Abstract
Iron is a nutrient metal, but excess iron promotes tissue damage. Since iron chelation therapies exhibit multiple off-target toxicities, there is a substantial demand for more specific approaches to decrease iron burden in iron overload. While the divalent metal transporter 1 (DMT1) plays a well-established role in the absorption of dietary iron, up-regulation of intestinal DMT1 is associated with iron overload in both humans and rodents. Hence, we developed a novel pH-sensitive multi-compartmental particulate (MCP) oral delivery system that encapsulates DMT1 siRNA and validated its efficacy in mice. Using the gelatin NPs coated with Eudragit® L100–55, we demonstrated that DMT1 siRNA-loaded MCPs down-regulated DMT1 mRNA levels in the duodenum, which was consistent with decreased intestinal absorption of orally-administered 59Fe. Together, the Eudragit® L100–55-based oral siRNA delivery system could provide an effective strategy to specifically down-regulate duodenal DMT1 and mitigate iron absorption.
Keywords: Eudragit® L100–55 oral delivery system, gelatin nanoparticles, DMT1 siRNA, iron overload, divalent metal transporter 1
BACKGROUND
Iron is required for many essential biological processes, including electron transfer in mitochondria, oxygen transport/storage, DNA synthesis, drug metabolism and neural activities. However, high iron stores are associated with the pathogenesis of various diseases, including heart failure, liver cirrhosis, arthritis, and diabetes (1). For example, hereditary hemochromatosis (HH) is one of the most common genetic iron overload disorders frequently found in the Caucasian population (2). HH is characterized by excessive intestinal absorption of dietary iron and progressive deposition of excess iron in parenchymal tissues, leading to multi-organ dysfunction (3). Iron overload also occurs due to multiple blood transfusions that are required for several hemoglobinopathies (4).
Although the chelation therapy has been commonly used to ameliorate disease conditions in patients with iron overload, iron chelators have shown a number of serious adverse effects, including gastrointestinal bleeding, neutropenia, liver fibrosis and kidney damage (5–9). Consequently, there is an unmet demand for a new treatment strategy for iron overload disorders. Since body iron status is tightly regulated at the level of absorption (10), inhibition of iron uptake in the intestine could provide therapeutic benefits for iron overload. While the divalent metal transporter 1 (DMT1) is a major iron importer expressed in the mammalian duodenum (11–13), up-regulation of intestinal DMT1 is associated with HH in both humans and mice (14–16). However, DMT1 also plays an essential role in intracellular iron transport during red cell production in the bone marrow (17, 18). Hence, an intestine-specific suppression of DMT1 could selectively inhibit iron absorption and improve iron overload with no effect on erythropoiesis or systemic utilization of iron. Although a few small molecule-based DMT1 inhibitors have been examined (19–22), these compounds modify DMT1 function by indirect mechanisms, including alterations in redox status (19, 20). Furthermore, these inhibitors may exhibit unfavorable in vivo pharmacokinetic properties and likely systemic side effects, including off-target activities (22). Although phlebotomy is a common practice to ameliorate iron overload conditions, this invasive method is accompanied by complications, including pain and fatigue (23). Thus, there is a substantial need for direct and more specific therapy to decrease iron absorption and improve the quality of life of patients with iron overload.
Since the development of first gene transfer method in the early 1980s, the possibility of using nucleic acid-based drugs to treat various diseases has been explored (24). Most of the human diseases are caused by activity of one or more genes, which can be targeted using anti-sense strategies such as anti-sense DNA, oligonucleotides and ribozymes (25). In particular, the siRNA duplex molecules have been known to specifically silence a single gene expression at the post-transcriptional level (such as cancer-causing proteins and pro-inflammatory cytokines) and exhibit efficacy at low concentrations compared with conventional small molecule therapeutics, which is a beneficial factor for design of any therapeutic strategy (25–27).
Oral non-viral gene delivery is one of the most preferable routes of administration over the invasive intestinal therapeutics due to better patient compliance, convenience and direct administration to the diseased tissue (28–32). However, it is also accompanied by the challenges associated with the route of delivery due to the susceptibility of payload to the acidic pH conditions, enzymatic environment, mucus trapping, and other transport barriers of the gastrointestinal tract (32, 33). To address this issue, we have engineered multi-compartmental delivery systems, such as solid-in-solid, solid-in-liquid, solid-in-gel, and liquid-in-liquid formulations, for target-specific oral delivery and evaluated their efficacies (32–35). An example of solid-in-solid multi-compartment delivery systems include polymeric nanoparticles (NPs), which are further encapsulated and protected in a particulate system against degradation. For example, multi-compartmental particle (MCP)-based oral delivery system composed of siRNA-containing gelatin NPs as core and surrounded by a pH-sensitive polymethacrylate-based anionic polymer, Eudragit® L100–55 (Evonik Health Care) shell. To ensure the clinical translation of the formulation, Eudragit® L100–55, a pH-sensitive polymer which dissolves at pH >5.5 (equivalent to duodenum pH) was selected for the preparation of the particle shell since it is biocompatible and is FDA-approved generally recognized as safe (GRAS) material which has been used in various oral-based medical and pharmaceutical applications (36, 37).
In our previous studies, we have demonstrated that intestinal TNFα was significantly down-regulated in a mouse model of colitis after oral administration of TNFα siRNA-containing MCP, which further improved colitis condition (32, 35, 38). These results suggest that the oral gene silencing strategy could be exploited in the area of iron metabolism, especially in iron transport, to mitigate iron absorption and potentially ameliorate physiological complications associated with iron overload. Thus, to achieve the goal of the present study, we have developed and validated an orally-active DMT1 siRNA in MCP formulation and have evaluated if such formulation decreases gene expression of DMT1, specifically in the duodenum, and mitigates intestinal iron absorption.
METHODS
Nanoparticle Formulation and Characterization.
All of the biopolymers used for preparation of nanoparticles were confirmed to be endotoxin and nuclease free and were tested using Ambion® RNaseAlert® lab test kit (Thermo Fischer scientific). Gelatin NPs were formulated using ethanol-water solvent-displacement method as previously published (39) with minor modifications (Supplementary Information). The encapsulation efficiency of siRNA was determined using the protease digestion method (39). The released siRNA was quantified using RiboGreen® RNA assay reagent following manufacturer’s instructions. Protamine/siRNA complexed NPs were formed at a 1:1 w/w ratio. Protamine and siRNA solutions were prepared at a concentration of 1.0 and 0.5 mg/ml in RNase-free distilled water, respectively. Both solutions were gently vortexed for 1 min and were incubated for 10 min at room temperature to form self-assembled NPs. HA-PEI/HA-PEG/siRNA complexed NPs were prepared according to previously published method (40). Briefly, HA (20 kDa, Lifecore Biomedical Co.) was first conjugated to bPEI (10 kDa, Polysciences Inc.) and PEG-amine (2 kDa; Creative PEG works) polymers using the EDC/NHS reaction, and the final purified product was lyophilized and was stored at −20°C for further studies (40). For the NP formation, the HA-PEI and HA-PEG solutions were first mixed and vortexed at a 1:1 w/w in PBS buffer (pH 7.4) at a final concentration of 3 mg/ml. siRNA was added to the HA polymer solution at a mass ratio of 54:1 and was vortexed for 1 min with further incubation for 20 min at room temperature to form the self-assembled nano-systems. The un-encapsulated siRNA was separated from the NP suspension by centrifugation at 14,500 rpm for 60 min at 4°C. The encapsulation efficiency of protamine and HA NPs was determined by an indirect method measuring the amount of free siRNA in the supernatant using Quant-iT™ RiboGreen® reagent (40). All the NPs were freshly prepared prior to experiments.
