Summary
Nuclear interferon‐inducible protein 16 (IFI16) and anti‐IFI16 antibodies have been detected in subjects with several rheumatic diseases, often correlating with disease severity, and in this study we investigated their prevalence and clinical associations in psoriatic arthritis (PsA) compared to psoriasis (Pso). We tested sera and synovial fluids of patients with PsA for IFI16 protein levels by capture enzyme‐linked immunosorbent assay (ELISA) and for anti‐IFI16 immunoglobulin (Ig)G and IgA by ELISA, protein radio‐immunoprecipitation and immunoprecipitation‐Western blot of IgG. Sera from patients with Pso and healthy subjects were used as controls, and in a subgroup of patients with PsA we also studied sera after treatment with etanercept. IFI16 was detectable in the sera of 66% of patients with Pso, 46% with PsA and 19% of controls. Among PsA cases, 51% of IFI16‐positive cases had elevated levels of C‐reactive protein (CRP) compared to 31% of patients with undetectable IFI16. Anti‐IFI16 of both IgG and IgA isoforms were detected with significantly higher frequency in PsA and Pso compared to healthy controls, with higher IgG titres in patients with elevated C‐reactive protein (CRP) (P = 0·015). Immunoprecipitation confirmed the presence of anti‐IFI16 IgG antibodies and these preferentially recognized epitopes outside the N‐terminus of the protein. Lastly, IFI16 was detected in one of seven and anti‐IFI16 in three of seven synovial fluids from patients with PsA. Therefore, IFI16 and anti‐IFI16 are detectable in serum and synovial fluid of PsA patients, especially in cases of elevated CRP.
Keywords: IFI16, anti‐IFI16 antibodies, psoriatic arthritis
IFI16 and anti‐IFI16 are associated with higher disease activity and treatment response in psoriatic arthritis patients.
Introduction
Psoriatic arthritis (PsA) is a chronic inflammatory disease within the broader psoriatic disease encompassing musculoskeletal features (with enthesitis, synovitis and erosion with osteitis of the peripheral joints and/or the axial skeleton), skin and nail manifestations 1, estimated to affect approximately 0·5% of the general population 2. PsA is found in a variable proportion (15–40%) of the patients with psoriasis (Pso), and in 74% of cases it is preceded by skin manifestations 3.
Differing from rheumatoid arthritis (RA), both PsA and Pso lack serum biomarkers for an early diagnosis, while sharing a T cell‐mediated response based on the tumour necrosis factor (TNF)‐α/interleukin (IL)‐17 axis 4. As reported for other immune‐mediated diseases 5, an interferon (IFN) signature is observed in Pso, as self‐DNA‐engulfed plasmacytoid dendritic cells (pDC) produce a large amount of IFN‐α 6. Further, over‐expression of IFN‐γ and TNF‐α‐inducible genes is observed in Pso plaques due to T cell and macrophage recruitment and activation 7. Despite these observations, treatments targeting IFN‐α or IFN‐γ have proved ineffective in Pso, while TNF‐α and IL‐12/‐23 or IL‐17 blockers lead to clear skin in a large proportion of patients 8, 9.
The interferon‐inducible protein 16 (IFI16) is a DNA sensor involved in the inflammasome‐mediated defence against viral infections 10 that also displays proinflammatory and anti‐angiogenetic effects 11. In the case of psoriatic disease, IFI16 is over‐expressed in the affected skin 12, peripheral blood mononuclear cells and synovial tissues 13, 14. Previous studies have demonstrated that IFI16 is over‐expressed in different connective tissue diseases and in RA 5, and anti‐IFI16 antibodies have been detected in systemic lupus erythematosus (SLE) 15, Sjӧgren’s syndrome 16, 17, systemic sclerosis 18, 19, 20, inflammatory bowel diseases 21 and RA 22, possibly associated with disease severity and progression.
In this study, we report for the first time, to our knowledge, that IFI16 and anti‐IFI16 antibodies are detectable in the sera and synovial fluids of patients with PsA and may represent disease activity biomarkers.
Patients and methods
Patients
We investigated 158 consecutive patients with a diagnosis of PsA based on the CASPAR criteria 23 (Table 1), while 182 healthy subjects and 44 patients with skin or nail Pso but no evidence of PsA were used as controls. Serum samples were collected and stored at −20°C until used; demographic and clinical data (presence or absence of psoriasis, presence or absence of axial involvement and reported blood tests performed by the patient within 1 month) were recorded. Disease activity score 28 joint (DAS28) was available only for 30 PsA patients treated with biological agents.
Table 1.
