Abstract
Cells use different cell adhesion and communication structures to promote tissue development, maintenance of tissue integrity as well as repair and regenerative processes. Another recently discovered way of information exchange is long‐distance thin cellular processes called nanotubes (NTs), mainly studied in vitro. Information on the existence and relevance of NTs in vivo is sparse. Building on two references which hint at the potential existence of longitudinally directed cell processes resembling NTs, we investigated tendons from young (3 weeks) and adult (9 weeks, 4 and 8 months) Fisher rats. Whole mounts of rat tail tendon fascicles (RTTfs) and sections of Achilles, flexor, extensor and patellar tendons were stained with Deep Red plasma membrane and DAPI nuclear stain and immunolabelled with Connexin43 (Cx43). In addition, 3‐D reconstruction of serial semithin sections and TEM was used to verify the presence of NTs. We were able to demonstrate NTs as straight thin longitudinal processes (Ø 100–500 nm) reaching up to several 100 μm in length, mainly originating from lateral sheet‐like cell processes or cell bodies in all tendon types investigated. NTs were observed to distend between tenocyte rows at the same level but also connect cells of different rows, thus leading to a complex 3‐D cellular scaffold. Shorter NTs connected lateral cell sheets of tenocytes in the same row, omitting one or two cells. In addition, we detected links or potential branching of NTs. Cx43 immunostaining for the detection of gap junctions revealed Cx43‐positive foci at the end‐to‐end contacts of tenocyte cell bodies as well as along their contacting sheet‐like processes. Only rarely, we found clear Cx43 signals at their potential contact points between NTs and tendon cells as well as along the course of NTs, and most NTs appeared completely devoid of Cx43 signals. Therefore, we conclude that NTs in tendons could have a twofold function: long‐distance communication as well as stabilization of a mechanically challenged tissue. From in vitro studies it is known that NTs allow intercellular transmission of various cell components, offering potential protective effects for the respective tissue. Further studies on functional properties of NTs in tendons under changing mechanical loading regimens are required in the future. The fact that NTs are present in tendons may necessitate the reconsideration of our traditional understanding of cell‐to‐cell communication.
Keywords: confocal, gap junctions, rat, three‐dimensional reconstruction, transmission electron microscopy, tendon
Nanotubes (NTs), a recently discovered way of information exchange comprising long‐distance thin cellular processes, are present in tendons. Together with the well‐known flat sheet‐like lateral processes, NTs form a complex three‐dimensional cellular network. NTs in tendons could have a twofold function: long‐distance communication as well as stabilization of a mechanically challenged tissue, and may require the reconsideration of our traditional understanding of cell‐to‐cell communication.

Introduction
Cells are equipped with a diversity of cell adhesion and communication structures in order to promote tissue development, proper tissue function, maintenance of tissue integrity as well as repair and regenerative processes.
Cell communication is classically known to be established via gap junctions that consist of specific cell membrane proteins, connexins, forming tunnels which allow direct crosstalk between neighbouring cells. Lately, new ways of intercellular communication have been found and intensively studied: exosomes or extracellular vesicles (Braicu et al. 2015) and ultrathin intercellular structures called nanotubes (NTs) (for review see Davis & Sowinski, 2008; Sisakhtnezhad & Khosravi, 2015).
NTs, also known as tunneling NTs or membrane NTs, have been found in many types of cells in vitro including tumor cells (Rustom et al. 2004), immune cells (Önfelt et al. 2004), normal rat kidney cells (Wang et al. 2010), peritoneal mesothelial cells (Ranzinger et al. 2014) and neural cells (Wang et al. 2012). So far, the existence of NTs in vivo has been documented in mouse cornea myeloid cells (Chinnery et al. 2008), mouse kidney cells (Ranzinger et al. 2014), migratory neural crest cells in chicken embryo (McKinney et al. 2011), human malignant pleural mesothelioma and lung adenocarcinoma (Lou et al. 2012).
