Summary
The intricate host–microbial interaction and the overwhelming complexity of the mucosal immune system in the adult host raise the question of how this system is initially established. Here, we propose the implementation of the concept of the ‘postnatal window of opportunity’ into the model of a ‘layered immunity’ to explain how the newborn's mucosal immune system matures and how host–microbial immune homeostasis is established after birth. We outline the concept of a timed succession of non‐redundant phases during postnatal immune development and discuss the possible influence of external factors and conditions.
Keywords: layered immunity, mucosal immunology, neonatal window of opportunity, postnatal development
The intricate host–microbial interaction and the overwhelming complexity of the mucosal immune system raise the question of how this system is initially established. Here, we implement the concept of the ‘postnatal window of opportunity’ into the model of a ‘layered immunity’ and formulate a concept of a timed succession of non‐redundant phases during postnatal immune development. This concept explains how host–microbial immune homeostasis is established after birth and we outline and discuss the possible influence of external factors and conditions.
Abbreviations
- GAP
goblet cell‐associated passages
- IFN
interferon
- IL
interleukin
- NEC
necrotizing enterocolitis
- NK
natural killer
- Nod
nucleotide‐binding oligomerization domain‐like
- PAMP
pathogen‐associated molecular pattern
- SLO
secondary lymphoid tissue
- Th cell
T helper cell
- TLR
Toll‐like receptor
- Treg cell
regulatory T cell
Introduction
How do we cope with the massive encounter of microbial constituents and viable microorganisms immediately after birth? How do we ensure mucosal homeostasis during the postnatal emergence of a dense and diversifying microbiota but still maintain immune responsiveness to infectious pathogens? How do we mount regulatory responses after birth to the large spectrum of antigens from diet and beneficial commensal bacteria to provide protection from inappropriate immune stimulation? How do we generate a mature mucosal adaptive immune system during early life reactive against the appropriate microorganisms?
These questions are difficult to answer with our current knowledge on the adult innate and adaptive immune system. However, we are able to obtain testable hypotheses when we accept two assumptions. First, the neonatal immune system is unique and differs in many aspects from the immune system in the adult host. And second, the transition from the immediate postnatal period to the mature adult immune system represents a process of consecutive phases that are non‐redundant and follow a particular order of events. These two assumptions are not new and have led to the concept of ‘the neonatal window of opportunity’ and to the idea of a ‘layered immunity’. The concept of the ‘neonatal window of opportunity’ postulates a non‐redundant priming period of the innate and adaptive immune system after birth that sets the stage for immune homeostasis and subsequent host–microbial interaction.1 It has recently attracted much attention because functional studies in animal models indicate that it may provide an explanation for the ‘hygiene hypothesis’, an epidemiological association between the western lifestyle with enhanced hygiene standards and the increase in atopic and autoimmune diseases.2, 3, 4 ‘Layered immunity’ suggests that the maturation of the innate and adaptive immune system occurs in subsequent phases that (might) depend on each other to facilitate the establishment of a mature, homeostatic, but vigilant immune system. It has originally been developed to explain immune cell ontogeny but may be able to provide a broader framework to understand the maturation of the innate and adaptive immune system as a whole.5
Notably, the concept of a timed succession of non‐redundant phases during postnatal immune maturation can also include external factors and conditions. For example, the reduced exposure to pathogenic microorganisms during the immediate postnatal period that results from breast milk as a safe nutrient source, and the reduced social interaction, which both minimize transmission of pathogens. Also, the fact that breast milk as sole dietary substrate of the neonate host represents immunological self‐antigen and therefore does not require tolerance induction. Finally, the finding that the compositions of the pre‐ and post‐weaning enteric microbiota differ significantly. The initiation of immune tolerance to commensal bacteria may therefore be more efficient after weaning. The integration of external circumstances may further allow adaptation of the evolution‐shaped immune system to various geographical locations associated with the different availability of food sources and exposure to microorganisms. The non‐redundant nature of immune maturation might then provide an explanation for the long‐known fact that early environmental influences can have lasting consequences.6 It is also consistent with the observation that the effect of immunotoxicants, i.e. chemical agents such as halogenated aromatic hydrocarbons, polycyclic aromatic hydrocarbons, hormonal substances, therapeutic agents or heavy metals that exhibit developmental toxicity, is particularly pronounced during fetal and early postnatal development.7
In the following paragraphs, we will first summarize what is known about the development of the microbiota and the innate and adaptive immune system with particular focus on the intestinal mucosa after birth. We will then try to assemble those findings into a model of a timed succession of non‐redundant phases to generate a model that explains how host–microbial homeostasis and a mature immune system are established after birth.
Development of the neonatal microbiota
The healthy neonate is born sterile. Recent reports suggesting the presence of a microbiota in healthy placental and fetal tissue before birth have been challenged and most probably reflect the detection of free bacterial nucleic acid molecules derived from environmental sources.8, 9 Nevertheless, the fetal tissue is of course not completely shielded from microbial constituents and innate immune signals. A recent study in mice reported on the priming effect of aryl hydrocarbon receptor ligands of the maternal microbiota on the offspring's immune system with elevated numbers of type 3 innate lymphoid and myeloid cells known to participate in the antibacterial host response.10 Similarly, the allergy‐protective effect of farm exposure with accelerated T helper type 17 immune maturation in the human offspring was also observed upon prenatal maternal farm exposure.11 This idea is consistent with reports that suggest that microbiota‐derived microbial stimuli such as peptidoglycan and flagellin reach distant body sites,12, 13 and provides a possible explanation for the effect of microbial stimuli on the fetal tissue in the absence of prenatal colonization.
With rupture of the amniotic membranes and during the following transit through the birth canal, the newborn becomes exposed to bacteria colonizing the maternal reproductive tract. This process may be quite efficient given the narrowness of the birth canal and the prolonged time required to allow passage of the infant's head. In contrast, cesarean section circumvents contact with maternal mucosal surfaces and initially exposes the neonate to the (maternal) skin microbiota.14, 15 This scenario is consistent with studies of the early neonatal microbiome that describe a relatively homogeneous microbiota across different body sites early after birth and illustrate differences between the enteric microbiome of babies born by vaginal delivery or by elective cesarean section during the first weeks after birth.16, 17 Notably, this difference appears to be temporary. Also, the fact that some studies failed to observe a major effect of the mode of delivery suggests that its influence may depend on the absence of a compensatory mechanism of bacterial exposure. Nevertheless, as we will discuss later, it may still be functionally relevant, and, accordingly, attempts are being made to compensate for the lack of early exposure to maternal urogenital bacteria.18
The initial lack of bacteria competing for space and nutrients and the lactose‐rich milk diet support the rapid rise in bacterial numbers in the intestine during the early phase of colonization.19, 20 Three major categories of factors were identified to influence the infant microbiota composition.21, 22 First, factors that presumably provide additional bacterial exposure, such as geographical factors (that may in fact reflect cultural and habitual differences in the management of newborns) as well as the presence of siblings and household pets. Notably, the number of siblings was identified as a key factor in the first report on the influence of early‐life factors on allergy etiology.2 Second, mechanisms that promote or suppress the growth of specific bacteria and here particularly dietary factors such as breast‐milk constituents and antibiotics. Antibiotics may be given perinatally to the mother, for example, to prevent transmission of group B streptococci, the most frequent cause of neonatal sepsis. Antibiotics kill bacteria in a logarithmic fashion, so it is conceivable that they exert a particularly potent effect on the early, low‐density microbiota. Third, host factors such as gestational age and genetic factors can directly or indirectly influence the microbiota development.
