Abstract
Transgender women are impacted by elevated rates of HIV infection and drug use. This study investigated effects of drug use on HIV care outcomes among transgender women of color living with HIV who enrolled in a combined peer health navigation (PHN) and contingency management (CM) intervention (N = 129). At baseline, 71.3% reported any drug use in the past six months. Linkage to HIV care was delayed for users of any stimulant compared to non-users of stimulants, and for methamphetamine users compared to non-users of methamphetamine. Any drug use, relative to no drug use, was associated with fewer HIV care visits (IRR = 0.50, 95% CI [0.30, 0.85]), but did not significantly impact ART adherence, or attaining an undetectable viral load. PHN sessions were positively related to the number of HIV care visits (IRR = 1.20, 95% CI [1.07, 1.34]), especially for users of any stimulant and for methamphetamine users, to ART adherence (OR = 2.54, 95% CI [1.67, 3.86]), and to virological suppression (OR = 7.57, 95% CI [1.64, 34.94]). These findings demonstrate the value of assessing drug use as a possible barrier to HIV care.
Keywords: transgender, drug use, HIV Care Continuum, ART adherence
INTRODUCTION
Transgender women (hereafter: trans women) in the U.S. are impacted by multiple physical and mental health disparities. In particular, prior literature has demonstrated that trans women are at high risk for HIV infection and transmission (1,2). A meta-analysis of laboratory-confirmed HIV prevalence rates showed a pooled HIV prevalence of 21.7%, placing trans women at 34.2 times the risk of being HIV seropositive relative to non-trans adults (3). Several risk factors contribute to the disproportionate HIV disease burden among trans women, including being African-American/Black (2,4-6), experiencing homelessness or having unstable housing (7), engagement in sex work (8), and substance use (9-12).
Various studies have demonstrated that rates of drug use are high among trans women. A meta-analysis estimated that 20.2% of trans women used marijuana and 26.7% used crack or other illicit drugs within the past year (2). More recently, commensurate rates of recent marijuana (25.6%) and methamphetamine (21.5%) use were observed among high-risk trans women living in Los Angeles, California, nearly three quarters of whom (73.3%) also engaged in sex work (6). Empirical associations between trans women’s drug use and HIV infection and transmission are well-documented: Drug use increases the likelihood of unprotected sex with primary and casual partners (6,10,11) and mediates the effect of life stress on sexual risk behaviors (13).
By contrast, little is known about the effects of drug use on advancements along the HIV Care Continuum for trans women. Timely linkage to HIV clinical care after an initial diagnosis, early initiation of antiretroviral therapy (ART), retention in care, and ART adherence are essential for HIV treatment success (14). Evidence from the HIV literature at large shows that linkage to HIV care (15), initiation of and adherence to ART (16,17), and retention in care, especially among young adults, are suboptimal for persons with a history of drug use (14,18,19). These findings raise the question whether drug use has a similar, adverse impact on HIV care for trans women. The few studies to date that examined HIV treatment outcomes and related factors in trans women reported that decreased ART adherence was associated with perceived difficulties of integrating treatment into a daily routine (20,21). Other correlates of ART adherence by trans women include age, stress related to transphobic experiences, importance of gender affirmation, adherence to hormone therapy, and alcohol use (22). In addition, self-reported viral load (VL) was found to be related to stressfulness of transphobic experiences and being in a relationship (22). Qualitative research suggests that drug use interferes with trans women’s engagement in HIV care and medication adherence (23) but, thus far, no study has addressed the effects of drug use on HIV treatment outcomes for trans women.
This study fills a much needed gap in the literature as it is, to our knowledge, the first study to examine the associations between drug use and advancement along the HIV Care Continuum in a sample of trans women of color. Findings are presented from a demonstration study that monitored participants’ progression through the HIV Care Continuum for up to 36 months. The study was designed to optimize HIV health outcomes through a combined peer health navigation (PHN) and contingency management (CM) intervention. Detailed descriptions of both the PHN and CM components of the intervention are provided elsewhere (25). It was hypothesized that drug use at baseline would be negatively associated with indicators of HIV treatment progress, while the combined PHN/CM intervention would improve HIV treatment outcomes.
METHODS
Participants
From February 2014 through August 2016, 139 participants were enrolled. Inclusion criteria were: 1) identified as a trans woman; 2) assigned male sex at birth; 3) between the ages of 18 and 65 years; 4) racial/ethnic identity reported as not Caucasian/White; and, 5) HIV positive and currently not in HIV care or had not seen a HIV medical provider in the past six months or not prescribed ART medication or prescribed ART medication but not always adherent. Individuals were excluded if they did not meet all eligibility criteria. Results reported here were from a subsample of 129 participants who, at baseline, provided data on drug use in the past six months.
