Abstract
In this study, the effects of methyl jasmonate (MeJA) on the phytomass and triterpenoid production of diploid and tetraploid Centella asiatica hairy roots were investigated. Hairy root cultures were obtained from diploid and induced tetraploid plants of C. asiatica infected by Agrobacterium rhizogenes strain ATCC 43057. MeJA triggered triterpenoid production in both ploidy hairy roots, whereas triterpenoids were not produced in the untreated hairy roots. Among the treatments, the 50 µM MeJA treatment yielded the maximum triterpenoid production in diploid hairy roots of 27.25 ± 0.27 µg/mg Dry weight (DW) total triterpenoid at day 21. For the tetraploid hairy root cultures, the 28th-day hairy root culture produced a maximum amount of triterpenoids of 16.29 ± 6.32 µg/mg DW in response to the 50 µM MeJA treatment, whereas the 100 µM MeJA treatment produced a similar triterpenoid amount (16.31 ± 9.24 µg/mg DW) at day 14. Moreover, in response to 50 µM MeJA, we obtained different ratios of aglycone to glycoside, i.e., 1:7 and 1:2, between the diploid and tetraploid hairy root cultures. Asiaticoside was the dominant phytochemical, followed by asiatic acid and madecassic acid. This study provides valuable information for producing triterpenoids for C. asiatica commercial products and preparations by using hairy root cultures.
Subject terms: Plant biotechnology, Secondary metabolism
Introduction
Centella asiatica (L.) Urb. (Apiaceae) is a well-known medicinal plant in tropical and subtropical regions. The plant is a main ingredient of Ayurvedic preparations (India), traditional Chinese medicine (China), and commercial drugs in the European market1,2. Madecassoside, asiaticoside, madecassic acid and asiatic acid are the principle phytochemicals of C. asiatica and possess pharmacological activities such as wound healing3,4, memory improvement, cognition and mood modulation5. Although the demand for C. asiatica is increasing, conventional cultivation cannot guarantee phytomass and phytochemical production6,7. The triterpenoid amount of C. asiatica cultures varies among seasons, environmental conditions, cultivation regions and genotypes1,8.
In vitro hairy root culture, a disease caused by Agrobacterium rhizogenes infection, is an innovative platform for phytochemical production and a sustainable and economically feasible alternative to propagated plants9. The application of the elicitor, i.e., Methyl jasmonate (MeJA), is considered effective for enhancing terpenoid production in different plant cell, tissue and organ cultures, such as Panax ginseng cell culture10, P. ginseng hairy root culture11, Glycyrrhiza glabra cell culture12, and C. asiatica whole plant culture13,14. In addition, several studies have reported high phytomass and phytochemical production by tetraploid medicinal plants relative to the levels in normal diploid plants, such as Papaver somniferum15, Artemisia annua16 and Salvia miltiorrhiza17. Higher phytomass and triterpenoid production from tetraploid greenhouse-grown and field-grown C. asiatica plants have been reported18,19. Therefore, the aims of the present study were (1) to establish an efficient hairy root induction protocol for diploid and tetraploid C. asiatica and (2) to study the effects of MeJA on the phytomass and triterpenoid production of diploid and tetraploid C. asiatica hairy roots.
Materials and Methods
Plant materials
Diploid and tetraploid C. asiatica plantlets were obtained from a previous study by Kaensaksiri et al.18. The plantlets were subcultured at 30-day intervals in semisolid Murashige and Skoog media (MS media)20 supplemented with 3.0% sucrose and containing 5.5 g/l Agargel®, with a pH of 5.8. The plantlets were cultured at 25 °C under a 16 h light/8 h dark photoperiod. The 24-day-old in vitro diploid and tetraploid C. asiatica plantlets were separated into petioles and upper and lower parts of leaves (Fig. 1).
Figure 1.
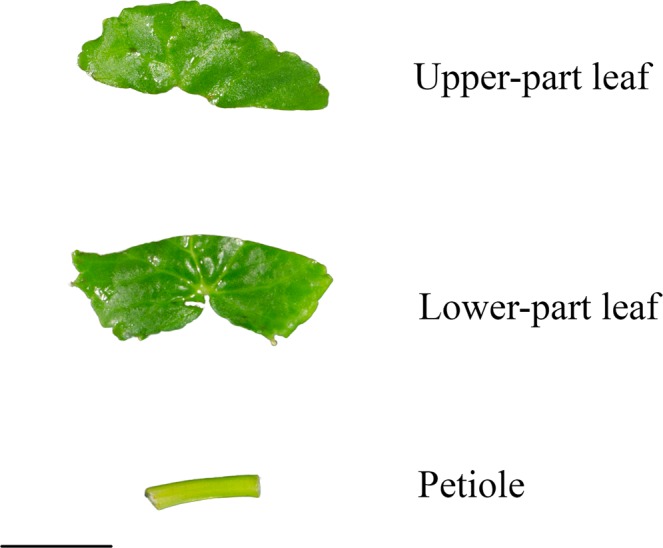
Explant types of C. asiatica for A. rhizogenes infection. Bar = 1.0 cm. (a) upper-part leaf explant, (b) lower-part leaf explant, (c) petiole explant.