A particularly interesting role may be attributed to genetic factors because their identification and characterization could help to understand the evolutionary benefit of a certain microbiota composition and to explain the adverse effects of an altered microbiota because of altered habits or medical interventions. The analysis of mouse intercross lines and human twin cohorts revealed that the host's genes shape approximately 9% of the enteric microbiota as a polygenic trait.23, 24 However, individual genes and regulatory circuits are only beginning to be identified. Benson et al., identified 18 quantitative trait loci in mice with significant linkage with the relative abundances of specific microbial taxa.23 The identified clusters are related to diet, metabolism, olfaction, barrier defense and self/non‐self recognition.23, 24 We have recently characterized an epithelial Toll‐like receptor 5 (TLR5) ‐mediated regulatory mechanism to favor early colonization by non‐flagellated bacteria in the mouse model.25
Whereas the adult‐like bacterial density (i.e. the number of bacterial microorganisms per gram of tissue) in the human intestinal tract is reached after birth within days,19 it takes 2–3 years in humans and 3–4 weeks in mice until an adult‐like richness (i.e. the number of bacterial species) and bacterial composition is established.26, 27 Major changes of the microbiota composition are observed during weaning, i.e. during the transition from breast milk or formula feeding to solid food, taking place in humans from 6 months after birth and in mice at 2–3 weeks after birth. Cessation of breastfeeding was identified as a major driving factor highlighting the critical influence of breast‐milk constituents on early host–microbial homeostasis.20 Beside lactose, human milk oligosaccharides, lactoferrin and secretory IgA may exert direct antibacterial effects whereas epidermal growth factor has a major influence on intestinal epithelial gene expression and immune development.28, 29, 30 Notably, major differences are found between breastfed and formula‐fed babies.20 The temporal changes of the bacterial composition are accompanied by non‐random transitions with different bacterial phyla dominating the enteric microbiota.20, 31 Although the precise underlying mechanisms are not clear, a microbiota development built upon a succession of dominating bacterial species might be particularly susceptible to transient insults. In other words, a temporary disturbance during childhood might induce a lasting influence on the adult microbiota with potential changes in the susceptibility to diseases later in life.
Postnatal maturation of the neonatal innate immune system
Although the neonatal organism is frequently referred to as immature and less prone to react to (innate) immune stimulation, neonatal sepsis, a highly prevalent and life‐threatening condition particularly in prematurely born babies, is associated with overwhelming inflammation. Similarly, necrotizing enterocolitis (NEC), a life‐threatening transmural intestinal disease in premature neonates is characterized by a strong inflammatory reaction. Hence, although significant differences in innate immune recognition may exist between the neonatal and adult hosts, the neonatal innate immune system is clearly able to recognize microbial exposure and to respond with the secretion of pro‐inflammatory cytokines. We should therefore refer to the neonatal innate immune system as ‘differentially adapted’ rather than ‘immature’ or ‘less developed’. However, the precise differences and their biological reasons are ill‐understood.
The epithelium lines the mucosal surface and separates the densely colonized gut lumen from the underlying tissue. To investigate the establishment of mucosal host–microbial homeostasis during the early postnatal period and the pathogenesis of NEC, the ability of the intestinal epithelium to recognize microbial stimuli has been studied in much detail. Intestinal epithelial cells express innate immune receptor molecules of the toll‐like receptor, Rig I‐like receptor, nucleotide‐binding oligomerization domain (NOD) ‐like receptor and inflammasome families.32, 33, 34 Consistent with these results, epithelial innate immune stimulation was shown to reinforce the epithelial barrier and contribute to host–microbial homeostasis.35, 36, 37 TLR5 expression in absorptive enterocytes is restricted to the pre‐weaning period.25, 32 Neonatal epithelial TLR5 exerts a significant influence on the composition of the neonatal microbiota.25 The altered microbiota in TLR5‐deficient mice persists throughout adulthood and confers enhanced susceptibility to a metabolic phenotype with obesity.38
Also, the peptidoglycan sensor NOD2 was shown to contribute to the resilience of the neonatal enteric microbiota with long‐term consequences. Whereas the influence of NOD2 on the adult microbiota composition has remained controversial, a recent study demonstrated a prolonged alteration of the microbiota in NOD2‐deficient mice after administration of ampicillin, an antibiotic frequently used in newborn humans.39 Interestingly, this prolonged alteration of the microbiota composition in NOD2‐deficient animals was linked to an enhanced susceptibility to colitis in adulthood. Of note, loss‐of‐function mutations in the NOD2 locus represent one of the most important genetic risk factors for inflammatory bowel disease.
Inappropriate immune stimulation, however, may be harmful, particularly in the neonate. Some years ago, the Hackam group described a decrease of the intestinal epithelial TLR4 expression before birth paralleled by enhanced TLR9 expression.40 As TLR9 has been suggested to play an immune regulatory role, this may dampen the overall susceptibility to immune stimulation in the term neonate.41 Similarly, TLR3 expression was shown to be low in the neonatal intestinal epithelium and was up‐regulated only in adult mice.42 Intestinal epithelial TLR5 expression could be beneficial in the neonate host as discussed above but the subsequent down‐regulation in absorptive enterocytes might help to prevent inappropriate stimulation in the adult host. Consistently, a loss‐of‐function polymorphism in TLR5 has been shown to protect whereas a gain‐of‐function was associated with an increased risk of inflammatory bowel disease in humans.43, 44 Additionally, soluble factors in breast milk such as epidermal growth factor, the local cytokine milieu and regulatory molecules such as A20 may help to avoid inappropriate innate immune stimulation during the immediate postnatal period.28, 45, 46
In addition to developmental factors, the innate immune system actively adapts to the situation after birth. This was first described for the intestinal epithelium, which upon first postnatal microbial exposure undergoes a transient TLR4‐mediated innate immune stimulation followed by a state of immune tolerance.47, 48 Notably, signs of postnatal innate immune stimulation have also been observed in humans.49, 50 Postnatal acquisition of transient innate immune unresponsiveness has also been described in cord‐blood‐derived leukocytes. Human neonatal immune cells express innate immune receptors and respond to microbial exposure, albeit in a different manner compared with adult cells.51 In contrast to the murine gut epithelium, postnatal reprogramming of human monocytes was mediated by high perinatal serum concentrations of the endogenous TLR4 ligand S100A8/9 (calprotectin).52 The S100A8/9 effect was also observed in cord blood macrophages mediated through inhibition of the mechanistic target of rapamycin pathway and associated with reduced glycolysis.53 Consistently, dynamic transcriptomic and metabolic changes have been described in human peripheral blood cells during the first week after birth. In both murine enterocytes and blood monocytes, innate immune tolerance was associated with cellular reprogramming and expression of an alternative set of genes.48, 52 It might, therefore, not only serve to reduce the susceptibility to inflammation but simultaneously to foster the establishment of a stable host–microbial interaction.
