Abstract
Objectives:
The aims of this study were to: (1) measure the rate of failure to provide defect-free postoperative venous thromboembolism (VTE) chemo-prophylaxis, (2) identify reasons for failure to provide defect-free VTE chemoprophylaxis, and (3) examine patient- and hospital-level factors associated with failure.
Summary Background Data:
Current VTE quality measures are inadequate. VTE outcome measures are invalidated for interhospital comparison by surveillance bias. VTE process measures (e.g., SCIP-VTE-2) do not comprehensively capture failures throughout patients’ entire hospitalization.
Methods:
We examined adherence to a novel VTE chemoprophylaxis process measure in patients who underwent colectomies over 18 months at 36 hospitals in a statewide surgical collaborative. This measure assessed comprehensive VTE chemoprophylaxis during each patient’s entire hospitalization, including reasons why chemoprophylaxis was not given. Associations of patient and hospital characteristics with measure failure were examined. Results: The SCIP-VTE-2 hospital-level quality measure identified failures of VTE chemoprophylaxis in 0% to 3% of patients. Conversely, the novel measure unmasked failure to provide defect-free chemoprophylaxis in 18% (736/4086) of colectomies. Reasons for failure included medication not ordered (30.4%), patient refusal (30.3%), incorrect dosage/frequency (8.2%), and patient off-unit (3.4%). Patients were less likely to fail the chemoprophylaxis process measure if treated at nonsafety net hospitals (OR 0.62, 95% CI 0.39–0.99, P = 0.045) or Magnet designated hospitals (OR 0.45, 95% CI 0.29–0.71, P = 0.001).
Conclusions:
In contrast to SCIP-VTE-2, our novel quality measure unmasked VTE chemoprophylaxis failures in 18% of colectomies. Most failures were due to patient refusals or ordering errors. Hospitals should focus improvement efforts on ensuring patients receive VTE prophylaxis throughout their entire hospitalization.
Keywords: chemoprophylaxis, deep vein thrombosis, Magnet, pulmonary embolism, quality, safety net, SCIP-VTE-2, venous thromboembolism
Venous thromboembolism (VTE), which includes pulmonary embolism (PE) and deep vein thrombosis (DVT), is a leading cause of potentially preventable hospital morbidity and mortality.1 Chemoprophylaxis has been shown to reduce the risk of symptomatic VTE by 70% to 80%, suggesting that many incidences of VTE are preventable.2,3 Accordingly, the Agency for Healthcare Research and Quality (AHRQ) and the Centers for Medicare and Medicaid Services (CMS) have prioritized VTE prevention as a key patient safety goal, and several quality measures emphasizing perioperative VTE prevention have been developed and utilized for public reporting and to drive quality improvement (QI) efforts.4 Unfortunately, these VTE quality indicators have significant flaws that limit their utility in these domains. The VTE outcome measures that include the actual presence of VTE (PSI-12, VTE-6) are susceptible to VTE surveillance bias based on local diagnostic practices and thus, while potentially serving as internal benchmarks of performance, they are not valid for publicly-reported performance comparisons between institutions.5–8 The VTE process measure (SCIP-VTE-2), while not susceptible to surveillance bias, only measures care provided in the 24hours around surgery and does not readily identify reasons why chemoprophylaxis failures occur.7,9,10 Most hospitals have maximized their performance on this recently retired publicly-reported process measure.11
The American College of Chest Physicians recommends VTE chemoprophylaxis throughout a patient’s entire inpatient hospitalization for surgical patients at moderate or high risk for VTE.1 Despite high rates of SCIP-VTE-2 adherence at most hospitals, single-institution studies have reported that up to one-third of patients undergoing inpatient surgery do not receive appropriate VTE chemoprophylaxis throughout their entire inpatient hospital stay.9,12–15 In trauma and surgical patient populations, missed doses of chemoprophylaxis are associated with increased incidence of VTE.12,15,16 Though some smaller single institution studies have identified reasons patients do not receive chemoprophylaxis,12–17 only 1 study9 accounted for evidence-based clinical exceptions to chemoprophylaxis administration (e.g., high-risk for postoperative bleeding).
The flaws in the current VTE quality measures highlight the need for clinically relevant quality measures that apply throughout a patient’s hospitalization with defined exclusion criteria and provide actionable data (e.g., specific reasons for missed doses) to meaningfully inform effective QI interventions focused on VTE chemoprophylaxis. We recently introduced a novel VTE quality measure across a statewide surgical collaborative. This process measure assesses comprehensive postoperative VTE chemoprophylaxis during each patient’s entire inpatient hospitalization, including the appropriateness of dose and frequency, and requires specific documentation of reasons why individual doses of chemoprophylaxis were not given to patients.9 The objectives of this statewide, multi-institutional study in patients undergoing colectomy were: (1) to examine adherence to this novel, comprehensive VTE chemoprophylaxis quality measure compared with the traditional VTE process measure, SCIP-VTE-2; (2) to categorize failures in inpatient postoperative VTE chemoprophylaxis; and (3) to assess patient- and hospital-level factors associated with failure to adhere to appropriate VTE chemoprophylaxis.