Procedure
Participants were recruited via 1) community-wide social network recruitment and engagement methodology (i.e., Respondent Driven Sampling); 2) venue- and street-based outreach; 3) project flyers; 4) in-reach at other programs conducted at the project site; 5) in-services conducted at local agencies; and, 6) collaborating HIV medical care clinics. Potential participants who were unable to provide documentation of their HIV-positive serostatus (e.g., medication prescription, laboratory results) were tested onsite for verification of a HIV-positive serostatus. Following consent, participants completed a baseline Computer Assisted Self Interview (CASI) assessment administered via REDCap (Research Electronic Data Capture) (24). Participants received a $10 gift card for eligibility screening and a $25 gift card at the completion of the baseline assessment. Immediately following baseline procedures, each participant met with a Peer Health Navigator and began the combined, 18-month, PHN/CM intervention. Follow-up assessments were conducted at 6-, 12-, 18-, 24-, 30- and 36-months post enrollment, or through the conclusion of the funding period. Thus, participants who enrolled toward the end of the enrollment period received fewer follow-up assessments; all participants were eligible for the 6- and 12-month follow-up assessment. The project was conducted at Friends Community Center in Hollywood, CA, the community research site of Friends Research Institute. The project was approved by the Institutional Review Board of Friends Research Institute, Inc.
Measures
Core Assessment
The CASI core assessment gathered information on sociodemographic characteristics, educational attainment, income, housing status, engagement in transactional sex, and incarceration.
Los Angeles Transgender Health Survey
Developed by the first author and colleagues in 1997, in consultation with trans women community members, and updated as community needs have changed, the Los Angeles Transgender Health Survey gathered information on drug use. Participants indicated for each of the following drugs whether they used the substance in the past six months by responding with “Yes,” “No,” or “Don’t know”: marijuana, methamphetamine, amphetamines (uppers, speed), barbiturates, ecstasy, tranquilizers (Valium, Xanax), hallucinogens, inhalants, powder cocaine, crack cocaine, heroin, ketamine, primos (marijuana mixed with cocaine).
Peer Health Navigation Sessions
The PHN sessions focused on the multiple and complex barriers that impede the participants’ ability to advance along the HIV Care Continuum. The PHN sessions included identifying the barrier(s) to HIV care, identifying and linking participants into other auxiliary needed services, and fostering self-efficacy and health literacy for working with HIV care providers. Peer Health Navigators did not provide counseling or psychotherapy; rather, they worked with participants to develop a specific client-centered treatment plan and successfully navigate their HIV care. If needed, the Peer Health Navigators provided transportation to HIV care appointments. Participants could attend an unlimited number of PHN sessions. All Peer Health Navigators were trans women of color living with HIV. A detailed description of the PHN component of the intervention is provided elsewhere (25).
Contingency Management and the HIV Care Continuum.
Contingency management, based on behavioral economics, applies contingent rewards to motivate behavioral change. For this project, CM was adapted to optimize outcomes along the HIV Care Continuum. The HIV Care Continuum was operationalized as: 1) Linkage to HIV care. The time in days between the date of enrollment in the study and the date of the initial HIV medical care appointment. 2) Retention in HIV medical visits. The number of HIV care visits attended in the course of the study. Optimum HIV care visits was defined as quarterly, per Centers for Disease Control and Prevention (CDC) guidelines (26). 3) VL at each HIV care visit and undetectable VL. ART adherence was operationalized as an ordinal measure based on decreases in plasma VL. VL was measured as four ordered categories: no VL reduction, 1-log10 VL reduction, 2-log10 VL reduction, VL undetectable (i.e., below the limits of assay detection, ≤200 copies/mL), and once undetectable, a patient should remain undetectable. Both ART adherence and undetectable VL were based on confirmed laboratory results and not self-reported data on ART adherence or VL. Participants received escalating CM points for attending quarterly HIV care visits or achieving a VL reduction. A detailed description of the CM component of the intervention is provided elsewhere (25). Of note, since participants did not receive CM points for attending PHN sessions, PHN and CM were two independent components of the combined PHN/CM intervention.
Data Analysis
Descriptive statistics were compiled separately for participants who self-reported at baseline that they did not use any drugs during the past six months and for participants who self-reported the use of at least one drug in the past six months. The independent-samples t-test was used to compare differences between group means, chi-square tests were used to evaluate associations between categorical variables, and the Wilcoxon rank-sum test was used to assess stochastic dominance between groups.
The association between drug use and time to linkage to HIV care was assessed with Cox proportional hazards regression analysis with robust coefficient-variance estimates. Multivariable regression analyses regressed the outcome variables “number of HIV care visits,” “ART adherence,” and “undetectable VL” on drug use status and the binary (i.e., base 2) logarithm of the number of PHN sessions. The type of regression analysis was determined by the scale of the regressand: For number of HIV care visits, Poisson regression analysis was carried out with an offset to control for individual differences in study exposure. The offset was 1 for participants who were enrolled at least 18 months (= 547.875 days) before the end of the study. For each remaining participant, the offset was calculated as the difference in days between the date of enrollment and the date of the last assessment of the participant, divided by 547.875 days. Robust standard errors (White’s estimator) were calculated for the parameter estimates to guard against mild violation of the Poisson distribution assumption that the mean and the variance are equal. Ordinal logistic regression was used for the outcome variable “ART adherence.” A review of the fit of the full model with two main effects and an interaction effect revealed that the parameter for the interaction effect could not be estimated reliably without violating the proportional-odds assumption. The interaction term was therefore dropped from the model. Intercepts (cutpoints) are not reported because they were not used in the interpretation of the results. Finally, the dichotomous outcome variable “undetectable VL” was submitted to logistic regression analysis. For each outcome variable, separate regression analyses were carried out for three types of drug use over the past six months at baseline: 1) use of any of the assessed drugs vs. no drug use; 2) use of any stimulant (methamphetamine, amphetamines, ecstasy, powder cocaine, crack cocaine, and/or primos) vs. no use of stimulants (irrespective of the use of drugs other than stimulants); and, 3) methamphetamine use vs. no use of methamphetamine (irrespective of the use of drugs other than methamphetamine). The significance level for all statistical tests was set to α = .05. All analyses were carried out using the R language and environment for statistical computing, version 3.4.3.