Hairy root culture induction
The hairy root induction of C. asiatica followed the protocol of Kim et al.21 with some modifications. One loop of each of the A. rhizogenes strains ATCC 43057 and ATCC 15834 (ATCC-USA) was cultured separately in 20 ml Yeast mannitol broth (YMB) medium for 3 days under a 16 h light/8 h dark photoperiod at 25 °C and agitated at 110 rpm. Three-day-old bacterial suspension cultures were used for infection.
The explants were immersed in the bacterial suspension for 40 min, placed in half-strength semisolid MS media supplemented with 50 µM acetosyringone and cultured at 25 °C in darkness. The coculture period spanned 3 days and was followed by decontamination of the bacteria with 500 mg/l cefotaxime for 14 days. The concentration of cefotaxime was reduced to 250 mg/l for the next 14 days and maintained in subculture in the same type of media until no bacteria were present.
The hairy roots were cultured in 50 ml half-strength liquid MS media, subcultured at 30-day intervals at 25 °C in the dark and agitated at 110 rpm.
Hairy root confirmation and ploidy determination
Total DNA of the putative hairy roots was extracted by using a DNeasy plant mini kit (Qiagen, Germany) following the manufacturer’s instructions. The primers for rolB amplification and the Polymerase chain reaction (PCR) conditions followed those in Furner et al.22: The primers were rolB-1 primer 5′-GCTCTTGCAGTGCTAGATTT-3′ and rolB-2 primer 5′-GAAGGTGCAAGCTACCTCTC-3′; and the PCR conditions were initial denaturation at 95 °C for 2 min, 30 cycles of denaturation at 95 °C for 30 min, annealing at 53.5 °C for 45 min and extension at 72 °C for 45 min, and a final extension at 72 °C for 6 min. The PCR products were transferred to a 1.2% (w/v) agarose gel for evaluation by gel electrophoresis. The data were recorded by a Gel Documentation instrument (Gene Genius, Syngene) under UV at 260 nm after staining with SYBR® Safe DNA gel stain (Fisher).
Ploidy-level determination followed the protocol of Dolezel et al.23. The data were recorded by a BD FACSCantoTM flow cytometer (BD Biosciences). Diploid hairy roots were used as a control group.
Elicitor treatments
Kim et al.13 demonstrated that asiaticoside content increased when a whole plant culture was elicited by 10–100 µM MeJA and that the optimal concentration for elicitation was 100 µM MeJA. However, they reported a negative effect of MeJA at high concentration on senescence of the whole plants. Mangas et al.24 reported that 50 µM MeJA did not elicit a whole plant culture, in contrast to the results of Kim et al.13, and that asiaticoside content increased with increasing MeJA concentration (up to 100 µM). Moreover, 200 µM MeJA treatment produced symptoms of root necrosis; thus, 100 µM MeJA was considered the optimum treatment concentration24. In this study, we studied the effects of MeJA on hairy root culture; as hairy root culture is more sensitive than whole plant culture, we selected a safe range of MeJA concentrations and predicted that these concentrations would produce effects. The selected concentrations of MeJA were 0 µM (as control), 50 µM and 100 µM (treatment).
A MeJA stock (Sigma-Aldrich) solution was diluted to different concentrations (i.e., 0.0 µM, 50 µM and 100 µM) by absolute ethanol (analytical grade). MeJA was applied to the hairy root cultures at the beginning of the experiment. Fresh weight (FW), Dry weight (DW) and triterpenoid production was recorded at 7-day intervals until day 28. The cultures of all treatments were cultured in the dark at 25 °C and agitated at 110 rpm.
