M, Majsterek I, Mik M, Dziki A, and Dziki L. 2016. Evaluation of Effect CAT −262C/T, SOD + 35A/C, GPx1 Pro197Leu Polymorphisms in Patients with IBD in the Polish Population. Pol Przegl Chir 88: 321–327. [DOI] [PubMed] [Google Scholar]
- 41.Zhang C, Guo L, and Qin Y. 2016. [Interaction of MIF gene −173G/C polymorphism and GPX1 gene 594C/T polymorphism with high-fat diet in ulcerative colitis]. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 33: 85–90. [DOI] [PubMed] [Google Scholar]
- 42.Sharma A, Yuen D, Huet O, Pickering R, Stefanovic N, Bernatchez P, and de Haan JB. 2016. Lack of glutathione peroxidase-1 facilitates a pro-inflammatory and activated vascular endothelium. Vascul Pharmacol 79: 32–42. [DOI] [PubMed] [Google Scholar]
- 43.Li Q, Sanlioglu S, Li S, Ritchie T, Oberley L, and Engelhardt JF. 2001. GPx-1 gene delivery modulates NFkappaB activation following diverse environmental injuries through a specific subunit of the IKK complex. Antioxid Redox Signal 3: 415–432. [DOI] [PubMed] [Google Scholar]
- 44.Morgan MJ, and Liu ZG. 2011. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res 21: 103–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tian T, Wang Z, and Zhang J. 2017. Pathomechanisms of Oxidative Stress in Inflammatory Bowel Disease and Potential Antioxidant Therapies. Oxid Med Cell Longev 2017: 4535194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lubos E, Kelly NJ, Oldebeken SR, Leopold JA, Zhang YY, Loscalzo J, and Handy DE. 2011. Glutathione peroxidase-1 deficiency augments proinflammatory cytokine-induced redox signaling and human endothelial cell activation. J Biol Chem 286: 35407–35417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang W, Wang G, Xu ZG, Tu H, Hu F, Dai J, Chang Y, Chen Y, Lu Y, Zeng H, Cai Z, Han F, Xu C, Jin G, Sun L, Pan BS, Lai SW, Hsu CC, Xu J, Chen ZZ, Li HY, Seth P, Hu J, Zhang X, Li H, and Lin HK. 2019. Lactate Is a Natural Suppressor of RLR Signaling by Targeting MAVS. Cell 178: 176–189 e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li T, Li X, Attri KS, Liu C, Li L, Herring LE, Asara JM, Lei YL, Singh PK, Gao C, and Wen H. 2018. O-GlcNAc Transferase Links Glucose Metabolism to MAVS-Mediated Antiviral Innate Immunity. Cell Host Microbe 24: 791–803 e796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zwicker S, Lira-Junior R, Hoog C, Almer S, and Bostrom EA. 2017. Systemic Chemokine Levels with “Gut-Specific” Vedolizumab in Patients with Inflammatory Bowel Disease-A Pilot Study. Int J Mol Sci 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matusiewicz M, Neubauer K, Bednarz-Misa I, Gorska S, and Krzystek-Korpacka M. 2017. Systemic interleukin-9 in inflammatory bowel disease: Association with mucosal healing in ulcerative colitis. World J Gastroenterol 23: 4039–4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arimura K, Takagi H, Uto T, Fukaya T, Nakamura T, Choijookhuu N, Hishikawa Y, Yamashita Y, and Sato K. 2017. Crucial role of plasmacytoid dendritic cells in the development of acute colitis through the regulation of intestinal inflammation. Mucosal Immunol 10: 957–970. [DOI] [PubMed] [Google Scholar]
- 52.Harbour SN, Maynard CL, Zindl CL, Schoeb TR, and Weaver CT. 2015. Th17 cells give rise to Th1 cells that are required for the pathogenesis of colitis. Proc Natl Acad Sci U S A 112: 7061–7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feagan BG, Sandborn WJ, D’Haens G, Panes J, Kaser A, Ferrante M, Louis E, Franchimont D, Dewit O, Seidler U, Kim KJ, Neurath MF, Schreiber S, Scholl P, Pamulapati C, Lalovic B, Visvanathan S, Padula SJ, Herichova I, Soaita A, Hall DB, and Bocher WO. 2017. Induction therapy with the selective interleukin-23 inhibitor risankizumab in patients with moderate-to-severe Crohn’s disease: a randomised, double-blind, placebo-controlled phase 2 study. Lancet 389: 1699–1709. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.