Summary
Transposon Tn7 is notable for the control it exercises over where transposition events are directed. One Tn7 integration pathways recognizes a highly conserved attachment (att) site in the chromosome, while a second pathway specifically recognizes mobile plasmids that facilitate transfer of the element to new hosts. In this review, I discuss newly discovered families of Tn7-like elements with different targeting pathways. Perhaps the most exciting examples are multiple instances where Tn7-like elements have repurposed CRISPR/Cas systems. In these cases, the CRISPR/Cas systems have lost their canonical defensive function to destroy incoming mobile elements; instead, the systems have been naturally adapted to use guide RNAs to specifically direct transposition into these mobile elements. The new families of Tn7-like elements also include a variety of novel att sites in bacterial chromosomes where genome islands can form. Interesting families have also been revealed where proteins described in the prototypic Tn7 element are fused or otherwise repurposed for new dual activities. This expanded understanding of Tn7-like elements broadens our view of how genetic systems are repurposed and provides potentially exciting new tools for genome modification and genomics. Future opportunities and challenges to understanding the impact of the new families of Tn7-like elements are discussed.
Keywords: Transposons, Genome evolution, target site selection, genomic islands, horizontal transfer, CRISPR, Cas
Abbreviated Summary
Recent studies show that Tn7-like transposons are far more numerous and diverse that previously appreciated. In multiple instances, Tn7-like elements were shown to make evolutionarily stable associations with CRISPR/Cas systems. Diverse lines of investigation suggest that in these cases, the CRISPR/Cas systems are not used for destroying invading mobile plasmids and bacteriophage, but instead targeting transposition into these DNAs via guide RNA to facilitate cell to cell transfer of the element.
Introduction
Transposons are mobile elements that can move between positions in a genome. Transposon Tn7 and similar elements are common reservoirs for antibiotic resistance and pathogenesis functions in clinical settings, as well as encoding other adaptive functions in natural environments (Parks & Peters, 2009, Peters et al., 2014). The success of these elements likely stems from the amount of control they have over when and where transposition occurs. The Tn7 system has evolved mechanisms to almost completely avoid integrating into important host genes, but also maximize dispersal of the element by recognizing mobile plasmids and bacteriophage capable of moving Tn7 between host bacteria (Li et al., 2013, Peters, 2014). While it has been clear for some time that Tn7-like elements have evolved as separate families with distinct pathways (Peters et al., 2014), research over the last couple of years has dramatically increased our appreciation of the widespread nature of these elements. It was found that over 20% of sequenced bacterial genomes have the signature proteins associated with these elements (over 10,000 putative elements from almost 49,000 partial and complete bacterial genomes) (Peters et al., 2017). Moreover, a phylogenetic analysis of the transposition genes of the Tn7-like elements revealed that they fell into over twenty phylogenetically distinct families possessing additional genes and gene configurations (Peters et al., 2017). An especially intriguing finding from this bioinformatic analysis was the observation that Tn7-like elements had recruited CRISPR/Cas systems on multiple occasions. It was hypothesized that in such cases the system was not used as a mechanism of defense for the host from invading DNA, but instead was adapted as a mechanism to target transposition into mobile plasmids and bacteriophage that would subsequently allow the transposon to transfer to new bacterial hosts (Peters et al., 2017, Faure et al., 2019). Excitingly, it has recently been confirmed that transposon-associated CRISPR/Cas systems are indeed being used as mechanisms for targeting transposition with the two most prolific transposon-associated CRISPR/Cas systems (Strecker et al., 2019, Klompe et al., 2019). This MicroReview explores the recent appreciation of the diversity of Tn7-like elements with a window into how the signature proteins found in these elements have adapted a variety of successful targeting strategies. Also covered are future directions that the field will need to take to fully understand the role Tn7-like elements naturally play in allowing bacteria to acquire new functions and to realize their full potential as future tools in genomics and as vectors for genome modification.
Tn7 and related elements encoding tnsABCDE
Tn7 was discovered over four decades ago and it remains the prototype for the many Tn7-like elements. Many of the molecular details responsible for Tn7 targeting are well-understood and have been covered in previous reviews (Craig, 2002, Parks & Peters, 2007, Li et al., 2013, Peters, 2014). Bacterial transposons that use a recombinase from the retroviral integrase superfamily are common, possessing a DDE-motif (rve, pfam 00665) in the active site (Nowotny, 2009). The majority of these elements have a relatively simple make up, often only encoding the transposase needed for excision and integration at a new position flanked by the cis-acting sequences it recognizes (Craig et al., 2015). While these elements can have highly adapted mechanisms of regulation for when transposition occurs, they tend to move with little control over the target sites they choose.
Tn7 and Tn7-like elements are notable for the control they have over when and where they insert, possessing one pathway that directs insertion into a single conserved position in bacterial genomes and a second pathway that appears to be adapted to maximizing targeting into mobile plasmids capable of transporting the element between bacteria (Figure 1). This ability in part stems from a heteromeric transposase consisting of TnsA and TnsB. The DDE-motif containing protein (TnsB) cleaves at the 3’ ends of the element and directly joins these strands to the target DNA, while the TnsA protein, an endonuclease (TnsA)(Tn7_Tnp_TnsA_N, pfam 08722), cleaves at the 5’ ends of the element allowing it to be completely excised from a donor DNA (Sarnovsky et al., 1996, May & Craig, 1996, Hickman et al., 2000). The mechanism involving complete removal of the transposon from the donor DNA and direct insertion of the DNA element into a new location is an atypical type of cut-and-paste transposition (Turlan & Chandler, 2000). TnsB is responsible for recognizing the cis-acting left and right ends of the element via a series of conserved TnsB-binding sites (Figure 2)(Arciszewska et al., 1989). With Tn7, the heteromeric TnsA+TnsB transposase further coordinates with additional proteins allowing transposition to only occur when a suitable target site has been identified (Bainton et al., 1993). Tn7 transposition will occur at a high frequency into a single site found in bacteria called its attachment site (attTn7) located downstream of the glmS gene, which encodes a highly conserved and essential protein (Figure 2). The attTn7 site is recognized by the Tn7-encoded sequence-specific DNA binding protein TnsD, a member of the larger TniQ (pfam 06527) family of proteins (Figure 2)(Bainton et al., 1993, Kuduvalli et al., 2001, Mitra et al., 2010). TnsD(TniQ) recruits TnsC, a AAA+ ATPase (Li et al., 2013) to the attTn7 site using a combination of protein-protein interactions and a distortion imposed by TnsD-binding that is recognized by TnsC (Kuduvalli et al., 2001, Mitra et al., 2010). TnsC is responsible for recruiting the element to the target site by interacting with the TnsA+TnsB transposase (Choi et al., 2013).
