Abstract
Time perception is an important ability that is related closely to humans’ and animals’ daily activities. It can be distorted by various emotional states. In human studies, experimental pain has been shown to prolong the perception of time. However, related animal studies are lacking. In this study, we used a temporal bisection task to investigate how acute inflammatory pain (induced by hind-paw formalin injection) and chronic neuropathic pain [induced by spinal nerve ligation (SNL)] affected time perception in rats. Rats were trained to recognize “short” (1200-ms) and “long” (2400-ms) anchor-duration pure tones and were rewarded for corresponding lever presses. During testing, rats perceived a series of intermediate-duration and anchor-duration pure tones, and selected levers corresponding to the “short” and “long” tones. After formalin injection, rats gave more “long” lever-press responses than after saline injection. The point of subjective equality after formalin injection also increased, suggesting that formalin-induced acute pain extended time perception. In contrast, rats that had undergone SNL gave fewer “long” lever-press responses compared with the sham surgery group. This animal study suggests that formalin-induced pain and neuropathic pain may have different effects on time perception.
Subject terms: Perception, Psychology
Introduction
Time estimation is a critical ability of animals and humans in their daily activities1,2. A leopard needs to choose the right time to attack its prey; a chef needs to estimate the cooking time to ensure that a dish has the best flavor. However, the subjective perception of time is not invariable; it depends on the environmental context and emotional state. Previous studies have suggested that time perception is regulated by many factors, including emotion, motivation, and attention2–4. When faced with a threat stimulus, such as an angry face5,6 or a frightening spider7,8, human subjects overestimate the amount of time passed. By contrast, in high approach motivation states, humans and animals perceive that time passes more quickly4,9. In addition, a series of studies showed that subjective time perception could be shortened when subjects were distracted from to-be-timed stimuli10–12.
Pain is a multidimensional subjective experience, with sensory-discriminative, affective-motivational, and cognitive-evaluational components13. It can affect psychological processes such as decision making14,15, attention16,17, and working memory18. Some human studies have also shown that time perception is affected by acute pain. Subjects have been found to overestimate the neutral visual stimulation time when feeling radiant heat pain19 and in a cold pressor test20. The influence of chronic pain on time perception is more complex. Many patients with chronic pain perceive that time passes more slowly in daily life21,22. In a study conducted with migraineurs, Anagnostou and Mitsikostas23 found that temporal estimation remained normal for most subjects, but was prolonged in a subgroup with depressive symptoms. The threshold of somatosensory time discrimination has also been found to be increased during migraine attacks24,25. However, animal studies of pain and time perception are lacking. Appropriate animal models will aid exploration of the neurological and pharmacological mechanisms of pain’s effects on time perception.
In this study, we assessed changes in time perception under acute inflammatory pain and chronic neuropathic pain in rats using a temporal bisection task. Formalin injection was used to induce acute inflammatory pain and spinal nerve ligation (SNL) was used to induce chronic neuropathic pain. The temporal bisection task is a widely accepted paradigm for the study of time perception in animals and humans9,20,26–28; we trained rats to discriminate “long” and “short” anchor durations of a pure tone, and to press corresponding levers. The psychometric function was used to fit the data obtained and to examine response bias and sensitivity29,30.
Materials and Methods
Animals
Male Sprague-Dawley rats (weight 230–250 g on arrival, 260–280 g before experiments; Charles River, Beijing, China) were used in this study. All rats were housed individually with food and water available ad libitum in a temperature- and humidity-controlled room (22°C, 65% humidity), maintained with a reverse 12:12-h light:dark cycle (light on at 8:00 pm). After arriving in the laboratory, the rats were adapted to the environment for at least 1 week. Before the experiments, they were handled daily to familiarize them with the manipulation of the experimenter. All the experimental procedures were approved by the Institutional Animal Care and Use Committee of Chinese Academy of Sciences. We confirm that all methods were performed in accordance with the relevant guidelines and regulations.
Temporal bisection task
The training sessions and temporal bisection task were carried out in the same operant box (21 cm W × 32.5 cm D × 42.5 cm H), which was located in a dim soundproof chamber. The walls of the operant box were made of acrylic, and the floor was made of acrylic strips and positioned above a catch pan. Two retractable levers (ENV-112CMP; MED Associates, American) were mounted symmetrically on one side wall of the box (9 cm above the floor), with a liquid dispenser (ENV-201A; MED Associates, American) and water receptacle located between and equidistant from them (2.5 cm above the floor) to provide water as the reinforcer. A tone generator (ENV-223AM; MED Associates, American) mounted on the wall was used to present the pure tone stimuli (2900 Hz, 65 dB). On the middle of the opposite wall, an illuminated infrared detective nose-poke hole (ENV-114BM; MED Associates, American) was mounted 2.5 cm above the floor, and an indicator lamp (ENV-221M; MED Associates, American) was mounted directly above it. All output and input devices mounted in the operant box were furnished by the experimenters.
Temporal discrimination training was conducted using a previously described procedure9,31,32 with modification. Right and left lever-press responses following tones with anchor durations of 1200 and 2400 ms were reinforced (Fig. 1a). The relationship of the two anchor durations with the two levers was counterbalanced. In the last three free-choice training sessions, the accuracy of all rats’ responses to the anchor-duration tones exceeded 85% and the average accuracy in each group of rats exceeded 90%.
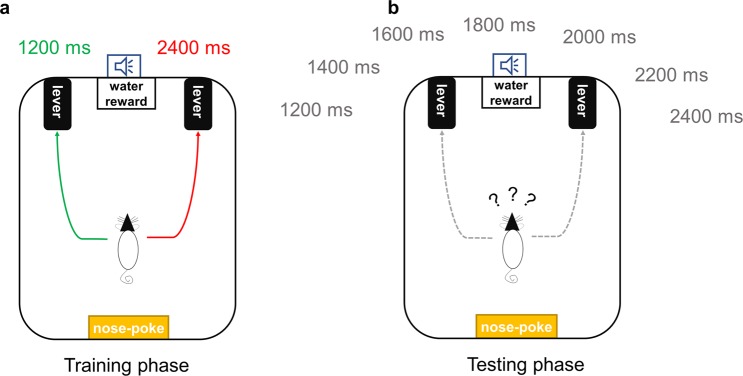
Figure 1.
Overview of temporal bisection training and task. (a) During the training stage, rats were trained to discriminate two anchor-duration pure tones and to press the corresponding levers. (b) During the test stage, several intermediate-duration tones were presented as well as the anchor-duration tones, and rats were free to select which lever to press.
