Summary
Kidney injury significantly increases overall mortality. Neutrophilic granulocytes (neutrophils) are the most abundant human blood leukocytes. They are characterized by a high turnover rate, chiefly controlled by granulocyte colony stimulating factor (G‐CSF). The role of kidney injury and uremia in regulation of granulopoiesis has not been reported. Kidney transplantation, which inherently causes ischemia–reperfusion injury of the graft, elevated human neutrophil expression of the surface glycoprotein CD177. CD177 is among the most G‐CSF‐responsive neutrophil genes and reversibly increased on neutrophils of healthy donors who received recombinant G‐CSF. In kidney graft recipients, a transient rise in neutrophil CD177 correlated with renal tubular epithelial G‐CSF expression. In contrast, CD177 was unaltered in patients with chronic renal impairment and independent of renal replacement therapy. Under controlled conditions of experimental ischemia–reperfusion and unilateral ureteral obstruction injuries in mice, renal G‐CSF mRNA and protein expression significantly increased and systemic neutrophilia developed. Human renal tubular epithelial cell G‐CSF expression was promoted by hypoxia and proinflammatory cytokine interleukin 17A in vitro. Clinically, recipients of ABO blood group‐incompatible kidney grafts developed a larger rise in neutrophil CD177. Their grafts are characterized by complement C4d deposition on the renal endothelium, even in the absence of rejection. Indeed, complement activation, but not hypoxia, induced primary human endothelial cell G‐CSF expression. Our data demonstrate that kidney injury induces renal G‐CSF expression and modulates granulopoiesis. They delineate differential G‐CSF regulation in renal epithelium and endothelium. Altered granulopoiesis may contribute to the systemic impact of kidney injury.
Keywords: CD177, emergency granulopoiesis, ischemia–reperfusion injury, neutrophilic granulocyte
Our prospective study in kidney graft recipients shows that human neutrophil CD177, a major G‐CSF target gene, significantly and reversibly increases after transplantation. Two mouse models demonstrate enhanced renal G‐CSF expression and granulopoiesis after acute kidney injury under controlled conditions in vivo. Experiments in primary human cells revealed differential regulation of G‐CSF in human renal tubular epithelium and vascular endothelium by hypoxia, IL‐17A and complement activation as possible underlying mechanisms.

Introduction
Acute kidney injury increases long‐term mortality, despite the fact that kidney functions in electrolyte and volume balance can be replaced by dialysis 1, 2, 3, 4. This correlates with the severity of injury and comorbid conditions, but is detectable also in patients with restored excretory kidney function. The underlying mechanisms are very incompletely understood. Recent studies have delineated long‐term alterations in renal gene expression after experimental murine and clinical human kidney allograft injury 5, 6, including numerous soluble mediators of inflammation.
Neutrophilic granulocytes (neutrophils) are the most abundant human blood leukocytes 7, 8. Despite their high turnover rate, individual baseline counts are remarkably stable. Neutrophils are positively controlled chiefly by granulocyte colony‐stimulating factor (G‐CSF) 8, 9, 10. It maintains their baseline levels and enhances maturation and release in inflammation, a process termed ‘emergency granulopoiesis’ 9. G‐CSF can be produced by a number of cell types, including bone marrow stroma and various epithelia. However, there are comparatively few data on the relative contributions of different cell types to G‐CSF production in pathophysiology 11. In kidney tissue, both injury 12 and tumors 13 can induce G‐CSF expression. Indeed, neutrophilia has been reported as a very rare paraneoplastic event in renal cancer 13. A role for renal G‐CSF in systemic granulopoiesis in humans with non‐malignant renal conditions has not been demonstrated.
CD177 is among the most G‐CSF‐regulated neutrophil genes (alternate names: NB1, PRV‐1) 14, 15. In human neutrophils, this GPI‐linked glycoprotein has a unique expression pattern that distinguishes two populations and is stable in individuals 14, 16, 17, 18. Up‐regulation of CD177 neutrophil surface levels and the proportion of positive neutrophils (CD177%) was found after G‐CSF injection. However, longitudinal data that determine whether neutrophil CD177% reverts to the individual baseline are lacking.
Cross‐sectional studies report elevated neutrophil CD177% in cord blood, during pregnancy and also in sepsis and vasculitides, mainly in anti‐neutrophil cytoplasmic antibodies (ANCA)‐associated vasculitis 14, 19, 20 and in Kawasaki disease 21. In both sepsis and vasculitides, a high neutrophil CD177% is consistently associated with worse overall and renal outcomes. It is not known whether, in reverse, uremia or kidney injury impact upon this neutrophil phenotype.
In this study, we tested whether kidney injury affects renal G‐CSF expression, systemic granulopoiesis and the human neutrophil CD177 phenotype in a longitudinal study in kidney transplant recipients, in uremic patients, mouse models of kidney injury and human cell culture.
Materials and methods
Patients and probands
The study was conducted in adult patients after informed consent according to the Declaration of Helsinki and local ethics board approval. Blood from stable hemodialysis out‐patients without active infection or previous bone marrow transplantation was analyzed after a long interval. Healthy human granulocyte donor leukocytes were analyzed on the day of, the day after and 40 ± 5 days after application of 263 μg recombinant human G‐CSF. Healthy blood donor leukocytes were recovered from volunteers and anonymized buffy coats. Adult patients scheduled for living donor kidney transplantation at Hannover Medical School conducted according to the Declaration of Istanbul were analyzed before surgery and on return to the out‐patient department 3 and 12 months after transplantation. Patients receiving a first dose of rituximab for non‐malignant nephrological conditions were recruited at MHH.
Mouse models of renal injury
Renal ischemia–reperfusion injury (IR) and unilateral ureteral obstruction (UUO) surgeries were performed according to the Directive 2010/63/EU of the European Parliament and approved by the state authority in male wild‐type (C57BL/6J) mice kept in specific pathogen‐free conditions. IR was induced by 27 min clamping; for UUO, proximal ureteral ligation was performed 22.
Leukocyte staining and flow cytometry
Murine full blood counts were obtained using an automated analyzer (VetABC; ScilVet, Viernheim, Germany). Mouse bone marrow cells were flushed from femurs. The following directly labeled antibodies were used: anti‐CD11b (M1/70), anti‐CD14 (HCD14), anti‐CD16 (3G8), anti‐CD115 (AFS98), anti‐CD49d (9F10), anti‐CD66b (G10F5), anti‐CXCR4 (12G5), anti‐Gr1 (RB6‐8C5) and anti‐CD177 (MEM166) (Biolegend, San Diego, CA, USA). Flow cytometry data obtained on a Becton‐Dickinson fluorescence activated cell sorter (FACS)Canto were analyzed using FlowJo software (Tree Star Inc., Ashland, OR, USA).