The pertinent literature revealed a considerable heterogeneity in structure, process of formation and functional properties of NTs in vitro, probably dependent on the cell types involved (Davis & Sowinski, 2008). Thin NTs were characterized as actin‐containing tubes with a diameter of 50–200 nm, whereas thick NTs of up to 800 nm diameter also contained microtubules (Davis & Sowinski, 2008). Also, the length of NTs can vary considerable, ranging from several cell diameters up to several 100 μm (Sisakhtnezhad & Khosravi, 2015).
Besides connecting only two cells, they can also establish complex cellular networks (Rustom et al. 2004). It has been shown that NTs form adhesion (Lokar et al. 2010) as well as communicating junctions (Wang et al. 2010) at their contact points with other cells. NTs can provide membrane continuity between connected cells, allowing exchange of molecules, cell organelles and transfer of plasma membrane components but can also exist as closed NTs which potentially play a role in mechanical stabilization (Davis & Sowinski, 2008).
Mechanosensitive tissues like tendons are able to detect and convey mechanical signals like tensile strain, fluid flow and compression via cell‐matrix interaction and cell‐to‐cell signalling via gap junctions as well as mechanosensitive voltage‐gated ion channels (Wall & Banes, 2005; Magra et al. 2007). Tenocytes can respond individually to these mechanobiological stimuli, but they can also coordinate and communicate this response between neighbouring cells through a three‐dimensional network of cell processes linked by gap junctions (McNeilly et al. 1996). Tenocytes are arranged with their cell bodies consecutively aligned in parallel rows. Furthermore, tenocytes of adjacent rows are connected by flat sheet‐like lateral processes. When studying tenocyte cell communication and its regulation upon transmission of mechanical signals, McNeilly et al. (1996) found long, thin longitudinal processes in rat deep flexor tendons that were assumed to provide long‐distance contact between cells.
The only other reference to the potential existence of NTs or similar structures in tendons was made by Ralphs et al. (2002), who described ‘stress fibres of considerable length’ (phalloidin positive structures) running longitudinally between cell rows in deep digital flexor tendons of chickens.
Based on these sparse indications as well as coincidental findings in our own research, we systematically investigated the potential existence of NTs in different tendon types in the rat.
We therefore examined the three‐dimensional tenocyte morphology in healthy rat tendons using different approaches (whole mount staining, 3‐D reconstruction of semithin sections and TEM). Furthermore, we performed immunostaining for the detection of gap junction protein to address potential functional aspects of NTs in tendons.
Materials and methods
Tendons were harvested from young (3 weeks) and adult (9 weeks, 4 and 8 months old) Fisher rats, respectively, immediately after euthanasia. Animals were euthanized for reasons unrelated to this study following institutional animal care and use approval.
For whole mount stainings, rat tail tendon fascicles (RTTfs) of all age groups were pulled using a forceps, cut in 2‐cm pieces and fixed in buffered formalin containing 4% formaldehyde (ACM, Vienna, Austria) for 10 min at room temperature. After washing with phosphate‐buffered saline (PBS; Morphisto, Frankfurt am Main, Germany), tendon fascicles were submerged in a 5 μg mL−1 solution of CellMask™ Deep Red plasma membrane stain (Molecular Probes, Eugene, OR, USA) in PBS and incubated for 15 min in the dark. Nuclei were counterstained with 4′,6′‐diamidino‐2‐phenylindole (DAPI, Sigma‐Aldrich, Vienna, Austria) and tendons were mounted with Mowiol 4‐88 (Polysciences Inc., Eppelheim, Germany) for microscopy. Image stacks were obtained using a confocal laser scanning microscope (LSM 510 Meta, Zeiss, Vienna, Austria) and visualized by multichannel 3‐D volume rendering. In addition, Achilles tendons, extensor and flexor tendons and patellar tendons were obtained from the 4‐month‐old animal, fixed in buffered formalin containing 4% formaldehyde and embedded in paraffin. Sections were cut at 6–8 μm thickness and stained as described above for the whole mount RTTfs.
