Summary
Immunity is shaped by commensal microbiota. From early life onwards, microbes colonize mucosal surfaces of the body and thereby trigger the establishment of immune homeostasis and defense mechanisms. Recent evidence reveals that the family of innate lymphoid cells (ILCs), which are mainly located in mucosal tissues, are essential in the maintenance of barrier functions as well as in the initiation of an appropriate immune response upon pathogenic infection. In this review, we summarize recent insights on the functional interaction of microbiota and ILCs at steady‐state and throughout life. Furthermore, we will discuss the interplay of ILCs and the microbiota in mucosal infections focusing on intestinal immunity.
Keywords: early life, infectious diseases, innate lymphoid cells, microbiota
Immunity is shaped by commensal microbiota. Recent insights will be discussed on the functional interaction of microbiota and innate lymphoid cells at steady‐state and throughout life in health and disease.
Abbreviations
- AhR
arylhydrocarbon receptor
- AOM
azoxymethane
- CCR6
CC chemokine receptor 6
- CD
cluster of differentiation
- CHILP
common helper ILC precursor
- CLP
common lymphoid progenitor
- DC
dendritic cell
- ES
excretory secreted
- FRC
fibroblastic reticular cells
- GF
germ‐free
- GM‐CSF
granulocyte monocyte colony‐stimulating factor
- HAART
highly active antiretroviral therapy
- HpARI
Heligmosomoides polygyrus alarmin release inhibitor
- IBD
inflammatory bowel disease
- Id2
inhibitor of DNA‐binding 2
- IFN
interferon
- IgA/G
immunoglobulin A/G
- IL
interleukin
- ILC
innate lymphoid cell
- ILF
intestinal lymphoid follicle
- Lgr5
leucine‐rich repeat‐containing G‐protein‐coupled receptor 5
- LTi cell
lymphoid tissue inducer cell
- MHC
major histocompatibility complex
- NCR
NK cell receptor
- NK cell
natural killer cell
- pDC
plasmacytoid dendritic cell
- RA
retinoic acid
- Rag
recombination‐activated gene
- Reg3
regenerating islet‐derived protein 3
- RORyt
retinoic acid‐related orphan receptor gT
- SFB
segmented filamentous bacteria
- SLO
secondary lymphoid organs
- SPF
specific pathogen‐free
- Tfh
T follicular helper cell
- Th
T helper
- TLR
Toll‐like receptor
- TNF
tumour necrosis factor
- Treg
regulatory T‐cell
- TRUC
Tbx21 − /− Rag2 − /− ulcerative colitis
- TSLP
thymic stromal lymphopoietin
- WT
wild‐type
Introduction
The role of the immune system is to protect our body from a variety of foreign and potentially harmful substances, which we are exposed to throughout life. This is especially true at the inner and outer body surfaces, such as the airways, the skin, the intestine and the urogenital tract, where the body constantly encounters non‐self‐antigens present in the food or the environment, potential pathogens, and microbes naturally colonizing these surfaces. The latter consist of bacteria, viruses, archaea and fungi, collectively referred to as commensal microbiota.
Colonization with commensal microbiota starts at birth, when the newborn leaves the sterile environment of the womb and gets in contact with the first colonizing bacteria in the birth canal. Throughout the first 1–2 years of life, the human microbiota is rather unstable and subject to change, before it reaches an astonishingly stable composition that stays throughout adulthood if external factors, such as diet, hygiene and health state are unaltered. It is only in the elderly, that the composition of the human microbiota commences to change again.1, 2 Commensal microbiota and the host live in a mutualistic relationship. While the microbiota contributes to digestive functions of the host, produces important vitamins, for example vitamin K and B group vitamins, and protects the body from invasion with pathogens, it also importantly matures the host immune system, both at mucosal and systemic sites (for reviews, see Refs3, 4). The presence of such high numbers of bacteria in and on the mammalian body is also potentially harmful, as they might induce inflammatory responses. Tight barriers and regulatory mechanisms need to be in place in order to prevent invasion of the microbes into the body and inappropriate inflammatory reactions, respectively.
Components of the innate immune system are predominantly present at mucosal sites and help maintain the host−commensal mutualism. Besides mechanical barriers, such as tight junctions between the intestinal epithelial cells and mucus produced by goblet cells, haematopoietic cells, such as mononuclear cells and innate lymphoid cells (ILCs), are important effector cells of the innate immune system.3 ILCs derive from the common lymphoid progenitor in the bone marrow and are by definition lymphocytes. They are predominantly found at mucosal sites, such as the intestine and the airways, react fast during an immune response, and lack antigen‐specific receptors – all typical features of innate immune cells.5 ILCs can be classified into five subsets, type 1 ILCs (ILC1s), type 2 ILCs (ILC2s), type 3 ILCs (ILC3s), lymphoid tissue inducer cells (LTi), which are often classified as a subset of ILC3s, and natural killer (NK) cells.6 ILC1s express the transcription factor T‐bet and produce interferon (IFN)‐γ and tumour necrosis factor (TNF)‐α. Similarly, NK cells also express T‐bet and produce IFN‐γ and TNF‐α. In addition, NK cells can lyse target cells in a cell‐contact‐dependent manner through the release of perforin and granzymes. Classical NK cells express the transcription factor Eomes and are developmentally distinct from ILC1s. Only a minor subset of Eomes‐positive, granzyme and perforin‐expressing ILC1‐like cells has been described to be involved in tumour surveillance in mice.7 In addition, human intraepithelial ILC1s express Eomes.8, 9 ILC2s are dependent on the expression of GATA‐3 and release mainly IL‐5 and IL‐13, thus contributing to T helper (Th)2‐responses. Both ILC3s and LTi cells are characterized by the expression of the transcription factor retinoic acid‐related orphan receptor gT (RORγt). While ILC3s produce IL‐22, IL17A and IL17F, LTi cells are mainly involved in lymphoid organ formation through the production of lymphotoxins.6, 10, 11 Recently, a regulatory ILC population, which also develops from the common lymphoid progenitor (CLP) in an Id3‐dependent way, was described to be the main source of immunomodulatory IL‐10 in the intestine following inflammation.12
Numerous functions of ILCs during homeostasis, immune responses and diseases have been described, and more are to be revealed. Interestingly, many of these either involve signals from the commensal microbiota or contribute to host−microbial mutualism. In the following, we aimed to summarize identified interactions between ILCs and the microbiota during health and disease throughout life.
ILC development and microbiota in prenatal and early life
Although adult ILCs derive from the CLP in the bone marrow giving rise to the inhibitor of DNA‐binding 2 (Id2)+ common helper ILC precursor (CHILP), which finally differentiates into all ILC subsets expect NK cells,13 certain fetal and perinatal ILCs can derive from a progenitor found in the fetal liver. RORyt‐expressing LTi cells were first identified at embryonic day E13·5–E15·5 in the lymph node and Peyer’s patch anlagen to be responsible for the induction of these secondary lymphoid organs (SLO).14, 15, 16, 17, 18 The formation of these early LTi cells seems to be independent of the presence of the commensal microbiota as germ‐free (GF) mice harbour normal numbers of lymph nodes and Peyer’s patches.16, 19 In contrast, maternal dietary components, such as retinoic acid (RA), were shown to be crucial to maintain this LTi cell population during fetal development and consequently for the correct induction of SLO.20 Interestingly, microbiota is directly regulating the availability of RA.21 Specifically, Clostridia species influence epithelial RA synthesis.22 A similar population of RORyt+ ILC3s is required to induce cryptopatches and intestinal lymphoid follicles (ILFs) after birth.23, 24, 25, 26 Lin− IL‐7Ra+ Flt3− a4b7 + progenitors present in the fetal liver can give rise to ILC3s that can be found in the intestinal lamina propria at the time of birth.27, 28 The population of RORyt‐expressing ILCs in the lamina propria shortly after birth consists of mainly CCR6+ c‐kithi LTi cells, which seed the intestinal lamina propria during fetal development, and low numbers of CCR6−c‐kitlo ILC3s that expand strongly within the first 2–4 weeks of birth and that can acquire NK cell markers.29, 30 The expansion of the latter was shown to be dependent on signals originating from the maternal microbiota as shown in a model of reversible gestational colonization during pregnancy.31 Arylhydrocarbon receptor (AhR) ligands produced by the maternal microbiota were transferred to the offspring postnatally through the milk and permanently increased the absolute number of NKp46+ ILC3s in the offspring small intestinal lamina propria starting at postnatal day 14. This is in line with three studies comparing adult specific pathogen‐free (SPF) and GF mice, which reported a reduced number of intestinal lamina propria NCR+ ILC3s in GF mice.32, 33, 34 This area is, however, subject to controversy as other studies showed a negative influence of missing microbiota on the different ILC3 subsets.35 In a separate study using RORc Cre × AhR fl/fl mice and experimentally purified AhR‐ligand‐enriched or depleted diets, it was demonstrated that CCR6− ILC3s were strongly reduced in numbers in the absence of any Ahr signalling, which resulted in the absence of intestinal cryptopatches and ILFs.36 Natural ligands of the AhR include agonists derived from cruciferous vegetables in the diet as well as indoles produced by members of the microbiota. Although, this study did not investigate the influence of microbiota‐derived Ahr ligands on the proliferation of CCR6− ILC3s, it is likely to speculate that AhR ligands derived from not only the maternal microbiota but also from the endogenous microbiota of the offspring contribute to the homeostasis of this ILC population. This is supported by the observation that GF mice show a delay in the maturation of cryptopatches into ILFs.37 A high degree of plasticity has been described for intestinal ILC3s. While the T‐bet induced upregulation of NK cell receptors (NCRs) on ILC3s was dependent on the presence of the commensal microbiota as described above,13, 32, 34 the loss of RORyt‐expression in NCR+ ILC3s and their switch to a more ILC1‐like phenotype was prevented in colonized compared with GF mice.33
A recent publication showed that perinatal signals leading to expansion of neonatal ILC2s are important to maintain even the adult pool of ILC2s.38 This perinatal expansion of ILC2s was independent of commensal microbiota as it occurred also in GF mice. As interactions between an individual SPF consortium and a defined diet are very complex, it is not unlikely that a combination of dietary and microbial signals contributes to this event. It is also known that ILC2 numbers in the intestine of GF adult mice are higher compared with those in colonized animals, reflecting the general shift towards Th2‐type immune responses in GF mice.39 In contrast to the ILC3s, transient maternal microbial exposure during gestation did not change the ILC2 numbers in the offspring intestine.31
The role of microbiota in the differentiation of ILC1s and NK cells during early life has not been investigated in detail. However, cell numbers in the different lymphoid organs were unaffected by the commensal microbiota both in young and adult mice wherever investigated.40 A study on the human ILC1 subset by Spits and colleagues has suggested that colonization of the neonatal intestine is triggered through microbiota colonization after birth as this subset seems absent in the fetal intestine. In addition, they show that this ILC1 subset has the potential to differentiate into RORγt‐expressing ILC3 during adulthood in the presence of IL‐23 and RA, the latter being partially regulated by the commensal microbiota.41
A study by Gury‐BenAri et al. has characterized 15 different existing intestinal ILC subsets at single cell level in regard to transcriptomic activity and chromatin landscape in SPF, GF and antibiotic‐treated mice. While antibiotic‐treated and GF mice exhibited ILC subsets with very similar expression profiles, colonized animals clustered separately in most subsets. ILC1 and ILC2 subsets were most affected by the absence of commensal microbiota and acquired a phenotype that more closely resembled ILC3 subsets.42 In conclusion, while ILC3s seem to be mainly influenced by microbial signals during early life, ILC2s and ILC1s are more shaped through microbiota during adulthood (Fig. 1).
