Abstract
Objectives
Treatment for osteoporotic vertebral fracture (OVF) with cord compression is challenging and it usually requires surgical interventions to decompress nerves and restore spinal sequences. To describe a novel surgical strategy for treating OVFs with cord compression.
Methods
This is a single‐center retrospective analysis. The inclusion criteria were Frankel grade C‐E, single level T10‐L2. Between January 2008 and December 2016, a total of 56 OVF patients (47 females and nine males, with an average age of 72 years (66–88 years), comprising of eight grade C, 23 grade D, and 25 grade E patients) were enrolled. The treatment algorithm included preoperative evaluation by MRI, extension CT, and radiography to classify the OVFs as type 1.1 (reducible, stable; n = 13), type1.2 (reducible, unstable; n = 16), type 2 (irreducible; n = 19) or type 2M (modifier; n = 8). Vertebroplasty (VP)/kyphoplasty (KP) was applied in type 1.1. VP/KP with posterior fixation and posterolateral fusion was applied in type 1.2. And additional laminectomy/osteotomy was used in type 2, except in a modifier group (2M) where same procedure as applied for type 1.2 was used. VAS, ODI, Cobb angle, Frankel functional grade, and complications were recorded.
Results
Thirteen cases were classified as type 1.1, 16 cases as type 1.2, 19 cases as type 2, and eight cases as type 2M. The follow‐up period was 38.9 months (range, 24–108 months). All patients were followed‐up in at least 24 months, in which time four patients died, two patients were lost at the last follow‐up, and 50 patients completed the full study. The total VAS and ODI improved from 8 (7, 9) and 75.5% (67.2%, 80.0%) preoperatively to 2 (1, 3) and 31% (24.0%, 37.0%) on conclusion, respectively (P < 0.01). The local kyphotic angle was corrected from 22.3° (17.1°, 33. 8°) preoperatively to 10.4° (6.4°, 15.3°) on conclusion (P < 0.01). Twenty‐three patients had achieved neurological recovery on conclusion (42E, 8D, P < 0.01). Asymptotic cement leakage was observed in 17/56 cases (30.4%), 6/56 in the affected vertebra (10.7%), and 24/330 in the screw trajectory (7.3%). At 2 years postoperatively, 11 new VFs had occurred in nine patients (16.1%), including VFs in nine adjacent segments that all occurred within 1 year after surgery. No cement migration or implant failure was noted.
Conclusion
The novel surgical strategy for treating OVFs with cord compression consists of the most tailored and least invasive treatment for each patient. The positive mid‐ and long‐term clinical and radiological outcomes observed could represent a step forward in devising the proposed algorithm.
Keywords: Spinal Cord Compression, CT Scan, Osteoporotic fracture, Surgery
Introduction
Approximately 1.4 mn patients suffer from osteoporotic vertebral fractures (OVFs) per year worldwide1. The majority of these patients experience chronic back pain, disability, and kyphotic deformity, which decrease their quality of life and can even cause death2, 3. Fortunately, most OVFs can heal or remain asymptomatic with conservative treatment4; however, such treatment fails in approximately 10%–30% of cases, leading to painful nonunion, kyphotic deformity, and neurological deficits5. Delayed post‐traumatic vertebral collapse is frequently associated with an intravertebral cleft, known as Kummell's disease6, and cases with neurological compression by posterior cortex breakage are classified into stage III which requires surgical interventions7, 8. Neurological complications or paraparesis that develops in an osteoporotic spine9 can be a major challenge in achieving a durable surgical fixation. Various surgical procedures have been proposed in the management of OVFs with cord compression. However, the optimal surgical procedures remain controversial because, in such cases, planning the surgical strategy is much more complicated due to various factors that need to be considered, including neurological status, spinal deformity, bone quality, and comorbidities of the patient, as well as the surgical risk related to the planned technique.
To date, surgical procedures proposed for the management of OVFs with delayed neurological deficits include vertebroplasty (VP) and instrumentation from an anterior, posterior or combined approach 7, 10, 11. Posterior Egg‐shell procedure12, 13 and instrumentation has been tried to correct deformity. However, the posterior Egg‐shell procedure is technically demanding and highly risky. To minimize surgical invasiveness in aging patients, vertebroplasty or kyphoplasty has appealed widely in the management of OVFs, and satisfactory outcomes have been reported in the treatment of painful OVFs with delayed neurological deficit14. However, to successfully treat unstable vertebral collapse with spinal canal occupation by posterior bony fragments inducing neurological deficits, direct decompression of the nerve tissue and reconstruction of the spinal column is unavoidable for some patients. Therefore, a one‐stage posterior instrumentation technique with vertebroplasty has been tried 7, 11, 15. By augmentation of the anterior column using vertebroplasty, more rigid fixation was achieved with posterior instrumentation than with traditional posterior fixation. Besides, vertebroplasty is proved to be an effective method to provide anterior load sharing based on fundamental biomechanical principles16. It has been reported that the vertebroplasty technique corrects angular deformity, reconstitutes body height, and reduces instrumentation failure rate compared to no vertebroplasty involved.
On the other hand, anterior instrumentation surgery is shown as superior for direct resection of the retropulsed bony fragment and the reconstruction of the stable anterior spinal column17, 18. However, anterior spinal fixation alone is not sufficient for restoring and maintaining spinal sequence: 20% to 30% of cases underwent revised surgery with posterior instrumentation due to progression of a kyphotic deformity or screw loosening within 3 months postoperatively7, 8. To avoid such unexpected additional surgery, anterior‐posterior combined surgery has been applied. It shows better maintenance of kyphotic correction, less implant‐related complications and pseudarthrosis; however, it has longer operative time, greater intraoperative blood loss and comparable neurological improvement or the angle of kyphosis correction in the comparison with posterior fixation with vertebroplasty12.
Previous studies on outcomes after anterior or posterior surgical approaches were based on heterogeneous groups of patients with respect to the type of vertebral collapse, number and level of collapsed vertebrae, degree of osteoporosis, and neurological status. However, none of these studies explored tailored surgeries for different patterns of OVFs with cord compression. Previous treatment strategies for non‐union OVFs have been based on bone morphology6, 19, pelvic parameters, sagittal alignment19, and neurological status20. However, few studies have ever described sufficiently comprehensive plans for different types of OVFs with cord compression that present different symptoms which require individualized surgeries. In addition, these aged patients usually have numerous medical comorbidities which adds high risks of complications into complicated surgeries with long surgical times and large amounts of blood loss, such as osteotomies and long‐segment fixations. To individually simplify the operation for each patient to reduce time and invasive techniques is necessary.