METHODS
Study Design, Data Source, and Participants
We performed a cross-sectional analysis utilizing 2 prospectively maintained databases, the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) and the 56-hospital Illinois Surgical Quality Improvement Collaborative (ISQIC) online data platform. In these databases, cases are abstracted by trained surgical clinical reviewers and undergo an audit process to promote data accuracy and quality in addition to the rigorous data reliability audits regularly performed by ACS NSQIP.18 Eligible patients were those 18 years or older who underwent a colectomy at an ISQIC hospital from January 1, 2015 through June 30, 2016. Cases that included a proctectomy were excluded. Patients from hospitals with missing or discontinuous abstraction were also excluded.
We merged the ACS NSQIP and ISQIC data with the 2014 American Hospital Association (AHA) Annual Survey (hospital characteristics) and the September 2015 update of the FY2015 Centers for Medicare & Medicaid Services (CMS) Payment Impact File. The CMS Impact file was used in a manner previously described19,20 to identify each hospital’s safety net status (defined as a hospital in the highest quartile receiving CMS disproportionate share payments), teaching status (defined as a hospital with a resident-to-bed ratio ≥ 0.001), and case mix index (CMI; calculated by summing the diagnosis-related group weights for all Medicare discharges and dividing by the number of Medicare discharges). A higher CMI indicates a hospital with more diverse, clinically complex, and, potentially, higher risk patient population. Magnet designation status for each hospital was obtained from The American Nurses Credentialing Center (ANCC) and is a rigorous review process that “recognizes health care organizations for quality patient care, nursing excellence and innovations in professional nursing practice.”21 The risk-adjusted VTE event rate for ISQIC hospitals (occurring within 30 d after the index operation) according to the standard ACS NSQIP definition was calculated for the time period relevant to this study using the standard ACS NSQIP modeling approach which has been previously well described and accounts for differences in patient comorbidities.22
SCIP-VTE-2 adherence rates were obtained from the Timely and Effective Care file from the 2016 CMS Hospital Compare data archive Annual Files. SCIP-VTE-2 is a process measure developed by CMS and the Joint Commission that reports the percentage of patients who receive appropriate VTE prophylaxis within the 24 hours prior to surgical incision time to 24hours after surgery end time.23 This measure was recently retired, but data are still available.11
Comprehensive VTE Chemoprophylaxis
Comprehensive VTE chemoprophylaxis was defined according to major consensus guidelines,1 and requires the appropriate medication to be ordered at the correct dose and frequency, and administered without missed doses throughout each patient’s entire inpatient hospitalization. Appropriate chemoprophylaxis agents (e.g., low molecular weight heparin, unfractionated heparin) were defined according to major consensus guidelines and FDA-approved indications.1 Failure was defined as a missed or incorrect dose of chemoprophylaxis at any time during each patient’s inpatient hospitalization for any reason not covered by an appropriate clinical exception. Clinically appropriate, evidence-based exceptions were allowed according to objective criteria for situations such as active bleeding, epidural or spinal anesthesia, and holding doses for additional procedures such as those performed in interventional radiology (Supplemental Appendix, http://links.lww.com/SLA/B534).9 Reasons for failure to provide comprehensive VTE chemoprophylaxis were captured in 5 categories: patient refusal, medication not ordered, incorrect dose or frequency, patient off unit, or other reasons for missed dose.
Statistical Analysis
Cluster-adjusted chi-square tests were used to examine bivariate relationships between failure to provide comprehensive VTE chemoprophylaxis and patient and hospital characteristics. We constructed hierarchical logistic regression models with hospital-level intercepts that accounted for clustering of patients within hospitals to further examine these relationships. Clinically relevant variables and variables with a P value less than 0.05 on bivariate analysis were included in the models. The following independent variables were included in the models: age, sex, race, body mass index, number of comorbidities, smoking status, surgical indication, whether the case was performed electively, safety net hospital status, Magnet designation, teaching hospital status, nurse-to-bed ratio, and case mix index. All analyses were performed at the patient level. All tests were 2 sided with statistical significance set at 0.05. Statistical analysis was performed using SAS 9.4 (SAS Institute Inc, Cary, NC) and Stata 14.1 (StataCorp LP, College Station, TX).
RESULTS
Patient and Hospital Cohort Characteristics
We identified 5845 patients who underwent colectomy at 52 ISQIC hospitals during the 18-month study period. After excluding patients who underwent a colectomy at a hospital that did not participate in the VTE process measure abstraction (n = 872), had observations that contained discontinuous abstraction (n = 152), were unable to be matched to the ACS NSQIP Semi-Annual Report dataset (n = 93), were duplicate observations (n = 45), or had missing process measure data (n = 597), the final cohort contained 4086 patients from 36 ISQIC hospitals. Patients were more commonly white (80.6%), female (53.2%), and 61 to 74 years of age (35.0%) (Table 1). Two-thirds of the colectomies were elective (67.1%). Neoplasm (47.4%) was the indication for surgery in nearly half of the patients. The median length of stay (LOS) was 5 days. Most patients had 1 or zero comorbidities (58.5%). Most colectomies were performed at teaching hospitals (68.6%). Nine hospitals (25%) were identified as safety net hospitals and 20 (55.6%) had Magnet designation (Table 2). Additional patient and hospital characteristics are detailed in Tables 1 and 2, respectively.
TABLE 1.