Also, the overall anatomical organization of the epithelium and the spectrum and localization of effector molecules of the innate immune system display striking age‐dependent differences.54 They are more pronounced in mice, because of their shorter gestational age (and hence lower tissue maturation at birth). In adult mice, small intestinal crypts provide a safe niche for the epithelial stem cells and the rapidly proliferating transit amplifying cells. Newly generated epithelial cells leave the crypts and migrate along the crypt–villus axis to be continuously exfoliated at the villus tip. Strikingly, this continuous epithelial turnover does not exist in neonates. Here, epithelial proliferation is relatively low and no directed cell migration and continuous exfoliation are observed. In addition, the expression of antimicrobial peptides in the adult small intestine is restricted to crypt‐based Paneth cells. The regulation occurs almost entirely at the post‐transcriptional level, i.e. by enzymatic processing and secretion.35 In contrast, the neonatal epithelium is largely devoid of mature Paneth cells and expresses the cathelicidin CRAMP.55 Also, the production of mucins strongly increases with age and so the mucus layer as an additional protective shield only establishes at weaning.56, 57 Overall, this suggests that a more intense interaction between the microbiota and the host's epithelium occurs in the neonatal host. In contrast, the adult tissue is characterized by a protected stem cell niche, an antimicrobial peptide‐enriched mucus layer, and rapid epithelial cell turnover to separate the two compartments.58
The maturation of the adaptive mucosal immune system
The adaptive immune system in adult sterile tissues is confined to secondary lymphoid organs and circulation, whereas the picture is markedly different at mucosal sites. Here, a vast number of antigen‐experienced T cells and plasma cells are found at steady‐state with the intestinal tract as a primary example, where more immune cells are found than in the rest of the organism taken together. Hence, the immunological state of the adult intestine was often referred to as ‘physiological inflammation’ – highlighting the presence of activated lymphocytes under homeostatic conditions.59 Further, it is assumed that the presence of activated lymphocytes reflects a steady cross‐talk of the adaptive immune system with the rich repertoire of luminal antigen derived from microbiota, diet and environment.
But how is this state of ‘physiological inflammation’ of the adult mucosa reached? Whereas microbial antigens become available shortly after birth, they greatly expand in diversity around weaning. At this time‐point, dietary antigens are added when the infant begins to ingest solid food. In mice, this transition starts at the end of the second week of life and is completed 3 weeks after the infants are separated from the dam (i.e. at approximately 6 weeks of age). B and T cells start to exit primary lymphoid tissues in great numbers around birth and quickly home to secondary lymphoid tissues, including the draining lymph nodes of the mucosa and gut‐associated lymphoid tissue anlagen.60, 61 However, activated lymphocytes and production of secretory immunoglobulins (IgA and IgG) by the young host are not detected until after weaning and adult levels of adaptive cellularity and IgA repertoire are not reached until adulthood.42, 62, 63
At mucosal surfaces, the establishment of tolerance to microbiota‐, diet‐ and environment‐derived antigens is characterized by an expansion of regulatory T (Treg) cells.29, 30, 60, 64, 65, 66 Interestingly, the expansion of Treg cells in skin and lung (week 2 and 3, respectively) induced by exposure to commensal bacteria precedes that in the gut (week 4).29, 30, 66 Also, the origin of the mucosal Treg cells is different between the three locations – the early Treg cell wave detected in the hair follicles of the skin is recruited in a CCR6‐dependent manner from the thymus. Lung Treg cells that mediate tolerance do not express the transcription factor Helios and are presumably induced in the periphery.64, 65 Whereas the neonatal host harbors thymus‐derived Treg cells in the secondary lymphoid tissues of the intestine, microbiota‐dependent peripherally generated Treg cells that express the transcription factor retinoic acid receptor‐related orphan receptor γt67, 68 only expand in the 4th week of life.30, 60, 67
In the colon, this process of tolerance induction might be initiated by a transient increase of antigen transport through goblet‐cell‐associated passages (GAPs) between days 10 and 20 after birth induced by decreasing epidermal growth factor ligands.30 This early antigen exposure of CD103+ lamina propria dendritic cells under homeostatic conditions can lead to activation of T cells and promotes the development of a tolerogenic response characterized by the induction of peripheral Treg cells during the 3rd week of life directed against microbial antigens. In the small intestine, follicular T helper cells are induced and aid the production of polyreactive immunoglobulin A. IgA is secreted to the intestinal lumen and coats microorganisms, promoting a tolerogenic immune response.62, 69 This microbial stimulation may also cause the reported transient interleukin‐22‐ (IL‐22) and IL‐23‐induced signal transducer and activator of transcription 3 phosphorylation in innate lymphoid type 3 cells and epithelial cells.70 Notably, the time‐point of the intestinal tolerization process and Treg cell generation is critical and delayed exposure to microbiota has far reaching consequences for health and disease in later life with increased susceptibility to immune‐mediated diseases such as inflammatory bowel disease, cancer, and allergic sensitization.29, 30
Other studies have highlighted the critical importance of microbial exposure during the early neonatal period for immune homeostasis. A diverse enteric microbiota early after birth is required to suppress IgE levels and prevent an exaggerated anaphylactic response.71 Similarly, microbial exposure during this time window reduces the number of invariant natural killer T cells in lung and colon tissue and protects from mucosal inflammation and allergic responses.72 Finally, antibiotic treatment of neonatal mice enhances the susceptibility towards food allergen sensitization and experimental psoriasis.73, 74 Also in humans, exposure to microbial constituents protects children from asthma development and specific bacterial species have been identified and shown to protect from food allergy.75, 76 Conversely, early‐life antibiotic treatment alters the metabolome through alteration of the microbiota and increases the risk of obesity and allergies.77, 78 Importantly, the underlying molecular mechanism of the protective effect of the microbiota is incompletely understood. The delivery of microbial constituents derived from a particular group of bacteria for tolerance induction, the supply with metabolic immunomodulators and/or the immunostimulatory capacity of microbial constituents may critically contribute.79, 80, 81, 82
On the other hand, premature activation of the adaptive immune system in the intestine can also lead to enhanced disease susceptibility. It can be induced by removing maternal immunoglobulin from breast milk or enforcing a premature transport of antigen to lamina propria dendritic cells via GAPs.29, 30, 60, 83, 84 The consequences of premature activation are under‐studied. A recent investigation in humans demonstrated that it may contribute to the etiology of NEC. Expansion of Enterobacteriaceae that are not coated by maternal IgA precedes the onset of NEC.85 Further consequences could reach from autoimmune disorders due to interference with the induction of peripheral tolerance to failure to induce tolerance towards antigens that become available at weaning only. The clinical manifestations of this premature immune activation, however, might only become overt over time.
Towards the concept of a temporally layered postnatal establishment of the innate and adaptive immune system
Over the last decades, it has become evident that the neonatal immune system is not just a ‘less developed’ version of the adult immune system – neonates are able to mount a strong and protective immune response in the event of an infectious challenge.86, 87, 88 Instead, the neonatal immune system is unique and optimally equipped to cope with the requirements of this ontogenetic time window. This time window is characterized by the need to support postnatal microbial colonization, the need to tolerate sudden exposure to high concentrations of microbial innate immune stimuli yet preserve a reactive innate immune system, the need to generate adaptive tolerance towards new antigens, the need to expand Treg cells, and the need to develop and mature effector T cells and plasma cells.
Within hours after birth, systemic immune cells and the intestinal epithelium acquire innate immune tolerance through TLR4 activation.47, 52 This activation may occur through exposure to exogenous endotoxin or by the elevated levels of the endogenous mediator S100A8/9 after birth (Fig. 1, left panel). Importantly, this innate immune tolerance is accompanied by transcriptional reprogramming, which may serve to provide some basic degree of mucosal and systemic antimicrobial host defense activation during the first days after birth.48, 52 During this early time window, the initial bacterial colonization takes place. Although still characterized by low richness, the bacterial density reaches high levels shortly after birth and so is expected to provide exposure to high concentrations of microbial innate immune ligands within days.19 Notably, this initial colonization is mainly based on bacteria transmitted by the healthy mother, i.e. represented by non‐pathogenic and beneficial commensal bacteria. The concomitant transfer of maternal IgA antibodies directed against the very same set of bacteria may help to restrict bacterial colonization to the intestinal lumen and avoid inappropriate immune stimulation.62, 85, 89 The rapid colonization by commensal bacteria and the low risk of infection because of breast milk being the sole food source may also allow the absence of antimicrobial mechanisms such as antimicrobial peptide‐producing Paneth cells or epithelial TLR3 expression.42, 55 Still, the composition of the very early microbiota is highly individual because of the low colonization resistance.19, 26 Extreme bacterial compositions may lead to adverse effects and the host may therefore try to restrict the growth of certain types of bacteria.25
Figure 1.
