Abstract
Objective
The purpose of this retrospective study was to evaluate the clinical and oncological results of combination treatment of short‐term preoperative denosumab (the receptor activator of nuclear factor kappa‐B ligand inhibitor) with surgery in unresectable or recurrent cases of giant cell tumor of the bone (GCTB).
Methods
Between 2016 and 2018, 11 eligible patients (1 man, 10 women, mean age 38.1 years) with grade 3 GCTB were treated with a combination of short‐term (six doses) preoperative denosumab and surgery in a single institution. The clinical, radiological, and pathological alteration after the denosumab treatment were compared. The oncological results of the combination therapy were also recorded. Meanwhile, adverse effects or complications of denosumab, if any, were reported.
Results
The median follow‐up time after surgical procedure was 30 months (range 13–45 months). After 3–4 denosumab injections, pain relief was observed in all patients. In two spine patients, the neurological status improved after four doses of treatment. Intraoperatively, the margin of the tumor became clear and the intensity of the tumor increased while the blood supply around and within the lesion decreased. Within the lesion, the typically soft and loose tissue were replaced by the tough and dense fibro‐osseous tissue. The mean diameter of the lesion before and after treatment was 61.55 ± 22.49 mm and 51.81 ± 21.12 mm, respectively, and the T‐score was 1.02 (P = 0.32). Variable calcification was observed at the periphery and within the lesion. A total of three patients experienced local recurrence in this study. In the resection group, only one extremity patient had soft tissue recurrence that was treated with en‐bloc excision. In the curettage group, two of three sacral tumor patients had local occurrence. Both refused re‐operation and restarted the monthly denosumab injection thereafter, and the lesions remained stable at the final follow up. Finally, no adverse effects or complications related to denosumab treatment were found.
Conclusion
For the unresectable or recurrent GCTB cases, short‐term (six doses) preoperative use of denosumab improved clinical symptoms, decreased the tumor size, and increased the tumor density. The changes in tumors, in turn, simplified the tumor removal manipulation and, subsequently, decreased the local recurrence for the resection surgery. For the curettage, the denosumab‐induced changes had mixed impacts, and shorter term (fewer than six doses) usage may be more appropriate. Our six‐dose regime was deemed safe, while the safety of long‐term use remains unknown.
Keywords: Bone, Denosumab, Giant cell tumor, Preoperative, Short‐term
Introduction
Giant cell tumors of the bones (GCTB) are benign, locally aggressive tumors that tend to recur. Giant cell tumors (GCT) typically arise at the end of long bones in skeletally mature individuals but can also occur in the axial skeleton and occasionally appear in children. The most commonly affected locations are the distal femur, the proximal tibia, and the distal radius. Patients with GCTB usually present with functional limitations, swelling at the affected site, and pain1, 2. GCTB account for approximately 3% to 6% of all biopsy‐analyzed primary bone tumors in Western countries, while in Asia, they comprise up to 20%3, 4, 5. If untreated, the clinical course is unpredictable, because of possible mechanical load failure and joint function compromise6. Therefore, whenever feasible, the standard treatment is surgery, with the aim of complete removal of the tumor while preserving the function7. For most GCTB, extended intralesional curettage with or without physical or surgical adjuvants is the management of choice. Adjuvant therapies include bone cement, phenol, ethanol, cryotherapy, and intravenous or oral bisphosphonates.
To be more specific, for the “expendable” bones, such as the proximal part of the fibula, en‐bloc resection is preferable. Nevertheless, some lesions are deemed to be unresectable, when the rate of morbidity and mortality predicted for a resection procedure would represent an unjustifiable risk. Typically, unresectable lesions refer to those of the axial skeleton, such as the sacrum and posterior elements of the spine3, 5, 8. Some recurrent GCTB are also considered to be inoperable. In these challenging cases, reported treatments include debulking surgery (incomplete removal) and/or the use of adjuvants such as embolization9, 10 and radiation therapy11, 12. However, the results for these options were less favorable, and high local occurrence was a major concern.
Fortunately, the molecular mechanism of GCTB has been recently discovered and provides a promising option for the management of the tumor. As is well known, histologically, there are three cell types in GCTB: mesenchymal stromal cells, mononuclear monocytes, and multinucleated osteoclast‐like giant cells. The stromal cells are the main neoplastic component of the tumor and express high levels of receptor activator of nuclear factor kappa‐B ligand (RANKL); the giant cells overexpress the receptor activator of nuclear factor kappa‐B (RANK) receptor13. The interaction between RANK and RANKL might be the main cause of robust osteoclastogenesis and extensive bone resorption in GCTB3, 7, 13.