Growth determination and triterpenoid analysis
Three flasks of hairy roots were harvested from each MeJA treatment. FW was measured after the roots were media absorbed by sterilized tissue paper. The harvested hairy roots were dried at 40 °C for 48 h in a hot-air oven to determine DW. The hairy roots were homogenized to powder and extracted twice with 80% methanol (extraction ratio: 5:1 w/v) by sonication for 15 min. The extract was analyzed by a Thermo Scientific Ultra high-performance liquid chromatography (UHPLC) model Dionex Ultimate 3000 with a diode array detector using a protocol developed and validated by our research group (Thong-On, et al.19). A LiChroCART® column (LiChrospher® 100 RP-18, 250 mm × 4.0 mm I.D., particle size: 5.0 µm) and an acetonitrile and water (containing 0.1% H3PO4) gradient system were used. The mobile phase system of acetonitrile was as follows: 20–35% (10 min), 35–65% (15 min), 65–80% (5 min), 80–20% (5 min) and 20% (10 min). The flow rate was 1.0 ml/min, the injection volume was 20 µl, and 206 nm was used to detect the 4 major phytochemicals.
Results and Discussion
Confirmation of hairy root transformation and ploidy determination
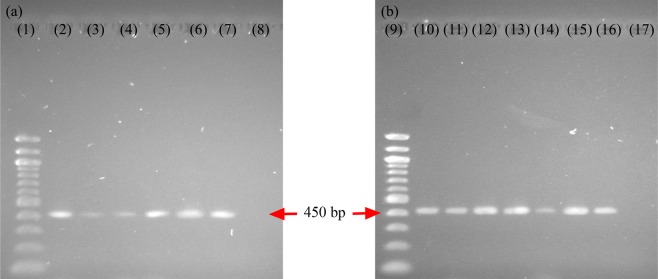
The transgenic state of the hairy root lines was confirmed by the presence of rolB in the putative hairy root genome. There were 4 diploid hairy root lines (HRD1–4) (Fig. 2a) and 5 tetraploid hairy root lines (HRT1-5) (Fig. 2b). The healthy diploid and tetraploid hairy root lines were chosen to conduct the elicitation experiment.
Figure 2.
PCR analysis of the rolB gene of A. rhizogenes. (a) diploid hairy root lines; (b) tetraploid hairy root lines, lanes 1 and 9 DNA marker; lanes 8 and 17 distilled water (negative control); lanes 2–5 (HRD1, HRD2, HRD3, HRD4) and 10–14 (HRT1, HRT2, HRT3, HRT4, HRT5) amplified bands of rolB from the DNA of hairy root lines; lanes 6–7 and 15–16 amplified bands of rolB from A. rhizogenes (positive control).
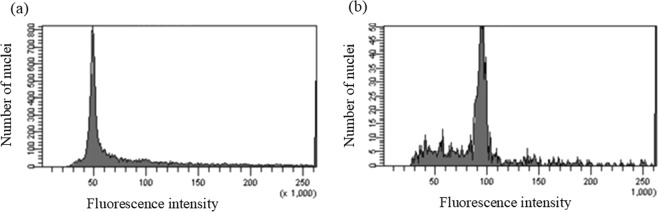
In addition, rolB was detected in these hairy root lines, confirming the transformation (Supplement Information). The ploidy levels of the hairy root lines obtained from the tetraploid explants were determined by flow cytometry. The peaks of the tetraploid hairy roots (4x) and diploid control hairy roots (2x) were set at the channels 100 and 50, respectively (Fig. 3).
Figure 3.
Flow cytometry histogram of C. asiatica hairy roots. (a) Histogram of a diploid control hairy root line, (b) histogram of a tetraploid hairy root line.
Effects of explant type and A. rhizogenes strain on hairy root induction
At both ploidy levels, hairy roots were induced from the lower part of the leaf explants after 15–17 days, whereas 29 days elapsed before the petiole explants produced hairy roots. There was no hairy root induction from the upper parts of the leaves of explants of either ploidy level of C. asiatica. Hairy roots were induced directly from the cut ends of the explants. The hairy roots induced from tetraploid explants were confirmed to be tetraploid by flow cytometry. A higher transformation rate was observed from the lower parts of the leaves of diploid (5–10%) and tetraploid explants (4.17–7.14%) (Table 1). The A. rhizogenes strain ATCC 43057 was more virulent than the strain ATCC 15834 and thus infected more explant types, i.e., the petioles and lower parts of leaves, and induced a higher transformation rate. Variation in A. rhizogenes strain and explant type can lead to different results because of differences among bacteria in the ability to induce hairy roots and the different responses of explants to bacteria25,26. The high transformation rate of the lower parts of the leaves may have been observed because of the larger midveins at the base of the leaves than in the upper parts; these might have allowed a large number of bacterial host cells to be integrated into the genome of the plant. Moreover, the lower parts of the leaves had 2 cut ends, which increased the infection area relative to that at the upper parts. Therefore, the use of the lower parts of the leaves for infection by A. rhizogenes strain ATCC 43057 for three days in the darkness was efficient for achieving a high successful transformation rate for C. asiatica hairy root induction.