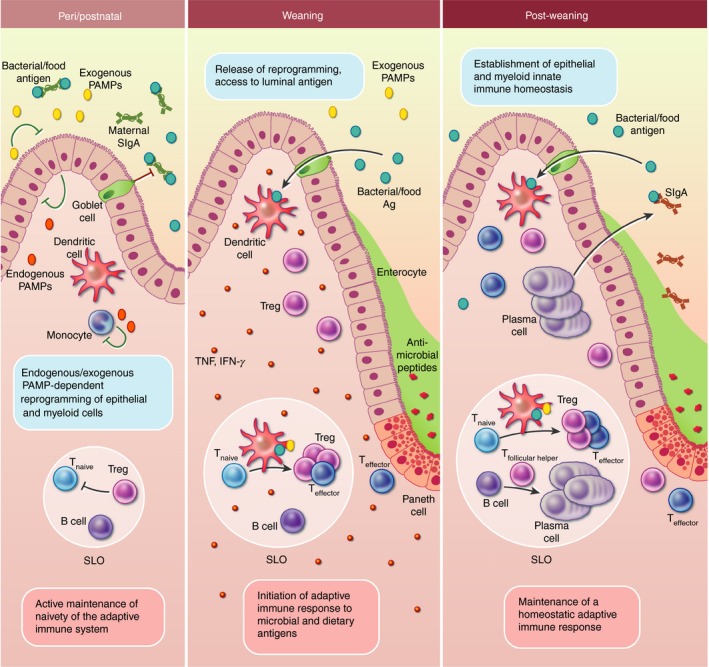
Development of the mucosal immune system in the intestine under homeostatic conditions. At birth, the small intestine becomes readily colonized by a low‐diversity microbiota and microbial antigen and microbiota‐derived pathogen‐associated molecular patterns (PAMPs) become available. Simultaneously, endogenous innate immune stimuli are produced and a perinatal Toll‐like receptor (TLR) stimulation induces innate hyporesponsiveness and reprogramming in the intestinal epithelium and myeloid cells. Around birth, T and B cells exit from the thymus and bone marrow, respectively, and home to secondary lymphoid tissues (SLO) including the mesenteric lymph nodes and gut‐associated lymphoid tissues (e.g. Peyer's patches and solitary intestinal lymphoid tissues). Microbiota – initially transferred from the mother at birth – is likely to be largely bound to breast‐milk‐derived maternal secretory IgA that shields microbial antigen from the adaptive immune system. Maternal secretory IgA and neonatal thymus‐derived regulatory T (tTreg) cells contribute to the naive state of the adaptive immune system throughout the postnatal phase. At weaning, the host starts to ingest solid food containing complex carbohydrates. This leads to an increased richness of the intestinal microbiota. The innate unresponsiveness of the epithelium is reversed and physiological tissue development is largely complete so that crypts with antimicrobial‐producing Paneth cells are found and mucus production is up‐regulated in goblet cells shielding the microbiota from the now responsive epithelium. At the same time, goblet cells start to transport luminal antigen to the underlying dendritic cells (DCs) in the lamina propria. In the SLOs, DCs present antigen to naive T cells and a transient (adaptive) immune activation is induced – the weaning reaction – characterized by pro‐inflammatory cytokines – tumor necrosis factor‐α (TNF‐α) and interferon‐γ (IFN‐γ). At the same time, retinoic acid receptor‐related orphan receptor γt‐positive Treg cells are induced that promote tolerance and tune the mucosal immune system for appropriate responsiveness to immune stimuli in later life, protecting the host from immune‐mediated diseases. After weaning, the microbiota composition stabilizes and is less sensitive to perturbations such as incoming pathogens or antibiotic treatment. Luminal antigen is facilitated to the antigen‐presenting cells in a highly controlled manner and the homeostatic immune response is dominated by tolerance promoting Treg cells that in turn induce the production of endogenous IgA by plasma cells.
Shortly after birth, T cells exit from the murine thymus and home to the secondary lymphoid organs.61 Despite the rapid colonization of the intestine and exposure to microbial antigen, however, they remain in a naive state until the 3rd week of life.60 Hence, in contrast to the situation in the adult intestine with a high proportion of antigen‐experienced effector T lymphocytes, the neonatal intestine lacks the hallmark traits of a pronounced mucosal immune system (Fig. 1, left panel). The localization and phenotype of the adaptive immune cells at this age rather resembles that of sterile tissues at non‐mucosal body sites. The underlying reasons are incompletely understood but breast‐milk‐derived maternal immunoglobulin and neonatal Treg cells were identified to contribute to the active suppression of T‐cell maturation before weaning.60, 62 In humans, T‐cell homing to the intestine already occurs in the fetus.90, 91 Yet fetal and adult T cells derive from hematopoietic stem cells that originate in different tissues (i.e. fetal liver versus adult bone marrow) and have distinct properties: Fetally derived cells exhibit higher proliferative capacity and are more prone to a tolerogenic response.92 Interestingly, subsequent studies revealed that differences in the transcriptome and functional phenotype between T lymphocytes generated in the neonate or adult also exist in mice. Perinatally generated, thymus‐derived Treg cells promote self‐tolerance and are critical for protection from autoimmune disorders.93, 94 Neonatally generated cytotoxic T cells exhibit more innate‐like effector functions whereas adult‐derived cells are skewed towards memory cells.95, 96 Consistent with a more innate immune function, neonatal T cells were shown to produce tumor necrosis factor (TNF) and CXCL8.91, 97 Age‐dependent qualitative differences have also been identified within the ILC compartment.98 EOMES+ natural killer cells with a robust effector phenotype populate the human neonatal intestine and might provide protection until T‐cell effector cells have matured.99
Clearly, developmental mechanisms contribute significantly to the initial adaptation to microbial exposure. Prematurity, i.e. a reduced maturity of the infant at birth due to a lower gestational age, might reduce the ability to undergo the above‐mentioned innate immune tolerization and reprogramming. Indeed, a significant number of prematurely born children develop NEC and the incidence and severity rise with a low gestational age at birth. Consistent with this hypothesis, an enhanced innate immune receptor expression and susceptibility to microbial ligands have been demonstrated in mouse fetal intestinal epithelium.34, 40 The protective effect of breast milk has long been described; a recent study by Gopalakrishna et al. now indicates that the lack of maternal IgA specific for the colonizing bacteria may contribute to the development of NEC.85 IgA may dampen the release of microbial stimuli and restrict access of bacteria to the tissue. On the other hand, tumor necrosis factor secretion by fetal T lymphocytes and the relatively low abundance of intestinal Treg cells may promote inflammation upon barrier disruption.91
In mice, intestinal epithelial innate immune tolerance is suspended approximately at weaning.48 Concomitantly, the mucosal barrier is reinforced, illustrated for example by the enhanced mucus production,56 the appearance of antimicrobial peptides producing Paneth cells,55 increased TLR3 expression,42 and the start of the continuous proliferation, crypt–villus axis migration and exfoliation of epithelial cells100 (Fig. 1, middle panel). Hence, the re‐establishment of a fully responsive epithelial innate immune system after this initial period of tolerance occurs when a physical separation between the luminal microbiota and the mucosal tissue is accomplished. Only the presence of a physico‐chemical barrier covering the surface may allow the coexistence of a sensitive mucosal innate immune system with the presence of the enteric microbiota.57, 58
Age‐dependent differences in the mucosal barrier formation, antigen uptake and presentation as well as immune cell determination may have implications for the neonatal immune responses both to pathogenic stimuli as well as to innocuous dietary antigens. Few studies have systematically analyzed responses to luminal antigens within the intestinal mucosa in an age‐dependent manner. However, oral tolerance, a major hallmark of the adult intestinal immune system, is more difficult to induce during the postnatal period.101, 102, 103 Similarly, the neonatal lung reacts much more strongly in a model of allergic asthma.64
At weaning, once the epithelial tissue maturation has occurred in mice and innate immune homeostasis is reached, the decreasing amounts of epidermal growth factor in breast milk open GAPs that provide luminal antigen to antigen‐presenting cells30(Fig. 1, middle panel). This in turn provokes a strong immune reaction, the so‐called weaning reaction that depends on the microbiota, microbial metabolites, and dietary factors and promotes the expansion of retinoic acid receptor‐related orphan receptor γt+ Treg cells and lasting protection from inflammatory disease.29 At this stage, GAPs co‐transport microbiota‐derived innate immune stimuli such as lipopolysaccharide and this process might significantly strengthen a tolerogenic adaptive immune response. In humans, the high abundance of low stimulatory lipopolysaccharide‐producing commensal bacteria found in children living in countries with a western lifestyle has been associated with increased susceptibility to autoimmune disease.79 Consistently, TLR‐mediated signals were suggested to directly promote Treg cell numbers and mucosal tolerance.104 The appearance of Treg cells subsequently terminates the weaning reaction and stimulatory effect of microbial exposure70 (Fig. 1, right panel).