Demographic, clinical and serological characteristics of PsA patients
| All PsA | IFI16 | Anti‐IFI16 IgG | Anti‐IFI16 IgA | ||||
|---|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | Positive | Negative | ||
| Patients (%) | 158 (100) | 23 (15) | 135 (85) | 57 (36) | 101 (64) | 26 (17) | 132 (83) |
| Women (%) | 93 (50·5) | 12 (52) | 67 (50) | 34 (60) | 45 (45) | 11 (42) | 68 (52) |
| Age, years (IQR) | 50 (43–61) | 51 (42–69) | 50 (43–60) | 49 (43–61) | 51 (43–60·5) | 63·5 (49–69) | 48 (42–58) ** |
| Disease duration, months (IQR) | 60 (16–119) | 82 (24–118) | 49 (15–120) | 72 (18–119·5) | 48 (15–118) | 60 (18–96) | 56 (16–132) |
| Disease duration < 12 months (%) | 33 (29) | 1 (4·3) | 32 (24) * | 8 (14) | 10 (10) | 2 (8) | 16 (12) |
| Disease duration > 60 months (%) | 76 (49) | 14 (61) | 62 (47) | 33 (60) | 43 (43) | 14 (54) | 62 (47) |
| Psoriasis (%) | 127 (80) | 17 (74) | 109 (81) | 45 (79) | 82 (81) | 24 (92) | 102 (77) |
| Axial involvement, (%) | 45 (29) | 7 (30) | 38 (29) | 16 (28) | 29 (29) | 4 (15) | 41 (31) |
| High CRP (%) | 63 (40) | 18 (78) | 45 (33) * | 23 (40) | 40 (40) | 12 (46) | 51 (39) |
| Ongoing treatments | |||||||
| No treatment | 64 (40·5) | 17 (74) | 77 (57) | 21 (39) | 43 (43) | 9 (35) | 55 (42) |
| Methotrexate | 64 (40·5) | 15 (71) | 49 (46) | 21 (37) | 43 (43) | 14 (54) | 50 (38) |
| Median MTX dose, weekly | 15·2 (range 12·5–17·5) | 12·7 (range 12·5–15) | 15·2 (range 12·5–17·5) | 15·2 (range 12·5–17·5) | 15·2 (range 12·5–17·5) | 12·7 (range 12·5–15) | 15·7 (range 15–17·5) |
| Anti‐TNF‐α | 30 (19) | 2 (9) | 28 (21) | 15 (26) | 15 (15) | 3 (12) | 27 (20) |
| Etanercept | 8 | 1 | 7 | 4 | 4 | 1 | 7 |
| Adalimumab | 2 | 0 | 2 | 1 | 1 | 0 | 2 |
| Infliximab | 20 | 1 | 19 | 9 | 11 | 2 | 18 |
Continuous variables are expressed as median and interquartile range (IQR) = binary as numbers and percentages.
PsA = psoriatic arthritis; IFI16 = interferon‐inducible protein 16; Ig = immunoglobulin; CRP = C‐reactive protein; TNF = tumour necrosis factor; MTX = methotrexate.
P < 0·01 positive versus negative patients,
P < 0·001 positive versus negative patients.
Values for significant associations are displayed in bold.
In a subgroup of eight patients with PsA, serum samples were also available at 3 months after starting weekly subcutaneous etanercept 50 mg. Synovial fluid samples from seven patients with PsA who underwent arthrocentesis for knee effusion and had not received intra‐articular medications (e.g. corticosteroids, hyaluronic acid) in the previous 6 months were also analysed (blood samples were collected on the same day). Synovial fluids were centrifuged and supernatants stored at −80°C until use. Clinical and serological records were collected at the time of enrolment. This study was approved by the local Institutional Review Board and written informed consents were obtained from patients and controls.
Determination of extracellular IFI16 protein by capture enzyme‐linked immunosorbent assay
A capture ELISA was employed for determination of circulating extracellular IFI16 protein following a procedure described elsewhere 24, and the threshold cut‐off value was defined as the 95th percentile of healthy controls as 27 ng/ml.
Determination of antibody titres towards human recombinant IFI16 by ELISA
To determine anti‐IFI16 antibody titres of IgG and IgA isotype in sera of patients, we performed in‐house ELISA as previously described 21. Accordingly, cut‐off values were calculated as the 95th percentile of healthy controls and the threshold values were set to 113 U/ml and 9·6 U/ml for IgG and IgA isotype, respectively.
Indirect immunofluorescence assay
The localization of cellular antigens recognized by autoantibodies was tested by indirect immunofluorescence (IIF) on HEp‐2 cells (Inova Diagnostics, San Diego, CA, USA) using a 1 : 80 dilution of human sera of patients and controls, followed by secondary antibodies marked with fluorochrome [AlexaFluor488 AffiniPure F(ab')2 fragment goat anti‐human IgG, Fcγ fragment‐specific; Jackson Immunoresearch Europe Ltd, Ely, UK], as previously described 25. Samples were acquired on an Olympus BX53 Upright fluorescence microscope.
Radioimmunoprecipitation assay
PsA sera were analysed by protein radio‐immunoprecipitation (IP) using marked 35S‐ HeLa cell extract. IP was used to identify autoantibodies directed against protein self‐antigens, as described elsewhere 26; briefly, sera were incubated with protein A sepharose (PAS) beads and after serial washes samples have been incubated with cell lysate (radioactively marked for protein‐IP). After forming immunocomplexes, samples were prepared for 8% sodium dodecyl sulphate‐polyacrylamide (SDS‐PAGE) electrophoresis for protein‐IP. To confirm data obtained with IP we performed IP‐Western blotting. In detail, human sera and 50 ng of mouse monoclonal anti‐human IFI16 as a positive control were cross‐linked with PAS beads to isolate human IgG directed against IFI16 27. IP was initially performed with cell extract from a 5 × 106 HeLa cells/sample, but as human foreskin fibroblast (HFF) expresses IFI16 at higher basal levels than HeLa, a control experiment was performed with HFF. Proteins were then fractionated by 8% SDS‐PAGE and transferred to a nitrocellulose membrane, probed with 1 : 500 of mouse monoclonal anti‐human IFI16 antibody (Novus Biologicals, Littleton, CO, USA) for band 88 kDa identification, followed by horseradish peroxidase (HRP)‐goat anti‐mouse IgG (1 : 10000 dilution; ThermoFisher, Waltham, MA, USA). Development was performed by Immobilon Western Chemiluminescent HRP substrate (Millipore, Darmstadt, Germany) and acquired using ChemiDoc (Bio‐Rad, Hercules, CA, USA).