For semithin and ultrathin sectioning, fresh RTTfs from 9‐week‐ and 8‐month‐old rats were cut in 0.5‐cm pieces and fixed in 2.5% glutaraldehyde, subjected to three washing sessions (10 min each) in Sørensen phosphate buffer (0.1 m, pH 7.4) and post‐fixed in 1% osmium tetroxide (Electron Microscopy Sciences, Hatfield, PA, USA). After dehydration in a series of ethanol dilutions (70–100%), samples were embedded in Epon resin (Serva, Mannheim, Germany).
Serial semithin sections were cut at 0.8 μm thickness at a speed of 1 mm s−1 with an ultramicrotome (Reichert Ultracut S, Leica, Vienna, Austria) using a Diatome Histo Jumbo diamond knife (Diatome Ltd., CH) following the instructions of Ruthensteiner (2008). Four bands of 15–20 sections each were collected on SuperFrost glass slides (Roth, Karlsruhe, Germany), dried on a 70 °C heat plate for at least 1 h and subsequently stained with toluidine blue for 20 s. Slides were finally mounted with Epon resin, covered with a coverslip and the resin was polymerized by placing the slides in an incubator at 60 °C overnight.
Of 200 serial semithin sections of each tendon sample, 20 sections (8 months) and 45 sections (9 weeks), respectively, were finally used for 3‐D reconstruction and one image per section was taken at 40× magnification. After serial section images were aligned, tenocytes and tenocyte nuclei were segmented using manual image segmentation tools. Based on segmented materials, polygon surface models were created and rendered. All 3‐D image processing and rendering procedures were done using the 3‐D software amira (FEI SAS, Mérignac Cedex, France; part of Thermo Fisher Scientific™).
For transmission electron microscopic (TEM) analysis, ultrathin sections were cut on a Reichert Ultracut S (Leica) at 70 nm thickness. The sections were mounted on copper grids (Gröpl, Tulln, Austria) and stained with uranyl acetate (Fluka, Buchs, Switzerland) and lead citrate (Merck, Darmstadt, Germany). TEM pictures were taken using an EM900 (Zeiss, Oberkochen, Germany).
Gap junctions were detected by immunofluorescence staining for connexin43 (Cx43) in whole mount RTTfs and sections of formalin‐fixed, paraffin‐embedded tendons mentioned above. After washing in PBS, tendon tissue was submerged in 1.5% goat serum (PAA, Pasching, Austria) for blocking. Afterwards, a rabbit polyclonal primary antibody against Cx43 (Sigma‐Aldrich, Vienna, Austria) at a dilution of 1 : 2000 in PBS was applied, which was subsequently detected with an anti‐rabbit secondary antibody conjugated with Alexa Fluor 488 (Molecular Probes, Eugene, OR, USA). Deep Red plasma membrane staining and detection of cell nuclei with DAPI were performed as described above.
Results
Deep Red membrane stain clearly revealed the presence of NTs in whole mounts of RTTfs as well as in thick sections of all other tendon types investigated. In addition to the flat, sheet‐like cytoplasmic processes, we found NTs as straight, thin (Ø 100–500 nm), longitudinal processes reaching up to several 100 μm in length, moving in and out of the section plane (Fig. 1a–c). They often originated from lateral cell sheets running parallel to the rows of cell bodies, or obliquely crossing to another cell row (Fig. 1a, arrows). Shorter NTs connected lateral sheet‐like processes of tenocytes in the same row, omitting one or two cells (Fig. 1b, arrowheads). In addition, we detected links or potential branches of NTs (Fig. 1c, arrowhead). No age difference was found in rat tendons older than 9 weeks. However, tenocytes formed multiple short processes in the highly cellular tendons of the 3‐week‐old rat (Supporting Information Figure S1). NTs appeared at similar frequency in all tendon types investigated.
Figure 1.