Which functional requirements determine the succession of the phases of postnatal immune maturation (Fig. 2)? Peripheral tolerance may need to be established, before an effective host response can be mounted without the risk of bystander autoreactivity. Consistently, a perinatal wave of thymus‐derived Treg cells with potent suppressory capacities protects the host from autoimmune diseases and induces anergy in self‐reactive T cells.93, 105
Figure 2.
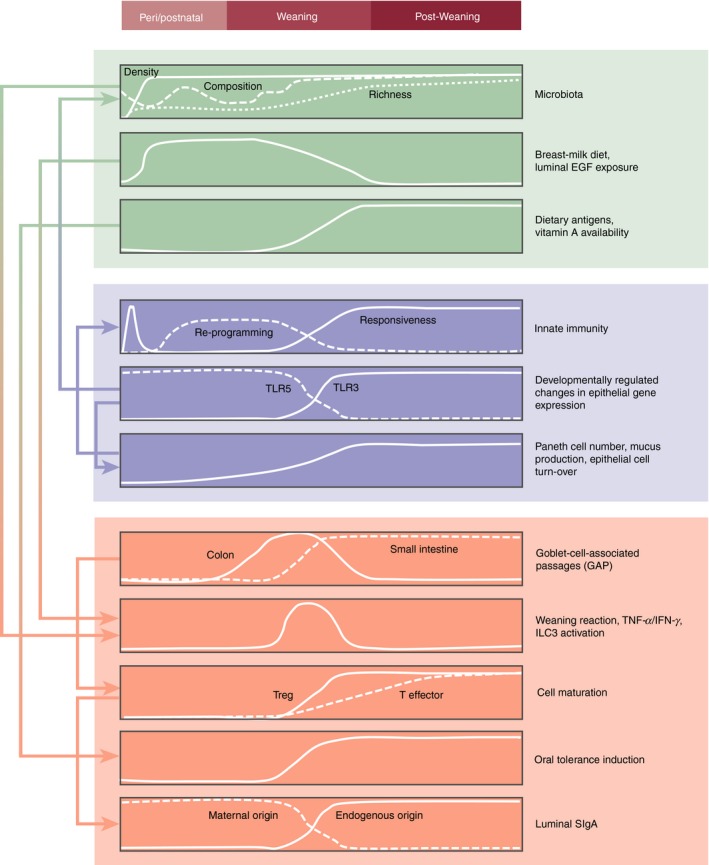
Approximate time kinetic and tentative functional interrelationship and directionality of the individual processes that govern the transition from the fetal to the neonatal, weaning and post‐weaning periods. Aspects of the microbiota (in green), and innate (blue) and adaptive (orange) immune system are depicted.
Interestingly, homeostatic signaling via the IL‐33/ST2 (IL1rl1) axis, which is usually associated with tissue damage, appears to balance and adjust the degree of T‐cell anergy in the neonatal host.105, 106 Also, the induction of peripheral tolerance to self‐antigen may have to precede the tolerance to microbial and dietary antigens at mucosal sites.29, 30, 60, 64, 66 Alternatively, the establishment of tolerance to dietary and microbial antigens (that change almost completely around weaning) may have to be postponed until a stable dietary and microbial exposure is reached. Interestingly, in contrast to the mouse, host effector lymphocytes are detected in the human intestine early during gestation. It is not clear, whether it is self‐antigen that stimulates this effector response and how (self) tolerance is maintained at this developmental stage.90 Finally, the layered development of the immune system is evolutionarily driven and follows a cost–benefit principle. The maintenance of an elaborate immune system may come at a price and may have to be adapted to the availability of energy resources that at this time‐point might rather be dedicated to growth and development.
In conclusion, we here propose to combine the concepts of a ‘layered immunity’ and the ‘postnatal window of opportunity’ to explain how the newborn establishes immune homeostasis after birth. Taking into account the published data on the establishment of microbiota and innate and adaptive immune maturation, we attempted to assemble a more general picture that sheds light on the biological requirements of the neonate host, the time course of events during the postnatal period, and possible functional links between individual mechanisms and the susceptibility to immune‐mediated diseases. Although still incomplete, we believe that this model may help to further dissect the regulatory mechanisms and their timed succession during postnatal immune maturation. Ultimately, this may allow the development of strategies to avoid adverse immune imprinting and subsequent manifestation of immune‐mediated diseases and improve the clinical management of newborns with immune malfunction or disruption of host–microbial homeostasis.
Disclosures
The authors have no conflicts of interest to disclose.
Acknowledgement
This work was supported by grants from the German Research Foundation (TO 1052/1‐1 to NT and HO 2236/14‐1, HO 2236/11‐1 and CRC1382, project B01 to MWH).