Denosumab, a monoclonal antibody to RANKL, has been proved to inhibit the RANKL pathway and, thus, prevent osteoclast activation. It has more recently been introduced clinically as a promising therapeutic option for GCTB14, 15, 16, 17, 18, since it was reported that denosumab could halt the progression of GCT, alleviate the symptoms, and promote the bone formation within and around the lesion, which may help to facilitate ease of surgery or decrease the morbidity of the surgical procedure in selected cases6, 16, 19.
Despite the encouraging results, a few questions still exit6. First, consensus regarding denosumab treatment dose and duration has not been reached, with published reports describing widely varying denosumab treatment durations3, 20, 21, 22. Second, very few studies have exclusively evaluated the combination regime of “neoadjuvant” denosumab with surgery without postoperative denosumab use, especially for those unresectable or recurrent GCTB.
Therefore, we decided to evaluate a single‐institution series of GCTB patients treated with preoperative denosumab and surgery without postoperative denosumab. In addition, we attempted to answer the following questions:
-
(i)
Does our combination strategy of “neoadjuvant” denosumab with surgery reduce the risk of local recurrence, especially for the unresectable or recurrent GCTB?
-
(ii)
Are there adverse effects of short‐term preoperative use of denosumab and, if any, what are they?
-
(iii)
Should we tailor the denosumab regime to fit different surgery strategies of resection and curettage?
Materials and Methods
Inclusion and Exclusion Criteria
Inclusion criteria were as follows: (i) patients with histologically confirmed GCTB by preoperative needle biopsy; (ii) patients treated with preoperative use of denosumab followed by definitive surgery without postoperative denosumab; (iii) outcomes include clinical improvement, radiographic parameters, pathologic evaluation, oncologic result, and complications; and (iv) postoperative follow‐up for at least 1 year, with clinical and radiological assessments performed.
Exclusion criteria were as follows: (i) patient with a history of embolism or bisphosphonate for treatment of GCT; (ii) patient had less than 12 months of follow up after surgery; and (iii) incomplete medical records and radiographic data, especially CT and MRI.
Basic Information
We searched the hospital records and images in our database for patients who were treated for a GCTB between January 2016 and December 2018. Our institute review board waived approval for the human protocol for this study.
In the present study, there were 11 patients (1 man and 10 woman). The mean age was 38.1 years (19 to 67). Eight patients had primary GCTB of axial and girdle bones. The other three presented with recurrent disease arising from the extremity bones, and they all had extensive soft tissue extension. To be more specific, the locations included the spine (n = 3), the sacrum (n = 3), the pelvis (n = 2), the proximal humerus (n = 1), the distal radius (n = 1), and the distal tibia (n = 1). A more detailed overview of the patients is shown in Table 1.
Table 1.
Patient profiles, clinical and oncologic results
| S. No. | Sex | Age (yrs) | Site | Primary or recurrent | Diameter (mm) | Surgery | Fx‐up (mths) | Local recurrence | ||
|---|---|---|---|---|---|---|---|---|---|---|
| pre | post | |||||||||
| 1 | F | 23 | Thoracic 6–8 | Primary | 34 | 24 | Resection | 40 | ‐ | |
| 2 | F | 25 | Lumbar 4 | Primary | 45 | 35 | Resection | 28 | ‐ | |
| 3 | F | 62 | Thoracic 10 | Primary | 34 | 20 | Resection | 26 | ‐ | |
| 4 | F | 52 | Acetabulum | Primary | 65 | 59 | Resection | 14 | ‐ | |
| 5 | F | 27 | Ilia&sacrum | Primary | 104 | 90 | Resection | 43 | ‐ | |
| 6 | F | 29 | Sacrum2‐4 | Primary | 64 | 55 | Curettage | 29 | ‐ | |
| 7 | M | 24 | Sacrum1‐3 | Primary | 71 | 64 | Curettage | 41 | Yes | |
| 8 | F | 55 | Sacrum1‐3 | Primary | 82 | 70 | Curettage | 45 | Yes | |
| 9 | F | 36 | Dx tibia | Recurrent | 41 | 33 | Resection | 13 | ‐ | |
| 10 | F | 67 | Dx radius | Recurrent | 54 | 47 | Resection | 14 | ‐ | |
| 11 | F | 19 | Px humerus | Recurrent | 83 | 73 | Resection | 37 | Yes | |
Dx, distal; Fx‐up, Follow‐up; post‐, posttreatment; pre, pretreatment; Px, proximal; S.No., series number.