Classification of spinal fractures to facilitate communication and encourage optimal treatment protocols has long been a focus of the spine community. Therefore, the purposes of this study were to: (i) introduce extension‐CT into assessment of OVFs with cord compression; (ii) describe a novel surgical strategy tailored to each patient for minimized invasiveness on the premise of sufficient neurological decompression and stable fixation; and (iii) analyze the clinical and radiological outcomes.
Patients and Methods
Patient Demographics
Institutional review board approval was obtained for medical record review. This study was a retrospective, single‐center study. The inclusion criteria were as follows: (i) patients no younger than 60 years old, diagnosed as single‐level nonunion VF with cord compression located from T10‐L2 which resulted from trivial injury, underwent spine surgeries; (ii) patients whose Frankel function status were at grade C, D, or E presented with back pain, weakness and/or numbness in lower limb(s) and/or dysfunction of bladder and bowel due to neurological compression, or without neurological injury in whom conservative treatment for at least 2 weeks failed, or who suffered from severe back pain caused by vertebral collapse and/or significant kyphotic deformity; (iii) bone mineral density (BMD) measured by dual‐energy x‐ray absorptiometry (DXA) at the lumbar vertebrae or femoral Ward area with T scores ≤‐2.5 SD; (iv) preoperative magnetic resonance imaging (MRI) and computed tomography (CT) scans and both preoperative and follow‐up radiographs; and (v) follow‐up time longer than 2 years. The exclusion criteria were as follows: (i) metastatic spinal tumors; (ii) degenerative spinal stenosis (defined as those who had a history of outpatient visits for spinal stenosis that had been diagnosed by radiographical and physical examinations); and (iii) previous spinal surgical history.
A total of 56 patients were enrolled between January 2008 and December 2016. There were 47 females and nine males, with an average age of 72 years (66–88 years) and average T scores of BMD at −3.8 SD (−2.9 to −5.4 SD). In 32 patients the injury was mild, and in 24 patients the injury was due to no memorized accident. The illness duration was 6.75 months on average (0.01–27 months). The medical disorder included hypertension in 18 cases, coronary disease in 15 cases, 2‐type diabetes in 18 cases and COPD in five cases. The affected vertebra was mainly at L1(23/56), T12 (15/56) and L2(14/56) level, 4/56 cases at T11.
Classifications and Surgical Strategies
Surgical treatment was indicated for the patients with neurological deficits and/or continuous severe lower back pain caused by vertebral instability and/or collapse.
Patients with nonunion OVF and cord compression confirmed by MRI underwent supine and extension CT scans (Fig. 1B). The scans were collected with the patient in the prone position with one hard pillow inserted beneath the chest and another inserted beneath the pelvis to determine the restorability of the posterior bony fragment and the height of the vertebra. The OVFs were classified into two main types and three subtypes, as follows:
Figure 1.
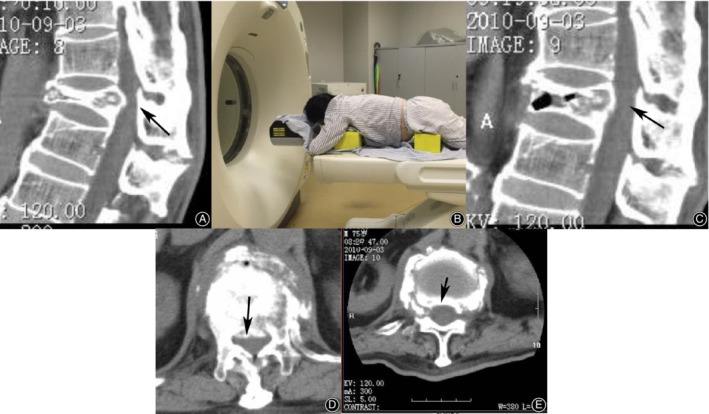
Comparison of vertebral height and posterior bony protrusion before and after extension CT. A supine CT scan shows that T12 is compressed by half (A), and the posterior bony fragment occupies the spinal canal (D); an image of a patient undergoing extension CT (B); observed on extension CT, the height is restored by more than 50% (C), and the spinal canal is decompressed (E).
Type 1
Reducible fracture. Observed on sagittal extension CT scans, the anterior height is restored by at least 50%, and the secondary spinal stenosis is decompressed more than 50% (Fig. 1). Furthermore, it is divided into two subtypes.
Type 1.1
Reducible and stable fracture. The bony fragment shows only one or two fracture lines, and the pedicles are intact. Vertebroplasty (VP)/kyphoplasty (KP) is applied.
Type 1.2
Reducible and instable fracture. Three or more fracture lines in posterior bony fragments and/or pedicle fracture are observed, and/or MRI shows injury to the posterior ligament complex. Fixation in situ using posterior augmented pedicle screws and posterolateral autologous bone fusion combined with VP is applied (Fig. 2).
Figure 2.
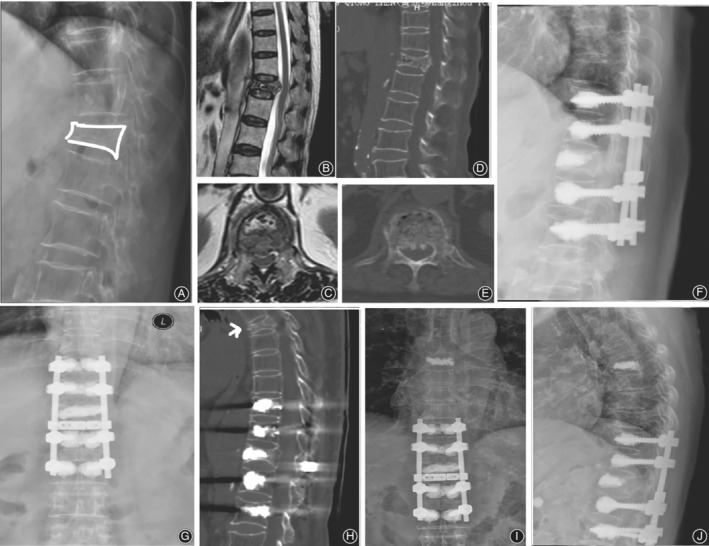
Representative case of type1.2 OVF. An 82‐year‐old female underwent VP at T12, posterolateral autologous bone fusion and instrumentation from T10 to L2 with augmented pedicle screw fixation. Preoperative lateral radiographs (A) and T2‐weighted MRI scans (B, C) show T12 fracture with dural compression. Height restoration and canal decompression are shown on extension CT (E, D), but multiple fracture lines are present in the posterior fragments (E). Postoperative lateral radiograph at 1 week (G). Six years later, she suffered another fracture at T6 (H) and underwent PVP. Postoperative radiographs (I, J).