References
- 1. Torow N, Hornef MW. The neonatal window of opportunity: setting the stage for life‐long host–microbial interaction and immune homeostasis. J Immunol 2017; 198:557–63. [DOI] [PubMed] [Google Scholar]
- 2. Strachan DP. Hay fever, hygiene, and household size. BMJ 1989; 299:1259–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med 2002; 347:911–20. [DOI] [PubMed] [Google Scholar]
- 4. Braun‐Fahrlander C, Riedler J, Herz U, Eder W, Waser M, Grize L et al Environmental exposure to endotoxin and its relation to asthma in school‐age children. N Engl J Med 2002; 347:869–77. [DOI] [PubMed] [Google Scholar]
- 5. Herzenberg LA, Herzenberg LA. Toward a layered immune system. Cell 1989; 59:953–4. [DOI] [PubMed] [Google Scholar]
- 6. Dubos R, Lee CJ, Costello R. Lasting biological effects of early environmental influences. V. Viability, growth, and longevity. J Exp Med 1969; 130:963–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Holladay SD, Smialowicz RJ. Development of the murine and human immune system: differential effects of immunotoxicants depend on time of exposure. Environ Health Perspect 2000; 108(Suppl 3):463–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de Goffau MC, Lager S, Sovio U, Gaccioli F, Cook E, Peacock SJ et al Human placenta has no microbiome but can contain potential pathogens. Nature 2019; 572:329–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hornef M, Penders J. Does a prenatal bacterial microbiota exist? Mucosal Immunol 2017; 10:598–601. [DOI] [PubMed] [Google Scholar]
- 10. Gomez de Aguero M, Ganal‐Vonarburg SC, Fuhrer T, Rupp S, Uchimura Y, Li H et al The maternal microbiota drives early postnatal innate immune development. Science 2016; 351:1296–302. [DOI] [PubMed] [Google Scholar]
- 11. Lluis A, Depner M, Gaugler B, Saas P, Casaca VI, Raedler D et al Increased regulatory T‐cell numbers are associated with farm milk exposure and lower atopic sensitization and asthma in childhood. J Allergy Clin Immunol 2014; 133:551–9. [DOI] [PubMed] [Google Scholar]
- 12. Oh JZ, Ravindran R, Chassaing B, Carvalho FA, Maddur MS, Bower M et al TLR5‐mediated sensing of gut microbiota is necessary for antibody responses to seasonal influenza vaccination. Immunity 2014; 41:478–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Clarke TB, Davis KM, Lysenko ES, Zhou AY, Yu Y, Weiser JN. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat Med 2010; 16:228–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dominguez‐Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N et al Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A 2010; 107:11971–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hill CJ, Lynch DB, Murphy K, Ulaszewska M, Jeffery IB, O'Shea CA et al Evolution of gut microbiota composition from birth to 24 weeks in the INFANTMET Cohort. Microbiome 2017; 5:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chu DM, Ma J, Prince AL, Antony KM, Seferovic MD, Aagaard KM. Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat Med 2017; 23:314–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fouhy F, Watkins C, Hill CJ, O'Shea CA, Nagle B, Dempsey EM et al Perinatal factors affect the gut microbiota up to four years after birth. Nat Commun 2019; 10:1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dominguez‐Bello MG, De Jesus‐Laboy KM, Shen N, Cox LM, Amir A, Gonzalez A et al Partial restoration of the microbiota of cesarean‐born infants via vaginal microbial transfer. Nat Med 2016; 22:250–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biol 2007; 5:e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Backhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva‐Datchary P et al Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 2015; 17:690–703. [DOI] [PubMed] [Google Scholar]
- 21. van Best N, Hornef MW, Savelkoul PH, Penders J. On the origin of species: factors shaping the establishment of infant's gut microbiota. Birth Defects Res C Embryo Today 2015; 105:240–51. [DOI] [PubMed] [Google Scholar]
- 22. Tamburini S, Shen N, Wu HC, Clemente JC. The microbiome in early life: implications for health outcomes. Nat Med 2016; 22:713–22. [DOI] [PubMed] [Google Scholar]
- 23. Benson AK, Kelly SA, Legge R, Ma F, Low SJ, Kim J et al Individuality in gut microbiota composition is a complex polygenic trait shaped by multiple environmental and host genetic factors. Proc Natl Acad Sci U S A 2010; 107:18933–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Goodrich JK, Davenport ER, Beaumont M, Jackson MA, Knight R, Ober C et al Genetic determinants of the gut microbiome in UK Twins. Cell Host Microbe 2016; 19:731–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fulde M, Sommer F, Chassaing B, van Vorst K, Dupont A, Hensel M et al Neonatal selection by Toll‐like receptor 5 influences long‐term gut microbiota composition. Nature 2018; 560:489–93. [DOI] [PubMed] [Google Scholar]
- 26. Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez‐Bello MG, Contreras M et al Human gut microbiome viewed across age and geography. Nature 2012; 486:222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pantoja‐Feliciano IG, Clemente JC, Costello EK, Perez ME, Blaser MJ, Knight R et al Biphasic assembly of the murine intestinal microbiota during early development. ISME J 2013; 7:1112–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Good M, Siggers RH, Sodhi CP, Afrazi A, Alkhudari F, Egan CE et al Amniotic fluid inhibits Toll‐like receptor 4 signaling in the fetal and neonatal intestinal epithelium. Proc Natl Acad Sci U S A 2012; 109:11330–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Al Nabhani Z, Dulauroy S, Marques R, Cousu C, Al Bounny S, Dejardin F et al A weaning reaction to microbiota is required for resistance to immunopathologies in the adult. Immunity 2019; 50:1276–88.e5. [DOI] [PubMed] [Google Scholar]
- 30. Knoop KA, Gustafsson JK, McDonald KG, Kulkarni DH, Coughlin PE, McCrate S et al Microbial antigen encounter during a preweaning interval is critical for tolerance to gut bacteria. Sci Immunol 2017; 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yassour M, Vatanen T, Siljander H, Hamalainen AM, Harkonen T, Ryhanen SJ et al Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Sci Transl Med 2016; 8:343ra81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Price AE, Shamardani K, Lugo KA, Deguine J, Roberts AW, Lee BL et al A map of toll‐like receptor expression in the intestinal epithelium reveals distinct spatial, cell type‐specific, and temporal patterns. Immunity 2018; 49:560–75.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hornef MW, Fulde M. Ontogeny of intestinal epithelial innate immune responses. Front Immunol 2014; 5:474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kempster SL, Belteki G, Forhead AJ, Fowden AL, Catalano RD, Lam BY et al Developmental control of the Nlrp6 inflammasome and a substrate, IL‐18, in mammalian intestine. Am J Physiol Gastrointest Liver Physiol 2011; 300:G253–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stockinger S, Albers T, Duerr CU, Menard S, Putsep K, Andersson M et al Interleukin‐13‐mediated Paneth cell degranulation and antimicrobial peptide release. J Innate Immun 2014; 6:530–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stockinger S, Duerr CU, Fulde M, Dolowschiak T, Pott J, Yang I et al TRIF signaling drives homeostatic intestinal epithelial antimicrobial peptide expression. J Immunol 2014; 193:4223–34. [DOI] [PubMed] [Google Scholar]