Nanoparticle Size and Surface Charge Analyses.
The hydrodynamic diameter and the surface charge of NPs were determined in water using Malvern’s Zetasizer Nano ZS 90 apparatus (Westborough, MA) at a 90° scattering angle. Gelatin NPs were evaluated for their size measurement and surface morphology with a Hitachi S4800 (Pleasanton, CA) field emission scanning electron microscope (SEM). The freeze-dried particles (in the presence of 3% sucrose) were sputter coated with gold-palladium to minimize surface charging for SEM imaging. Transmission electron microscope (TEM) images of protamine and HA-PEI/HA-PEG NPs stained using 2% uranyl acetate were assessed for morphology and size using JEOL JEM-1000 instrument (JEOL Ltd, Tokyo, Japan). All sizing measurements were conducted at room temperature.
Formulation and Characterization of Multi-Compartmental Particles.
The MCP formulation was prepared by utilizing the “double emulsion-like” technique, which is a frequently used method for production of particulate systems in the pharmaceutical industry (35, 41). Briefly, 4% (w/v) solution of Eudragit® L100–55 was prepared by dissolving in absolute ethanol. Next, 500 μl of gelatin NP suspension (5.0 mg/ml) containing desired amount of siRNA was added to 5 ml of Eudragit® L100–55 solution. The solution was then homogenized at 8,000 rpm using a Silverson lab mixture (Model L4RT-A, Silverson Lab Machines, Bucks, England) for 10 min. To form double emulsion system, 10 ml of 0.5% (w/v) polyvinyl alcohol (PVA) dissolved in deionized water was added to the above solution and the resulting emulsion was stirred for 10 h for evaporation of ethanol and further hardening of the particles. The suspension was then centrifuged at 8,000 rpm for 40 min and washed three times with water for removal of excess PVA, followed by storage at 4°C until used. The evaluation of siRNA loading in MCPs was carried out by adding MCP samples to phosphate buffer (pH 8.0) to completely dissolve the Eudragit® matrix and release gelatin NPs. The gelatin NPs were then further digested using protease to release siRNA (38). The final siRNA loading efficiency in the MCPs was evaluated using the Quant-iT™ RiboGreen® assay. MCP samples were lyophilized and were sputter coated with gold-palladium to minimize surface charging and were evaluated for their size measurement and surface morphology with a Hitachi S4800 field emission SEM.
Cell Culture Conditions and Transfection.
Caco-2 epithelial cells (American Type Culture Collection; VA) were cultured in DMEM supplemented with 20% FBS, 1 mM sodium pyruvate, 1 mM non-essential amino acids and 1% antibiotics (penicillin/streptomycin) and maintained in a 37°C and 5% CO2 humidified incubator. The Caco-2 cell line was used for selection of the NP delivery system for GAPDH and DMT1 siRNA transfection. Details of DMT1/GAPDH siRNA sequences and manufacturer are provided in the Supplementary Information. Experiments were conducted using cell passages 10–20. Briefly, 200,000 cells were incubated with NPs containing 100 nM siRNA in serum-free media for 6 h at 37°C. After 6 h, the cell media was replaced with serum-containing media and cells were further incubated for 48 h. The three NP systems, including gelatin, protamine and HA-PEI/HA-PEG NPs loaded with GAPDH siRNA, were tested at 48 h post-transfection based on our previous publications (39, 42). The untreated cells, cells treated with blank (empty) NPs and negative siRNA loaded NPs served as control groups. The DharmaFECT4 (Dharmacon Inc., CO) loaded siRNA served as the positive control group. After the selection of gelatin NPs for DMT1 siRNA studies, the optimization for time kinetics was performed at 12, 24, and 48 h post-transfection. Total RNA was isolated using Quick-RNA™ isolation kit as per manufacturer’s instructions (Zymo Research, CA). RNA (1 µg) was reversely transcribed into cDNA and the expression of GAPDH and DMT1 mRNA was quantified by 7300 Real-Time PCR System from Applied Biosystems using the cycle program for SYBR® green reaction (sequences of primers listed in Suppl. Table 1). The comparative Ct method (43) was used to analyze expression of mRNA. β-actin was used as a house-keeping gene.
In Vivo Evaluation of GAPDH and DMT1 Gene Silencing.
The experiments involving use of animals were approved by Northeastern University’s Institutional Animal Care and Use Committee. CD-1 male mice (6–7 weeks old; Charles River Laboratories, Wilmington, MA) were given facility chow and water ad libitum and randomly assigned to the different treatment groups (n=5–6). In order to reduce the effect of food on the transport of NPs, the mice were fasted overnight, followed by intragastric gavage of blank MCPs (i.e. siRNA unloaded particles), GAPDH siRNA-loaded MCPs or DMT1 siRNA-loaded MCPs with a total of 2 or 5 mg siRNA/kg body weight. Vehicle, which refers to ‘empty or blank particles’, were suspended in distilled water for oral gavage. An additional untreated group was also included in the study; mice were fasted overnight and received no oral treatment throughout the study. The GAPDH siRNA-treated group was used as a negative control for the DMT1 siRNA silencing experiment, and the DMT1 siRNA group was used as a negative control for the GAPDH siRNA silencing experiment. After the gavage, the mice were provided access to regular facility chow and water ad libitum.
For the time course of gene silencing effect, mice treated with blank MCPs or GAPDH siRNA-loaded MCPs (2 mg siRNA/kg) were euthanized at 24 h and 48 h post-gavage by isoflurane overdose, followed by exsanguination to harvest blood, duodenum, jejunum, ileum and colon. To evaluate the gene silencing efficacy of GAPDH and DMT1, mice treated with blank MCPs, GAPDH siRNA-loaded MCPs (5 mg siRNA/kg) or DMT1 siRNA-loaded MCPs (5 mg siRNA/kg) were euthanized 24 h post-administration. All tissues were flash-frozen in liquid nitrogen and stored at −80°C until further analysis. Total RNA was isolated from frozen tissues using TRI reagent following the manufacturer’s instructions. RNA (1 µg) was reversely transcribed into cDNA, which was used for real-time PCR assay using SYBR® green reagent. The target genes for qPCR were GAPDH and DMT1, and β-actin was used as a house-keeping gene (primers listed in Suppl. Table 1).
In Vivo 59Fe Absorption and Distribution.