Patient Characteristics and Comprehensive VTE Chemoprophylaxis Failure Rates
| Patient Characteristic | Patients n (%) | Any VTE Chemoprophylaxis Failure* n (%) | P Value† |
|---|---|---|---|
| Overall | 4086 (100) | 734 (18.0) | |
| Age | 0.49 | ||
| ≤40 yrs | 351 (8.6) | 100 (28.5) | |
| 41–60 yrs | 1,378 (33.7) | 256 (18.6) | |
| 61–74 yrs | 1430 (35.0) | 223 (15.6) | |
| ≥75 yrs | 927 (22.7) | 155 (16.7) | |
| Sex | 0.85 | ||
| Male | 1911 (46.8) | 352 (18.4) | |
| Female | 2175 (53.2) | 382 (17.6) | |
| Race | 0.58 | ||
| Asian | 103 (2.5) | 15 (14.6) | |
| African American | 482 (11.8) | 127 (26.3) | |
| White | 3294 (80.6) | 553 (16.8) | |
| Other/unknown | 207 (5.1) | 39 (18.8) | |
| Body Mass Index | 0.79 | ||
| Underweight | 141 (3.5) | 34 (24.1) | |
| Normal | 1172 (28.7) | 226 (19.3) | |
| Overweight/obese | 2773 (67.9) | 474 (17.1) | |
| Diabetes | 0.77 | ||
| Yes | 637 (15.6) | 105 (16.5) | |
| No | 3449 (84.4) | 629 (18.2) | |
| Comorbidities | 0.96 | ||
| 0 | 985 (24.1) | 157 (15.9) | |
| 1 | 1406 (34.4) | 257 (18.3) | |
| 2 | 905 (22.2) | 174 (19.2) | |
| ≥3 | 790 (19.3) | 146 (18.5) | |
| Active smoker | 0.98 | ||
| Yes | 746 (18.3) | 135 (18.1) | |
| No | 3340 (81.7) | 599 (17.9) | |
| Surgical indication | 0.28 | ||
| Neoplasm | 1935 (47.4) | 299 (15.5) | |
| Benign | 2151 (52.6) | 435 (20.2) | |
| Elective surgery | 0.19 | ||
| Yes | 2741 (67.1) | 437 (15.9) | |
| No | 1345 (32.9) | 297 (22.1) | |
| Length of Stay (LOS) | 0.15 | ||
| Quartile 1 (≤3 d) | 957 (23.4) | 105 (11.0) | |
| Quartile 2 (4–5 d) | 1239 (30.3) | 207 (16.7) | |
| Quartile 3 (6–8 d) | 974 (23.8) | 185 (19.0) | |
| Quartile 4 (≥9 d) | 915 (22.4) | 237 (25.9) | |
| Median LOS, d (IQR) | 5 (4–8) | ||
Patients that failed comprehensive VTE chemoprophylaxis for any reason. The percentage is out of the total patients in the corresponding subgroup.
Clustered chi-square test of association.
TABLE 2.
Comparison of Comprehensive VTE Chemoprophylaxis Failure Rates by Hospital Characteristic
| Hospital Characteristics |
Hospital Frequency n (%) |
Patient Frequency n (%) |
Patient-Level VTE Prophylaxis Failure n (%) |
P Value§ |
|---|---|---|---|---|
| Overall | 36 (100) | 4086 (100) | 734 (18.0) | |
| Hospital ownership | 0.69 | |||
| For-profit | 1 (2.8) | 99 (2.4) | 10 (10.1) | |
| Not-for-profit | 32 (88.9) | 3751 (91.8) | 667 (17.8) | |
| Government nonfederal | 3 (8.3) | 236 (5.8) | 57 (24.2) | |
| Hospital bed size | 0.91 | |||
| Quartile 1 [≤242] | 15 (41.6) | 1065 (26.1) | 193 (18.1) | |
| Quartile 2 [243–371] | 10 (27.8) | 958 (23.4) | 155 (16.2) | |
| Quartile 3 [372–509] | 7 (19.4) | 1116 (27.3) | 190 (17.0) | |
| Quartile 4 [>509] | 4 (11.1) | 947 (23.2) | 196 (20.7) | |
| Total hospital admissions | 0.87 | |||
| Quartile 1 [≤13,062] | 15 (41.6) | 1040 (25.5) | 166 (16.0) | |
| Quartile 2 [13,063–19,238] | 11 (30.6) | 975 (23.9) | 185 (19.0) | |
| Quartile 3 [19,239–23,558] | 6 (16.7) | 1124 (27.5) | 187 (16.6) | |
| Quartile 4 [>23,558] | 4 (11.1) | 947 (23.2) | 196 (20.7) | |
| Case mix index* | 0.78 | |||
| Quartile 1 [1.54] | 14 (38.9) | 1073 (26.3) | 207 (19.3) | |
| Quartile 2 [1.55–1.65] | 10 (27.8) | 1033 (25.3) | 180 (17.4) | |
| Quartile 3 [1.66–1.82] | 8 (22.2) | 999 (24.5) | 144 (14.4) | |
| Quartile 4 [>1.82] | 4 (11.1) | 981 (24.0) | 203 (20.7) | |
| Safety Net Hospital† | 0.009 | |||
| Yes | 9 (25.0) | 808 (19.8) | 240 (29.7) | |