Type 2
Irreducible fracture. Observed on sagittal extension CT scans, the anterior height is restored by less than 50%, and the secondary spinal stenosis is not decompressed well. Osteotomy/laminectomy and bone graft fusion combined with posterior augmented pedicle screw fixation is applied (Figs 3 and 4).
Figure 3.
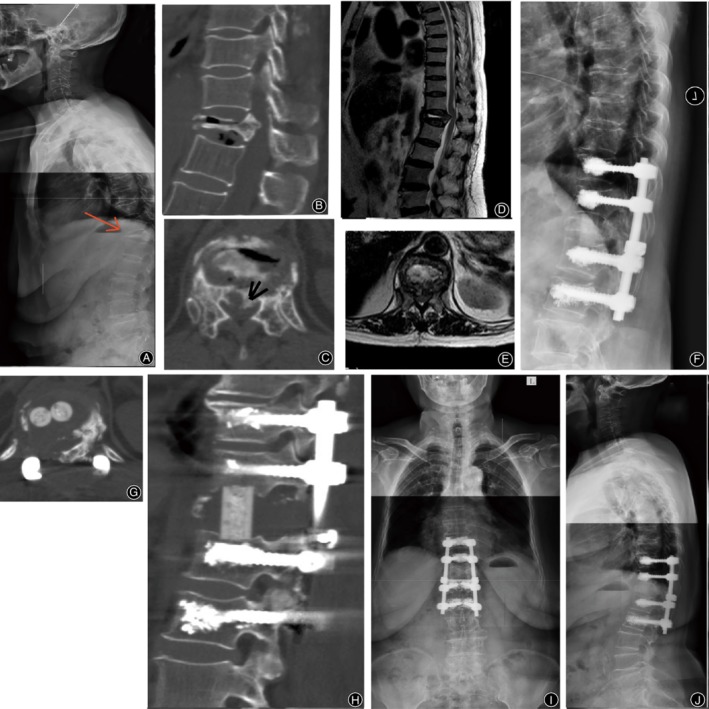
Representative case of type 2 OVF. A 77‐year‐old female underwent corpectomy at T12, reconstruction with a hydroxyapatite cage and intervertebral autologous bone fusion combined with instrumentation from T10 to L2 with augmented pedicle screw fixation. Preoperative lateral radiographs (A) and T2‐weighted MRI scans (D, E) show significant T12 collapse with dural compression. Barely no height restoration or canal decompression are shown on extension CT (B, C). Postoperative lateral radiograph (F) and CT scans (G, H) at 1 week (F) and 2 years (I, J) do not show instrumentation failure.
Figure 4.
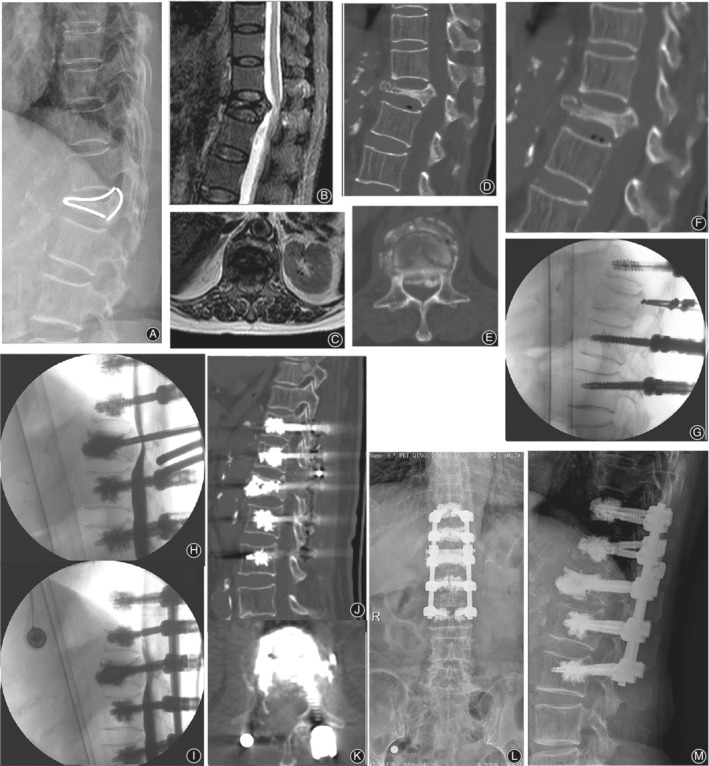
Representative case of type 2 OVF. A 76‐year‐old female underwent VP and limited laminectomy at L1, combined with posterolateral bone fusion and instrumentation from T11 to L3 with augmented pedicle screw fixation. Preoperative lateral radiographs (A) and T2‐weighted MRI scans (B,C) show that significant T12 collapse with dural compression. Barely any height restoration or canal decompression is shown on extension CT (D, E). Intraoperative fluoroscopy (G) shows restoration of the vertebral height without dural decompression on myelography (H, I). Postoperative CT scans (J, K) and lateral radiographs (L, I).
Type 2M
Modifier. In cases in this subgroup of type 2 cases, an anterior height reduction of more than 50% is achieved in the extended prone position after general anesthesia, and secondary spinal decompression is confirmed by myelography performed via L4,5 lumbar puncture using nonionic contrast medium (Omnipaque 300, GE Healthcare, Shanghai, China). Then, the procedure is the same as applied for type 1.2 fractures.
The anterior vertebral body height ratio was also measured as the percentage of the anterior and posterior vertebral height of the collapsed vertebra with respect to average heights of two adjacent vertebrae. The extent of bony fragment encroachment in the spinal canal was calculated as the ratio (percentage) of the anteroposterior diameter of bony fragments to the anteroposterior diameter of the spinal canal.
