Abstract
Objectives
To describe the epidemiological characteristics of patients with spinal metastases between 2007 and 2019.
Methods
Patients with spinal metastases were identified from several clinical centers in China between January 2007 and July 2019. Demographics, primary tumor types, spinal involvement, and Clinical indicators of each patient were reviewed.
Results
A total of 1196 patients were included in this study, 717 males (59.95%) and 479 females (40.05%), with a male to female ratio of 1.50:1. Most patients (63.71%) were in the ages range of 50 to 69 years. The mean age was 58.6 ± 11.6 (range 13–89) years and the median age was 59.0 years. The average age of females was younger than that of males, and the difference was statistically significant. The proportion of male patients over 60 years old was higher than that of females, and the difference was statistically significant. The most common primary tumor was lung cancer (n = 437, 36.54%), followed by unknown origin (n = 194, 16.22%), kidney cancer (n = 78, 6.52%), breast cancer (n = 76, 6.35%), and liver/biliary cancer (n = 75, 6.27%). The most common primary tumor was lung cancer in both males and females, followed by unknown origin in males and breast cancer in females. There were 730 patients (61.04%) in the subgroup of the number<3; the highest level was lumbar vertebrae, with 250 patients (34.25%). The remaining 466 patients (38.96%) were included in the subgroup of the number ≥ 3; the highest level was tumor metastasis of multiple‐level of spine, with 334 patients (71.67%). Among the 1196 patients, spinal cord injury occurred in 54.01% of patients, 76.34% of patients developed moderate and above pain, 55.69% of patients had metastatic spinal cord compression, and only 26.67% of patients had a clear history of primary tumors.
Conclusion
This study provided a relatively detailed description of epidemiological characteristics in spinal metastases in China, which could assist orthopaedic surgeons to understand the clinical characteristics of spinal metastases, and is of great significance in guiding clinical diagnoses and scientific research.
Keywords: Epidemiological, Metastasis, Population, Spine
Introduction
Improvements in clinical anti‐tumor multimodality therapies and palliative therapy have prolonged patients' survival, but the incidence of distant metastasis is still increasing1. The vertebral column is the third most frequent metastasis site after the lungs and liver, and accounts for approximately 50% of bone metastases2, 3, 4. Overall, 40% to 70% of patients with advanced neoplasia will eventually develop spinal metastases, and metastatic spinal cord compression (MSCC) will develop in 10% to 20% of these patients5, 6, 7. Of note, its main clinical manifestations are not only associated with severe pain but also with paralysis, sensory loss, sexual dysfunction, and urinary and fecal incontinency, especially when the neurologic elements are compressed, which are important factors leading to seriously reduced quality of life and even death8. The incidence rates and distributions of spinal metastases not only show significant geographic differences but also vary in age, gender, and ethnicity. Hence, understanding the epidemiologic characteristics of spinal metastases is fundamental and necessary for decisions regarding medical intervention and prediction of patients' prognosis.
In recent years, the number of epidemiological studies on spinal metastases has been gradually increasing worldwide. A spinal metastases epidemiology study that compared surgery trends across two decades and three continents concluded that surgical habits had been fairly consistent among countries worldwide and over time9. Moreover, the study of Sohn et al.10 was the first nationwide analysis of spine tumors, including metastatic spine tumors, in Asia. It reported epidemiology and healthcare utilization of 1600 patients with primary and metastatic spine tumors between 1 January 2009 and 31 December 2012. Horn et al.11 analyzed 333 patients <20 years old with spinal metastases undergoing spinal surgery from the Kid Inpatient Database in the United States and concluded that surgical treatment for spinal metastasis in the past decade has increased, although the complication rates, in‐hospital mortality, and length of stay remained stable.
However, some problems remain with epidemiological studies on spinal metastases in China. There are relatively few studies with large‐scale epidemiological analyses of spinal metastases. In addition, many studies focus on spinal tumors but not on spinal metastases specifically12, 13.