| No | 27 (75.0) | 3278 (80.2) | 494 (15.1) | |
| Inpatient surgeries per bed | 0.86 | |||
| Quartile 1 [≤11.59] | 14 (39) | 1059 (25.9) | 182 (17.2) | |
| Quartile 2 [11.60–16.05] | 9 (25.0) | 1141 (27.9) | 240 (21.0) | |
| Quartile 3 [16.06–18.03] | 5 (13.9) | 740 (18.1) | 122 (16.5) | |
| Quartile 4 [>18.03] | 8 (22.2) | 1146 (28.1) | 190 (16.6) | |
| Teaching Hospital‡ | 0.14 | |||
| Non-Teaching | 12 (33.3) | 1285 (31.4) | 169 (13.2) | |
| Teaching | 24 (66.7) | 2801 (68.6) | 565 (20.2) | |
| Transplant services | 0.69 | |||
| Yes | 12 (33.3) | 2036 (49.8) | 384 (18.9) | |
| No | 24 (66.7) | 2050 (50.2) | 350 (17.1) | |
| Level 1 trauma center | 0.34 | |||
| Yes | 9 (25.0) | 1491 (36.5) | 310 (20.8) | |
| No | 27 (75.0) | 2595 (63.5) | 424 (16.3) | |
| Joint Commission accreditation | 0.95 | |||
| Yes | 32 (88.9) | 3755 (91.9) | 676 (18.0) | |
| No | 4 (11.1) | 331 (8.1) | 58 (17.5) | |
| Commission on Cancer accreditation | 0.82 | |||
| Yes | 31 (86.1) | 3862 (94.5) | 689 (17.8) | |
| No | 5 (13.9) | 224 (5.5) | 45 (20.1) | |
| ACGME accreditation | 0.45 | |||
| Yes | 26 (72.2) | 3151 (77.1) | 595 (18.9) | |
| No | 10 (27.8) | 935 (22.9) | 139 (14.9) | |
| Council of Teaching Hospitals | 0.29 | |||
| Yes | 10 (27.8) | 1763 (43.1) | 364 (20.6) | |
| No | 26 (72.2) | 2323 (56.9) | 370 (15.9) | |
| Magnet hospital | 0.008 | |||
| Yes | 20 (55.6) | 2790 (68.3) | 388 (13.9) | |
| No | 16 (44.4) | 1296 (31.7) | 346 (26.7) | |
| Nurse-to-bed ratio | 0.49 | |||
| Quartile 1 [≤1.51] | 15 (41.6) | 1129 (27.6) | 157 (13.9) | |
| Quartile 2 [1.52–1.893] | 8 (22.2) | 1014 (24.8) | 167 (16.5) | |
| Quartile 3 [1.893–2.40] | 7 (19.4) | 956 (23.4) | 181 (18.9) | |
| Quartile 4 [>2.40] | 6 (16.7) | 987 (24.2) | 229 (23.2) | |
| SCIP-2 VTE failure (%) | 0.98 | |||
| 0 | 31 (86.1) | 3625 (88.7) | 659 (18.2) | |
| 1 | 3 (8.3) | 413 (10.1) | 69 (16.7) | |
| 2 | 1 (2.8) | 17 (0.4) | 1 (5.9) | |
| 3 | 1 (2.8) | 31 (0.8) | 5 (16.1) | |
The case mix index (CMI) is calculated by summing the diagnosis-related group weights for all Medicare discharges and dividing by the number of Medicare discharges (https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Acute-Inpatient-Files-for-Download-Items/CMS022630.html). A higher CMI indicates more diverse, clinically complex, and, potentially, sicker patient population.
Safety Net Hospital was defined as a hospital in the highest quartile of the disproportionate share payments.
Teaching hospital was defined as a hospital with a resident-to-bed ratio ≥0.001 from the FY 2015 CMS Impact File.
Cluster chi-square test of association performed at the patient level.
Failures of VTE Chemoprophylaxis
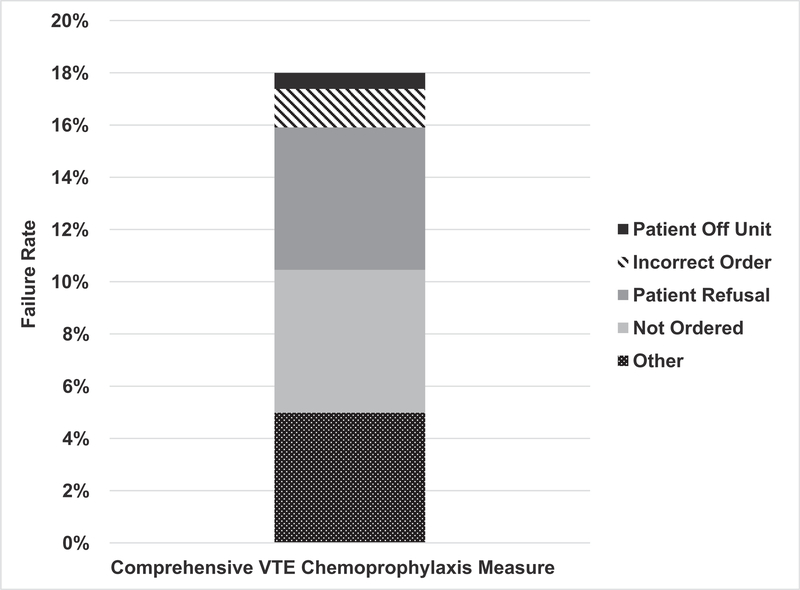
Failure to provide comprehensive postoperative VTE chemoprophylaxis occurred in 18% of patients (n = 736; Fig. 1). Medication not ordered (30.4%) and patient refusal (30.3%) were the most documented reasons for failure to provide chemoprophylaxis (Fig. 1). Failures due to incorrect dosage/frequency (8.2%) and the patient being off unit at the time of administration (3.4%) were less common. “Other reason for missed dose” was selected as the failure mode in 27.7% of cases. Of the 82% of patients who were considered to have received appropriate postoperative VTE chemoprophylaxis, 728 (22%) were considered to have passed the measure with an appropriate clinical exception (Supplemental Appendix, http://links.lww.com/SLA/B534).
