Abstract
Transgender women, particularly racial/ethnic minority transgender women, evidence disproportionately high rates of untreated HIV infection and disproportionately low rates of HIV viral suppression. The Alexis Project was a combined Peer Health Navigation (PHN) and Contingency Management (CM) intervention that targeted HIV milestones associated with advancement along the HIV Care Continuum. From February 2014 through August 2016, 139 transgender women of color (TWOC) enrolled and received unlimited PHN sessions and an escalating CM rewards schedule for confirmed achievement of both behavioral (e.g., HIV care visits) and biomedical (e.g., viral load reductions and achieved/sustained viral suppression) HIV milestones. Results demonstrated that increased attendance to PHN sessions was associated with significant achievement of both behavioral (coef. range 0.12–0.38) and biomedical (coef. = 0.10) HIV milestones (all p ≤ 0.01); 85% were linked to HIV care, and 83% who enrolled detectable and achieved the minimum 1 log viral load reduction advanced to full viral suppression. The combined PHN and CM intervention successfully promoted advancement along the HIV Care Continuum, with particularly robust effects for behavioral HIV milestones.
Keywords: transgender, HIV, contingency management, peer health navigation, HIV Care Continuum
INTRODUCTION
Transgender women (hereafter: trans women) bear a disproportionate burden of HIV infection in the U.S., with trans women of color (TWOC) being especially impacted [1]. Furthermore, there is a wide divergence between mean self-reported rates of HIV among trans women (~12%) and actual population testing estimates (~28%), implying that a substantial percentage of HIV-positive trans women are unaware of their status [2, 3]. The rate of unaware and undiagnosed HIV infection among trans women is more than twice the national average (57% vs. 27%) [2, 4–6]. Nationally, trans women evidence suboptimal advancement through the HIV Care Continuum [7–9], including poor access and attendance to medical care [2], and reduced rates of viral suppression [9–11]. Among those in care, medication adherence has been shown to be suboptimal [12], a result associated with a lack of self-efficacy (i.e., feelings of empowerment and personal agency) surrounding HIV care and HIV health-related behaviors [13]. Clinical observations of trans women living with HIV suggest that many trans-specific challenges (e.g., gender discrimination at healthcare providers, fears of interactions between HIV medications and hormone therapies) interfere with adherence to HIV care and treatment regimens [14]. As a result of low medication adherence, rates of viral suppression have been shown to be lower among trans women than other HIV-positive populations [11].
Health deficits are particularly accentuated among racial/ethnic minority trans women living in the U.S. [2] TWOC experience particularly extreme stigma [15], given the increased transphobia and heteronormativity expressed in many minority cultures, and the effect of intersecting stigmatized statuses [16, 17]. Many of the health disparities that trans women face are the result of a number of psychosocial challenges specific to their gender identification, including discrimination, prejudice, stigmatization, and social/economic marginalization [18, 19]. The stigma experienced by trans women is particularly intense, often more severe and psychologically damaging than that experienced by cisgender men and women who identify as gay, lesbian, and/or bisexual [20]. Such issues stand as obstacles to proper medical, mental health, and social service engagement and care, as trans women report discrimination and/or blatant verbal abuse at standard health care facilities [21, 22], with evidence of medical providers perpetuating stigma in their interactions with trans patients [23] including denying the provision of medical care [24]. Distrust and avoidance of proper medical facilities can lead to failure to disclose relevant information about risk behaviors and increased risk of delaying or avoiding care [25], including refusal to be tested for HIV [20]. Meta-analytic data has demonstrated that approximately half of all trans persons are without health insurance [2], and past national survey data has indicated that only 30–40% utilize regular medical care [26]. However, since the Affordable Care Act was implemented in 2014, one California study found an increase from 35% to 77% in trans women reporting current healthcare coverage [27].
Developing a Culturally Competent HIV Care Intervention for TWOC
Two significant challenges to developing effective interventions to link and retain TWOC living with HIV into HIV care, and to sustain medication adherence to achieve and maintain virological suppression, are: 1) overcoming the structural and individual barriers to HIV care; and, 2) providing a powerful operant conditioning paradigm that can serve to motivate trans women to remain engaged in HIV care. The Alexis Project used two distinctly different evidence-based behavioral interventions to link and retain TWOC living with HIV into HIV care: Peer Health Navigation and Contingency Management.
Peer Health Navigation (PHN) was first developed and implemented in 1990 to enhance treatment outcomes among cancer patients [28] and emerged as a promising intervention for HIV care coordination in the early 2000s [29]. PHN is based on the mechanisms of Social Cognitive Theory, a theory that posits interactive causal relationships among personal determinants, behavior, and environmental influences [30, 31], and is designed to improve participant self-efficacy. Low self-efficacy is associated with increased HIV risk among sexual and gender minority populations [32] and suboptimal HIV healthcare outcomes in trans women [33]. Client-centered PHN helps to: (1) identify the barriers to HIV care for each particular participant, (2) identify and link participants into needed auxiliary services, and (3) increase participants’ self-efficacy in working with HIV care providers and other social service and treatment facilities. Peer Health Navigators do not provide counseling or psychotherapy; rather, they work with each participant to successfully navigate complicated health care and social service systems. In short, Peer Health Navigators identify, and then help participants overcome, whatever barriers stand between them and proper HIV primary care. As the name implies, a PHN intervention is staffed by “peers,” thus making it an ideal intervention to apply to a resource-limited project and, still, yield high outcomes.
Contingency Management (CM) is guided by the theoretical framework of behavioral economics. The theoretical base of behavioral economics is the application of contingencies to motivate individuals toward health-promoting behavior change. CM has become an increasingly popular intervention for achieving desired health outcomes that are in the participant’s self-interest but have been otherwise difficult to accomplish [34, 35]. A behavioral economics intervention has particular promise when adapted for low/no income, marginalized, and disenfranchised populations [36]. Behavioral economics incorporates reinforcement – the primary construct in the operant form of learning theory – whereby behaviors are learned (i.e., reinforced) through rewards. The primary element of behavioral economics captured in CM involves the provision of direct and immediate reinforcement for healthy or health-promoting behaviors. The principle of contingencies, i.e., making the reward contingent upon the operant behavior change, distinguishes CM from just providing a traditional incentive [34]. CM interventions have been successfully implemented in domestic and international research, and community public health settings, targeting a wide variety of operant behaviors such as HIV medication adherence [37], smoking cessation [38], weight loss [39], medication adherence [40], follow-up for abnormal pap-smears [41], drug abstinence [42–44], hepatitis vaccines [45], TB screening and adherence [46], school attendance [36], employment [47], and routine medical checkups [48, 49].
The existing literature indicates that distrust/stigma/prejudice, lack of HIV testing and/or utilization of HIV care, engagement in sex work, hormone misuse, housing instability, lack of stable transportation, and lack of peer support are all issues that increase health risks and reduce HIV testing, linkage, and retention in care for TWOC [50]. The adaptation of PHN was designed to provide TWOC with informed, culturally sensitive peer support, with a specific focus on providing services, resources, and information to increase health care utilization, engagement, and retention in HIV care and reduce high-risk behaviors (e.g., engagement in sex work, hormone misuse). The individualized plan provided by the Peer Health Navigators for each participant was further designed to link participants to numerous ancillary services (e.g., transportation, legal services, housing) that are so integral when working with TWOC who experience multiple health disparities. The coupling of PHN and CM into one combined intervention was designed to increase health care engagement to reach and sustain HIV milestones including viral load suppression.