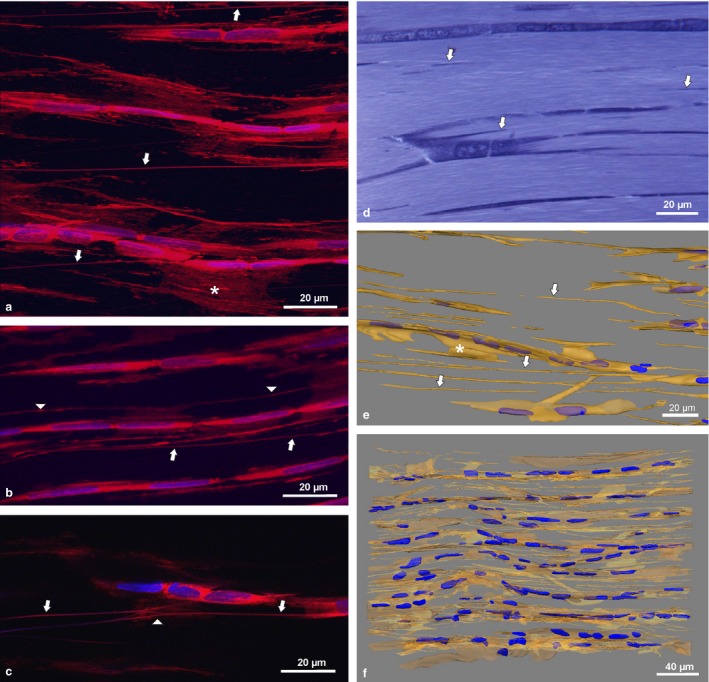
Confocal images of whole mount adult rat tail tendon fascicles (RTTfs) stained with Deep Red and DAPI nuclear stain (a–c) showing sheet‐like processes (*) and NTs (arrows) connecting tenocytes. Arrowheads depict shorter NTs connecting cell sheets of tenocytes in the same cell row (b) and links or potential branching of NTs (c), respectively. Semithin section of RTTfs of a 9‐week‐old rat after toluidine blue staining (d) and 3‐D reconstruction (e,f) showing NTs (arrows). For detailed display of the 3‐D reconstruction see Video S1.
In toluidine blue‐stained semithin sections, short cuts of NTs were visible (Fig. 1d, arrows). They became apparent and clearly discernible from flat sheet‐like processes after segmentation‐based 3‐D reconstruction (Fig. 1e, arrows). Total 3‐D reconstruction of a 300‐μm‐long, 200‐μm‐high (containing eight vertical cell rows) and 36‐μm‐thick (three cell layers) segment of a RTTf from a 9‐week‐old rat finally revealed the intertwined network of cellular processes between tenocytes (Fig. 1f, Supporting Information Video S1). NTs distended between tenocyte rows at the same level but also connected cells of different rows, thus leading to a complex 3‐D cellular scaffold. The same pattern of cellular organization was found in the tendon of the 8‐month‐old rat (data not shown).
TEM clearly confirmed the existence of NTs between tenocytes (Fig. 2). NTs preferentially originated (or potentially ended) from lateral cell sheets or cell bodies, and while those showed irregular shape and surface structure, NTs appeared very straight with smooth surface (Fig. 2a,c). NTs ran parallel between the longitudinally arranged collagen fibrils and were very hard to trace over a longer distance as they moved in and out of the section plane (Fig. 2b). In spite of this challenge, one NT end‐to‐end contact, where two NTs met and formed a short overlapping contact area, was found (Fig. 2d).
Figure 2.
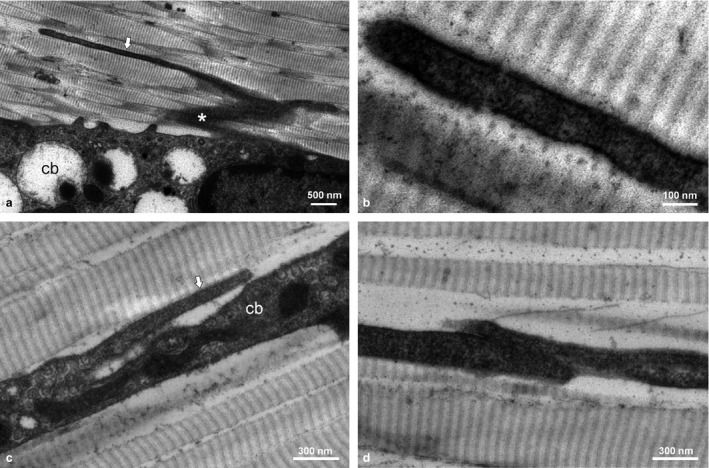
TEM micrographs of adult rat tendons showing cell bodies (cb) of tenocytes with a lateral sheet‐like process (*) and a longitudinally directed NT (arrow) originating from the cell sheet (a) and the cell body (c); detail of NT (b) and closed NT end‐to‐end contact (d).