FIGURE 1.
Patient refusal and ordering errors comprise the majority of chemoprophylaxis failures.
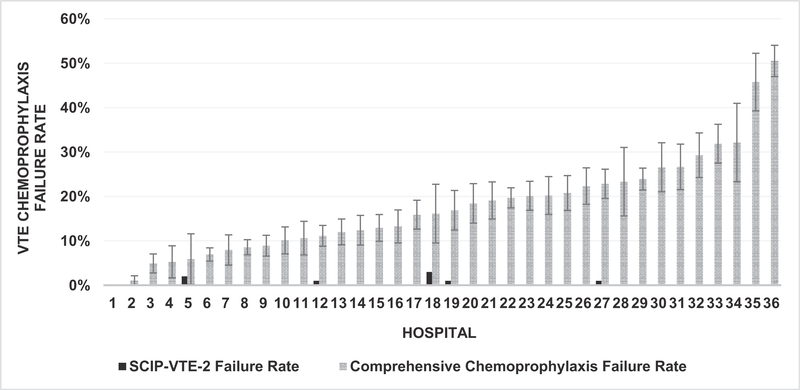
Comprehensive chemoprophylaxis failure rates by hospital ranged from 0% to 50.5% (median 95 cases per hospital; interquartile range 64 to 141 cases) (Fig. 2). Of the 36 hospitals, only 1 hospital provided defect-free care (ie, no measure failures), while 4 hospitals (11%) failed to provide comprehensive VTE chemoprophylaxis in more than 30% of cases. Conversely, the hospital perioperative VTE chemoprophylaxis failure rate captured by the SCIP-VTE-2 measure ranged from 0% to 3.0% (Table 2 and Fig. 2). The VTE event rate for the hospitals in ISQIC decreased from 3.1% in the baseline period (January to August 2015) to 2.5% (September 2016 to June 2017) after implementation of quality improvement projects at each hospital specifically focused on improving adherence to postoperative VTE prophylaxis.
FIGURE 2.
SCIP-VTE-2 inadequately captures VTE chemoprophylaxis quality compared with the ISQIC comprehensive VTE chemoprophylaxis measure.
Factors Associated With Comprehensive VTE Chemoprophylaxis Failure
In the unadjusted analysis, no patient-level factors were significantly associated with overall chemoprophylaxis failure (Table 1). Hospital-level characteristics associated with lower failure rates were nonsafety net status hospitals or Magnet designation hospitals (Table 2). Notably, SCIP-VTE-2 performance was not associated with failure of the ISQIC comprehensive VTE chemoprophylaxis process measure (P = 0.98).
In the adjusted analysis, patients were less likely to fail the comprehensive VTE chemoprophylaxis measure if they received treatment at a nonsafety net hospital (OR 0.62, 95% CI 0.39–0.99, P = 0.045 vs. safety net hospital; Table 3) or a Magnet designation hospital (OR 0.45, 95% CI 0.29–0.71, P = 0.001 vs. hospital without Magnet designation), or underwent an elective colectomy (OR 0.76, 95% CI 0.63–0.93, P = 0.007 vs. nonelective colectomy). Patients who experienced a length of stay in the highest quartile (≥9 d) were more likely to fail the comprehensive VTE chemoprophylaxis measure (OR 2.64, 95% CI 1.98–3.51, P < 0.001); LOS was otherwise not associated with measure failure (Table 3).
TABLE 3.