Treatment Strategy
Before initiation of denosumab treatment, all patients were evaluated with plain radiographs, CT, and MRI of the involved region, CT (Figs. 1A–H and 2A,B) of the chest, and bone scans. According to the Campanacci classification23, all the tumors were classified as aggressive stage 3 lesions. Moreover, the baseline laboratory tests, such as blood count, renal function, liver function, and electrolytes, were also collected. Dental radiographs were routinely reviewed to exclude risk factors of jaw osteonecrosis.
Figure 1.
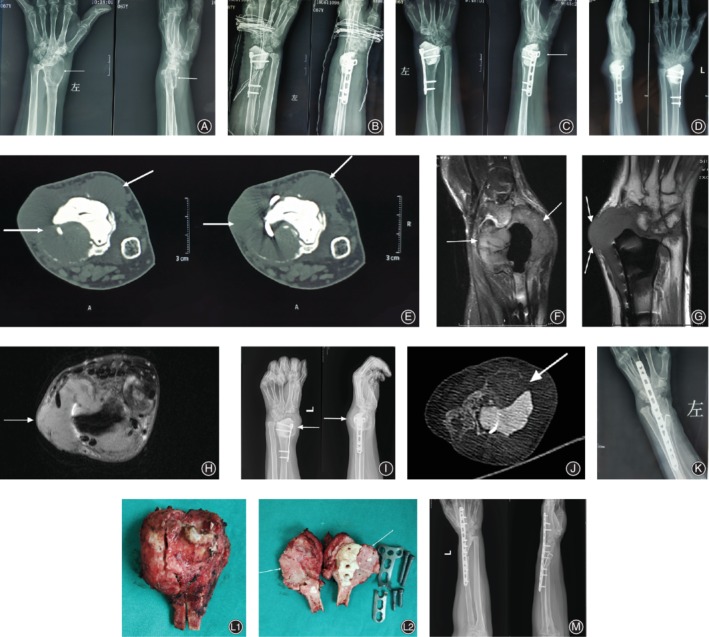
A 67‐year‐old female with a recurrent left distal radius GCTB. (A‐C) Images of the primary curettage surgery in outer hospital. (A) Plain radiograph (before surgery) of a typical GCTB demonstrating a lytic and expansile lesion (arrows) in the distal radius. (B) The immediately postoperative radiographs of the surgery of curettage, cementation and internal fixation. (C) Plain radiograph showing local recurrence (arrows) 2 months after the surgery. 4 months after the primary curettage surgery, the patient was referred to our hospital. (D‐H) Plain radiograph(D), CT (axial E), and MRI (sagittal, coronal, axial F‐H) showing local recurrence of the distal radius with cortical discontinuity and massive soft tissue component (arrows). (I‐J) Plain radiograph (I) and CT (J axial) following denosumab treatment demonstrating significant shrinkage of tumor size, and calcified sclerotic rim(arrows) and central sclerosis. (K) Immediately postoperative radiographs following a resection procedure with allograft bone reconstruction and wrist arthrodesis. (L) Resection material: 1. cross section of the resected tumor. 2. the new formed tissue after denosumab treatment (arrows). (M) Plain radiograph showing no signs of local recurrence 12months after the 2nd surgery.
Figure 2.
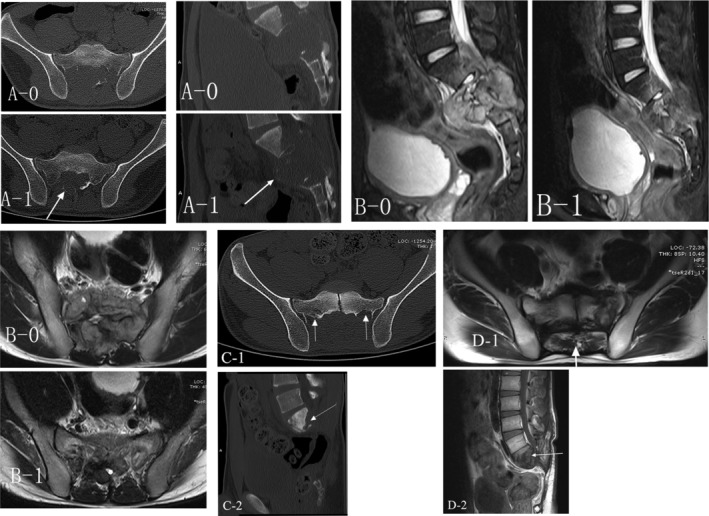
A 24‐year‐old male with primary sacral (S1‐3) GCTB treated by definitive curettage surgery. Local recurrence was observed radiologically 18 months following curettage surgery A (CT) and B (MRI) demonstrating the comparison between before (A‐0, B‐0) and after (A‐1, B‐1) the denosumab treatment. Arrows showing the new formed calcified sclerotic rim. C (CT 18 months after surgery) demonstrating local recurrence and arrows indicating the recurrent lesion and D (MRI 41 months after surgery) showing no signs of worsening of local recurrence.