Table 1.
Effect of explant type and A. rhizogenes strain on hairy root induction (%) of C. asiatica.
| Ploidy level | Explant types | A. rhizogenes strain | ||
|---|---|---|---|---|
| Control | ATCC 43057 | ATCC 15834 | ||
| Diploid plant | Upper-leaf | — | — | — |
| Lower-leaf | — | 10.00% | 5.00% | |
| Petiole | — | 2.38% | — | |
| Tetraploid plant | Upper-leaf | — | — | — |
| Lower-leaf | — | 7.14% | 4.17% | |
| Petiole | — | 5.26% | — | |
The characteristics of the hairy roots of differed between the diploid and tetraploid C. asiatica explants (Fig. 4). The diploid hairy roots displayed high lateral branch formation and rapid growth. Moreover, the dimensions of the primary hairy roots were similar to those of the lateral branches. However, the tetraploid hairy roots grew slowly and produced fewer lateral roots. The dimensions of the primary roots were larger than those of the lateral branches in tetraploid C. asiatica hairy roots.
Figure 4.
Diploid (a) and tetraploid (b) plants and derived diploid (c) and tetraploid (d) hairy root cultures of C. asiatica. Bar = 1.0 cm.
Effects of MeJA on the growth of hairy root cultures
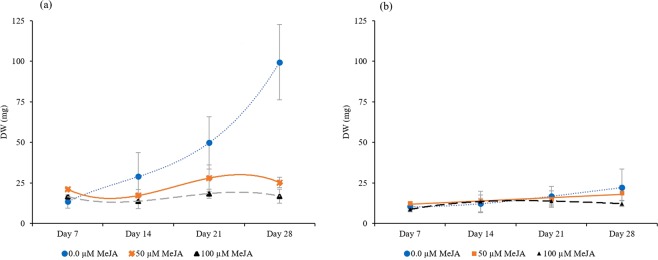
Figure 5 demonstrates the growth patterns of diploid and tetraploid hairy root cultures treated with different MeJA treatments. For the diploid hairy root cultures, the control treatment produced higher DW at day 28 than the other MeJA treatments, reaching 99.44 ± 23.2 mg/flask. The hairy root cultures of the 50 µM MeJA treatment and the 100 µM MeJA treatment showed maximum DW of 28.02 ± 8.05 mg/flask and 18.50 ± 2.78 mg/flask, respectively, on day 21. MeJA inhibited the growth of diploid hairy roots, especially those treated with high MeJA concentrations. The maximum DW for the tetraploid hairy root cultures was 22.14 ± 11.48 mg/flask at day 28 for the control treatment, 18.86 ± 4.58 mg/flask at day 28 for the 50 µM MeJA treatment and 13.88 ± 2.34 mg/flask at day 21 for the 100 µM MeJA treatment. The nonelicited tetraploid hairy roots grew but at a slower growth rate than the nonelicited diploid hairy roots. The growth of the nonelicited diploid hairy roots was 4.5-fold higher than that of nonelicited tetraploid hairy roots at day 28. Inhibitory effects of MeJA have previously been reported on hairy root cultures of Rhinacanthus nasutus27 and Glycine max28. The reasons may be related to the phenolic compounds secreted from hairy roots, which are stimulated by the stress from MeJA, or the toxic effect of MeJA itself on the hairy roots29. Moreover, polyploidy plants may have a small number of cell divisions during growth and development that result in a lower growth rate than in normal diploid plants30,31. De Jesus-Gonzalez and Weathers32 reported similar results for Artemisia annua.
Figure 5.
Growth patterns of diploid (a) and tetraploid (b) C. asiatica hairy root cultures. The data are presented as mean ± SD.