After discrimination training, the temporal bisection task was conducted (Fig. 1b). This task comprised 140 trials (30 s/trial; total duration, 70 min): 14 trials each with tones of five intermediate durations (1400, 1600, 1800, 2000, and 2200 ms) without reinforcement, and 35 trials each with the two anchor-duration tones (1200 and 2400 ms) with reinforcement. The order of trials was random, but with the constraint of no more than three consecutive intermediate-duration trials. Sessions began with illumination of the indicator light. The to-be-timed tone was initiated by activation of the nose-poke hole. Its termination was followed by immediate presentation of the two levers. In the anchor-duration trials, correct responses were reinforced with drips of water. For each test session, the trials were presented in the same order for all animals.
Establishment of the pain model
Acute inflammatory pain was induced by injection of 50 μl 1% formalin solution into the hind paw. Saline injection was used as the control. Each rat was returned to the operant box immediately after injection, and the nociceptive behavior of the injected paw was recorded for 70 min. After the test stage, rats that received the formalin injection were placed in the operant box again without the temporal bisection task. We calculated the durations of lifting and licking of the injected paw in 5-min epochs.
L5 SNL was used to establish an animal model of neuropathic pain. According to the procedure of Kim and Chung33, rats were placed in a prone position and anesthetized with 50 mg/kg sodium pentobarbital (i.p). The fur on the left side of the spine was then shaved to expose the skin. The skin was cut parallel to the spine and the paraspinal muscles were separated. The L5 transverse process was removed until the L5 spinal nerve was exposed. The L5 nerve was isolated and ligated with silk thread. Finally, the muscle and skin incisions were sutured and the wound was disinfected with iodophor and 75% ethyl alcohol. In the sham group, rats underwent the same surgical procedure without the nerve ligation.
Behavioral tests
The von Frey test with the up-down method described by Dixon et al.34 was used to measure the 50% paw withdrawal threshold (PWT) of rats in the neuropathic pain experiment. All rats were allowed to acclimatize to the environment for at least 10 min before the test. In brief, the rats were placed on an iron metal grid, restricted and separated with plastic covers. von Frey filament (0.008–15.0 g) was applied vertically to the plantar surfaces of the hind paw ipsilateral or contralateral to the SNL/sham surgery. The test position was on the rear hind paw (avoiding the pads), as described previously35. Sharp paw lifting within 4 s was defined as a positive response.
Radiant heat was used to examine thermal hyperalgesia of rats in the neuropathic pain experiment. As described in our previous studies36, the rats were placed on a transparent plexiglass plate and separated with plastic covers to restrict their activity. A radiant heat source beneath the floor was aimed at the plantar surface of the hind paw ipsilateral or contralateral to the SNL/sham surgery. In each test, the heat source was turned on until the rat quickly withdrew its hind paw or until the cutoff time of 25 s. Paw withdrawal latency (PWL) was defined as the time between heat onset and paw lifting. Five trials were conducted on each hind paw at 5-min intervals. PWL values from the last 3 trials were averaged.
Experimental procedure
We adopted a within-subjects design to assess the effect of formalin-induced pain on time perception (n = 10). After the temporal discrimination training stage, all rats received two injections (one of saline and one of formalin) at a 7-day interval. To balance the order effect, half of the rats received saline injections first, and the other half received formalin injections first. The rats performed the bisection task immediately after saline/formalin injection.
Two groups of rats (SNL and sham; n = 10 each) were used to assess the effect of neuropathic pain on time perception in this experiment. The von Frey test and thermal paw withdrawal test were performed before surgery (baseline) and 14 days after surgery. Two days before surgery, the temporal bisection task was performed as a baseline measure. After surgery, the rats were allowed to recover for 7 days. On days 8–13, the rats underwent five training sessions to restore their temporal discrimination ability. On postoperative days 14 and 15, during the period of sensitivity to neuropathic pain37–39, the temporal bisection task was administered again (Once per day, to ensure the stability of the results).
Data analysis
The proportion of long response (PL) to each tone duration in the temporal bisection task was used to assess time perception. The function related to PL and the duration is a typically sigmoidal, as an example of psychometric function. Following widespread practice29–31, we adopted a sigmoidal cumulative Gaussian function to fit the experimental data from the temporal bisection tasks. This function can be expressed as:
| 1 |
where ƒ(t) represents the expected PL when the duration is equal to a given sample t, µ represents the mean and σ represents the standard deviation (SD), a represents the low asymptote of the function, and b represents the range. The point of subjective equality (PSE), defined as a tone duration that animals have 50% chance of perceiving as “short” or “long,” is equal to µ. An increase or decrease in the PSE value is an index of the response bias to a “long” or “short” tone, which was interpreted generally as the subjective under- or overestimation of time40. The SD, equal to σ, represents the slope of the function and was calculated as a measure of temporal sensitivity. A decrease in the SD indicates temporal sensitivity to the durations increase29–31. Curve fitting and parameter calculation were performed using Prism software (version 5, GraphPad software, Inc).
For the temporal bisection task, trials with no lever-press response were omitted from the analysis. Three-way and two-way analyses of variance, and independent-sample and paired-sample t tests, were used with appropriate factors for data analysis. The Duncan test was adopted as a post-hoc test, and p < 0.05 was considered to indicate significance. All data are expressed as means ± standard errors of the mean. The statistical analyses were conducted with Prism (version 5, GraphPad software, Inc) and STATISTICA (version 6, StatSoft.Inc) software.
Results
Effect of formalin-induced acute inflammatory pain on time perception
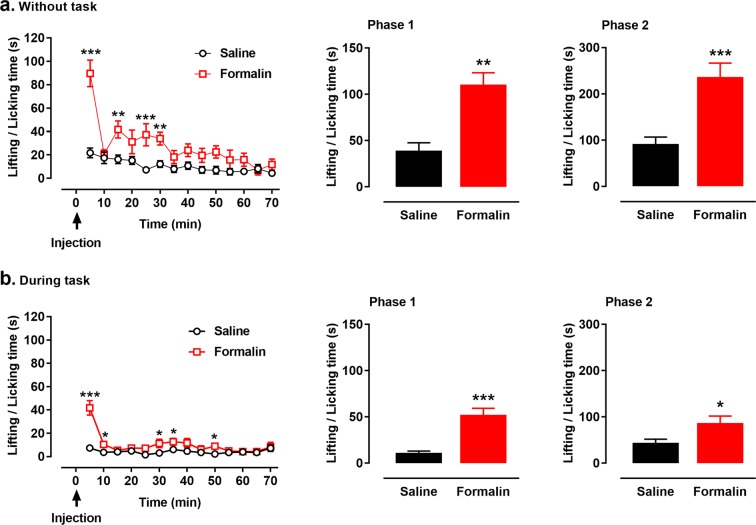
A typical biphasic pattern of nociceptive behavior was observed after formalin injection into the hind paw. With no temporal bisection task, rats injected with formalin exhibited much more paw lifting and licking behavior than did rats injected with normal saline [interactive effect: F(13, 117) = 5.211, p < 0.001; treatment effect: F(1, 9) = 41.394, p < 0.001; time effect: F(13, 117) = 12.331, p < 0.001; Fig. 2a, left panel]. The cumulative time spent in paw lifting and licking was significantly increased in phase 1 [0–10 min; t(9) = 6.668, p = 0.001; Fig. 2a, middle panel] and phase 2 [20–60 min; t(9) = 5.911, p < 0.001, Fig. 2a, right panel] after formalin injection compared with the time spent after saline injection.
