Abstract
Objective
To compare and analyze the clinical outcomes of the proximal humeral internal locking system (PHILOS) alone and the PHILOS combined with fibular allograft in the treatment of Neer three‐ and four‐part proximal humerus fractures (PHF) in the elderly.
Methods
From January 2014 to January 2018, a total of 42 elderly patients with Neer three‐ or four‐part PHF admitted to our hospital were randomly divided into observation group and control group, with 21 patients in each group. The observation group was treated with the PHILOS combined with fibular allograft. The control group was treated with the PHILOS alone. Perioperative parameters and fracture classification were recorded in the two groups. Function results were assessed by Visual Analog Scale (VAS), Constant‐Murley score (CMS), American Shoulder and Elbow Surgeons (ASES) score, and the Disability of Arm‐Shoulder‐Hand (DASH) score. Radiological results were evaluated using the neck‐shaft angle (NSA) and humeral head height (HHH), and complications were also recorded in each group.
Results
There were no significant differences between the two groups in terms of preoperative status, age, gender, cause of trauma, fracture site, and fracture classification. The average follow‐up time was 12 months. At the last follow‐up, the VAS and DASH observation groups were lower than the control group, and there was significant difference between the two groups (P < 0.05). The CMS and ASES were higher in the observation group than the control group, and there was significant difference between the two groups (P < 0.05). The mean difference in the NSA and HHH were lower in the observation group than the control group, and there was a significant difference between the two groups (P < 0.05). There was one postoperative complication in the observation group, which was humeral head avascular necrosis (AVN). There were seven postoperative complications in the control group, including three cases of humeral head collapse and three cases of screw cutout and one case of humeral head AVN. The incidence of postoperative complications in the observation group was significantly lower than the control group (P < 0.05), there was a significant difference between the two groups.
Conclusions
For Neer three‐ or four‐part PHF in the elderly patients, PHILOS fixation with fibular allograft shows satisfactory short‐term results with respect to humeral head support and maintenance of reduction, and may reduce the incidence of complications associated with fixation using a PHILOS alone.
Keywords: Anatomical allograft, Elderly, Fibular shaft, Proximal humeral internal locking system, Proximal humerus fractures
Introduction
Proximal humeral fractures (PHF) account for 4%–5% of the whole body bone fractures1, and the incidence of PHF is increasing due to a high number of elderly osteoporotic patients2, making PHF the third most common injury among older people3. It has been reported that more than 80% of patients with minimally displaced PHF can be managed by nonoperation4, but approximately 20% of patients with displaced and comminuted fractures require surgery5. These fractures are difficult to treat, as it is unpredictable whether they will achieve stable fixation that maintains intra‐operative reduction. Operative management of PHF still remains challenging for orthopedic surgeons in the world6.
Although various surgical techniques have been described for the unstable PHF, proximal humeral internal locking systems (PHILOS) are increasingly popular for treating these fractures because they offer improved biomechanical properties by providing divergent and convergent fixed‐angle screws that improve fixation and pullout strength in osteoporotic bone7. It is also known to be clinically and biomechanically effective in elderly patients with PHF8. However, it is difficult to obtain stable fixation in osteoporotic patients even with PHILOS9. Some authors have also reported some complications, such as avascular necrosis (AVN), screw cutout, implant failure, plate impingement, head collapse, and infection10, 11. Good outcomes have been reported following the use of an associated intramedullary allograft9, 12. However, most previous studies are case series, and no comparative study has evaluated the clinical and radiological outcomes of PHILOS with and without an associated fibular allograft in elderly patients.
The aim of this study was to compare and analyze the clinical outcomes of the PHILOS alone and the PHILOS combined with fibular allograft in the treatment of Neer three‐ or four‐part PHF in the elderly. The hypothesis was that patients treated using the PHILOS combined with fibular allograft would have better clinical and radiological outcomes with lower complication rates than those treated using only the PHILOS alone.
Patients and Methods
This research was a retrospective study and was approved by the Ethics Committee of Nanjing First Hospital, Nanjing Medical University. Written informed consents were obtained from all enrolled patients.
From January 2014 to January 2018, a total of 42 elderly patients with Neer three‐ or four‐part PHF admitted to Nanjing First Hospital were randomly divided into observation group and control group, with 21 patients in each group. The observation group was treated with the PHILOS combined with fibular allograft. The control group was treated with the PHILOS alone. Perioperative parameters and fracture classification were recorded in the two groups. The demographic characteristics of the patients in the two groups are shown in Table 1.
Table 1.