Renal histology
Three months’ surveillance biopsies were scored using the current Banff criteria 23 in 3‐μm formaldehyde‐fixed paraffin‐embedded serial sections stained with hematoxylin and eosin, periodic acid–Schiff, Jones’ methenamine silver and anti‐C4d and anti‐SV40 stainings by two nephropathologists unaware of all other parameters. For G‐CSF staining, 3‐µm sections were dewaxed and rehydrated. After endogenous peroxidase activity (3% H2O2) and protein blocking (Zytomed Systems, Berlin, Germany) and antigen retrieval in 0·01 M sodium citrate buffer (pH 6.0) for 30 min at 98°C, murine kidneys were stained with polyclonal rabbit anti‐G‐CSF antibody (60 min, dilution 1 : 500; Bioss, Woburn, MA, USA). Human samples were stained with polyclonal rabbit anti‐G‐CSF antibody (60 min, dilution 1 : 50; Sigma Aldrich, Darmstadt, Germany). Secondary antibody horseradish peroxidase (HRP)‐conjugated polyclonal donkey anti‐rabbit immunoglobulin (30 min, 1 : 100; Jackson ImmunoResearch Laboratories, Inc., West Baltimore Pike, PA, USA) was applied prior to 3,3′‐diaminobenzidine detection (Zytomed Systems, Berlin, Germany). For negative controls, primary antibodies were omitted. Sections were evaluated using an Olympus U‐DO3 microscope after mild hemalum counterstain. Representative photomicrographs were taken using a Zeiss Axioplan microscope with an MRT digital camera and Axiovision Software (Zeiss, Oberkochen, Germany). Renal tubuli were counted and assessed for G‐CSF positivity in cortex and medulla separately. Values are expressed as percentage of positive tubuli.
Human renal tubular and endothelial cell culture
HK‐2 human tubular cells (American Type Culture Collection, Manassas, VA, USA) and human umbilical vein endothelial cells (HUVEC), isolated after informed consent and ethics approval, were cultured under normal and hypoxic conditions as described previously 24. Recombinant human interleukin (IL)‐17A (Peprotech, London, UK) was applied at the indicated concentrations; no effects were seen for heat‐degraded IL‐17A used as control (data not shown). To test complement activation, HUVEC were exposed to normal human serum with and without anti‐CD59 (BRIC229; International Blood Group Reference Laboratory, NHS Blood and Transplant, Bristol, UK) to block complement inactivation or immunoglobulin (Ig)G isotype control, as described previously 25.
RNA isolation and real time–polymerase chain reaction (RT–PCR)
RNA isolation, reverse transcription and quantitative PCR (qPCR) were performed as described previously 24. Primers were as follows; mouse: Csf3: forward: CAGGCTCTATCGGGTATTT, reverse: GGAAGGCAGAAGTGAAGG; Hprt: forward: CAGTCCCAGCGTCGTGATTA, reverse: AGCAAGTCTTTCAGTCCTGTC; human: Csf3: forward: GCTGCTTGAGCCAACTCCATA, reverse: GAACGCGGTACGACACCTC; and Hprt: forward: TGACACTGGCAAAACAATGCA, reverse: GGTCCTTTTCACCAGCAAGCT. Products were confirmed by melting curve analysis and gel electrophoresis. Data were analyzed with hypoxanthine–guanine phosphoribosyltransferase (HPRT) as a reference gene using LinRegPCR software.
Renal ischemia–reperfusion gene expression data set
In agreement with the Creative Commons Attribution 4.0 International License, expression data were extracted from the supplemental gene expression data set of 6.
Statistical analysis
Two‐tailed Student’s t‐test or Mann–Whitney test for non‐normally distributed samples was used to compare two conditions. If more than two conditions were compared, Bonferroni’s or Dunnett’s test was applied after analysis of variance (anova), as indicated. P‐values < 0·05 were considered significant. In the figures, data are expressed as mean ± standard error of the mean (s.e.m.). P‐values are indicated as follows: *P < 0·05, **P < 0·01, ***P < 0·001.
Results
G‐CSF transiently increases human neutrophil CD177 expression
Cd177 is a highly G‐CSF regulated gene 14, 15, 26. The presence of the CD177 protein distinguishes two human neutrophil subsets. Surface CD177+ cells also contain intracellular protein, while surface negative cells do not contain the protein in their intracellular granules, as illustrated by flow cytometry and confocal microscopy of cells from four individuals (Fig. 1a,b).
Figure 1.
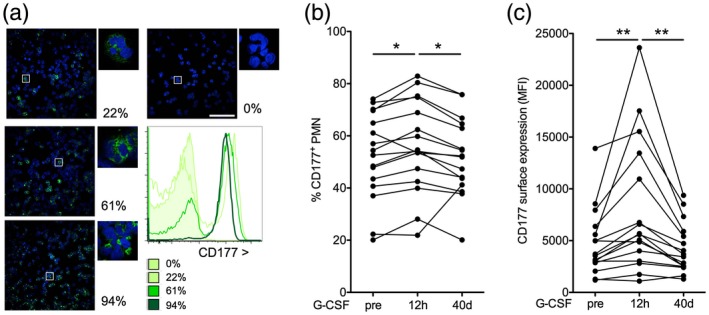
Granulocyte colony‐stimulating factor (G‐CSF) increases human neutrophil CD177 expression. (a,b) In human blood neutrophils, CD177 is expressed in intracellular granules and on the surface of a subset in most individuals. Four healthy donors with different percentages of CD177+ cells are shown as examples [a: confocal microscopy of density gradient enriched neutrophils, green: CD177 and blue, 4′,6‐diamidino‐2‐phenylindole (DAPI), 40× and 63× original magnification, bars indicate 100 μm; (b) flow cytometric CD177 assessment in blood of the same donors gated for CD11b+CD16high large granular cells]. (c,d) CD177+ neutrophils before (pre), 12 h and 40 ± 5 days after G‐CSF administration to healthy volunteers were assessed as depicted in (b). The proportion (c) and mean fluorescence intensity (MFI, d) of CD177+ polymorphonuclear neutrophils (PMN) are shown [statistical analysis of n = 19, Bonferroni after analysis of variance (anova)].
Other markers, including the beta‐2 integrin subunit CD11b, which is considered a neutrophil activation marker 27, the Fc receptor CD16, which is higher in mature than immature neutrophils but again shed in activation or cell death 27, 28, CD66b, which is a GPI‐linked protein as CD177 29, bone marrow homing receptor CXCR4 (CD184) 30 and CD49d, which is a reported marker of human myeloid derived suppressor cells 31, stained very similarly on the subsets (Supporting information, Fig. S1).