Effects of MeJA on the triterpenoid production of hairy root cultures
There was no triterpenoid production in nonelicited C. asiatica hairy root culture, whereas MeJA led to triterpenoid production in both diploid and tetraploid hairy root cultures (Tables 2 and 3). For the diploid hairy root cultures, the highest triterpenoid production under the 50 and 100 µM MeJA treatments was 27.25 ± 0.27 µg/mg DW at day 21 and 6.17 ± 2.30 µg/mg DW at day 14, respectively. When treated on the same day, the 50 µM MeJA treatment produced more triterpenoids than the 100 µM MeJA treatment. For the tetraploid hairy root cultures, in the 50 µM MeJA treatment, the hairy root culture at day 28 produced the maximum amount of triterpenoids, 16.29 ± 6.32 µg/mg DW, whereas the 100 µM MeJA treatment produced a similar triterpenoid amount (16.31 ± 9.24 µg/mg DW) at day 14 (Tables 2 and 3). Ploidy level (p = 0.0334), MeJA treatment (p = 0.0000) and number of days of culture (p = 0.0003) were the main factors that significantly affected the total triterpenoid production of C. asiatica hairy roots, as determined by 3-way analysis of variance. Furthermore, the triterpenoid production patterns in each ploidy level for all MeJA treatments differed in their production characteristics. Asiaticoside was dominant, followed by asiatic acid and madecassic acid. Madecassoside was not detected in hairy roots of either ploidy level. Interestingly, there were different ratios of aglycones (i.e., asiatic acid and madecassic acid) to glycoside (i.e., asiaticoside) when the hairy roots were treated with MeJA. The ratios in diploid and tetraploid hairy roots treated with 50 µM MeJA at day 28 were 1:7 and 1:2, respectively. However, the ratios under 100 µM MeJA treatment were similar between the diploid and tetraploid hairy roots, e.g., 1:1 on day 28. These results showed that the ratio of aglycones to glycoside in diploid hairy roots was affected by the concentration of MeJA, whereas the ratio was largely stable in the tetraploid hairy roots, even at high MeJA concentrations. Aziz et al.33 and Kim et al.34 reported that there was no triterpenoid production from nonelicited C. asiatica hairy root cultures. This result may be related to organ-specific biosynthesis, which involves interactions between plant organs and plant metabolic precursors produced in roots and transported to aerial parts for bioconversion33. Moreover, the genes associated with triterpenoid biosynthesis may be poorly expressed in transgenic roots34. In contrast, the addition of MeJA to the hairy root cultures resulted in triterpenoid production, possibly because of the upregulation of CaSQS (Centella asiatica squalene synthase) and CabAS (Centella asiatica β-amyrin synthase) genes21,35. Previous studies reported different proportions of triterpenoids in 4-month-old field-grown diploid and tetraploid C. asiatica plants18,19. The ratios of aglycones to glycosides were 1:13 and 1:9 in diploid and tetraploid leaves, respectively. In the present study, the tetraploid hairy roots produced more glycones than did the diploid hairy roots, which increased the aglycone/glycoside ratio to 1:2 and 1:1 under treatment with 50 and 100 µM MeJA, respectively, at day 28. The downregulation of UDP-glucosyltransferases in the tetraploid hairy roots by MeJA may have contributed to the different proportions between the plants of different ploidies36. Commercial drugs produced from C. asiatica and other preparations consist of 60% aglycones and 40% asiaticoside (equivalent to a 3:2 ratio)1,2. The manufacturing processes of these C. asiatica drugs and preparations are complex and involve chemical treatments that cannot maintain the proportions of natural components. Purified chemicals are also added to enrich the extract, which may affect the price of C. asiatica products2. By using elicited tetraploid hairy roots, these production obstacles may be solved. The findings indicate that C. asiatica hairy roots cannot produce the four major triterpenoids without intervention, which may be related to organ-specific biosynthesis. The application of MeJA resulted in not only triterpenoid production but also differences in triterpenoid proportion between diploid and tetraploid hairy root cultures. The diploid hairy roots were suitable for glycoside production, and the tetraploid hairy roots were suitable for aglycone production. Moreover, 50 µM MeJA was the optimum concentration for maximizing the amount of triterpenoids per flask from C. asiatica hairy roots of both ploidy levels.
Table 2.