The patients underwent spinal surgery based on the protocol shown in the flow chart (Fig. 5).
Figure 5.
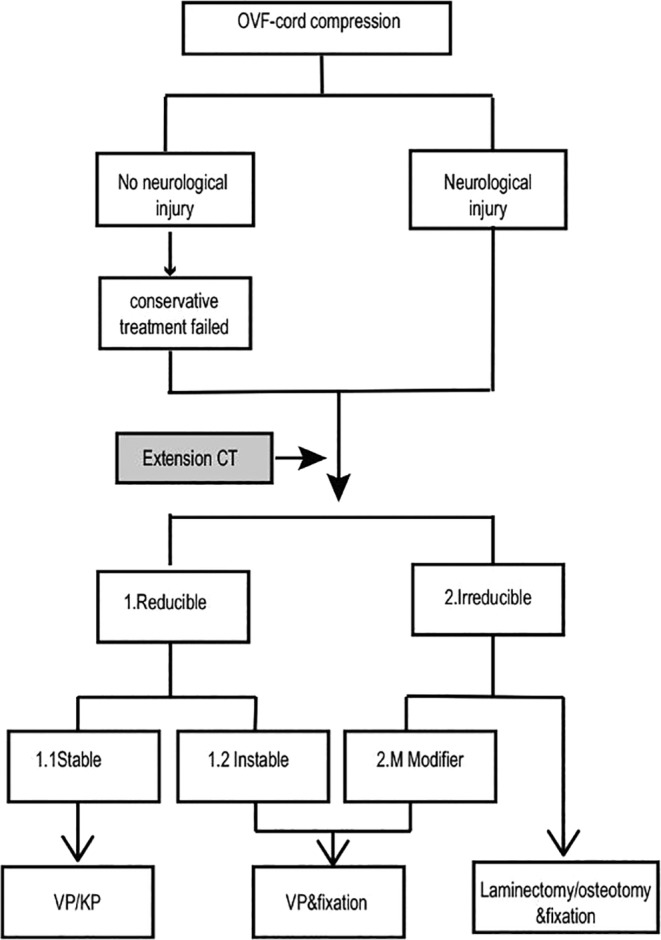
Algorithm of the surgical strategy for treating OVFs with cord compression. Modifier: Patients in this subgroup achieved >50% anterior height restoration in the extended prone position under general anesthesia; OVF: osteoporotic vertebral fracture; PLF: posterolateral fusion.
Methods
All surgeries were performed by three teams at the same center. The demographic, clinical, and radiological data were obtained retrospectively from the patient's case file and subsequently during follow‐up. The outcome measures included the following:
Clinical data preoperatively, 1 week postoperatively, 2 years postoperatively, and at the last follow‐up were measured.
1. Visual Analogue Scale (VAS)
The pain VAS is self‐completed by the respondent. Respondents mark the location on the 10 cm line corresponding to the amount of pain they experienced. Zero is no pain and 10 is severest pain.
2. Oswestry Disability Index (ODI)
ODI is a principal condition‐specific outcome measure used in the management of spinal disorders, and to assess patient progress in routine clinical practice. Zero percent to 20% is considered mild dysfunction, 21%–40% is moderate dysfunction, 41%–60% is severe dysfunction, and 61%–80% is considered as disability. For cases with score of 81%–100%, the patient is either long‐term bedridden, or exaggerating the impact of pain on their life.
3. Frankel Functional Grade
The Frankel Grade classification provides an assessment of spinal cord function and is used as a tool in spinal cord injury, as follows:
Grade A: Complete neurological injury ‐ No motor or sensory function detected below level of lesion.
Grade B: Preserved sensation only ‐ No motor function detected below level of lesion, some sensory function below level of lesion preserved.
Grade C: Preserved motor, nonfunctional ‐ Some voluntary motor function preserved below level of lesion but too weak to serve any useful purpose, sensation may or may not be preserved.
Grade D: Preserved motor, functional ‐ Functionally useful voluntary motor function below level of injury is preserved.
Grade E: Normal motor function ‐ Normal motor and sensory function below level of lesion, abnormal reflexes may persist.
Radiological Data
1. Local kyphotic (Cobb) angle
The Cobb angle was measured on X‐ray films as the angle between the upper endplate and the lower endplate of the index vertebra before and after surgery.
2. Cement leakage and migration
The cement leakage was located at paravertebral site, adjacent disc, spinal canal, and/or in vein which was detected from X‐ray or CT scan after surgery. The cement migration was that cement block was found partially migrating outside from vertebral body.
3. Screw loosening
Radiolucent halo around the pedicle screw was observed from X‐rays or CT scans.
4. New vertebral fracture
New vertebral fracture found from postoperative X‐rays or CT scans
Statistical Analysis
IBM SPSS Statistics 25 was used for analysis. Non‐normally distributed quantitative data are represented as the median (25th, 75th quartiles). Statistical analysis included the Kruskal‐Wallis test to compare differences in pre‐ and postoperative VAS scores, ODI and local kyphotic angles, a Dunn‐Bonferroni post hoc test, and a Pearson χ2 test for comparing Frankel functional grades. All P values ≤0.05 were considered statistically significant.
Results
Demographics Data
All patients underwent individualized procedures according to the algorithm. Thirteen cases were classified as type 1.1, 16 cases as type 1.2, 19 cases as type 2, and eight cases as type 2M. Autologous bone fusion was applied in type 1.2, 2 and 2M cases. Six osteotomy and 13 laminectomy were applied in type 2 cases, respectively.
Postoperative Management
Patients with type 1.1 fractures were ambulatory with a brace the day after surgery and were discharged 2 days after surgery. The postoperative management of the remaining patients included antibiotic treatment, wound drainage for 24 to 48 hours and ambulation with a brace at 2 or 3 days postoperatively. A deep wound infection occurred in one patient, who recovered after wound debridement, drainage and antibiotic treatment for 2 weeks. Another patient reported no improvement in bilateral thigh pain for 1 week after undergoing Smith‐Peterson osteotomy (SPO) at T12 and fixation at T10‐L2. She underwent a revision corpectomy at T12 with reconstruction and autologous bone fusion using a titian cage and a lateral approach and subsequently recovered. All patients were treated with a brace for 12 weeks and anti‐osteoporotic agents, including oral calcium 1200 mg/d and vitamin D 1200 iu/d. Additionally, female patients (creatinine clearance rate ≤36%) were scheduled to receive bisphosphonates intravenously once a year.