Cd177 mRNA, as many neutrophil genes, is functionally expressed in the progenitors in the bone marrow rather than in peripheral blood cells 14, 15, 26. We therefore tested whether CD177 protein expression on blood neutrophils can serve as dynamic indicators of G‐CSF action in humans. Indeed, a single dose of G‐CSF significantly increased the percentage of CD177 neutrophils (CD177%) and the CD177 expression level on positive neutrophils in healthy human granulocyte donors (Fig. 1c,d), as described previously 14, 32, 33. We further performed individual follow‐up. This determined that CD177% reverted to baseline within 40 days after a single dose of G‐CSF.
These data demonstrate that CD177 reversibly increased in response to G‐CSF propose CD177% as a dynamic indicator of G‐CSF action in vivo.
Kidney transplantation, but not uremia, enhances human neutrophil CD177 expression
To investigate the impact of kidney function and kidney damage, CD177 expression was studied in chronic renal impairment before and after kidney transplantation.
In a cohort of 51 patients receiving maintenance hemodialysis, CD177% and expression level did not differ from healthy blood donors (Supporting information, Fig. S2; clinical characteristics in Supporting information, Table S1). CD177 did not associate with age, gender, dialysis vintage, total leukocyte count or the use of erythropoietic agents in this cohort. Also, in a cohort of 48 patients scheduled for living donor kidney transplantation (clinical characteristics in Supporting information, Table S2), CD177% and expression level were very similar in patients already receiving dialysis for renal replacement or not (Supporting information, Fig. S3). The proportion of completely CD177‐deficient individuals did not differ from healthy controls for either of the cohorts. CD177% did not correlate with any of the studied laboratory or clinical values that are reported in Supporting information, Tables S1 and S2. It was longitudinally stable in subgroup of 11 patients evaluated twice before transplantation (Supporting information, Fig. S4), as described in healthy donors 14. Taken together, these results exclude a significant impact of uremia and type of renal replacement therapy on the neutrophil CD177 phenotype.
However, after kidney transplantation, CD177% and its surface expression level significantly increased (Fig. 2a). At this time‐point, 3 months after transplantation, plasma G‐CSF levels were elevated compared to reported normal values (n = 23, mean = 152 pg/ml, range = 6–724, literature normal range = 34–54 pg/ml) 34, 35. In the presence of intense immunosuppression, total white blood counts and absolute neutrophils were in the lower range [6 ± 0·4/nl (normal = 3·9–10·2) and 4·2 ± 0·3/nl (normal = 1·5–7·7)]. The increase in CD177% was unrelated to age, gender, excretory renal function or immunosuppressive regimen (data not shown). Expressed as a change from baseline, the increase in CD177% reverted within 1 year (Fig. 2b). A similar trend was observed for its surface expression level (Fig. 2c).
Figure 2.
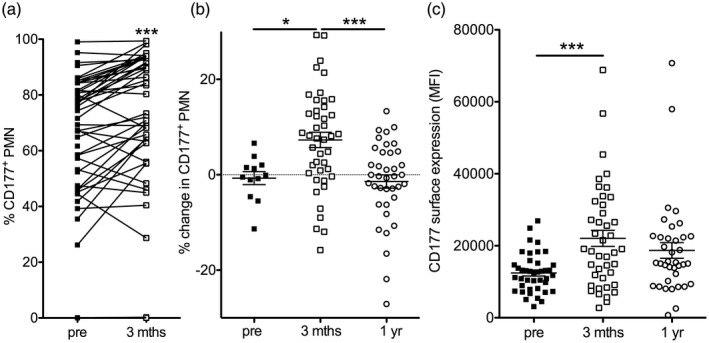
Kidney transplantation transiently increases human neutrophil CD177%. (a–c) The proportion of CD177+ blood neutrophils (PMN) was measured in a cohort of 48 living donor kidney recipients before (pre) and 3 months after kidney transplantation (a, paired t‐tests). In all patients with a CD177+ neutrophil subpopulation, the change in CD177% compared to the baseline sample was assessed in additional samples before transplantation if available (pre) and 3 months and 1 year after transplantation [b, Bonferroni after analysis of variance (anova)]. The mean fluorescence intensity (MFI) on positive cells is shown before, 3 months and 1 year after transplantation (c, Bonferroni after anova).
These results introduce kidney transplantation as a novel inducer of a reversible up‐regulation of human neutrophil CD177% in vivo.
Experimental kidney injury enhances renal G‐CSF production and introduces neutrophilia
To determine renal G‐CSF production as a major inducer of CD177 14, 15 under defined conditions in vivo, murine kidney injury models of ischemia–reperfusion injury and unilateral ureteral obstruction were employed. G‐CSF was below detection level on both protein and mRNA level at rest (Fig. 3). However, both G‐CSF protein and mRNA markedly increased in both types of injury (Fig. 3a–c), while G‐CSF mRNA was below detection level in nearly all heart, lung, liver, spleen and bone marrow samples after injury (Supporting information, Fig. S5). Long‐term follow‐up data after ischemia–reperfusion injury demonstrated elevated renal G‐CSF expression for at least 1 month after the event (Fig. 3d) 6.
Figure 3.