To clarify functional aspects of NTs between tenocytes, we performed Cx43 immunostaining for the detection of gap junctions. We found Cx43‐positive foci at the end‐to‐end contacts of tenocyte cell bodies as well as along their contacting sheet‐like processes (Fig. 3). Only rarely did we find clear Cx43 signals at the potential contact points between NTs and tendon cells (Fig. 3a, arrow) or along the course of NTs (Fig. 3b, arrow). Most NTs appeared completely devoid of Cx43 signals.
Figure 3.
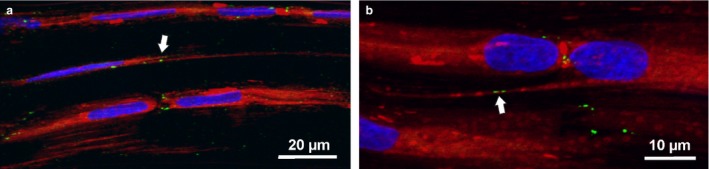
Confocal images of whole mount adult rat tail tendon fascicles stained with Deep Red, DAPI nuclear stain and immunolabelling for connexin43 (green) showing gap junctions at contact points (arrows) between NTs and tendon cells (a) as well as along the course of NTs (b)
Discussion
Using confocal and electron microscopy and 3‐D reconstruction of serial sections, we were able clearly to demonstrate the presence of NTs in different types of rat tendons, connecting tenocytes longitudinally over a long distance in all tendon types investigated. With these results, we confirm previous hints at the potential existence of NTs in tendons made by McNeilly et al. (1996) and Ralphs et al. (2002). McNeilly et al. (1996) first described long, thin longitudinal cell processes ‘which make contact with cell processes or cell bodies of cells some distance away’ in deep flexor tendons of adult rats, but did not discuss their incidental finding any further. Ralphs et al. (2002) described ‘long oriented rows of actin stress fibres running along the rows of tendon cells’. However, the authors did not attribute the actin stress fibre arrangement to specific cellular structures such as cell protrusions and mainly focused on the contractile functional aspect as an active mechanism for protection and recovery from stretch. Additionally, NTs are inadvertently depicted in RT tendons stained for tubulin to study the role of tendon cell cilia (Gardner et al. 2011).
Regarding the structure, NTs found in our study showed a diameter range of 100–500 nm and therefore comprise thin as well as thick NTs (Davis & Sowinski, 2008). Detailed analysis of quantity of thin and thick NTs in different tendon types and their potential content of cytoskeletal components should be addressed in future studies. The length of NTs is known to be heterogeneous (Sisakhtnezhad & Khosravi, 2015), as confirmed by the present study, where we found NTs of different length in rat tendons in vivo. Our findings of NT branching support the results of Rustom et al. (2004) that NTs can connect more than two cells, thus establishing complex cellular networks.
Two major ways of NT formation have been postulated from in vitro experiments: by an actin‐driven protrusion connecting with a nearby cell or separation of neighbour cells pulling out NTs as they move apart (Davis & Sowinski, 2008). Based on morphological changes observed during tendon development and maturation (Fig. S1), we postulate that tenocytes establish NTs by initial cell‐cell contact and subsequent separation by collagen fibrils production and mechanical signals along the main direction of tension, maintaining their cellular connection with other tenocytes over long distances via longitudinally arranged NTs. Especially for tendon tissue, formation of NTs via separation seems far more likely as the density of cells, which are connected via a multitude of short thin cellular processes, is much higher during tendon development compared with adult tendons (Fig. S1) and active protrusion of cell processes between already existing bundles of collagen fibrils over a long distance seems implausible.