Follow‐up Outcomes
The follow‐up time was 38.9 ± 29.0 months (24–108 months). Two patients in the type 1.2 group died of coronary disease at 3 and 4 years after surgery, and one patient in the type 2M group died of pneumonia at 27 months after surgery. Among patients in the type 2 group, one patient died from a stroke 4 years after surgery, two patients were lost to follow‐up after 2 years due to a change in telephone number.
Clinical Outcomes
VAS
Pain was significantly relieved from preoperative mean VAS = 8 (7, 9), to mean VAS = 4 (2, 5) at 1 week postoperatively, and continuously reduced to a mean VAS = 2 (1, 3) at final follow‐up (P < 0.01, Table 2).
Table 2.
Pre‐and postoperative clinical outcomes
| Indexes | Preoperative | One week postoperative | Two years postoperative | Last follow‐up | Value | P value |
|---|---|---|---|---|---|---|
| VAS | 8 (7,9) | 4 (2, 5)* | 2 (1,3)* † | Z = 111.31 | <0.01 | |
| ODI (%) | 75.5 (67.2,80.0) | 45.1 (28.2,56.8)* | 31 (24.0,37.0)* † | Z = 102.46 | <0.01 | |
| Kyphotic angle (°) | 22.3 (17.1,33.8) | 7.4 (3.0, 10.9)* | 8.5 (6.0,12.7)* | 10.4 (6.4,15.3)* | Z = 98.98 | <0.01 |
Compared to preoperation, P < 0.05.
Compared to 1 week postoperation, P < 0.05.
For the χ2 test, the data of Frankel grades D and C were pooled.
ODI
Functional Disability was improved from preoperative mean ODI = 75.5% (67.2%, 80.0%), to ODI = 45.1% (28.2%, 56.8%) at 1 week postoperatively, and ODI = 31% (24.0%, 37.0%) at final follow‐up (P<0.01,Table 2).
Neurological Evaluation
Twenty three patients had achieved neurological recovery at conclusion of the study from preoperative 25 Frankel E, 23 Frankel D, 8 Frankel C to 42 E, 8 D at the final follow‐up (P < 0.01, Table 3).
Table 3.
Neurological evaluation by Frankel's grade before surgery and at final follow‐up
| Frankel's grade | Pre‐op No. of cases | Final follow‐up | ||
|---|---|---|---|---|
| C | D | E | ||
| C | 8 | 0 | 3 | 5 |
| D | 23 | 0 | 5 | 15 |
| E | 25 | 0 | 0 | 22 |
| Sum | 56 | 0 | 8 | 42 |
Significance of neurological recovery at the last follow‐up compared to preoperation. Value (X2) = 17.594, P < 0.01.
Radiological Data
Local Kyphotic Angle on Plain Radiograph Before and After Surgery
Mean local kyphotic angles were 22.3° (17.1°, 33. 8°) preoperatively, 7.4° (3.0°, 10.9°) at 1 week postoperatively, 8.5° (6.0°, 12.7°) at 2 years after surgery, and 10.4° (6.4°, 15.3°) at last (P < 0.01)
The clinical data for the cases of each fracture type are summarized in Table 1. The total VAS score, ODI, neurologic deficits and local Cobb angle significantly improved postoperatively. The outcomes are summarized in Table 2.
Table 1.
Clinical data of each type
| No. of cases | FS | RF | Local kyphotic angle (°) | |||
|---|---|---|---|---|---|---|
| Type | Preoperative (n = 56) | FU (n = 50) | Preoperative (n = 56) | FU (n = 50) | ||
| 1.1 | 13 | 13 | — | 5 | 20.0 (14.7,24.2) | 14.2 (7.8, 16.7) |
| 1.2 | 16 | 14 | 2 | 3 | 20.32 (14.9,25.8) | 8.0 (5.5, 13.0) |
| 2M | 8 | 7 | 3.35 | 3 | 33.8 (19.5, 39.6) | 7.84 (5.7, 15.4) |
| 2 | 19 | 16 | 4.6 | — | 34.8 (17.8, 42.7) | 14.3 (7.3, 16.6) |
FU, final follow‐up; FS, fixed segment; RF, refracture vertebrae.
Complications
Cement Leakage and Migration
Asymptotic cement leakage was observed in 17/56 cases (30.4%), 6/56 in the affected vertebra (10.7%), and 24/330 in the screw trajectory (7.3%). No cement migration was detected.
New vertebral Fracture
At 2 years postoperatively, 11 new VFs had occurred in nine patients (16.1%, Table 1), including VFs in nine adjacent segments that all occurred within 1 year after surgery. All patients with new fractures underwent VP.
Screw Loosening
No screw loosening was detected postoperatively.
Discussion
We innovatively proposed a surgical strategy for treating OVFs with cord compression based on the stability and restorability of the vertebra and replenishes our previous classification21 system applied for stage III Kümmell's disease. We propose three kinds of procedures tailored to four types of OVFs with cord compression; this method is succinct, practical, creative, and effective in the mid to long term. Other studies have also discussed VP22, fixation alone23, and fixation plus decompression7 for OVFs with compression; however, in these studies, the different types, which may require individualized interventions, were not recognized.
It is the first time we proposed to use an extension CT for assistance. This is based on the idea that manual reduction allows safe reduction of the fractured vertebra and decompression of the spinal cord in cases of acute non‐osteoporotic burst fracture24. Li et al.23 reported the application of manual reduction in treating stage III Kümmell's disease to avoid a subsequent decompression procedure. Similarly, extension CT allows determination of not only the reduced condition of the posterior bony fragment but also the stability of the fracture (e.g. the presence of a broken endplate or pedicle). With this information, some cases showing moderate to severe occupation into spinal canal by posterior bony fragments or severe collapse of vertebral body on routine CT scan and MRI, which seems probably requiring a laminectomy or osteotomy, could show height restored and spinal canal decompressed partially on extension CT so that less invasive surgical strategies could be available as alternatives.