RESULTS
Sociodemographics
Table I compares subgroups of participants who did (n = 92, 71.3%) and did not (n = 37, 28.7%) use any drugs (including marijuana) at baseline on sociodemographic and other variables. Recent drug use was associated with greater proportions of non-Hispanic (64.1% vs. 48.6%; χ2(1) = 4.1, p = .044) and recently homeless (60.9% vs. 37.8%; χ 2(1) = 4.7, p = .03) participants. Trans women who used drugs tended to be younger, more educated, and more likely to engage in sex work, but these effects were only marginally significant (.05 < ps ≤ .1).
Table I.
Baseline Sociodemographic Characteristics by Drug Use Status (N = 129)
| Any drug use in the past 6 months | |||
|---|---|---|---|
| Characteristic | No n = 37 |
Yes n = 92 |
Significance |
| Age (years), M (SD) | 38.7 (10.5) | 35.4 (9.4) | t(60) = 1.67✝ |
| Ethnic identity, n (%) | |||
| Non-Hispanic | 18 (48.6%) | 59 (64.1%) | χ2(1) = 4.1* |
| Hispanic/Latina | 19 (51.4%) | 28 (30.4%) | |
| Education, n (%) | |||
| < High School | 19 (51.4%) | 32 (34.8%) | χ2(1) = 2.7✝ |
| ≥ High School | 18 (48.6%) | 58 (63.0%) | |
| Income (annual), n (%) | |||
| < $600 | 19 (51.4%) | 41 (44.6%) | χ2(1) = 1.0 |
| ≥ $600 | 10 (27.0%) | 34 (37.0%) | |
| Homelessnessa, n (%) | |||
| No | 18 (48.6%) | 29 (31.5%) | χ2(1) = 4.7* |
| Yes | 14 (37.8%) | 56 (60.9%) | |
| Sex worka, n (%) | |||
| No | 20 (54.1%) | 42 (45.7%) | χ2(1) = 3.1✝ |
| Yes | 9 (24.3%) | 42 (45.7%) | |
| Incarcerationa, n (%) | |||
| No | 26 (70.3%) | 67 (72.8%) | χ2(1) = 0.0 |
| Yes | 5 (13.5%) | 14 (15.2%) | |
| Linkage to HIV care (days), Med (range; IQR) | 285 (2-338; 12-52)b |
29.5 (2-464; 10.75-95.25)c |
W = 930 |
Note. A sample size of less than 37 for non-drug users and 92 for drug users for any sociodemographic characteristic was due to missing data on the respective variable.
In the past 6 months.
n = 29.
n = 68.
p ≤ .1.
p ≤ .05.
HIV Treatment Progress
Linkage to HIV Care
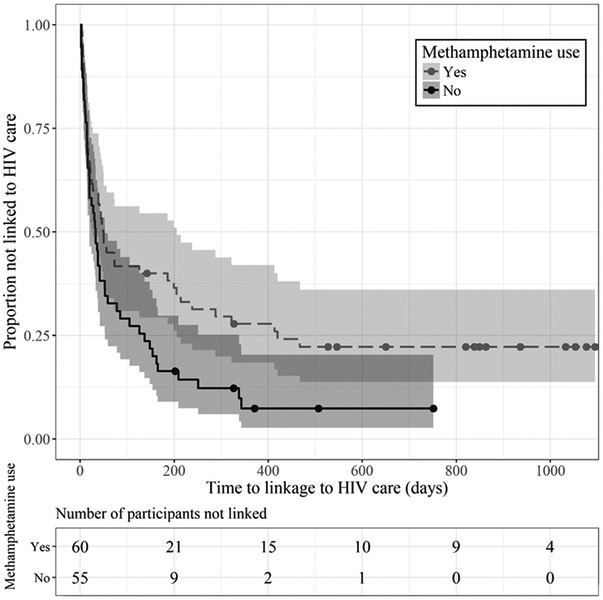
Cox proportional hazards regression analyses were conducted separately for three types of drug use at baseline (Table II, Models I-III). Any stimulant use (Model II) significantly reduced the probability of linkage to HIV care by 33% (HR = 0.67, 95% CI [0.44, 1.00]). Similarly, methamphetamine use (Model III) conferred a significant 37% decrease in the probability of linkage to HIV care (HR = 0.63, 95% CI [0.42, 0.94]). The Kaplan-Meier survival curves shown in Figure I graph the distribution of time to linkage for participants who did and did not use methamphetamine.