Recombinant IFI16 domains
The coding region of the three IFI16 domains was amplified from full‐length human IFI16 (isoform b) cDNA using primers containing BamHI and XhoI restriction sites (DAPIN – forward: 5′‐GGCGGATCCATGGGAAAAAAATACAAGAAC‐3′, reverse: 5′‐GCCTCGAGTCATTTTAACTTTTCTTTTTTAAG‐3′; HINA – forward: 5′‐GGCGGATCCCCGAAAACAGTGGCCAAATG‐3′, reverse: 5′‐GCCTCGAGTCATTTCTTTATCTGGATAAAACTA‐3′; HINB – forward: 5′‐GGCGGATCCGAACCTGAAGAAGTTTCCATA‐3′, reverse: 5′‐GCCTCGAGTCAGATGACCTTGATGTGACTATG‐3′). The amplified inserts were digested with BamHI and XhoI and cloned in pET30a expression vector containing an N‐terminal histidine tag (Novagen, Madison, WI, USA). Expression and affinity purification of the recombinant proteins were performed according to standard procedures, and purity assessed by 12% SDS‐PAGE.
IFI16 epitope mapping
IFI16 protein domains were diluted in phosphate‐buffered saline (PBS) to a concentration of 2 μg/ml. Polystyrene microwell plates (Nunc‐Immuno Maxisorp/Thermo Scientific, Nunc, Roskilde, Denmark) were coated with 100 µl diluted protein per well and incubated overnight at 4°C. After blocking, sera were added in duplicate. After washing, HRP‐conjugated rabbit anti‐human IgG (DakoCytomation, Glostrup, Denmark) was added. Following the addition of the substrate (tetramethylbenzidine, KPL), absorbance was measured at 450 nm using a microplate reader (SpectraCount, Packard BioScience, Meriden, CT). The background reactivity of the reference mixture was subtracted to calculate the results. Cut‐off values were calculated as the 95th percentile of the absorbance of healthy controls and the threshold values were set to 0·395 for DAPIN, 0·900 for HINA and 0·776 for HINB.
Statistical analysis
All data analyses were performed using stata for Macintosh version 13.1 (StataCorp, College Station, TX, USA). As detailed throughout, χ2 test, Mann–Whitney U‐test, Kruskal‐Wallis test with Dunn’s tests for multiple comparison post hoc or Wilcoxon’s test were employed to compare groups based on the data distribution. Linear regression and Spearman’s test were used to correlate IFI16 and anti‐IFI16 levels with continuous variables such as disease duration and age. C‐reactive protein (CRP) absolute values were not comparable due to different cut‐offs between laboratories; therefore, we used the single cut‐off of each centre to discriminate high CRP levels.
Multivariate analysis adjusted for demographic and clinical data (age, sex and disease duration) was also performed. All comparisons were two‐tailed and P‐values below 0·05 were considered statistically significant.
Results
Circulating IFI16 in different disease groups
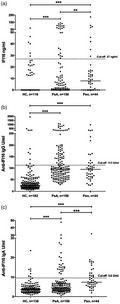
The levels of IFI16 were significantly higher in PsA and Pso patients compared to healthy controls (PsA median = 0, range = 0–131 versus healthy controls 0, 0–67·6, P = 0·0004; Pso 7·93, 0–223·5 versus healthy controls P < 0·0001; Fig. 1a). Moreover, IFI16 concentrations were higher in Pso patients compared to PsA (P = 0·0006). According to the established 27 ng/ml threshold, 23 of 158 (15%) of PsA and eight of 44 (18%) Pso sera were positive for circulating IFI16, compared to six of 116 (5%) healthy controls (PsA versus healthy controls, P = 0·002; Pso versus healthy controls, P = 0·003), confirming a higher prevalence of free IFI16 circulating protein in PsA and Pso patients in comparison with healthy controls.
Figure 1.

Serum interferon‐inducible protein 16 (IFI16) and anti‐IFI16 immunoglobulin (Ig)G and IgA levels in patients with psoriatic arthritis (PsA), psoriasis and healthy controls (HCs). (a) IFI16 protein levels in patients’ and controls’ sera were determined using an in‐house capture enzyme‐linked immunosorbent assay (ELISA). Each dot represents the concentration of IFI16 protein (expressed in ng/ml on a linear scale) in each individual subject. The horizontal bar represents the median values. Values over the dotted line indicate the percentage of subjects with IFI16 protein levels above the cut‐off value (27 ng/ml). (b) Serum IgG and (c) IgA specific for IFI16 were quantified by ELISA in HC and patients suffering from psoriatic arthritis and psoriasis. Each dot represents the autoantibody level for each subject sample expressed in arbitrary units on a linear scale. The horizontal bars in each group represent the median values. Values over the dotted line indicate the percentage of subjects with antibody titres above the cut‐off value (113 U/ml for anti‐IFI16 IgG and 9·6 U/mL for anti‐IFI16 IgA, calculated as the 95th percentile of the control population). Statistical significance: ***P < 0·0001 (Mann–Whitney U‐tests).