Triterpenoid Production of diploid hairy root cultures (Mean ± SD (µg/mg DW)).
| MeJA | Harvest Date | AD | MA | AA | TT |
|---|---|---|---|---|---|
| 0 µM | Day 7 | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a |
| Day 14 | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | |
| Day 21 | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | |
| Day 28 | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | |
| 50 µM | Day 7 | 15.35 ± 9.71bc | 0.00 ± 0.00a | 0.00 ± 0.00a | 15.35 ± 9.71c |
| Day 14 | 11.67 ± 3.49b | 0.00 ± 0.00a | 0.00 ± 0.00a | 11.67 ± 3.49bc | |
| Day 21 | 25.87 ± 0.93d | 0.47 ± 0.16bc | 0.91 ± 0.53abc | 27.25 ± 0.27d | |
| Day 28 | 18.82 ± 5.90c | 0.79 ± 0.50c | 2.24 ± 0.92d | 22.85 ± 7.32d | |
| 100 µM | Day 7 | 4.20 ± 0.76a | 0.00 ± 0.00a | 0.00 ± 0.00a | 4.20 ± 0.76a |
| Day 14 | 5.20 ± 2.10a | 0.35 ± 0.15ab | 0.62 ± 0.33ab | 6.17 ± 2.30ab | |
| Day 21 | 3.04 ± 1.60a | 0.44 ± 0.50bc | 1.28 ± 1.43bc | 4.76 ± 3.44ab | |
| Day 28 | 2.76 ± 0.63a | 0.55 ± 0.11bc | 1.66 ± 0.30 cd | 4.96 ± 1.03ab |
Means within each column with different letters are significantly different at P < 0.05.
TT: Total triterpenoid, AD: Asiaticoside, MA: Madecassic Acid, AA: Asiatic Acid.
Table 3.
Triterpenoid Production of tetraploid hairy root cultures (Mean ± SD (µg/mg DW)).
| MeJA | Harvest Date | AD | MA | AA | TT |
|---|---|---|---|---|---|
| 0 µM | Day 7 | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a |
| Day 14 | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | |
| Day 21 | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | |
| Day 28 | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | |
| 50 µM | Day 7 | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a |
| Day 14 | 0.00 ± 0.00a | 0.37 ± 0.20ab | 0.00 ± 0.00a | 0.37 ± 0.20a | |
| Day 21 | 5.83 ± 3.37abc | 0.65 ± 0.23ab | 1.84 ± 0.29ab | 8.32 ± 2.87abc | |
| Day 28 | 10.72 ± 4.25cd | 1.40 ± 0.69cd | 4.17 ± 1.38c | 16.29 ± 6.32c | |
| 100 µM | Day 7 | 3.49 ± 3.03ab | 0.00 ± 0.00a | 0.00 ± 0.00a | 3.49 ± 3.03ab |
| Day 14 | 14.39 ± 7.86d | 0.51 ± 0.21ab | 1.41 ± 1.22ab | 16.31 ± 9.24c | |
| Day 21 | 7.60 ± 7.19bc | 0.82 ± 0.58bc | 2.20 ± 1.9b | 10.62 ± 9.34bc | |
| Day 28 | 7.95 ± 2.06bc | 1.85 ± 1.04d | 5.69 ± 1.96c | 15.49 ± 4.93c |
Means within each column with different letters are significantly different at P < 0.05.
TT: Total triterpenoid, AD: Asiaticoside, MA: Madecassic Acid, AA: Asiatic Acid.
Supplementary information
Author contributions
K.V.N. conducted all experiments, collected and analyzed data, and drafted the manuscript. B.P., T.P. and U.V. participated in data analysis, discussion and recommendation. S.P. participated in research conception and design, data analysis, and manuscript revision.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-019-54460-z.
References
- 1.Brinkhaus B, Lindner M, Schuppan D, Hahn EG. Chemical, pharmacological and clinical profile of the East Asian medical plant Centella asiatica. Phytomedicine. 2000;7:427–448. doi: 10.1016/S0944-7113(00)80065-3. [DOI] [PubMed] [Google Scholar]
- 2.Delbò, M. & Calapai, G. Assessment report on Centella asiatica (L.) Urban, herba. (European Medicines Agency, United Kingdom, 2010).