Figure 1.
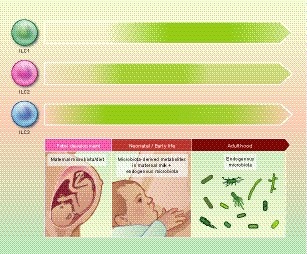
Influence of the commensal microbiota on innate lymphoid cells (ILCs) throughout life. Although mammals get colonized with commensal microbiota at the time of birth, microbial signals originating from the maternal microbiota can reach the offspring already during the fetal stage via the placenta and during neonatal life through maternal milk. In addition, both ILC3s and ILC2s have been described in the fetal liver. Microbial influences on ILC development, maintenance and function are thus possible throughout life. The current knowledge on microbial influences on ILC numbers and functions was used to estimate the importance of commensal microbiota‐derived signals on intestinal ILCs at the different stages of life (fetal, neonatal, adult). The strength/importance of the microbial influence is indicated here by the shade of green colour used in the arrows. ILC3s seem to be the ILC population that is most strongly dependent on microbial signals, and these are especially important in establishing the intestinal ILC3 pool during early life through signals originating also from the maternal microbiota during pregnancy. ILC2s are generally slightly less dependent on the presence of commensal microbiota, especially in early life. However, a slight increase in cell numbers has been described in adult germ‐free (GF) mice. ILC1s are believed to be absent during fetal life, and the influence of microbiota appears to be stronger the more mature and possibly pro‐inflammatory the intestinal microbiota has become.
Microbiota and ILC interactions during steady‐state in adulthood
Contribution of microbiota to the establishment of a physical barrier: epithelial integrity
The different ILC subsets are predominantly found at mucosal sites, and the intestinal lamina propria is home to all three main subsets of ILCs: ILC1s, ILC2s and ILC3s. One of the first discovered functions of ILCs is their role in maintaining a tight and healthy intestinal barrier, and thus contributing to control the intestinal microbiota. ILC3 produce the cytokine IL‐22, which can act on the IL‐22 receptor expressed exclusively on non‐haematopoietic cells in the gut, mainly intestinal epithelial cells.43 IL‐22 predominantly acts on Paneth cells to induce the release of the antimicrobial proteins Reg3b, Reg3y, S100A8, S100A9.44 IL‐22 produced by ILCs was also shown to enhance proliferation of Lgr5+ intestinal epithelial stem cells in murine and human organoids and in an in vivo mouse model.45 Recently, Gronke et al. demonstrated that ILC3‐derived IL‐22 induces the DNA damage response in intestinal epithelial cells, protecting the epithelial layer from exogenous genotoxic stress, thus limiting tumorigenesis.46 The production of IL‐22 by both CCR6− and CCR6+ ILC3s is stimulated by commensal microbiota as shown by the use of GF and antibiotic‐treated mice.29 ILC3‐derived IL‐22 induces the expression of various other factors in the epithelium, including tight and gap junction proteins, mucins, and cytokine receptors that contribute to the homeostasis and barrier function of the epithelium (for review, see Ref.47). Two reports illustrated that ILC3s are crucial in preventing dissemination of intestinal commensals to peripheral tissues: the titres of serum IgG specific for intestinal commensal bacteria were elevated in RORyt‐deficient mice,48 and Rag−/− mice exhibited systemic translocation of Alcaligenes species and systemic inflammation upon depletion of ILCs using anti‐Thy1 antibodies.49
Epithelial‐derived cytokines, such as IL‐25, IL‐33 and thymic stromal lymphopoietin (TSLP), are regulated by commensal microbiota and act on ILC2 maintenance and function.35, 50 IL‐13 produced by ILC2s can act on intestinal epithelial stem cells and bias their differentiation into goblet cells and tuft cells, promoting mucus production by goblet cells and environmental sensing capacities of the intestinal epithelium through tuft cells. A positive feedback loop exists between ILC2s and Tuft cells as ILC2‐derived IL‐13 induces further IL‐25 secretion by Tuft cells.51 In addition, IL‐13 secreted by ILC2s supports self‐renewal of intestinal epithelial stem cells.52
IFN‐γ and TNF‐α, which are also produced by T‐bet+ ILC1s, are known to increase permeability of the intestinal epithelial cell barrier as shown in bacterial translocation studies using monolayers of human intestinal epithelial cells.53, 54 The absence of T‐bet in the innate immune system also directly affects microbiota composition as presented further down.
As outlined above, one main function of ILCs lies within the strengthening of epithelial barriers at mucosal surfaces through various mechanisms and thus regulating the translocation of microbial members into the body. This is supported by the location of most ILCs within mucosal tissues and the resulting proximity to such epithelial barriers.
Microbiota, ILCs and other members of the innate immune system
Innate lymphoid cells (ILCs) carry several cytokine receptors and can thus react to cytokines released by other innate immune cells. Intestinal mononuclear phagocytes produce IL‐23, IL‐1α and IL‐1β in a microbiota‐dependent manner, which induce IL‐22 and IL‐17 production by ILC3s.55, 56, 57, 58 These cytokines are mainly released by CX3CR1+ macrophages and CD103+ CD11b+ dendritic cells (DCs) resident in the intestinal lamina propria.57, 59, 60 Microbiota‐induced IL‐1β also induces the release of granulocyte monocyte colony‐stimulating factor (GM‐CSF) by ILC3, which in turn promotes mononuclear phagocytes to produce regulatory components, such as IL‐10 and RA.57 The latter are important to promote regulatory T‐cell (Treg) differentiation and expansion in the intestine to ensure intestinal homeostasis.