Table II.
Effects of Different Types of Drug Use at Baseline on Time to Linkage to HIV Care
| Model parameter | n | HR [95% CI] |
|---|---|---|
| Model I | ||
| Any drug | ||
| No | 33 | Ref. Cat. |
| Yes | 83 | 0.77 [0.50, 1.18] |
| Model II | ||
| Any stimulant | ||
| No | 47 | Ref. Cat. |
| Yes | 69 | 0.67 [0.44, 1.00]* |
| Model III | ||
| Methamphetaminea | ||
| No | 55 | Ref. Cat. |
| Yes | 60 | 0.63 [0.42, 0.94]* |
Note. Data from participants who were in HIV care prior to enrollment in the study was excluded from the survival analyses (n = 13).
Data from one participant who did not provide data on methamphetamine use was missing.
p ≤ .05.
Figure I:
Kaplan-Meier estimates of the probability of linkage to HIV care by methamphetamine use status. Shaded areas represent 95% CIs for pointwise probability estimates. Quantitatively similar results were obtained for any stimulant use.
Number of HIV Care Visits
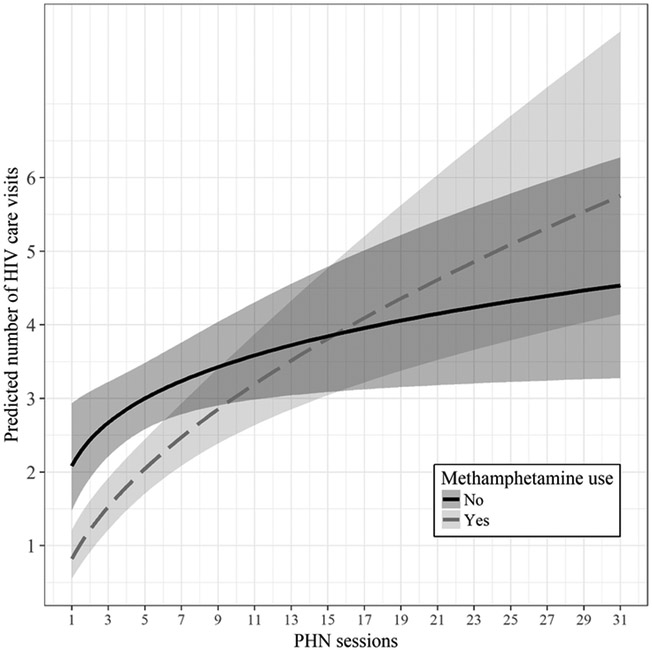
Table III summarizes the results of multivariable Poisson regression analyses for each type of drug use (Models IV-VI). Model IV estimated that participants who reported using any drugs attended half as many HIV care visits as participants who did not use drugs (IRR = 0.50, 95% CI [0.30, 0.85]). The PHN/CM intervention yielded a significant effect: a one-unit increase in the binary logarithm of the number of PHN sessions—corresponding to a two-fold increase in the number of PHN sessions—was associated with a 20% increase in medical visits (IRR = 1.20, 95% CI [1.07, 1.34]). Model V revealed that users of any stimulant (n = 75, 58.1%) had significantly fewer HIV care visits (IRR = 0.40, 95% CI [0.24, 0.67] than participants who did not use stimulants (n = 54, 41.9%). The effect of the PHN/CM intervention was also significant: attending PHN sessions increased the number of HIV care visits (IRR = 1.19, 95% CI [1.07, 1.32]). A significant interaction between any stimulant use and PHN sessions (IRR = 1.22, 95% CI [1.05, 1.42]) indicated that stimulant users benefited more from PHN sessions than non-stimulant users. According to Model VI, methamphetamine users (n = 66, 51.2%) made significantly fewer medical visits (IRR = 0.39, 95% CI [0.23, 0.67]) than non-methamphetamine users (n = 62, 48.1%), and the frequency of attending PHN sessions was positively associated with HIV care visits (IRR = 1.17, 95% CI [1.05, 1.30]. The interaction between methamphetamine use and PHN sessions was also significant (IRR = 1.27, 95% CI [1.08, 1.48]. Figure II displays the predicted number of HIV care visits for methamphetamine users and non-methamphetamine users as a function of number of PHN sessions attended. In sum, drug use of any type was associated with approximately 50% fewer HIV care visits, while attending PHN sessions was related to an increase in HIV care visits, especially for participants who reported using any stimulant or methamphetamine at baseline.
Table III.