The Alexis Project1 was an innovative and novel adaptation of PHN and CM to link and retain TWOC living with HIV into HIV care and to achieve and maintain virological suppression. The combined PHN plus CM intervention period was 18 months, with semiannual follow-up assessments through 36 months post-enrollment. The project was guided by two main research questions: (1) Will PHN prove successful in identifying and addressing the barriers to linkage and retention in HIV medical care among HIV-positive TWOC? And, (2) Will a combined PHN plus CM intervention successfully link HIV-positive TWOC into HIV care and retain them in care to reach and sustain HIV milestones?
METHOD
Participants
From February 2014 through August 2016, 139 participants enrolled in the project. Inclusion criteria for participation were 1) identified as a trans woman; 2) assigned the male sex on her original birth certificate; 3) between the ages of 18 and 65 years; 4) reported her racial/ethnic identity as other than Caucasian/White; and, 5) a: HIV positive and currently not in HIV care, or b: had not seen a HIV medical provider in the previous six months, or c: not prescribed ART medication, or d: prescribed ART medication but not always adherent. Individuals were excluded if they did not meet all eligibility criteria.
Procedure
Participants were recruited via 1) a community-wide social network recruitment and engagement methodology (i.e., Respondent Driven Sampling); 2) venue- and street-based outreach at food lines, bars, street corners, and other locations where trans women congregate; 3) dissemination of project flyers, including a postcard-sized flyer and a business card-sized flyer that folded over to fit into a person’s wallet or pocket; 4) in-reach at other programs conducted at the project site; 5) in-services conducted at local agencies that provide services to trans women; and, 6) collaborating HIV medical care clinics. Potential participants who were unable to provide documentation of their HIV-positive serostatus (e.g., medication prescription, laboratory results) were tested onsite for verification of a positive HIV status. Thirty-five participants (25%) reported trouble adhering to ART and/or maintaining their HIV primary care regimen, but tested undetectable upon enrolling. To achieve biomedical CM outcomes, these participants were instructed to maintain their undetectable status; behavioral CM targets for these participants were identical to participants who did not enroll with an undetectable viral load. Following consent, participants completed a baseline Computer Assisted Self Interview (CASI) assessment administered via REDCap [51], followed by similar assessments every six months. Participants were compensated with a $10 gift card for eligibility screening, a $25 gift card at the completion of the baseline assessment, a $50 gift card for completing the 6- and 12-month follow-up assessments, and a $100 gift card for completing the 18-, 24-, 30-, and 36-month follow-up assessments. By study design, enrollment closed on August 31, 2016 while follow-up assessments continued through August 31, 2017, allowing for a maximum of two follow-up assessments (6- and 12- months) for the newest enrolled participants. Participants enrolled toward the beginning of the study had a greater number of follow-up assessments than those enrolled later in the study. Additionally, those enrolled after May 2016 (n = 9) were not eligible to achieve the 6th HIV care visit CM milestone. The project was conducted at Friends Community Center in Hollywood, CA, the community research site of Friends Research Institute. The project was approved by the Institutional Review Board of Friends Research Institute, Inc.
Intervention
Peer Health Navigation
The PHN intervention for The Alexis Project utilized an individualized case management approach but with the specific goal of removing barriers that can impede linkage to and retention in HIV care, as well as the sustainment of medication adherence to achieve and maintain virological suppression. All Peer Health Navigators were TWOC living with HIV. Each participant worked with a Peer Health Navigator to develop a client-centered treatment plan and to directly link to HIV care and/or other needed auxiliary physical, mental health, and psychosocial services (e.g., hormone therapy, dental care, hepatitis testing/care, TB testing/care, mental health counseling and/or psychotropic medication, substance abuse treatment, legal services, job training/development). Each PHN session focused on the multiple and complex barriers that made it difficult for the participant to be linked and/or retained in HIV care.
Participants were encouraged to have ongoing contact with their Peer Health Navigator. In most cases, the frequency of contacts titrated down after the first quarter of care. When the Peer Health Navigator-participant relationship was well-established with a consistently maintained treatment plan as well as the removal of barriers to auxiliary services, Peer Health Navigators then began to work with the participant to become more self-sufficient and establish self-efficacy regarding her treatment plan. Participants were able to contact a Peer Health Navigator at any time for information, guidance, and support or if another service was required. Peer Health Navigators always provided up-to-date, trans-competent referrals. The Peer Health Navigator also reminded the participant of her upcoming appointments and, if necessary, transported and accompanied her to and from scheduled HIV medical care appointments (provision of transportation fell under the purview of PHN activities).
The initial PHN training was conducted by a PsyD pre-doc student; this training included creating and setting boundaries, writing progress notes, maintaining confidentiality, developing active listening communication skills, understanding countertransference, and self-care. Thereafter, the Peer Health Navigators received semimonthly clinical supervision from a Ph.D.-level clinician. A HIV infectious disease physician served as the medical advisor. The medical advisor worked closely with the Peer Health Navigators by providing an annual training on HIV care and the interpretation of medical records, ongoing individualized interpretation of medical records, the review of hormone treatment profiles and ART choices as needed, and providing ongoing guidance in how to best coach participants to achieve and sustain HIV milestones.
Contingency Management
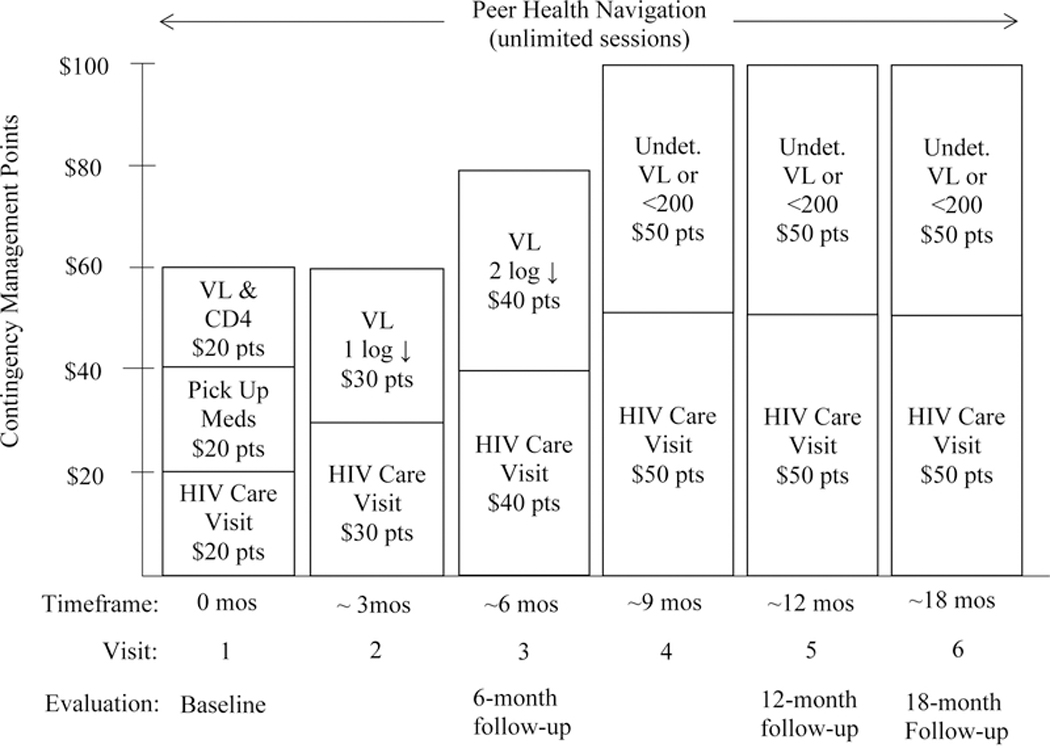
In The Alexis Project, a CM intervention was adapted and structured to increase linkage and retention of TWOC living with HIV into HIV care, and to sustain medication adherence to achieve and maintain virological suppression by specifically targeting: 1) HIV care visits; and, 2) HIV milestones. Increasingly valuable reinforcers were connected with HIV care visits and reaching and sustaining HIV milestones with the ultimate goal of reaching an undetectable viral load. The escalating reinforcement schedule of the CM intervention was structured to serve as a motivator for HIV care-seeking behavior, such that HIV milestones were expected to be achieved with regard to retention in regular HIV care visits, treatment, and medication adherence.