Splenic NK cells in GF and antibiotic‐treated mice are largely unresponsive when stimulated to produce IFN‐γ or exert specific lysis of target cells following Toll‐like receptor (TLR) ligand exposure in vivo. This was due to functionally impaired DCs in the absence of microbiota that were unable to produce the NK priming cytokines IFN‐I and IL‐15.40
Microbiota, ILCs and adaptive immune responses
While ILC function is influenced by commensal microbiota, ILCs themselves can influence adaptive immune cells, which in return help keeping the host−microbial mutualism in check. The largest set of data is available on the role of ILC3s in regulating T‐ and B‐cell responses. The CCR6+ subset of ILC3s present in the colonic lamina propria and mesenteric lymph nodes expresses major histocompatibility complex (MHC)‐II and can act as antigen‐presenting cells to negatively select commensal bacteria‐specific CD4+ T‐cells. The elimination of microbiota‐specific T‐cells was very important to prevent low‐grade systemic and spontaneous intestinal inflammation.61, 62 An independent study demonstrated that splenic but not intestinal MHC‐II‐expressing ILC3s can be activated through microbiota‐derived IL‐1β to express co‐stimulator molecules and thus drive CD4+ T‐cell and B‐cell responses in vivo.63 Similarly, microbiota‐derived signals during colitis induced the expression of the co‐stimulatory molecule OX40 on MHC‐II‐expressing ILC3s, which could subsequently activate intestinal T‐cells.64 ILC3s were also shown to localize within the MLN to the interface between the T‐ and B‐cell zone to regulate T follicular helper cell (Tfh)‐dependent germinal centre reactions.65 This was dependent on MHC‐II expression and antigen presentation by ILC3s and significantly reduced colonic IgA+ B‐cell responses to commensal microbiota, thereby contributing to host−commensal mutualism.66 At the same time, lymphotoxin alpha expression by ILC3s was critical to control T‐cell homing to the gut, representing another influence of ILC3s on T‐dependent IgA production.67 However, ILC3s not only affect T‐dependent, but also T‐independent IgA induction via the production of BAFF and APRIL in the spleen,68 and through expression of the lymphotoxin beta receptor and interaction with DCs in intestinal lymphoid follicles.69
ILC2s can likewise influence CD4+ T‐cell responses. ILC2s were shown to skew CD4+ T‐cell differentiation and activation towards Th2 instead of Th1 in a cell−cell contact dependent manner.70, 71, 72 A subset of ILC2s also express MHC‐II and can present antigens to CD4+ T‐cells.70, 71 ILC2s could potentially also affect the generation of intestinal IgA, and therefore influence commensal microbiota, through the release of IL‐5, which is favourable for IgA induction in B cells, and through IL‐6 that promotes B‐1 cell proliferation.73
Impact of ILCs on microbiota composition
Knowing that ILCs have direct effects on intestinal barrier integrity on several levels, including the regulation of intestinal epithelial cells, of innate immune populations and of adaptive immune responses, it is expected that the absence of one or several ILC populations goes along with an altered host−microbial mutualism and ultimately with microbial dysbiosis. Still, only few studies are available that have addressed commensal microbiota composition in mice deficient in ILC populations or with altered ILC function. Such studies are also challenging as cage effects are prone to affect the results, and the requirement for littermates can be a limiting factor and is often neglected.74, 75 While microbiota diversity and phylum composition were unaltered in the absence of ILC3s (RORc Cre × Id2 fl/fl), these mice exhibited higher levels of segmented filamentous bacteria (SFB) as well as Clostridiales species.76 Mice lacking the AhR in ILC3s, which goes along with reduced numbers of ILC3s, or lymphotoxin alpha in ILC3s, also carried more SFB,67, 77 corroborating the hypothesis that SFB, which are associated with a Th‐17‐mediated inflammatory phenotype,78 are under control of ILC3s. Several studies addressed microbiota composition in models of IL‐22 deficiencies. IL‐22‐deficient mice harboured a dysbiotic colonic microbiota with colitogenic potential compared with wild‐type (WT) control mice, which was transmissible to WT animals if adult animals of the two strains were co‐housed.79 Unfortunately, no littermates were addressed to understand the role of IL‐22 in protecting from the acquisition of a colitogenic microbiota in early life as it has been shown for the presence of TLR5 in the neonatal period.80 Another study demonstrated that Id2 expression in ILC3s was important for the generation of IL‐22, which maintained a healthy microbiota that exhibited early colonization resistance to Citrobacter rodentium.76 ILC3s in the skin were recently shown to control the growth of the sebaceous glands and consequently the release of antimicrobial lipids, enabling the establishment of a healthy skin microbiota.81
T‐bet depletion in Rag −/− mice exhibited commensal dysbiosis associated with susceptibility to colitis in one but not another mouse colony.82, 83 Since a subset of ILC3s, ILC1s, and NK cells express or depend on the transcription factor T‐bet, it is difficult to know which ILC subset is responsible for this phenotype. T‐bet depletion in the innate immune system in the colony harbouring the colitic microbiota went along with stronger IL‐17 expression by the remaining ILC3s.83 In a later study, if T‐bet was depleted ubiquitously (Tbx21 −/−) or in NCR+ ILCs (Ncr1 iCreTg × Tbx21 fl/fl) in either immunocompetent or compromised mice, ILC2 frequency and function in the colonic lamina propria, spleen and lymph nodes were increased, promoting protective mucosal immunity during worm infections.84 This was mediated through increased IL7Rα expression by ILC2s in the absence of T‐bet. To our knowledge, intestinal microbiota composition in ILC2‐deficient mice has not yet been investigated.
Microbiota – ILC axis during infectious diseases
Microbiota and ILCs in parasitic infections
Helminth infections
Approximately one‐third of the world’s population is infected with helminths.85 Helminth infections greatly influence the host’s immune response and dampen immunity to co‐infections.86 Type 2 signature cytokines are induced triggering goblet cell differentiation, mucin release and smooth muscle contraction commonly referred to as ‘weep and sweep’ mechanism to expel helminths. Not surprisingly, helminth infections disturb the microbial composition of the host. Depending on the infecting helminth, different shifts of the microbiota towards specific bacterial species have been reported with an imbalance of Lactobacilli species in C57BL/6 laboratory mice.87, 88 Helminths are also able to act directly on the host’s immune response by the release of excretory secreted (ES) vesicles carrying several different immunomodulators.89 Upon helminth infections, intestinal ILC2s are activated mainly by alarmins such as IL‐25 and IL‐33.90, 91 Indeed, ILC2s have been firstly identified in detail upon infection with the intestinal helminth Nippostrongylus brasiliensis (N. brasiliensis).73, 92, 93 Due to their fast release of type 2 cytokines, such as IL‐13, ILC2s accelerate worm expulsion by triggering epithelial immunity. Release of tuft cell‐derived IL‐25 activates ILC2s to increase IL‐13 expression, which then again acts on the epithelium. This positive feedback loop of cooperate action of ILC2s and epithelial cells is key to expel and eliminate the pathogen.51, 94, 95 However, helminth‐derived Heligmosomoides polygyrus alarmin release inhibitor (HpARI), which is able to neutralize ILC2 activating IL‐33, dampens protective type 2 immunity.96 Whether ILC populations and specifically ILC2s are able to directly sense and react to helminth‐derived ES vesicles will be of great interest for future studies. Helminth infections can trigger malnutrition and worsen disorders including vitamin A deficiency. The vitamin A metabolite RA is essential for the intestinal immune response upon infection: decreased ILC3 levels but increased number and activity of ILC2s, such as increased IL‐13 secretion, have been reported in helminth infections (Trichuris muris; T. muris) in vitamin A‐insufficient mice.97 The metabolism of ILC2 relies highly on fatty acid metabolism at steady‐state as well as upon T. muris infection on RA‐triggered malnutrition.98 AhR‐deficient ILC2s show enhanced activity and thereby acceleration of clearance of helminths (Heligmosomoides polygyrus bakeri).99 This is due to decreased recruitment of the transcriptional determinant Gfi1 to the Il1rl1 locus in genetically induced AhR‐deleted ILC2s.
Toxoplasma gondii
The intracellular parasite Toxoplasma gondii (T. gondii) is mainly transmitted in the cyst stage, and induces dysbiosis and a shift of the microbiota to Enterobacteriaceae.100 A strong type 1 immune response is generated by IFN‐γ producing innate and adaptive lymphoid cells. Indeed, T‐bet‐dependent ILC1s are able to control T. gondii infections by their release of IFN‐γ and TNF‐α.13 An additional T‐bet‐dependent population of intraepithelial lymphocytes with an ILC1 profile has been reported recently.101 These NKp46− CD8αα− Ly49E+ IELs express IFN‐γ upon T. gondii infection, and thereby promote the type 1 immune response to eliminate T. gondii. A recent report identified plasticity of NK cells to ILC1s using Eomes‐fate‐map mice in T. gondii infection highlighting how closely related these populations are.102 Moreover, not only parasitic but also bacterial and viral infections impact on microbiota composition and ILCs functionality (Fig. 2), which will be discussed in the next paragraphs.
Figure 2.
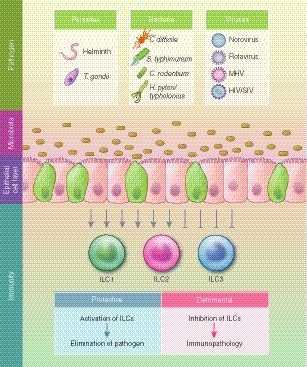
Intestinal infections lead to perturbations of the microbiota and alter innate lymphoid cell (ILC) activity. Parasitic, bacterial and viral infections influence microbiota composition and function as well as the activity of ILCs. Depending on microbial components and immunomodulators induced by pathogens, the ILC activation can be protective or detrimental, resulting in either pathogen elimination or immunopathology, respectively.
Microbiota and ILCs in bacterial infections
Gram‐positive bacteria – Clostridium difficile infections
Microbiota is severely reduced and colonization resistance lost upon broad‐spectrum antibiotics treatment, which increases the susceptibility to infection by the Gram‐positive bacterium Clostridium difficile (C. difficile). In North America, C. difficile infects several hundred thousand people every year, and represents an important health threat especially for immune‐compromised and hospitalized patients. Adaptive immune responses and innate immunity cooperate to eliminate C. difficile, which establishes its infection by the toxin‐controlled inhibition of epithelial GTPases thereby destroying the epithelial barrier.103 Innate lymphoid populations are important in the defense against C. difficile reported by studies in ILC‐deficient mice.104, 105 Transfer experiments of ILCs revealed that especially ILC1s and ILC3s contribute through the secretion of IFN‐γ and IL‐22 in the acute phase of C. difficile infection.104 In a recent report, an additional mechanism based on IL‐33 and its induction of ILC2s in C. difficile infection was described: upregulation of IL‐33 during infection induces ILC2s thereby acting as a protective immune mechanism. Furthermore, in human fecal transplant patients, the transfer of microbiota induced IL‐33 and thereby triggered a protective immune response.106 These reports indicate that all helper ILC populations are involved in resolving C. difficile infections; however, their importance may be dependent on the phase of the infection. As mentioned earlier, C. difficile infections are successfully treated by the therapeutic approach of fecal transplants to restore microbiota and eliminate the ecological niche for C. difficile. 107 Whereas this therapeutic approach has had great success, a current trial has led to the death of one patient by transferring microbiota including multi‐resistant bacteria,108 underlining the need for accurate and detailed screening of potential pathogens in transplants. Conversely to the immune response in C. difficile infections, it is still unknown whether and to which extent ILCs contribute to the short‐ and long‐term changes upon fecal transplant in humans.109 Moreover, susceptibility to C. difficile increases with age; however, direct links to microbiota dysbiosis and/or ILC populations have not yet been reported in these conditions.