Comparison of general data between the two groups of patients
| Variables | Observation group(n = 21) | Control group(n = 21) | χ2/t‐value | P‐value | |
|---|---|---|---|---|---|
| Gender | Male | 11 | 12 | 0.096 | 0.757 |
| Female | 12 | 9 | |||
| Mean age | 68.8 ± 6.3 | 69.0 ± 7.2 | 0.114 | 0.901 | |
| Preoperative time (days) | 4.2 ± 1.1 | 4.3 ± 1.2 | 0.281 | 0.780 | |
| Mechanism of injury | F | 15 | 17 | 0.525 | 0.469 |
| TA | 6 | 4 | |||
| Fracture site | Left | 9 | 11 | 0.382 | 0.537 |
| Right | 12 | 10 | |||
| Neer classification | Neer 3 | 14 | 15 | 0.111 | 0.739 |
| Neer 4 | 7 | 6 |
Observation group: PHILOS combined with fibular allograft; Control group: PHILOS alone.
Inclusion criteria were as follows: (i) patients over 60 years of age; (ii) unilateral acute shift of Neer classification13 of three‐ or four‐part of the proximal humerus fracture; (iii) patient's fragments were either displaced more than 1.0 cm or angulated more than 45° and were preoperatively conformed by radiograph or computed tomography (CT) with three‐dimensional (3D) reconstructions; and (iv) there were no obvious surgical contraindications.
Exclusion criteria were as follows: (i) a history of shoulder surgery or chronic bone nonunion, there was severe soft tissue injury at the surgical site or systemic or local infection; (ii) pathological fracture or open fracture; (iii) complications of serious nervous or vascular injury; (iv) hypertensive patients with poor blood pressure control and diabetic patients with poor glycemic control; and (v) patients who refused to participate or failed to cooperate during the trial.
Surgical Technique
A proximal humeral locking plate (PHILOS; DePuy Synthes, Pennsylvania, America) was used in all patients. A fibular allograft (Datsing, Beijing, China) was used in the observation group.
Step 1: All patients were placed in the beach chair position on a radiolucent operating table followed by cervical plexus anesthesia.
Step 2: A deltopectoral approach was performed for all patients. An approximate 15 cm skin incision was made. The insertion of the deltoid muscle was one‐half detached posteriorly subperiosteally.
Step 3: Non‐absorbable sutures were passed through the junction of the greater tuberosity and the rotator cuff to promote mobilization and reduce fragmented tuberosities. A 2.0 mm Kirschner‐wire (K‐wire) was inserted into the humeral head to control rotation, and a periosteal elevator was used as a joystick at the fracture site to obtain reduction. After reduction of the greater tuberosity and humeral head, one or two 1.5 mm K‐wires were used for temporary fixation. The plate was placed in a position that did not impinge on the acromion. Indirect reduction was performed by inserting a cortical screw into the humeral shaft following the shape of the plate. In the observation group, fibular allograft (12 mm × 60 mm, Fig. 1) was inserted into the intramedullary canal distal to fracture site and was then driven back to the proximal humeral bone. Intramedullary fibular allograft was pushed onto the medial calcar to support the humeral head for prevention of varus displacement and deformity of the humeral head. The position varied depending on the configuration of the fracture. The graft was positioned vertically in valgus fractures and at a slightly oblique angle along the inferior cortex in the varus fractures. If anatomical reduction was achieved, locking screws were placed through the fibula into the humeral head and shaft to secure its position. Rotator cuff sutures were passed through the proximal humeral locking plate, and this was then slid from proximal to distal along the lateral aspect of the shaft, under the axillary nerve. The rotator cuff sutures were tied into place through eyelets on the plate, fixation was then obtained with screws and the wound was closed (Fig. 2).
Figure 1.
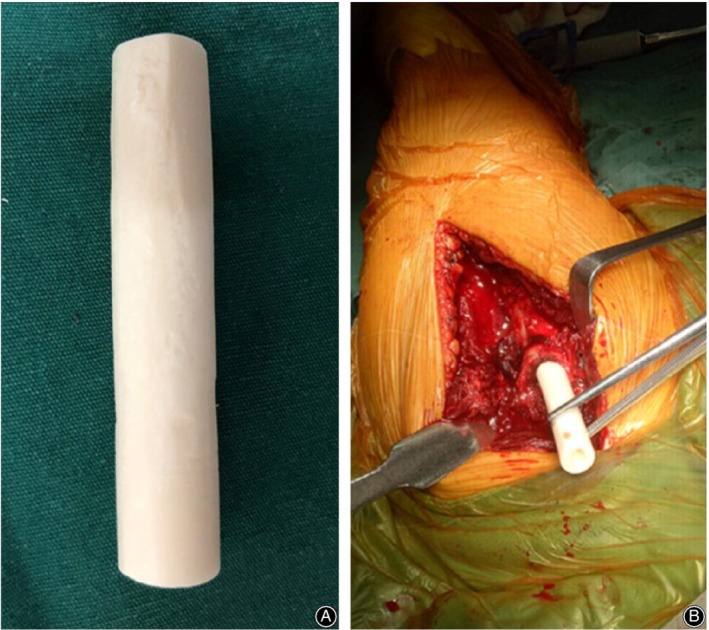
(A) Fibular allograft (12 mm×60 mm); (B) Insertion of fibular allograft.
Figure 2.