Circulating IFI16 and PsA clinical features at baseline
The clinical features of the PsA cases included disease duration, peripheral or axial involvement and skin disease analysed according to the positivity/negativity for IFI16 protein and are illustrated in Table 1. We identified a significant association between elevated CRP levels and the presence of the circulating IFI16 protein (P < 0·0001). We subsequently evaluated whether IFI16 serum concentration, rather than its presence, could be correlated to any clinical variable. As expected, IFI16 levels were significantly higher in PsA cases with high CRP levels (median = 23·5 ng/ml, IQR = 10·4–65·4) compared with patients with low CRP levels (2·6 ng/ml, IQR = 1·4–10·6; P < 0·001). IFI16 levels were also higher in PsA cases with Pso compared to PsA cases without Pso, albeit not significantly (11·4 ng/ml, IQR = 2·6–31·9 versus 6·4 ng/ml, IQR = 1·8–67·6). No differences in IFI16 levels were observed with regard to ongoing therapies. We further performed a multivariate analysis with prespecified confounders, and this failed to identify statistically significant differences after adjustment for age, sex and disease duration.
Circulating IFI16 and PsA response to etanercept
Samples at 3 months of etanercept treatment were also available for eight subjects positive for IFI16 at baseline, and we could observe a decrease in IFI16 levels in five of eight patients (baseline median = 81·7 ng/ml, IQR = 63·2–102·1 versus 31·8 ng/ml, 22·1–85 at 3 months; P = 0·16). Similarly, according to DAS28‐CRP, disease activity reached a minimal clinically significant reduction in four of eight IFI16‐positive patients, with a median reduction of 1·25 points. Lastly, circulating IFI16 was measured in the synovial fluid of seven PsA patients and detected in one case at low concentration (data not shown).
Circulating anti‐IFI16 IgG and IgA and PsA clinical features at baseline
Significantly higher levels of anti‐IFI16 IgG antibodies were present in PsA and Pso patients in comparison with healthy controls (PsA median = 96, IQR = 65·3–151·7 versus healthy controls 28·9, IQR = 17·3–45·3, P < 0·001; Pso 94·9, 62·8–126 versus healthy controls, P < 0·001), while the titres were comparable between PsA and Pso sera. According to the cut‐off value of 113 U/ml, 57 of 158 (36%) PsA and 14 of 44 (32%) Pso sera were positive for anti‐IFI16 IgG, compared to 11 of 182 (6%) of the healthy controls (P < 0·001 versus PsA; P < 0·001 versus Pso; Fig. 1b).
When the autoantibody status was analysed according to sex, age, disease duration, skin, axial involvement or ongoing therapy (Table 1), we observed a significant correlation between anti‐IFI16 IgG levels and disease duration after adjusting for age (beta coefficient 0·38; P = 0·036). Moreover, by considering anti‐IFI16 IgG serum concentrations, anti‐IFI16 IgG titres were increased in subjects with high CRP levels compared with subjects with low CRP levels (median = 268·1 U/ml, IQR = 162·8–562·4 versus 162 U/ml, 142·1–199·7; P = 0·015). However, there were no differences in the percentage of patients with high CRP and anti‐IFI16 positive versus negative subjects (Table 1).
Circulating anti‐IFI16 IgG and IgA in different disease groups
Median anti‐IFI16 IgA titres were increased in PsA and Pso sera compared to healthy controls (PsA = 4·7, IQR = 3·3–7·1 versus healthy controls = 3·7, 2·5–5·2, P < 0·0001; Pso 7·4, 0–16·5 versus healthy controls, P < 0·0001); moreover, anti‐IFI16 IgA were higher in Pso compared to PsA sera (P < 0·0001). According to the 9·6 U/ml threshold, 25 of 158 (16%) of PsA and 16 of 44 (36%) of Pso sera were positive, compared to seven of 138 (5%) of healthy controls (P < 0·001 versus PsA; P < 0·001 versus Pso; P = 0·004 PsA versus Pso) (Fig. 1c). As reported in Table 1, anti‐IFI16 IgA‐positive PsA cases were significantly older compared to anti‐IFI16 IgA‐negative (median age = 63·5, IQR = 49–69 versus 48·5, IQR = 42–58; P < 0·001).
Circulating anti‐IFI16 IgG and IgA and PsA response to etanercept
Six subjects positive for anti‐IFI16 IgG at baseline were tested also during etanercept therapy without significant changes in autoantibody titres after 3 months of therapy (baseline median = 197 U/ml, IQR = 188–269 versus 3 months 223 U/ml, 194–269; P = 0·9). Of these, four of six (67%) did not reach a minimal clinically significant reduction of DAS28‐CRP and the median DAS28 change was –0·85 after 3 months of therapy. Four subjects positive for anti‐IFI16 IgA were subsequently tested after 3 months of etanercept therapy without significant changes in antibody titres (basal median = 7·56, IQR = 5·18, 12·72 versus 3 months 6·55, 5·46–12·6; P = 0·2), while three of four (75%) of these reached a minimal clinically significant reduction of DAS28, with a median DAS28 change of –2·25 (data not shown).