Gram‐negative bacteria – Salmonella, Citrobacter and Helicobacter infections
Non‐typhoidal Salmonella (Gram‐negative) species such as Salmonella typhimurium are transmitted by contaminated food. Intestinal infections occur fast, accompanied by stomach pain and diarrhoea, and are usually cleared within several days in healthy individuals. Direct links to ILCs during Salmonella infections in humans have not been reported, but it has been suggested that ILC1s play a role based on the observation that ILC1s are not present in the fetal human gut in contrast to ILC2s and ILC3s, and thus may be triggered by commensal bacteria acquired upon birth, which include Gram‐negative bacteria.110, 111 In mice, S. typhimurium is only able to establish an intestinal infection upon loss of the host’s colonization resistance experimentally induced by a one‐time treatment with the antibiotic streptomycin.112 Upon Salmonella infection, adaptive and innate immunity including epithelial cells and ILCs work in concert to eliminate the bacteria. IFN‐y is key to fight the infection, and its expression has been reported to be shared by conventional NK cells as well as ILC3s during Salmonella infection in mice with T‐bet+ CCR6− RORγt+ ILC3s being the strongest IFN‐y producers.29 In addition, intestinal IL‐22 expression triggered by intestinal epithelial cell‐derived RA affects antimicrobial peptide expression and bacterial load in S. typhimurium infection.22
Enterohaemorrhagic Escherichia coli (EHEC) infections, which are often detrimental in humans, are modelled by infection with the Gram‐negative bacterium C. rodentium in mice. ILCs are essential in the defense against C. rodentium on several levels. ILC‐derived IL‐22 acts on non‐haematopoietic cells to initiate an innate immune defense program against C. rodentium. 113, 114 CD4+ LTi cells have been identified as an important source of IL‐22 upon C. rodentium infection in mice115 as well as ILC3s, specifically, CCR6low/− ILC3s. Interestingly, the latter population is dependent on ligand‐induced stimulation of AhR, as discussed previously. Whereas ILC3s are supported by AhR stimulation and needed to fight off C. rodentium infection, ILC2s are inhibited by AhR signalling in helminth infections.99 Retinoic acid is essential for ILC3 activity during C. rodentium infection. Vitamin A‐insufficient mice are more susceptible to C. rodentium due to a reduction in IL‐22‐expressing ILC3s.97
Antigen presentation by ILC3s via MHC‐II is important to maintain the mucosal barrier at steady‐state, but also upon infection. In mice lacking MHC‐II in ILC3s, infection with C. rodentium resulted in an increased Tfh response, elevated levels of IgA and C. rodentium‐specific IgA underlining that LTi‐like ILC3s are key in triggering the adaptive immune response via regulation of IgA and pathogen infections.66
In humans, the Gram‐negative bacteria Helicobacter pylori colonizes the stomach and intestine, but can also trigger colon cancer when becoming pathogenic. Li et al. reported an increase in Lin+ GATA3+ as well as Lin− GATA3+ ILC2‐like populations together with a more pronounced type 2 signature limiting the protective type 1 immune response in the development of cancers.116 In mice, Helicobacter typhlonius infection triggers IL‐17A expressing ILCs by synergizing DCs derived TNF‐α and IL‐23 in the Tbx21 −/− Rag2 −/− ulcerative colitis (TRUC) model.83
Microbiota and ILCs in viral infections
ssRNA viruses: Norovirus, mouse hepatitis virus and influenza virus
Norovirus (family of Caliciviridae, ssRNA) infections are highly contagious, and typical symptoms include diarrhoea, nausea, vomiting and abdominal pain in humans. In mice, Murine Norovirus (MNV) triggers changes of epithelial innate immunity of the small intestine in mice hypomorphic for ATG16L expression.117, 118 Because ATG16L is a risk gene for Crohn’s disease in humans, this report highlights the close link of genetic predisposition and microbial exposure. Interestingly, infections of MNV in GF mice have identified an important role of virus ‘colonization’ on the host immune system. Here, MNV mimics beneficial immune modulatory function of the microbiota and influences intestinal immune responses as well as ILC numbers and function.39 Whether and how MNV infection impact on microbiota and thereby immunity of ILC populations in SPF colonized mice still needs to be investigated. However, upon antibiotic treatment, MNV infection or sensing of viral ssRNA by TLR7 is able to trigger antimicrobial peptide expression as well as IL‐23 release, and thus tightening of the epithelial barrier together with increased ILC3 function in the defense against vancomycin‐resistant Enterococcus faecium (VRE).119 Interestingly, treatment of psoriasis with the TLR7‐activating drug imiquimod has been linked to colonic dysbiosis and thus to increased susceptibility to DSS colitis.120 However, this was not ILC3 dependent in contrast to reports from the previously discussed study.119
The pathogenic Mouse Hepatitis Virus (MHV; family of Coronaviridae, ssRNA) targets the intestine and liver, and is able to cause severe systemic disease. It is feared in animal facilities as a contamination but also used successfully to study viral pathogenicity. Here, upon oral MHV infection, fibroblastic reticular cells (FRCs) secrete IL‐15 in a MyD88‐dependent manner and thereby control ILC1s and intestinal inflammation. Dysbiosis is observed in the absence of innate immune stimulation, showing that tissue circuits between non‐haematopoietic cells, such as FRCs and haematopoietic cells including ILC1s, are key in intestinal immune responses against viruses.121
Although respiratory viruses, including influenza virus (ssRNA), are limited to the airways during infection and have been reported to impact systemic immunity and the intestinal microbiota.122, 123 Indeed, immunity and microbiota of the gut and lungs are closely interlinked.124 In humans, the microbiome of the upper respiratory tract, including nose and throat, has been shown to change upon influenza infection.125 Whether intestinal ILC populations are affected in influenza infection in humans has not yet been addressed. However, ILCs, specifically ILC2s, are increased upon infection with influenza A virus (IAV) in the respiratory tract in mice.126, 127, 128 IAV‐induced ILC2s can be detrimental for the host by inducing excessive type 2 immune responses and airway hyperreactivity independently of the adaptive immune system. Control of ILC2s by IFN is thus an important mechanism to restrain ILC2s upon IAV infection.127 However, ILC2s can also support wound healing upon infection by the secretion of AREG in the respiratory tract as well as in the gut during inflammation, and thereby be beneficial for the host.126, 129 Experimental factors might be involved in the different outcome of the ILC2 character including the use of different IAV subtypes in the studies, namely H3N1 and H1N1, or general variations in experimental procedures such as the different experimental time points analysed as previously discussed.130, 131
Antibiotic treatment influences not only the microbial composition in the gut (discussed above), but also has an important impact on ILC plasticity and function,42 as well as on immunity to respiratory infections, such as influenza virus.132 Here, reduced signalling via the inflammasome and TLRs leads to decreased activation and migration of DCs into the mediastinal lymph node. Thereby, the priming of T‐cells is reduced and the initiation of the immune response hampered. Moreover, reduced levels of virus‐specific T‐cells to influenza virus were reported by Abt and colleagues due to changes in macrophage function in antibiotic‐treated mice.133 Conversely, IAV infection has been reported to not be influenced by antibiotic treatment in immature mice.134 However, how antibiotic treatment influences pulmonary ILCs in pathogenic infections remains to be elucidated.
Lentiviruses: Human immunodeficiency virus and simian immunodeficiency virus
Infection by human immunodeficiency virus (HIV; family of Retroviridae, ssRNA) leads to acquired immunodeficiency syndrome (AIDS), an immunodeficiency characterized by a severe reduction of CD4 T‐cells. Highly active antiretroviral therapy (HAART) is used successfully to restrict viral load and maintain CD4 T‐cells. Studies to decipher the underlying mechanism in HIV infections include studying simian immunodeficiency virus (SIV)‐infected non‐human primates, HIV‐infected humanized mice and peripheral blood, as well as biopsies of HIV‐infected human individuals. HIV and SIV infection break down the intestinal barrier leading to disruption of the intestinal integrity and transition of intestinal antigens followed by systemic inflammation.135 Patients with HIV infections have been reported with perturbations of the bacterial microbiota together with expansion of the virome.136 Upon HIV/SIV infection, intestinal CD4 T‐cells as well as ILC populations, specifically ILC3s, are massively decreased.137 HIV is not able to directly infect ILCs as they lack both co‐receptors used by HIV for viral docking, namely CCR5 and CD4. However, human CD45+ Lin− CD117− CRTH2− CD127+ CD56− CD4+ cells can still be infected by HIV.138 In acute HIV infection, ILC1s, ILC2s and ILC3s are significantly decreased in peripheral blood and only early HAART is able to preserve ILCs. Late HAART therapy was not able to reconstitute ILCs levels.139 Decrease in ILC subsets in HIV infection is further confirmed by a recent report showing the reduction of intestinal ILC subsets upon chronic HIV‐1 infection and furthermore reporting an increase in CD94+ memory‐like NK cells.140 Indeed, intestinal ILCs in patients with HIV change their phenotypic appearance such as expression of the IL‐7Rα chain (CD127), which is needed to sense IL‐7, an important survival and proliferation factor.141 Moreover, upregulation of apoptotic signals has been reported in ILC3s induced by plasmacytoid dendritic cell (pDC)‐derived interferon upon HIV in humanized mice. Microbiota triggered IL‐2 production by ILC3 has just recently been shown to support Tregs.142 It is tempting to speculate that loss of IL‐2 producing ILC populations may have a direct effect on T‐cell homeostasis in HIV infection.