CD-1 male mice were fasted overnight, followed by intragastric gavage of either blank MCPs or DMT1 siRNA-loaded MCPs with a total of 5 mg siRNA/kg. An additional untreated group was also included in the study; these mice were fasted overnight and received no oral administration of MCPs. After MCP administration, the mice were given regular facility chow and water ad libitum. At 20 h post-MCP administration, the mice were fasted for 4 h and intragastrically gavaged with 59FeCl2 (200 µCi/kg; Perkin Elmer, MA). At 4 h post-59Fe gavage, the mice were euthanized by isoflurane overdose to harvest blood, urine, bile, and tissues, including GI segments (stomach, whole intestine), liver, lung, heart, spleen, brain, kidney, femur and upper thigh (quadricep) muscle. Radioactivity in the tissues was quantified using a gamma counter (Packard Cobra) and calculated as the percentage of dose.
Statistical Analysis.
Data were presented as means ± standard error of the mean (SE). Statistical significance was determined by the Student’s t-test (for two-group comparisons) or one-way ANOVA followed by Tukey’s post-hoc analysis (more than two-group comparisons) to evaluate the silencing effect of siRNA-containing NP groups. Data values in cell experiments are presented as mean ± SE, n=4–6 individual experiments per group. Differences were considered significant at p < 0.05.
RESULTS
Physicochemical Characterization of GAPDH siRNA-Loaded NPs.
Since the absence of RNase is important to ensure the stability of siRNA in the NP formulations, we first tested inherent RNase activity of the polymers used for NP formulations (Suppl. Fig. 1) by a fluorescence measurement-based assay, which showed absence of RNase in the selected polymers. We then characterized the particle size, zeta potential and morphology of GAPDH siRNA-loaded NPs prepared using fish gelatin, protamine and HA-PEI/HA-PEG (Fig. 1). Gelatin/GAPDH siRNA NPs showed an average mean hydrodynamic diameter of 230.3 ± 6.4 nm and a slight negative surface charge of −4.5 ± 0.5 mV (Fig. 1A). SEM analysis demonstrated the presence of uniform, spherical-shaped particles with a smooth surface morphology (Fig. 1B). The gelatin NPs also showed a favorable encapsulation efficiency of siRNA, 85.0 ± 4.0%.
Fig. 1. Size and surface charge distribution of GAPDH/siRNA encapsulated gelatin, protamine and HA-PEI/HA-PEG NPs.
(A) Malvern Zetasizer (n=3; mean ± SE); SEM image of (B) gelatin/GAPDH siRNA NPs; TEM image of (C) protamine/GAPDH siRNA, and (D) HA-PEI/HA-PEG/GAPDH siRNA NPs, respectively.
Protamine/GAPDH siRNA NPs showed an average size of 198.0 ± 8.3 nm with a slightly positive surface charge (+3.6 ± 0.2 mV) and high encapsulation of siRNA (98.7 ± 0.3%) (Fig. 1A), indicating the strong electrostatic interaction between positive-charged protamine and negative-charged siRNA. In addition, since HA-PEI/HA-PEG NPs encapsulate siRNA owing to the electrostatic interaction between the positively charged PEI and negatively charged siRNA, we also developed GAPDH siRNA-containing HA-PEI/HA-PEG NPs (Fig. 1A). HA-PEI/HA-PEG/GAPDH siRNA NPs exhibited an average size of 144.9 ± 5.9 nm and negative surface charge (−11.5 ± 0.3 mV), which was likely contributed by both HA and PEG. The TEM image of protamine- and HA-based NPs showed spherical shaped particles with dark core, which could be attributed to the high contrast arising from the uranyl acetate stained protamine or PEI-condensed siRNA (Fig. 1C and 1D). The high encapsulation efficiency (>98.0 %) of GAPDH siRNA in HA-NPs likely results from the optimum ratio between the polymer and siRNA.
In Vitro Gene Silencing Effects of GAPDH or DMT1 siRNA-loaded NPs.
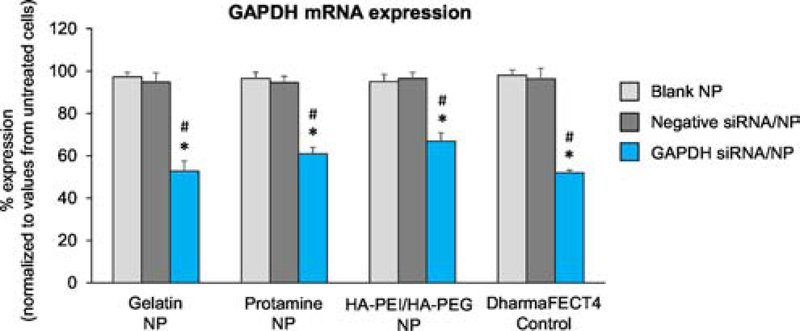
To determine the gene silencing effect of siRNA using the three NP platforms, Caco-2 cells were exposed to NPs, followed by real-time PCR assay to quantify the expression levels of GAPDH mRNA. The GAPDH mRNA expression was significantly decreased in cells exposed to all three GAPDH siRNA/NPs compared with untreated cells over 48 h post-exposure (Fig. 2). There were no significant changes in the GAPDH gene expression levels in blank NP-exposed cells or negative siRNA/NP-exposed cells compared with untreated cells. Moreover, the expression levels of GAPDH in cells exposed to GAPDH siRNA-loaded gelatin, protamine and HA-PEI/HA-PEG NPs significantly decreased by 46%, 37%, and 30%, respectively, when compared with the blank NP-exposed cells (Fig. 2). Positive control (DharmaFECT4/siRNA transfected cells) showed a 49% decrease in GAPDH mRNA expression compared with blank NP-exposed cells. The decreases in GAPDH mRNA expression were also consistently significant in the GAPDH siRNA/NP groups when compared with respective negative siRNA control groups.
Fig. 2. GAPDH expression in Caco-2 cells transfected with 100 nM GAPDH siRNA-loaded gelatin, protamine, and HA-PEI/HA-PEG NPs.
DharmaFECT4-loaded siRNA was used as a positive control group. qPCR was performed for quantification of GAPDH mRNA level 48 h post-incubation of NPs. GAPDH mRNA expression from test groups was normalized to that from untreated cells. Data values are presented as mean ± SE, n=4 individual experiments per group. One-way ANOVA was employed, followed by Tukey’s post-hoc analysis. *p<0.05 vs. blank NP. #p<0.05 vs. negative siRNA/NP.