Synovial fluid anti‐IFI16 IgG and IgA and PsA clinical features
Anti‐IFI16 IgG and IgA antibodies were measured also in synovial fluids of seven PsA patients (data not shown). Three of seven samples were positive for IgG subtype and two of seven were positive for IgA subtype, and only one patient was positive for both Ig subtypes. We failed to detect correlations between antibody titres and any of the clinical parameters analysed.
Anti‐IFI16 confirmation tests
Protein‐IP and IP‐WB were used to confirm the presence of anti‐IFI16 IgG antibodies, as these techniques are considered the gold standard for the identification of autoantibodies due to their high sensitivity and specificity. As shown in Fig. 2a, we performed protein‐IP on 19 cases and identified an IP pattern of PsA sera recognizing a 90‐kDa protein (black arrows, Fig. 2a). This band corresponds to the IFI16 protein, as shown by IP‐WB (Fig. 2b). IIF showed a nuclear fluorescence that seems in accordance with the nuclear prevalent IFI16 function (Fig. 2c).
Figure 2.

Analysis of protein component of autoantigen interferon‐inducible protein 16 (IFI16) [8% sodium dodecyl sulphate‐polyacrylamide (SDS‐PAGE)] by radio‐immunoprecipitation (IP) (a), IP‐Western blotting (WB) (b) and indirect immunofluorescence (c). The IP pattern of psoriatic arthritis (PsA) sera recognizing a 90 kDa protein (black arrows, a). IP‐WB confirming the identity of the IP bands corresponding to IFI16 (b). The nuclear pattern for a representative sample positive for ani‐IFI16 IgG in IIF (larger image, c) compared to an anti‐IFI16‐negative patient (small image in the upper right side, c) and to a normal subject (small image in the bottom right side, c).
Circulating IFI16 and anti‐IFI16 IgG and IgA in the study populations
No correlations were found between IFI16 and anti‐IFI16 IgG or IgA levels when IFI16 and anti‐IFI16 data were combined. However, we note that among the 57 PsA patients positive for anti‐IFI16 IgG, 13 (23%) were positive also for circulating IFI16. In contrast, 57% of the 23 patients positive for IFI16 were also positive for IgG autoantibodies (Table 2). Among the 25 PsA patients positive for anti‐IFI16 IgA, five (20%) were positive for the circulating protein. A significant correlation between anti‐IFI16 IgG and IgA antibody titres was observed in PsA (Spearman’s r = 0·1802, P = 0·0235). Moreover, while only 21% of the 57 PsA patients positive for anti‐IFI16 IgG were also positive for the IgA subtype, 48% of 25 IgA‐positive patients also displayed high titres of anti‐IFI16 IgG antibodies.
Table 2.
Association of IFI16‐based biomarkers in the PsA cohort
| PsA, n = 158 | IFI16‐positive, n = 23 (15%) | Anti‐IFI16 IgG‐positive, n = 57 (36%) | Anti‐IFI16 IgA‐positive, n = 25 (16%) |
|---|---|---|---|
| IFI16‐positive, n = 23 (15%) | – | 13/57 (23%) | 5/25 (20%) |
| Anti‐IFI16 IgG‐positive, n = 57 (36%) | 13/23 (57%) | – | 12/25 (48%) |
| Anti‐IFI16 IgA‐positive, n = 25 (16%) | 5/23 (22%) | 12/57 (21%) | – |
Variables are expressed as numbers (n) and percentages.
PsA = psoriatic arthritis; IFI16 = interferon‐inducible protein 16; Ig = immunoglobulin.
Anti‐IFI16 IgG epitope mapping
To determine whether anti‐IFI16 IgG antibodies detected in sera were directed against specific IFI16 domains, we cloned the DAPIN (spanning from aa 1 to 88), HINA (from aa 131 to 337) and HINB (from aa 506 to 705) domains of IFI16 in a pET30a expression vector. The purity and specificity of the recombinant domains were then established by blue Coomassie and Western blot analyses, and finally recombinant DAPIN (rDAPIN), rHINA and rHINB were used as immobilized surfaces in ELISA for testing the sera of the 57 patients positive for anti‐IFI16 IgG. As shown in Fig. 3, significantly higher anti‐DAPIN, anti‐HINA and anti‐HINB absorbance were detected in anti‐IFI16‐positive than in anti‐IFI16‐negative samples. However, with cut‐off levels for each domain corresponding to the 95th percentile of the distribution in the control population, 40% of anti‐IFI16‐positive patients were positive for HINA and 28% were also positive for HINB when compared to anti‐IFI16‐negative patients (12 and 7%, respectively; P < 0.0001 and P = 0·007, χ2 with Yates’ correction). In contrast, only 19% of the samples were positive for both anti‐IFI16 and anti‐DAPIN (versus 8%, P = 0·063). These data suggest that anti‐IFI16 IgGs are mainly directed against epitopes outside the N‐terminus of the protein.
Figure 3.