All patients were treated with subcutaneous injections of 120 mg denosumab (Xgeva; Amgen, CA, USA) every 4 weeks, with additional doses on days 8 and 15 of the first cycle. All patients received three cycles of treatment. That is, six doses of denosumab were administered preoperatively. No additional denosumab was given postoperatively. Meanwhile, all patients were given supplements with calcium (500 mg/day) and vitamin D (400 IU/day). Laboratory tests were repeated very 4 weeks.
Two weeks after final administration of denosumab, the lesions of interest were routinely re‐evaluated with MRI and CT (Figs. 1I,J and 2A,B). Subsequently, definitive surgical procedures were carried out. Eight patients underwent resection surgery. The other three sacral tumor patients, had curettage surgery because morbidity of resection was deemed too high for such a benign tumor. Curettage/resection specimens obtained were routinely sent for histopathology.
Follow‐Up Contents
After surgery, patients had a clinical and radiographic review at 3–4 monthly intervals for the first 2 years, at 6‐month intervals for the following 3 years, and annually thereafter2. A chest radiograph and bone scan were performed once a year. Survival status was evaluated according to both local and distant tumor control. The data of the most recent follow up was used for this series.
Evaluation Criteria
Visual Analogue Scale
The patient pain level was evaluated with the visual analogue scale (VAS) scoring system. The scoring system standard (scores from 0 to 10) was as follows: 0 = painless; less than 3 = mild pain that the patient could endure; 4–6 = patient was in pain that could be endured and was able to sleep; and 7–10 = patient had intense pain and was unable to tolerate the pain.
Frankel Grade System
Before and after administration of denosumab, the neurological status of spine patients was determined based on the Frankel grade system. The system provides an assessment of spinal cord function. There are five grades: Grade A, complete neurological injury; Grade B, preserved sensation only; Grade C: preserved motor, nonfunctional; Grade D: preserved motor, functional; and Grade E: normal motor function.
Oncological Outcome
Oncological results were evaluated based on both local recurrence and distal metastasis. The patients were routinely reviewed with local MRI/CT and chest CT. The clinical and radiological assessments were used to determine the survival status. If malignant transformation was suspected, biopsy was routinely performed.
Complications
The complications related to denosumab were as follows: hematologic, liver, and renal function abnormalities, jaw osteonecrosis, and malignant transformation of GCTB. Oncological failure was not regarded as a complication.
Statistical Analysis
We performed the statistical analyses using the software of SPSS Statistics 19.0 (IBM SPSS, Chicago, IL, USA). Mean and standard deviation were calculated for quantitative variables consistent with the normal distribution. Differences of the lesion parameter before and after treatment were analyzed using the t‐test. A P‐value less than 0.05 was considered significant.
Results
The median follow‐up time after the surgical procedure was 30 months (range 13–45 months). All patients were followed for longer than 1 year.
Clinical outcome
After three to four injections, all patients experienced pain relief and the VAS score ranged from 0 to 2. The three extremity patients and two pelvic patients demonstrated a detectable reduction in volume during treatment. In addition, the neurological status of two spine patients improved from Frankel grade C to Frankel grade D after four doses of treatment.
Intraoperatively, for the resection surgery, the margin of the tumor was clear and could be more easily identified from the surrounding tissue. The intensity of the tumor increased and the blood supply around the lesion decreased. However, for the curettage group, the typically soft and loose neoplastic tissue was not observed; instead, the lesion was filled with tough and dense fibro‐osseous tissue. Although the blood loss was also reduced, the identification of the “safe margin” was more challenging.
Radiologic Evaluation
The mean diameter of the lesion measured on CT/MRI before and after treatment was 61.55 ± 22.49 mm and 51.81 ± 21.12 mm, respectively, and the P‐value was 0.32 (T‐score = 1.02). All patients had a complete or close to complete calcified sclerotic rim (Fig. 1I,J, Fig. 2A,B). In all cases, variable newly formed bone was observed inside the lesion.
Pathologic Evaluation
Macroscopically, in all cases, the typical GCT tissue was replaced by tough or firm fibro‐osseous tumor with a variable reaction shell. On the cross‐section, the newly formed tissue was grayish (Fig. 1L).
Microscopically, all cases showed remarkable depletion of giant cells, and even no giant cell was detected in one case. Proliferation of mononuclear cells and varying degrees of ossification were also observed. A dramatic decrease of neoplastic angiogenesis was another important finding.