Figure 2.
Nociceptive behaviors after formalin/saline injection during and without the temporal bisection task. Rats received single injections of 1% formalin solution (50 μl) into the hind paw. Nociceptive behavior was measured as the time spent in lifting and licking the injected paw, which varied as a function of time. Formalin injection induced more nociceptive behaviors during 70 min without the task (a) and during the task (b). Cumulative paw-licking scores in phases 1 (middle panel of a, b) and 2 (left panel of a, b) after formalin injection were significantly higher than these after saline injection. *p < 0.05, **p < 0.01, ***p < 0.001 vs. saline injection; n = 10. Results are shown as means ± SEM.
Similarly, during the temporal bisection task, more nociceptive behavior was observed after formalin injection than after saline injection [interactive effect: F(13, 117) = 8.212, p < 0.001; treatment effect: F(1, 9) = 22.029, p = 0.001; time effect: F(13, 117) = 10.371, p < 0.001; Fig. 2b, left panel]. Formalin injection significantly increased the cumulative time spent in paw lifting and licking in phase 1 [0–10 min; t(9) = 4.663, p < 0.001; Fig. 2b, middle panel] and phase 2 [20–60 min; t(9) = 2.836, p = 0.020; Fig. 2b, right panel] compared with that spent after saline injection. By comparing the cumulative time spent in paw lifting and licking in 0–70 min, less nociceptive behavior was observed during the temporal bisection task than without the task after formalin injection [during task vs. without task: 144.4 ± 57.0 s vs. 389.0 ± 122.7 s, t(9) = 5.654, p < 0.001]. Besides, the cumulative time spent in paw lifting and licking after formalin injection during the task were significantly shorter than that without task [phase 1: t(9) = 4.015, p = 0.003; phase 2: t(9) = 5.015, p = 0.001].
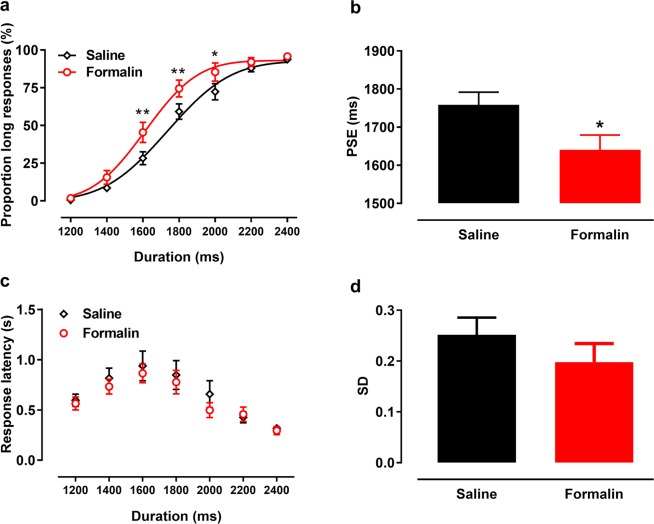
During the test phase, PLs increased with increasing tone duration, forming nearly standard sigmoid curves (Fig. 3a). The goodness of fit of the Gaussian function for individual rats was ≥0.90. Under the effect of 1% formalin, the fitted curve shifted to the left and upward; rats gave more “long” anchor-duration lever-press responses after formalin injection than after saline injection [interactive effect: F(6, 54) = 1.454, p = 0.212; treatment effect: F(1, 9) = 6.186, p = 0.035; Fig. 3a]. Specifically, formalin injection increased the PLs for 1600-ms (p = 0.003), 1800-ms (p = 0.009), and 2000-ms (p = 0.026) tones. Formalin injection also decreased rats’ PSE compared with saline injection [t(9) = −2.646, p = 0.027; Fig. 3b], indicating subjective overestimation of time. The average response latency to each tone duration took an inverted U shape (Fig. 3c). Response latency did not differ according to treatment, suggesting that physical activity was not affected by formalin injection. Formalin injection did not produce a significant difference in the SD [t(9) = 0.135, p = 0.304; Fig. 3d], indicating no effect on temporal sensitivity. We also compared data from omitted trials performed after saline (average, 2.3 ± 1.0 trials) and formalin (average, 2.5 ± 0.5 trials) injections, and found no difference between treatments [t(9) = 0.169, p = 0.869].
Figure 3.
Response patterns in temporal bisection tasks after formalin/saline injection [Proportion of long response (PL) by duration and psychometric fitting curves]. (a) After formalin injection, the fitting curve shifted left compared with saline injection and baseline. (b) Formalin injection significantly decreased the point of subjective equality (PSE). Response latency (c) and the standard deviations (SD) of fitting function (d) did not differ between conditions. *p < 0.05, **p < 0.01 vs. saline injection; n = 10. Results are shown as means ± SEM.
Effects of SNL-induced neuropathic pain on interval timing behaviors
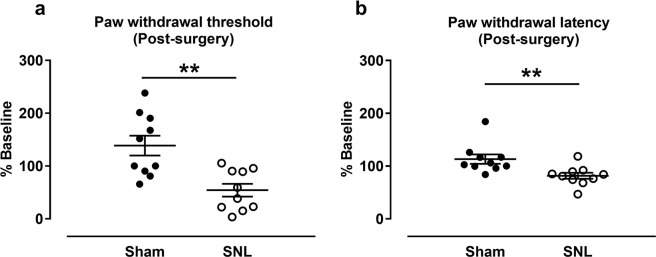
In 14 days after SNL surgery, paw withdrawal threshold [PWT, relative to the baseline, (Baseline: Sham group, 4.0 ± 0.5 g, SNL group: 5.7 ± 1.3 g; Day 14: Sham group, 4.0 ± 0.7 g, SNL group: 2.0 ± 0.1 g)] in the von Frey test was significantly lower in the SNL group than in the sham group [t(18) = 3.788, p = 0.001; Fig. 4a]. Paw withdrawal latency [PWL, relative to the baseline. (Baseline: Sham group: 13.7 ± 0.5 s, SNL group: 13.6 ± 0.7 s; Day 14: Sham group: 15.4 ± 1.1 s, SNL group, 10.8 ± 0.4 s)] in the thermal test was also significantly lower in the SNL group than in the sham group [t(18) = 2.999, p = 0.008; Fig. 4b]. These results confirmed that the rats had allodynia and thermal hyperalgesia 14 days after SNL surgery.