HIV Care Visits (Behavioral) CM Points: A participant received $20 in CM points for attending her first HIV care visit, another $20 in CM points for picking up her HIV medications, and another $20 in CM points for bringing her laboratory records to a Peer Health Navigator. Optimum HIV care visits were defined as quarterly, per Centers for Disease Control and Prevention (CDC) guidelines. Attendance at the second and third HIV care visit earned $30 and $40 in CM points, respectively; HIV care visits 4 through 6 each earned $50 in CM points.
HIV Milestones (Biomedical) CM Points: Assuming an appropriate treatment regimen (i.e., potent and to which the virus is susceptible), the trajectory of HIV RNA decline should be clear and continuous. Department of Health and Human Services guidelines note that virologic “failure” should be defined by HIV RNA >200 copies/mL measured twice after 24 weeks (6 months) on therapy, and once “undetectable” (below the limits of assay detection, variably 20–75 copies/mL), should remain undetectable. Following the baseline HIV care visit, participants received $30 in CM points for a 1-log viral load reduction at approximately 3 months, $40 in CM points for a 2-log viral load reduction at approximately 6 months, $50 in CM points for viral suppression (<200 copies/mL) at approximately 9 months, and $50 in CM points for maintaining virological suppression at approximately 12 and 18 months.
The maintenance of an undetectable viral load durably over time has been demonstrated to reduce morbidity and mortality associated with HIV infection [52]. Even when such parameters are carefully controlled, failure to adhere to regular HIV care clinic attendance has been associated with increased mortality [53]. Therefore, it was critical that the CM point schedule provided reinforcement for both HIV care clinic attendance and HIV milestones. Each CM point was equivalent to $1 in purchasing power. CM points were exchanged for any goods or services, of the participant’s choosing, that promoted a healthy, pro-social lifestyle such as gift cards to grocery stores or department stores, clothing, wigs and make-up, electrolysis and other skin care services or other gender-promoting services, and computer equipment. No cash was provided. Figure I illustrates the combined PHN and CM intervention.
Figure I: Combined Peer Health Navigation plus Contingency Management Intervention*.
*total possible = $500 in CM points
Measures
Core Assessment
The CASI core assessment gathered information on sociodemographic characteristics, educational attainment, income, and housing status.
Peer Health Navigation
A Peer Health Navigator met with each participant at the completion of the baseline assessment and, thus, each participant received a minimum of one PHN session. PHN sessions were unlimited; each participant received as few or as many sessions as needed throughout the 18-month intervention period, or 12-month intervention period for those who enrolled after May 2016. At the first session, Peer Health Navigators conducted an assessment of participants’ health care history (including past HIV care), unmet service needs and barriers to health care, developed a client-centered treatment plan, decided how they would correspond (text, email, phone), and answered any questions regarding the PHN intervention.
Contingency Management
CM target points were allocated for reaching and/or maintaining HIV behavioral and biomedical milestones. Behavioral CM target points were allocated for attending HIV care visits and biomedical CM target points were allocated for reaching and subsequently maintaining HIV milestones. Participants who achieved all of the behavioral and biomedical HIV health-promoting targets accumulated $500 in CM points. Both HIV care visits and HIV milestones were validated by confirmed laboratory results such as medical record extraction, and/or print-outs from service providers confirming medical outcomes; these data were never self-reported.
Statistical Analysis
Descriptive statistics were provided for participant sociodemographic characteristics, as well as intervention outcomes, with the specific metric chosen based on the distributional properties of the variable in question. Four separate inferential models estimated associations between attendance to PHN sessions and achievement of CM targets. Model 1 employed a partial proportional odds ordinal logistic regression, as a post hoc Brant test after a proportional odds ordinal logistic regression indicated differential effects across behavioral CM targets. Model 2 employed a proportional odds ordinal logistic regression, as the post hoc Brant test indicated consistent effects of PHN session attendance across biomedical CM targets. Model 3 combined the total number of behavioral and biomedical CM targets achieved per participant and regressed this count onto the number of PHN sessions completed using the negative binomial family, after a significant post hoc likelihood-ratio test indicated a superior fit relative to the Poisson alternative. Model 4 moved from examining “targets achieved” to “points earned,” and robustly regressed each participant’s total CM earnings onto the number of PHN sessions they achieved, via iteratively reweighted least squares regression. Across all four analytical Models, significant coefficient estimates related to attendance at PHN sessions indicate observed treatment effects related to intervention exposure. Model fit statistics were provided for all models. Where possible, significance tests defaulted to two-tailed, and results were flagged beginning at p ≤ 0.05.