Figure 1 –
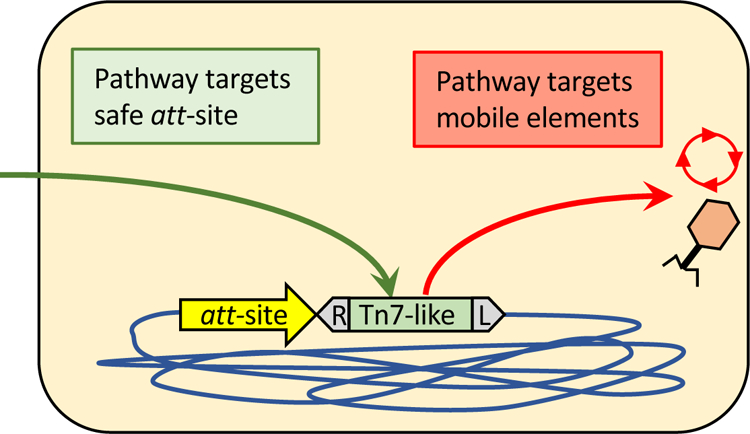
Representation of the two pathways found in Tn7 and Tn7-like elements. Tn7-like transposons newly entering the host on a plasmid, virus, or other DNA can transpose at a high frequency with the TnsABC + TnsD(TniQ) pathway into a host attachment site (attTn7/glmS in the case of Tn7)(green arrow). Different attachment sites are recognized by other Tn7-like elements as described in the text. Tn7 preferentially directs transposition events into mobile plasmids or filamentous phages with a second pathway using TnsABC + TnsE (Red arrow), a pathway that would facilitate rapid cell-to-cell transfer across bacterial populations. Tn7-CRISPR/Cas elements can utilize a TnsABC + TniQ + guide RNA complex to target protospacers recognized by spacers in the element-encoded CRISPR array. As described in the text, the majority of spacer matches identified from CRISPR arrays in CRISPR/Cas associated transposons are from mobile plasmids indicating that these systems likely facilitate distribution of the transposon via mobile plasmids. Insertion into the att site always occur with the right (R) and left (L) ends oriented as indicated for the Tn7-like element in the diagram.
Figure 2 –
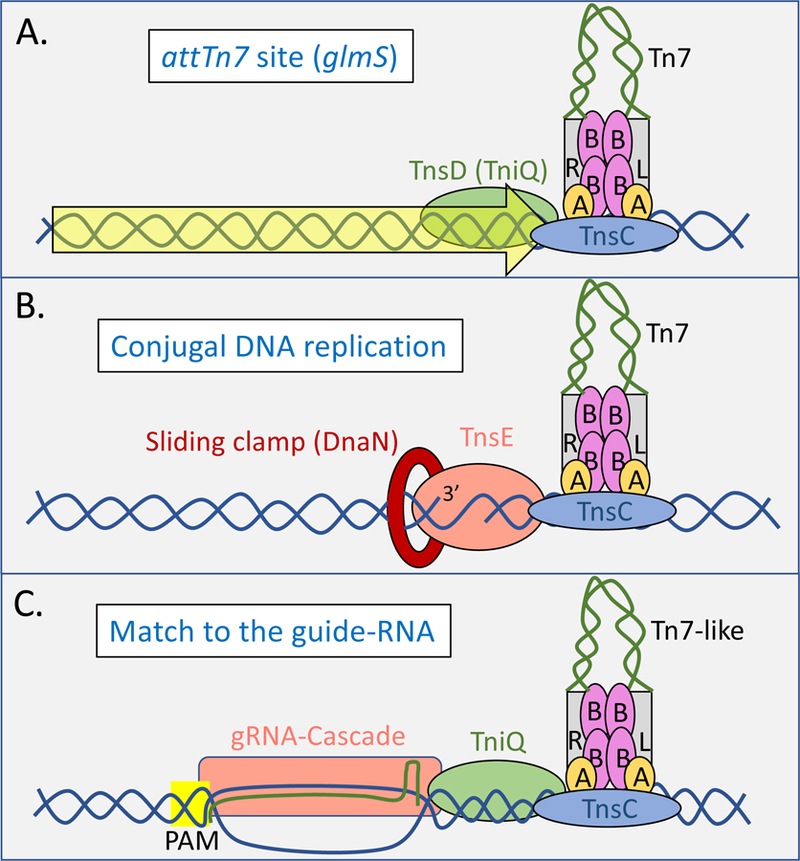
Models for how target complexes are assembled at various preferred target sites with Tn7 and Tn7-like elements. The Left (L) and Right (R) ends of the Tn7/Tn7-like elements are indicated with grey rectangles and the body of the element in green. The ends of the element are synapsed with TnsB (purple ovals, labeled B) and shown interfaced at the blue target DNA with TnsC (blue oval) and TnsA (yellow ovals, labeled A) to the site of insertion as recruited by three different targeting mechanisms. A. Prototypic Tn7 transposition into the attTn7 site uses the sequence specific DNA binding protein TnsD(TniQ) (green oval) that recognizes the 3’ coding region of the glmS gene (yellow block arrow) to recruit and activate transposition. Insertion occurs at a single position with the right end of the element integrated closest to glmS. Tn7-like elements may use a similar process for recognizing other att sites using TniQ as described in the text. B. Prototypic Tn7 transposition that recognizes active DNA replication found during plasmid conjugation uses the TnsE proteins which recognizes two features of discontinuous DNA replication in recipient cell bacteria; interaction with the sliding clamp (DnaN – red ring) and 3’ recessed-end substrates to direct transposon integration. Tn7 insertion events occur at multiple positions, but in a single origination with the direction of discontinuous DNA replication in recipient bacteria. C. Tn7-like elements with the I-F variant CRISPR/Cas system use a guide RNA(gRNA) complex to recognize a protospacer with the correct PAM sequence as predominantly found in mobile plasmids to direct transposition events ~49 bp from the protospacer with the right end of the element proximal to the end of the protospacer through an association with TniQ.