Figure 4.
Results of the von-Frey and thermal withdrawal tests at 14 days after SNL. SNL surgery significant decreased the paw withdrawal threshold relative to baseline (a) and paw withdrawal latency relative to baseline (b) compared with the sham group. **p < 0.01 vs. sham group. n = 10/group. Results are shown as means ± SEM.
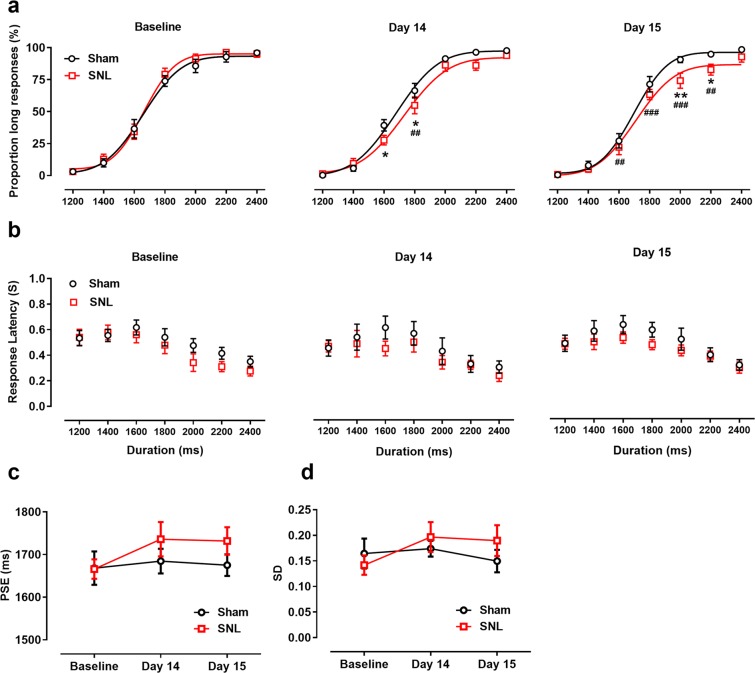
Results from temporal bisection tasks performed before and day 14 and day 15 after surgery are presented in Fig. 5. The fitting curve explains most variation in these experimental data. The goodness of fit of the Gaussian function for individual rats was ≥0.87. After surgery, the fitted function for the SNL group shifted to the right and downward compared with those for the sham group and baseline. On days 14 and 15, the PLs to some tone durations were lower after surgery [interactive effect: F(12, 216) = 2.468, p = 0.005; time effect: F(1, 18) = 3.073, p = 0.059, Fig. 5a]. Specifically, on day 14, rats with SNL had lower PLs for the 1600-ms (p = 0.034) and 1800-ms (p = 0.030) tones compared with the sham group, and a lower PL for the 1800-ms tone (p < 0.001) compared with baseline; on day 15, rats with SNL had lower PLs for the 2000-ms (p = 0.003) and 2200-ms (p = 0.043) tones compared with the sham group, and lower PLs for the 1600-ms (p = 0.009), 1800-ms (p < 0.001), and 2200-ms (p = 0.008) tones compared with baseline. Values for response latency during the temporal bisection task exhibited an inverted U shape (Fig. 5b). Response latency in the SNL group did not differ from that in the sham group or from baseline [interactive effect: F(2, 36) = 0.184, p = 0.833; time effect: F(2, 36) = 2.626, p = 0.086; group effect: F(1, 18) = 0.048, p = 0.829].
Figure 5.
Response patterns in temporal bisection tasks [Proportion of long response (PL) by duration and psychometric fitting curves] at baseline and on days 14 and 15 after SNL surgery. SNL surgery significantly reduced the PL in days 14 (middle panel) and days 15 (right panel) compared with the sham group and baseline (left panel) (a), but did not affect response latency in baseline (left panel) and days 14 (middle panel) and days 15 (right panel) (b). The point of subjective equality (PSE) (c) and standard deviations (SD) of fitting function (d) did not differ among the three sessions of the temporal bisection task. *p < 0.05, **p < 0.01 vs. sham group; ##p < 0.01, ###p < 0.001 vs. baseline; n = 10/group. Results are shown as means ± SEM.
Baseline and postoperative PSEs in the two groups did not differ significantly [interactive effect: F(2, 36) = 0.36, p = 0.700; Fig. 5c]. In addition, the SD of the fitting function did not differ across the three test sessions [interactive effect: F(2, 36) = 0.711, p = 0.498; Fig. 5d]. Average values from omitted trials did not differ from baseline (SNL group, 0.8 ± 0.2 trials; sham group, 0.9 ± 0.2 trials) to 14 days (SNL group, 1.3 ± 0.3 trials; sham group, 1.0 ± 0.15 trials) and 15 days (SNL group, 1.2 ± 0.2 trials; sham group, 1.0 ± 0.3 trials) after surgery [interactive effect: F(2, 36) = 1, p = 0.377].
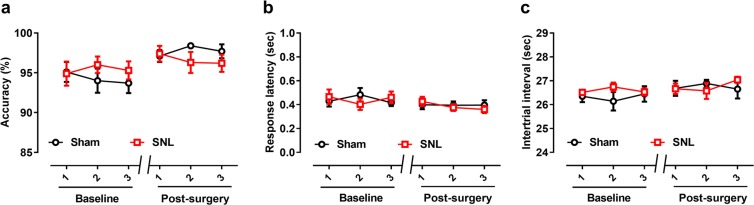
The two groups of rats showed no significant difference across six training sessions (three preoperative free-choice training sessions, three postoperative sessions) in response accuracy [interactive effect: F(5, 90) = 1.227, p = 0.303; Fig. 6a], response latency [interactive effect: F(5, 90) = 1.238, p = 0.298; Fig. 6b], or the ITI [interactive effect: F(5, 90) = 0.913, p = 0.476; Fig. 6c]. These results indicate that SNL surgery did not affect the rats’ basic physical activity or time discrimination ability.
Figure 6.
Comparison of response accuracy, the intertrial interval (ITI), and response latency in the last three training sessions before and three sessions after SNL surgery. (a) Before the test stage, the average response accuracy exceeded 90%, with no difference between groups. Response latency (b) and the ITI (c) did not differ between the SNL and sham groups. Results are shown as means ± SEM.