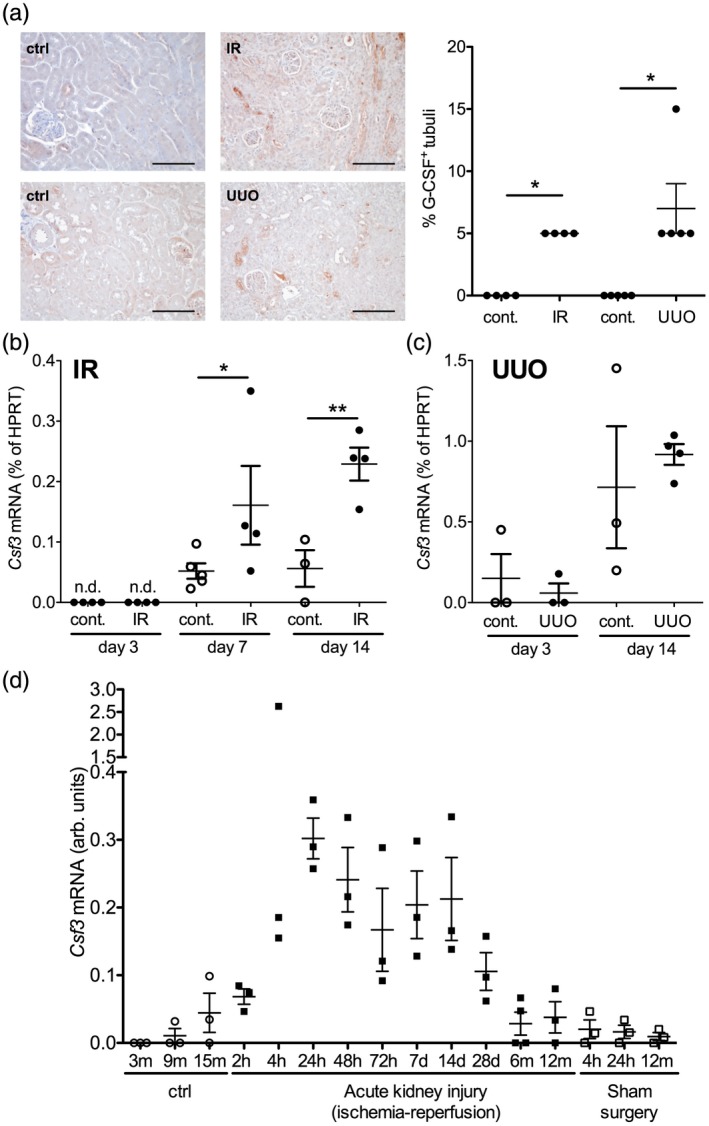
Regulation of renal granulocyte colony‐stimulating factor (G‐CSF) expression after experimental kidney injury. (a–c) Renal G‐CSF expression was assessed after experimental ischemia–reperfusion injury (IR) and unilateral ureteral obstruction (UUO) in mice. G‐CSF protein in the renal tubules was quantified on day 14 after injury in injured and contralateral (cont.) kidneys (a: examples, bars = 100 μm, and quantification of n = 4–5/group, Mann–Whitney tests). G‐CSF (Csf3) mRNA was quantified by quantitative polymerase chain reaction (qPCR) in ischemic (IR) and contralateral (cont.) (b, n = 3–5, Bonferroni after analysis of variance (anova), n.d. = not detectable) and UUO kidneys (c, n = 3–4, Bonferroni after anova). (d) Long‐term regulation of renal G‐CSF gene expression was determined at the indicated time‐points after renal ischemia–reperfusion injury, after sham surgery and in control mice 6.
Assessment of blood neutrophil counts revealed a significant increase in absolute leukocyte counts with relative and absolute neutrophil counts (Fig. 4a–d). This phenotype is consistent with emergency granulopoiesis 9.
Figure 4.
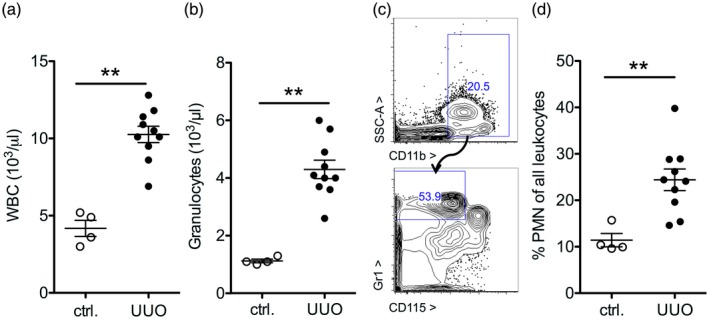
Increased neutrophil counts after experimental kidney injury. (a,b) On day 14 after unilateral ureteral obstruction (UUO) or in untreated control animals (ctrl), granulopoiesis was assessed. Total white blood count (WBC, a) and granulocytes (b) in peripheral blood were measured by an automated analyzer (A,B) and by flow cytometry as a complementary method by gating for CD11b+gr1highCD115– cells [polymorphonuclear neutrophils (PMN), examples in (c,d): statistical analysis of n = 4,10].
These results show that kidney injury increases renal tubular G‐CSF production with systemic effects on granulocytes in vivo.
Human kidney graft tubular G‐CSF expression correlates with the increase in neutrophil CD177 expression
G‐CSF was stained in human kidney grafts in surveillance biopsy samples obtained at the time of blood neutrophil assessment 3 months after transplantation. G‐CSF protein was detected in renal tubuli in both cortex and medulla of the samples (Fig. 5a,b). The proportion of G–CSF expressing tubuli in both compartments closely correlated with the CD177% rise at this time‐point (Fig. 5c,d)
Figure 5.
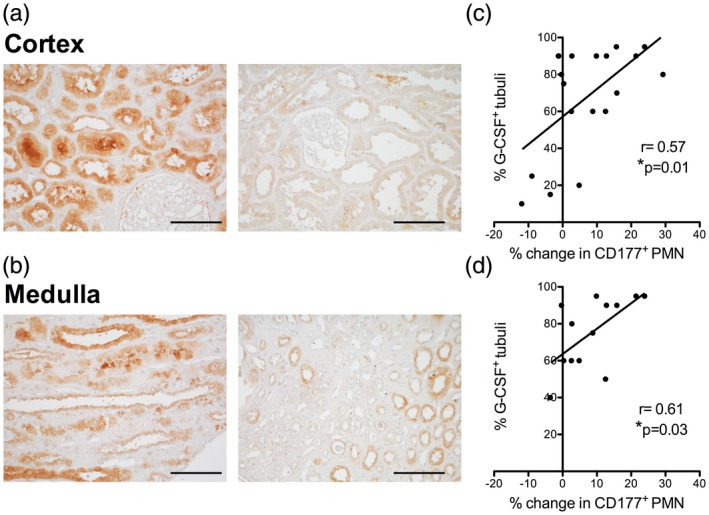
Tubular granulocyte colony‐stimulating factor (G‐CSF) protein expression correlates with the increase in neutrophil CD177% 3 months after kidney transplantation. (a–d) G‐CSF protein was detected in the renal allograft in 3 months surveillance biopsies in cortex and medulla (a,b, examples of medulla and cortex in grafts with high and low expression, bars = 100 μm). The percentage of positive tubuli was in relation to polymorphonuclear neutrophils (PMN) CD177% change was analyzed (c,d, n = 19 cortex and 13 medulla samples, Pearson’s r).
This correlation is consistent with a mechanistic function of renal G‐CSF for the neutrophil CD177 phenotype.
Hypoxia and IL‐17A up‐regulate G‐CSF in renal tubular cells
To determine the underlying mechanisms of renal tubular G‐CSF production we first performed hypoxia experiments, as ischemia was present in experimental and clinical kidney injury. We found that transient hypoxia indeed significantly increased human tubular cell G‐CSF expression, while it had no detectable effect on endothelial cells (Fig 6a,b). IL‐17A is a known strong inducer of G‐CSF and has been detected in damaged human allografts 36. It was also up‐regulated from undetectable levels after isolated ischemia–reperfusion injury in the controlled murine system (Fig. 6c). In human tubular cells, it promoted G‐CSF expression dose‐ and time‐dependently (Fig. 6d,e). IL‐17A also promoted human endothelial cell G‐CSF expression, albeit only at a very high concentration (Fig. 6f).
Figure 6.