Prototypic Tn7 has a second pathway of transposition capable of preferentially targeting transposition into mobile plasmids and filamentous bacteriophage, facilitating the spread of Tn7 between bacterial hosts (Wolkow et al., 1996, Finn et al., 2007). This second pathway uses TnsE, a dedicated target selecting protein that functions along with the core transposition machinery (TnsABC). TnsE recognizes features of discontinuous DNA replication during conjugation, 3’ recessed ends and the sliding clamp protein (DnaN) (Peters & Craig, 2001, Parks et al., 2009)(Figure 2). A structural analysis of the C-terminal portion of TnsE indicates that the protein has a completely novel DNA binding mechanism without discernable sequence or structural homology with known proteins, including TnsD(TniQ)(Shi et al., 2015). The large family of Tn7 elements possessing TnsABCDE have diverged significantly and are found in a broad variety of bacteria which all appear to use the glmS attTn7 site as recognized by TnsD(TniQ)(Peters et al., 2017) and presumably benefit from maximizing horizontal transfer via mobile plasmids targeted by TnsE.
While the origional Tn7 element has been well-studied, structural information is still needed for most of the Tn7 transposition components to gain a complete understanding for how the heteromeric transposase functions (something only found in Tn7-like elements) and how target information is conveyed from dedicated target site selecting proteins through TnsC. As the model for the larger group of elements this should also provide insight into how the conserved proteins seem to be primed for adapting to new targeting pathways across the many families of Tn7-like elements.
There are numerous families of Tn7-like elements with novel transposition pathways including unique att sites
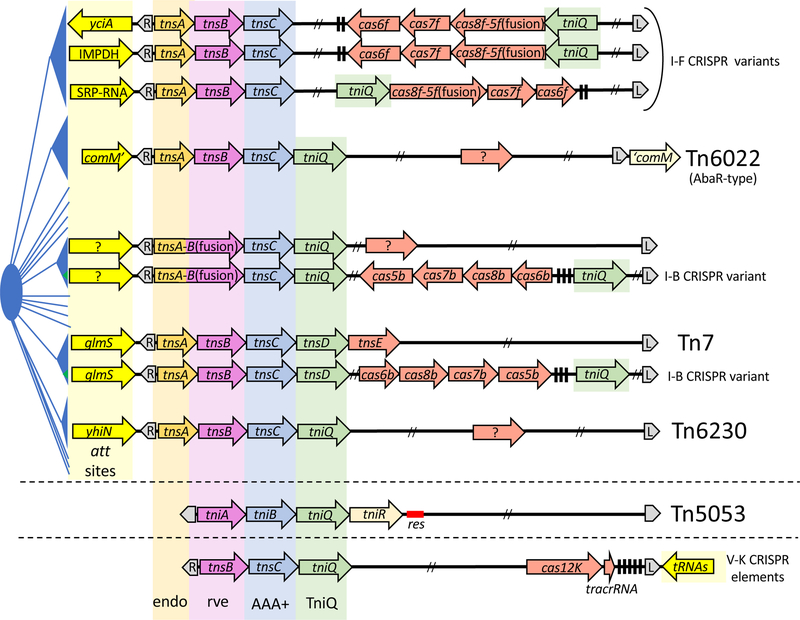
Based on the phylogeny of TnsA, Tn7-like elements are estimated to fall into over twenty distinct families and all of these transposons appear to encode TnsA, TnsB, TnsC, and TnsD(TniQ) (Peters et al., 2017) (Figure 3). Dedicated att sites that allow targeted integration into the bacterial genome at a specific site is one of the defining features of Tn7 and Tn7-like elements. The use of specific att sites allows Tn7-like elements to safely integrate into the host genome by allowing the element to avoid insertion into host genes that would compromise the host. Work with TnsD(TniQ) explains how an essential and conserved gene can be recognized as a transposition target without killing the host through insertional inactivation of the gene. This is because the point of integration is in the glmS transcriptional terminator, offset from the DNA sequence in the glmS coding region that is recognized by TnsD(TniQ) (Bainton et al., 1993, Kuduvalli et al., 2001)(Figure 2). Tn7 transposition into the highly conserved attTn7 has provided an important genetic tool for the single-copy insertion of genetic information into the chromosome in a single orientation at high efficiency, including the efficient delivery of a mobile-CRISPRi system for genomics analysis in non-model bacteria (McKenzie & Craig, 2006, Choi et al., 2005, Peters et al., 2018). The attTn7 site recognition sequences from archaea and eukaryotes can also be recognized at the DNA level by TnsD(TniQ) suggesting there is potential promise for Tn7-based systems outside of bacteria (Kuduvalli et al., 2005). Each of the Tn7-like families appears to use att sites that are specific for the group and recognized by a TniQ family protein (Figure 3)(Peters et al., 2014, Peters et al., 2017, Faure et al., 2019). In some cases it is clear that like the glmS gene, the att sites similarly stem from recognizing essential genes, such as the gene encoding the SRP-RNA required for the signal recognition particle pathway of protein transport or in another case the gene encoding the rate limiting step in GTP biosynthesis, Inosine monophosphate dehydrogenase (IMPDH) (Peters et al., 2017).
Figure 3 –
Representation of the major Tn7-like element families identified in sequenced bacterial genomes. Schematic representation of Tn7 and Tn7-like elements as situated with the attachment site(s) used within each family (bright yellow block arrow)(named where known or indicated with a ?). Transposons are shown with their transposon end sequences (grey polygons) with the right (R) and left (L) ends indicated where relevant. Signature Tn7 proteins families are indicated; the endonuclease TnsA (orange block arrow – endo), the transposase TnsB (purple block arrow – rve), the regulator protein TnsC (blue block arrow - AAA+) and the TnsD/TniQ family protein (green block arrow). Note: one unnamed family of Tn7-like elements has naturally fused TnsA and TnsB proteins, TnsA-B(fusion). Where known, accessory proteins for targeting mobile DNA elements capable of cell-cell transport are indicated (hypothetical activities are indicated with a ?). The Tn5053 site-specific recombination system is indicated (with the tniR-encoded resolvase and the resolution site it recognizes for resolving cointegrates found following replicative transposition indicated (res - red)). Black rectangles indicate spacers found in the CRISPR arrays. Two slashes indicate where cargo genes have been omitted to simplify the figure. An artistic representation of the TnsA phylogenetic tree is shown (Blue), but is available in Peters et. al. 2017 along with information for downloading via ftp. See text for details.