Oncologic Outcome
All patients were alive at the final follow up. The patient with a proximal humerus GCT developed a local soft tissue recurrence 18 months after the resection surgery. She underwent en‐bloc excision of the soft tissue recurrence and remained disease‐free for 9 months. Two of the three sacral tumor patients had local occurrence at 6 and 18 months following curettage surgery, respectively (Fig. 2C,D). Both refused surgery and restarted the monthly denosumab injection thereafter; the lesion remained stable at the latest follow up. No metastases were observed in our series. No malignant transformation of GCTB occurred during and after the treatment.
Complications
No adverse effects or any complications related to denosumab treatment were found. No hematologic, liver, and renal function abnormalities were detected during or after treatment. No signs of jaw osteonecrosis, thus far, have developed.
Discussion
In the history of treating GCTB, the introduction of denosumab was regarded as “revolutionary,” because it greatly altered the clinical course of GCTB and the whole approach. In 2013, the U.S. Food and Drug Administration approved the use of denosumab for treating adults and skeletally mature adolescents with unresectable GCTB or when resection is likely to result in severe morbidity5. In 2017, the NCCN Guideline also recommended that denosumab be given in cases of inoperable axial (spine, pelvic, and sacrum) lesions and metastatic lesions24.
However, controversy regarding the use of denosumab in GCTB has remained. Some researchers have reported that the preoperative use of denosumab may be associated with an increased risk of local recurrences of GCTB6, 25, 26. In addition, the high cost of denosumab had to be borne by the patient themselves in the less developed area in which our study was performed, which means there was a heavy economic burden. Therefore, after thorough discussion with the multidisciplinary board in our institute, a stricter indication for denosumab, thus indirectly the inclusion in this study, was eventually made. Only patients with locally aggressive spine tumor lesions and recurrent extremity lesions in whom resection or amputation might be the only option were enrolled. We also recommended that three cycles (six doses) of preoperative denosumab treatment should be used in all patients.
In our study, after the denosumab treatment, varying reduction of the lesion volume were palpated for the extremity and pelvic patients. Radiologically, the lesion diameter was also somewhat decreased in all cases, although the difference between the pretreatment and posttreatment diameter was not statistically significant. The shrinkage of tumor volume may be an important reason for the neurological improvement of the two spine patients in the present study. In addition, pain relief was observed in all patients. Our beneficial results compared favorably with those in the literature6, 8, 27. However, “non‐responders,” whose lesions were progressive under denosumab treatment were also reported by Müller DA et al.6. In their 25‐case series, a total of four patients (16%) were classified as non‐responders. Fortunately, the patients had no negative consequences except the risk of possible worsening of the local situation with the delaying of definite surgery. Therefore, the possibility of non‐responders should be kept in mind when using the drug. Clinical and radiological surveillance during the denosumab treatment was necessary, and the risk factors and molecular mechanisms for non‐responders warranted further investigation.
A total of three patients experienced local recurrence in our 11‐case study. In the resection group, only one extremity patient had recurrence. The remaining three spine and two pelvic patients had no recurrence, which was a very encouraging result. A high recurrence rate of approximately 25%–50% after the mobile spine GCT surgery had been reported28, 29, 30, 31. The reported local recurrence rate of pelvic GCT was 7% to 75%32, 33, 34, 35. Our favorable result was mainly due to changes after denosumab treatment. First, as was observed in surgery, the blood supply of tumors and the surrounding tissue decreased considerably, which simplified the procedure. Second, the newly formed osseous rim after denosumab made it easier for the surgeon to identify the safe margin, therefore decreasing the possibility of injury to important adjacent neurovascular structures, especially the spinal cord and major blood vessels. Third, the increased tumor density facilitated effective separation procedures with less concern about inadvertent tumor cells spilling. Our results are in accord with previous research25, 28, 31, 36. Of note, compared with the research, our denosumab duration was shorter. Based on our findings, we assumed that short‐term duration (six doses) may be enough to achieve the desired result for the resection. We should keep in mind that less drug usage means less risk of side effects and complications.
In the curettage group, two‐thirds of sacral tumor patients developed local occurrence following surgery. The high recurrence could be explained by the fact that the lesion involved sacrum 1–3. Resection surgery was not chosen because the morbidity of sacrificing of sacral 1–3 nerves was too severe given the benign nature of GCTB. Based on our experience, for resection surgery, preoperative denosumab had a double‐edged sword effect on tumor indentification and resection. On one hand, the decrease of microvascular density induced by the drug remarkably reduced intraoperative bleeding. On the other hand, the typical friable tumor tissue is replaced with gritty fibro‐osseous tissue, making it very difficult to separate the nerve root from the surrounding tissue. The newly formed ossified rim and gritty fibro‐osseous tissue make it extremely hard for the surgeon to identifying the safe margin.