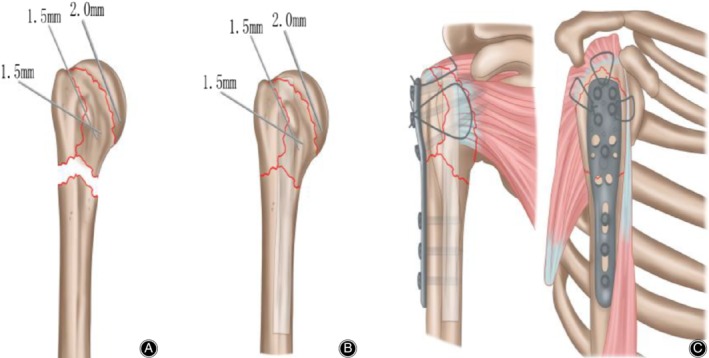
(A) (The illustration of the PHILOS combined with fibular allograft technology): A 2.0 mm Kirschner‐wire (K‐wire) was inserted into the humeral head to control rotation, and a periosteal elevator was used as a joystick at the fracture site to obtain reduction. After reduction of the greater tuberosity and humeral head, one or two 1.5 mm K‐wires were used for temporary fixation;(B) The allograft humerus is placed in the distal medullary cavity of the fracture, and the medial support is used to prevent the deformity and collapse of the humeral head in the long term; (C) Rotator cuff sutures were passed through the proximal humeral locking plate, and this was then slid from proximal to distal along the lateral aspect of the shaft, under the axillary nerve. The rotator cuff sutures were tied into place through eyelets on the plate, fixation was then obtained with screws and the wound was closed.
Postoperative Rehabilitation
The shoulders were immobilized in functional brace for between 4 and 6 weeks postoperatively. Patients received passive exercises immediately after the operation. About 4 weeks and 6 weeks later, active exercises including external and internal rotation were performed by/on all patients.
Clinical and Radiological Evaluation
Radiological Measurements
Radiological evaluation was performed immediately after the operation and at the final follow‐up by measuring the neck‐shaft angle (NSA) and humeral head height (HHH) on anteroposterior views (Fig. 3).
Figure 3.
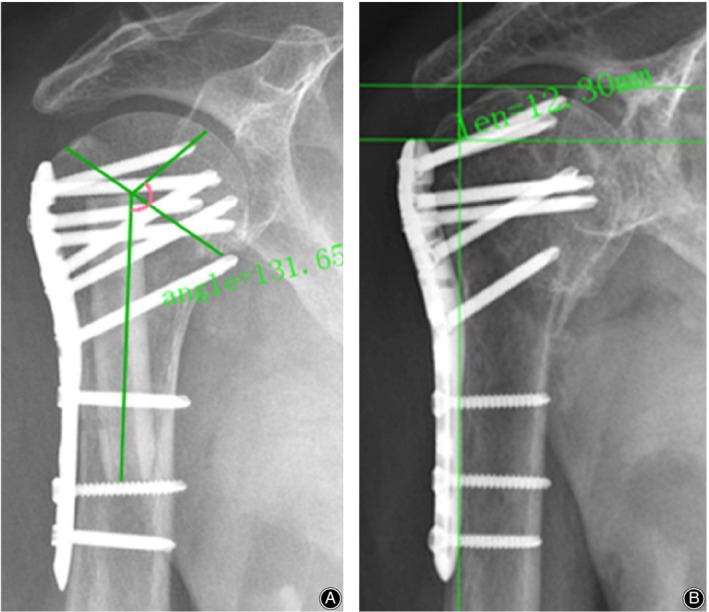
(A) The humeral neck‐shaft angle (NSA) was defined as the angle between a line that is perpendicular to the articular segment or atomic neck of the humerus and a line that bisects the humeral shaft; (B) The humeral head height (HHH) was defined as the distance between uppermost edge of the plate and the uppermost part of the humeral head.
The humeral neck‐shaft angle (NSA) was defined as the angle between a line that is perpendicular to the articular segment or atomic neck of the humerus and a line that bisects the humeral shaft by the measurement tools of the hospital's PACS system. The change in NSA was measured to assess whether the humerus had varus deformity.
HHH was defined as the distance between the upper end of the plate and the upper end of the humeral head by the measurement tools of the hospital's PACS system. The change in HHH was measured to assess the collapse of the humeral head. Comparing the immediate postoperative radiological findings with those at the final follow‐up, loss of fixation was defined if the varus NSA change was >5° or if HHH change was >3 mm14, 15.
Postoperative Patient Follow‐Up
Clinical evaluation was assessed at the final follow‐up using a Visual Analog Scale (VAS)16 score for pain, Constant‐Murley score (CMS)17, American Shoulder and Elbow Surgeons (ASES) score18, and Disability of Arm‐Shoulder‐Hand (DASH)19 score.
The VAS was used to assess the pain of the shoulder joint of the subject. The VAS total score is 10 points, with zero points indicating no pain and 10 points indicating unbearable pain.