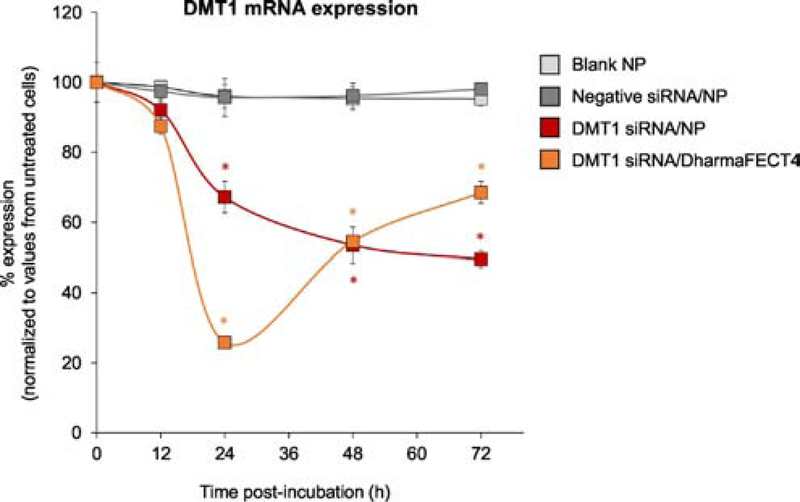
Since the gelatin/GAPDH siRNA NPs showed the highest gene silencing effect among NPs tested (Fig. 2), we selected gelatin as a preferred NP platform for further studies involving DMT1 silencing. The characterization of gelatin/DMT1 NPs showed an average mean hydrodynamic diameter of 220.0 ± 10.0 nm, a slight negative surface charge of −3.6 ± 0.8 mV and ~85.0 % encapsulation of DMT1 siRNA. While there was no significant change in DMT1 mRNA expression in Caco-2 cells exposed to blank NPs or negative siRNA/NPs compared with untreated cells, the expression levels of DMT1 mRNA in cells exposed to DMT1 siRNA-loaded gelatin NPs significantly decreased in a time-dependent manner (Fig. 3). The half-life of DMT1 mRNA was 109 h.
Fig. 3. DMT1 expression in Caco-2 cells exposed to gelatin NPs loaded with DMT1 siRNA.
qPCR was performed for quantification of DMT1 mRNA levels at 12, 24, 48 and 72 h post incubation of NPs. DMT1 mRNA expression in each test group was normalized to untreated cells. Data values are presented as mean ± SE, n=4 individual experiments per group. A mono-exponential kinetic model was employed to fit the gelatin NP-loaded DMT1 data and calculate the half-life. One-way ANOVA was employed, followed by Tukey’s post-hoc analysis. *p<0.05 vs. blank NP at each time point.
Characterization of Eudragit® L100–55 based MCP System.
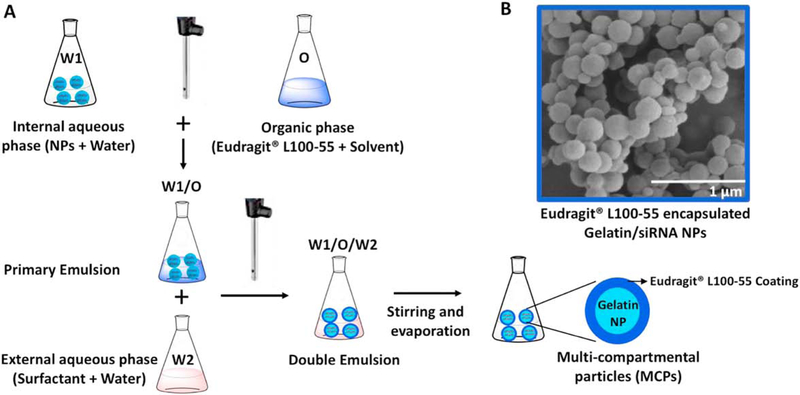
After the selection of NP platform, we prepared the Eudragit® L100–55-coated MCPs with gelatin NPs using the double-emulsion technique as illustrated in Fig. 4. The SEM image showed fairly uniform spherical MCPs with smooth surface morphology and an average size of ~300 nm. The size of the MCPs was greater than that of the gelatin NPs (~230 nm), indicating uniform coating of Eudragit® L100–55 around gelatin NPs. The siRNA encapsulation efficiency of the MCP was 80 ± 4.5%.
Fig. 4. Schematic representation of Eudragit® L100–55 multi-compartment particles (MCPs) with encapsulated gelatin/siRNA NPs.
(A) Schematics of the MCP formation process. (B) Representative SEM image of the MCPs formed using Eudragit® L100–55 encapsulated gelatin/siRNA NPs.
In Vivo Intestinal GAPDH Silencing.
To gain insight into the region-specific silencing efficacy of MCP formulation, we first investigated silencing of GAPDH gene in various regions of intestine by real-time qPCR. There was no silencing effect on GAPDH expression in the gut from animals treated with blank MCP when compared with untreated animals (Suppl. Fig. 2). However, the GAPDH mRNA levels in the duodenum from the MCP/GAPDH siRNA-treated group (2 mg siRNA/kg) were significantly decreased by 25 ± 6% compared with the blank MCP group at 24 h post-administration (Suppl. Fig. 2A). In addition, there was no gene silencing effect on the GAPDH expression in the jejunum, ileum and colon.
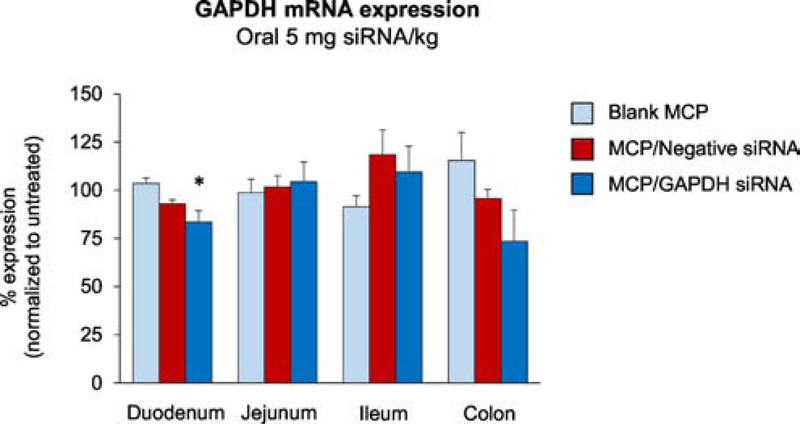
To test the duration of gene silencing effect, we measured GAPDH gene expression at 48 h time point post-administration (Suppl. Fig. 2B). There was no significant effect on GAPDH gene expression in the duodenum and other regions of the intestines, which suggests that 24 h is an optimal time for in vivo silencing effect in the duodenum after a single dose. Next, to explore the dose effect on the GAPDH gene expression, we increased the dose of GAPDH siRNA to 5 mg/kg. The GAPDH mRNA expression was decreased by 20 ± 3% (p = 0.010) in the duodenum compared with the blank MCP group at 24 h post-administration (Fig. 5). We also included a negative control group to address potential non-specific gene silencing effects, which showed no significant effect on GAPDH gene expression. Although there was a trend of decrease in GAPDH levels in the colon of the MCP/GAPDH siRNA group, the difference was not statistically significant.
Fig. 5. GAPDH expression in the intestine of mice exposed to Eudragit® L100–55 MCPs encapsulating gelatin/GAPDH siRNA NPs.
Mice were orally administered with blank MCP, MCP/negative control siRNA and MCP/GAPDH siRNA with 5 mg siRNA/kg. At 24 h post-administration, GAPDH mRNA levels in the intestine of the test groups were quantified by qPCR and normalized to those of the untreated group. Mean ± SE, n=5–6/group. One-way ANOVA was employed, followed by Tukey’s post-hoc analysis. *p<0.05 vs. blank MCP.
In Vivo Intestinal DMT1 Silencing.