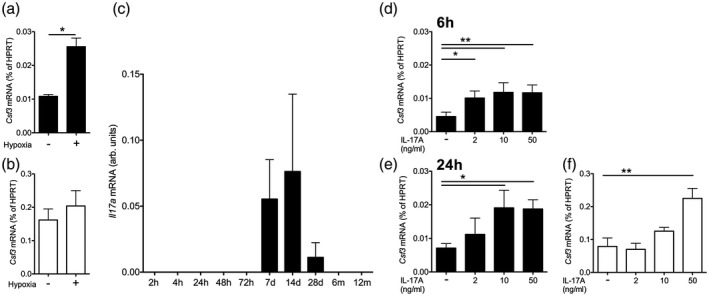
Mechanisms of granulocyte colony‐stimulating factor (G‐CSF) regulation in renal tubular epithelial cells and endothelium. (a,b) G‐CSF (Csf3) mRNA expression in human renal tubular epithelial HK2 cells (a) and human endothelial cells (HUVEC, b) after 24 h hypoxia (a,b, n = 3 for each cell type, t‐tests). (c) Regulation of renal interleukin (IL)‐17A gene expression after renal ischemia–reperfusion injury in mice (n = 3 per time‐point 6). (d–f) G‐CSF (Csf3) mRNA 6 and 24 h after stimulation with the indicated concentrations of recombinant human interleukin (IL)‐17A in HK2 [d,e, n = 3, Dunnett’s after analysis of variance (anova)] and HUVEC after 24 h (f, n = 3, Dunnett’s after anova).
These data propose tubular damage mechanisms for G‐CSF production.
Increased rise of neutrophil CD177% in recipients of blood group incompatible grafts
We next investigated clinical factors that may further modulate the CD177% rise after human kidney transplantation.
CD177% did not correlate with human leucocyte antigen (HLA) mismatch number or donor‐recipient relationship. There were no significant differences in the changes in CD177% or of tubular G‐CSF in grafts with and without rejection (Supporting information, Table S3 and data not shown). The rise in CD177% was significantly higher in the ABO blood group‐incompatible graft recipients (Fig. 7a). The therapeutic regimen of ABO blood group‐incompatible graft recipients differed in B cell depletion by rituximab and plasma exchange and/or immunoadsorption before transplantation. We analyzed the effect of B cell depletion in patients with other kidney conditions, where it did not induce a significant change (Supporting information, Fig. S6). Also, in our cohort there was no differential effect of plasma‐exchange or immunoadsorption (Supporting information, Fig. S7a). We also tested the effect of rejection therapies, including high‐dose intravenous steroids and anti‐thymocyte globulin as well as post‐transplantation plasma exchange (Supporting information, Fig. S7b). None of these were significantly associated with the change in neutrophil CD177.
Figure 7.
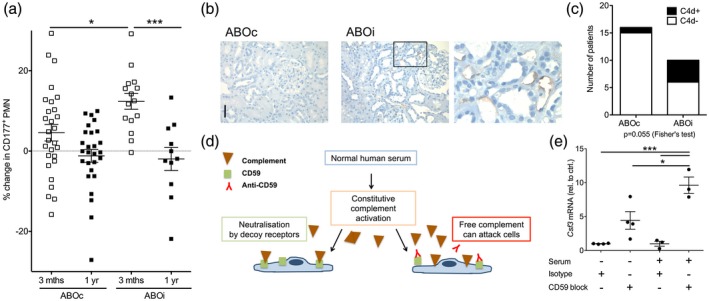
Complement activation upregulates granulocyte colony‐stimulating factor (G‐CSF) in endothelial cells. (a) Analysis of polymorphonuclear neutrophils (PMN) CD177% in ABO blood group‐compatible (ABOc, n = 28) and ‐incompatible (ABOi, n = 15) renal transplant recipients. Changes 3 and 12 months after transplantation are shown relative to the pre‐transplant assessment in all CD177‐expressing patients [Bonferroni after analysis of variance (anova)]. (b,c) Endothelial complement C4d deposition in peritubular capillaries in 3‐month surveillance biopsies (b, examples, 40× original magnification, bar = 50 μm and c, evaluation according to the Banff classification, n = 16 ABOc and 10 ABOi grafts, Fisher’s exact test). (d,e) Complement is constitutively activated in human serum and blocked by decoy receptors, including CD59 (d). Change in G‐CSF (Csf3) mRNA was analyzed in human endothelium with blockade of the decoy CD59 receptor and control cells incubated with isotype in the presence and absence of normal human serum (e, n = 3–4, Bonferroni after anova).
In summary, blood group‐incompatible kidney grafts were associated with an increased neutrophil CD177%.
Complement activation increases endothelial G‐CSF production
In ABO‐incompatible grafts, endothelial complement C4d deposition is regularly detected 37. This phenotype was also present in our patient cohort (Fig. 7b,c). We therefore tested endothelial G‐CSF production in response to complement (Fig. 7d). Indeed, blockade of complement decoy receptor CD59 significantly enhanced endothelial G‐CSF mRNA expression in the presence of normal human serum (Fig. 7e). In mice, expression of the protective CD59b isoform 38 decreased after ischemia–reperfusion injury (Supporting information, Fig. S8). As complement is activated and CD59 over‐expression protects from damage in this murine model 39, endothelial activation by complement may also promote G‐CSF in this setting.
These data propose complement‐mediated endothelial injury as an additional source of renal G‐CSF.
Discussion
This study demonstrates that kidney injury enhances renal G‐CSF expression and regulates granulopoiesis. Our experiments delineate differential G‐CSF regulation in renal tubuli and endothelium and show its impact on the human neutrophil CD177 phenotype in vivo.
CD177 that was discovered in alloimmune neutropenia 40, and again in myeloproliferative disease 41, is peculiar for its bimodal expression pattern on human neutrophils 42, and was recently found to follow a previously unknown unique monoallelic expression pattern 43. For the present project, we made use of the fact that CD177 is among the most G‐CSF regulated genes in human neutrophils 15. Our longitudinal data in healthy subjects treated with exogenous G‐CSF add to this knowledge by demonstrating that the individual CD177% setpoint is maintained after excursion.
We subsequently used the CD177 protein as a sensor of G‐CSF action, as absolute neutrophil counts in peripheral blood may not reflect the full G‐CSF impact on the bone marrow in complex conditions that include the use of anti‐proliferative substances, e.g. autoimmune disease and organ transplantation, and most neutrophil mRNA is silenced 7, 8, 26. The neutrophil CD177 up‐regulation after renal transplantation with inherent ischemia–reperfusion injury described here parallels the response to exogenous G‐CSF, and indeed correlated with renal G‐CSF protein. The experimental murine data demonstrate up‐regulation of granulopoiesis and long‐term renal G‐CSF expression after a single episode of ischemia–reperfusion injury. Consistently in patients 3 months after kidney transplantation, neutrophil CD177% significantly increased.