Our findings are similar with those of previous studies6, 26, 27. Moreover, histologically, after denosumab the giant cells disappeared almost completely, but the true neoplastic cells of stromal cells harbored in the newly formed bone persisted26, 37. Mak et al.13. found that once the giant cell tumor tissue was no longer exposed to denosumab, the stromal cells continued to proliferate in vitro, albeit to a lesser degree. Errani et al. reported that the local recurrence rates were 60% (15) of 25 GCTB patients treated with curettage and denosumab and 16% (36) of 222 patients treated with curettage alone26. Therefore, for the curettage surgery, whether or not to use denosumab is still debatable. We do think that preoperative denosumab use should be beneficial if used appropriately. Some authors have recommended that ideally curettage should be done within 3 to 4 months of starting denosumab7, 14. Based on our limited experience, we supposed that a shorter duration (fewer than six doses) may be more appropriate for the curettage, especially for axial lesions. When curettage surgery was indicated, whether or not using denosumab, and how to use if necessary, must be taken into consideration to better balance the risks and benefits.
No complications of preoperative denosumab treatment were observed in our small series. We caution readers that complications may not be found in our series with a relatively short‐term follow up. In other studies, several adverse effects have been reported. Of note, osteonecrosis of the jaw (ONJ) is an uncommon but potentially serious and debilitating complication38, 39, 40. Fortunately, this adverse event can be significantly reduced by dental screening and treatments of oral disease prior to treatment40, 41. Malignant transformation to sarcoma, a very rare but very serious complication, has also been reported42, 43. Together with the reported findings, we made a tentative conclusion that short course denosumab was relatively safe and close survillance was mandatory.
Our study has several limitations. The major limitation was selection bias. The study was retrospective and randomization was not possible. The indication for denosumab treatment was made in a multidisciplinary setting. Only patients locally aggressive, extended tumor axial lesions or difficult recurrent extremity lesions were enrolled. Our results may not be entirely applicable for the purpose of “downstaging” in the primary extremities aggressive GCTB. Another limitation was the small sample size, which was due to the fact that aggressive GCTB is not a common disease and the cases that met our inclusion criteria were relatively scare. With the limited numbers available, although high local recurrence was observed in the curettage group, we could not conclude that preoperative denosumab increases the risk of recurrence of curettage surgery. A multi institutional, prospective study of a larger number of patients is necessary to further determine the efficacy and safety of denosumab in treating GCTB.
Nevertheless, compared with other studies, the major strength of this study was that standardization on the number of doses and the frequency of administration of denosumab. In our series, all patients were treated preoperatively with the same regime and were evaluated using the same methods. To the best of our knowledge, this kind of standardization has seldom been seen in published reports. recurrent) GCTB cases, preoperative use of denosumab alleviated clinical symptoms, decreased the tumor size, and increased the tumor density. Those changes, in turn, greatly simplified the tumor removal manipulation and the local recurrence was decreased for the resection surgery. However, for the curettage surgery, the denosumab‐induced changes had a double‐edged sword impact on the procedure. Of note, the changes made it more difficult to identify the safe margin during the operation, and whether denosumab reduces local recurrence warrants further investigation. According to our experience, short‐term (six doses) preoperative denosumab may be enough for resection surgery; a shorter course (fewer than six doses) may be more appropriate for the curettage surgery. Our six‐dose regime was deemed to be safe, while the safety of long‐term remains unknown.
Acknowledgments
Without our patients this study would not have been possible, and we thank them for all their support and willingness to participate. This work was supported by a grant from the Aiding Project for Introduction of Overseas Students in Hebei Province 2018 (No. C201853) and the Key R & D Plan Project of Hebei Science and Technology Department (No. 18277798D).
Disclosure: The authors declare no conflict of interest.