Hence, the purpose of this study is to: (i) describe the epidemiological characteristics of spinal metastases in China; (ii) focus attention on the distribution of age, gender, and primary tumor type in patients with spinal metastases; and (iii) provide guidance for clinical work and further research on spinal metastases.
Patients and Methods
Patients Inclusion
Patients with spinal metastases were identified from several clinical centers in China between January 2007 and July 2019. We retrieved and screened eligible cases through the hospital case management system by using disease codes, which were standardized according to the International Classification of Disease, Tenth Revision (ICD‐10), and patient information was obtained through analysis of inpatient medical records. A unified database was developed using an epidemiological method, including the patient's name, gender, age, primary tumor types, and level and number of involved vertebrae.
Inclusion criteria: (i) patients diagnosed with spinal metastases based on clinical symptoms, radiographic examinations, and/or histopathology; (ii) hematological malignancies including myeloma and lymphoma were also included; and (iii) patients whose observation indicators could be retrospectively analyzed.
Exclusion criteria: (i) patients with impaired spinal cord function due to severe spinal degenerative diseases; (ii) patients with other spinal diseases such as primary spinal tumors or spinal tuberculosis at the same time; and (iii) outpatients.
Recorded Indicators
The following indicators were retrospectively recorded: (i) patient demographics, including age and gender; (ii) primary tumor types (origin of spinal metastases); and (iii) spinal involvement, including level and number of involved vertebrae (the sacral vertebrae and caudal vertebra were defined as one vertebra); and (iv) clinical indicators, including Frankel grade, visual analog scale (VAS) for pain, MSCC, and tumor history.
The Frankel grade classification provides an assessment of spinal cord function. There are five grades (A, B, C, D, and E) based on the degree of spinal cord injury14, as follows:
Grade A. Complete neurological injury: No motor or sensory function detected below the level of the lesion.
Grade B. Preserved sensation only: No motor function detected below the level of the lesion; some sensory function below the level of the lesion preserved.
Grade C. Preserved motor, nonfunctional: Some voluntary motor function preserved below the level of the lesion but too weak to serve any useful purpose; sensation may or may not be preserved.
Grade D. Preserved motor, functional: Functionally useful voluntary motor function below level of injury is preserved.
Grade E. Normal motor function: Normal motor and sensory function below level of lesion; abnormal reflexes may persist.
The VAS is a measure of pain intensity. It is a continuous scale comprised of a horizontal (called a horizontal visual analog scale) or vertical (called vertical visual analog scale), usually 10 cm or 100 mm length. For pain intensity, the scores can be from 0–10, which is determined by measuring the distance (mm) on the 10‐cm line between the “no pain” anchor and the patient's mark15.
Metastatic spinal cord compression is defined radiographically as an epidural metastatic lesion causing true displacement of the spinal cord from its normal position in the spinal canal16. It is an important source of morbidity (including paralysis and bowel and bladder disorders) in patients with systemic cancer.
Tumor history is defined as that the primary tumor has been clearly diagnosed before the diagnosis of spinal metastases.
Statistical Analysis
The age and VAS were described using mean and median values, while gender, primary tumor, level and number of involved vertebrae, Frankel grade, VAS, MSCC, and tumor history were described using the composition ratios. The mean age of male and female patients was compared using Student t‐test. The difference in gender distribution among different age groups was statistically compared by χ2‐test. All statistical analyses were performed using IBM SPSS Statistics 22.0 (IBM, Armonk, NY, USA); a two‐tailed P < 0.05 was statistically significant.