Induction of G‐CSF production as observed in our study in renal tubular cells parallels findings in epithelium of other organs, e.g. the lung 44. We found that, in renal tubular cells, both hypoxia and IL‐17A, which was also induced after isolated ischemia–reperfusion injury in vivo in the absence of an alloimmune stimulus, increased G‐CSF expression. In vitro, these actions were non‐additive. This may explain why rejection, despite being a known IL‐17A inducer in the kidney 36, did not have a detectable effect on renal G‐CSF production and CD177% in our cohort. However, the present study evaluated relatively early surveillance biopsies with remnant tubular injury from transplantation in all and rejection in only very few patients. The balance of hypoxia‐ versus cytokine‐induced renal G‐CSF may differ in patients with older grafts. An evaluation of this will need to include prospective neutrophil analysis in a sufficiently sized longitudinal cohort.
We found that neutrophil CD177% increased more in patients who received ABO blood group‐incompatible grafts, where endothelial C4d complement deposition occurs; indeed, complement activation induced primary human endothelial cell G‐CSF production in vitro. Elevated neutrophil CD177% has been reported in other conditions with endothelial stress or injury, including ANCA‐associated vasculitis, sepsis and pregnancy 20, 45, 46, 47. CD177 interacts with endothelial CD31 [platelet endothelial cell adhesion molecule (PECAM‐1)] 48 and was reported to mediate neutrophil adhesion 49 and possibly migration 50, 51, 52. It also displays proteinase 3, a main autoantigen in ANCA‐associated vasculitis, on the neutrophil surface 53, 54. Other studies and the present work show that G‐CSF is also an emergency signal that promotes this neutrophil subtype 9, 10. G‐CSF promotes neutrophil mobilization as well as differentiation in the bone marrow, resulting in a number of alterations of circulating neutrophil phenotype and function. It is therefore conceivable that the surplus CD177+ cells in the presence of G‐CSF stimulation differ from CD177+ cells under homeostatic conditions. While understanding of the clinical impact of baseline and induced human neutrophil subsets progresses 16, 17, 55, the fact that the dichotomous human neutrophil CD177 phenotype and the mechanisms of human CD177 interaction with proteinase 3 and integrins do not apply to its murine homologue have impaired functional studies of this specific subtype 56. Very recent data on a differential role in migration depending on the underlying stimulus for murine Ly6G 57, a protein of similar structure as CD177, should be considered when planning prospective human studies with parallel assessment of systemic and local CD177 phenotypes in inflammation. Renal transplant recipients may be considered for such a study, as the kidney graft is frequently biopsied and different forms of inflammation are observed. Both tubulointerstitial inflammation in infection and glomerulitis in antibody‐mediated rejection are sufficiently frequent and contain significant numbers of neutrophils, and their CD177 expression could be compared to peripheral blood at the same time‐point.
Defective erythropoiesis due to lack of erythropoietin and altered monocyte numbers and composition are well‐known systemic hematopoietic effects of uremia 1, 58, 59. Also, neutrophil functional changes have been associated with the degree of uremia 60. Our findings in neutrophils differ from this: uremia or dialysis did not have a detectable effect on neutrophil CD177%. In contrast, renal transplantation, which transfers a kidney with ischemia–reperfusion injury into the body significantly transiently enhanced CD177. Our data from experimental kidney injury show that, indeed, the damaged kidney produces G‐CSF and kidney damage increases granulopoiesis. As local long‐term up‐regulation has been shown for other cytokines after renal injury 6, effects of a ‘toxic kidney’ should also be investigated for other immune cells and in disease conditions.
In summary, our data are consistent with a model of G‐CSF as a systemic injury signal emitted by the kidney parenchyma.
Disclosures
None.
Author contributions
J. V., J. S., J. H. B., R. S., H. H. and S. v. V. designed research; J. V., J. S., J. N., L. D., A. H., P. S. and J. H. B. performed experiments; J. V., S I., W. B., W. G. and S. v. V. recruited patients; J. V., J. S., J. N., L. D., J. H. B. and S. v. V. analyzed data; S. v. V. wrote the manuscript with J. V., J. S. and P. S.; all authors read and approved the manuscript.
Supporting information
Table S1. Characterization of the dialysis patient cohort (n = 51)
Table S2. Characterization of kidney transplant recipients (n = 48)
Table S3. Pathologic diagnoses of three‐month surveillance biopsies (n = 34)
Figure S1. Characterization of CD177+ and CD177‐ human neutrophils
Figure S2. Neutrophil CD177 expression is unaffected by end stage renal disease
Figure S3. Neutrophil CD177 expression in patients with and without renal replacement therapy
Figure S4. Longitudinal assessment of CD177 expression before kidney transplantation
Figure S5. G‐CSF expression after renal injury is detected in the injured kidney, but not other organs
Figure S6. B cell depletion does not affect neutrophil CD177 expression
Figure S7. Role of immunosuppressive regimens in neutrophil CD177 expression
Figure S8. Regulation of complement regulator CD59 after murine ischemia‐reperfusion injury
Acknowledgements
We thank the staff at MHH blood donation service for help with blood collection, M. Ausmeier, M. Flechsig, B. Hertel, C. Kessemeier and R. Lohmann for expert technical assistance and Dr S. Rong for mouse surgeries. J. S., J. H. B. and S. v. V. were supported by Jackstädt Stiftung; S. I., H. H. and S. v. V. were supported by IFBTx.