Factors Associated With Comprehensive VTE Chemoprophylaxis Failure
| Factor | Any Failure Reason OR (95% CI) | P Value | Patient Refusal OR (95% CI) | P Value |
|---|---|---|---|---|
| Age | ||||
| ≥75 yrs | 1.0 (REF) | 1.0 (REF) | ||
| 61–74 yrs | 0.95 (0.75–1.21) | 0.68 | 1.20 (0.76–1.89) | 0.45 |
| 41–60 yrs | 1.20 (0.93–1.55) | 0.16 | 1.69 (1.06–2.69) | 0.027 |
| ≤40 yrs | 1.56 (1.11–2.19) | 0.011 | 2.10 (1.21–3.66) | 0.009 |
| Sex | ||||
| Male | 1.0 (REF) | 1.0 (REF) | ||
| Female | 0.97 (0.82–1.15) | 0.75 | 1.16 (0.86–1.54) | 0.33 |
| Race | ||||
| White | 1.0 (REF) | 1.0 (REF) | ||
| Asian | 0.65 (0.36–1.18) | 0.16 | 0.63 (0.22–1.81) | 0.39 |
| African American | 1.06 (0.81–1.39) | 0.68 | 1.27 (0.85–1.90) | 0.24 |
| Other/unknown | 0.88 (0.60–1.31) | 0.53 | 1.05 (0.59–1.86) | 0.86 |
| Body Mass Index | ||||
| Overweight/obese | 1.0 (REF) | 1.0 (REF) | ||
| Normal | 1.14 (0.94–1.38) | 0.17 | 1.35 (0.99–1.85) | 0.06 |
| Underweight | 1.46 (0.95–2.26) | 0.09 | 2.34 (1.27–4.33) | 0.006 |
| Comorbidities | ||||
| 0 | 1.0 (REF) | 1.0 (REF) | ||
| 1 | 1.10 (0.87–1.40) | 0.41 | 1.12 (0.76–1.66) | 0.57 |
| 2 | 1.08 (0.82–1.42) | 0.58 | 1.04 (0.66–1.63) | 0.87 |
| ≥3 | 0.93 (0.68–1.26) | 0.62 | 0.60 (0.34–1.04) | 0.07 |
| Active smoker | ||||
| No | 1.0 (REF) | 1.0 (REF) | ||
| Yes | 0.87 (0.69–1.11) | 0.27 | 0.99 (0.67–1.47) | 0.98 |
| Surgical indication | ||||
| Neoplasm | 1.0 (REF) | 1.0 (REF) | ||
| Benign | 1.14 (0.94–1.38) | 0.17 | 1.37 (0.99–1.89) | 0.06 |
| Elective procedure | ||||
| No | 1.0 (REF) | 1.0 (REF) | ||
| Yes | 0.92 (0.75–1.13) | 0.43 | 0.84 (0.60–1.18) | 0.32 |
| Length of stay | ||||
| Quartile 1 (≤3 d) | 1.0 (REF) | 1.0 (REF) | ||
| Quartile 2 (4–5 d) | 1.43 (1.10–1.86) | 0.008 | 1.58 (0.99–2.52) | 0.06 |
| Quartile 3 (6–8 d) | 1.72 (1.30–2.28) | <0.001 | 1.45 (0.88–2.40) | 0.15 |
| Quartile 4 (≥9 d) | 2.64 (1.98–3.51) | <0.001 | 2.46 (1.51–4.03) | <0.001 |
| Safety Net Hospital | ||||
| No | 0.64 (0.40–1.03) | 0.07 | 0.47 (0.25–0.91) | 0.026 |
| Yes | 1.0 (REF) | 1.0 (REF) | ||
| Magnet Hospital | ||||
| No | 1.0 (REF) | 1.0 (REF) | ||
| Yes | 0.44 (0.28–0.69) | <0.001 | 0.70 (0.36–1.34) | 0.28 |
| Teaching Hospital | ||||
| No | 1.0 (REF) | 1.0 (REF) | ||
| Yes | 0.92 (0.59–1.45) | 0.72 | 2.04 (1.01–4.12) | 0.048 |
Adjusted odds ratios were estimated with hierarchical logistic regression models with hospital-level random intercepts that accounted for clustering of patients within hospitals. In addition, the model adjusted for case mix index and quartile of nursing-to-bed ratio. Safety Net Hospital was defined as a hospital in the highest quartile of the disproportionate share patients in FY 2013. Teaching status was defined as those with a resident-to-bed ratio ≥ 0.001 from the FY 2015 CMS Impact File. n = 4086.
Failure to deliver comprehensive VTE chemoprophylaxis due to patient refusal was more likely in patients who were: ≤ 40 years old (OR1.91, 95% CI1.10–3.32; P = 0.022vs.age ≥ 75yrs;Table 3);had normal or underweight BMI (normal BMI OR 1.37, 95% CI 1.00–1.87, P = 0.048, and underweight BMI OR2.24,95% CI1.22–4.13, P = 0.009 vs. patients with overweight/obese BMI); underwent colectomy for a benign indication (OR 1.41, 95% CI 1.02–1.94, P = 0.039 vs. neoplastic indication);had an LOS in the highest quartile(LOS ≥ 9 d OR 2.64, 95% CI 1.98–3.51, P < 0.001); or received treatment at a teaching hospital (OR 2.15, 95% CI 1.05–4.38, P = 0.036 vs. nonteaching hospital). Patients receiving treatment at a nonsafety net hospital were less likely to refuse VTE chemoprophylaxis (OR 0.46 95% CI 0.23–0.88, P = 0.021 vs. safety net hospital).
DISCUSSION
VTE remains a significant, potentially preventable clinical problem that can result in serious patient harm. Our large-scale, multi-institutional study of VTE chemoprophylaxis practices utilized a novel process measure of comprehensive VTE chemoprophylaxis that evaluates chemoprophylaxis throughout each patient’s entire inpatient hospital stay. We found that hospitals failed to provide defect-free chemoprophylaxis in 18% of colectomies. This figure stands in glaring contrast to the same hospitals’ SCIP-VTE-2 failure rates, which were only 0% to 3%, confirming the shortcomings of using SCIP-VTE-2 as a quality measure or for QI due to its lack of comprehensiveness and granularity to inform QI efforts. Furthermore, we found that very few hospitals provide defect-free comprehensive VTE chemoprophylaxis, and, in fact, only 1 hospital out of our cohort of 36 hospitals had zero failures during the study period. Thus, there is ample opportunity at most hospitals to improve the quality of care in postoperative VTE chemoprophylaxis.