Results
Patient Demographics
A total of 1196 patients were included in this study, 717 males (59.95%) and 479 females (40.05%), with a male to female ratio of 1.50:1. Most patients (63.71%) were aged from 50 to 69 years. The mean age was 58.6 ± 11.6 (range 13–89) years and the median age was 59.0 years. The mean age of males and females was 59.4 ± 11.9 (range 16–89) years and 57.4 ± 11.1 (range 13–83) years, respectively, which showed that the onset time of spinal metastases was 2 years earlier in females than in males, and the difference was statistically significant (t = 2.96, P = 0.03). The proportion of male patients over 60 years old (52.02%) was 7.76% higher than that of females (44.26%), and the difference was statistically significant (χ2 = 6.926, P = 0.008). The distribution of age and gender in 1196 patients is shown in Table 1 and Fig. 1.
Table 1.
Distribution of gender and age in 1196 patients with spinal metastases
| Age | Male | Female | Total | |||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| <20 | 2 | 0.28 | 1 | 0.21 | 3 | 0.25 |
| 20‐24 | 4 | 0.56 | 1 | 0.21 | 5 | 0.42 |
| 25‐29 | 6 | 0.84 | 9 | 1.88 | 15 | 1.25 |
| 30‐34 | 9 | 1.26 | 7 | 1.46 | 16 | 1.34 |
| 35‐39 | 18 | 2.51 | 8 | 1.67 | 26 | 2.17 |
| 40‐44 | 33 | 4.60 | 27 | 5.64 | 60 | 5.02 |
| 45‐49 | 65 | 9.07 | 53 | 11.06 | 118 | 9.87 |
| 50‐54 | 90 | 12.55 | 74 | 15.45 | 164 | 13.71 |
| 55‐59 | 117 | 16.32 | 87 | 18.16 | 204 | 17.06 |
| 60‐64 | 133 | 18.55 | 80 | 16.70 | 213 | 17.81 |
| 65‐69 | 103 | 14.37 | 78 | 16.28 | 181 | 15.13 |
| 70‐74 | 62 | 8.65 | 29 | 6.05 | 91 | 7.61 |
| 75‐79 | 48 | 6.69 | 19 | 3.97 | 67 | 5.60 |
| 80‐84 | 21 | 2.93 | 6 | 1.25 | 27 | 2.26 |
| 85‐89 | 6 | 0.84 | 0 | 0.00 | 6 | 0.50 |
| Total | 717 | 100.00 | 479 | 100.00 | 1196 | 100.00 |
N = number of cases.
Figure 1.
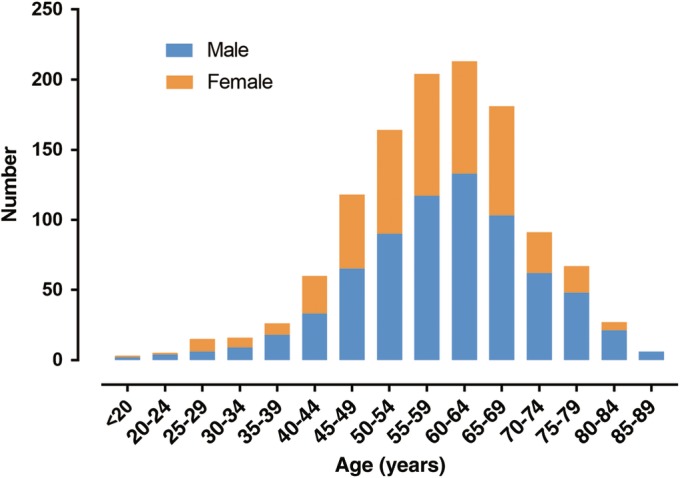
Distribution of gender and age in 1196 patients with spinal metastases. There were a total of 1196 patients in this study, including 717 males and 479 females, with a male to female ratio of 1.50:1; most patients were in the age range of 50–69 years.
Primary Tumor Types
The most common primary tumor was lung cancer (n = 437, 36.54%), followed by unknown origin (n = 194, 16.22%), kidney cancer (n = 78, 6.52%), breast cancer (n = 76, 6.35%), liver/biliary cancer (n = 75, 6.27%), gastrointestinal cancer (n = 53,4.43%), myeloma (n = 53, 4.43%), prostate cancer (n = 53, 4.43%), thyroid cancer (n = 37, 3.09%), sarcoma (n = 33, 2.76%), other origin (n = 107, 8.95%) such as esophageal cancer, lymphoma, and cervical cancer. The distribution of gender and primary tumors in 1196 patients is shown in Table 2 and Fig. 2.