Type 1 OVFs are characterized by a reducible posterior bony protrusion and body height, which allows surgical decompression to be avoided. Patients with these fractures often have normal neurology in the supine position, when the spine is not loaded. Nonunion collapse on standing results in “dynamic” spinal cord compression25 and neurological deficits. The intact pedicle and simple posterior fracture line(s) are the highlights of type 1.1 fractures. Therefore, intravertebral instability is the dominant cause of posterior bony protrusion. Posterior breakage is a contraindication for VP/KP due to the possibility of neural complications induced by epidural cement leakage 26; however, our previous work27 and other studies10, 20 have shown satisfactory outcomes with a thorough operation. In this study, VP/KP also appeared to be an effective, safe and minimally invasive treatment for reducible and stable OVFs in elderly patients. On the other hand, pedicle nonunion and/or multiple posterior fracture lines and/or posterior ligament complex injuries are components of type 1.2 fractures. These factors contribute to the instability of the middle and posterior columns of the vertebral body. A high risk of cement migration, progressive kyphosis and chronic back pain have been reported when VP/KP alone is applied to treat this type of OVF28. Additionally, it may not be feasible to fuse multiple bony fragments with cement. Thus, posterior fixation is inevitably needed to stabilize the spine. Similar benign outcomes have been reported in several studies20, 23, 25, 29.
Type 2 OVFs usually present as severe collapse and neural compression manifesting as irreducible anterior height of the vertebral body or the lack of spinal canal decompression on extension CT. Laminectomy or osteotomy is required to decompress the spinal canal or correct the kyphotic deformity. To plan a decompression procedure, the collapse rate and kyphotic deformity may be taken into consideration. Formica19 considered that, in cases with a collapse rate greater than 75%, anterior column reconstruction could not be achieved by VP/KP or interbody fusion but instead required corpectomy and bone fusion with a mesh cage to guarantee anterior support. Patil et al.20 reported that PSO could be performed in cases of significant kyphotic deformity (>30°) at the deformed level to transform a vertebral body from kyphotic to vertebra plana, restoring the adequate local sagittal profile. Takahashi et al.30 reported that patients with an occupation rate of >45% or a supine local kyphotic angle less than ‐25° tended to need PVO. However, in our study, only six patients underwent osteotomy, and most patients with type 2 and 2M OVFs had a kyphotic angle greater than 30°.In our experience, kyphotic deformity greater than 30° is not a boundary indicating to proceed with osteotomy because it is mainly induced by wedge‐type compression and is not stiff. In that situation, secondary correction of kyphosis may be acquired through prone and extended patient positioning and instrumentation. Generally, osteotomy is more invasive and associated with a higher risk of perioperative neurovascular complications than other posterior surgical techniques, and osseous resection will result in a loss of stability, which needs to be carefully planned.
Compared to our previous study, the refinement of type 2 with the modifier category saved some patients from undergoing aggressive procedures. The extended position could facilitate some vertebral reduction, but tight paraspinal muscle is resistant and impedes the extension process when the patient is awake and in pain. Some patients with irreducible OVFs can achieve more height restoration through extension under general anesthesia, such that the anterior column can be supported with cement or an intravertebral bone graft. Li et al.23 reported the use of manual reduction under general anesthesia to restore the height and decompress the spinal canal in stage III Kümmell's disease, as confirmed by myelography. In that case, he could apply short‐segment fixation with posterior body reconstruction augmented by TpBA and a bone graft to avoid laminectomy or corpectomy, as we have. However, in our opinion, manual reduction should be cautiously performed in elderly patients due to the high risk of iatrogenic fracture under traction, elevation and compression of the trunk. Regarding cases with cord compression confirmed by myelography, local laminectomy is sufficient to decompress the spinal canal.
Loosening and pull‐out of pedicle screws is frequently seen in osteoporotic spine20, 31. But in this present study, no instrumentation failure was found assisted with application of augmented pedicle screw with PMMA. It has been proved that cement augmentation can increase pullout strength and biomechanical stability from 96% to 278%32. Although bone cement augmentation of pedicle screw bares a risk of cement leakage ranging from 0% to 73.3%33, 34, 35, 36, 37, the majority of them are asymptomatic. Only 7.3% of asymptomatic cement leakage of pedicle screw occurred in this study. There is a positive correlation between the applied cement volume and the likelihood of cement leakage38, 39. The cement volume should be kept to the smallest volume needed and should not exceed 2.8 mL per pedicle screw40.
Adjacent vertebral fracture is commonly seen after instrumentation and VP/KP. In our study, 19.6% new vertebral fracture occurred postoperatively. Almost half new vertebral fractures occurred in type 1, however, a recent meta‐analysis revealed no evidence of an increased risk of vertebral fractures, especially those adjacent to the treated vertebrae following augmentation with either method compared with conservative treatment41. No new fracture was found in type 2 probably due to long segment fixation within thoracolumbar levels where vertebral fracture mainly occurs42. Similarly, Toyone et al. 43 disclosed that postmenopausal female patients who underwent lumbar spinal instrumentation surgery were susceptible to develop subsequent vertebral fractures within 2 years after surgery. Systemic and regular anti‐osteoporosis after surgery is essential for preventing new fractures44.
Limitations
Because this is a single‐center retrospective analysis, its limitations should be mentioned. The patient sample for each type was small due to restricted inclusion criteria and low incidence of OVFs with cord compression. Patients in this series were elderly, and it was impossible to follow four patients due to death from other diseases and another two patients due to telephone number changes. All patients were assessed regarding the final follow‐up data from medical records or a telephone interview. Evaluations were not blinded in this study, which would impart observer bias to the final report. The clinical results were evaluated by the authors, who were not blinded. The mean follow‐up period in these patients was only 38.9 months (24–108 months). We did not illuminate further divisions for type 2 to guide situations in which laminectomy, osteotomy or corpectomy could be applied due to only 19 patients enrolled. As such, further investigation of a large number of cases is required to assess the surgical outcomes and to design a validated algorithm.
Conclusions
We revised our previous classification and propose a novel surgical strategy that is the most tailored and least invasive for treating OVFs with cord compression based on the reducibility and stability of the affected vertebrae. Accordingly, the retrospective analysis of 56 elderly patients shows satisfactory mid‐ and long‐term clinical and radiological outcomes. Further study is still ongoing with a larger sample and longer follow‐up time to design a validated algorithm.