Interestingly, in a number of cases, the genes used as attachment sites do not appear to be essential or broadly conserved and could potentially limit the ability of these Tn7-like elements to spread across more diverse hosts (Figure 3). Tn6230 and related elements insert into the very C-terminal encoding region of the yhiN gene (Peters et al., 2014, Moreno Switt et al., 2012) and one sub-family of elements inserts adjacent to the yciA gene (Peters et al., 2017). Tn6022 and related elements insert well within the comM gene, presumably inactivating the normal gene product (Fournier et al., 2006, Hamidian & Hall, 2011). The comM gene product appears to play a role in integrating horizontally acquired DNA (Nero et al., 2018), but any advantage that the transposon may gain by inactivating this gene remains unclear. Tn6022 related elements also account for the AbaR-type genomic islands that are common in pathogenic Acinetobacter baumannii (Bi et al., 2019), although as indicated previously, relatives of this element are found broadly across bacteria and outside of clinical settings (Peters et al., 2014).
Understanding the att sites used across the many families of Tn7-like elements will be important to understand the impact of Tn7-like elements in their capacity to form genomic islands. Genomic islands are blocks of genes in a given strain of bacteria that are not found in other members of the species. These regions can be highly variable indicating a specific mechanism was involved in accruing genetic information at the locus. In some cases, genetic features may be present in a genome island indicating how they were originally formed, such as identifiable bacteriophage genes. However, in most cases any signature genes that could suggest the mechanism used to deposit genetic information has been lost leaving the origin enigmatic. Previously it was shown that genomic islands can form at the glmS attTn7 site with Tn7-like elements (Parks & Peters, 2007, Peters et al., 2014). When transposons become immobilized through loss of end sequences or mutations in the Tns proteins, recognizable transposon features will slowly be lost, but cargo genes that provide a selective advantage to the host will be maintained as genomic islands. It is reasonable to assume that these islands will be more numerous than actual functional Tn7-like elements and genome annotation work would generally benefit from a better understanding of these features. Further investigation into new att sites in novel and poorly characterized genera of bacteria should also provide the basis for new tools for inserting genetic information at neutral positions in bacteria which currently lack genetic systems.
Tn7-like elements recruited CRISPR/Cas systems on multiple separate occasions for guide RNA-directed transposition
CRISPR/Cas systems are well-known as adaptive immune systems found in most archaea and many bacteria (Makarova et al., 2015). The memory of the adaptive immune system is in the form of short stretches of DNA sequence called spacers that were captured from bacteriophages, plasmids or transposons that previously invaded the host. Spacers are found between DNA repeats in a CRISPR array that function with associated proteins called Cas proteins. CRISPR/Cas systems have multiple evolutionally distinct origins, falling into two large classes and six types based on signature proteins. There are also several sub groups within each type. For example, type I elements have seven subtypes, I-A through I-F plus I-U.
The three major steps in the immunity process are shared between all of the major CRISPR/Cas systems; spacer acquisition, pre-crRNA processing, and interference (Koonin et al., 2017b). In the spacer acquisition step (also known as adaptation) Cas1 and Cas2, and in some cases Cas4, preferentially harvest spacer DNAs from bacteriophage and other mobile elements which are subsequently integrated into the CRISPR array (Xiao et al., 2017). Cas1 and Cas2 are conserved across CRISPR/Cas systems that function as adaptive immune systems. In the pre-crRNA processing step, the CRISPR array is transcribed and individual guide RNAs (crRNAs) are processed from the larger transcript. In the final step in the immunity process called interference, the guide RNA is used to recognize an invading bacteriophage or other genetic element which is destroyed with a nuclease activity found in one of the Cas proteins. DNA sequences are capable of becoming spacers, called protospacers, if they are associated with a short DNA motif called a Protospacer Adjacent Motif (PAM). The PAM is important for the system to function because during the interference stage this allows the guide RNA complex to recognize the protospacer with its PAM in the invading element (and the element destroyed) while the spacer in the array is ignored because it lacks the PAM.
Several specific features of the transposon-associated CRISPR/Cas systems support the idea that they have been adapted for targeting transposition primarily into mobile plasmids, replacing a function provided by TnsE in prototypic Tn7 (Peters et al., 2017). For one, transposon-associated CRISPR/Cas systems lack the Cas3 nuclease that is normally recruited to DNAs recognized by the guide RNA complex for degradation (Figure 4). Comparing the phylogeny of the signature transposon and Cas proteins in the elements also indicates that they are congruent and in each case the specific variant CRISPR/Cas systems are only found with a specific family of Tn7-like elements. This finding indicates that they evolved together, supporting the hypothesis that they participate in a shared function. Furthermore, analysis of the spacers encoded in the small CRISPR arrays found in these elements indicates a near complete bias for mobile plasmids found in the same genera of bacteria, whereas spacer matches with canonical I-F systems are almost exclusively to bacteriophages (Shmakov et al., 2017b). The strongest piece of evidence for guide RNA-directed transposition at the time of the 2017 Peters et. al. paper was the finding that in two cases a spacer encoded in the transposon CRISPR array matched a position immediately adjacent to the element (a position to the right of the element where att sites are also recognized for TnsD(TniQ)-mediated transposition, Figure 2). Of additional interest was the finding that CRISPR/Cas recruitment had similarly occurred on three separate occasions, one large group with a I-F variant system forming its own phylogenetically distinct family, and two smaller subgroups with I-B systems (Figure 3). The way the CRISPR/Cas systems were adapted into Tn7-like elements was also the same. In all cases the Cas3 nuclease was missing, as well as the Cas1-Cas2 spacer acquisition system (Figure 4).