Discussion
In this study, we used a temporal bisection task to assess rats’ time perception under formalin-induced acute pain and SNL-induced neuropathic pain. We found that acute inflammatory pain prolonged the estimation of time in the range of 1.2–2.4 s, whereas neuropathic pain exhibited a complex effect on time perception. Neuropathic pain lowered the PLs to the intermediate durations within this range, but not affected the PSE significantly. Neither pain type affected temporal sensitivity. These results suggest that the acute and neuropathic pain states have different effects on time perception.
Our results for formalin-induced acute pain are consistent with those of human studies, which have demonstrated that subjects overestimate stimulus duration under acute pain19,20. One explanation for this overestimation is acceleration of the internal clock mechanism, as conceptualized via the pacemaker-accumulator (PA) model. This model assumes that the internal clock generates “pulses” in the process of time perception, and that the estimation of time is based on the cumulative number of these pulses1,41. High arousal can accelerate pulse accumulation, leading to a subjectively longer experience2. Human subjects under experimental pain exhibit symptoms of high physiological arousal, such as increased heart rate and blood pressure42,43. Formalin-induced acute inflammatory pain, used widely in animal studies44, also induces a high-arousal state by facilitating the release of noradrenaline45–48. Increased heart and respiratory rates have been observed in rats after formalin injection49,50. Therefore, according to the PA model, the high arousal associated with formalin-induced pain contributes to the overestimation of time.
In contrast, after SNL surgery, rats’ PLs were lower when judging tone duration, with no change in the PSE or temporal sensitivity, in this study. Time perception is more complex in patients with chronic pain21,22,51. The impairment of arousal and attention caused by chronic pain may co-contribute to the effect of this type of pain on time perception. In the framework of the PA model, attention plays the key role of a “switch”1,41. When attention is distracted, the internal clock mechanism may not be activated, leading to the underestimation of temporal duration10–12. Chronic pain was also found to induce attentional bias toward pain-related stimuli, thereby influencing attention-related temporal dynamics52. Patients with neuropathic pain exhibit impairment of attentional resources53,54, and rats with such pain show poor performance in attentional tasks55,56. Abnormal activity in the medial prefrontal cortex (mPFC), which is related to attentional function, has also been identified in human patients57 and in animals58 with chronic pain. These results suggest that neuropathic pain’s impairment of attentional function may contributes to the change of time perception. In the neuropathic pain experiment, we did not measure time perception during the acute stage because rats needed to recover from SNL surgery for a few days. Therefore, this study did not compare the impact of acute and chronic pain on time perception in the same model. This is a limitation of this study.
Thus, whereas formalin injection induced acute pain (i.e., due to high arousal) leads to time overestimation, the SNL surgery induced chronic neuropathic pain has a complex effect on time perception. Different forms of chronic pain may be accompanied by different arousal states, with different effect on time perception. Our findings for neuropathic pain are not consistent with those of previous human studies of migraineurs21,22,51. These subjects may maintain high arousal states for some time, especially during pain attacks59. They exhibit abnormal temporal discrimination during migraine attacks, but normal discrimination during headache-free periods24,25. We thus consider that high arousal during migraine attacks contributes to time overestimation. On the contrary, neuropathic pain can lead to noradrenergic injury in rats60, reduce arousal, and thus fail to produce the prolongation of time estimation.
In addition, dopamine (DA) neurons in the midbrain may also be an underlying mechanism of the abnormal changes of time perception during chronic pain. It has been proved that DA system plays an important role in the process of time perception61,62. Soares et al. found that activation or inhibition of midbrain DA neurons was sufficient to slow down or speed up time estimation in mice, respectively9. Meanwhile, chronic pain may influence DA neurons in the ventral tegmental area (VTA). It has been demonstrated that partial sciatic nerve ligation induced neuropathic pain significantly decreased the proportion of activated DA neurons in the lateral VTA63. However, Fu et al., found that that spared nerve injury (SNI) can significantly increase the firing rate of VTA DA neurons64. These opposite effects may be due to the heterogeneity of DA neurons in VTA and the opposite functions of medial and lateral neurons in VTA65. Therefore, the activities of VTA-DA neurons may play a complex role in mediating the effect of chronic pain on time perception.
The lengthening effect of acute pain on time perception may have an evolutionary basis. Under acute pain, time overestimation caused by high arousal and concentration can reduce injury by leading to withdrawal from a (potentially) harmful environment66. Droit-Volet67 suggested that the dynamic perception of time involves the defensive system, with the temporal lengthening effect when facing a threat associated with a state of alertness, mobility, and readiness to act (fight back or run away). Therefore, acute pain–induced temporal lengthening may be associated with the organism’s preparation to avoid (even inevitable) pain. Chronic pain, in contrast, is caused by a different set of physiological and pathological changes68. Patients have characterized neuropathic pain as widespread and inexplicable, with pain attacks occurring seemingly without provocation69. In the absence of an obvious source of injury to avoid, temporal lengthening may not be important under chronic pain.
Acute pain can also cause distraction. Previous behavioral studies have shown that formalin injection significantly disrupts attentional tasks in rats, with increased response latency and omissions16,17. Acute inflammatory pain can alter neuronal activity in the mPFC in rats18,70,71. In our preliminary experiments, we injected rats with 5% formalin, which resulted in a significantly higher omission rate during the task (data not shown), suggesting that acute pain caused strong distraction. To complete the experiment smoothly, we chose to use 1% formalin, which may not be a sufficient concentration to disrupt the temporal bisection task. This formalin dose caused less nociceptive behavior and fewer omissions, suggesting the absence of strong distraction. Meanwhile, the cumulative time spent in paw lifting and licking after formalin injection during the task were significantly shorter than that without task. This phenomenon suggests that temporal bisection task distracted some attention resource from the formalin pain. According to the limited attentional capacity theory, the more attentional resources used by distraction, the less resources are available for perceiving pain72.
We also took measures to rule out the effects of other confusing factors. Chronic pain was found depress the activity of the mesolimbic dopamine system and lead to decreased reward-seeking motivation in animals37. To exclude this factor, we examined temporal bisection task trial omission. Neither neuropathic nor formalin-induced pain increased the omission rate, indicating that the animals maintained reward-seeking motivation during the temporal bisection task. In addition, pain may impair physical activity73 and perceptual accuracy74. In this study, we found that SNL surgery had no effect on response latency, the ITI, or response accuracy, and that response latency did not increase after formalin injection. These results indicate that the SNL surgery and formalin injection did not affect physical activity during the task. Furthermore, some studies have suggested that depression associated with chronic pain mediates changes in time perception in human patients22,23. Depressed patients exhibit poor sensitivity to long-duration stimuli, but normal sensitivity to short-duration stimuli75,76. Therefore, we performed open field and sucrose preference tests, but did not find significant depressive-like behavior in the rats after SNL surgery (Supplementary information Fig. S1). Some studies have observed depressive symptoms at 2 weeks77 or 4–6 weeks64 after spare nerve injury (SNI) surgery in rats. These studies used the same species of animal as our present study, but a different neuropathic pain model. Different pain models, pain duration and different laboratory environment may jointly affect the induction of depressive-like behavior. In the future, longer observation time or other model attempts, such as SNI, may be helpful to further explore the effect of pain depression comorbidity on time perception.