Both ways of NT formation can lead to either open‐ended or closed NTs depending on whether membrane fusion occurs upon contact (Davis & Sowinski, 2008). Our TEM results clearly show the existence of closed NTs in tendons meeting other NTs (Fig. 2d). Whether NTs that end at tenocyte cell bodies or their sheet‐like processes are closed or open‐ended is, however, not evident from our results. In cell culture, closed NTs (i.e. NTs without membrane fusion) connect with cell bodies or NTs of other cells via anchoring proteins such as N‐cadherin and β‐catenin (Lokar et al. 2010) and/or communicational proteins such as Cx43 (Wang et al. 2010). Our finding that gap junctions are only rarely established along NTs in tendons is consistent with results of Wang et al. (2010), who found that not all NTs possess Cx43 in normal rat kidney cells and therefore do not display electrical coupling. However, some of the Cx43‐positive signals seen at cell bodies might represent endings of NTs with gap junctions. We therefore conclude that NTs in tendons could have a twofold function: long‐distance communication (open‐ended NTs and closed NTs with gap junctions) and stabilization of a mechanically challenged tissue (closed NTs without gap junctions). The longitudinal organization of NTs might be ideal for sensing load along the main direction of tensile stress of tendons (Ralphs et al. 2002).
The existence of NTs mediating functional connectivity between cells in vivo has only recently been discovered (Chinnery et al. 2008). From in vitro studies it is known that NTs allow intercellular transmission of various cell components (Rustom et al. 2004), offering potential protective effects for the respective tissue. Further studies on structural and functional properties of NTs in tendons under changing mechanical loading regimens as well as in tendinopathies are required in the future. The fact that NTs are present in tendons (and potentially in many other mammalian tissues) may necessitate the reconsideration of our traditional understanding of cell‐to‐cell communication.
Conflict of interest
The authors have no conflict of interest to declare.
Author contributions
M. Egerbacher conceptualized the study, contributed to the acquisition and interpretation of the data and wrote the manuscript. S. Handschuh contributed to the imaging design of the study and performed the 3‐D reconstruction. S. Battisti contributed to the acquisition of data. S. Gabner contributed to the acquisition and interpretation of the data and provided critical contributions to the manuscript. All authors approved the final manuscript.
Supporting information
Video S1. Three‐dimensional reconstruction of semithin section of RTTfs of a 9 weeks old rat after toluidin blue staining.
Fig. S1. Light microscopic images of H&E‐stained rat tail tendon sections showing the age difference in cellularity between a young (3 weeks old, A) and an adult (8 months old rat, C). Confocal images of whole mount rat tail tendon fascicles stained with Deep Red and DAPI nuclear stain show the multiple cell processes (arrows) in the immature tendon of the 3‐week‐old animal (B).
Acknowledgements
The authors wish to acknowledge the technical support from the Histology and Embryology (University of Veterinary Medicine, Vienna) lab staff, especially of Irina Preining, who performed immunohistochemical stainings, and express special thanks to Waltraud Tschulenk for the elaborate work on serial semithin sectioning and Stefan Gabriel for laborious work on image processing. This research was supported using resources of the VetCore Facility (Imaging) of the University of Veterinary Medicine Vienna.