Epitope mapping for anti‐interferon‐inducible protein 16 (IFI16) immunoglobulin (Ig)G. To determine the target of the anti‐IFI16 IgG antibodies found in the sera of psoriatic arthritis (PsA) patients, the DAPIN (spanning from aa 1 to 88), HINA (from aa 131 to 337) and HINB (from aa 506 to 705) domains of IFI16 were purified as recombinant peptides and 2 μg/ml each used to perform enzyme‐linked immunosorbent assay (ELISA). Each dot represents the value of the absorbance at 450 nm for 158 sera from study patients (PsA) and 101 healthy controls (CTRL) tested against the three domains. The horizontal bars in each scatter represent the median values. Values over the dotted line indicate the percentage of subjects with antibody titres above the cut‐off value calculated as the 95th percentile of the control population (0·395, 0·900 and 0·776 for DAPIN, HINA and HINB, respectively). Statistical significance: ***P < 0·0001, **P < 0·001, *P < 0·05 (Mann–Whitney U‐tests).
Discussion
A significant role of DNA sensing peptides in the pathogenesis of Pso has been recently suggested, largely based on data concerning LL37, a fragment of the anti‐microbial protein cathelicidin, capable of activating dendritic cells by complexing self‐DNA, and T cells by acting as a self‐antigen 28, 29. Similar to other autoimmune diseases characterized by serum reactivity to intracellular antigens, we cannot speculate on the pathogenetic mechanisms linking IFI16 autoantibodies to tissue damage. However, we suggest that the DNA sensor IFI16 is involved as a self‐antigen in the humoral response and in the inflammatory response possibly via the inflammasome in PsA.
In the present study, we observed that IFI16 and anti‐IFI16 seem to be associated with higher disease activity in PsA and possibly with response to anti‐TNF‐α treatment, thus possibly being candidate biomarkers of this seronegative condition and representing a different clinical phenotype. Furthermore, these markers are also detected in the synovial fluids for the first time, and may thus represent a mechanistic player in the PsA pathogenesis. These findings may be promising in filling the diagnostic gap of PsA where no serum biomarker for the diagnosis or management is available 30.
IFI16 is an IFN‐induced protein and a DNA sensor able to start an innate immune response against pathogenic microorganism by activating the inflammasome and the production of type I IFNs 31. It has been proposed that the large amount of self‐DNA secondary to keratinocyte proliferation in Pso plaques can activate pDC via LL37/Toll‐like receptor (TLR)‐9 to produce IFN‐α and recruit T cells and, at the same time, can activate the inflammasome with IL‐1β production 31, 32. The over‐expression of IFI16 in keratinocytes could be explained by an attempt to control excessive cell proliferation, resulting, however, in a chronic activation of the inflammasome. Furthermore, extracellular IFI16 can spread inflammation, as it has been demonstrated that the protein is able to bind the membrane of endothelial cells and to induce the production of proinflammatory cytokines in these cells 24, 33. The hypothesis, based on an accelerated cell turnover, seems to be supported by the high IFI16 levels observed in patients with Pso and in PsA with skin PsO and IFI16 over‐expression in PsA circulating mononuclear cells and synovial tissue 13. In fact, in PsA sera IFI16 is significantly higher compared to healthy controls, particularly when associated with an active disease represented by elevated CRP, and mechanisms may include changes similar to that observed in systemic lupus erythematosus models 34 or NETosis in a self‐perpetuating fashion 35.
Type I IFN inducible genes are elevated in the serum of patients with different systemic autoimmune diseases, albeit with inconclusive evidence and frequently conflicting results in different rheumatic conditions 36. IFI16 is among the five most important IFN inducible genes being over‐expressed in connective tissue diseases and RA, raising the coined ‘IFN‐signature’ 5. Different from PsA, in which IFI16 significantly correlates with inflammation, this protein is not a biomarker of disease activity in RA, but is associated with pulmonary involvement and with the presence of rheumatoid factor and anti‐cyclic citrullinated peptide antibodies. IFI16 in RA synovial fluid is not associated with joint disease activity, disease duration or erosion 22. In the case of SLE, IFI16 is associated with disease activity, particularly lupus nephritis, and is found over‐expressed in skin lesions 18, 37, 38.
The inflammatory context in which an intracellular protein is released is thought to be one of the mechanisms leading to the breakdown of tolerance and the recognition of self‐antigens; this hypothesis could explain the antibody response against IFI16 observed in PsA and Pso, particularly with the intriguing observation of a prevalent IgA response compared to IgG response in PsO versus PsA. As previously mentioned, and different from PsA (with anti‐IFI16 IgG titres slightly increased in subjects with elevated CRP levels), anti‐IFI16 antibodies are not correlated to RA activity, but rather with serum autoantibody positivity, while no association between anti‐IFI16 and disease activity or erosions has been found 22. In SLE, anti‐IFI16 antibodies were first reported to be associated with anti‐double‐stranded DNA antibodies 17, 18, but this was not confirmed by our group 15. Anti‐IFI16 antibody‐positive patients with systemic sclerosis were more likely to have limited cutaneous systemic sclerosis, a longer disease duration and more severe vascular and pulmonary manifestations, i.e. digital ischaemia and a low carbon monoxide diffusing capacity 18, 20. An association with disease severity, as for PsA, has been documented in Sjӧgren’s syndrome, with lower tear and saliva production, higher focus score in minor salivary glands biopsies, germinal centre‐like structures in the labial salivary gland lymphocytic infiltrates and higher IgG and anti‐nuclear antibody levels being associated with anti‐IFI16 16, 17. The epitope mapping also provided somewhat surprising evidence compared to other rheumatic conditions. In systemic sclerosis, anti‐IFI16 reacted with either N‐ or C‐terminal fragments or both 18, while in 70% of Sjӧgren’s syndrome anti‐IFI16 were directed against an epitope outside the N‐terminus 17 and in SLE antibodies are directed predominantly against N‐terminus 17. We can hypothesize that, in SLE, anti‐IFI16 blocking the N‐terminus allows the formation of self‐DNA/IFI16 complexes which favour nucleic acid clearance and are protective in terms of SLE nephritis. Our data in PsA are in agreement with those obtained in Sjӧgren’s syndrome, as the DAPIN domain was poorly detected by anti‐IFI16 IgG. Accordingly, this would have left free the moiety of the protein mainly involved in the inflammatory activity.