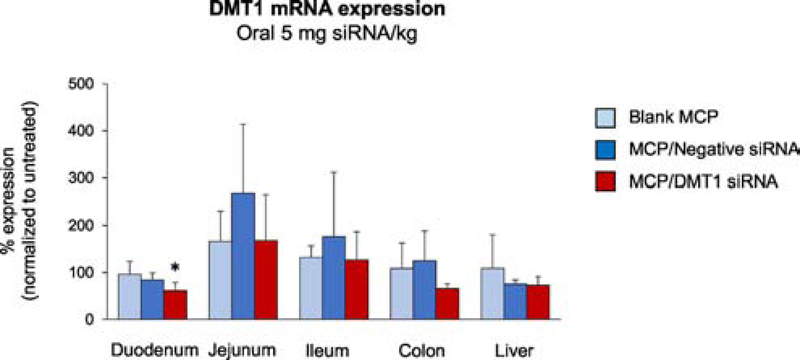
To demonstrate duodenum specific gene silencing, we selected the DMT1 gene, which is a major iron transporter and abundantly expressed in the duodenum. We first conducted in vitro screening of different sequences of mouse-specific DMT1 siRNA using DharmaFECT4 reagent in J774A.1 murine macrophages (Suppl. Fig. 3). Among four sequences, the sequence (GGACCUUUCUGACGAUGAA) showed the maximum DMT1 silencing effect (68% down-regulation) at 48 h post-transfection and hence was selected for in vivo studies. We then administered Eudragit® L100–55 MCPs-loaded with DMT1 siRNA at 5 mg/kg siRNA dose by intragastric gavage. The DMT1 gene expression was significantly decreased by 40% in the duodenum (p = 0.039), but not in other tissues (e.g. jejunum, ileum, colon, and liver), of the MCP/DMT1 siRNA group at 24 h post-administration compared with the blank MCP control group (Fig. 6), indicating no significant off-target effects of MCP/DMT1 siRNA. In contrast, the MCP/negative siRNA demonstrated no change in DMT1 expression in the intestine or liver.
Fig. 6. DMT1 expression in the intestine and liver of mice administered with Eudragit® L100–55 MCPs encapsulating gelatin/DMT1 siRNA NPs.
Mice were orally administered with blank MCP, MCP/negative control siRNA and MCP/DMT1 siRNA with 5 mg siRNA/kg. At 24 h post-administration, DMT1 mRNA levels in the intestine and liver of the test groups were quantified by qPCR and normalized to those of the untreated group. Mean ± SE, n=5–6/group. One-way ANOVA was employed, followed by Tukey’s post-hoc analysis. *p<0.05 vs. blank MCP.
Intestinal Absorption of 59Fe in MCP/DMT1 siRNA-Treated Mice.
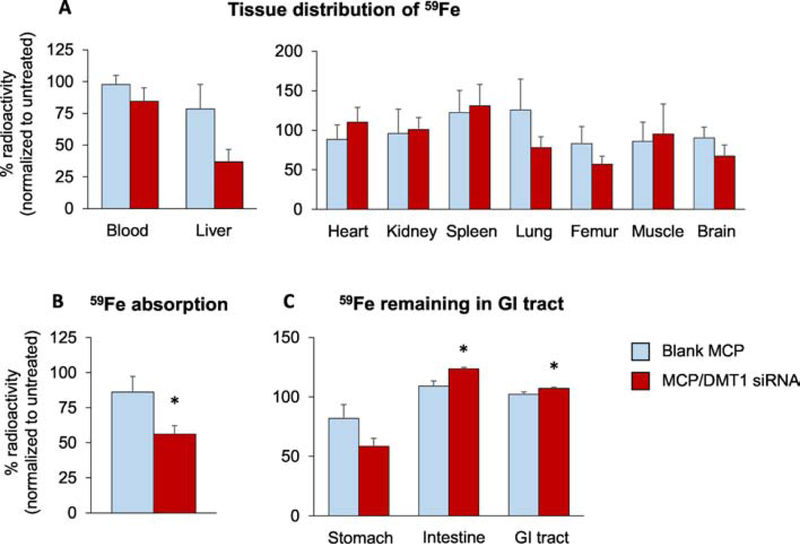
To confirm the effect of DMT1 silencing on the iron absorption, MCP/DMT1 siRNA-treated mice were intragastrically gavaged with 59Fe, followed by tissue collection 4 h post-gavage. The amounts of 59Fe in blood, liver and other tissues at 4 h post-administration showed a trend of decrease in MCP/DMT1 siRNA-treated mice compared with blank MCP-treated mice (Fig. 7, A and B). In particular, DMT1 gene silencing decreased liver 59Fe levels by 53%, although statistically insignificant (Fig. 7A). These could be due to inter-individual differences in the distribution of 59Fe into various tissues after intestinal absorption. Hence, we combined 59Fe amounts recovered in all tissues, except the GI tracts and GI contents, and presented as the amounts of 59Fe remaining in the body (Fig. 7B). These quantities can represent the extent of gut absorption since iron excretion is slow and limited (see Discussion). The total 59Fe absorption was significantly decreased by 44% in mice treated with MCP/DMT1 siRNA compared with blank MCP-treated mice. We also calculated the unabsorbed fraction of 59Fe remaining in the whole GI tract, including stomach and small and large intestines (Fig. 7C). Consistent with tissue-based assessment (Fig. 7B), significantly more 59Fe was found in the GI tract from the MCP/DMT1 siRNA group as compared with the blank MCP group (Fig. 7C), suggesting that MCP/DMT1 siRNA decreases 59Fe absorption after oral administration.
Fig. 7. In vivo absorption and distribution of orally-administered 59Fe.
Following overnight fasting, CD-1 mice were intragastrically-gavaged with blank MCP and MCP/DMT1 siRNA (5 mg siRNA/kg). The radioactivity in blood, liver and other tissues (A) was determined using a gamma counter and normalized to the radioactivity of the untreated mice. (B) Radioactivity recovered in major tissues other than the GI tract was combined to calculate the amount absorbed during 4 h after oral administration of 59Fe. (C) The amount of 59Fe remaining in the stomach, whole intestine and total GI tract at 4 h after oral administration of 59Fe. Mean ± SE. n=7–8/group. The Student’s t-test was employed for two-group comparison. *p<0.05 vs. blank MCP.
DISCUSSION
The RNAi therapy has been considered as a highly selective treatment, especially for intestine-related disorders because it can potentially reduce specific gene expression involved in disease progression (44). Since DMT1 is the main iron transporter that is frequently up-regulated in the duodenum in various iron overload disorders (14–16), we hypothesized that duodenal DMT1 gene silencing would mitigate dietary iron absorption and thereby provide therapeutic benefits for individuals with iron overload. However, the challenging part of the oral delivery system is to safely and efficiently deliver nucleic acids to the targeted intestine region after circumventing the harsh environment of the GI tract, including low pH and high nuclease activities. In this report, we have designed a Eudragit® L100–55-coated NP system which dissolves at pH >5.5 and releases siRNA-containing NPs, thereby allowing for duodenum-specific gene silencing.