The CMS score was 100 points, which consisted of pain (15 points), muscle strength (25 points), functional activity (20 points), and shoulder mobility (40 points). Higher scores indicate better functionality. Among them, objective evaluation indicators included shoulder mobility and muscle strength (65 points), and subjective evaluation indicators included pain and functional activities (35 points).
The rating scale of the ASES, which includes pain (50%) and life function (50%), is 100 points. The higher the score, the better the shoulder function.
The DASH scoring system consists of 30 items. Each of the five options corresponds to one to five points. When there are more than three missing items, the score is not calculated. The better the activity, the lower the score.
In addition, complications, such as AVN, screw cutout, implant failure, plate impingement, head collapse, and infection, were also recorded.
Statistical Analysis
Statistical analysis was performed using SPSS v. 19.0 (SPSS Inc., Chicago, Illinois, US). Continuous variables, presented as the mean and standard deviation (SD), were compared by the Student t test to detect the group differences. Qualitative data of groups was compared by the χ2 test. P value <0.05 was considered as significant difference.
Results
Intraoperative Radiological Outcomes
The radiological outcomes are shown in Table 2. The average NSA immediately postoperatively in the observation group was 138.9° (118.9°–156.6°), the average NSA at the last follow‐up was 135.8° (118.4°–155.5°), and the mean difference in the NSA was 3.42 ± 0.71°. The average NSA immediately postoperatively in the control group was 135.4° (109.0°–155.8°), the average NSA at the last follow‐up was 125.8° (97.0°–149.7°), and the mean difference in the NSA was 9.82 ± 1.02°. The mean difference in the NSA between the values immediately postoperatively and those at the final follow‐up were significantly greater in the control group (P = 0.000).
Table 2.
Radiological outcomes of the two groups
| Outcomes | Observation group (n = 21) | Control group (n = 21) | t‐value | P‐value |
|---|---|---|---|---|
| Mean difference of NSA (°) | 3.42 ± 0.71 | 9.82 ± 1.02 | 23.599 | <0.001 |
| Mean difference of HHH (mm) | 2.14 ± 0.33 | 4.54 ± 0.42 | 2.429 | <0.001 |
Observation group: PHILOS combined with fibular allograft; Control group: PHILOS alone; NSA, neck‐shaft angle; HHH, humeral head height.
The average HHH immediately postoperatively in the observation group was 12.3 mm (6.7 mm–15.7 mm), the average HHH at the last follow‐up was 10.2 mm (6.5 mm–13.5 mm), and the mean difference in the HHH was 2.14 ± 0.33 mm. The average HHH immediately postoperatively in the observation group was 12.2 mm (7.0 mm–18.5 mm), the average HHH at the last follow‐up was 9.0 mm (1 mm–15.1 mm), and the mean difference in the HHH was 4.54 ± 0.42 mm. The mean difference in the HHH between the values immediately postoperatively and those at the final follow‐up were significantly greater in the control group (P = 0.000).
Follow‐up Results
The clinical outcomes are shown in Table 3. No patients were lost to follow‐up. The average follow‐up time was 12 months. All fractures healed clinically and radiologically. The VAS (P = 0.734) score was not significantly different between the two groups. The CMS (P = 0.020) score, ASES (P = 0.024) score, and DASH (P = 0.023) score were significantly different between the two groups at the final follow‐up.
Table 3.
Clinical outcomes in the two groups
| Outcomes | Observation group (n = 21) | Control group (n = 21) | χ2/t‐value | P‐value |
|---|---|---|---|---|
| VAS | 1.14 ± 0.96 | 1.25 ± 1.12 | 0.342 | 0.734 |
| CMS | 86.00 ± 7.56 | 79.71 ± 9.14 | 2.429 | 0.020 |
| ASES | 87.76 ± 7.15 | 81.62 ± 9.62 | 2.349 | 0.024 |
| DASH | 17.95 ± 7.47 | 28.14 ± 8.27 | 4.190 | <0.001 |
| Complications | 1 | 7 | 3.860 | 0.018 |
Observation group: PHILOS combined with fibular allograft; Control group: PHILOS alone; VAS, Visual Analog Scale; CMS, Constant‐Murley score; ASES, American Shoulder and Elbow Surgeons; DASH, Disability of Arm‐Shoulder‐Hand score.
Complications
There were seven postoperative complications in the control group (Fig. 4), including three cases of humeral head collapse, three cases of screw cutout, and one case of humeral head AVN. There was one postoperative complication in the observation group (Fig. 5, 6, 7, 8), which was AVN.
Figure 4.