References
- 1. Agarwal MG, Gundavda MK, Gupta R, Reddy R. Does denosumab change the giant cell tumor treatment strategy? Lessons learned from early experience. Clin Orthop Relat Res, 2018, 476: 1773–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Qi D, Wang P, Ye Z, et al Clinical and radiographic results of reconstruction with fibular autograft for distal radius giant cell tumor. Orthop Surg, 2016, 8: 196–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thornley P, Habib A, Bozzo A, Evaniew N, Ghert M. The role of denosumab in the modern treatment of giant cell tumor of bone. JBJS Rev, 2017, 5: e4. [DOI] [PubMed] [Google Scholar]
- 4. Brodowicz T, Hemetsberger M, Windhager R. Denosumab for the treatment of giant cell tumor of the bone. Future Oncol, 2015, 11: 1881–1894. [DOI] [PubMed] [Google Scholar]
- 5. López‐Pousa A, Broto J, Garrido T, Vázquez J. Giant cell tumour of bone: new treatments in development. Clin Transl Oncol, 2015, 17: 419–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Müller DA, Beltrami G, Scoccianti G, Campanacci DA, Franchi A, Capanna R. Risks and benefits of combining denosumab and surgery in giant cell tumor of bone‐a case series. World J Surg Oncol, 2016, 14: 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gaston CL, Grimer RJ, Parry M, et al Current status and unanswered questions on the use of denosumab in giant cell tumor of bone. Clin Sarcoma Res, 2016, 6: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ueda T, Morioka H, Nishida Y, et al Objective tumor response to denosumab in patients with giant cell tumor of bone: a multicenter phase II trial. Ann Oncol, 2015, 26: 2149–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. He S, Xu W, Sun Z, Liu WB, et al Selective arterial embolization for the treatment of sacral and pelvic giant cell tumor: a systematic review. Orthop Surg, 2017, 9: 139–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ji T, Yang Y, Wang Y, Sun K, Guo W. Combining of serial embolization and denosumab for large sacropelvic giant cell tumor: case report of 3 cases. Medicine (Baltimore), 2017, 96: e7799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shi W, Indelicato DJ, Reith J, et al Radiotherapy in the management of giant cell tumor of bone. Am J Clin Oncol, 2013, 36: 505–508. [DOI] [PubMed] [Google Scholar]
- 12. Kriz J, Eich HT, Mücke R, et al Radiotherapy for giant cell tumors of the bone: a safe and effective treatment modality. Anticancer Res, 2012, 32: 2069–2073. [PubMed] [Google Scholar]
- 13. Mak IWY, Evaniew N, Popovic S, Tozer R, Ghert M. A translational study of the neoplastic cells of giant cell tumor of bone following neoadjuvant denosumab. J Bone Joint Surg Am, 2014, 96: e127. [DOI] [PubMed] [Google Scholar]
- 14. Rutkowski P, Gaston L, Borkowska A, et al Denosumab treatment of inoperable or locally advanced giant cell tumor of bone – multicenter analysis outside clinical trial. Eur J Surg Oncol, 2018, 44: 1384–1390. [DOI] [PubMed] [Google Scholar]
- 15. Engellau J, Seeger L, Grimer R, et al Assessment of denosumab treatment effects and imaging response in patients with giant cell tumor of bone. World J Surg Oncol, 2018, 16: 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chawla S, Henshaw R, Seeger L, et al Safety and efficacy of denosumab for adults and skeletally mature adolescents with giant cell tumour of bone: interim analysis of an open‐label, parallel‐group, phase 2 study. Lancet Oncol, 2013, 14: 901–908. [DOI] [PubMed] [Google Scholar]
- 17. Errani C, Tsukamoto S, Mavrogenis AF. How safe and effective is denosumab for bone giant cell tumour? Int Orthop, 2017, 41: 2397–2400. [DOI] [PubMed] [Google Scholar]
- 18. Mukaihara K, Suehara Y, Kohsaka S, et al Protein expression profiling of giant cell tumors of bone treated with denosumab. PLoS One, 2016, 11: e0148401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thomas D, Henshaw R, Skubitz K, et al Denosumab in patients with giant‐cell tumour of bone: an open‐label, phase 2 study. Lancet Oncol, 2010, 11: 275–280. [DOI] [PubMed] [Google Scholar]
- 20. Palmerini E, Chawla NS, Ferrari S, et al Denosumab in advanced/unresectable giant‐cell tumour of bone (GCTB): for how long? Eur J Cancer, 2017, 76: 118–124. [DOI] [PubMed] [Google Scholar]
- 21. Boye K, Jebsen NL, Zaikova O, et al Denosumab in patients with giant‐cell tumor of bone in Norway: results from a nationwide cohort. Acta Oncol, 2017, 56: 479–483. [DOI] [PubMed] [Google Scholar]
- 22. Li S, Chen P, Yang Q. Denosumab versus zoledronic acid in cases of surgically unsalvageable giant cell tumor of bone: a randomized clinical trial. J Bone Oncol, 2019, 15: 100217. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23. Campanacci M, Baldini N, Boriani S, Sudanese A. Giant‐cell tumor of bone. J Bone Joint Surg Am, 1987, 69: 106–114. [PubMed] [Google Scholar]