RESULTS
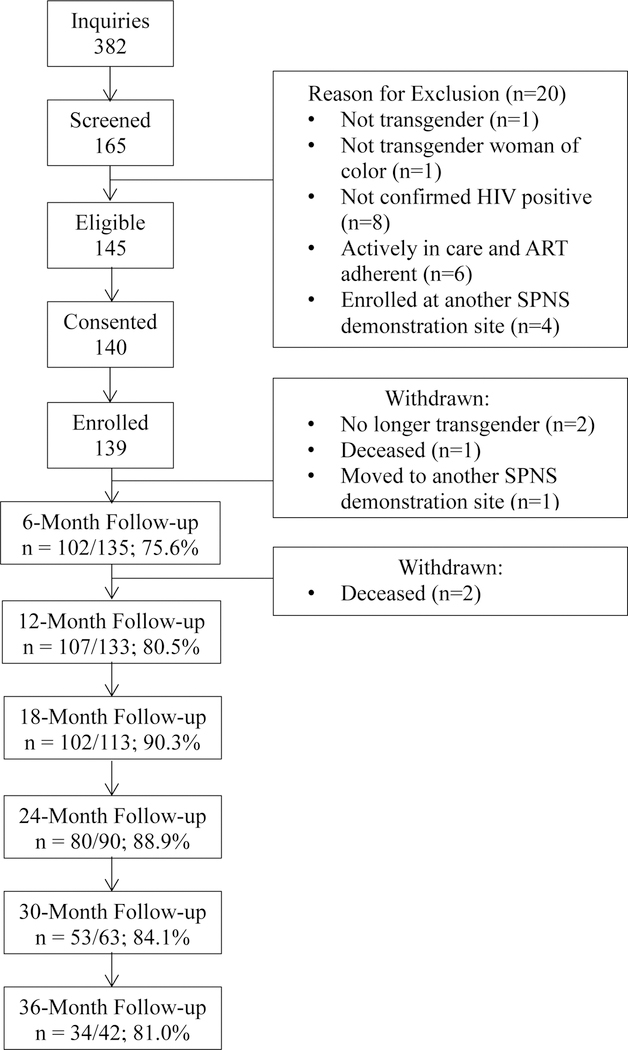
Figure II illustrates the progress and retention from initial screening through 36-month follow-up evaluations. A total of 165 potential participants screened, 140 provided informed consent, and 139 participants enrolled in The Alexis Project. Follow-up rates ranged from 76% at the 6-month follow-up point to 90% at the 18-month follow-up point, with a mean follow-up rate of 83%. Participants who enrolled toward the beginning of the study had a greater number of follow-up assessments than those enrolled later in the study.
Figure II:
Progress and Retention
As shown in Table I, most participants identified as either African American/Black (39%) or Hispanic/Latina (38%), as either heterosexual/straight (47%) or gay (23%), and 38% reported less than a high school diploma or GED. The age range was 19 through 59 years, with the mean age of 36.2 years (Standard Deviation [SD] = 9.7). Two-thirds of participants earned less than $3,000 a year, slightly more than a quarter (29%) indicated that sex work was a main source of their income, and nearly a third (32%) were currently experiencing homelessness or living in a homeless shelter. At baseline, 11 participants were unaware of their HIV positivity and, thus, the project’s new positivity rate was 7.9%. Time from enrollment to linkage to HIV care ranged from 0 to 467 days (median = 20 days; mean = 67 days; SD = 103 days).
Table I:
Participant Sociodemographics (N = 139)
| Mean (SD) or N (%) | ||
|---|---|---|
| Age | years | 36.2 (9.7) |
| range | 19–59 | |
| Racial/Ethnic Identity | ||
| Black/African American | 54 (38.9%) | |
| Hispanic/Latina | 53 (38.1%) | |
| Native American | 10 (7.2%) | |
| Asian/Pacific Islander | 3 (2.2%) | |
| Multiracial/Other | 19 (13.7%) | |
| Sexual Identity | ||
| Heterosexual/Straight | 65 (46.8%) | |
| Gay | 32 (23.0%) | |
| Bisexual | 8 (5.8%) | |
| Lesbian | 4 (2.9%) | |
| Other | 15 (10.8%) | |
| Refused | 15 (10.8%) | |
| Newly Diagnosed with HIV | 11 (7.9%) | |
| Educational Attainment | ||
| Grade 8 or Less | 12 (8.6%) | |
| Grades 9–11 | 41 (29.5%) | |
| HS Diploma or GED | 45 (32.4%) | |
| Some College | 36 (25.9%) | |
| Bachelor’s Degree | 2 (1.4%) | |
| Graduate Degree | 1 (0.7%) | |
| Refused | 2 (1.4%) | |
| Income (past year) | ||
| Less than $600 | 66 (47.5%) | |
| $600-$2,999 | 28 (20.1%) | |
| $3,000-$5,999 | 3 (2.2%) | |
| $6,000-$11,490 | 9 (6.5%) | |
| $11,491-$15,282 | 4 (2.9%) | |
| $15,283-$35,999 | 2 (1.4%) | |
| Refused | 27 (19.4%) | |
| Main Source(s) of Incomea | ||
| Employment | 16 (11.5%) | |
| Sex Work | 40 (28.8%) | |
| Food Stamps | 25 (18.0%) | |
| General Assistance/Relief | 34 (24.5%) | |
| Social Security/Disability | 30 (21.6%) | |
| Current Living Situation | ||
| Rent/Own | 38 (27.3%) | |
| Staying with Family/Friends | 15 (10.8%) | |
| Staying with Partner | 2 (1.4%) | |
| Hotel/Motel/SRO | 10 (7.2%) | |
| Homeless/Homeless Shelter | 44 (31.7%) | |
| Hospital/Jail/Sober Living | 5 (3.6%) | |
| Other/Don’t Know | 22 (15.8%) | |
| Refused | 3 (2.2%) | |
Includes all responses endorsed by at least 10% of participants; responses are not mutually exclusive.
Table II provides summary outcomes for the PHN and CM components of the intervention. On average, The Alexis Project participants attended 6.6 PHN sessions (SD = 6.5; Median = 4; IQR = 2; 9), for a total of 919 sessions over the course of the 18-month intervention. The Alexis Project participants also achieved 648 verified CM targets and earned a total of $19,960 CM points (Mean = $143.60, Median = $90.00; Mean = 4.7 targets, Median = 4 targets). A majority (85%) of The Alexis Project participants attended the first HIV care visit, 71% received ART medications, 69% received viral load and CD4 tests, and 57% returned for a second HIV care visit. Based on the CM reward schedule, more than a third (37%) of all participants reduced their viral load by a minimum of one full log, a quarter (27%) reduced their viral load by a minimum of two full logs, and one-in-seven (14%) escalated through the entire CM schedule to achieve undetectable status. It is important to note, however, that participants were always incentivized in the escalating manner prescribed in the CM schedule, even if biological outcomes outpaced CM milestones. Thus, participants who enrolled undetectable or reached undetectable more quickly than anticipated still progressed at pace through the “≤ 1 log reduction” and “≤ 2 log reduction” incentives prior to reaching the “undetectable” incentives. A detailed tally of biologic outcomes follows: Over the course of the intervention, 25 of the 104 participants who enrolled with a detectable viral load were confirmed to have reached and/or maintained full virological suppression (i.e., VL ≤ 200 copies/mL), and 21 of the 35 participants who enrolled undetectable provided evidence of having maintained their undetectable status during the intervention, and an additional 14 of the 35 participants who enrolled undetectable did not provide further viral load lab results. Post-hoc Chi-square and Fisher’s Exact analyses revealed that participants who did not achieve an undetectable viral load were not significantly different in terms of their sociodemographics (i.e., gender identity, racial/ethnic identity, sexual identity, age, educational attainment, housing status, or income) from participants who achieved and/or maintained viral suppression.