Table 2.
Distribution of gender and primary tumor in 1196 patients with spinal metastases
| Primary tumor | Male | Female | Total | |||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Lung cancer | 258 | 35.98 | 179 | 37.37 | 437 | 36.54 |
| Unknown origin | 121 | 16.88 | 73 | 15.24 | 194 | 16.22 |
| Kidney cancer | 70 | 9.76 | 8 | 1.67 | 78 | 6.52 |
| Breast cancer | 0 | 0.00 | 76 | 15.87 | 76 | 6.35 |
| Liver/biliary cancer | 61 | 8.51 | 14 | 2.92 | 75 | 6.27 |
| Gastrointestinal cancer | 38 | 5.30 | 15 | 3.13 | 53 | 4.43 |
| Myeloma | 28 | 3.91 | 25 | 5.22 | 53 | 4.43 |
| Prostate cancer | 53 | 7.39 | 0 | 0.00 | 53 | 4.43 |
| Thyroid cancer | 11 | 1.53 | 26 | 5.43 | 37 | 3.09 |
| Sarcoma | 13 | 1.81 | 20 | 4.18 | 33 | 2.76 |
| Other origin | 64 | 8.93 | 43 | 8.98 | 107 | 8.95 |
| Total | 717 | 100.00 | 479 | 100.00 | 1196 | 100.00 |
N = number of cases.
Figure 2.
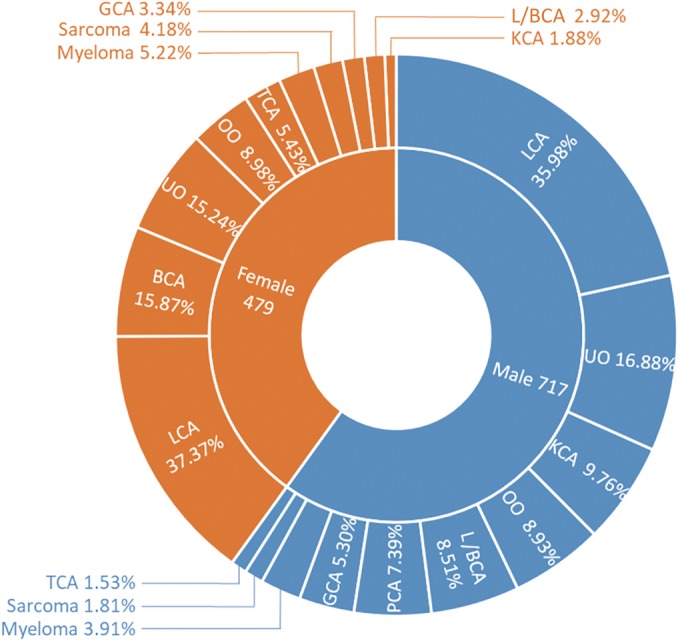
Distribution of gender and primary tumors in 1196 patients with spinal metastases. The most common primary tumor was lung cancer in both males and females, followed by unknown origin in males and breast cancer in females. BCA, breast cancer; GCA, gastrointestinal cancer; L/BCA, liver/biliary cancer; KCA, kidney cancer; LCA, lung cancer; OO, other origin; PCA, prostate cancer; TCA, thyroid cancer; UO, unknown origin.
According to the Tomita score, primary tumors can be divided into rapid growth tumors, moderate growth tumors, and slow growth tumors17. Among metastatic spine tumors in this study, 813 patients (67.98%) had rapid growth tumors, 129 patients (10.78%) had moderate growth tumors, and 254 patients (21.24%) had slow growth tumors.