In conclusion, we found that formalin-induced and neuropathic pain have different effects on time perception. An important issue in the field of pain research is the manner in which acute pain translates into chronic pain68. Our research suggests that the neurological mechanisms associated with time perception are related to the occurrence of chronic pain. Most importantly, findings from this study lay a foundation for further research on the mechanism by which pain regulation affects time perception.
Supplementary information
Acknowledgements
This work was funded by an NNSF (National Natural Science Foundation of China) grants (31671140) to N.W. an NNSFgrant (31271092) to J.-Y.W., an NNSF grant (31471061) to F.L., grants from CAS Key Laboratory of Mental Health, Institute of Psychology (KLMH 2014G01, KLMH2016K02) and a grant from the initiation fund of the CAS/SAFEA International Partnership Program for Creative Research Teams (Y2CX131003).
Author contributions
The idea for this research was conceived by N.W., J.Y.W. and F.L.; X.H.L. performed the experiments and analyzed the data. This paper was written primarily by X.H.L. and N.W. All authors have read and approved the final manuscript.
Data availability
The experimental generated and analyzed during this study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-019-55168-w.
References
- 1.Buhusi CV, Meck WH. What makes us tick? Functional and neural mechanisms of interval timing. Nature reviews. Neuroscience. 2005;6:755–765. doi: 10.1038/nrn1764. [DOI] [PubMed] [Google Scholar]
- 2.Lake JI, LaBar KS, Meck WH. Emotional modulation of interval timing and time perception. Neuroscience and biobehavioral reviews. 2016;64:403–420. doi: 10.1016/j.neubiorev.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gil S, Droit-Volet S. Time perception, depression and sadness. Behavioural processes. 2009;80:169–176. doi: 10.1016/j.beproc.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 4.Gable PA, Poole BD. Time flies when you’re having approach-motivated fun: effects of motivational intensity on time perception. Psychological science. 2012;23:879–886. doi: 10.1177/0956797611435817. [DOI] [PubMed] [Google Scholar]
- 5.Gil S, Droit-Volet S. “Time flies in the presence of angry faces”… depending on the temporal task used! Acta psychologica. 2011;136:354–362. doi: 10.1016/j.actpsy.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 6.Gil S, Niedenthal PM, Droit-Volet S. Anger and time perception in children. Emotion (Washington, D.C.) 2007;7:219–225. doi: 10.1037/1528-3542.7.1.219. [DOI] [PubMed] [Google Scholar]
- 7.Watts FN, Sharrock R. Fear and time estimation. Perceptual and motor skills. 1984;59:597–598. doi: 10.2466/pms.1984.59.2.597. [DOI] [PubMed] [Google Scholar]
- 8.Buetti S, Lleras A. Perceiving control over aversive and fearful events can alter how we experience those events: an investigation of time perception in spider-fearful individuals. Frontiers in psychology. 2012;3:337. doi: 10.3389/fpsyg.2012.00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soares S, Atallah BV, Paton JJ. Midbrain dopamine neurons control judgment of time. Science (New York, N.Y.) 2016;354:1273–1277. doi: 10.1126/science.aah5234. [DOI] [PubMed] [Google Scholar]
- 10.Macar F, Grondin S, Casini L. Controlled attention sharing influences time estimation. Memory & cognition. 1994;22:673–686. doi: 10.3758/BF03209252. [DOI] [PubMed] [Google Scholar]
- 11.Casini L, Macar F. Effects of attention manipulation on judgments of duration and of intensity in the visual modality. Memory & cognition. 1997;25:812–818. doi: 10.3758/BF03211325. [DOI] [PubMed] [Google Scholar]
- 12.Chen Z, O’Neill P. Processing demand modulates the effects of spatial attention on the judged duration of a brief stimulus. Percept Psychophys. 2001;63:1229–1238. doi: 10.3758/BF03194536. [DOI] [PubMed] [Google Scholar]
- 13.Melzack R, Wall PD. Pain mechanisms: a new theory. Science (New York, N.Y.) 1965;150:971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- 14.Tamburin S, et al. Cognition and emotional decision-making in chronic low back pain: an ERPs study during Iowa gambling task. Frontiers in psychology. 2014;5:1350. doi: 10.3389/fpsyg.2014.01350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pais-Vieira M, Aguiar P, Lima D, Galhardo V. Inflammatory pain disrupts the orbitofrontal neuronal activity and risk-assessment performance in a rodent decision-making task. Pain. 2012;153:1625–1635. doi: 10.1016/j.pain.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 16.Freitas KC, Hillhouse TM, Leitl MD, Negus SS. Effects of acute and sustained pain manipulations on performance in a visual-signal detection task of attention in rats. Drug development research. 2015;76:194–203. doi: 10.1002/ddr.21255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyette-Davis JA, Thompson CD, Fuchs PN. Alterations in attentional mechanisms in response to acute inflammatory pain and morphine administration. Neuroscience. 2008;151:558–563. doi: 10.1016/j.neuroscience.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 18.Cardoso-Cruz H, Sousa M, Vieira JB, Lima D, Galhardo V. Prefrontal cortex and mediodorsal thalamus reduced connectivity is associated with spatial working memory impairment in rats with inflammatory pain. Pain. 2013;154:2397–2406. doi: 10.1016/j.pain.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 19.Ogden RS, Moore D, Redfern L, McGlone F. The effect of pain and the anticipation of pain on temporal perception: A role for attention and arousal. Cognition & emotion. 2015;29:910–922. doi: 10.1080/02699931.2014.954529. [DOI] [PubMed] [Google Scholar]
- 20.Rey AE, et al. Pain dilates time perception. Scientific reports. 2017;7:15682. doi: 10.1038/s41598-017-15982-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang J, et al. The study of time perception in migraineurs. Headache. 2012;52:1483–1498. doi: 10.1111/j.1526-4610.2012.02222.x. [DOI] [PubMed] [Google Scholar]
- 22.van Laarhoven HW, Schilderman J, Verhagen CA, Prins JB. Time perception of cancer patients without evidence of disease and advanced cancer patients in a palliative, end-of-life-care setting. Cancer nursing. 2011;34:453–463. doi: 10.1097/NCC.0b013e31820f4eb7. [DOI] [PubMed] [Google Scholar]