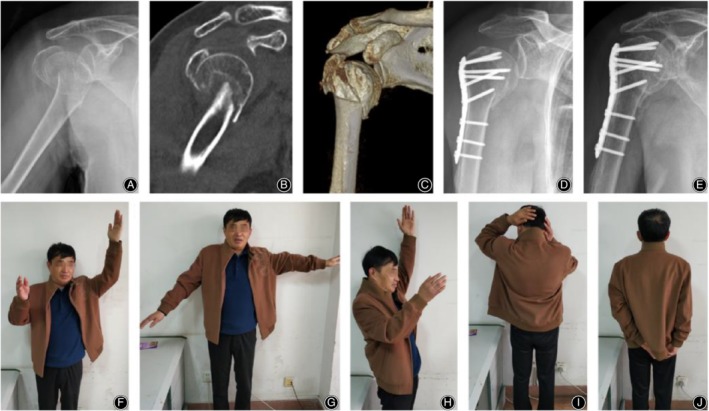
PHILOS fixation alone in a 65‐year‐old male patient with a four‐part proximal humeral fracture on the right side. (A) X‐ray film before surgery; (B) CT before surgery; (C) 3D CT before surgery; (D) X‐ray film after surgery; (E) X‐ray film 3 months after surgery; (F‐J) Function of patient's upper arm at the last follow‐up.
Figure 5.
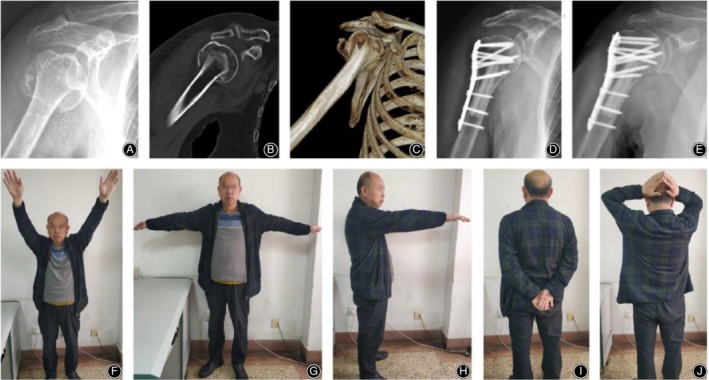
PHILOS fixation with fibular allograft in a 76‐year‐old male patient with a four‐part proximal humeral fracture on the right side. (A) X‐ray film before surgery; (B) CT before surgery; (C) 3D CT before surgery; (D) X‐ray film after surgery; (E) X‐ray film 3 months after surgery; (F–J) Function of patient's upper arm at the last follow‐up.
Figure 6.
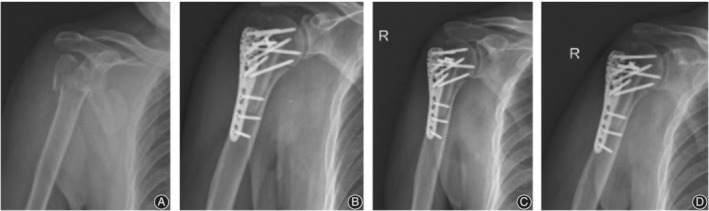
PHILOS fixation with fibular allograft in three patients with a four‐part proximal humeral fracture on the right side. (A) X‐ray film before surgery; (B) X‐ray film after surgery; (C) X‐ray film 1 month after surgery; (D) X‐ray film 3 months after surgery.
Figure 7.
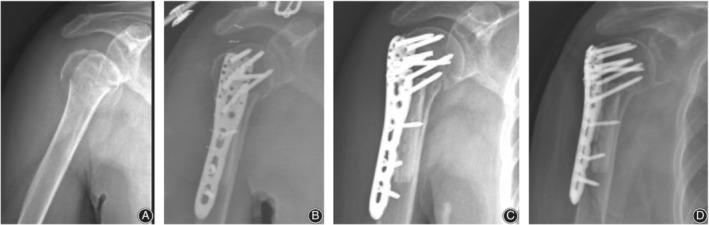
PHILOS fixation with fibular allograft in three patients with a four‐part proximal humeral fracture on the right side. (A) X‐ray film before surgery; (B) X‐ray film after surgery; (C) X‐ray film 1 month after surgery; (D) X‐ray film 3 months after surgery.
Figure 8.
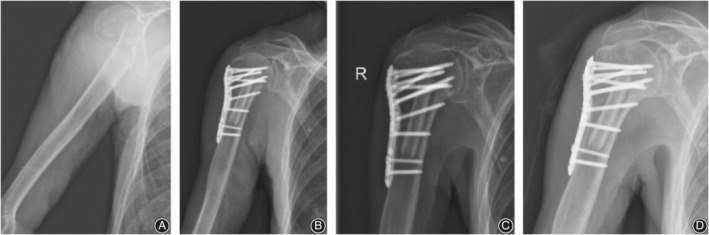
PHILOS fixation with fibular allograft in three patients with a four‐part proximal humeral fracture on the right side. (A) X‐ray film before surgery; (B) X‐ray film after surgery; (C) X‐ray film 1 month after surgery; (D) X‐ray film 3 months after surgery.