References
- 1. Betjes MG. Immune cell dysfunction and inflammation in end‐stage renal disease. Nat Rev Nephrol 2013; 9:255–65. [DOI] [PubMed] [Google Scholar]
- 2. Druml W. Systemic consequences of acute kidney injury. Curr Opin Crit Care 2014; 20:613–9. [DOI] [PubMed] [Google Scholar]
- 3. Sawhney S, Mitchell M, Marks A, Fluck N, Black C. Long‐term prognosis after acute kidney injury (AKI): what is the role of baseline kidney function and recovery? A systematic review. BMJ Open 2015; 5:e006497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Venkatachalam MA, Weinberg JM, Kriz W, Bidani AK. Failed tubule recovery, AKI‐CKD transition, and kidney disease progression. J Am Soc Nephrol 2015; 26:1765–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cippa PE, Sun B, Liu J, Chen L, Naesens M, McMahon AP. Transcriptional trajectories of human kidney injury progression. JCI Insight 2018; 3:e123151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu J, Kumar S, Dolzhenko E et al. Molecular characterization of the transition from acute to chronic kidney injury following ischemia/reperfusion. JCI Insight 2017; 2:e94716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol 2006; 6:173–82. [DOI] [PubMed] [Google Scholar]
- 8. von Vietinghoff S, Ley K. Homeostatic regulation of blood neutrophil counts. J Immunol 2008; 181:5183–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boettcher S, Gerosa RC, Radpour R et al. Endothelial cells translate pathogen signals into G‐CSF‐driven emergency granulopoiesis. Blood 2014; 124:1393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhao JL, Baltimore D. Regulation of stress‐induced hematopoiesis. Curr Opin Hematol 2015; 22:286–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Panopoulos AD, Watowich SS. Granulocyte colony‐stimulating factor: molecular mechanisms of action during steady state and ‘emergency’ hematopoiesis. Cytokine 2008; 42:277–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang Y, Woodward VK, Shelton JM et al. Ischemia‐reperfusion induces G‐CSF gene expression by renal medullary thick ascending limb cells in vivo and in vitro . Am J Physiol Renal Physiol 2004; 286:F1193–201. [DOI] [PubMed] [Google Scholar]
- 13. Kyono Y, Takayama T, Kinoshita M et al. Combination therapy with sorafenib and S‐1 for renal cell carcinoma producing granulocyte colony‐stimulating factor. Int J Clin Oncol 2011; 16:275–8. [DOI] [PubMed] [Google Scholar]
- 14. Stroncek DF. Neutrophil‐specific antigen HNA‐2a, NB1 glycoprotein, and CD177. Curr Opin Hematol 2007; 14:688–93. [DOI] [PubMed] [Google Scholar]
- 15. Drewniak A, van Raam BJ, Geissler J et al. Changes in gene expression of granulocytes during in vivo granulocyte colony‐stimulating factor/dexamethasone mobilization for transfusion purposes. Blood 2009; 113:5979–98. [DOI] [PubMed] [Google Scholar]
- 16. Grieshaber‐Bouyer R, Nigrovic PA. Neutrophil heterogeneity as therapeutic opportunity in immune‐mediated disease. Front Immunol 2019; 10:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Deniset JF, Kubes P. Neutrophil heterogeneity: bona fide subsets or polarization states? J Leukoc Biol 2018; 103:829–38. [DOI] [PubMed] [Google Scholar]
- 18. Silvestre‐Roig C, Hidalgo A, Soehnlein O. Neutrophil heterogeneity: implications for homeostasis and pathogenesis. Blood 2016; 127:2173–81. [DOI] [PubMed] [Google Scholar]
- 19. Schreiber A, Otto B, Ju X et al. Membrane proteinase 3 expression in patients with Wegener's granulomatosis and in human hematopoietic stem cell‐derived neutrophils. J Am Soc Nephrol 2005; 16:2216–24. [DOI] [PubMed] [Google Scholar]
- 20. Witko‐Sarsat V, Lesavre P, Lopez S et al. A large subset of neutrophils expressing membrane proteinase 3 is a risk factor for vasculitis and rheumatoid arthritis. J Am Soc Nephrol 1999; 10:1224–33. [DOI] [PubMed] [Google Scholar]
- 21. Ko TM, Chang JS, Chen SP et al. Genome‐wide transcriptome analysis to further understand neutrophil activation and lncRNA transcript profiles in Kawasaki disease. Sci Rep 2019; 9:328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Susnik N, Sorensen‐Zender I, Rong S et al. Ablation of proximal tubular suppressor of cytokine signaling 3 enhances tubular cell cycling and modifies macrophage phenotype during acute kidney injury. Kidney Int 2014; 85:1357–68. [DOI] [PubMed] [Google Scholar]
- 23. Haas M, Rastaldi MP, Fervenza FC. Histologic classification of glomerular diseases: clinicopathologic correlations, limitations exposed by validation studies, and suggestions for modification. Kidney Int 2014; 86:648. [DOI] [PubMed] [Google Scholar]
- 24. Helmke A, Casper J, Nordlohne J et al. Endothelial‐to‐mesenchymal transition shapes the atherosclerotic plaque and modulates macrophage function. FASEB J 2019; 33:2278–89. [DOI] [PubMed] [Google Scholar]
- 25. Noone DG, Riedl M, Pluthero FG et al. Von Willebrand factor regulates complement on endothelial cells. Kidney Int 2016; 90:123–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Borregaard N, Sorensen OE, Theilgaard‐Monch K. Neutrophil granules: a library of innate immunity proteins. Trends Immunol 2007; 28:340–5. [DOI] [PubMed] [Google Scholar]
- 27. Nauseef WM, Borregaard N. Neutrophils at work. Nat Immunol 2014; 15:602–11. [DOI] [PubMed] [Google Scholar]
- 28. Kerst JM, de Haas M, van der Schoot CE et al. Recombinant granulocyte colony‐stimulating factor administration to healthy volunteers: induction of immunophenotypically and functionally altered neutrophils via an effect on myeloid progenitor cells. Blood 1993; 82:3265–72. [PubMed] [Google Scholar]
- 29. Zhao L, Xu S, Fjaertoft G, Pauksen K, Hakansson L, Venge P. An enzyme‐linked immunosorbent assay for human carcinoembryonic antigen‐related cell adhesion molecule 8, a biological marker of granulocyte activities in vivo. J Immunol Methods 2004; 293:207–14. [DOI] [PubMed] [Google Scholar]
- 30. Casanova‐Acebes M, Pitaval C, Weiss LA et al. Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell 2013; 153:1025–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Haile LA, Gamrekelashvili J, Manns MP, Korangy F, Greten TF. CD49d is a new marker for distinct myeloid‐derived suppressor cell subpopulations in mice. J Immunol 2010; 185:203–10. [DOI] [PubMed] [Google Scholar]
- 32. Stroncek DF, Jaszcz W, Herr GP, Clay ME, McCullough J. Expression of neutrophil antigens after 10 days of granulocyte‐colony‐stimulating factor. Transfusion 1998; 38:663–8. [DOI] [PubMed] [Google Scholar]
- 33. Gohring K, Wolff J, Doppl W, et al. Neutrophil CD177 (NB1 gp, HNA‐2a) expression is increased in severe bacterial infections and polycythaemia vera. Br J Haematol 2004; 126:252–4. [DOI] [PubMed] [Google Scholar]