- 3.Liu M, et al. Madecassoside isolated from Centella asiatica herbs facilitates burn wound healing in mice. Planta Med. 2008;74:809–815. doi: 10.1055/s-2008-1074533. [DOI] [PubMed] [Google Scholar]
- 4.Shukla A, et al. In vitro and in vivo wound healing activity of asiaticoside isolated from Centella asiatica. J. Ethnopharmacol. 1999;65:1–11. doi: 10.1016/S0378-8741(98)00141-X. [DOI] [PubMed] [Google Scholar]
- 5.Wattanathorn J, et al. Positive modulation of cognition and mood in the healthy elderly volunteer following the administration of Centella asiatica. J. Ethnopharmacol. 2008;116:325–332. doi: 10.1016/j.jep.2007.11.038. [DOI] [PubMed] [Google Scholar]
- 6.Tiwari KM, Sharma NC, Tiwari V, Singh BD. Micropropagation of Centella asiatica (L.), a valuable medicinal herb. Plant Cell Tiss. Org. Cult. 2000;63:179–185. doi: 10.1023/A:101069060. [DOI] [Google Scholar]
- 7.Thomas MT, et al. Elite genotypes/chemotypes, with high contents of madecassoside and asiaticoside, from sixty accessions of Centella asiatica of south India and the Andaman Islands: For cultivation and utility in cosmetic and herbal drug applications. Ind. Crop. Prod. 2010;32:545–550. doi: 10.1016/jandcrop.2010.07.003. [DOI] [Google Scholar]
- 8.Randriamampionona D, et al. Comparative analysis of active constituents in Centella asiatica samples from Madagascar: application for ex situ conservation and clonal propagation. Fitoterapia. 2007;78:482–489. doi: 10.1016/j.fitote.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 9.Eibl, R. & Eibl, D. In Plant Biotechnology and Transgenic Plants (eds Kirsi-Marja Oksmah-Caldentey & Wolfgang H. Barz) Ch. 8, 163–199 (Marcel Dekker, 2002).
- 10.Lu MB, Wong HL, Teng WL. Effects of elicitation on the production of saponin in cell culture of Panax ginseng. Plant Cell Rep. 2001;20:674–677. doi: 10.1007/s002990100378. [DOI] [Google Scholar]
- 11.Kim OT, et al. Upregulation of ginsenoside and gene expression related to triterpene biosynthesis in ginseng hairy root cultures elicited by methyl jasmonate. Plant Cell Tiss. Org. Cult. 2009;98:25–33. doi: 10.1007/s11240-009-9535-9. [DOI] [Google Scholar]
- 12.Hayashi H, Huang P, Inoue K. Up-regulation of soyasaponin biosynthesis by methyl jasmonate in cultured cells of Glycyrrhiza glabra. Plant Cell Physiol. 2003;44:404–411. doi: 10.1093/pcp/pcg054. [DOI] [PubMed] [Google Scholar]
- 13.Kim OT, Kim MY, Hong MH, Ahn JC, Hwang B. Stimulation of asiaticoside accumulation in the whole plant cultures of Centella asiatica (L.) Urban by elicitors. Plant Cell Rep. 2004;23:339–344. doi: 10.1007/s00299-004-0826-7. [DOI] [PubMed] [Google Scholar]
- 14.Yoo NH, et al. Enhancement of centelloside production from cultured plants of Centella asiatica by combination of thidiazuron and methyl jasmonate. Plant Biotechnol. Rep. 2011;5:283–287. doi: 10.1007/s11816-011-0173-4. [DOI] [Google Scholar]
- 15.Mishra BK, Pathak S, Sharma A, Trivedi PK, Shukla S. Modulated gene expression in newly synthesized auto-tetraploid of Papaver somniferum L. S. Afr. J. Bot. 2010;76:447–452. doi: 10.1016/j.sajb.2010.02.090. [DOI] [Google Scholar]
- 16.Wallaart TE, Pras N, Quax WJ. Seasonal variations of artemisinin and its biosynthetic precursors in tetraploid Artemisia annua plants compared with the diploid wild-type. Planta Med. 1999;65:723–728. doi: 10.1055/s-1999-14094. [DOI] [PubMed] [Google Scholar]
- 17.Gao SL, Zhu DN, Cai ZH, Xu DR. Autotetraploid plants from colchicine-treated bud culture of Salvia miltiorrhiza Bge. Plant Cell Tiss. Org. Cult. 1996;47:73–77. doi: 10.1007/bf02318968. [DOI] [Google Scholar]
- 18.Kaensaksiri T, Soontornchainaksaeng P, Soonthornchareonnon N, Prathanturarug S. In vitro induction of polyploidy in Centella asiatica (L.) Urban. Plant Cell Tiss. Org. Cult. 2011;107:187–194. doi: 10.1007/s11240-011-9969-8. [DOI] [Google Scholar]