Furthermore, decreased ILC2 function and thus reduced IL‐13 production upon ex vivo stimulation of SIV‐infected non‐human primate samples has been reported.143 It is not yet known whether impaired ILC2 activity in HIV/SIV parallels the restraint of ILC2s in IAV by interferon, and thereby triggers the loss of intestinal barrier and the increased transition of microbial ligands from the gut. IFN regulatory mechanisms upon HIV infection may be triggered by HIV itself as well as by the expansion of the enteric virome, but this hypothesis still needs to be confirmed. Interestingly, virome expansion has been reported upon SIV infections.144
dsRNA viruses: Rotavirus
Rotavirus (family of Reoviridae, dsRNA) can cause gastroenteritis, with young children being predominantly affected. All three groups of IFNs are important in the defense against Rotavirus, but type III IFNs (IFN‐λ) are only acting on and triggering intestinal epithelial cell immunity.145 Moreover, synergy between IL‐22 produced by ILC3s and IFN‐γ has been identified,146 further highlighting the strong interaction of ILCs and epithelial cells during infection. Data are limited on the link between rotavirus infection and microbiota; however, the efficacy of a rotavirus vaccine (Rotarix: live, attenuated human rotavirus vaccine used in infants) and microbiota has been noted.147 To which extend this is linked to ILC3 activity has not yet been addressed, but would be of great interest.
Microbiota − ILCs, cancer and aging
In this last paragraph of our review we want to highlight key aspects that are known about microbiota development and ILC function with age, and highlight their contributions to the development and defense against cancers – one of the most common age‐related diseases. As outlined previously, microbiota composition and function dramatically changes in aging individuals.148 This by itself may already alter ILC function, although one could also imagine that altered ILC activity with age149, 150 can impact the composition of the commensal microbiota through various mechanisms that we have reviewed above. Several studies have reported that both dysbiosis and altered ILC functionality can be involved in tumorigenesis and also in the anti‐tumour immune response. These aspects have recently been discussed in excellent reviews by Wagner and Koyasu,151 Panda and Colonna,152 Mattiola and Diefenbach,153 and Atreya et al. 154 Thus, we will only briefly touch on the role of ILCs in colon cancer, focusing on ILC3s, the ILC population with the strongest link to cancer so far.
Innate lymphoid cells can be both pro‐ and anti‐tumorigenic. Upon induction of intestinal inflammation by Helicobacter hepaticus and of cancer formation with the carcinogen azoxymethane (AOM), ILC‐derived IL‐22 acts on epithelial cells and induces cancer formation. Blocking of IL‐22 or ILCs by anti‐Thy1 antibody reduces colorectal cancer.155 IL‐22 can also be regulated by an endogenous mechanism, namely by the decoy IL‐22BP. Increased expression of IL‐22BP by T‐cells in inflammatory bowel disease (IBD) patients promotes cancer development.156, 157 Intestinal inflammation in IBD patients regularly triggers the development of cancer,158, 159 further confirming that inflammation is an additional hallmark of cancer.160 However, the protective role of IL‐22 in intestinal homeostasis can be extended to colon cancer. Recently, AhR‐induced IL‐22 in ILC3s was shown to reduce formation of epithelial stem cells in the colon upon AOM treatment. The underlying mechanism was elucidated by using the Confetti system enabling to trace IL‐22 receptor expressing or non‐expressing crypts. IL‐22‐deficient crypts lost the control of apoptosis through the DNA damage response. This dysregulation triggered the formation of colon cancer upon AOM treatment, highlighting the importance of IL‐22 in colon cancer carcinogenesis.46 In the same study, they could also demonstrate that dietary‐derived AhR ligands directly induce genotoxic stress in intestinal epithelial cells, while at the same time promoting IL‐22 production in ILC3s, which in return switches on the DNA damage response in intestinal epithelial cells. These studies further underline that ILC3s are key players in homeostatic but also in inflammatory conditions at the interface between the environment and the body. For certain, aging and cancers will be of interest in future studies looking at microbiota−ILC interactions.
Summary and outlook
The tremendous impact of the microbiota on health and thus the immune system of the host has been appreciated for a long time. The recently discovered field of ILC biology and its importance in the establishment and regulation of the host immune system provides novel insights into the regulation and function of the host−microbial mutualism. In this review, we aimed to give an overview of recent findings on the regulatory interplay between microbiota and ILC function throughout life at steady‐state and during disease. The microbiota is continuously adapting to its environment throughout the lifetime. The synergism of commensals and immune cells has a tremendous impact on the host’s health and immune defense. Just recently, Godinho‐Silva et al. deciphered that the circadian clock activator Antl is essential for intestinal ILC3 function at steady‐state and upon infection by C. rodentium,161 revealing that ILC3s are controlled by additional environmental factors. We are just beginning to understand the complexity of these networks, and are excited to decipher this close interaction of microbiota and ILCs during the complete lifespan in the future.
Disclosures
The authors declare no conflict of interest.
Acknowledgement
CUD acknowledges support by a Rahel Hirsch Habilitationsstipendium.
Contributor Information
Stephanie C. Ganal‐Vonarburg, Email: stephanie.ganal@dbmr.unibe.ch.
Claudia U. Duerr, Email: claudia.duerr@charite.de.
References
- 1. Claesson MJ, Cusack S, O'Sullivan O et al Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci USA 2011; 108(Suppl 1):4586–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ottman N, Smidt H, de Vos WM et al The function of our microbiota: who is out there and what do they do? Front Cell Infect Microbiol 2012; 2:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol 2010; 10:159–69. [DOI] [PubMed] [Google Scholar]
- 4. Rowland I, Gibson G, Heinken A et al Gut microbiota functions: metabolism of nutrients and other food components. Eur J Nutr 2018; 57:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eberl G, Colonna M, Di Santo JP et al Innate lymphoid cells: a new paradigm in immunology. Science 2015; 348:aaa6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vivier E, Artis D, Colonna M et al Innate lymphoid cells: 10 years on. Cell 2018; 174:1054–66. [DOI] [PubMed] [Google Scholar]
- 7. Dadi S, Chhangawala S, Whitlock BM et al Cancer immunosurveillance by tissue‐resident innate lymphoid cells and innate‐like T cells. Cell 2016; 164:365–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fuchs A, Vermi W, Lee JS et al Intraepithelial type 1 innate lymphoid cells are a unique subset of IL‐12‐ and IL‐15‐responsive IFN‐gamma‐producing cells. Immunity 2013; 38:769–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Robinette ML, Colonna M. Immune modules shared by innate lymphoid cells and T cells. J Allergy Clin Immunol 2016; 138:1243–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Klose CS, Diefenbach A. Transcription factors controlling innate lymphoid cell fate decisions. Curr Top Microbiol Immunol 2014; 381:215–55. [DOI] [PubMed] [Google Scholar]
- 11. Artis D, Spits H. The biology of innate lymphoid cells. Nature 2015; 517:293–301. [DOI] [PubMed] [Google Scholar]
- 12. Wang S, Xia P, Chen Y et al Regulatory innate lymphoid cells control innate intestinal inflammation. Cell 2017; 171:201–16 e18. [DOI] [PubMed] [Google Scholar]
- 13. Klose CSN, Flach M, Mohle L et al Differentiation of type 1 ILCs from a common progenitor to all helper‐like innate lymphoid cell lineages. Cell 2014; 157:340–56. [DOI] [PubMed] [Google Scholar]
- 14. Adachi S, Yoshida H, Honda K et al Essential role of IL‐7 receptor alpha in the formation of Peyer's patch anlage. Int Immunol 1998; 10:1–6. [DOI] [PubMed] [Google Scholar]
- 15. Adachi S, Yoshida H, Kataoka H et al Three distinctive steps in Peyer's patch formation of murine embryo. Int Immunol 1997; 9:507–14. [DOI] [PubMed] [Google Scholar]
- 16. Mebius RE, Rennert P, Weissman IL. Developing lymph nodes collect CD4+CD3‐ LTbeta+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity 1997; 7:493–504. [DOI] [PubMed] [Google Scholar]
- 17. Yoshida H, Naito A, Inoue J et al Different cytokines induce surface lymphotoxin‐alphabeta on IL‐7 receptor‐alpha cells that differentially engender lymph nodes and Peyer's patches. Immunity 2002; 17:823–33. [DOI] [PubMed] [Google Scholar]
- 18. Eberl G, Marmon S, Sunshine MJ et al An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol 2004; 5:64–73. [DOI] [PubMed] [Google Scholar]
- 19. van de Pavert SA, Mebius RE. New insights into the development of lymphoid tissues. Nat Rev Immunol 2010; 10:664–74. [DOI] [PubMed] [Google Scholar]