Effects of Different Types of Drug Use at Baseline and PHN Sessions on Number of HIV Care Visits, ART Adherence, and Undetectable VL
| Model parameters | No. of HIV care visits Coef. [95% CI] |
ART adherence Coef. [95% CI] |
Undetectable VL Coef. [95% CI] |
|---|---|---|---|
| Any drug | |||
|
Model IV n = 128b |
Model VII n = 93b,d |
Model X n = 93b,d |
|
| Any drug | −0.69 [−1.21, −0.16]* | −1.00 [−2.11, 0.11]✝ | 2.69 [−1.88, 7.26] |
| PHN sessionsa | 0.18 [0.07, 0.29]** | 0.93 [0.51, 1.35]*** | 2.02 [0.49, 3.55]** |
| Any drug × PHN sess.a | 0.15 [0.00, 0.31]✝ | _e | −1.38 [−2.99, 0.23]✝ |
| Intercept | −0.28 [−0.65, 0.10] | _f | −5.61 [−9.92, −1.30]* |
| Any Stimulant | |||
|
Model V n = 128b |
Model VIII n = 93b,d |
Model XI n = 93b,d |
|
| Any stimulant | −0.92 [−1.42, −0.41]*** | −0.52 [−1.52, 0.48] | 0.88 [−2.21, 3.97] |
| PHN sessionsa | 0.17 [0.07, 0.27]** | 0.87 [0.46, 1.28]*** | 1.17 [0.32, 2.01]** |
| Any stim. × PHN sess.a | 0.20 [0.05, 0.35]* | -e | −0.46 [−1.47, 0.54] |
| Intercept | −0.24 [−0.58, 0.11] | _f | −3.90 [−6.54, −1.27]** |
| Methamphetamine | |||
|
Model VI n = 127 b,c |
Model IX n = 92b,c,d |
Model XII n = 92b,c,d |
|
| Methamphetamine | −0.93 [−1.46, −0.40]*** | −0.19 [−1.18, 0.79] | −0.07 [−2.97, 2.83] |
| PHN sessionsa | 0.16 [0.05, 0.26]** | 0.88 [0.47, 1.29]*** | 0.91 [0.19, 1.62]* |
| Meth. × PHN sess.a | 0.24 [0.08, 0.39]** | _e | −0.05 [−0.98, 0.89] |
| Intercept | −0.27 [−0.60, 0.07] | _f | −3.37 [−5.60, −1.14]** |
Note. PHN = peer health navigation. VL = viral load.
base-2 log transformed.
PHN session data was missing for one participant.
One participant did not provide data on methamphetamine use.
Data of participants were excluded who were undetectable at the time of enrollment (n = 35) or whose lab work was missing (n = 4).
The interaction term was dropped due to model estimation problems.
Intercepts (cutpoints) are not reported.
p ≤ .1.
p ≤ .05.
p ≤ .01.
p ≤ .001.
Figure II:
Predicted number of HIV care visits by methamphetamine use status. Initial group differences in the number of predicted HIV care visits are reduced as the number of PHN sessions increases. Shaded areas represent 95% CIs. Quantitatively similar results were obtained for any stimulant use.
ART Adherence
Effects of drug use on ART adherence, as operationalized by VL reduction, were examined in multivariable ordered logistic regression analyses (Table III, Models VII-IX). Model VII, comparing users of any drugs with non-users, yielded a marginally significant effect of drug use. By contrast, the PHN/CM intervention was significantly related to ART adherence: When the number of PHN sessions was doubled, the odds of achieving a higher level of VL reduction were 2.5 times higher (OR = 2.54, 95% CI [1.67, 3.86]). Similarly, comparing any stimulant users with non-stimulant users (Model VIII), a doubling of the number of PHN sessions was associated with a 2.4-fold increase in the likelihood of VL reduction (OR = 2.39, 95% CI [1.58, 3.60]). An effect of similar magnitude for the PHN/CM intervention was also obtained in the analysis that distinguished between methamphetamine users and non-methamphetamine users (Model IX; OR = 2.41, 95% CI [1.60, 3.63]). In sum, the regression analyses did not yield significant effects of drug use of any type, but attending PHN sessions was associated with increased odds of ART adherence.
Undetectable VL
The likelihood of achieving a reduction in VL to undetectable levels was examined in multivariable logistic regression analyses (Table III, Models X-XII). The results were commensurate with those reported for ART adherence. Drug use of any type was not significantly related to the likelihood of having an undetectable VL. By contrast, significant main effects were obtained for the PHN/CM intervention: Doubling the number of PHN sessions was associated with a 2.5 to 7.6-fold increase in the likelihood of having an undetectable VL, depending on the particular type of drug use (Model X: OR = 7.57, 95% CI [1.64, 34.94]; Model XI: OR = 3.22, CI [1.38. 7.49]; Model XII: OR = 2.47, CI [1.22, 5.03]).