- 23.Anagnostou E, Mitsikostas DD. Time perception in migraine sufferers: an experimental matched-pairs study. Cephalalgia. 2005;25:60–67. doi: 10.1111/j.1468-2982.2004.00809.x. [DOI] [PubMed] [Google Scholar]
- 24.Boran HE, Cengiz B, Bolay H. Somatosensory temporal discrimination is prolonged during migraine attacks. Headache. 2016;56:104–112. doi: 10.1111/head.12734. [DOI] [PubMed] [Google Scholar]
- 25.Vuralli D, Boran HE, Cengiz B, Coskun O, Bolay H. Somatosensory temporal discrimination remains intact in tension-type headache whereas it is disrupted in migraine attacks. Cephalalgia. 2017;37:1241–1247. doi: 10.1177/0333102416677050. [DOI] [PubMed] [Google Scholar]
- 26.Fayolle S, Gil S, Droit-Volet S. Fear and time: Fear speeds up the internal clock. Behavioural processes. 2015;120:135–140. doi: 10.1016/j.beproc.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 27.Meck WH. Selective adjustment of the speed of internal clock and memory processes. Journal of experimental psychology. Animal behavior processes. 1983;9:171–201. doi: 10.1037/0097-7403.9.2.171. [DOI] [PubMed] [Google Scholar]
- 28.Tipples J. When time stands still: fear-specific modulation of temporal bias due to threat. Emotion (Washington, D.C.) 2011;11:74–80. doi: 10.1037/a0022015. [DOI] [PubMed] [Google Scholar]
- 29.McClure EA, Saulsgiver KA, Wynne CD. Effects of D-amphetamine on temporal discrimination in pigeons. Behavioural pharmacology. 2005;16:193–208. doi: 10.1097/01.fbp.0000171773.69292.bd. [DOI] [PubMed] [Google Scholar]
- 30.Ward RD, Odum AL. Disruption of temporal discrimination and the choose-short effect. Learning & behavior. 2007;35:60–70. doi: 10.3758/BF03196075. [DOI] [PubMed] [Google Scholar]
- 31.Deane AR, Millar J, Bilkey DK, Ward RD. Maternal immune activation in rats produces temporal perception impairments in adult offspring analogous to those observed in schizophrenia. PloS one. 2017;12:e0187719. doi: 10.1371/journal.pone.0187719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Callu D, El Massioui N, Dutrieux G, Brown BL, Doyere V. Cognitive processing impairments in a supra-second temporal discrimination task in rats with cerebellar lesion. Neurobiology of learning and memory. 2009;91:250–259. doi: 10.1016/j.nlm.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 33.Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- 34.Dixon WJ. Efficient analysis of experimental observations. Annual review of pharmacology and toxicology. 1980;20:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- 35.Savage S, Ma D. Experimental behaviour testing: pain. British journal of anaesthesia. 2015;114:721–724. doi: 10.1093/bja/aeu346. [DOI] [PubMed] [Google Scholar]
- 36.Wei X, Sun Y, Luo F. Impaired Spinal Glucocorticoid Receptor Signaling Contributes to the Attenuating Effect of Depression on Mechanical Allodynia and Thermal Hyperalgesia in Rats with Neuropathic. Pain. Frontiers in cellular neuroscience. 2017;11:145. doi: 10.3389/fncel.2017.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwartz N, et al. Chronic pain. Decreased motivation during chronic pain requires long-term depression in the nucleus accumbens. Science (New York, N.Y.) 2014;345:535–542. doi: 10.1126/science.1253994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dang YH, et al. The role of dopamine receptors in ventrolateral orbital cortex-evoked anti-nociception in a rat model of neuropathic pain. Neuroscience. 2010;169:1872–1880. doi: 10.1016/j.neuroscience.2010.06.050. [DOI] [PubMed] [Google Scholar]
- 39.Wei L, et al. Activation of alpha1 adrenoceptors in ventrolateral orbital cortex attenuates allodynia induced by spared nerve injury in rats. Neurochemistry international. 2016;99:85–93. doi: 10.1016/j.neuint.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 40.Meck WH. Neuropharmacology of timing and time perception. Brain research. Cognitive brain research. 1996;3:227–242. doi: 10.1016/0926-6410(96)00009-2. [DOI] [PubMed] [Google Scholar]
- 41.Gibbon J, Church RM, Meck WH. Scalar timing in memory. Annals of the New York Academy of Sciences. 1984;423:52–77. doi: 10.1111/j.1749-6632.1984.tb23417.x. [DOI] [PubMed] [Google Scholar]
- 42.Roberts MH, Klatzkin RR, Mechlin B. Social Support Attenuates Physiological Stress Responses and Experimental Pain Sensitivity to Cold Pressor Pain. Annals of behavioral medicine: a publication of the Society of Behavioral Medicine. 2015;49:557–569. doi: 10.1007/s12160-015-9686-3. [DOI] [PubMed] [Google Scholar]
- 43.Sambo CF, Howard M, Kopelman M, Williams S, Fotopoulou A. Knowing you care: effects of perceived empathy and attachment style on pain perception. Pain. 2010;151:687–693. doi: 10.1016/j.pain.2010.08.035. [DOI] [PubMed] [Google Scholar]
- 44.Tjolsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. The formalin test: an evaluation of the method. Pain. 1992;51:5–17. doi: 10.1016/0304-3959(92)90003-T. [DOI] [PubMed] [Google Scholar]
- 45.Martins I, et al. Noradrenaline increases pain facilitation from the brain during inflammatory pain. Neuropharmacology. 2013;71:299–307. doi: 10.1016/j.neuropharm.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 46.Sajedianfard J, Khatami S, Semnanian S, Naghdi N, Jorjani M. In vivo measurement of noradrenaline in the locus coeruleus of rats during the formalin test: a microdialysis study. European journal of pharmacology. 2005;512:153–156. doi: 10.1016/j.ejphar.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 47.Berridge CW, Schmeichel BE, Espana RA. Noradrenergic modulation of wakefulness/arousal. Sleep medicine reviews. 2012;16:187–197. doi: 10.1016/j.smrv.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berridge CW. Noradrenergic modulation of arousal. Brain research reviews. 2008;58:1–17. doi: 10.1016/j.brainresrev.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Santuzzi CH, et al. High-frequency transcutaneous electrical nerve stimulation reduces pain and cardio-respiratory parameters in an animal model of acute pain: participation of peripheral serotonin. Physiotherapy theory and practice. 2013;29:630–638. doi: 10.3109/09593985.2013.774451. [DOI] [PubMed] [Google Scholar]
- 50.Barr GA. Maturation of the biphasic behavioral and heart rate response in the formalin test. Pharmacology, biochemistry, and behavior. 1998;60:329–335. doi: 10.1016/S0091-3057(97)00602-3. [DOI] [PubMed] [Google Scholar]