We first characterized and evaluated GAPDH silencing efficacy of three different NP platforms, including gelatin-, HA-, and protamine-NPs. All the three NP platforms showed favorable physicochemical characteristics, including small and uniform size and slightly negative or positive charge, suggesting reasonable cell transfection conditions (45–47). However, the three NP formulations differed in the transfection efficiency, with highest gene silencing effect observed in cells exposed to gelatin NPs. These differences could be attributed to distinct characteristics of cell internalization and/or capability of endosomal escape of individual NPs (39, 40). We then selected gelatin NPs to further investigate the DMT1 gene silencing effects; the gelatin NPs showed a sustained DMT1 silencing effect up to 72 h compared with the positive control group DharmaFECT4 particles (24 h). While the shorter duration of effect of DharmaFECT4 particles likely results from lack of protective barrier and thereby rapid degradation of the siRNA within the cell, the gelatin matrix in a non-condensing environment could act as a protective physical barrier, and hence the release of siRNA from gelatin NPs is dependent on degradation of NPs over time (39, 42). Moreover, the kinetic pattern of DMT1 mRNA knockdown after NP exposure was in accordance with silencing efficacy of transglutaminase siRNA-loaded gelatin NPs in Caco-2 cells in our previous report (39). Combined, these results support the idea that the gelatin NPs promote their endocytosis in Caco-2 cells and prolong the suppression of DMT1 expression by slow and sustained release of siRNA in the cytosol, providing favorable multiple-dose regimen for oral gene delivery.
We then developed an effective method to protect gelatin NPs from degradation at an acidic pH in the stomach and from enzymatic degradation during the GI transit by coating the gelatin NPs with Eudragit® L100–55 polymer (36, 37). Eudragit is an FDA-approved polymer for oral drug delivery. It has been widely used as nanoparticle carriers in oral drug delivery system (48, 49). Moreover, the Eudragit® coating provides favourable in vivo oral delivery conditions since it not only helps in safe-guarding gelatin NPs from low pH conditions of the stomach, but also promotes the release of NPs in the duodenum owing to the unique property of Eudragit® L100–55 coating that starts to dissolve in duodenum pH (>5.5), which hence would be beneficial for gene delivery to the duodenum (32, 33, 38). We first used GAPDH siRNA since it is universally expressed throughout the intestine (50). Overall, the results showed similar decreases in duodenal GAPDH mRNA expression at both 2 and 5 mg/kg, suggesting a saturation effect in gene silencing, likely due to insufficient dissolution of high dose of Eudragit® L100–55 during the rapid duodenal transit. In addition, we observed decreased GAPDH levels, although statistically insignificant, in the colon following 5 mg/kg dose. These results suggest that some fraction of Eudragit® L100–55 MCPs after high dose could have passed through the duodenum as intact (undissolved) forms and eventually been dissolved (51), followed by a release of siRNA/NPs in the colon, which could be favored by a long colonic transit time (52, 53). However, this was not observed in DMT1 siRNA when administered the same high dose (5 mg siRNA/kg). This could be due to the different amount of expression of each gene in the intestine; GAPDH, as an established house-keeping gene, is highly expressed in all regions of the intestine (50), whereas DMT1 expression is region-specific with the highest expression in the duodenum (13). Thus, it is feasible that the release of siRNA by colonic dissolution of MCP could down-regulate GAPDH more effectively than DMT1 gene. Together, these results indicate that Eudragit® L100–55 MCPs safely deliver siRNA-containing NPs to the duodenal epithelial cells and provides duodenum-specific DMT1 gene silencing effects, while the amount of dose plays an important role in determining both the site and the extent of silencing. Further, the DMT1 siRNA/MCPs showed target specificity by only showing a decrease in DMT1 levels in the duodenum with no off-target effects observed in the liver.
To verify our model of duodenal DMT1 gene silencing by another method, we employed a functional assay in which mice pretreated with MCP/DMT siRNA or blank MCP were administered 59Fe via intragastric gavage. Since 70% of intravenously-injected 59Fe disappears from the blood over 4 h post-injection due to rapid distribution into various tissues (54), the radioactivity of 59Fe remaining only in blood after oral administration would not serve as an accurate measure of iron absorption. Instead, the extent of 59Fe absorption after intragastric gavage would be more quantitatively assessed by a sum of 59Fe remaining in blood and tissues (except the GI contents) as well as 59Fe that had been excreted over 4 h. In a separate pilot study, we found 59Fe levels in feces (0.5% of dose) and urine (< 1%) were negligible during 4 h after intravenous injection. Hence, the amounts of 59Fe recovered in blood and major tissues provides the extent of iron absorption. We also determined and compared 59Fe radioactivity remaining (unabsorbed) in the GI tract between MCP/DMT1 siRNA-treated and blank MCP-treated mice. Both results (i.e. decreased 59Fe in internal tissues and increased 59Fe in GI contents) support the idea that MCP-assisted DMT1 oral gene delivery efficiently down-regulates DMT1 expression in the gut and mitigates intestinal iron absorption.
While there has been increased interest in the application of oral gene silencing in various diseases, Wang et al. (55) have recently demonstrated the influence of DMT1 gene silencing on the inhibition of intestinal iron absorption using ginger nanoparticles-derived lipid vectors (GDLV). Although this is an important advancement of oral gene silencing therapies in iron disorders, the authors did not characterize the exact site of gene silencing within the GI tract (i.e. duodenum vs. jejunum, ileum and colon). In contrast, the benefit of our nanosystem is the duodenum-specific silencing since the Eudragit polymer (L100–55) used for coating of nanoparticles dissolves at duodenum pH which helps in boosting the gene silencing effect by maximum delivery of nanoparticles into the duodenum. Thus, our nanosystem efficiently modulates duodenal iron uptake and could also be useful for other disease conditions where duodenum specificity is required. Moreover, we have optimized the MCP delivery system using two different genes, i.e. GAPDH and DMT1, which vary in terms of expression and specificity in different regions of the intestine, to better understand its applicability in region-specific delivery of payload. Another important aspect is that Eudragit polymers are already FDA-approved and currently used for coating of tablets for oral delivery, which reduces concerns about safety. Additionally, our nanosystem is homogenous in comparison to GDLV nanoparticles which are highly heterogenous and typical of natural nanosystems.
In conclusion, we have successfully developed and optimized a Eudragit® L100–55 based MCP delivery system for intestine-specific gene silencing by both in vitro and in vivo approaches. Our oral gene silencing strategy could provide a specific and effective method to mitigate intestinal uptake of dietary iron with no significant off-target effects. Future studies will be focused on long-term therapeutic efficacy of oral gene silencing which could be combined with existing iron chelation therapies for synergistic effects, as well as clarifications of potential safety issues.
Supplementary Material
Acknowledgements.
We thank Evonik (Darmstadt, Germany) for providing a sample of Eudragit® L100–55 polymer. Electron microscopy was performed at the Electron Microscopy Center of Northeastern University with the assistance of Mr. William Fowle.
This work was partially supported by a research grant EB023025 from the National Institute of Biomedical Imaging and Bioengineering, National Institute of Health.