DISCUSSION
At baseline, 71.3% of the study participants reported the use of at least one drug in the past six months. Drug use of any type was associated with fewer HIV care visits. Use of any stimulant, and methamphetamine use in particular, delayed linkage to HIV care. By contrast, drug use of any type did not impair ART adherence or decrease the likelihood of achieving VL reduction to undetectable levels. Both drug users and non-drug users benefited from the combined PHN/CM intervention. The frequency of PHN sessions was positively related to the number of HIV care visits, to ART adherence, and to the likelihood of achieving an undetectable VL. Of note, as the number of PHN sessions increased, any stimulant users and methamphetamine users increased HIV care visits at a greater rate than participants who did not use stimulants, including methamphetamine. For these participants, the PHN/CM intervention was thus instrumental in offsetting the HIV treatment disparity, i.e., fewer HIV care visits, that was observed for all types of drug use. Specifically, although drug users, in general, attended fewer HIV care visits than non-drug users, interaction effects included in the regression models predicted that any stimulant users and methamphetamine users would achieve parity with their non-drug using counterparts after attending approximately 15 PHN sessions (see Figure II).
The finding that drug use by trans women of color living with HIV was associated with fewer HIV care visits is consistent with research that demonstrated poorer treatment retention among MSM and male and cisgender female HIV patients who use drugs (14,18). Lifestyle instabilities and the exigencies of drug use can cause drug users to miss scheduled medical appointments. Additionally, trans women have reported reluctance to seek medical care due to stigma and previous negative experiences with health care providers related to their gender identity or expression (21,27). This reluctance can be exacerbated by mutual mistrust and suspicion that frequently characterizes the relationship between drug users and health care providers (28). Regular HIV care visits, however, are an important component of successful HIV treatment as patients who miss HIV care visits generally exhibit a greater risk of mortality (29,30).
The lack of effects of any type of drug use on ART adherence and the likelihood of attaining an undetectable VL was surprising given numerous previous studies that have documented such effects among cisgender populations (31-33). There is, however, considerable heterogeneity of results both between and within studies, depending on factors such as the type of drug used, the timeframe of the drug use (e.g., past versus current), route of administration, timeframe of adherence monitoring, and adherence metric (e.g., self-report, electronic monitoring, plasma VL). To illustrate, Mathews and colleagues found lifetime amphetamine use, but not current use (i.e., in the past 30 days), to be predictive of medication adherence if it was measured with an electronic Medication Event Monitoring System (MEMS) that tracked the times and dates of pill-bottle openings, but not if it was self-reported (34). For cocaine users in the Mathews et al. study, however, the pattern was reversed: Current, but not lifetime use, was associated with self-reported adherence, but not with pill-bottle openings. Arnsten and colleagues, by contrast, identified current cocaine use as the strongest predictor of pill bottle openings (35). In a study by Cofrancesco and colleagues, current and past users of crack cocaine or powder cocaine were less likely to be virologically suppressed, whereas past amphetamine use actually increased the likelihood of viral suppression (31). These findings are difficult to reconcile and indicate that more research is needed to understand how drug use affects ART medication adherence and virological response in HIV patients in general, and among trans women, in particular.
The promising findings from the combined PHN/CM intervention observed in this study were consistent with the effects of PHN programs on HIV health outcomes reported in the literature for cisgender populations. For example, two recent observational studies that enrolled persons who had fallen out of HIV care into PHN interventions found significant increases in the proportion of participants with an undetectable VL (36,37). Of particular interest is a study with substance-using, hospitalized participants living with HIV who received either standard care or patient navigation (PN) with or without CM that targeted HIV care engagement and substance use (38). No differences in VL suppression were observed between the treatment groups at the primary 12-month endpoint (i.e., six months after intervention completion), but at six months, the rate of VL suppression was significantly higher in the PN-plus-CM condition compared to standard care. Moreover, follow-up analyses revealed that the more PN sessions participants attended (with or without CM), the more likely they were to attain viral suppression at 12 months, and that CM increased the number of PN sessions attended (39). The present study not only demonstrated that a combined PHN/CM intervention was associated with improvements in HIV care outcomes in a sample of trans women of color, most of whom used drugs, but that the intervention helped reduce a HIV treatment disparity (i.e., fewer HIV care visits) associated with drug use.
Limitations
These findings were derived from a sample of trans women of color living with HIV in a major west coast urban center in the U.S. and may not be generalizable to other populations with different characteristics or living in another geographic location. The lack of significant effects of drug use on ART adherence and the likelihood of achieving an undetectable VL may be attributable to the reduction in sample size and concomitant loss of statistical power that resulted from excluding data from participants who enrolled in the study with an undetectable VL (n = 35).
Drug use in the past six months was assessed in a binary manner. Participants were classified, depending on the analysis, as users or non-users of any drug, users of any stimulant or non-users of stimulants (irrespective of the use of drugs other than stimulants) and methamphetamine users or non-users of methamphetamine (irrespective of the use of drugs other than methamphetamine). The frequency, severity, or duration of use were not taken into account, and no diagnostic criteria for drug use disorders were available. Aggregating over different types of drugs and/or drug use patterns likely attenuated any differences in HIV treatment outcomes between users and non-users. The findings reported here, therefore, provide a conservative estimate of the negative impact of drug use on HIV care outcomes among this sample of trans women of color. It is possible, for example, that future research that disambiguates outcomes by drug use disorders (none, mild, moderate, severe) may demonstrate negative effects of drug use on ART adherence and VL status that were absent in this study among those with severe drug use disorder(s).