Figure 4 –
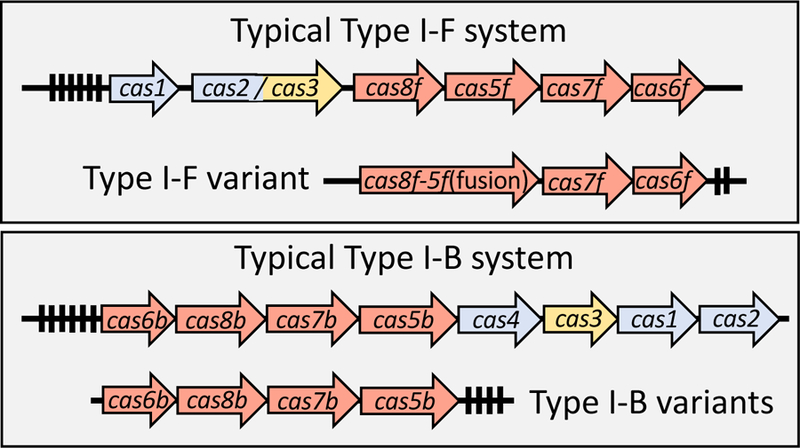
Representation of typical examples of the type I-F and I-B CRISPR/Cas systems and the variants found in Tn7-like elements. CRISPR associated (cas) genes are indicated with block arrows. Black rectangles indicate spacers found in the CRISPR arrays. Genes maintained in the typical system and the transposon encoded systems are indicated in red, the spacer acquisition system is indicated in blue, and the nuclease component shown in orange. See text for details.
Amazingly in subsequent genomics analyses, a fourth example of CRISPR/Cas recruitment was also identified, this time with a Class 2 type V system with a single effector protein (Cas12)(Faure et al., 2019)(Figure 3). Phylogenetic analysis indicated that the entire distinct Cas12k (formally type V-U5) sub type is exclusively found in this specific type of transposon (Shmakov et al., 2017a, Faure et al., 2019). As in the case of the variant I-F and I-B systems, the effector complex was not proficient for cleavage, in this system because it lacked residues that normally allow Cas12 cleavage in type V elements. These Cas12k elements, also lacked the Cas1 Cas2 spacer acquisition system (Faure et al., 2019)(Figure 3). A computational search for spacer matches from Cas12k elements only revealed a few targets, which matched plasmids and a distinct different class of transposon (Faure et al., 2019).
While bioinformatics analysis of these elements supported the hypothesis that the CRISPR/Cas systems were being used for guide RNA targeted transposition (Peters et al., 2017) the final proof came from experiments in a heterologous E. coli system. Remarkably, by expressing the transposition and CRISPR/Cas guide RNA systems in E. coli, targeting could be specifically directed with the I-F variant (Klompe et al., 2019) and Cas12k (Strecker et al., 2019) systems. In both cases, the specific transposition systems only worked with the I-F variant or Cas12k CRISPR/Cas systems that coevolved together, and not with other native CRISPR/Cas systems that were tested. In both, the I-F variant and Cas12k systems, it was confirmed that similar to standard CRISPR/Cas systems of the same type, a small PAM sequence motif needed to be found adjacent to the spacer match (protospacer) (i.e. the systems had PAM requirements as expected for type I and V systems). All of transposition proteins, including TniQ were also needed for the guide RNA-directed process to occur efficiently (although there did not appear to be an absolute TniQ requirement with the Cas12k system in vitro). This was unexpected because in the prototypical Tn7 system, the TnsD(TniQ) protein is not involved in the TnsE-pathway that targets mobile plasmids. In the I-F variant system, a guide RNA complex consisting of the three Cas proteins and TniQ was also isolated supporting a relevant role for this complex in target site selection and showing how the CRISPR/Cas system could interface with the transposition system (Klompe et al., 2019). This finding is further supported by a preliminary cryo-EM structure indicating that TniQ associates with the Cas proteins in the guide RNA complex proximal to the point of insertion of the transposon (Halpin-Healy et al., 2019)(Figure 2c). With the Cas12k system, an in vitro recombination system was reconstituted using purified proteins, and plasmid insertion products directed by the guide RNA were recovered following transformation into E. coli (Strecker et al., 2019). In total, these two papers conclusively showed that transposon ends could be directed by guide RNAs to join to a target DNA containing a match to a spacer in a programable way.
Additional characterization and optimizing guide RNA-directed transposition systems
The molecular details that underlie the process of guide RNA-directed transposition with Tn7-like elements are still unclear. Details of the targeting process will be essential for future work adapting these systems as genetic tools. The current assay systems were sufficient to show that transposon ends can be joined to target DNAs in a guide RNA-programmed way in vivo (Strecker et al., 2019, Klompe et al., 2019). However, the atypical genetic assays used in these studies were also highly reliant on PCR-based methods which often poorly assess full integration events. Additionally, these assays are not obviously amenable to the same kind of experiments used for the analysis and optimization previously done with IS200/IS605, IS911, Tn5, Tn7, Tn10, Tn4430, bacteriophage Mu, Himar1 and numerous other transposition systems which have been analyzed in great detail (Craig et al., 2015). Establishing these genetic tools for accurately analyzing transposition frequency and targeting, with mechanisms of screening for mutations will be needed to realize the full potential of CRISPR/Cas-directed transposition. It also remains incompletely resolved whether unexpected attributes of each system stem from the assay system in E. coli. For example, a strict orientation bias naturally found with Tn7-like elements was also observed with the Cas12k elements in the heterologous E. coli system (Strecker et al., 2019), but insertions could be identified in both orientation with the I-F system that was similarly analyzed (Klompe et al., 2019). Furthermore, differences in off-site targeting were noted between the two systems, but it was unclear if this results from the E. coli overexpression systems or a natural behavior of the elements outside the native host.
The Cas12k-based transposons in particular raise important and interesting questions. These elements are further diverged from the other Tn7-like elements. For example, the Cas12k elements recognize targets at the left junction of the element and not the right as found with Tn7 and the other Tn7-like elements (Peters et al., 2017). This is true, for example, for its att site downstream of tRNA genes and guide RNA directed insertions (Figure 3). The greatest outstanding question with the Cas12k elements involves the lack of any obvious TnsA-like activity in these elements (Faure et al., 2019)(Figure 3). With Tn7, TnsA plays an essential role for allowing cut-and-paste transposition, while elements lacking this endonuclease utilize replicative transposition requiring extensive host-initiated repair replication and also some secondary type of cointegrate resolution system (like Tn5053, Figure 3)(Nicolas et al., 2015), which has not been identifiable across the Cas12k elements (Faure et al., 2019). Alternatively, the Cas12k elements could use a more elaborate process involving double strand break repair and the host replication machinery as found with bacteriophage Mu integration, which despite lacking a TnsA-like activity can still produce a simple insertion product (Choi et al., 2014, Jang & Harshey, 2015).