- 24. Biermann JS, Chow W, Reed DR, et al NCCN guidelines insights: bone cancer. J Natl Compr Canc Netw, 2017, 15: 155–167. [DOI] [PubMed] [Google Scholar]
- 25. Scoccianti G, Totti F, Scorianz M, et al Preoperative denosumab with curettage and cryotherapy in giant cell tumor of bone: is there an increased risk of local recurrence? Clin Orthop Relat Res, 2018, 476: 1783–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Errani C, Tsukamoto S, Leone G, et al Denosumab may increase the risk of local recurrence in patients with giant‐cell tumor of bone treated with curettage. J Bone Joint Surg Am, 2018, 100: 496–504. [DOI] [PubMed] [Google Scholar]
- 27. Puri A, Gulia A, Hegde P, Verma V, Rekhi B. Neoadjuvant denosumab: its role and results in operable cases of giant cell tumour of bone. Bone Joint J, 2019, 101‐B: 170–177. [DOI] [PubMed] [Google Scholar]
- 28. Rutkowski P, Ferrari S, Grimer RJ, et al Surgical downstaging in an open‐label phase II trial of denosumab in patients with giant cell tumor of bone. Ann Surg Oncol, 2015, 22: 2860–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Luksanapruksa P, Buchowski JM, Singhatanadgige W, Rose PC, Bumpass DB. Management of spinal giant cell tumors. Spine J, 2016, 16: 259–269. [DOI] [PubMed] [Google Scholar]
- 30. Ma Y, Li J, Pan J, et al Treatment options and prognosis for repeatedly recurrent giant cell tumor of the spine. Eur Spine J, 2016, 25: 4033–4042. [DOI] [PubMed] [Google Scholar]
- 31. de Carvalho Cavalcante RA, Silva Marques RA, dos Santos VG, et al Spondylectomy for giant cell tumor after denosumab therapy. Spine (Phila Pa 1976), 2016, 41: E178–E182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Guo W, Sun X, Zang J, Qu H. Intralesional excision versus wide resection for giant cell tumor involving the acetabulum: which is better? Clin Orthop Relat Res, 2012, 470: 1213–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Leggon RE, Zlotecki R, Reith J, Scarborough MT. Giant cell tumor of the pelvis and sacrum: 17 cases and analysis of the literature. Clin Orthop Relat Res, 2004, 423: 196–207. [DOI] [PubMed] [Google Scholar]
- 34. Zheng K, Wang Z, Wu S, et al Giant cell tumor of the pelvis: a systematic review. Orthop Surg, 2015, 7: 102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tang X, Guo W, Yang R, Yan T, Tang S, Li D. Acetabular reconstruction with femoral head autograft after Intraarticular resection of periacetabular tumors is durable at short‐term followup. Clin Orthop Relat Res, 2017, 475: 3060–3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Deveci MA, Paydaş S, Gönlüşen G, Özkan C, Biçer ÖS, Tekin M. Clinical and pathological results of denosumab treatment for giant cell tumors of bone: prospective study of 14 cases. Acta Orthop Traumatol Turc, 2017, 51: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Girolami I, Mancini I, Simoni A, et al Denosumab treated giant cell tumour of bone: a morphological, immunohistochemical and molecular analysis of a series. J Clin Pathol, 2016, 69: 240–247. [DOI] [PubMed] [Google Scholar]
- 38. Fusco V, Rossi M, De Martino I, Alessio M, Fasciolo A, Numico G. Incidence of osteonecrosis of the jaw (ONJ) in cancer patients with bone metastases treated with bisphosphonates and/or denosumab: some comments and questions. Acta Clin Belg, 2018, 73: 163–164. [DOI] [PubMed] [Google Scholar]
- 39. Turner B, Drudge‐Coates L, Ali S, et al Osteonecrosis of the jaw in patients receiving bone‐targeted therapies: an overview–part I. Urol Nurs, 2016, 111–116: 36. [PubMed] [Google Scholar]
- 40. Di Fede O, Panzarella V, Mauceri R, et al The dental management of patients at risk of medication‐related osteonecrosis of the jaw: new paradigm of primary prevention. Biomed Res Int, 2018, 2018: 2684924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Saad F, Brown JE, Van Poznak C, et al Incidence, risk factors, and outcomes of osteonecrosis of the jaw: integrated analysis from three blinded active‐controlled phase III trials in cancer patients with bone metastases. Ann Oncol, 2012, 23: 1341–1347. [DOI] [PubMed] [Google Scholar]
- 42. Broehm CJ, Garbrecht EL, Wood J, Bocklage T. Two cases of sarcoma arising in giant cell tumor of bone treated with denosumab. Case Rep Med, 2015, 2015: 767198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Aponte‐Tinao LA, Piuzzi NS, Roitman P, Farfalli GL. A high‐grade sarcoma arising in a patient with recurrent benign giant cell tumor of the proximal tibia while receiving treatment with denosumab. Clin Orthop Relat Res, 2015, 473: 3050–3055. [DOI] [PMC free article] [PubMed] [Google Scholar]