Operationalizing ART adherence as change from baseline plasma VL provided a reliable measure of adherence but it must be noted that changes in a drug regimen can take weeks or months to affect virologic response (34). Thus, the measure of ART adherence as plasma VL may not have been able to detect more short-lived variations in medication taking that were potentially associated with drug use status. In addition, drug use was assessed at baseline and related to HIV treatment progress during the study period. Some studies suggest that current drug use is a stronger predictor of ART non-adherence and inferior virological response to ART than past drug use (32), as missing scheduled ART doses may be a result of acute intoxication, rather than an expression of a stable characteristic related to drug use (16). Other consequences of current drug use such as withdrawal or the exacerbation of psychiatric symptoms may further compromise ART adherence.
CONCLUSION
Trans women and drug users are among the most socially vulnerable populations that experience multiple barriers to health care. These findings demonstrated that drug use further delayed linkage to HIV care and reduced retention in HIV care, and strongly emphasize the need for an integrated treatment approach. HIV patients should be assessed for drug use at baseline so that the unique health care needs of these underserved populations can be better served.
ACKNOWLEDGEMENTS
This project was supported by the Health Resources and Services Administration (HRSA) of the U.S. Department of Health and Human Services (HHS) under grant number H97HA24968 in the last annual award amount of $285,757 awarded to Friends Research Institute (PI: C. Reback). No percentage of this project was financed with non-governmental sources. This information or content and conclusions are those of the authors and should not be construed as the official position or policy of, nor should any endorsements be inferred by HRSA, HHS or the U.S. Government. Dr. Reback acknowledges additional support from the National Institute of Mental Health (P30 MH58107).
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent: Informed consent was obtained from all individual participants included in the study.
REFERENCES
- 1).Clements-Nolle K, Marx R, Guzman R, Katz M. HIV prevalence, risk behaviors, health care use, and mental health status of transgender persons: implications for public health intervention. Am J Public Health. 2001;91(6):915–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Herbst JH, Jacobs ED, Finlayson TJ, et al. Estimating HIV prevalence and risk behaviors of transgender persons in the United States: a systematic review. AIDS Behav. 2008;12(1):1–17. [DOI] [PubMed] [Google Scholar]
- 3).Baral SD, Poteat T, Strömdahl S, Wirtz AL, Guadamuz TE, Beyrer C. Worldwide burden of HIV in transgender women: a systematic review and meta-analysis. Lancet Infect Dis. 2013;13(3):214–22. [DOI] [PubMed] [Google Scholar]
- 4).James SE, Herman JL, Rankin S, Keisling M, Mottet L, Anafi M. The Report of the 2015 U.S. Transgender Survey. Washington, DC: National Center for Transgender Equality; 2016. 297 p. [Google Scholar]
- 5).Nuttbrock L, Hwahng S, Bockting W, et al. Lifetime risk factors for HIV/sexually transmitted infections among male-to-female transgender persons. J Acquir Immune Defic Syndr. 2009;52(3):417–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Reback CJ, Fletcher JB. HIV prevalence, substance use, and sexual risk behaviors among transgender women recruited through outreach. AIDS Behav. 2014;18(7):1359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Fletcher JB, Kisler KA, Reback CJ. Housing status and HIV risk behaviors among transgender women in Los Angeles. Arch Sex Behav. 2014;43(8):1651–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Operario D, Soma T, Underhill K. Sex work and HIV status among transgender women. J Acquir Immune Defic Syndr. 2008;48(1):97–103. [DOI] [PubMed] [Google Scholar]
- 9).Hoffman BR. The interaction of drug use, sex work, and HIV among transgender women. Subst Use Misuse. 2014;49(8):1049–53. [DOI] [PubMed] [Google Scholar]
- 10).Nemoto T, Operario D, Keatley J, Han L, Soma T. HIV risk behaviors among male-to-female transgender persons of color in San Francisco. Am J Public Health. 2004;94(7):1193–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Operario D, Nemoto T, Iwamoto M, Moore T. Unprotected sexual behavior and HIV risk in the context of primary partnerships for transgender women. AIDS Behav. 2011;15(3):674–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Santos G-M, Rapues J, Wilson EC, et al. Alcohol and substance use among transgender women in San Francisco: prevalence and association with human immunodeficiency virus infection. Drug Alcohol Rev. 2014;33(3):287–95. [DOI] [PubMed] [Google Scholar]
- 13).Hotton AL, Garofalo R, Kuhns LM, Johnson AK. Substance use as a mediator of the relationship between life stress and sexual risk among young transgender women. AIDS Educ Prev. 2013;25(1):62–71. [DOI] [PubMed] [Google Scholar]
- 14).Ulett KB, Willig JH, Lin H-Y, et al. The therapeutic implications of timely linkage and early retention in HIV care. AIDS Patient Care STDS. 2009;23(1):41–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Torian LV, Wiewel EW, Liu K-L, Sackoff JE, Frieden TR. Risk factors for delayed initiation of medical care after diagnosis of human immunodeficiency virus. JAMA Intern Med. 2008;168(11):1181–7. [DOI] [PubMed] [Google Scholar]