- 51.Vicario CM, Gulisano M, Martino D, Rizzo R. The perception of time in childhood migraine. Cephalalgia. 2014;34:548–553. doi: 10.1177/0333102413517774. [DOI] [PubMed] [Google Scholar]
- 52.Zheng C, Wang JY, Luo F. Painful faces-induced attentional blink modulated by top-down and bottom-up mechanisms. Frontiers in psychology. 2015;6:695. doi: 10.3389/fpsyg.2015.00695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sharpe L, Haggman S, Nicholas M, Dear BF, Refshauge K. Avoidance of affective pain stimuli predicts chronicity in patients with acute low back pain. Pain. 2014;155:45–52. doi: 10.1016/j.pain.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 54.Schoth DE, Nunes VD, Liossi C. Attentional bias towards pain-related information in chronic pain; a meta-analysis of visual-probe investigations. Clinical psychology review. 2012;32:13–25. doi: 10.1016/j.cpr.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 55.Higgins GA, et al. Enduring attentional deficits in rats treated with a peripheral nerve injury. Behavioural brain research. 2015;286:347–355. doi: 10.1016/j.bbr.2015.02.050. [DOI] [PubMed] [Google Scholar]
- 56.Leite-Almeida H, et al. Differential effects of left/right neuropathy on rats’ anxiety and cognitive behavior. Pain. 2012;153:2218–2225. doi: 10.1016/j.pain.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 57.Shi H, Yuan C, Dai Z, Ma H, Sheng L. Gray matter abnormalities associated with fibromyalgia: A meta-analysis of voxel-based morphometric studies. Seminars in arthritis and rheumatism. 2016;46:330–337. doi: 10.1016/j.semarthrit.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 58.Radzicki D, Pollema-Mays SL, Sanz-Clemente A, Martina M. Loss of M1 Receptor Dependent Cholinergic Excitation Contributes to mPFC Deactivation in Neuropathic Pain. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2017;37:2292–2304. doi: 10.1523/jneurosci.1553-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Engstrom M, et al. Sleep quality, arousal and pain thresholds in migraineurs: a blinded controlled polysomnographic study. The journal of headache and pain. 2013;14:12. doi: 10.1186/1129-2377-14-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alba-Delgado C, et al. Chronic pain leads to concomitant noradrenergic impairment and mood disorders. Biological psychiatry. 2013;73:54–62. doi: 10.1016/j.biopsych.2012.06.033. [DOI] [PubMed] [Google Scholar]
- 61.Meck WH. Neuroanatomical localization of an internal clock: a functional link between mesolimbic, nigrostriatal, and mesocortical dopaminergic systems. Brain research. 2006;1109:93–107. doi: 10.1016/j.brainres.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 62.Maricq AV, Church RM. The differential effects of haloperidol and methamphetamine on time estimation in the rat. Psychopharmacology. 1983;79:10–15. doi: 10.1007/bf00433008. [DOI] [PubMed] [Google Scholar]
- 63.Kami K, Tajima F, Senba E. Activation of mesolimbic reward system via laterodorsal tegmental nucleus and hypothalamus in exercise-induced hypoalgesia. Scientific reports. 2018;8:11540. doi: 10.1038/s41598-018-29915-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fu B, et al. Gabapentin regulates dopaminergic neuron firing and theta oscillation in the ventral tegmental area to reverse depression-like behavior in chronic neuropathic pain state. Journal of pain research. 2018;11:2247–2256. doi: 10.2147/jpr.S170167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lammel S, Lim BK, Malenka RC. Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacology. 2014;76(Pt B):351–359. doi: 10.1016/j.neuropharm.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baliki MN, Apkarian AV. Nociception, Pain, Negative Moods, and Behavior Selection. Neuron. 2015;87:474–491. doi: 10.1016/j.neuron.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Droit-Volet S. Time perception, emotions and mood disorders. Journal of physiology, Paris. 2013;107:255–264. doi: 10.1016/j.jphysparis.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 68.Kuner R, Flor H. Structural plasticity and reorganisation in chronic pain. Nature reviews. Neuroscience. 2016;18:20–30. doi: 10.1038/nrn.2016.162. [DOI] [PubMed] [Google Scholar]
- 69.Campbell JN, Meyer RA. Mechanisms of neuropathic pain. Neuron. 2006;52:77–92. doi: 10.1016/j.neuron.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li AL, Yang X, Chiao JC, Peng YB. Reduced local field potential power in the medial prefrontal cortex by noxious stimuli. Brain research bulletin. 2016;127:92–99. doi: 10.1016/j.brainresbull.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 71.Luongo L, et al. Role of metabotropic glutamate receptor 1 in the basolateral amygdala-driven prefrontal cortical deactivation in inflammatory pain in the rat. Neuropharmacology. 2013;66:317–329. doi: 10.1016/j.neuropharm.2012.05.047. [DOI] [PubMed] [Google Scholar]
- 72.Johnson MH. How does distraction work in the management of pain? Current pain and headache reports. 2005;9:90–95. doi: 10.1007/s11916-005-0044-1. [DOI] [PubMed] [Google Scholar]
- 73.Simons LE, Elman I, Borsook D. Psychological processing in chronic pain: a neural systems approach. Neuroscience and biobehavioral reviews. 2014;39:61–78. doi: 10.1016/j.neubiorev.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wiech K, et al. Influence of prior information on pain involves biased perceptual decision-making. Current biology: CB. 2014;24:R679–681. doi: 10.1016/j.cub.2014.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Msetfi RM, Murphy RA, Kornbrot DE. The effect of mild depression on time discrimination. Quarterly journal of experimental psychology (2006) 2012;65:632–645. doi: 10.1080/17470218.2011.608908. [DOI] [PubMed] [Google Scholar]
- 76.Sevigny MC, Everett J, Grondin S. Depression, attention, and time estimation. Brain and cognition. 2003;53:351–353. doi: 10.1016/S0278-2626(03)00141-6. [DOI] [PubMed] [Google Scholar]
- 77.Wang J, et al. A single subanesthetic dose of ketamine relieves depression-like behaviors induced by neuropathic pain in rats. Anesthesiology. 2011;115:812–821. doi: 10.1097/ALN.0b013e31822f16ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The experimental generated and analyzed during this study are available from the corresponding author on reasonable request.