- 20. van de Pavert SA, Ferreira M, Domingues RG et al Maternal retinoids control type 3 innate lymphoid cells and set the offspring immunity. Nature 2014; 508:123–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bhattacharya N, Yuan R, Prestwood TR et al Normalizing microbiota‐induced retinoic acid deficiency stimulates protective CD8(+) T cell‐mediated immunity in colorectal cancer. Immunity 2016; 45:641–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Grizotte‐Lake M, Zhong G, Duncan K et al Commensals suppress intestinal epithelial cell retinoic acid synthesis to regulate interleukin‐22 activity and prevent microbial dysbiosis. Immunity 2018; 49:1103–15 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eberl G, Littman DR. Thymic origin of intestinal alphabeta T cells revealed by fate mapping of RORgammat+ cells. Science 2004; 305:248–51. [DOI] [PubMed] [Google Scholar]
- 24. Eberl G. Inducible lymphoid tissues in the adult gut: recapitulation of a fetal developmental pathway? Nat Rev Immunol 2005; 5:413–20. [DOI] [PubMed] [Google Scholar]
- 25. Hamada H, Hiroi T, Nishiyama Y et al Identification of multiple isolated lymphoid follicles on the antimesenteric wall of the mouse small intestine. J Immunol 2002; 168:57–64. [DOI] [PubMed] [Google Scholar]
- 26. Kanamori Y, Ishimaru K, Nanno M et al Identification of novel lymphoid tissues in murine intestinal mucosa where clusters of c‐kit+ IL‐7R+ Thy1+ lympho‐hemopoietic progenitors develop. J Exp Med 1996; 184:1449–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cherrier M, Sawa S, Eberl G. Notch, Id2, and RORgammat sequentially orchestrate the fetal development of lymphoid tissue inducer cells. J Exp Med 2012; 209:729–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Possot C, Schmutz S, Chea S et al Notch signaling is necessary for adult, but not fetal, development of RORgammat(+) innate lymphoid cells. Nat Immunol 2011; 12:949–58. [DOI] [PubMed] [Google Scholar]
- 29. Klose CS, Kiss EA, Schwierzeck V et al A T‐bet gradient controls the fate and function of CCR6‐RORgammat+ innate lymphoid cells. Nature 2013; 494:261–5. [DOI] [PubMed] [Google Scholar]
- 30. Sawa S, Cherrier M, Lochner M et al Lineage relationship analysis of RORgammat+ innate lymphoid cells. Science 2010; 330:665–9. [DOI] [PubMed] [Google Scholar]
- 31. Gomez de Aguero M, Ganal‐Vonarburg SC, Fuhrer T et al The maternal microbiota drives early postnatal innate immune development. Science 2016; 351:1296–302. [DOI] [PubMed] [Google Scholar]
- 32. Sanos SL, Bui VL, Mortha A et al RORgammat and commensal microflora are required for the differentiation of mucosal interleukin 22‐producing NKp46+ cells. Nat Immunol 2009; 10:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vonarbourg C, Mortha A, Bui VL et al Regulated expression of nuclear receptor RORgammat confers distinct functional fates to NK cell receptor‐expressing RORgammat(+) innate lymphocytes. Immunity 2010; 33:736–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Satoh‐Takayama N, Vosshenrich CA, Lesjean‐Pottier S et al Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity 2008; 29:958–70. [DOI] [PubMed] [Google Scholar]
- 35. Sawa S, Lochner M, Satoh‐Takayama N et al RORgammat+ innate lymphoid cells regulate intestinal homeostasis by integrating negative signals from the symbiotic microbiota. Nat Immunol 2011; 12:320–6. [DOI] [PubMed] [Google Scholar]
- 36. Kiss EA, Vonarbourg C, Kopfmann S et al Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science 2011; 334:1561–5. [DOI] [PubMed] [Google Scholar]
- 37. Pabst O, Herbrand H, Friedrichsen M et al Adaptation of solitary intestinal lymphoid tissue in response to microbiota and chemokine receptor CCR7 signaling. J Immunol 2006; 177:6824–32. [DOI] [PubMed] [Google Scholar]
- 38. Schneider C, Lee J, Koga S et al Tissue‐resident group 2 innate lymphoid cells differentiate by layered ontogeny and in situ perinatal priming. Immunity 2019; 50:1425–38 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kernbauer E, Ding Y, Cadwell K. An enteric virus can replace the beneficial function of commensal bacteria. Nature 2014; 516:94–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ganal SC, Sanos SL, Kallfass C et al Priming of natural killer cells by nonmucosal mononuclear phagocytes requires instructive signals from commensal microbiota. Immunity 2012; 37:171–86. [DOI] [PubMed] [Google Scholar]
- 41. Bernink JH, Krabbendam L, Germar K et al Interleukin‐12 and ‐23 control plasticity of CD127(+) group 1 and group 3 innate lymphoid cells in the intestinal lamina propria. Immunity 2015; 43:146–60. [DOI] [PubMed] [Google Scholar]
- 42. Gury‐BenAri M, Thaiss CA, Serafini N et al The spectrum and regulatory landscape of intestinal innate lymphoid cells are shaped by the microbiome. Cell 2016; 166:1231–46 e13. [DOI] [PubMed] [Google Scholar]
- 43. Wolk K, Kunz S, Witte E et al IL‐22 increases the innate immunity of tissues. Immunity 2004; 21:241–54. [DOI] [PubMed] [Google Scholar]
- 44. Zheng Y, Valdez PA, Danilenko DM et al Interleukin‐22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med 2008; 14:282–9. [DOI] [PubMed] [Google Scholar]
- 45. Lindemans CA, Calafiore M, Mertelsmann AM et al Interleukin‐22 promotes intestinal‐stem‐cell‐mediated epithelial regeneration. Nature 2015; 528:560–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gronke K, Hernandez PP, Zimmermann J et al Interleukin‐22 protects intestinal stem cells against genotoxic stress. Nature 2019; 566:249–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sonnenberg GF, Fouser LA, Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL‐22. Nat Immunol 2011; 12:383–90. [DOI] [PubMed] [Google Scholar]
- 48. Lochner M, Ohnmacht C, Presley L et al Microbiota‐induced tertiary lymphoid tissues aggravate inflammatory disease in the absence of RORgamma t and LTi cells. J Exp Med 2011; 208:125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sonnenberg GF, Monticelli LA, Alenghat T et al Innate lymphoid cells promote anatomical containment of lymphoid‐resident commensal bacteria. Science 2012; 336:1321–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mosconi I, Geuking MB, Zaiss MM et al Intestinal bacteria induce TSLP to promote mutualistic T‐cell responses. Mucosal Immunol 2013; 6:1157–67. [DOI] [PubMed] [Google Scholar]
- 51. von Moltke J, Ji M, Liang HE, Locksley RM. Tuft‐cell‐derived IL‐25 regulates an intestinal ILC2‐epithelial response circuit. Nature 2016; 529:221–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhu P, Zhu X, Wu J et al IL‐13 secreted by ILC2s promotes the self‐renewal of intestinal stem cells through circular RNA circPan3. Nat Immunol 2019; 20:183–94. [DOI] [PubMed] [Google Scholar]
- 53. Mullin JM, Snock KV. Effect of tumor necrosis factor on epithelial tight junctions and transepithelial permeability. Cancer Res 1990; 50:2172–6. [PubMed] [Google Scholar]
- 54. Clark E, Hoare C, Tanianis‐Hughes J et al Interferon gamma induces translocation of commensal Escherichia coli across gut epithelial cells via a lipid raft‐mediated process. Gastroenterology 2005; 128:1258–67. [DOI] [PubMed] [Google Scholar]
- 55. Takatori H, Kanno Y, Watford WT et al Lymphoid tissue inducer‐like cells are an innate source of IL‐17 and IL‐22. J Exp Med 2009; 206:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Buonocore S, Ahern PP, Uhlig HH et al Innate lymphoid cells drive interleukin‐23‐dependent innate intestinal pathology. Nature 2010; 464:1371–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mortha A, Chudnovskiy A, Hashimoto D et al Microbiota‐dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science 2014; 343:1 249 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Branzk N, Gronke K, Diefenbach A. Innate lymphoid cells, mediators of tissue homeostasis, adaptation and disease tolerance. Immunol Rev 2018; 286:86–101. [DOI] [PubMed] [Google Scholar]
- 59. Longman RS, Diehl GE, Victorio DA et al CX(3)CR1(+) mononuclear phagocytes support colitis‐associated innate lymphoid cell production of IL‐22. J Exp Med 2014; 211:1571–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kinnebrew MA, Buffie CG, Diehl GE et al Interleukin 23 production by intestinal CD103(+)CD11b(+) dendritic cells in response to bacterial flagellin enhances mucosal innate immune defense. Immunity 2012; 36:276–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hepworth MR, Fung TC, Masur SH et al Immune tolerance. Group 3 innate lymphoid cells mediate intestinal selection of commensal bacteria‐specific CD4(+) T cells. Science 2015; 348:1031–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hepworth MR, Monticelli LA, Fung TC et al Innate lymphoid cells regulate CD4+ T‐cell responses to intestinal commensal bacteria. Nature 2013; 498:113–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. von Burg N, Chappaz S, Baerenwaldt A et al Activated group 3 innate lymphoid cells promote T‐cell‐mediated immune responses. Proc Natl Acad Sci USA 2014; 111:12 835–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Castellanos JG, Woo V, Viladomiu M et al Microbiota‐induced TNF‐like ligand 1A drives group 3 innate lymphoid cell‐mediated barrier protection and intestinal T cell activation during colitis. Immunity 2018; 49:1077–89 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mackley EC, Houston S, Marriott CL et al CCR7‐dependent trafficking of RORgamma(+) ILCs creates a unique microenvironment within mucosal draining lymph nodes. Nat Commun 2015; 6:5862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Melo‐Gonzalez F, Kammoun H, Evren E et al Antigen‐presenting ILC3 regulate T cell‐dependent IgA responses to colonic mucosal bacteria. J Exp Med 2019; 216:728–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kruglov AA, Grivennikov SI, Kuprash DV et al Nonredundant function of soluble LTalpha3 produced by innate lymphoid cells in intestinal homeostasis. Science 2013; 342:1243–6. [DOI] [PubMed] [Google Scholar]