Discussion
PHF has been ranked as the third most frequent fracture and has a strong correlation with osteoporosis20. The management of these fractures depends on the vascular status, bone quality, fracture pattern, degree of commination, and patient factors. Non‐operative management is preferred for elderly patients and those with major comorbidities and for undisplaced fractures21. However, treating these fractures using non‐operative method requires a high level of patient compliance and it is associated with complications, like stiff shoulder and Sudeck's osteodystrophy22.
Although there are many surgical options available, PHILOS have consistently demonstrated biomechanical superiority over other forms of fixation in osteoporotic bone23, 24. Proponents of PHILOS fixation often cite better fixation, early mobilization, head preservation, restoration of range of motion, and satisfactory function as some of the major advantages of PHILOS construct25. But multiple studies have reported on the implant‐related complications associated with PHILOS fixation, most commonly, intra‐articular screw penetration, postoperative fracture displacement, and AVN26, 27, 28. A meta‐analysis of 12 studies with a total of 514 proximal humerus fractures treated with PHILOS fixation showed an overall complication rate of 49% and a 13.8% reoperation rate. The most common indication for reoperation involved intra‐articular screw perforation. The most common complications were varus malunion (16%), osteonecrosis (10%), intra‐articular screw penetration (8%), subacromial impingement (6%), and infection (4%)29.
The importance of reducing and maintaining the medial calcar to provide biomechanical support for a laterally placed plate has been recognized30, 31, 32. Researchers concluded that medial support screws played a key role in PHILOS fixation of displaced PHF, but a biomechanical study showed the addition of medial support screws had no effect on the stiffness of the medial cortex in cases with varus malunion33. In addition, a clinical study demonstrated that the place of calcar screws might lead to a high risk of humeral head necrosis34. Some investigators tried to use bone cement to strengthen the stability of PHILOS in PHF, and the data showed that a good clinical result with a decreasing complication rate35, but cement‐related heat injuries may exist36. Autologous bone grafting might be an alternative method for overcoming varus collapse. However, autologous bone grafting harvested from the patients themselves has some complications, such as vascular or neurologic injuries, deep infections at the donor site, and deep hematoma formation37.
Use of an endosteal strut allograft can re‐establish medial support, even in the comminuted osteoporotic bone commonly found in these patients. We considered that the fibula might be the most suitable donor bone for reconstructing the medial support in these types of fractures. Its length, geometrical shape, and mechanical strength might also be appropriate for these fractures. The cortical bone of the fibula provides immediate structural continuity and stability at the fracture site. When used as an intramedullary bone graft, it also has some osteogenic potential in addition to acting like a strut across the fracture site. Allograft is cancellous or corticocancellous chips or tricortical graft used as osteoconductive filler for metaphyseal defects. The fibular strut allograft may also minimize postoperative osteonecrosis by increasing the biomechanical strength of the construct and resisting a loss of reduction. Maintenance of reduction may permit revascularization of head pieces rendered ischemic at the time of injury. An important finding of this study is that the rate of varus malalignment and loss of HHH was significantly less when plate fixation was accompanied by a strut allograft.
The similarly good clinical outcomes in our study might be related with some factors. Fibular allograft used as volumetric filling in the bone void formed after reduction of humeral neck‐shaft angle could push the humeral head resistance to the force from the scapular fossa along with the screws, preventing the screw penetrating into the articular surface. Besides, this medial strut could prevent the varus placement of the head to diminish humeral head varus collapse and reduce the incidence of malunion. Anatomical medial strut with allograft bone has more potential to prevent humeral head varus displacement compared with the isolated fibula allograft. Anatomical allograft should be modified into a specific shape to fill the bone void according to the intramedullary geometry of the proximal humerus through computer virtual design with Pro‐E software. This kind of structural allograft provides enough medial stability and allows the formation of osteogenic tissue across a fracture site along with the surface of the allograft followed by bone formation. In addition, faster fracture healing could minimize articular segment AVN or collapse. Anatomical allograft is a plane contacted with the humeral head, and the support position could be pushed to the inferior medial point. However, isolated fibula was just a point‐to‐point support of the humeral head, and the support point is just at the line of extension of the intramedullary canal direction.
This study has several limitations. Firstly, it is not a randomized trial because the two techniques were performed at different times. Secondly, the study was limited by its retrospective design, which could introduce selection bias and the potential for confounding. Thirdly, although a large number of patients were included, this study had relatively low power to detect differences in forward elevation between the two groups. Subtle differences between the groups may not be identified in a single‐center study. A large‐scale, multicenter study would be required to investigate these clinical outcomes further.
Conclusions
In conclusion, the present results showed that that patients treated by PHILOS combined with fibular allograft had a better functional outcome and a lower complication rate comparted to patients treated by PHILOS alone. Suitable void filler in the proximal humerus for supporting the head fragment, medial cortical bone and greater tuberosity might play a key role in reducing the incidence of complications in elderly patients, especially with osteoporosis.
Acknowledgments
This study was supported by a grant from scientific Research Project of Health Department of Jiangsu Province (H201439). We obtained all patients' consent for publication of their medical data, including medical records, photographs, and images.