- 37. Vaishnava S, Yamamoto M, Severson KM, Ruhn KA, Yu X, Koren O et al The antibacterial lectin RegIIIγ promotes the spatial segregation of microbiota and host in the intestine. Science 2011; 334:255–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vijay‐Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S et al Metabolic syndrome and altered gut microbiota in mice lacking Toll‐like receptor 5. Science 2010; 328:228–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Goethel A, Turpin W, Rouquier S, Zanello G, Robertson SJ, Streutker CJ et al Nod2 influences microbial resilience and susceptibility to colitis following antibiotic exposure. Mucosal Immunol 2019; 12:720–32. [DOI] [PubMed] [Google Scholar]
- 40. Gribar SC, Sodhi CP, Richardson WM, Anand RJ, Gittes GK, Branca MF et al Reciprocal expression and signaling of TLR4 and TLR9 in the pathogenesis and treatment of necrotizing enterocolitis. J Immunol 2009; 182:636–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee J, Mo JH, Katakura K, Alkalay I, Rucker AN, Liu YT et al Maintenance of colonic homeostasis by distinctive apical TLR9 signalling in intestinal epithelial cells. Nat Cell Biol 2006; 8:1327–36. [DOI] [PubMed] [Google Scholar]
- 42. Pott J, Stockinger S, Torow N, Smoczek A, Lindner C, McInerney G et al Age‐dependent TLR3 expression of the intestinal epithelium contributes to rotavirus susceptibility. PLoS Pathog 2012; 8:e1002670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gewirtz AT, Vijay‐Kumar M, Brant SR, Duerr RH, Nicolae DL, Cho JH. Dominant‐negative TLR5 polymorphism reduces adaptive immune response to flagellin and negatively associates with Crohn's disease. Am J Physiol Gastrointest Liver Physiol 2006; 290:G1157–63. [DOI] [PubMed] [Google Scholar]
- 44. Bank S, Andersen PS, Burisch J, Pedersen N, Roug S, Galsgaard J et al Polymorphisms in the toll‐like receptor and the IL‐23/IL‐17 pathways were associated with susceptibility to inflammatory bowel disease in a Danish Cohort. PLoS ONE 2015; 10:e0145302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang X, Deriaud E, Jiao X, Braun D, Leclerc C, Lo‐Man R. Type I interferons protect neonates from acute inflammation through interleukin 10‐producing B cells. J Exp Med 2007; 204:1107–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vereecke L, Sze M, Mc Guire C, Rogiers B, Chu Y, Schmidt‐Supprian M et al Enterocyte‐specific A20 deficiency sensitizes to tumor necrosis factor‐induced toxicity and experimental colitis. J Exp Med 2010; 207:1513–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lotz M, Gutle D, Walther S, Menard S, Bogdan C, Hornef MW. Postnatal acquisition of endotoxin tolerance in intestinal epithelial cells. J Exp Med 2006; 203:973–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chassin C, Kocur M, Pott J, Duerr CU, Gutle D, Lotz M et al miR‐146a mediates protective innate immune tolerance in the neonate intestine. Cell Host Microbe 2010; 8:358–68. [DOI] [PubMed] [Google Scholar]
- 49. Orlikowsky TW, Trug C, Neunhoeffer F, Deperschmidt M, Eichner M, Poets CF. Lipopolysaccharide‐binding protein in noninfected neonates and those with suspected early‐onset bacterial infection. J Perinatol 2006; 26:115–9. [DOI] [PubMed] [Google Scholar]
- 50. Rouge C, Butel MJ, Piloquet H, Ferraris L, Legrand A, Vodovar M et al Fecal calprotectin excretion in preterm infants during the neonatal period. PLoS ONE 2010; 5:e11083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kollmann TR, Levy O, Montgomery RR, Goriely S. Innate immune function by Toll‐like receptors: distinct responses in newborns and the elderly. Immunity 2012; 37:771–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ulas T, Pirr S, Fehlhaber B, Bickes MS, Loof TG, Vogl T et al S100‐alarmin‐induced innate immune programming protects newborn infants from sepsis. Nat Immunol 2017; 18:622–32. [DOI] [PubMed] [Google Scholar]
- 53. Dreschers S, Ohl K, Lehrke M, Mollmann J, Denecke B, Costa I et al Impaired cellular energy metabolism in cord blood macrophages contributes to abortive response toward inflammatory threats. Nat Commun 2019; 10:1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rakoff‐Nahoum S, Kong Y, Kleinstein SH, Subramanian S, Ahern PP, Gordon JI et al Analysis of gene‐environment interactions in postnatal development of the mammalian intestine. Proc Natl Acad Sci U S A 2015; 112:1929–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Menard S, Forster V, Lotz M, Gutle D, Duerr CU, Gallo RL et al Developmental switch of intestinal antimicrobial peptide expression. J Exp Med 2008; 205:183–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhang K, Dupont A, Torow N, Gohde F, Leschner S, Lienenklaus S et al Age‐dependent enterocyte invasion and microcolony formation by Salmonella . PLoS Pathog 2014; 10:e1004385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Johansson ME, Hansson GC. Immunological aspects of intestinal mucus and mucins. Nat Rev Immunol 2016; 16:639–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dupont A, Kaconis Y, Yang I, Albers T, Woltemate S, Heinbockel L et al Intestinal mucus affinity and biological activity of an orally administered antibacterial and anti‐inflammatory peptide. Gut 2015; 64:222–32. [DOI] [PubMed] [Google Scholar]
- 59. Haller D, Jobin C. Interaction between resident luminal bacteria and the host: can a healthy relationship turn sour? J Pediatr Gastroenterol Nutr 2004; 38:123–36. [DOI] [PubMed] [Google Scholar]
- 60. Torow N, Yu K, Hassani K, Freitag J, Schulz O, Basic M et al Active suppression of intestinal CD4+TCRα β + T‐lymphocyte maturation during the postnatal period. Nat Commun 2015; 6:7725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Garcia AM, Fadel SA, Cao S, Sarzotti M. T cell immunity in neonates. Immunol Res 2000; 22:177–90. [DOI] [PubMed] [Google Scholar]
- 62. Koch MA, Reiner GL, Lugo KA, Kreuk LS, Stanbery AG, Ansaldo E et al Maternal IgG and IgA antibodies dampen mucosal T helper cell responses in early life. Cell 2016; 165:827–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lindner C, Wahl B, Fohse L, Suerbaum S, Macpherson AJ, Prinz I et al Age, microbiota, and T cells shape diverse individual IgA repertoires in the intestine. J Exp Med 2012; 209:365–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gollwitzer ES, Saglani S, Trompette A, Yadava K, Sherburn R, McCoy KD et al Lung microbiota promotes tolerance to allergens in neonates via PD‐L1. Nat Med 2014; 20:642–7. [DOI] [PubMed] [Google Scholar]
- 65. Scharschmidt TC, Vasquez KS, Pauli ML, Leitner EG, Chu K, Truong HA et al Commensal microbes and hair follicle morphogenesis coordinately drive Treg migration into neonatal skin. Cell Host Microbe 2017; 21:467–77.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Scharschmidt TC, Vasquez KS, Truong HA, Gearty SV, Pauli ML, Nosbaum A et al A wave of regulatory T cells into neonatal skin mediates tolerance to commensal microbes. Immunity 2015; 43:1011–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sefik E, Geva‐Zatorsky N, Oh S, Konnikova L, Zemmour D, McGuire AM et al Individual intestinal symbionts induce a distinct population of RORγ + regulatory T cells. Science 2015; 349:993–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ohnmacht C, Park JH, Cording S, Wing JB, Atarashi K, Obata Y et al The microbiota regulates type 2 immunity through RORγt+ T cells. Science 2015; 349:989–93. [DOI] [PubMed] [Google Scholar]
- 69. Bunker JJ, Erickson SA, Flynn TM, Henry C, Koval JC, Meisel M et al Natural polyreactive IgA antibodies coat the intestinal microbiota. Science 2017; 358:eaan6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Mao K, Baptista AP, Tamoutounour S, Zhuang L, Bouladoux N, Martins AJ et al Innate and adaptive lymphocytes sequentially shape the gut microbiota and lipid metabolism. Nature 2018; 554:255–9. [DOI] [PubMed] [Google Scholar]