Abbreviations:
- DMT1
divalent metal transporter 1
- DMEM
Dulbecco’s Minimum essential medium modified Eagle medium
- EDC
N-(3-dimethylaminopropyl)-N’-ethylcarbodiimide hydrochloride
- FBS
fetal bovine serum
- GI
gastrointestinal
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- HH
hereditary hemochromatosis
- HA
hyaluronic acid
- MCPs
multi-compartmental particles
- NPs
nanoparticles
- NHS
N-hydroxysuccinimide
- PDI
poly-dispersity index
- PEG
poly(ethylene glycol)
- PEI
poly(ethylenimine)
- PVA
poly(vinyl alcohol)
- qPCR
quantitative polymerase chain reaction
- SEM
scanning electron microscope
- siRNA
small interfering ribonucleic acid
- TEM
transmission electron microscope
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest.
REFERENCES
- 1.Pietrangelo A. Hereditary hemochromatosis--a new look at an old disease. N Engl J Med. 2004;350(23):2383–97. [DOI] [PubMed] [Google Scholar]
- 2.Bacon BR, Powell LW, Adams PC, Kresina TF, Hoofnagle JH. Molecular medicine and hemochromatosis: at the crossroads. Gastroenterology. 1999;116(1):193–207. [DOI] [PubMed] [Google Scholar]
- 3.Pietrangelo A. Hereditary hemochromatosis: pathogenesis, diagnosis, and treatment. Gastroenterology. 2010;139(2):393–408, e1–2. [DOI] [PubMed] [Google Scholar]
- 4.Wood JC, Enriquez C, Ghugre N, Otto-Duessel M, Aguilar M, Nelson MD, et al. Physiology and pathophysiology of iron cardiomyopathy in thalassemia. Ann N Y Acad Sci. 2005;1054:386–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taher A, El-Beshlawy A, Elalfy MS, Al Zir K, Daar S, Habr D, et al. Efficacy and safety of deferasirox, an oral iron chelator, in heavily iron-overloaded patients with beta-thalassaemia: the ESCALATOR study. Eur J Haematol. 2009;82(6):458–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porter JB, Hoyes KP, Abeysinghe RD, Brooks PN, Huehns ER, Hider RC. Comparison of the subacute toxicity and efficacy of 3-hydroxypyridin-4-one iron chelators in overloaded and nonoverloaded mice. Blood. 1991;78(10):2727–34. [PubMed] [Google Scholar]
- 7.Koren G, Bentur Y, Strong D, Harvey E, Klein J, Baumal R, et al. Acute changes in renal function associated with deferoxamine therapy. Am J Dis Child. 1989;143(9):1077–80. [DOI] [PubMed] [Google Scholar]
- 8.Olivieri NF, Brittenham GM, McLaren CE, Templeton DM, Cameron RG, McClelland RA, et al. Long-term safety and effectiveness of iron-chelation therapy with deferiprone for thalassemia major. N Engl J Med. 1998;339(7):417–23. [DOI] [PubMed] [Google Scholar]
- 9.Diaz-Garcia JD, Gallegos-Villalobos A, Gonzalez-Espinoza L, Sanchez-Nino MD, Villarrubia J, Ortiz A. Deferasirox nephrotoxicity-the knowns and unknowns. Nat Rev Nephrol. 2014;10(10):574–86. [DOI] [PubMed] [Google Scholar]
- 10.Hentze MW, Muckenthaler MU, Andrews NC. Balancing acts: molecular control of mammalian iron metabolism. Cell. 2004;117(3):285–97. [DOI] [PubMed] [Google Scholar]
- 11.Mackenzie B, Takanaga H, Hubert N, Rolfs A, Hediger MA. Functional properties of multiple isoforms of human divalent metal-ion transporter 1 (DMT1). Biochem J. 2007;403(1):59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garrick MD, Singleton ST, Vargas F, Kuo HC, Zhao L, Knopfel M, et al. DMT1: which metals does it transport? Biol Res. 2006;39(1):79–85. [DOI] [PubMed] [Google Scholar]
- 13.Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, et al. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388(6641):482–8. [DOI] [PubMed] [Google Scholar]
- 14.Fleming RE, Migas MC, Zhou X, Jiang J, Britton RS, Brunt EM, et al. Mechanism of increased iron absorption in murine model of hereditary hemochromatosis: increased duodenal expression of the iron transporter DMT1. Proc Natl Acad Sci U S A. 1999;96(6):3143–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffiths WJ, Sly WS, Cox TM. Intestinal iron uptake determined by divalent metal transporter is enhanced in HFE-deficient mice with hemochromatosis. Gastroenterology. 2001;120(6):1420–9. [DOI] [PubMed] [Google Scholar]
- 16.Zoller H, Koch RO, Theurl I, Obrist P, Pietrangelo A, Montosi G, et al. Expression of the duodenal iron transporters divalent-metal transporter 1 and ferroportin 1 in iron deficiency and iron overload. Gastroenterology. 2001;120(6):1412–9. [DOI] [PubMed] [Google Scholar]
- 17.Andrews NC. Disorders of iron metabolism. N Engl J Med. 1999;341(26):1986–95. [DOI] [PubMed] [Google Scholar]
- 18.Gunshin H, Fujiwara Y, Custodio AO, Direnzo C, Robine S, Andrews NC. Slc11a2 is required for intestinal iron absorption and erythropoiesis but dispensable in placenta and liver. J Clin Invest. 2005;115(5):1258–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wetli HA, Buckett PD, Wessling-Resnick M. Small-molecule screening identifies the selanazal drug ebselen as a potent inhibitor of DMT1-mediated iron uptake. Chem Biol. 2006;13(9):965–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie L, Zheng W, Xin N, Xie JW, Wang T, Wang ZY. Ebselen inhibits iron-induced tau phosphorylation by attenuating DMT1 up-regulation and cellular iron uptake. Neurochem Int. 2012;61(3):334–40. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Z, Kodumuru V, Sviridov S, Liu S, Chafeev M, Chowdhury S, et al. Discovery of benzylisothioureas as potent divalent metal transporter 1 (DMT1) inhibitors. Bioorg Med Chem Lett. 2012;22(15):5108–13. [DOI] [PubMed] [Google Scholar]
- 22.Cadieux JA, Zhang Z, Mattice M, Brownlie-Cutts A, Fu J, Ratkay LG, et al. Synthesis and biological evaluation of substituted pyrazoles as blockers of divalent metal transporter 1 (DMT1). Bioorg Med Chem Lett. 2012;22(1):90–5. [DOI] [PubMed] [Google Scholar]
- 23.Bacon BR, Adams PC, Kowdley KV, Powell LW, Tavill AS, American Association for the Study of Liver D. Diagnosis and management of hemochromatosis: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011;54(1):328–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwok WW, Schuening F, Stead RB, Miller AD. Retroviral transfer of genes into canine hemopoietic progenitor cells in culture: a model for human gene therapy. Proc Natl Acad Sci U S A. 1986;83(12):4552–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rayburn ER, Zhang R. Antisense, RNAi, and gene silencing strategies for therapy: mission possible or impossible? Drug Discov Today. 2008;13(11–12):513–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet. 2003;4(5):346–58. [DOI] [PubMed] [Google Scholar]