We are aware of the limitations of our study, including the limited clinical data available, but we report for the first time, to our knowledge, that IFI16 and anti‐IFI16 are detectable in the sera of patients with PsA and seem to correlate with the degree of inflammation and possibly with treatment response. We cannot exclude a role of this protein and the elicited antibody in the pathogenesis of PsA; moreover, IFI16 and anti‐IFI16 may represent new candidate biomarkers for PsA diagnosis and monitoring when data are confirmed in larger and longitudinal studies.
Disclosures
The authors have no competing interests to declare.
References
- 1. Sakkas LI, Bogdanos DP. Are psoriasis and psoriatic arthritis the same disease? The IL‐23/IL‐17 axis data. Autoimmun Rev 2017; 16:10–5. [DOI] [PubMed] [Google Scholar]
- 2. Ogdie A, Weiss P. The epidemiology of psoriatic arthritis. Rheum Dis Clin North Am 2015; 41:545–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barnas JL, Ritchlin CT. Etiology and pathogenesis of psoriatic arthritis. Rheum Dis Clin North Am 2015; 41:643–63. [DOI] [PubMed] [Google Scholar]
- 4. Marinoni B, Ceribelli A, Massarotti MS, Selmi C. The Th17 axis in psoriatic disease: pathogenetic and therapeutic implications. Auto Immun Highlights 2014; 5:9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Higgs BW, Liu Z, White B et al Patients with systemic lupus erythematosus, myositis, rheumatoid arthritis and scleroderma share activation of a common type I interferon pathway. Ann Rheum Dis 2011; 70:2029–36. [DOI] [PubMed] [Google Scholar]
- 6. Boehncke WH. Etiology and pathogenesis of psoriasis. Rheum Dis Clin North Am 2015; 41:665–75. [DOI] [PubMed] [Google Scholar]
- 7. Yao Y, Richman L, Morehouse C et al Type I interferon: potential therapeutic target for psoriasis? PLOS ONE 2008; 3:e2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bissonnette R, Papp K, Maari C et al A randomized, double‐blind, placebo‐controlled, phase I study of MEDI‐545, an anti‐interferon‐alfa monoclonal antibody, in subjects with chronic psoriasis. J Am Acad Dermatol 2010; 62:427–36. [DOI] [PubMed] [Google Scholar]
- 9. Harden JL, Johnson‐Huang LM, Chamian MF, Lee E, Pearce T, Leonardi CL, Haider A, Lowes MA, Krueger JG. Humanized anti‐IFN‐gamma (HuZAF) in the treatment of psoriasis. J Allergy Clin Immunol 2015; 135:553–6. [DOI] [PubMed] [Google Scholar]
- 10. Dell’Oste V, Gatti D, Giorgio AG, Gariglio M, Landolfo S, De Andrea M. The interferon‐inducible DNA‐sensor protein IFI16: a key player in the antiviral response. New Microbiol 2015; 38:5–20. [PubMed] [Google Scholar]
- 11. Caposio P, Gugliesi F, Zannetti C et al A novel role of the interferon‐inducible protein IFI16 as inducer of proinflammatory molecules in endothelial cells. J Biol Chem 2007; 282:33515–29. [DOI] [PubMed] [Google Scholar]
- 12. Tervaniemi MH, Katayama S, Skoog T et al NOD‐like receptor signaling and inflammasome‐related pathways are highlighted in psoriatic epidermis. Sci Rep 2016; 6:22745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dolcino M, Ottria A, Barbieri A et al Gene expression profiling in peripheral blood cells and synovial membranes of patients with psoriatic arthritis. PLOS ONE 2015; 10:e0128262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Belasco J, Louie JS, Gulati N et al Comparative genomic profiling of synovium versus skin lesions in psoriatic arthritis. Arthritis Rheumatol 2015; 67:934–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Caneparo V, Cena T, De Andrea M et al Anti‐IFI16 antibodies and their relation to disease characteristics in systemic lupus erythematosus. Lupus 2013; 22:607–13. [DOI] [PubMed] [Google Scholar]
- 16. Alunno A, Caneparo V, Carubbi F et al Interferon gamma‐inducible protein 16 in primary Sjogren's syndrome: a novel player in disease pathogenesis? Arthritis Res Ther 2015; 17:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Baer AN, Petri M, Sohn J, Rosen A, Casciola‐Rosen L. Association of antibodies to interferon‐inducible protein‐16 with markers of more severe disease in primary Sjogren’s syndrome. Arthritis Care Res (Hoboken) 2016; 68:254–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mondini M, Vidali M, De Andrea M et al A novel autoantigen to differentiate limited cutaneous systemic sclerosis from diffuse cutaneous systemic sclerosis: the interferon‐inducible gene IFI16. Arthritis Rheum 2006; 54:3939–44. [DOI] [PubMed] [Google Scholar]