- 71. Cahenzli J, Koller Y, Wyss M, Geuking MB, McCoy KD. Intestinal microbial diversity during early‐life colonization shapes long‐term IgE levels. Cell Host Microbe 2013; 14:559–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Olszak T, An D, Zeissig S, Vera MP, Richter J, Franke A et al Microbial exposure during early life has persistent effects on natural killer T cell function. Science 2012; 336:489–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Stefka AT, Feehley T, Tripathi P, Qiu J, McCoy K, Mazmanian SK et al Commensal bacteria protect against food allergen sensitization. Proc Natl Acad Sci U S A 2014; 111:13145–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zanvit P, Konkel JE, Jiao X, Kasagi S, Zhang D, Wu R et al Antibiotics in neonatal life increase murine susceptibility to experimental psoriasis. Nat Commun 2015; 6:8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kirjavainen PV, Karvonen AM, Adams RI, Taubel M, Roponen M, Tuoresmaki P et al Farm‐like indoor microbiota in non‐farm homes protects children from asthma development. Nat Med 2019:25:1089–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Feehley T, Plunkett CH, Bao R, Choi Hong SM, Culleen E, Belda‐Ferre P et al Healthy infants harbor intestinal bacteria that protect against food allergy. Nat Med 2019; 25:448–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Cox LM, Yamanishi S, Sohn J, Alekseyenko AV, Leung JM, Cho I et al Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 2014; 158:705–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ahmadizar F, Vijverberg SJH, Arets HGM, de Boer A, Lang JE, Garssen J et al Early‐life antibiotic exposure increases the risk of developing allergic symptoms later in life: a meta‐analysis. Allergy 2018; 73:971–86. [DOI] [PubMed] [Google Scholar]
- 79. Vatanen T, Kostic AD, d'Hennezel E, Siljander H, Franzosa EA, Yassour M et al Variation in microbiome LPS immunogenicity contributes to autoimmunity in humans. Cell 2016; 165:1551. [DOI] [PubMed] [Google Scholar]
- 80. Trompette A, Gollwitzer ES, Pattaroni C, Lopez‐Mejia IC, Riva E, Pernot J et al Dietary fiber confers protection against flu by shaping Ly6c– patrolling monocyte hematopoiesis and CD8+ T cell metabolism. Immunity 2018; 48:992–1005.e8. [DOI] [PubMed] [Google Scholar]
- 81. Tan J, McKenzie C, Vuillermin PJ, Goverse G, Vinuesa CG, Mebius RE et al Dietary fiber and bacterial SCFA enhance oral tolerance and protect against food allergy through diverse cellular pathways. Cell Rep 2016; 15:2809–24. [DOI] [PubMed] [Google Scholar]
- 82. Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P et al Metabolites produced by commensal bacteria promote peripheral regulatory T‐cell generation. Nature 2013; 504:451–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Jiang HQ, Bos NA, Cebra JJ. Timing, localization, and persistence of colonization by segmented filamentous bacteria in the neonatal mouse gut depend on immune status of mothers and pups. Infect Immun 2001; 69:3611–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Harris NL, Spoerri I, Schopfer JF, Nembrini C, Merky P, Massacand J et al Mechanisms of neonatal mucosal antibody protection. J Immunol 2006; 177:6256–62. [DOI] [PubMed] [Google Scholar]
- 85. Gopalakrishna KP, Macadangdang BR, Rogers MB, Tometich JT, Firek BA, Baker R et al Maternal IgA protects against the development of necrotizing enterocolitis in preterm infants. Nat Med 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Sarzotti M, Robbins DS, Hoffman PM. Induction of protective CTL responses in newborn mice by a murine retrovirus. Science 1996; 271:1726–8. [DOI] [PubMed] [Google Scholar]
- 87. Forsthuber T, Yip HC, Lehmann PV. Induction of TH1 and TH2 immunity in neonatal mice. Science 1996; 271:1728–30. [DOI] [PubMed] [Google Scholar]
- 88. Ridge JP, Fuchs EJ, Matzinger P. Neonatal tolerance revisited: turning on newborn T cells with dendritic cells. Science 1996; 271:1723–6. [DOI] [PubMed] [Google Scholar]
- 89. Lindner C, Thomsen I, Wahl B, Ugur M, Sethi MK, Friedrichsen M et al Diversification of memory B cells drives the continuous adaptation of secretory antibodies to gut microbiota. Nat Immunol 2015; 16:880–8. [DOI] [PubMed] [Google Scholar]
- 90. Li N, van Unen V, Abdelaal T, Guo N, Kasatskaya SA, Ladell K et al Memory CD4+ T cells are generated in the human fetal intestine. Nat Immunol 2019; 20:301–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Schreurs R, Baumdick ME, Sagebiel AF, Kaufmann M, Mokry M, Klarenbeek PL et al Human fetal TNF‐α‐cytokine‐producing CD4+ effector memory T cells promote intestinal development and mediate inflammation early in life. Immunity 2019; 50:462–76 e8. [DOI] [PubMed] [Google Scholar]
- 92. Mold JE, Venkatasubrahmanyam S, Burt TD, Michaelsson J, Rivera JM, Galkina SA et al Fetal and adult hematopoietic stem cells give rise to distinct T cell lineages in humans. Science 2010; 330:1695–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Yang S, Fujikado N, Kolodin D, Benoist C, Mathis D. Immune tolerance. Regulatory T cells generated early in life play a distinct role in maintaining self‐tolerance. Science 2015; 348:589–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Lahl K, Loddenkemper C, Drouin C, Freyer J, Arnason J, Eberl G et al Selective depletion of Foxp3+ regulatory T cells induces a scurfy‐like disease. J Exp Med 2007; 204:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Smith NL, Patel RK, Reynaldi A, Grenier JK, Wang J, Watson NB et al Developmental origin governs CD8+ T cell fate decisions during infection. Cell 2018; 174:117–130.e14 [DOI] [PubMed] [Google Scholar]
- 96. Zens KD, Chen JK, Guyer RS, Wu FL, Cvetkovski F, Miron M et al Reduced generation of lung tissue‐resident memory T cells during infancy. J Exp Med 2017; 214:2915–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Gibbons D, Fleming P, Virasami A, Michel ML, Sebire NJ, Costeloe K et al Interleukin‐8 (CXCL8) production is a signatory T cell effector function of human newborn infants. Nat Med 2014; 20:1206–10. [DOI] [PubMed] [Google Scholar]
- 98. Schneider C, Lee J, Koga S, Ricardo‐Gonzalez RR, Nussbaum JC, Smith LK et al Tissue‐resident group 2 innate lymphoid cells differentiate by layered ontogeny and in situ perinatal priming. Immunity 2019; 50:1425–38.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sagebiel AF, Steinert F, Lunemann S, Korner C, Schreurs R, Altfeld M et al Tissue‐resident Eomes+ NK cells are the major innate lymphoid cell population in human infant intestine. Nat Commun 2019; 10:975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Drozdowski LA, Clandinin T, Thomson AB. Ontogeny, growth and development of the small intestine: understanding pediatric gastroenterology. World J Gastroenterol 2010; 16:787–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Turfkruyer M, Rekima A, Macchiaverni P, Le Bourhis L, Muncan V, van den Brink GR et al Oral tolerance is inefficient in neonatal mice due to a physiological vitamin A deficiency. Mucosal Immunol 2016; 9:479–91. [DOI] [PubMed] [Google Scholar]
- 102. Hanson DG. Ontogeny of orally induced tolerance to soluble proteins in mice. I. Priming and tolerance in newborns. J. Immunol. 1981; 127:1518–24. [PubMed] [Google Scholar]
- 103. Strobel S, Ferguson A. Immune responses to fed protein antigens in mice. 3. Systemic tolerance or priming is related to age at which antigen is first encountered. Pediatr. Res. 1984; 18:588–94. [DOI] [PubMed] [Google Scholar]
- 104. Abdel‐Gadir A, Stephen‐Victor E, Gerber GK, Noval Rivas M, Wang S, Harb H et al Microbiota therapy acts via a regulatory T cell MyD88/RORγt pathway to suppress food allergy. Nat Med 2019;25:1164–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Tuncel J, Benoist C, Mathis D. T cell anergy in perinatal mice is promoted by T reg cells and prevented by IL‐33. J Exp Med 2019; 216:1328–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Cayrol C, Girard JP. Interleukin‐33 (IL‐33): a nuclear cytokine from the IL‐1 family. Immunol Rev. 2018; 281:154–68. [DOI] [PubMed] [Google Scholar]