Multiple other questions remain with Tn7-CRISPR/Cas elements, especially relating to CRISPR array usage. It is unclear why the spacer acquisition system is not present in any of the four elements that captured these systems (Figures 3 and 4). Notably, the type IV CRISPR/Cas systems which are found exclusively on plasmids and prophage also lack spacer acquisition systems (and lack guide RNA-directed degradation function) (Faure et al., 2019). One possibility is that it may be too risky for CRISPR/Cas-associated transposons to maintain a spacer acquisition system because acquisition systems specifically evolved to harvest spacers from mobile elements. Therefore maintaining the spacer acquisition system at all times would increase the chances that self-targeting spacers may be acquired, leading to the destruction of the transposon or the mobile plasmids where it resides. Borrowing the acquisition system in trans from other systems may be less detrimental because spacers are only collected episodically (i.e. when in a host that maintains the acquisition system and where the element likely resides in a chromosomal att site). It is also unknown if CRISPR/Cas-associated transposons can use guide RNAs from other CRISPR arrays in the cell allowing additional options for targeting. Presumably, there would be complications if other fully functional CRISPR/Cas systems could use guide RNAs from the transposon-associated CRISPR array, because these systems could destroy potential plasmids that the element could otherwise target for transposition.
Other evolutionary relationships between CRISPR/Cas systems and transposons
The evolution of CRISPR/Cas systems is interwoven with that of transposons and other mobile genetic elements in multiple ways (Faure et al., 2019). The Cas1 protein involved in spacer acquisition was originally derived from the transposase of a group of elements now known as casposons (Krupovic et al., 2014, Krupovic et al., 2017). It has even been suggested that the first CRISPR/Cas system could have been used for targeting transposition with ancient casposon elements, a process that later re-evolved with Tn7-like elements (Koonin & Makarova, 2017). In another example of exchange, spacer acquisition in one subtype within the type III CRISPR/Cas systems gained the capacity to harvest spacer information from RNA through the apparent capture of reverse transcriptase activity from Group II introns (Silas et al., 2016, Silas et al., 2017). Finally, the Cas12 effector proteins of type V CRISPR/Cas systems appears to have been derived from a RuvC-like nuclease activity from IS605-like transposons on multiple occasions (Koonin & Makarova, 2017).
Identifying novel systems in Tn7-like elements including new mechanisms for facilitating horizontal transfer
The TnsE-mediated pathway and transposon-associated CRISPR/Cas systems should facilitate transfer into new hosts by directing transposition into mobile plasmids and bacteriophage. Future research will be needed to establish if novel proteins exist that are capable of preferentially directing transposition with the other families of Tn7-like elements into mobile plasmids and possibly bacteriophage. One exciting idea, is that in some cases a second pathway capable of targeting mobile DNA elements may not require a stand-alone additional protein, but instead that there may be examples of dual function TniQ family proteins capable of recognizing an att site and possessing a distinct mechanism for recognizing mobile elements. For example, there is good evidence for TniQ possessing a dual function in the I-F CRISPR/Cas variant systems. The TniQ proteins are predicted to recognize specific att sites based on the congruence between the phylogeny of TniQ and the att sites recognized by the same element (Peters et al., 2017). However, as described above, it was also shown that the TniQ protein is involved in the guide RNA-directed transposition processes. Interestingly, the I-B variant systems appear to always encode two TniQ-family proteins which could suggest one functions like TnsD(TniQ) to recognizes the att site, while the second may be solely committed to functioning with the Cas proteins (Figure 3)(Peters et al., 2017). Previous work suggests that at least one TniQ family member may have evolved to direct transposition based on a novel plasmid features. The TniQ-encoding Tn5053 element shows a novel form of transposition targeting where insertion events appear to be specifically directed at plasmid resolution sites (Minakhina et al., 1999). This would allow such elements to recognize episomal elements that could facilitate transfer to other bacterial hosts. Interesting, the TniQ pfam (PF06527) domain organization entry indicates a diversity of architectures with the TniQ domain found fused with a wide variety of other protein domains consistent with the idea that functional plasticity may be common across the Tn7-like element families (http://pfam.xfam.org/family/PF06527).
Understanding the cargo Tn7-like elements maintain and how it was acquired
As described above, in addition to conserved sets of transposition genes in the various families of Tn7-like elements, they also maintain novel, so-called cargo genes. An incredible variety of genes can be found as cargo in Tn7-like elements, even in Tn7-like elements with very closely related transposition genes. Generally the cargo found in a Tn7-like element can provide insight into how the bacterial host adapted to its current environment. For example, as mentioned, Tn7-like elements are common reservoirs for antibiotic resistance and for pathogenesis functions in clinical settings. A better understanding of the cargo found in these elements could provide clinically-relevant information in pathogenic bacteria. Another particularly fascinating type of cargo that Tn7-like elements commonly encode are defense systems such as restriction and modification systems allowing protection from horizontally transferred DNA (Koonin et al., 2017a). DNA methylation systems are commonly encoded as cargo in Tn7-like elements (Peters et al., 2017, Faure et al., 2019), suggesting these elements could also be productive places to screen for novel anti-phage defense systems (Doron et al., 2018). It is reasonable to suggest that anti-phage systems could be broadly useful as cargo genes because they should be beneficial in almost all environments because of the ubiquity of bacteriophages. It also remains unknown how new cargo genes become associated with Tn7-like elements or if specific mechanisms exist that allow these elements to diversify the genetic cargo they carry in some settings.
Conclusions
Tn7-like elements offer a unique view into how bacteria gain new functions and should provide a mechanism that accounts for multiple hitherto unknown genomic islands in a variety of bacteria. Knowing the fundamentals of the processes used by Tn7 and Tn7-like elements should also allow these systems to be optimized as tools for genomics in a variety of bacteria and organisms in the other domains of life.