Current VTE quality measures are problematic because they lack the granularity to identify reasons why failures of prophylaxis occur. As a result, it can be difficult for hospitals and providers who want to improve VTE prophylaxis to use quality data from current metrics to inform their QI interventions. Our comprehensive VTE chemoprophylaxis quality measure improves upon existing quality measures in that the data include a specific cited reason when a dose of chemoprophylaxis is not provided. These reasons provide actionable direction to hospitals and providers who want to reduce failures of VTE chemoprophylaxis. Patient refusals combined with physician ordering errors accounted for two-thirds of chemoprophylaxis failures in our statewide surgical QI collaborative. Patient refusal and ordering errors are highly actionable modes of failure.13,17,24 QI interventions focused on improving performance in both of these modes of failure are ongoing in our statewide collaborative.
Finally, given the power afforded to us by this multi-institutional study, we sought to identify patient- and hospital-level predictors of failure in providing comprehensive VTE chemoprophylaxis in postoperative patients. Patients treated at nonsafety net hospitals or Magnet designation hospitals were less likely to experience failures of VTE chemoprophylaxis. Educating patients about the importance of VTE prophylaxis and setting patient expectations about chemoprophylaxis, particularly in the preoperative setting, may be one way to reduce patient refusals.24,25 Additionally, nurses play an important role in addressing patient refusals as reflected in the association between Magnet designation (a measure of nursing quality) and VTE chemoprophylaxis adherence. Focused efforts to equip nurses to counsel patients when they refuse chemoprophylaxis could be another effective way to reduce patient refusals. These efforts become even more important when patients have prolonged lengths of stay since there are more opportunities for doses of chemoprophylaxis to be missed or refused.
This study has several limitations. First, this analysis was focused on colectomy procedures only and results may not be generalizable to patients undergoing other procedures. Second, 1759 of the 5845 colectomies performed by ISQIC hospitals during the study time period were excluded from the analysis. Though the excluded cases could be a source of bias, the study cohort still includes 70% of the colectomies performed by 36 ISQIC hospitals, which we believe to be a highly representative sample, particularly since the overall measure failure rate (18%) is consistent with previous, smaller studies.9,13–15 Third, “Other reason for missed dose” was identified as the mode of failure in 27.7% of cases and did not offer any actionable methods to address this mode of failure. In the spirit of iterative improvement in QI, the measure has been updated and currently requires the healthcare professional to provide additional explanation regarding the reason for the missed dose when making this selection. As a result, we expect more granular and potentially actionable data in the future. Finally, while we observed a contemporaneous decrease in VTE event rates for ISQIC hospitals over a similar time period, this time period also included efforts by the hospitals to improve adherence to the comprehensive VTE chemoprophylaxis quality measure. While it is encouraging that improvements in adherence to the measure were reflected by a decrease in the aggregate VTE event rate, it is more difficult to definitively associate failures of prophylaxis to individual patient VTE events given the relative rarity of this event (~1% of surgical cases). Though assessing the effect of process measure adherence on outcomes is important, there may be other factors beyond inpatient prophylaxis practices, such as surveillance bias in VTE outcomes reporting and VTE events that occurred after discharge, that affect 30-day VTE event rates and will be a subject of future study. Improved adherence to this process measure reflects provision of the best evidence-based guideline recommended care possible for postoperative inpatient VTE chemoprophylaxis, and we believe that this is a relevant and worthy patient care goal.
In conclusion, this is the first multi-institution study examining failure patterns in providing comprehensive postoperative VTE chemoprophylaxis utilizing a novel, comprehensive VTE chemoprophylaxis quality measure. In contrast to SCIP-VTE-2, our measure unmasked chemoprophylaxis failures in 18% of colectomies in a statewide surgical collaborative. Most chemoprophylaxis failures were due to patient refusals or medication ordering errors, both of which are actionable targets for QI. Hospitals need to focus improvement efforts on ensuring patients receive VTE prophylaxis throughout their entire hospitalization.
Supplementary Material
ACKNOWLEDGMENTS
The authors acknowledge the work of all of the ISQIC hospital teams (Surgical Clinical Reviewers, Surgeon Champions, and Quality Improvement Designees), Surgeon Champion mentors, the process improvement coaches, and the staff of the ISQIC Coordinating Center.
Sources of Funding: American College of Surgeons (Thomas R. Russell Faculty Research Fellowship [ADY]), National Institutes of Health (5T32HS000078 [DBH], 5T32HL094293 [EB] and R01HS024516 [KYB]), Health Care Services Corporation/Blue Cross Blue Shield of Illinois.
Presented at the 13th Annual Academic Surgical Congress Clinical Outcomes Plenary Session, February 1, 2018, Jacksonville, FL.
Footnotes
The authors report no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.annalsofsurgery.com).