Table II:
Peer Health Navigator and Contingency Management Outcomes (N = 139)
|
Peer Health Navigator Sessions Attended
(per participant) |
Mean = 6.6; Median = 4 |
SD = 6.5 | Min. = 1 | Max. = 31 | |||||
| Peer Health Navigation Totals | 919 Sessions Attended Interquartile Range = 2 thru 9 | ||||||||
|
Contingency Management Points Earned
(per participant) |
Mean = $143.60; Median = $90.00 |
SD = $139.63 | Min.= $0.00 | Max. = $500.00 | |||||
| Contingency Management Behavioral Targets | |||||||||
| Target |
1st
HIV Care Visit |
Received ART
Medication |
Returned for
VL/CD4 |
2nd
HIV Care Visit |
3rd
HIV Care Visit |
4th
HIV Care Visit |
5th
HIV Care Visit |
6th
HIV Care Visit |
TOTALS |
| Achieved | 118 (84.9%) | 98 (70.5%) | 96 (69.1%) | 79 (56.8%) | 59 (42.5%) | 40 (28.8%) | 29 (20.9%) | 16 (11.5%) | 525 Targets Achieved |
| Reward | $20 pts | $20 pts | $20 pts | $30 pts | $40 pts | $50 pts | $50 pts | $50 pts | $280 Pts Possible |
| Total | $2,360 pts | $1,960 pts | $1,920 pts | $2,370 pts | $2,360 pts | $2,000 pts | $1,450 pts | $800 pts | $15,220 Pts Earned |
| Contingency Management Biomedical Targets | |||||||||
| Target |
Reduce VL
≤ 1 Log |
Reduce VL
≤ 2 Logs |
Undetectable
VL (1) |
Undetectable VL
(2) |
Undetectable
VL (3) |
||||
| Achieved | 52 (37.4%) | 37 (26.6%) | 19 (13.7%) | 12 (8.6%) | 3 (2.2%) | 123 Achieved | |||
| Reward | $30 pts | $40 pts | $50 pts | $50 pts | $50 pts | $220 Pts Possible | |||
| Total | $1,560 pts | $1,480 pts | $950 pts | $600 pts | $150 pts | $4,740 Pts Earned | |||
| Contingency Management Totals | 648 Targets Achieved Mean = 4.7 targets (Median = 4; SD = 3.7) Total = $19,960 Pts Earned | ||||||||
12 participants were not eligible to reach their 6th HIV care visit, due to the late date of their enrollment
Table III provides associations between attendance to PHN sessions and achievement of CM outcomes. Model 1 demonstrated that increased attendance to PHN sessions was associated with increased probability of achieving all behavioral CM targets except attendance to the 6th HIV care visit as participants who enrolled after May 2016 were ineligible to receive. Stronger effect estimates were observed for earliest behavioral targets, with effects waning in magnitude for distal targets. Model 2 demonstrated that attendance to PHN sessions was associated with significantly increased probability of achieving all biomedical CM targets, though estimated effects are equivalent in magnitude to the smallest effects observed in the behavioral targets model. Model 3 indicated that regardless of target, attendance to PHN sessions was associated with increased rate of HIV milestone achievements. Model 4 indicated that each PHN session attended was associated with an estimated increase of $8.52 in expected CM point earnings. All models demonstrated a good fit to the data (all p ≤ 0.0001).
Table III:
Associations between Attendance to Peer Health Navigator Sessions and Achievement of Contingency Management Targets (N = 139)
| Coef. (95% CI) | Coef. (95% CI) | Coef. (95% CI) | Coef. (95% CI) | Coef. (95% CI) | Coef. (95% CI) | Coef. (95% CI) | Coef. (95% CI) | |
|---|---|---|---|---|---|---|---|---|
| Model 1-- Behavioral Targets (Ordinal Logistic Regression; proportional odds assumption not met) | ||||||||
|
1st
HIV Care Visit |
Received ART Medication |
Returned for
VL/CD4 Lab Work |
2nd
HIV Care Visit |
3rd
HIV Care Visit |
4th
HIV Care Visit |
5th
HIV Care Visit |
6th
HIV Care Visita |
|
| PHN Sessions Attended | 0.38**
(0.09–0.67) |
0.43***
(0.24–0.62) |
0.28***
(0.14–0.41) |
0.20***
(0.11–0.29) |
0.13***
(0.07–0.20) |
0.12***
(0.06–0.18) |
0.10***
(0.04–0.16) |
0.03 (−0.06–0.11) |
| Model 2-- Biomedical Targets (Ordinal Logistic Regression; proportional odds assumption met) | ||||||||
|
Reduced VL
≤ 1 Log |
Reduced VL
≤ 2 Logs |
Undetectable
VL (1) |
Undetectable
VL (2) |
Undetectable
VL (3) |
||||
| PHN Sessions Attended | 0.10***
(0.05–0.14) |
0.10***
(0.05–0.14) |
0.10***
(0.05–0.14) |
0.10***
(0.05–0.14) |
0.10***
(0.05–0.14) |
|||
| Model 3-- Behavioral and Biomedical Targets (Negative Binomial Regression) | ||||||||
| # Targets Achieved | ||||||||
| PHN Sessions Attended | 0.05***
(0.03–0.07) |
|||||||
| Model 4-- Contingency Management Payouts (Robust Ordinary Least Squares Regression) | ||||||||
| Points Earned ($) | ||||||||
| PHN Sessions Attended | $8.52***
($5.60-$11.44) |
|||||||
12 participants were not eligible to reach their 6th HIV care visit, due to the late date of their enrollment
p ≤ 0.01
p ≤ 0.001
Model 1: χ2(8) = 53.31; Model 2: χ2(1) =16.71; Model 3: χ2(1) =21.23; Model 4: F(1,137) = 33.30 (all p < 0.0001)
DISCUSSION
The Alexis Project targeted TWOC living with HIV who were currently not in HIV care, or had not seen a HIV medical provider in the previous six months, or had not been prescribed ART, or had been prescribed ART but had not always been adherent. The sample, which was recruited from a large urban west coast city, proved to be highly impacted by several structural and individual barriers. Fully 76% reported living below the 2017 annual Federal poverty level ($12,060/yr). Only 12% reported being legally employed, 29% reported sex work as a main source of income, and 43% reported experiencing homelessness or unstable housing (hotel/motel/SRO: 7%; homeless/shelter: 32%; hospital/jail/sober living: 4%). Given the barriers that impacted the participants’ daily lives, a HIV care intervention designed specifically for this population had to be intense enough to overcome initial obstacles (PHN) as well as potent enough to motivate behavioral and biomedical outcomes (CM).
In this sample of TWOC living with HIV, increased exposure to the combined PHN plus CM intervention designed for The Alexis Project was associated with increased linkage to and retention in HIV care, and the sustainment of medication adherence to the achievement and maintenance of virological suppression. The study progress and retention rates demonstrated that The Alexis Project was both feasible and acceptable among the participants, and ultimately proved successful in reaching important HIV milestones associated with advancement along the HIV Care Continuum. Furthermore, the remarkably high new positivity rate (8%) among the participants demonstrated the project’s ability to recruit and enroll the desired target population.
Engagement and retention in The Alexis Project was high (88% of enrolled participants attended at least two PHN sessions, and the average number of PHN sessions attended was 6.6 sessions), thus demonstrating the feasibility of conducting a PHN intervention with TWOC living with HIV. The high rate of acceptability of the PHN intervention may be attributed to the trust and rapport effectively built and maintained between participants and the Peer Health Navigators. Given that The Alexis Project employed “true” peers as Peer Health Navigators (i.e., TWOC living with HIV), it was likely that participants felt safe to honestly discuss their barriers to linkage and retention in HIV care and medication adherence. Peer Health Navigators who have successfully navigated the HIV healthcare system and had maintained their own HIV care are in the unique position to serve as intermediaries between the participant and a seemingly overwhelming healthcare system. Furthermore, previous studies with trans women living with HIV have demonstrated that gender-confirming health care has been critical to successful engagement and retention in HIV care [50, 54], therefore, cultural competency trainings and booster sessions were provided at all clinics.