Acknowledgements
I thank Tobi Doerr, Alba Guarnè, Ailong Ke, and Mike Petassi for insightful comments on the manuscript. Work in the Peters lab is supported by Cornell University and NIH grant R01 GM129118. Cornell University has filed a US provisional patent application for CRISPR/Cas transposition systems with JEP as the inventor.
References
- Arciszewska LK, Drake D, and Craig NL (1989) Transposon Tn7 cis-acting sequences in transposition and transposition immunity. J. Mol. Biol 207: 35–52. [DOI] [PubMed] [Google Scholar]
- Bainton RJ, Kubo KM, Feng JN, and Craig NL (1993) Tn7 transposition: target DNA recognition is mediated by multiple Tn7-encoded proteins in a purified in vitro system. Cell 72: 931–943. [DOI] [PubMed] [Google Scholar]
- Bi D, Xie R, Zheng J, Yang H, Zhu X, Ou HY, and Wei Q (2019) Large-Scale Identification of AbaR-Type Genomic Islands in Acinetobacter baumannii Reveals Diverse Insertion Sites and Clonal Lineage-Specific Antimicrobial Resistance Gene Profiles. Antimicrob Agents Chemother 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KH, Gaynor JB, White KG, Lopez C, Bosio CM, Karkhoff-Schweizer RR, and Schweizer HP (2005) A Tn7-based broad-range bacterial cloning and expression system. Nat Methods 2: 443–448. [DOI] [PubMed] [Google Scholar]
- Choi KY, Li Y, Sarnovsky R, and Craig NL (2013) Direct interaction between the TnsA and TnsB subunits controls the heteromeric Tn7 transposase. Proceedings of the National Academy of Sciences 110: E2038–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi W, Jang S, and Harshey RM (2014) Mu transpososome and RecBCD nuclease collaborate in the repair of simple Mu insertions. Proc Natl Acad Sci U S A 111: 14112–14117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig NL, (2002) Tn7. In: Mobile DNA II Craig Nancy L, Craigie R, Gellert M & Lambowitz-Alan M (eds). Washington, DC: ASM Press, pp. 423–456. [Google Scholar]
- Craig NL, Chandler M, Gellert M, Lambowitz AM, Rice PA, and Sandmeyer SB, (2015) Mobile DNA III, p. 1100 ASM Press, Washington DC. [Google Scholar]
- Doron S, Melamed S, Ofir G, Leavitt A, Lopatina A, Keren M, Amitai G, and Sorek R (2018) Systematic discovery of antiphage defense systems in the microbial pangenome. Science 359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure G, Shmakov SA, Yan WX, Cheng DR, Scott DA, Peters JE, Makarova KS, and Koonin EV (2019) CRISPR-Cas in mobile genetic elements: counter-defence and beyond. Nat Rev Microbiol 17: 513–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn JA, Parks AR, and Peters JE (2007) Transposon Tn7 directs transposition into the genome of filamentous bacteriophage M13 using the element-encoded TnsE protein. J Bacteriol 189: 9122–9125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier P-E, Vallenet D, Barbe V, Audic S, Ogata H, Poirel L, Richet H, Robert C, Mangenot S, Abergel C, Nordmann P, Weissenbach J, Raoult D, and Claverie J-M, (2006) Comparative Genomics of Multidrug Resistance in Acinetobacter baumannii. In: PLoS Genet, pp. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpin-Healy TS, Klompe SE, Sternberg SH, and Fernandez IS (2019) Structural basis of DNA targeting by a transposon-encoded CRISPR-Cas system. bioRxiv [DOI] [PubMed]
- Hamidian M, and Hall RM (2011) AbaR4 replaces AbaR3 in a carbapenem-resistant Acinetobacter baumannii isolate belonging to global clone 1 from an Australian hospital. Journal of Antimicrobial Chemotherapy 66: 2484–2491. [DOI] [PubMed] [Google Scholar]
- Hickman AB, Li L, Mathew SV, May EW, Craig NL, and Dyda F (2000) Unexpected structural diversity in DNA recombination: the restriction endonuclease connection. Mol. Cell 5: 1025–1034. [DOI] [PubMed] [Google Scholar]
- Jang S, and Harshey RM (2015) Repair of transposable phage Mu DNA insertions begins only when the E. coli replisome collides with the transpososome. Mol Microbiol 97: 746–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klompe SE, Vo PLH, Halpin-Healy TS, and Sternberg SH (2019) Transposon-encoded CRISPR-Cas systems direct RNA-guided DNA integration. Nature 517: 219–225. [DOI] [PubMed] [Google Scholar]
- Koonin EV, and Makarova KS (2017) Mobile Genetic Elements and Evolution of CRISPR-Cas Systems: All the Way There and Back. Genome biology and evolution 9: 2812–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonin EV, Makarova KS, and Wolf YI (2017a) Evolutionary Genomics of Defense Systems in Archaea and Bacteria. Annu Rev Microbiol 71: 233–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonin EV, Makarova KS, and Zhang F (2017b) Diversity, classification and evolution of CRISPR-Cas systems. Curr Opin Microbiol 37: 67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupovic M, Beguin P, and Koonin EV (2017) Casposons: mobile genetic elements that gave rise to the CRISPR-Cas adaptation machinery. Curr Opin Microbiol 38: 36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupovic M, Makarova KS, Forterre P, Prangishvili D, and Koonin EV (2014) Casposons: a new superfamily of self-synthesizing DNA transposons at the origin of prokaryotic CRISPR-Cas immunity. BMC Biol 12: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuduvalli P, Rao JE, and Craig NL (2001) Target DNA structure plays a critical role in Tn7 transposition. EMBO J 20: 924–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuduvalli PN, Mitra R, and Craig NL (2005) Site-specific Tn7 transposition into the human genome. Nucleic Acids Res 33: 857–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Craig NL, and Peters JE, (2013) Transposon Tn7. In: Bacterial Integrative Mobile Genetic Elements Roberts AP & Mullany P (eds). Landes Bioscience, pp. 1–32. [Google Scholar]