REFERENCES
- 1.Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic-surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e227S–e277S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mismetti P, Laporte S, Darmon JY, et al. Meta-analysis of low molecular-weight heparin in the prevention of venous thromboembolism in general surgery. Br J Surg. 2001;88:913–930. [DOI] [PubMed] [Google Scholar]
- 3.Rasmussen MS, Jorgensen LN, Wille-Jorgensen P. Prolonged thromboprophylaxis with low molecular weight heparin for abdominal or pelvic surgery. Cochrane Database Syst Rev. 2009;(1):CD004318. [DOI] [PubMed] [Google Scholar]
- 4.Shojania KGMK, Wachter RM, Owens DK. Closing The Quality Gap: A Critical Analysis of Quality Improvement Strategies, Volume 1—Series Overview and Methodology Technical Review 9 (Contract No. 290–020017 to the Stanford University–UCSF Evidence based Practices Center). Agency for Healthcare Research and Quality. [PubMed] [Google Scholar]
- 5.Bilimoria KY, Chung J, Ju MH, et al. Evaluation of surveillance bias and the validity of the venous thromboembolism quality measure. JAMA. 2013;310:1482–1489. [DOI] [PubMed] [Google Scholar]
- 6.Ju MH, Chung JW, Kinnier CV, et al. Association between hospital imaging use and venous thromboembolism events rates based on clinical data. Ann Surg. 2014;260:558–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kinnier CV, Barnard C, Bilimoria KY. The need to revisit VTE quality measures. JAMA. 2014;312:286–287. [DOI] [PubMed] [Google Scholar]
- 8.Minami CA, Bilimoria KY. Are higher hospital venous thromboembolism rates an indicator of better quality?: Evaluation of the validity of a hospital quality measure. Adv Surg. 2015;49:185–204. [DOI] [PubMed] [Google Scholar]
- 9.Kinnier CV, Ju MH, Kmiecik T, et al. Development of a novel composite process measure for venous thromboembolism prophylaxis. Med Care. 2016;54:210–217. [DOI] [PubMed] [Google Scholar]
- 10.Yang AD, Bilimoria KY. Accurately measuring hospital venous thromboembolism prevention efforts. JAMA. 2016;315:2113–2114. [DOI] [PubMed] [Google Scholar]
- 11.Commission TJ. The Joint Commission Measure Sets Effective January 1,2015. Available at: https://www.jointcommission.org/assets/1/6/TJC_Measures_2015__11_15.pdf. Accessed June 10, 2017.
- 12.Louis SG, Sato M, Geraci T, et al. Correlation of missed doses of enoxaparin with increased incidence of deep vein thrombosis in trauma and general surgery patients. JAMA Surg. 2014;149:365–370. [DOI] [PubMed] [Google Scholar]
- 13.Shermock KM, Lau BD, Haut ER, et al. Patterns of non-administration of ordered doses of venous thromboembolism prophylaxis: implications for novel intervention strategies. PLoS One. 2013;8:e66311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fanikos J, Stevens LA, Labreche M, et al. Adherence to pharmacological thromboprophylaxis orders in hospitalized patients. Am J Med. 2010;123:536–541. [DOI] [PubMed] [Google Scholar]
- 15.Salottolo K, Offner P, Levy AS, et al. Interrupted pharmocologic thromboprophylaxis increases venous thromboembolism in traumatic brain injury. J Trauma. 2011;70:19–24. [DOI] [PubMed] [Google Scholar]
- 16.Ramanathan R, Gu Z, Limkemann AJ, et al. Association between interruptions in chemical prophylaxis and VTE formation. Am Surg. 2015;81:732–737. [PubMed] [Google Scholar]
- 17.Elder S, Hobson DB, Rand CS, et al. Hidden barriers to delivery of pharmacological venous thromboembolism prophylaxis: the role of nursing beliefs and practices. J Patient Safety. 2016;12:63–68. [DOI] [PubMed] [Google Scholar]
- 18.Hall BL, Hamilton BH, Richards K, et al. Does surgical quality improve in the American College of Surgeons National Surgical Quality Improvement Program: an evaluation of all participating hospitals. Ann Surg. 2009;250:363–376. [DOI] [PubMed] [Google Scholar]
- 19.Services CfMM. Case Mix Index. Available at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Acute-InpatientFiles-for-Download-Items/CMS022630.html. Accessed June 10, 2017.
- 20.Rajaram R, Chung JW, Kinnier CV, et al. Hospital characteristics associated with penalties in the centers for medicare & medicaid services hospital acquired condition reduction program. JAMA. 2015;314:375–383. [DOI] [PubMed] [Google Scholar]
- 21.Center ANC. ANCC Magnet Recognition Program. Available at: http://www.nursecredentialing.org/Magnet. Accessed June 10, 2017.
- 22.Cohen ME, Ko CY, Bilimoria KY, et al. Optimizing ACS NSQIP modeling for evaluation of surgical quality and risk: patient risk adjustment, procedure mix adjustment, shrinkage adjustment, and surgical focus. J Am Coll Surg. 2013;217:336.e1–346.e1. [DOI] [PubMed] [Google Scholar]
- 23.SCIP-venous-thromboembolism-2. Specifications Manual for Joint Commission National Quality Core Measures (2010B) 2010; Available at: https://manual.jointcommission.org/releases/archive/TJC2010B1/MIF0061.html. Accessed June 10, 2017.
- 24.Piazza G, Nguyen TN, Morrison R, et al. Patient education program for venous thromboembolism prevention in hospitalized patients. Am J Med. 2012;125:258–264. [DOI] [PubMed] [Google Scholar]
- 25.Lau BD, Shaffer DL, Hobson DB, et al. Effectiveness of two distinct web-based education tools for bedside nurses on medication administration practice for venous thromboembolism prevention: a randomized clinical trial. PLoS One. 2017;12:e0181664. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.