Acknowledgments
Thanks to Professor Xiao‐bin Jiang, Professor Da‐xiang Jin, Professor Zhi‐dong Yang, Professor Zhen‐song Yao, Professor Jin‐yong Ding from Spine Surgery Department, The First Affiliated Hospital of Guangzhou University of Chinese Medicine as participating investigators.
Grant Sources: The author(s) received financial support from the Traditional Chinese Medicine Bureau of Guangdong Province (20191098), Natural Science Foundation of Guangdong Province (2016A030313641), and Guangdong Science and Technology Department (2016A020215137) for the research, authorship, and/or publication of this article.
Disclosure: The authors declare that they have no conflict of interest.
Dan‐qing Guo and Miao Yu contributed equally to this article.
Contributor Information
Shun‐cong Zhang, Email: spinezsc@126.com.
De Liang, Email: ld_drspine@126.com.
References
- 1. Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int, 2006, 17: 1726–1733. [DOI] [PubMed] [Google Scholar]
- 2. Brunton S, Carmichael B, Gold D, et al Vertebral compression fractures in primary care: recommendations from a consensus panel. J Fam Pract, 2005, 54: 781–788. [PubMed] [Google Scholar]
- 3. Gold DT. Osteoporosis and quality of life psychosocial outcomes and interventions for individual patients. Clin Geriatr Med, 2003, 19: 271–280 vi. [DOI] [PubMed] [Google Scholar]
- 4. Roush K. Prevention and treatment of osteoporosis in postmenopausal women: a review. Am J Nurs, 2011, 111: 26–35 quiz 6‐7. [DOI] [PubMed] [Google Scholar]
- 5. Cooper C, Atkinson EJ, O'Fallon WM, Melton LJ 3rd. Incidence of clinically diagnosed vertebral fractures: a population‐based study in Rochester, Minnesota, 1985‐1989. J Bone Miner Res, 1992, 7: 221–227. [DOI] [PubMed] [Google Scholar]
- 6. Kung‐Chia Li T‐UW, Kung F‐C, Li A, Hsieh C‐H. Staging Of Kümmell's Disease. J Musculoskeletal Res, 2004, 8: 43–55. [Google Scholar]
- 7. Sudo H, Ito M, Kaneda K, et al Anterior decompression and strut graft versus posterior decompression and pedicle screw fixation with vertebroplasty for osteoporotic thoracolumbar vertebral collapse with neurologic deficits. Spine J, 2013, 13: 1726–1732. [DOI] [PubMed] [Google Scholar]
- 8. Kanayama M, Ishida T, Hashimoto T, et al Role of major spine surgery using Kaneda anterior instrumentation for osteoporotic vertebral collapse. J Spinal Disord Tech, 2010, 23: 53–56. [DOI] [PubMed] [Google Scholar]
- 9. O'Connor PA, Eustace S, O'Byrne J. Spinal cord injury following osteoporotic vertebral fracture: case report. Spine (Phila Pa 1976), 2002, 27: E413–E415. [DOI] [PubMed] [Google Scholar]
- 10. Uchida K, Nakajima H, Yayama T, et al Vertebroplasty‐augmented short‐segment posterior fixation of osteoporotic vertebral collapse with neurological deficit in the thoracolumbar spine: comparisons with posterior surgery without vertebroplasty and anterior surgery. J Neurosurg Spine, 2010, 13: 612–621. [DOI] [PubMed] [Google Scholar]
- 11. Nakashima H, Imagama S, Yukawa Y, et al Comparative study of 2 surgical procedures for osteoporotic delayed vertebral collapse: anterior and posterior combined surgery versus posterior spinal fusion with vertebroplasty. Spine (Phila Pa 1976), 2015, 40: E120–E126. [DOI] [PubMed] [Google Scholar]
- 12. Mochida J, Toh E, Chiba M, Nishimura K. Treatment of osteoporotic late collapse of a vertebral body of thoracic and lumbar spine. J Spinal Disord, 2001, 14: 393–398. [DOI] [PubMed] [Google Scholar]
- 13. Nakamae T, Fujimoto Y, Yamada K, Takata H, Shimbo T, Tsuchida Y. Percutaneous vertebroplasty for osteoporotic vertebral compression fracture with intravertebral cleft associated with delayed neurologic deficit. Eur Spine J, 2013, 22: 1624–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lehmer SM, Keppler L, Biscup RS, Enker P, Miller SD, Steffee AD. Posterior transvertebral osteotomy for adult thoracolumbar kyphosis. Spine (Phila Pa 1976), 1994, 19: 2060–2067. [DOI] [PubMed] [Google Scholar]
- 15. Matsuyama Y, Goto M, Yoshihara H, et al Vertebral reconstruction with biodegradable calcium phosphate cement in the treatment of osteoporotic vertebral compression fracture using instrumentation. J Spinal Disord, 2004, 17: 291–296. [DOI] [PubMed] [Google Scholar]
- 16. Lu WW, Cheung KM, Li YW, et al Bioactive bone cement as a principal fixture for spinal burst fracture: an in vitro biomechanical and morphologic study. Spine (Phila Pa 1976), 2001, 26: 2684–2690 discussion 90‐1. [DOI] [PubMed] [Google Scholar]
- 17. Uchida K, Kobayashi S, Matsuzaki M, et al Anterior versus posterior surgery for osteoporotic vertebral collapse with neurological deficit in the thoracolumbar spine. Eur Spine J, 2006, 15: 1759–1767. [DOI] [PubMed] [Google Scholar]
- 18. Kaneda K, Asano S, Hashimoto T, Satoh S, Fujiya M. The treatment of osteoporotic‐posttraumatic vertebral collapse using the Kaneda device and a bioactive ceramic vertebral prosthesis. Spine (Phila Pa 1976), 1992, 17: S295–S303. [DOI] [PubMed] [Google Scholar]
- 19. Formica M, Zanirato A, Cavagnaro L, et al Vertebral body osteonecrosis: proposal of a treatment‐oriented classification system. Eur Spine J, 2018, 27: 190–197. [DOI] [PubMed] [Google Scholar]
- 20. Patil S, Rawall S, Singh D, et al Surgical patterns in osteoporotic vertebral compression fractures. Eur Spine J, 2013, 22: 883–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang SC, Jiang XB, Liang D, et al CT classification at extension and its significance for stage III Kümmell's disease. Chin J Spine Spinal Cord, 2012, 5: 387–392. [Google Scholar]