- 19.Thong-On W, Arimatsu P, Pitiporn S, Soonthornchareonnon N, Prathanturarug S. Field evaluation of in vitro-induced tetraploid and diploid Centella asiatica (L.) Urban. J. Nat. Med. 2014;68:267–273. doi: 10.1007/s11418-013-0761-4. [DOI] [PubMed] [Google Scholar]
- 20.Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- 21.Kim OT, et al. Enhanced production of asiaticoside from hairy root cultures of Centella asiatica (L.) Urban elicited by methyl jasmonate. Plant Cell Rep. 2007;26:1941–1949. doi: 10.1007/s00299-007-0400-1. [DOI] [PubMed] [Google Scholar]
- 22.Furner IJ, et al. An Agrobacterium transformation in the evolution of the genus Nicotiana. Nature. 1986;319:422–427. doi: 10.1038/319422a0. [DOI] [Google Scholar]
- 23.Dolezel J, Greilhuber J, Suda J. Estimation of nuclear DNA content in plants using flow cytometry. Nat. Protoc. 2007;2:2233–2244. doi: 10.1038/nprot.2007.310. [DOI] [PubMed] [Google Scholar]
- 24.Mangas S, et al. The effect of methyl jasmonate on triterpene and sterol metabolisms of Centella asiatica, Ruscus aculeatus and Galphimia glauca cultured plants. Phytochemistry. 2006;67:2041–2049. doi: 10.1016/j.phytochem.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 25.Danphitsanuparn P, Boonsnongcheep P, Boriboonkaset T, Chintapakorn Y, Prathanturarug S. Effects of Agrobacterium rhizogenes strains and other parameters on production of isoflavonoids in hairy roots of Pueraria candollei Grah. ex Benth. var. candollei. Plant Cell Tiss. Org. Cult. 2012;111:315–322. doi: 10.1007/s11240-012-0196-8. [DOI] [Google Scholar]
- 26.Ha LT, et al. Hairy root cultures of Panax vietnamensis, a promising approach for the production of ocotillol-type ginsenosides. Plant Cell Tiss. Org. Cult. 2016;126:93–103. doi: 10.1007/s11240-016-0980-y. [DOI] [Google Scholar]
- 27.Cheruvathur MK, Thomas TD. Effect of plant growth regulators and elicitors on rhinacanthin accumulation in hairy root cultures of Rhinacanthus nasutus (L.) Kurz. Plant Cell Tiss. Org. Cult. 2014;118:169–177. doi: 10.1007/s11240-014-0473-9. [DOI] [Google Scholar]
- 28.Theboral J, et al. Enhanced production of isoflavones by elicitation in hairy root cultures of Soybean. Plant Cell Tiss. Org. Cult. 2014;117:477–481. doi: 10.1007/s11240-014-0450-3. [DOI] [Google Scholar]
- 29.Sivanandhan G, et al. Optimization of elicitation conditions with methyl jasmonate and salicylic acid to improve the productivity of withanolides in the adventitious root culture of Withania somnifera (l.) Dunal. Appl. Biochem. Biotechnol. 2012;168:681–696. doi: 10.1007/s12010-012-9809-2. [DOI] [PubMed] [Google Scholar]
- 30.Lewis, W. H. In Polyploidy: Biological Relevance (ed. Walter H. Lewis) 103–144 (Springer US, 1980).
- 31.Otto SP, Whitton J. Polyploid incidence and evolution. Annu. Rev. Genet. 2000;34:401–437. doi: 10.1146/annurev.genet.34.1.401. [DOI] [PubMed] [Google Scholar]
- 32.De Jesus-Gonzalez L, Weathers PJ. Tetraploid Artemisia annua hairy roots produce more artemisinin than diploids. Plant Cell Rep. 2003;21:809–813. doi: 10.1007/s00299-003-0587-8. [DOI] [PubMed] [Google Scholar]
- 33.Aziz ZA, et al. Production of asiaticoside and madecassoside in Centella asiatica in vitro and in vivo. Biol. Plant. 2007;51:34–42. doi: 10.1007/s10535-007-0008-x. [DOI] [Google Scholar]
- 34.Kim OT, et al. Upregulation of phytosterol and triterpene biosynthesis in Centella asiatica hairy roots overexpressed ginseng farnesyl diphosphate synthase. Plant Cell Rep. 2010;29:403–411. doi: 10.1007/s00299-010-0831-y. [DOI] [PubMed] [Google Scholar]
- 35.Kim OT, Seong NS, Kim MY, Hwang B. Isolation and characterization of squalene synthase cDNA from Centella asiatica (L.) Urban. Journal of Plant Biology. 2005;48:263–269. doi: 10.1007/Bf03030521. [DOI] [Google Scholar]
- 36.Kim OT, et al. Analysis of expressed sequence tags from Centella asiatica (L.) Urban hairy roots elicited by methyl jasmonate to discover genes related to cytochrome P450s and glucosyltransferases. Plant Biotechnol. Rep. 2014;8:211–220. doi: 10.1007/s11816-013-0311-2. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.