- 68. Magri G, Miyajima M, Bascones S et al Innate lymphoid cells integrate stromal and immunological signals to enhance antibody production by splenic marginal zone B cells. Nat Immunol 2014; 15:354–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Tsuji M, Suzuki K, Kitamura H et al Requirement for lymphoid tissue‐inducer cells in isolated follicle formation and T cell‐independent immunoglobulin A generation in the gut. Immunity 2008; 29:261–71. [DOI] [PubMed] [Google Scholar]
- 70. Oliphant CJ, Hwang YY, Walker JA et al MHCII‐mediated dialog between group 2 innate lymphoid cells and CD4(+) T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity 2014; 41:283–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mirchandani AS, Besnard AG, Yip E et al Type 2 innate lymphoid cells drive CD4+ Th2 cell responses. J Immunol 2014; 192:2442–8. [DOI] [PubMed] [Google Scholar]
- 72. Drake LY, Iijima K, Kita H. Group 2 innate lymphoid cells and CD4+ T cells cooperate to mediate type 2 immune response in mice. Allergy 2014; 69:1300–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Moro K, Yamada T, Tanabe M et al Innate production of T(H)2 cytokines by adipose tissue‐associated c‐Kit(+)Sca‐1(+) lymphoid cells. Nature 2010; 463:540–4. [DOI] [PubMed] [Google Scholar]
- 74. Mooser C, Gomez de Aguero M, Ganal‐Vonarburg SC. Standardization in host‐microbiota interaction studies: challenges, gnotobiology as a tool, and perspective. Curr Opin Microbiol 2018; 44:50–60. [DOI] [PubMed] [Google Scholar]
- 75. Mamantopoulos M, Ronchi F, McCoy KD et al Inflammasomes make the case for littermate‐controlled experimental design in studying host‐microbiota interactions. Gut Microbes 2018; 9:374–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Guo X, Liang Y, Zhang Y et al Innate lymphoid cells control early colonization resistance against intestinal pathogens through ID2‐dependent regulation of the microbiota. Immunity 2015; 42:731–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Qiu J, Heller JJ, Guo X et al The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity 2012; 36:92–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ivanov II, Atarashi K, Manel N et al Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 2009; 139:485–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zenewicz LA, Yin X, Wang G et al IL‐22 deficiency alters colonic microbiota to be transmissible and colitogenic. J Immunol 2013; 190:5306–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Fulde M, Sommer F, Chassaing B et al Early posnatal selection by intestinal epithelial Toll‐like receptor 5 influences long‐term microbiota composition. Nature 2018; 560:489–93. [DOI] [PubMed] [Google Scholar]
- 81. Kobayashi T, Voisin B, Kim DY et al Homeostatic control of sebaceous glands by innate lymphoid cells regulates commensal bacteria equilibrium. Cell 2019; 176:982–97 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Garrett WS, Lord GM, Punit S et al Communicable ulcerative colitis induced by T‐bet deficiency in the innate immune system. Cell 2007; 131:33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Powell N, Walker AW, Stolarczyk E et al The transcription factor T‐bet regulates intestinal inflammation mediated by interleukin‐7 receptor+ innate lymphoid cells. Immunity 2012; 37:674–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Garrido‐Mesa N, Schroeder JH, Stolarczyk E, Gallagher AL, Lo JW, Bailey C et al T‐bet controls intestinal mucosa immune responses via repression of type 2 innate lymphoid cell function. Mucosal Immunol 2019; 12:51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. WHO (2019).Soil‐transmitted helminth infections [WWW document] URL https://www.who.int/news-room/fact-sheets/detail/soil-transmitted-helminth-infections [accessed on 3 August 2019].
- 86. Salgame P, Yap GS, Gause WC. Effect of helminth‐induced immunity on infections with microbial pathogens. Nat Immunol 2013; 14:1118–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Holm JB, Sorobetea D, Kiilerich P et al Chronic Trichuris muris infection decreases diversity of the intestinal microbiota and concomitantly increases the abundance of Lactobacilli . PLoS ONE 2015; 10:e0125495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Reynolds LA, Smith KA, Filbey KJ et al Commensal‐pathogen interactions in the intestinal tract: Lactobacilli promote infection with, and are promoted by, helminth parasites. Gut Microbes 2014; 5:522–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Tritten L, Geary TG. Helminth extracellular vesicles in host‐parasite interactions. Curr Opin Microbiol 2018; 46:73–9. [DOI] [PubMed] [Google Scholar]
- 90. Shimokawa C, Kanaya T, Hachisuka M et al Mast cells are crucial for induction of group 2 innate lymphoid cells and clearance of helminth infections. Immunity 2017; 46:863–74 e4. [DOI] [PubMed] [Google Scholar]
- 91. Molofsky AB, Savage AK, Locksley RM. Interleukin‐33 in tissue homeostasis, injury, and inflammation. Immunity 2015; 42:1005–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Price AE, Liang HE, Sullivan BM et al Systemically dispersed innate IL‐13‐expressing cells in type 2 immunity. Proc Natl Acad Sci USA 2010; 107:11 489–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Neill DR, Wong SH, Bellosi A et al Nuocytes represent a new innate effector leukocyte that mediates type‐2 immunity. Nature 2010; 464:1367–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Gerbe F, Sidot E, Smyth DJ et al Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature 2016; 529:226–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Howitt MR, Lavoie S, Michaud M et al Tuft cells, taste‐chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science 2016; 351:1329–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Osbourn M, Soares DC, Vacca F et al HpARI protein secreted by a helminth parasite suppresses interleukin‐33. Immunity 2017; 47:739–51 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Spencer SP, Wilhelm C, Yang Q et al Adaptation of innate lymphoid cells to a micronutrient deficiency promotes type 2 barrier immunity. Science 2014; 343:432–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Wilhelm C, Harrison OJ, Schmitt V et al Critical role of fatty acid metabolism in ILC2‐mediated barrier protection during malnutrition and helminth infection. J Exp Med 2016; 213:1409–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Li S, Bostick JW, Ye J et al Aryl hydrocarbon receptor signaling cell intrinsically inhibits intestinal group 2 innate lymphoid cell function. Immunity 2018; 49:915–28 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Raetz M, Hwang SH, Wilhelm CL et al Parasite‐induced TH1 cells and intestinal dysbiosis cooperate in IFN‐gamma‐dependent elimination of Paneth cells. Nat Immunol 2013; 14:136–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Van Acker A, Gronke K, Biswas A et al A Murine intestinal intraepithelial NKp46‐negative innate lymphoid cell population characterized by group 1 properties. Cell Rep 2017; 19:1431–43. [DOI] [PubMed] [Google Scholar]
- 102. Park E, Patel SJ, Wang Q et al Toxoplasma gondii infection drives conversion 1 of NK cells into ILC1s. bioRxiv. 10.1101/642017. Online First: 17 May 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Voth DE, Ballard JD. Clostridium difficile toxins: mechanism of action and role in disease. Clin Microbiol Rev 2005; 18:247–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Abt MC, Lewis BB, Caballero S et al Innate immune defenses mediated by two ILC subsets are critical for protection against acute Clostridium difficile infection. Cell Host Microbe 2015; 18:27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Geiger TL, Abt MC, Gasteiger G et al Nfil3 is crucial for development of innate lymphoid cells and host protection against intestinal pathogens. J Exp Med 2014; 211:1723–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Frisbee AL, Saleh MM, Young MK et al IL‐33 drives group 2 innate lymphoid cell‐mediated protection during Clostridium difficile infection. Nat Commun 2019; 10:2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Gianotti RJ, Moss AC. Fecal microbiota transplantation: from Clostridium difficile to inflammatory bowel disease. Gastroenterol Hepatol 2017; 13:209–13. [PMC free article] [PubMed] [Google Scholar]
- 108. FDA (2019) FDA warns about potential risk of serious infections caused by multi-drug resistant organisms related to the investigational use of Fecal Microbiota for Transplantation. [WWW document]. URL https://www.fda.gov/news-events/fda-brief/fda-brief-fda-warns-about-potential-risk-serious-infections-caused-multi-drug-resistant-organisms [accessed on 3 August 2019].