Yi‐min Qi and Lei Zhao are co‐first authors.
Disclosure: The authors declare that they have no competing interests.
Contributor Information
Bin Liang, Email: ib15366110173@163.com.
Chun‐zhi Jiang, Email: jcz604@163.com.
References
- 1. Vachtsevanos L, Hayden L, Desai AS, Dramis A. Management of proximal humerus fractures in adults. World J Orthop, 2014, 5: 685–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang MQ, Youssef T, Smerdely P. Incidence and outcomes of humeral fractures in the older person. Osteoporos Int, 2018, 29: 1601–1608. [DOI] [PubMed] [Google Scholar]
- 3. Cai M, Tao K, Yang C, Li S. Internal fixation versus shoulder hemiarthroplasty for displaced 4‐part proximal humeral fractures in elderly patients. Orthopedics, 2012, 35: 748. [DOI] [PubMed] [Google Scholar]
- 4. Chivot M, Lami D, Bizzozero P, Galland A, Argenson JN. Three‐ and four‐part displaced proximal humeral fractures in patients older than 70 years: reverse shoulder arthroplasty or nonsurgical treatment. J Shoulder Elbow Surg, 2019, 28: 252–259. [DOI] [PubMed] [Google Scholar]
- 5. Lee SH, Han SS, Yoo BM, Kim JW. Outcomes of locking plate fixation with fibular allograft augmentation for proximal humeral fractures in osteoporotic patients. Bone Joint J., 2019, 101‐B: 260–265. [DOI] [PubMed] [Google Scholar]
- 6. Rabi S, Evaniew N, Sprague SA, Bhandari M, Slobogean GP. Operative vs non‐operative management of displaced proximal humeral fractures in the elderly: a systematic review and meta‐analysis of randomized controlled trials. World J Orthop, 2015, 6: 838–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gönç U, Atabek M, Teker K, Tanrıöver A. Minimally invasive plate osteosynthesis with PHILOS plate for proximal humerus fractures. Acta Orthop Traumatol Turc, 2017, 51: 17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Poole WEC, Wilson DGG, Guthrie HC, et al 'Modern' distal femoral locking plates allow safe, early weight‐bearing with a high rate of union and low rate of failure: five‐year experience from a United Kingdom major trauma centre. Bone Joint J, 2017, 99‐B: 951–957. [DOI] [PubMed] [Google Scholar]
- 9. Matassi F, Angeloni R, Carulli C, et al Locking plate and fibular allograft augmentation in unstable fractures of proximal humerus. Injury, 2012, 43: 1939–1942. [DOI] [PubMed] [Google Scholar]
- 10. Ricchetti ET, Warrender WJ, Abboud JA. Use of locking plates in the treatment of proximal humerus fractures. J Shoulder Elbow Surg, 2010, 19 Suppl: 66–75. [DOI] [PubMed] [Google Scholar]
- 11. Frima H, Michelitsch C, Beks RB, Houwert RM, Acklin YP, Sommer C. Long‐term follow‐up after MIPO Philos plating for proximal humerus fractures. Arch Orthop Trauma Surg, 2019, 139: 203–209. [DOI] [PubMed] [Google Scholar]
- 12. Hardeman F, Bollars P, Donnelly M, Bellemans J, Nijs S. Predictive factors for functional outcome and failure in angular stable osteosynthesis of the proximal humerus. Injury, 2012, 43: 153–158. [DOI] [PubMed] [Google Scholar]
- 13. Neer CS 2nd. Four‐segment classification of proximal humeral fractures: purpose and reliable use. J Shoulder Elbow Surg, 2002, 11: 389–400. [DOI] [PubMed] [Google Scholar]
- 14. Neviaser AS, Hettrich CM, Beamer BS, Dines JS, Lorich DG. Endosteal strut augment reduces complications associated with proximal humeral locking plates. Clin Orthop Relat Res, 2011, 469: 3300–3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Acklin YP, Stoffel K, Sommer C. A prospective analysis of the functional and radiological outcomes of minimally invasive plating in proximal humerus fractures. Injury, 2013, 44: 456–460. [DOI] [PubMed] [Google Scholar]
- 16. Katz J, Melzack R. Measurement of pain. Surg Clin North Am, 1999, 79: 231–252. [DOI] [PubMed] [Google Scholar]
- 17. Richards RR, An KN, Bigliani LU, et al A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg, 1994, 3: 347–352. [DOI] [PubMed] [Google Scholar]