- 34. Kawakami M, Tsutsumi H, Kumakawa T et al. Levels of serum granulocyte colony‐stimulating factor in patients with infections. Blood 1990; 76:1962–4. [PubMed] [Google Scholar]
- 35. Kleiner G, Marcuzzi A, Zanin V, Monasta L, Zauli G. Cytokine levels in the serum of healthy subjects. Mediators Inflamm 2013; 2013:434010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Van Kooten C, Boonstra JG, Paape ME et al. Interleukin‐17 activates human renal epithelial cells in vitro and is expressed during renal allograft rejection. J Am Soc Nephrol: JASN 1998; 9:1526–34. [DOI] [PubMed] [Google Scholar]
- 37. Haas M, Rahman MH, Racusen LC et al. C4d and C3d staining in biopsies of ABO‐ and HLA‐incompatible renal allografts: correlation with histologic findings. Am J Transplant 2006; 6:1829–40. [DOI] [PubMed] [Google Scholar]
- 38. Qian YM, Qin X, Miwa T, Sun X, Halperin JA, Song WC. Identification and functional characterization of a new gene encoding the mouse terminal complement inhibitor CD59. J Immunol 2000; 165:2528–34. [DOI] [PubMed] [Google Scholar]
- 39. Bongoni AK, Lu B, Salvaris EJ et al. Overexpression of human CD55 and CD59 or treatment with human CD55 protects against renal ischemia‐reperfusion injury in mice. J Immunol 2017; 198:4837–45. [DOI] [PubMed] [Google Scholar]
- 40. Lalezari P, Murphy GB, Allen FH Jr. NB1, a new neutrophil‐specific antigen involved in the pathogenesis of neonatal neutropenia. J Clin Invest 1971; 50:1108–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Klippel S, Strunck E, Busse CE, Behringer D, Pahl HL. Biochemical characterization of PRV‐1, a novel hematopoietic cell surface receptor, which is overexpressed in polycythemia rubra vera. Blood 2002; 100:2441–8. [DOI] [PubMed] [Google Scholar]
- 42. Goldschmeding R, van Dalen CM, Faber N et al. Further characterization of the NB 1 antigen as a variably expressed 56–62 kD GPI‐linked glycoprotein of plasma membranes and specific granules of neutrophils. Br J Haematol 1992; 81:336–45. [DOI] [PubMed] [Google Scholar]
- 43. Eulenberg‐Gustavus C, Bahring S, Maass PG, Luft FC, Kettritz R. Gene silencing and a novel monoallelic expression pattern in distinct CD177 neutrophil subsets. J Exp Med 2017; 214:2089–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ye P, Rodriguez FH, Kanaly S et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony‐stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med 2001; 194:519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schreiber A, Jannerjahn A, Kettritz R. CD177/NB1 receptor expression is dynamically regulated in sepsis patients. Immunohematology 2015; 31:128–9. [PubMed] [Google Scholar]
- 46. Taniguchi K, Nagata H, Katsuki T et al. Significance of human neutrophil antigen‐2a (NB1) expression and neutrophil number in pregnancy. Transfusion 2004; 44:581–5. [DOI] [PubMed] [Google Scholar]
- 47. von Vietinghoff S, Eulenberg C, Wellner M, Luft FC, Kettritz R. Neutrophil surface presentation of the anti‐neutrophil cytoplasmic antibody–antigen proteinase 3 depends on N‐terminal processing. Clin Exp Immunol 2008; 152:508–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sachs UJ, Andrei‐Selmer CL, Maniar A et al. The neutrophil‐specific antigen CD177 is a counter‐receptor for platelet endothelial cell adhesion molecule‐1 (CD31). J Biol Chem 2007; 282:23603–12. [DOI] [PubMed] [Google Scholar]
- 49. Bai M, Grieshaber‐Bouyer R, Wang J et al. CD177 modulates human neutrophil migration through activation‐mediated integrin and chemoreceptor regulation. Blood 2017; 130:2092–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bayat B, Bein G, Sachs UJ. A sequence‐specific polymerase chain reaction method for HNA‐2 genotyping: homozygous c.843A>T mutation predicts the absence of CD177. Transfusion 2016; 56:2127–32. [DOI] [PubMed] [Google Scholar]
- 51. Kuckleburg CJ, Tilkens SB, Santoso S, Newman PJ. Proteinase 3 contributes to transendothelial migration of NB1‐positive neutrophils. J Immunol 2012; 188:2419–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang L, Ge S, Agustian A, Hiss M, Haller H, von Vietinghoff S. Surface receptor CD177/NB1 does not confer a recruitment advantage to neutrophilic granulocytes during human peritonitis. Eur J Haematol 2013; 90:436–7. [DOI] [PubMed] [Google Scholar]
- 53. Korkmaz B, Kuhl A, Bayat B, Santoso S, Jenne DE. A hydrophobic patch on proteinase 3, the target of autoantibodies in Wegener granulomatosis, mediates membrane binding via NB1 receptors. J Biol Chem 2008; 283:35976–82. [DOI] [PubMed] [Google Scholar]
- 54. von Vietinghoff S, Tunnemann G, Eulenberg C et al. NB1 mediates surface expression of the ANCA antigen proteinase 3 on human neutrophils. Blood 2007; 109:4487–93. [DOI] [PubMed] [Google Scholar]
- 55. Carlucci PM, Purmalek MM, Dey AK et al. Neutrophil subsets and their gene signature associate with vascular inflammation and coronary atherosclerosis in lupus. JCI insight 2018; 3:e99276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Schreiber A, Eulenberg‐Gustavus C, Bergmann A, Jerke U, Kettritz R. Lessons from a double‐transgenic neutrophil approach to induce antiproteinase 3 antibody‐mediated vasculitis in mice. J Leukoc Biol 2016; 100:1443–52. [DOI] [PubMed] [Google Scholar]
- 57. Cunin P, Lee PY, Kim E et al. Differential attenuation of beta2 integrin‐dependent and ‐independent neutrophil migration by Ly6G ligation. Blood Adv 2019; 3:256–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Eckardt KU, Kurtz A. Regulation of erythropoietin production. Eur J Clin Invest 2005; 35(Suppl 3):13–9. [DOI] [PubMed] [Google Scholar]
- 59. Heine GH, Ortiz A, Massy ZA et al. Monocyte subpopulations and cardiovascular risk in chronic kidney disease. Nat Rev Nephrol 2012; 8:362–9. [DOI] [PubMed] [Google Scholar]
- 60. Rossaint J, Oehmichen J, Van Aken H et al. FGF23 signaling impairs neutrophil recruitment and host defense during CKD. J Clin Investig 2016; 126:962–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Characterization of the dialysis patient cohort (n = 51)
Table S2. Characterization of kidney transplant recipients (n = 48)
Table S3. Pathologic diagnoses of three‐month surveillance biopsies (n = 34)
Figure S1. Characterization of CD177+ and CD177‐ human neutrophils
Figure S2. Neutrophil CD177 expression is unaffected by end stage renal disease
Figure S3. Neutrophil CD177 expression in patients with and without renal replacement therapy
Figure S4. Longitudinal assessment of CD177 expression before kidney transplantation
Figure S5. G‐CSF expression after renal injury is detected in the injured kidney, but not other organs
Figure S6. B cell depletion does not affect neutrophil CD177 expression
Figure S7. Role of immunosuppressive regimens in neutrophil CD177 expression
Figure S8. Regulation of complement regulator CD59 after murine ischemia‐reperfusion injury