PHN dosage was critical, as increased PHN sessions was significantly positively associated with increased probability of achieving all but one of the behavioral CM targets (attendance at the 6th HIV care visit, of which 6.5% [9/139] of the sample enrolled too late to receive), including linkage to care, picking up ART medications, and receiving lab results. Participants may have attended an increased number of PHN sessions and successfully linked to and retained in HIV care because PHN sessions offered both instrumental support (e.g., transportation to and from medical appointments, assistance with obtaining a photo ID) and social support, both of which have been found to be of importance when addressing barriers to engagement and retention in HIV care among trans women [54]. However, it is also important to note that the Peer Health Navigators provided intensive and specific instrumental support beyond the warm hand-off to a service that is typically conducted as part of most interventions. For example, the Peer Health Navigators often sat with participants for several hours at a Department of Motor Vehicles office to secure a government-issued identification card or, if requested, were present in an examination room during a HIV care visit to enhance communication between the participant and her medical provider. Peer Health Navigators met with each participant as often as the participant deemed necessary, even if just to hear about her day, which provided social support without limitations on time or content. While previous studies have identified PHN as an important method for engaging and retaining trans women [55], and specifically TWOC in HIV care [54], The Alexis Project indicated the type, quality, and quantity of navigation necessary to assist with successful linkage to and continued retention in HIV care for this particular population.
PHN session attendance was associated with a significantly increased probability of achieving all biomedical CM targets including a one- and two-log reduction in viral load and reaching and sustaining an undetectable viral load. Furthermore, each PHN visit attended was associated with an $8.52 increase in expected CM point earnings. The proportional odds assumption was not violated for the analyses associated with these findings implying that The Alexis Project worked equally well for each of the biomedical outcomes associated with advancement through the HIV Care Continuum (i.e., one- and two-log reductions; reaching and sustaining an undetectable viral load). While this is extremely encouraging, the magnitude of the observed effects was much smaller than those observed in the behavioral outcomes implying the results are indeed reliable, but modest in size. Ultimately, it appears that while a combined PHN plus CM intervention like The Alexis Project is effective at promoting proximal behavioral and biomedical outcomes, it is most effective at affecting near-term behavioral outcomes (i.e., linkage to care, picking up HIV medications, attending the first few HIV care appointments). However, prolonged behavioral changes (i.e., forming a strong and steady relationship with a HIV care provider as part of long-term retention in care; taking HIV medications consistently over time resulting in long-term adherence) were less likely to be impacted. Perhaps this is because HIV care appointments can be rescheduled, but a missed dose of ART cannot.
Over the course of the intervention, 25 of the 104 (24%) participants who enrolled with a detectable viral load achieved full viral suppression; some outpaced the CM schedule and, thus, are not fully represented in Table II. The observed likelihood of confirmed maintenance of viral suppression was 60%, while observed likelihood of confirmed achievement of full viral suppression was 24%. Interestingly, a full 83% of the participants who enrolled detectable and achieved the minimum 1 log viral load reduction target went on to achieve full viral suppression. This implies that among TWOC, establishing initial ART adherence is a larger obstacle than the subsequent task of maintaining sufficient adherence to become virally suppressed. One quarter of The Alexis Project sample (n = 35) enrolled with an undetectable viral load; among these participants 40% (n = 14) did not engage with the biomedical component of the CM intervention (i.e., did not return any further viral load lab results for confirmation of undetectable status), all others (60%; n = 21) returned with subsequent lab work confirming maintenance of undetectable status (with the exception of one participant whose treatment-resistant strain of HIV occasionally evidenced a detectable VL count near the threshold of undetectable). The Alexis Project participants represented many TWOC already struggling to initiate or maintain proper HIV care and, thus, interventions able to assist any proportion of such highly impacted individuals in advancing through the HIV Care Continuum could have critical impact on their expected health outcomes.
While The Alexis Project demonstrated notable increases in linkage to and retention in care, medication adherence, and reaching and sustaining an undetectable viral load, this was not a randomized controlled trial (RCT). Thus, without a control group it was not possible to establish what unique proportion of the variance in outcomes in regards to linkage to and retention in HIV medical care and reaching and sustaining an undetectable viral load could be attributed to each of the interventions (PHN versus CM). However, it has been well-established that trans women experience numerous obstacles when accessing and retaining in HIV care; thus, it seems theoretically probable that PHN, which addresses the key issues of instrumental and social support previously identified in the literature as impeding linkage to and retention in HIV care, could prove an effective intervention for this population. In addition, the participants in The Alexis Project attended, on average, over six PHN sessions, and none of these were incentivized. Participants voluntarily attended PHN sessions, and attendance at more sessions led to better outcomes clearly demonstrating that PHN appears to be a promising intervention for linking and retaining TWOC in HIV care.
Furthermore, given that CM interventions have proven efficacious in promoting a number of healthy behaviors it was reasonable to suggest that CM could work within the context of HIV care. In fact, many of the CM milestones were reached by a sizable proportion of the participants, thus indicating that CM may have been a motivating factor for participants to achieve both behavioral and biomedical targets.
Limitations
As previously noted, a significant limitation of this study was that it used a non-random study design without a control group. Without a RCT design, it was impossible to determine if and to what extent either the PHN or CM component of the combined intervention was independently responsible for participants’ positive outcomes. Only joint effects could be determined; partial effects were not derivable. Furthermore, both interventions have implementation limitations: PHN can be time-consuming and, thus, requires staff whose sole responsibility is to deliver PHN sessions; and, CM interventions are generally more expensive to conduct than many other types of behavioral interventions. The time required to deliver PHN and the cost associated with CM [56] may limit the scalability of such a combined intervention. The sample also included limitations in that it was comprised of highly marginalized TWOC living with HIV and, thus, findings may not be generalizable to trans women more broadly. Data was also self-reported, which may include errors in recall, deliberate falsification, and/or intoxication during the data collection process [57]. Finally, it is important to note that there were many factors not measured as part of the evaluation of The Alexis Project that could have influenced outcomes such as a participant’s readiness for change, risk acuity, and/or other unrecognized barriers to attendance. However, for those participants who had the motivation and ability to attend PHN sessions, The Alexis Project was associated with significant beneficial biomedical and behavioral effects.
Conclusions
Despite its limitations and the fact that intervention participants were a more heavily impacted group of TWOC, the intervention was associated with positive effects on both behavioral and biomedical outcomes associated with the HIV Care Continuum. However, The Alexis Project was most successful in addressing the more proximal behavioral outcomes such as linking to care, retaining in care for the first five HIV medical visits, obtaining viral load and CD4 lab work, and picking up HIV medications. While The Alexis Project also assisted participants in reaching and sustaining the HIV milestones associated with biomedical outcomes, such as reducing viral load and reaching and sustaining virological suppression, the results were less strong than the behavioral outcomes. Thus, future interventions targeting longer-term biomedical outcomes for TWOC are needed.