- 109. Weingarden A, Gonzalez A, Vazquez‐Baeza Y et al Dynamic changes in short‐ and long‐term bacterial composition following fecal microbiota transplantation for recurrent Clostridium difficile infection. Microbiome 2015; 3:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Bernink JH, Peters CP, Munneke M et al Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat Immunol 2013; 14:221–9. [DOI] [PubMed] [Google Scholar]
- 111. Hazenberg MD, Spits H. Human innate lymphoid cells. Blood 2014; 124:700–9. [DOI] [PubMed] [Google Scholar]
- 112. Barthel M, Hapfelmeier S, Quintanilla‐Martinez L et al Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect Immun 2003; 71:2839–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Satoh‐Takayama N, Dumoutier L, Lesjean‐Pottier S et al The natural cytotoxicity receptor NKp46 is dispensable for IL‐22‐mediated innate intestinal immune defense against Citrobacter rodentium . J Immunol 2009; 183:6579–87. [DOI] [PubMed] [Google Scholar]
- 114. Rankin LC, Girard‐Madoux MJ, Seillet C et al Complementarity and redundancy of IL‐22‐producing innate lymphoid cells. Nat Immunol 2016; 17:179–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Sonnenberg GF, Monticelli LA, Elloso MM et al CD4(+) lymphoid tissue‐inducer cells promote innate immunity in the gut. Immunity 2011; 34:122–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Li R, Jiang XX, Zhang LF et al Group 2 innate lymphoid cells are involved in skewed type 2 immunity of gastric diseases induced by Helicobacter pylori infection. Mediators Inflamm 2017; 2017:4927964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Cadwell K, Patel KK, Maloney NS et al Virus‐plus‐susceptibility gene interaction determines Crohn's disease gene Atg16L1 phenotypes in intestine. Cell 2010; 141:1135–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Cadwell K, Liu JY, Brown SL et al A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature 2008; 456:259–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Abt MC, Buffie CG, Susac B et al TLR‐7 activation enhances IL‐22‐mediated colonization resistance against vancomycin‐resistant enterococcus. Sci Transl Med 2016; 8:327ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Kiyohara H, Sujino T, Teratani T et al Toll‐Like Receptor 7 agonist‐induced dermatitis causes severe dextran sulfate sodium colitis by altering the gut microbiome and immune cells. Cell Mol Gastroenterol Hepatol 2019; 7:135–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Gil‐Cruz C, Perez‐Shibayama C, Onder L et al Fibroblastic reticular cells regulate intestinal inflammation via IL‐15‐mediated control of group 1 ILCs. Nat Immunol 2016; 17:1388–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Yildiz S, Mazel‐Sanchez B, Kandasamy M et al Influenza A virus infection impacts systemic microbiota dynamics and causes quantitative enteric dysbiosis. Microbiome 2018; 6:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Wang J, Li F, Wei H et al Respiratory influenza virus infection induces intestinal immune injury via microbiota‐mediated Th17 cell‐dependent inflammation. J Exp Med 2014; 211:2397–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Budden KF, Gellatly SL, Wood DL et al Emerging pathogenic links between microbiota and the gut‐lung axis. Nat Rev Microbiol 2017; 15:55–63. [DOI] [PubMed] [Google Scholar]
- 125. Lee KH, Gordon A, Shedden K et al The respiratory microbiome and susceptibility to influenza virus infection. PLoS ONE 2019; 14:e0207898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Monticelli LA, Sonnenberg GF, Abt MC et al Innate lymphoid cells promote lung‐tissue homeostasis after infection with influenza virus. Nat Immunol 2011; 12:1045–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Duerr CU, McCarthy CD, Mindt BC et al Type I interferon restricts type 2 immunopathology through the regulation of group 2 innate lymphoid cells. Nat Immunol 2016; 17:65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Chang YJ, Kim HY, Albacker LA et al Innate lymphoid cells mediate influenza‐induced airway hyper‐reactivity independently of adaptive immunity. Nat Immunol 2011; 12:631–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Monticelli LA, Osborne LC, Noti M et al IL‐33 promotes an innate immune pathway of intestinal tissue protection dependent on amphiregulin‐EGFR interactions. Proc Natl Acad Sci USA 2015; 112:10 762–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Panda SK, Colonna M. Innate lymphoid cells in mucosal immunity. Front Immunol 2019; 10:861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Mindt BC, Fritz JH, Duerr CU. Group 2 innate lymphoid cells in pulmonary immunity and tissue homeostasis. Front Immunol 2018; 9:840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Ichinohe T, Lee HK, Ogura Y et al Inflammasome recognition of influenza virus is essential for adaptive immune responses. J Exp Med 2009; 206:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Abt MC, Osborne LC, Monticelli LA et al Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity 2012; 37:158–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Fuglsang E, Pizzolla A, Krych L et al Changes in gut microbiota prior to influenza A virus infection do not affect immune responses in pups or juvenile mice. Front Cell Infect Microbiol 2018; 8:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Brenchley JM, Price DA, Schacker TW et al Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 2006; 12:1365–71. [DOI] [PubMed] [Google Scholar]
- 136. Vujkovic‐Cvijin I, Dunham RM, Iwai S et al Dysbiosis of the gut microbiota is associated with HIV disease progression and tryptophan catabolism. Sci Transl Med 2013; 5:193ra91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Li H, Richert‐Spuhler LE, Evans TI et al Hypercytotoxicity and rapid loss of NKp44+ innate lymphoid cells during acute SIV infection. PLoS Pathog 2014; 10:e1004551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Zhao J, Cheng L, Wang H et al Infection and depletion of CD4+ group‐1 innate lymphoid cells by HIV‐1 via type‐I interferon pathway. PLoS Pathog 2018; 14:e1006819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Kloverpris HN, Kazer SW, Mjosberg J et al Innate lymphoid cells are depleted irreversibly during acute HIV‐1 infection in the absence of viral suppression. Immunity 2016; 44:391–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Wang Y, Gellatly K, Derr A et al HIV‐1 unmasks the plasticity of innate lymphoid cells. bioRxiv. 10.1101/221010. Online First: 4 January 2018 [DOI] [Google Scholar]
- 141. Kramer B, Goeser F, Lutz P et al Compartment‐specific distribution of human intestinal innate lymphoid cells is altered in HIV patients under effective therapy. PLoS Pathog 2017; 13:e1006373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Zhou L, Chu C, Teng F et al Innate lymphoid cells support regulatory T cells in the intestine through interleukin‐2. Nature 2019; 568:405–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Mudd JC, Busman‐Sahay K, DiNapoli SR et al Hallmarks of primate lentiviral immunodeficiency infection recapitulate loss of innate lymphoid cells. Nat Commun 2018; 9:3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Handley SA, Thackray LB, Zhao G et al Pathogenic simian immunodeficiency virus infection is associated with expansion of the enteric virome. Cell 2012; 151:253–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Pott J, Mahlakoiv T, Mordstein M et al IFN‐lambda determines the intestinal epithelial antiviral host defense. Proc Natl Acad Sci USA 2011; 108:7944–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Hernandez PP, Mahlakoiv T, Yang I et al Interferon‐lambda and interleukin 22 act synergistically for the induction of interferon‐stimulated genes and control of rotavirus infection. Nat Immunol. 2015; 16:698–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Parker EPK, Praharaj I, Zekavati A et al Influence of the intestinal microbiota on the immunogenicity of oral rotavirus vaccine given to infants in south India. Vaccine 2018; 36:264–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. O'Toole PW, Jeffery IB. Gut microbiota and aging. Science 2015; 350:1214–5. [DOI] [PubMed] [Google Scholar]
- 149. Yudanin NA, Schmitz F, Flamar AL et al Spatial and temporal mapping of human innate lymphoid cells reveals elements of tissue specificity. Immunity 2019; 50:505–19 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. D'Souza SS, Shen X, Fung ITH et al Compartmentalized effects of aging on group 2 innate lymphoid cell development and function. Aging Cell 2019; 18:e13019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Wagner M, Koyasu S. Cancer immunoediting by innate lymphoid cells. Trends Immunol 2019; 40:415–30. [DOI] [PubMed] [Google Scholar]
- 152. Panda SK, Colonna M. Innate lymphoid cells: a potential link between microbiota and immune responses against cancer. Semin Immunol 2019; 41:101271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Mattiola I, Diefenbach A. Innate lymphoid cells and cancer at border surfaces with the environment. Semin Immunol 2019; 41:101 278. [DOI] [PubMed] [Google Scholar]
- 154. Atreya I, Kindermann M, Wirtz S. Innate lymphoid cells in intestinal cancer development. Semin Immunol 2019; 41:101 267. [DOI] [PubMed] [Google Scholar]
- 155. Kirchberger S, Royston DJ, Boulard O et al Innate lymphoid cells sustain colon cancer through production of interleukin‐22 in a mouse model. J Exp Med 2013; 210:917–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Pelczar P, Witkowski M, Perez LG et al A pathogenic role for T cell‐derived IL‐22BP in inflammatory bowel disease. Science 2016; 354:358–62. [DOI] [PubMed] [Google Scholar]
- 157. Huber S, Gagliani N, Zenewicz LA et al IL‐22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature 2012; 491:259–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Kappelman MD, Farkas DK, Long MD et al Risk of cancer in patients with inflammatory bowel diseases: a nationwide population‐based cohort study with 30 years of follow‐up evaluation. Clin Gastroenterol Hepatol 2014; 12:265–73 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Lakatos PL, Lakatos L. Risk for colorectal cancer in ulcerative colitis: changes, causes and management strategies. World J Gastroenterol 2008; 14:3937–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646–74. [DOI] [PubMed] [Google Scholar]
- 161. Godinho‐Silva C, Domingues RG, Rendas M et al Light‐entrained and brain‐tuned circadian circuits regulate ILC3s and gut homeostasis. Nature 2019; 574:254–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