- 18. Dabija DI, Jain NB. Minimal clinically important difference of shoulder outcome measures and diagnoses: a systematic review. Am J Phys Med Rehabil, 2019, 98: 671–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Prodinger B, Hammond A, Tennant A, Prior Y, Tyson S. Revisiting the disabilities of the arm, shoulder and hand (DASH) and QuickDASH in rheumatoid arthritis. BMC Musculoskelet Disord, 2019, 20: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Launonen AP, Fjalestad T, Laitinen MK, et al Nordic Innovative Trials to Evaluate osteoPorotic Fractures (NITEP) Collaboration: The Nordic DeltaCon Trial protocol‐non‐operative treatment versus reversed total shoulder arthroplasty in patients 65 years of age and older with a displaced proximal humerus fracture: a prospective, randomised controlled trial. BMJ Open, 2019, 9: e024916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Koljonen AR, Fang C, Lau TW, et al Minimally invasive plate osteosynthesis for proximal humeral fractures. J Orthop Surg (Hong Kong), 2015, 23: 160–163. [DOI] [PubMed] [Google Scholar]
- 22. VL N, N B. Complex proximal humeral fracture fixation with PHILOS plate using Minimal Invasive Percutaneous Plate Osteosynthesis (MIPPO) technique: a series of 30 Patients. Malays Orthop J, 2018, 12: 20–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hettrich CM, Neviaser A, Beamer BS, Paul O, Helfet DL, Lorich DG. Locked plating of the proximal humerus using an endosteal implant. J Orthop Trauma, 2012, 26: 212–215. [DOI] [PubMed] [Google Scholar]
- 24. Foruria AM, Carrascal MT, Revilla C, Munuera L, Sanchez‐Sotelo J. Proximal humerus fracture rotational stability after fixation using a locking plate or a fixed‐angle locked nail: the role of implant stiffness. Clin Biomech, 2010, 25: 307–311. [DOI] [PubMed] [Google Scholar]
- 25. Olerud P, Ahrengart L, Ponzer S, Saving J, Tidermark J. Internal fixation versus nonoperative treatment of displaced 3‐part proximal humeral fractures in elderly patients: a randomized controlled trial. J Shoulder Elbow Surg, 2011, 20: 747–755. [DOI] [PubMed] [Google Scholar]
- 26. Schliemann B, Siemoneit J, Theisen C, et al Complex fractures of the proximal humerus in the elderly—outcome and complications after locking plate fixation. Musculoskelet Surg, 2012, 96: S3–S11. [DOI] [PubMed] [Google Scholar]
- 27. Micic ID, Kim KC, Shin DJ, et al Analysis of early failure of the locking compression plate in osteoporotic proximal humerus fractures. J Orthop Sci, 2009, 14: 596–601. [DOI] [PubMed] [Google Scholar]
- 28. Clavert P, Adam P, Bevort A, Bonnomet F, Kempf JF. Pitfalls and complications with locking plate for proximal humerus fracture. J Shoulder Elbow Surg, 2010, 19: 489–494. [DOI] [PubMed] [Google Scholar]
- 29. Sproul RC, Iyengar JJ, Devcic Z, Feeley BT. A systematic review of locking plate fixation of proximal humerus fractures. Injury, 2011, 42: 408–413. [DOI] [PubMed] [Google Scholar]
- 30. Jung WB, Moon ES, Kim SK, Kovacevic D, Kim MS. Does medial support decrease major complications of unstable proximal humerus fractures treated with locking plate. BMC Musculoskelet Disord, 2013, 14: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Krappinger D, Bizzotto N, Riedmann S, Kammerlander C, Hengg C, Kralinger FS. Predicting failure after surgical fixation of proximal humerus fractures. Injury, 2011, 42: 1283–1288. [DOI] [PubMed] [Google Scholar]
- 32. Lescheid J, Zdero R, Shah S, Kuzyk PRT, Schemitsch EH. The biomechanics of locked plating for repairing proximal humerus fractures with or without medial cortical support. J Trauma, 2010, 69: 1235–1242. [DOI] [PubMed] [Google Scholar]
- 33. Bai L, Fu Z, An S, Zhang P, Zhang D, Jiang B. Effect of calcar screw use in surgical neck fractures of the proximal humerus with unstable medial support: a biomechanical study. J Orthop Trauma, 2014, 28: 452–457. [DOI] [PubMed] [Google Scholar]
- 34. Osterhoff G, Ossendorf C, Wanner GA, Simmen HP, Werner CM. The calcar screw in angular stable plate fixation of proximal humeral fractures–a case study. J Orthop Surg Res, 2011, 6: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Egol KA, Sugi MT, Ong CC, Montero N, Davidovitch R, Zuckerman JD. Fracture site augmentation with calcium phosphate cement reduces screw penetration after open reduction‐internal fixation of proximal humeral fractures. J Shoulder Elbow Surg, 2012, 21: 741–748. [DOI] [PubMed] [Google Scholar]
- 36. Schliemann B, Wähnert D, Theisen C, et al How to enhance the stability of locking plate fixation of proximal humerus fractures? An overview of current biomechanical and clinical data. Injury, 2015, 46: 1207–1214. [DOI] [PubMed] [Google Scholar]
- 37. Chen H, Ji X, Zhang Q, Liang X, Tang P. Clinical outcomes of allograft with locking compression plates for elderly four‐part proximal humerus fractures. J Orthop Surg Res., 2015, 10: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]