Due to the significant structural and individual barriers experienced by TWOC, interventions must be intensive, creative, and address the wide range of critical, unmet needs experienced by this population. Peer-delivered PHN in concert with CM provided the intensity, referrals, and resources required to help this extremely vulnerable population achieve HIV milestones associated with successful advancement along the HIV Care Continuum.
ACKNOWLEDGMENTS
This project was supported by the Health Resources and Services Administration (HRSA) of the U.S. Department of Health and Human Services (HHS) under grant number H97HA24968 in the last annual award amount of $285,757 awarded to Friends Research Institute (PI: C. Reback). No percentage of this project was financed with non-governmental sources. This information or content and conclusions are those of the authors and should not be construed as the official position or policy of, nor should any endorsements be inferred by HRSA, HHS or the U.S. Government. Dr. Reback acknowledges additional support from the National Institute of Mental Health (P30 MH58107). The authors would like to thank the Peer Health Navigators, Angelina Alamilla and Miranda Ramirez, for their sincere commitment to improve the health outcomes of The Alexis Project participants.
Footnotes
COMPLIANCE WITH ETHICAL STANDARDS
Conflict of Interest: The authors declare that they have no conflict of interest.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent: Informed consent was obtained from all individual participants included in the study.
The Alexis Project was named after Alexis Rivera who died on March 28, 2012, at the age of 34, from complications related to HIV. Alexis was a proud Latina trans woman, a community activist, a peer advocate, and a gatekeeper.
REFERENCES
- 1.Poteat T, Reisner SL, Radix A. HIV epidemics among transgender women. Curr Opin HIV AIDS. 2014;9(2):168–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herbst JH, Jacobs ED, Finlayson TJ, McKleroy VS, Neumann MS, Crepaz N. Estimating HIV prevalence and risk behaviors of transgender persons in the United States: a systematic review. AIDS Behav. 2008. January;12(1):1–17. [DOI] [PubMed] [Google Scholar]
- 3.Operario D, Nemoto T, Iwamoto M, Moore T. Risk for HIV and unprotected sexual behavior in male primary partners of transgender women. Arch Sex Behav. 2011;40(6):1255–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sevelius JM, Keatley J, Gutierrez-Mock L. HIV/AIDS programming in the United States: considerations affecting transgender women and girls. Women’s Health Issues. 2011;21(6):S278–S82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. HIV Among Transgender People in the United States:−−2013 November 2013. [Google Scholar]
- 6.Institute of Medicine. The Health of Lesbian, Gay, Bisexual, and Transgender People: Building a Foundation for Better Understanding. Washington, D.C.: National Institute of Health; 2011. [PubMed] [Google Scholar]
- 7.Santos G-M, Wilson EC, Rapues J, Macias O, Packer T, Raymond HF. HIV treatment cascade among transgender women in a San Francisco respondent driven sampling study. Sex Transm Infect. 2014. April 8, 2014. [DOI] [PubMed] [Google Scholar]
- 8.Radix A, Sevelius J, Deutsch MB. Transgender women, hormonal therapy and HIV treatment: a comprehensive review of the literature and recommendations for best practices. J Int AIDS Soc 2016;19(3Suppl 2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalichman SC, Hernandez D, Finneran S, Price D, Driver R. Transgender women and HIV-related health disparities: falling off the HIV treatment cascade. Sex Health. 2017;14(5):469–76. [DOI] [PubMed] [Google Scholar]
- 10.Doshi RK, Milberg J, Isenberg D, Matthews T, Malitz F, Matosky M, et al. High rates of retention and viral suppression in the US HIV safety net system: HIV care continuum in the Ryan White HIV/AIDS Program, 2011. Clin Infect Dis. 2014;60(1):117–25. [DOI] [PubMed] [Google Scholar]
- 11.Mizuno Y, Frazier EL, Huang P, Skarbinski J. Characteristics of transgender women living with HIV receiving medical care in the United States. LGBT Health. 2015;2(3):228–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melendez RM, Exner TA, Ehrhardt AA, Dodge B, Remien RH, Rotheram-Borus M-J, et al. Health and health care among male-to-female transgender persons who are HIV positive. Am J Public Health. 2006. 2006/June/01;96(6):1034–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sevelius JM, Carrico A, Johnson MO. Antiretroviral therapy adherence among transgender women living with HIV. J Assoc Nurses AIDS care. 2010;21(3):256–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baguso GN, Gay CL, Lee KA. Medication adherence among transgender women living with HIV. AIDS care. 2016;28(8):976–981. doi: 10.1080/09540121.2016.1146401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lombardi E Varieties of transgender/transsexual lives and their relationship with transphobia. J Homosex 2009. 2009/October/30;56(8):977–92. [DOI] [PubMed] [Google Scholar]
- 16.de Vries KM. Transgender people of color at the center: conceptualizing a new intersectional model. Ethnicities. 2015;15(1):3–27. [Google Scholar]
- 17.Reback CJ, Larkins S. HIV risk behaviors among a sample of heterosexually identified men who occasionally have sex with another male and/or a transwoman. J Sex Res. 2011:1–13. [DOI] [PubMed] [Google Scholar]
- 18.De Santis JP. HIV infection risk factors among male-to-female transgender persons: a review of the literature. J Assoc Nurses AIDS care. 2009;20(5):362–72. [DOI] [PubMed] [Google Scholar]
- 19.Norton AT, Herek GM. Heterosexuals’ attitudes toward transgender people: Findings from a national probability sample of US adults. Sex Roles. 2013;68(11–12):738–53. [Google Scholar]
- 20.Bockting WO, Robinson BE, Rosser BR. Transgender HIV prevention: a qualitative needs assessment. AIDS Care. 1998;10:505–25. [DOI] [PubMed] [Google Scholar]
- 21.Nemoto T, Sausa LA, Operario D, Keatley J. Need for HIV/AIDS Education and Intervention for MTF Transgenders. J Homosex. 2006. 2006/September/25;51(1):183–201. [DOI] [PubMed] [Google Scholar]
- 22.Grant JM, Mottet LA, Tanis J, Harrison J, Herman JL, Keisling M. Injustice at Every Turn: A Report of the National Transgender Discrimination Survey. Washington, D.C.: National Center for Transgender Equality, and the National Gay and Lesbian Task Force; 2011. [Google Scholar]
- 23.Poteat T, German D, Kerrigan D. Managing uncertainty: A grounded theory of stigma in transgender health care encounters. Soc Sci Med. 2013;84(0):22–9. [DOI] [PubMed] [Google Scholar]
- 24.Bradford J, Reisner SL, Honnold JA, Xavier J. Experiences of transgender-related discrimination and implications for health: results from the Virginia Transgender Health Initiative Study. Am J Public Health. 2013;103(10):1820–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grossman AH, D’Augelli AR. Transgender youth: Invisible and vulnerable. J Homosex. 2006;51(1):111–28. [DOI] [PubMed] [Google Scholar]
- 26.Feldman J, Bockting W. Transgender health. Minn Med. 2003;86(7):25–32. [PubMed] [Google Scholar]