- 22. Song X, Wang W, Yan Y, Zuo J, Yao N, Lin H. Clinical effect evaluation of percutaneous vertebroplasty combined with the spinal external fixator for the treatment of osteoporotic compressive fractures with posterior vertebral defect. Eur Spine J, 2014, 23: 2711–2717. [DOI] [PubMed] [Google Scholar]
- 23. Li KC, Li AF, Hsieh CH, Liao TH, Chen CH. Another option to treat Kummell's disease with cord compression. Eur Spine J, 2007, 16: 1479–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li KC, Hsieh CH, Lee CY, Chen TH. Transpedicle body augmenter: a further step in treating burst fractures. Clin Orthop Related Res, 2005, 436: 119–125. [PubMed] [Google Scholar]
- 25. Ataka H, Tanno T, Yamazaki M. Posterior instrumented fusion without neural decompression for incomplete neurological deficits following vertebral collapse in the osteoporotic thoracolumbar spine. Eur Spine J, 2009, 18: 69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Boszczyk BM, Bierschneider M, Schmid K, Grillhosl A, Robert B, Jaksche H. Microsurgical interlaminary vertebro‐ and kyphoplasty for severe osteoporotic fractures. J Neurosurg, 2004, 100: 32–37. [DOI] [PubMed] [Google Scholar]
- 27. Zhang SC, Mo L, Liang D, et al Classification and treatment strategies of symptomatic severe osteoporotic vertebral fracture and collapse. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi, 2016, 30: 189–196. [PubMed] [Google Scholar]
- 28. Trouillier HH, Birkenmaier C, Seidl T, Jansson V. Complications following kyphoplasty in unstable osteoporotic vertebral body fractures. A guide to correct fracture analysis. Acta orthopaedica Belgica, 2013, 79: 488–494. [PubMed] [Google Scholar]
- 29. Huang YS, Hao DJ, Feng H, et al Comparison of percutaneous kyphoplasty and bone cement‐augmented short‐segment pedicle screw fixation for management of kummell disease. Med Sci Monit, 2018, 24: 1072–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Takahashi T, Hanakita J, Kawaoka T, Ohtake Y, Adachi H, Shimizu K. Indication for partial vertebral osteotomy and realignment in posterior spinal fixation for osteoporotic thoracolumbar vertebral collapse with neurological deficits. Neurol Med Chir (Tokyo), 2016, 56: 485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ponnusamy KE, Iyer S, Gupta G, Khanna AJ. Instrumentation of the osteoporotic spine: biomechanical and clinical considerations. Spine J, 2011, 11: 54–63. [DOI] [PubMed] [Google Scholar]
- 32. Bullmann V, Liljenqvist UR, Rodl R, Schulte TL. Pedicle screw augmentation from a biomechanical perspective. Orthopade, 2010, 39: 673–678. [DOI] [PubMed] [Google Scholar]
- 33. Mueller JU, Baldauf J, Marx S, Kirsch M, Schroeder HW, Pillich DT. Cement leakage in pedicle screw augmentation: a prospective analysis of 98 patients and 474 augmented pedicle screws. J Neurosurg Spine, 2016, 25: 103–109. [DOI] [PubMed] [Google Scholar]
- 34. Janssen I, Ryang YM, Gempt J, et al Risk of cement leakage and pulmonary embolism by bone cement‐augmented pedicle screw fixation of the thoracolumbar spine. Spine J, 2017, 17: 837–844. [DOI] [PubMed] [Google Scholar]
- 35. Aydogan M, Ozturk C, Karatoprak O, Tezer M, Aksu N, Hamzaoglu A. The pedicle screw fixation with vertebroplasty augmentation in the surgical treatment of the severe osteoporotic spines. J Spinal Disord Tech, 2009, 22: 444–447. [DOI] [PubMed] [Google Scholar]
- 36. Chang MC, Kao HC, Ying SH, Liu CL. Polymethylmethacrylate augmentation of cannulated pedicle screws for fixation in osteoporotic spines and comparison of its clinical results and biomechanical characteristics with the needle injection method. J Spinal Disord Tech, 2013, 26: 305–315. [DOI] [PubMed] [Google Scholar]
- 37. Moon BJ, Cho BY, Choi EY, Zhang HY. Polymethylmethacrylate‐augmented screw fixation for stabilization of the osteoporotic spine: a three‐year follow‐up of 37 patients. J Korean Neurosurg Soc, 2009, 46: 305–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Amendola L, Gasbarrini A, Fosco M, et al Fenestrated pedicle screws for cement‐augmented purchase in patients with bone softening: a review of 21 cases. J Orthop Traumatol, 2011, 12: 193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Venmans A, Klazen CA, van Rooij WJ, de Vries J, Mali WP, Lohle PN. Postprocedural CT for perivertebral cement leakage in percutaneous vertebroplasty is not necessary‐results from VERTOS II. Neuroradiology, 2011, 53: 19–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Folsch C, Goost H, Figiel J, Paletta JR, Schultz W, Lakemeier S. Correlation of pull‐out strength of cement‐augmented pedicle screws with CT‐volumetric measurement of cement. Biomed Tech (Berl), 2012, 57: 473–480. [DOI] [PubMed] [Google Scholar]
- 41. Zhang H, Xu C, Zhang T, Gao Z, Zhang T. Does percutaneous vertebroplasty or balloon kyphoplasty for osteoporotic vertebral compression fractures increase the incidence of new vertebral fractures? a meta‐analysis. Pain Physician, 2017, 20: E13–E28. [PubMed] [Google Scholar]
- 42. Lin X, Xiong D, Peng YQ, et al Epidemiology and management of osteoporosis in the People's Republic of China: current perspectives. Clin Interv Aging, 2015, 10: 1017–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Toyone T, Ozawa T, Kamikawa K, et al Subsequent vertebral fractures following spinal fusion surgery for degenerative lumbar disease: a mean ten‐year follow‐up. Spine (Phila Pa 1976), 2010, 35: 1915–1918. [DOI] [PubMed] [Google Scholar]
- 44. Silverman SL, Kupperman ES, Bukata SV, Members of IOFFWG . Fracture healing: a consensus report from the International Osteoporosis Foundation Fracture Working Group. Osteoporos Int, 2016, 27: 2197–2206. [DOI] [PubMed] [Google Scholar]