- 19. Costa S, Mondini M, Caneparo V et al Detection of anti‐IFI16 antibodies by ELISA: clinical and serological associations in systemic sclerosis. Rheumatology (Oxf) 2011; 50:674–81. [DOI] [PubMed] [Google Scholar]
- 20. McMahan ZH, Shah AA, Vaidya D, Wigley FM, Rosen A, Casciola‐Rosen L. Anti‐interferon‐inducible protein 16 antibodies associate with digital gangrene in patients with scleroderma. Arthritis Rheumatol 2016; 68:1262–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Caneparo V, Pastorelli L, Pisani LF et al Distinct anti‐IFI16 and anti‐GP2 antibodies in inflammatory bowel disease and their variation with infliximab therapy. Inflamm Bowel Dis 2016; 22:2977–87. [DOI] [PubMed] [Google Scholar]
- 22. Alunno A, Caneparo V, Bistoni O et al Circulating interferon‐inducible protein IFI16 correlates with clinical and serological features in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2016; 68:440–5. [DOI] [PubMed] [Google Scholar]
- 23. Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H, the Caspar Study Group . Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 2006; 54:2665–73. [DOI] [PubMed] [Google Scholar]
- 24. Gugliesi F, Bawadekar M, De Andrea M et al Nuclear DNA sensor IFI16 as circulating protein in autoimmune diseases is a signal of damage that impairs endothelial cells through high‐affinity membrane binding. PLOS ONE 2013; 8:e63045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ceribelli A, Fredi M, Taraborelli M et al Anti‐MJ/NXP‐2 autoantibody specificity in a cohort of adult Italian patients with polymyositis/dermatomyositis. Arthritis Res Ther 2012; 14:R97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ceribelli A, Isailovic N, De Santis M et al Myositis‐specific autoantibodies and their association with malignancy in Italian patients with polymyositis and dermatomyositis. Clin Rheumatol 2017; 36:469–75. [DOI] [PubMed] [Google Scholar]
- 27. Yamasaki Y, Narain S, Yoshida H et al Autoantibodies to RNA helicase A: a new serologic marker of early lupus. Arthritis Rheum 2007; 56:596–604. [DOI] [PubMed] [Google Scholar]
- 28. Lande R, Gregorio J, Facchinetti V et al Plasmacytoid dendritic cells sense self‐DNA coupled with antimicrobial peptide. Nature 2007; 449:564–9. [DOI] [PubMed] [Google Scholar]
- 29. Lande R, Botti E, Jandus C et al The antimicrobial peptide LL37 is a T‐cell autoantigen in psoriasis. Nat Commun 2014; 5:5621. [DOI] [PubMed] [Google Scholar]
- 30. McArdle A, Pennington S, FitzGerald O. Clinical features of psoriatic arthritis: a comprehensive review of unmet clinical needs. Clin Rev Allergy Immunol 2017. [DOI] [PubMed] [Google Scholar]
- 31. Zhao H, Gonzalezgugel E, Cheng L, Richbourgh B, Nie L, Liu C. The roles of interferon‐inducible p200 family members IFI16 and p204 in innate immune responses, cell differentiation and proliferation. Genes Dis 2015; 2:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chiliveru S, Rahbek SH, Jensen SK et al Inflammatory cytokines break down intrinsic immunological tolerance of human primary keratinocytes to cytosolic DNA. J Immunol 2014; 192:2395–404. [DOI] [PubMed] [Google Scholar]
- 33. Bawadekar M, De Andrea M, Lo Cigno I et al The extracellular IFI16 protein propagates inflammation in endothelial cells via p38 MAPK and NF‐kappaB p65 activation. J Interferon Cytokine Res 2015; 35:441–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shin JI, Lee KH, Joo YH et al Inflammasomes and autoimmune and rheumatic diseases: a comprehensive review. J Autoimmun 2019:102299. [DOI] [PubMed] [Google Scholar]
- 35. Lee KH, Kronbichler A, Park DD et al Neutrophil extracellular traps (NETs) in autoimmune diseases: a comprehensive review. Autoimmun Rev 2017; 16:1160–73. [DOI] [PubMed] [Google Scholar]
- 36. Ronnblom L. The importance of the type I interferon system in autoimmunity. Clin Exp Rheumatol 2016; 34:21–4. [PubMed] [Google Scholar]
- 37. Costa S, Borgogna C, Mondini M et al Redistribution of the nuclear protein IFI16 into the cytoplasm of ultraviolet B‐exposed keratinocytes as a mechanism of autoantigen processing. Br J Dermatol 2011; 164:282–90. [DOI] [PubMed] [Google Scholar]
- 38. Cao T, Shao S, Li B et al Up‐regulation of Interferon‐inducible protein 16 contributes to psoriasis by modulating chemokine production in keratinocytes. Sci Rep 2016; 6:25381. [DOI] [PMC free article] [PubMed] [Google Scholar]