- Makarova KS, Wolf YI, Alkhnbashi OS, Costa F, Shah SA, Saunders SJ, Barrangou R, Brouns SJ, Charpentier E, Haft DH, Horvath P, Moineau S, Mojica FJ, Terns RM, Terns MP, White MF, Yakunin AF, Garrett RA, van der Oost J, Backofen R, and Koonin EV (2015) An updated evolutionary classification of CRISPR-Cas systems. Nat Rev Microbiol 13: 722–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May EW, and Craig NL (1996) Switching from cut-and-paste to replicative Tn7 transposition. Science 272: 401–404. [DOI] [PubMed] [Google Scholar]
- McKenzie GJ, and Craig NL (2006) Fast, easy and efficient: site-specific insertion of transgenes into enterobacterial chromosomes using Tn7 without need for selection of the insertion event. BMC Microbiol 6: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minakhina S, Kholodii G, Mindlin S, Yurieva O, and Nikiforov V (1999) Tn5053 family transposons are res site hunters sensing plasmidal res sites occupied by cognate resolvases. Mol. Microbiol 33: 1059–1068. [DOI] [PubMed] [Google Scholar]
- Mitra R, McKenzie GJ, Yi L, Lee CA, and Craig NL (2010) Characterization of the TnsD-attTn7 complex that promotes site-specific insertion of Tn7. Mobile DNA 1: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno Switt AI, den Bakker HC, Cummings CA, Rodriguez-Rivera LD, Govoni G, Raneiri ML, Degoricija L, Brown S, Hoelzer K, Peters JE, Bolchacova E, Furtado MR, and Wiedmann M, (2012) Identification and characterization of novel Salmonella mobile elements involved in the dissemination of genes linked to virulence and transmission. In: PLoS ONE pp. e41247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nero TM, Dalia TN, Wang JC, Kysela DT, Bochman ML, and Dalia AB (2018) ComM is a hexameric helicase that promotes branch migration during natural transformation in diverse Gram-negative species. Nucleic Acids Res 46: 6099–6111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas E, Lambin M, Dandoy D, Galloy C, Nguyen N, Oger CA, and Hallet B (2015) The Tn3-family of Replicative Transposons. Microbiol Spectr 3. [DOI] [PubMed] [Google Scholar]
- Nowotny M (2009) Retroviral integrase superfamily: the structural perspective. EMBO Rep 10: 144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks AR, Li Z, Shi Q, Owens RM, Jin MM, and Peters JE (2009) Transposition into replicating DNA occurs through interaction with the processivity factor. Cell 138: 685–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks AR, and Peters JE (2007) Transposon Tn7 is widespread in diverse bacteria and forms genomic islands. Journal of Bacteriology 189: 2170–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks AR, and Peters JE (2009) Tn7 elements: engendering diversity from chromosomes to episomes. Plasmid 61: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JE (2014) Tn7. Microbiology Spectrum 2: 1–20. [DOI] [PubMed] [Google Scholar]
- Peters JE, and Craig NL (2001) Tn7 recognizes target structures associated with DNA replication using the DNA binding protein TnsE. Genes & Dev 15: 737–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JE, Fricker AD, Kapili BJ, and Petassi MT (2014) Heteromeric transposase elements: generators of genomic islands across diverse bacteria. Mol Microbiol 93: 1084–1092. [DOI] [PubMed] [Google Scholar]
- Peters JE, Makarova KS, Shmakov S, and Koonin EV (2017) Recruitment of CRISPR-Cas systems by Tn7-like transposons. Proc Natl Acad Sci U S A 114: E7358–E7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JM, Koo B-M, Patino R, Heussler GE, Hearne CC, Inclan Y, Hawkins JS, Lu CHS, Harden MM, Osadnik H, Peters JE, Engel JN, Dutton RJ, Grossman AD, Gross CA, and Rosenberg OS (2018) Mobile-CRISPRi: Enabling Genetic Analysis of Diverse Bacteria. bioRxiv [DOI] [PMC free article] [PubMed]
- Sarnovsky R, May EW, and Craig NL (1996) The Tn7 transposase is a heteromeric complex in which DNA breakage and joining activities are distributed between different gene products. EMBO J 15: 6348–6361. [PMC free article] [PubMed] [Google Scholar]
- Shi Q, Straus MR, Caron JJ, Wang H, Chung YS, Guarne A, and Peters JE (2015) Conformational toggling controls target site choice for the heteromeric transposase element Tn7. Nucleic Acids Res [DOI] [PMC free article] [PubMed]
- Shmakov S, Smargon A, Scott D, Cox D, Pyzocha N, Yan W, Abudayyeh OO, Gootenberg JS, Makarova KS, Wolf YI, Severinov K, Zhang F, and Koonin EV (2017a) Diversity and evolution of class 2 CRISPR-Cas systems. Nat Rev Microbiol 15: 169–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmakov SA, Sitnik V, Makarova KS, Wolf YI, Severinov KV, and Koonin EV (2017b) The CRISPR Spacer Space Is Dominated by Sequences from Species-Specific Mobilomes. MBio 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silas S, Makarova KS, Shmakov S, Paez-Espino D, Mohr G, Liu Y, Davison M, Roux S, Krishnamurthy SR, Fu BXH, Hansen LL, Wang D, Sullivan MB, Millard A, Clokie MR, Bhaya D, Lambowitz AM, Kyrpides NC, Koonin EV, and Fire AZ (2017) On the Origin of Reverse Transcriptase-Using CRISPR-Cas Systems and Their Hyperdiverse, Enigmatic Spacer Repertoires. MBio 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silas S, Mohr G, Sidote DJ, Markham LM, Sanchez-Amat A, Bhaya D, Lambowitz AM, and Fire AZ (2016) Direct CRISPR spacer acquisition from RNA by a natural reverse transcriptase-Cas1 fusion protein. Science 351: aad4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strecker J, Ladha A, Gardner Z, Schmid-Burgk JL, Makarova KS, Koonin EV, and Zhang F (2019) RNA-guided DNA insertion with CRISPR-associated transposases. Science 365: 48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turlan C, and Chandler M, (2000) Playing second fiddle: second-strand processing and liberation of transposable elements from donor DNA. In: Trends Microbiol pp. 268–274. [DOI] [PubMed] [Google Scholar]
- Wolkow CA, DeBoy RT, and Craig NL (1996) Conjugating plasmids are preferred targets for Tn7. Genes & Dev 10: 2145–2157. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Ng S, Nam KH, and Ke A (2017) How type II CRISPR-Cas establish immunity through Cas1-Cas2-mediated spacer integration. Nature 550: 137–141. [DOI] [PMC free article] [PubMed] [Google Scholar]