References
- Braicu C, Tomuleasa C, Monroig P, et al. (2015) Exosomes as divine messengers: are they the Hermes of modern molecular oncology? Cell Death Differ 22, 34–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnery HR, Pearlman E, Paul G, et al. (2008) Cutting edge: membrane nanotubes in vivo: a feature of MHC Class II+ cells in the mouse cornea. J Immunol 180, 5779–5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis DM, Sowinski S (2008) Membrane nanotubes: dynamic long‐distance connections between animal cells. Nat Rev Mol Cell Biol 9, 431–436. [DOI] [PubMed] [Google Scholar]
- Gardner K, Arnoczky SP, Lavagnino M (2011) Effect of in vitro stress‐deprivation and cyclic loading on the length of tendon cell cilia in situ. J Orthop Res 29, 582–587. [DOI] [PubMed] [Google Scholar]
- Lokar M, Iglič A, Veranič P (2010) Protruding membrane nanotubes: attachment of tubular protrusions to adjacent cells by several anchoring junctions. Protoplasma 246, 81–87. [DOI] [PubMed] [Google Scholar]
- Lou E, Fujisawa S, Morozov A, et al. (2012) Tunneling nanotubes provide a unique conduit for intercellular transfer of cellular contents in human malignant pleural mesothelioma. PLoS ONE 7, e33093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magra M, Hughes S, El Haj AJ, et al. (2007) VOCCs and TREK‐1 ion channel expression in human tenocytes. Am J Physiol Cell Physiol 292, C1053–C1060. [DOI] [PubMed] [Google Scholar]
- McKinney MC, Stark DA, Teddy J, et al. (2011) Neural crest cell communication involves an exchange of cytoplasmic material through cellular bridges revealed by photoconversion of KikGR. Dev Dyn 240, 1391–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeilly CM, Banes AJ, Benjamin M, et al. (1996) Tendon cells in vivo form a three dimensional network of cell processes linked by gap junctions. J Anat 189, 593–600. [PMC free article] [PubMed] [Google Scholar]
- Önfelt B, Nedvetzki S, Yanagi K, et al. (2004) Cutting edge: membrane nanotubes connect immune cells. J Immunol 173, 1511–1513. [DOI] [PubMed] [Google Scholar]
- Ralphs JR, Waggett AD, Benjamin M (2002) Actin stress fibres and cell‐cell adhesion molecules in tendons: organisation in vivo and response to mechanical loading of tendon cells in vitro. Matrix Biol 21, 67–74. [DOI] [PubMed] [Google Scholar]
- Ranzinger J, Rustom A, Heide D, et al. (2014) The receptor for advanced glycation end‐products (RAGE) plays a key role in the formation of nanotubes (NTs) between peritoneal mesothelial cells and in murine kidneys. Cell Tissue Res 357, 667–679. [DOI] [PubMed] [Google Scholar]
- Rustom A, Saffrich R, Markovic I, et al. (2004) Nanotubular highways for intercellular organelle transport. Science 303, 1007–1010. [DOI] [PubMed] [Google Scholar]
- Ruthensteiner B (2008) Soft Part 3D visualization by serial sectioning and computer reconstruction. Zoosymposia 1, 63–100. [Google Scholar]
- Sisakhtnezhad S, Khosravi L (2015) Emerging physiological and pathological implications of tunnelling nanotubes formation between cells. Eur J Cell Biol 94, 429–443. [DOI] [PubMed] [Google Scholar]
- Wall ME, Banes AJ (2005) Early responses to mechanical load in tendon: role for calcium signaling, gap junctions and intercellular communication. J Musculoskelet Neuronal Interact 5, 70–84. [PubMed] [Google Scholar]
- Wang X, Veruki ML, Bukoreshtliev NV, et al. (2010) Animal cells connected by nanotubes can be electrically coupled through interposed gap‐junction channels. PNAS 107, 17194–17199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Bukoreshtliev NV, Gerdes HH (2012) Developing neurons form transient nanotubes facilitating electrical coupling and calcium signaling with distant astrocytes. PLoS ONE 7, e47429. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1. Three‐dimensional reconstruction of semithin section of RTTfs of a 9 weeks old rat after toluidin blue staining.
Fig. S1. Light microscopic images of H&E‐stained rat tail tendon sections showing the age difference in cellularity between a young (3 weeks old, A) and an adult (8 months old rat, C). Confocal images of whole mount rat tail tendon fascicles stained with Deep Red and DAPI nuclear stain show the multiple cell processes (arrows) in the immature tendon of the 3‐week‐old animal (B).