- 27.Guo J, Fisher KA, Darcy R, Cryan JF, O’Driscoll C. Therapeutic targeting in the silent era: advances in non-viral siRNA delivery. Mol Biosyst. 2010;6(7):1143–61. [DOI] [PubMed] [Google Scholar]
- 28.Liu F, Ranmal S, Batchelor HK, Orlu-Gul M, Ernest TB, Thomas IW, et al. Patient-centred pharmaceutical design to improve acceptability of medicines: similarities and differences in paediatric and geriatric populations. Drugs. 2014;74(16):1871–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu G, Franssen E, Fitch MI, Warner E. Patient preferences for oral versus intravenous palliative chemotherapy. J Clin Oncol. 1997;15(1):110–5. [DOI] [PubMed] [Google Scholar]
- 30.Forbes DC, Peppas NA. Oral delivery of small RNA and DNA. J Control Release. 2012;162(2):438–45. [DOI] [PubMed] [Google Scholar]
- 31.Lautenschlager C, Schmidt C, Fischer D, Stallmach A. Drug delivery strategies in the therapy of inflammatory bowel disease. Adv Drug Deliv Rev. 2014;71:58–76. [DOI] [PubMed] [Google Scholar]
- 32.Kriegel C, Attarwala H, Amiji M. Multi-compartmental oral delivery systems for nucleic acid therapy in the gastrointestinal tract. Adv Drug Deliv Rev. 2013;65(6):891–901. [DOI] [PubMed] [Google Scholar]
- 33.Attarwala H, Han M, Kim J, Amiji M. Oral nucleic acid therapy using multicompartmental delivery systems. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2018;10(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhavsar MD, Amiji MM. Gastrointestinal distribution and in vivo gene transfection studies with nanoparticles-in-microsphere oral system (NiMOS). J Control Release. 2007;119(3):339–48. [DOI] [PubMed] [Google Scholar]
- 35.Bhavsar MD, Tiwari SB, Amiji MM. Formulation optimization for the nanoparticles-in-microsphere hybrid oral delivery system using factorial design. J Control Release. 2006;110(2):422–30. [DOI] [PubMed] [Google Scholar]
- 36.Calija B, Cekic N, Savic S, Daniels R, Markovic B, Milic J. pH-sensitive microparticles for oral drug delivery based on alginate/oligochitosan/Eudragit((R)) L100–55 “sandwich” polyelectrolyte complex. Colloids Surf B Biointerfaces. 2013;110:395–402. [DOI] [PubMed] [Google Scholar]
- 37.Nollenberger K, Albers J. Poly(meth)acrylate-based coatings. Int J Pharm. 2013;457(2):461–9. [DOI] [PubMed] [Google Scholar]
- 38.Kriegel C, Amiji M. Oral TNF-alpha gene silencing using a polymeric microsphere-based delivery system for the treatment of inflammatory bowel disease. J Control Release. 2011;150(1):77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Attarwala H, Clausen V, Chaturvedi P, Amiji MM. Cosilencing Intestinal Transglutaminase-2 and Interleukin-15 Using Gelatin-Based Nanoparticles in an in Vitro Model of Celiac Disease. Mol Pharm. 2017;14(9):3036–44. [DOI] [PubMed] [Google Scholar]
- 40.Ganesh S, Iyer AK, Morrissey DV, Amiji MM. Hyaluronic acid based self-assembling nanosystems for CD44 target mediated siRNA delivery to solid tumors. Biomaterials. 2013;34(13):3489–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tracy MA. Development and scale-up of a microsphere protein delivery system. Biotechnol Prog. 1998;14(1):108–15. [DOI] [PubMed] [Google Scholar]
- 42.Kaul G, Amiji M. Cellular interactions and in vitro DNA transfection studies with poly(ethylene glycol)-modified gelatin nanoparticles. J Pharm Sci. 2005;94(1):184–98. [DOI] [PubMed] [Google Scholar]
- 43.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–8. [DOI] [PubMed] [Google Scholar]
- 44.Liu L, Zheng M, Librizzi D, Renette T, Merkel OM, Kissel T. Efficient and Tumor Targeted siRNA Delivery by Polyethylenimine-graft-polycaprolactone-block-poly(ethylene glycol)-folate (PEI-PCL-PEG-Fol). Mol Pharm. 2016;13(1):134–43. [DOI] [PubMed] [Google Scholar]
- 45.Frohlich E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int J Nanomedicine. 2012;7:5577–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov. 2010;9(8):615–27. [DOI] [PubMed] [Google Scholar]
- 47.Wang CQ, Wu JL, Zhuo RX, Cheng SX. Protamine sulfate-calcium carbonate-plasmid DNA ternary nanoparticles for efficient gene delivery. Mol Biosyst. 2014;10(3):672–8. [DOI] [PubMed] [Google Scholar]
- 48.Lopedota A, Trapani A, Cutrignelli A, Chiarantini L, Pantucci E, Curci R, et al. The use of Eudragit RS 100/cyclodextrin nanoparticles for the transmucosal administration of glutathione. Eur J Pharm Biopharm. 2009;72(3):509–20. [DOI] [PubMed] [Google Scholar]
- 49.Ubrich N, Schmidt C, Bodmeier R, Hoffman M, Maincent P. Oral evaluation in rabbits of cyclosporin-loaded Eudragit RS or RL nanoparticles. Int J Pharm. 2005;288(1):169–75. [DOI] [PubMed] [Google Scholar]
- 50.Kouadjo KE, Nishida Y, Cadrin-Girard JF, Yoshioka M, St-Amand J. Housekeeping and tissue-specific genes in mouse tissues. BMC Genomics. 2007;8:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thakral S, Thakral NK, Majumdar DK. Eudragit: a technology evaluation. Expert Opin Drug Deliv. 2013;10(1):131–49. [DOI] [PubMed] [Google Scholar]
- 52.Schwarz R, Kaspar A, Seelig J, Kunnecke B. Gastrointestinal transit times in mice and humans measured with 27Al and 19F nuclear magnetic resonance. Magn Reson Med. 2002;48(2):255–61. [DOI] [PubMed] [Google Scholar]
- 53.Padmanabhan P, Grosse J, Asad AB, Radda GK, Golay X. Gastrointestinal transit measurements in mice with 99mTc-DTPA-labeled activated charcoal using NanoSPECT-CT. EJNMMI Res. 2013;3(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heilig E, Molina R, Donaghey T, Brain JD, Wessling-Resnick M. Pharmacokinetics of pulmonary manganese absorption: evidence for increased susceptibility to manganese loading in iron-deficient rats. Am J Physiol Lung Cell Mol Physiol. 2005;288(5):L887–93. [DOI] [PubMed] [Google Scholar]
- 55.Wang X, Zhang M, Flores SRL, Woloshun RR, Yang C, Yin L, et al. Oral Gavage of Ginger Nanoparticle-Derived Lipid Vectors Carrying Dmt1 siRNA Blunts Iron Loading in Murine Hereditary Hemochromatosis. Mol Ther. 2019;27(3):493–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.