- 27.Reback CJ, Clark K, Holloway IW, Fletcher JB. Health Disparities, Risk Behaviors and Healthcare Utilization among Transgender Women in Los Angeles County: A Comparison from 1998–1999 to 2015–2016. AIDS Behav. 2018;In Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hede K Agencies look to patient navigators to reduce cancer care disparities. J Natl Cancer Inst. 2006;98(3):157–9. [DOI] [PubMed] [Google Scholar]
- 29.Vargas RB, Cunningham WE. Evolving trends in medical care-coordination for patients with HIV and AIDS. Curr HIV/AIDS Rep. 2006;3(4):149–53. [DOI] [PubMed] [Google Scholar]
- 30.Bandura A. Social cognitive theory and exercise of control over HIV infection. Preventing AIDS: Springer; 1994. p. 25–59. [Google Scholar]
- 31.Bandura A Social cognitive theory: An agentic perspective. Annu Rev Psychol. 2001;52(1):1–26. [DOI] [PubMed] [Google Scholar]
- 32.Safren SA, Traeger L, Skeer MR, O’Cleirigh C, Meade CS, Covahey C, et al. Testing a social-cognitive model of HIV transmission risk behaviors in HIV-infected MSM with and without depression. Health Psychol. 2010;29(2):215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sugano E, Nemoto T, Operario D. The Impact of Exposure to Transphobia on HIV Risk Behavior in a Sample of Transgendered Women of Color in San Francisco. AIDS Behav. 2006;10(2):217–25. [DOI] [PubMed] [Google Scholar]
- 34.De Walque D, Dow WH, Nathan R, Abdul R, Abilahi F, Gong E, et al. Incentivising safe sex: a randomised trial of conditional cash transfers for HIV and sexually transmitted infection prevention in rural Tanzania. BMJ open. 2012;2(1):e000747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loewenstein G, Volpp KG, Asch DA. Incentives in health: different prescriptions for physicians and patients. JAMA. 2012;307(13):1375–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baird SJ, Garfein RS, McIntosh CT, Özler B. Effect of a cash transfer programme for schooling on prevalence of HIV and herpes simplex type 2 in Malawi: a cluster randomised trial. Lancet. 2012;379(9823):1320–9. [DOI] [PubMed] [Google Scholar]
- 37.Sorensen JL, Haug NA, Delucchi KL, Gruber V, Kletter E, Batki SL, et al. Voucher reinforcement improves medication adherence in HIV-positive methadone patients: a randomized trial. Drug Alcohol Depend. 2007;88(1):54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Volpp KG, Troxel AB, Pauly MV, Glick HA, Puig A, Asch DA, et al. A randomized, controlled trial of financial incentives for smoking cessation. N Engld J Med. 2009;360(7):699–709. [DOI] [PubMed] [Google Scholar]
- 39.John LK, Loewenstein G, Volpp KG. Empirical observations on longer-term use of incentives for weight loss. Prev Med. 2012;55:S68–S74. [DOI] [PubMed] [Google Scholar]
- 40.Volpp KG, Loewenstein G, Troxel AB, Doshi J, Price M, Laskin M, et al. A test of financial incentives to improve warfarin adherence. BMC Health Serv Res. 2008;8(1):272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marcus AC, Kaplan CP, Crane LA, Berek JS, Bernstein G, Gunning JE, et al. Reducing loss-to-follow-up among women with abnormal Pap smears: results from a randomized trial testing an intensive follow-up protocol and economic incentives. Med Care. 1998;36(3):397–410. [DOI] [PubMed] [Google Scholar]
- 42.Reback CJ, Peck JA, Dierst-Davies R, Nuno M, Kamien JB, Amass L. Contingency Management Among Homeless, Out-of-Treatment Men who have Sex with Men. J Sub Abuse Treat. 2010;39:255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shoptaw S, Klausner JD, Reback CJ, Tierney S, Stansell J, Hare CB, et al. A public health response to the methamphetamine epidemic: the implementation of contingency management to treat methamphetamine dependence. BMC Public Health. 2006;6:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reback CJ, Shoptaw S, Peck JA, Larkins S, Freese TE, Rawson RA Getting Off: A Behavioral Treatment Intervention for Gay and Bisexual Male Methamphetamine Users. Los Angeles, CA: Friends Research Institute, Inc.; 2005. [Google Scholar]
- 45.Seal KH, Kral AH, Lorvick J, McNees A, Gee L, Edlin BR. A randomized controlled trial of monetary incentives vs. outreach to enhance adherence to the hepatitis B vaccine series among injection drug users. Drug Alcohol Depend. 2003;71(2):127–31. [DOI] [PubMed] [Google Scholar]
- 46.Malotte CK, Hollingshead JR, Larro M. Incentives vs outreach workers for latent tuberculosis treatment in drug users. Am J Prev Med. 2001;20(2):103–7. [DOI] [PubMed] [Google Scholar]
- 47.Silverman K, Wong CJ, Needham M, Diemer KN, Knealing T, Crone‐Todd D, et al. A randomized trial of employment‐based reinforcement of cocaine abstinence in injection drug users. J Appl Behav Anal. 2007;40(3):387–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kirby KC, Carpenedo CM, Stitzer ML, Dugosh KL, Petry NM, Roll JM, et al. Is exposure to an effective contingency management intervention associated with more positive provider beliefs? J Subst Abuse Treat. 2012;42(4):356–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Riccio J, Dechausay N, Greenberg D, Miller C, Rucks Z, Verma N. Toward reduced poverty across generations: Early findings from New York City’s conditional cash transfer program. 2010. [Google Scholar]
- 50.Reback CJ, Ferlito D, Kisler KA, Fletcher JB. Recruiting, linking, and retaining high-risk transgender women into HIV prevention and care services: an overview of barriers, strategies, and lessons learned. Int J Transgend. 2015;16(4):209–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG, Research electronic data capture (REDCap) – A metadata-driven methodology and workflow process for providing translational research informatics support, J Biomed Inform. 2009. April;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.May MT, Costagliola D, Sabin CA, Phillips AN, Justice AC, Dabis F, et al. HIV treatment response and prognosis in Europe and North America in the first decade of highly active antiretroviral therapy: A collaborative analysis. Lancet. 2006;368(9534):451–8. [DOI] [PubMed] [Google Scholar]
- 53.Brennan AT, Maskew M, Sanne I, Fox MP. The importance of clinic attendance in the first six months on antiretroviral treatment: a retrospective analysis at a large public sector HIV clinic in South Africa. J Int AIDS Soc. 2010;13(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sevelius J, Patouhas E, Keatley J, Johnson M. Barriers and Facilitators to Engagement and Retention in Care among Transgender Women Living with Human Immunodeficiency Virus. Ann Behav Med. 2014. 2014/February/01;47(1):5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reisner SL, Radix A, Deutsch MB. Integrated and gender-affirming transgender clinical care and research. J Acquir Immune Defic Syndr (1999). 2016;72(Suppl 3):S235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sindelar J, Elbel B, & Petry NM (2007). What do we get for our money? Cost‐effectiveness of adding contingency management. Addiction, 102(2), 309–316. [DOI] [PubMed] [Google Scholar]
- 57.Jaccard J, McDonald R, Wan CK, Guilamo-Ramos V, Dittus P, Quinlan S. Recalling sexual partners: the accuracy of self-reports. J Health Psychol. 2004;9(6):699–712. [DOI] [PubMed] [Google Scholar]