Spinal Involvement
The most common level of involved vertebrae was multi‐level of spine (n = 432, 36.12%), which means that tumor had involved 2 or more levels., followed by thoracic vertebrae (n = 316, 26.42%), lumbar vertebrae (n = 281, 23.50%), sacral vertebrae (n = 86, 7.19%), and cervical vertebrae (n = 81, 6.77%).
There were 730 patients (61.04%) in the subgroup of the number<3; the highest level was lumbar vertebrae, with 250 patients (34.25%). The remaining 466 patients (38.96%) were included in the subgroup of the number ≥ 3; the highest level was multiple‐level of spine, with 334 patients (71.67%). The distribution of involved vertebrae in 1196 patients with spinal metastasis is shown in Table 3.
Table 3.
Distribution of involved vertebrae in 1196 patients with spinal metastasis
| Involved vertebrae | <3 | ≥3 | Total | |||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Cervical vertebrae | 67 | 9.18 | 14 | 3.01 | 81 | 6.77 |
| Thoracic vertebrae | 229 | 31.37 | 87 | 18.67 | 316 | 26.42 |
| Lumbar vertebrae | 250 | 34.25 | 31 | 6.65 | 281 | 23.50 |
| Sacral vertebrae | 86 | 11.78 | 0 | 0.00 | 86 | 7.19 |
| Multiple‐ level of spine | 98 | 13.42 | 334 | 71.67 | 432 | 36.12 |
| Total | 730 | 100.00 | 466 | 100.00 | 1196 | 100.00 |
N = number of cases.
Clinical Indicators
There were 79 patients (6.60%) with Frankel grade A–B, 567 patients (47.41%) with Frankel grade C–D, and 550 patients (45.99%) with Frankel grade E. In total, there were 880 patients (54.01%) with Frankel grade A–D, which indicated that they a spine cord injury.
There were 283 patients (23.66%) with a VAS score of 0–3, 531 patients (44.40%) with a score of 4–6, and 382 patients (31.94%) with a score of 7–10 score. The mean VAS score was 5.2 ± 2.0 and the median score was 6.0 There were 913 patients (76.34%) aggregately with a VAS score of 4 or above, which indicated that they had developed moderate and above pain.
Among the 1196 patients, 666 patients (55.69%) had MSCC, and only 319 patients (26.67%) had a clear history of primary tumors.
Discussion
This study included 717 males and 479 females with a male to female ratio of 1.50:1, and most patients were between the ages of 50 and 69 years. The average age of female patients was lower than that of male patients, and the difference was statistically significant. The proportion of male patients over 60 years old was higher than that of females, and the difference was statistically significant. For primary tumors, the most common site was lung cancer. When the number of involved vertebrae was fewer than three, the most level of which was lumbar vertebra. When the number of involved vertebrae was greater than three, the most level of which was multi‐level of spine. Among the 1196 patients, spinal cord injury occurred in 54.01% of patients, 76.34% of patients developed moderate and above pain, 55.69% of patients had MSCC, and only 26.67% of patients had a clear history of primary tumor.
Patient Demographics
The study of Bollen et al.18 reported 1143 patients with spinal metastases, including 542 males (52%) and 501 females (48%), with an average age of 64.8 ± 12.5 years. Another study19 of spine metastases included 544 patients, including 287 males (52.8%) and 200 females (47.2%), with an average age of 63 years. Karhade et al.20 reported 732 patients with spinal metastases, including 426 males (58.2%) and 306 females (41.8%), with an average age of 61 years. In general, there were more men than women with spinal metastases, with an average age of approximately 60 years, which resembled the results of our study.
Primary Tumor Types
Spinal metastases were mostly derived from epithelial tissue or glands, and a few were derived from mesenchymal tissue. Bollon et al.18 analyzed patients with spinal metastases from the Netherlands and found that the most common primary tumor was breast cancer, followed by lung cancer, prostate cancer, kidney cancer, and others. Similarly, an epidemiological study9 of spinal metastases across two decades and three continents showed that the most common primary tumor was breast cancer, followed by prostate cancer, lung cancer, kidney cancer, and others. A nationwide epidemiological study21 of adult Koreans with spinal metastases showed that the most common primary tumor was lung cancer, followed by liver cancer, breast cancer, colon cancer, and others. In general, the most common types of primary tumors varied greatly from country to country.