- 16).Hinkin CH, Barclay TR, Castellon SA, et al. Drug use and medication adherence among HIV-1 infected individuals. AIDS Behav. 2006;11(2):185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Tegger MK, Crane HM, Tapia KA, Uldall KK, Holte SE, Kitahata MM. The effect of mental illness, substance use, and treatment for depression on the initiation of highly active antiretroviral therapy among HIV-infected individuals. AIDS Patient Care STDS. 2008;22(3):233–43. [DOI] [PubMed] [Google Scholar]
- 18).Hartzler B, Dombrowski JC, Williams JR, et al. Influence of substance use disorders on 2-year HIV care retention in the United States. AIDS Behav. 2017;21(4):1138–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Rebeiro P, Althoff KN, Buchacz K, et al. Retention among North American HIV-infected persons in clinical care, 2000–2008. J Acquir Immune Defic Syndr. 2013;62(3):356–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Melendez RM, Exner TA, Ehrhardt AA, et al. Health and health care among male-to-female transgender persons who are HIV positive. Am J Public Health. 2006;96(6):1034–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Sevelius JM, Carrico A, Johnson MO. Antiretroviral therapy adherence among transgender women living with HIV. J Assoc Nurses AIDS Care. 2010;21(3):256–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Sevelius JM, Saberi P, Johnson MO. Correlates of antiretroviral adherence and viral load among transgender women living with HIV. AIDS Care. 2014;26(8):976–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Sevelius JM, Patouhas E, Keatley JG, Johnson MO. Barriers and facilitators to engagement and retention in care among transgender women living with human immunodeficiency virus. Ann Behav Med. 2013;47(1):5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Reback CJ, Kisler KA, Fletcher JB. A novel adaptation of peer health navigation and contingency management for advancement along the HIV care continuum among transgender women of color. AIDS Behav Under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Workowski KA, Bolan GA, Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1–137. [PMC free article] [PubMed] [Google Scholar]
- 27).Sanchez NF, Sanchez JP, Danoff A. Health care utilization, barriers to care, and hormone usage among male-to-female transgender persons in New York City. Am J Public Health. 2009;99(4):713–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Bruce RD, Altice FL. Clinical care of the HIV-infected drug user. Infect Dis Clin North Am. 2007;21(1):149–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Giordano TP, Gifford AL, White AC, et al. Retention in care: a challenge to survival with HIV infection. Clin Infect Dis. 2007;44(11):1493–9. [DOI] [PubMed] [Google Scholar]
- 30).Mugavero MJ, Lin H, Willig JH, et al. Missed visits and mortality among patients establishing initial outpatient HIV treatment. Clin Infect Dis. 2009;48(2):248–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31).Cofrancesco J, Scherzer R, Tien PC, et al. Illicit drug use and HIV treatment outcomes in a US cohort. AIDS. 2008;22(3):357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32).Lucas GM, Cheever LW, Chaisson RE, Moore RD. Detrimental effects of continued illicit drug use on the treatment of HIV-1 infection. J Acquir Immune Defic Syndr. 2001;27(3):251–9. [DOI] [PubMed] [Google Scholar]
- 33).Tucker JS, Burnam MA, Sherbourne CD, Kung F-Y, Gifford AL. Substance use and mental health correlates of nonadherence to antiretroviral medications in a sample of patients with human immunodeficiency virus infection. Am J Med. 2003;114(7):573–80. [DOI] [PubMed] [Google Scholar]
- 34).Mathews WC, Mar-Tang M, Ballard C, et al. Prevalence, predictors, and outcomes of early adherence after starting or changing antiretroviral therapy. AIDS Patient Care STDS. 2002;16(4):157–72. [DOI] [PubMed] [Google Scholar]
- 35).Arnsten JH, Demas PA, Grant RW, et al. Impact of active drug use on antiretroviral therapy Adherence and viral suppression in HIV-infected drug users. J Gen Intern Med. 2002;17(5):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).Wohl AR, Dierst-Davies R, Victoroff A, et al. The Navigation Program: an intervention to reengage lost patients at 7 HIV clinics in Los Angeles County, 2012–2014. J Acquir Immune Defic Syndr. 2016;71(2):e44–e50. [DOI] [PubMed] [Google Scholar]
- 37).Shacham E, López JD, Brown TM, Tippit K, Ritz A. Enhancing adherence to care in the HIV care continuum: the Barrier Elimination and Care Navigation (BEACON) project evaluation. AIDS Behav. 2018;22(1):258–64. [DOI] [PubMed] [Google Scholar]
- 38).Metsch LR, Feaster DJ, Gooden L, et al. Effect of patient navigation with or without financial incentives on viral suppression among hospitalized patients with HIV infection and substance use. JAMA. 2016;316(2):156–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Stitzer M, Matheson T, Cunningham C, et al. Enhancing patient navigation to improve intervention session attendance and viral load suppression of persons with HIV and substance use: a secondary post hoc analysis of the Project HOPE study. Addict Sci Clin Pract. 2017;12:16. [DOI] [PMC free article] [PubMed] [Google Scholar]