In this study, the most common primary tumor was lung cancer, followed by unknown origin, kidney cancer, breast cancer, liver/biliary cancer, and other. In males, it was followed by lung cancer, unknown origin, kidney cancer, liver/biliary cancer, prostate cancer, and others. In females, it was followed by lung cancer, breast cancer, unknown origin, thyroid cancer, and others. The results were similar to those for studies conducted in China and quite different from those reported in other countries. On the one hand, this may be due to the differences in the environment and ethnic origins, which resulted in different incidences of primary tumors. In China, lung cancer is the most common malignant tumor in both males and females. However, in Western countries, prostate cancer is the most common malignant tumor in males and breast cancer in females22. On the other hand, unknown origin accounted for 16.22% among the total patients in this study, which was quite different from 2%–4% in foreign countries23, 24, 25. This was related to the low economic level in some areas of China and the lack of knowledge about malignant tumors among the people. When some patients were diagnosed with spinal metastases, they would refuse further examinations to confirm primary tumors, leading to difficulty in identifying primary tumors.
Spinal Involvement
The level and number of involved vertebrae in this study were similar to those in other studies. In the study of Bollen et al.18, the level of involved vertebrae was more common in multi‐level of spine, thoracic vertebrae and lumbar vertebrae, and the number of involved vertebrae was fewer than three in 517 patients (49.57%). Soon Bum et al.26 reported on 217 patients with spinal metastases, including 100 patients with thoracic metastasis, 65 patients with lumbosacral metastasis, and 32 patients with cervical metastasis.
Clinical Indicators
The results for clinical indicators were similar to those of other studies27, 28. In this study, 73.33% of patients had no clear history of primary tumors when diagnosed with spinal metastases, and most of them saw a doctor because of local pain or MSCC. Therefore, for patients over 50 years old and without a history of tumors, there were no obvious cause of severe spinal pain, limb weakness, or sensory loss. When the conservative treatment effect was not obvious, it was necessary to alert the patients about the possibility of spinal metastases.
Limitations
Several limitations of this study should be noted. First, approximately 50% of cases of spinal metastases would occur with symptoms, and approximately 10% of them would need surgical intervention29. Most of these patients without spine‐related symptoms were treated in oncology departments and rarely underwent surgical intervention. However, most of the patients included in this study incurred spine‐related symptoms and there were few patients who did not require surgical intervention. Therefore, the actual number of patients with spinal metastases in the population should be much higher than the number of patients included in this study. Second, the onset time, spine‐related symptoms, and the level and number of involved vertebrae in patients with spinal metastases were closely related to the biological behavior of primary tumors. This study only provides a general analysis and description of the patients and the spinal metastases characteristics. If the researchers focus on a single type of primary tumor, the results may be biased.
Conclusion
The present study provides a relatively detailed description of epidemiological characteristics in spinal metastases in China, which could assist orthopaedic surgeons in understanding the clinical characteristics of spinal metastases and is of great significance in guiding clinical diagnoses and the scientific research.
Acknowledgements
The authors are grateful for support from the Library of Tianjin Medical University. We would also like to thank the friends who helped us in the creation and revision of the article.
Disclosure: This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
Reference
- 1. Luksanapruksa P, Buchowski JM, Zebala LP, Kepler CK, Singhatanadgige W, Bumpass DB. Perioperative complications of spinal metastases surgery. Clin Spine Surg, 2017, 30: 4–13. [DOI] [PubMed] [Google Scholar]
- 2. Atkinson RA, Jones A, Ousey K, Stephenson J. Management and cost of surgical site infection in patients undergoing surgery for spinal metastasis. J Hosp Infect, 2017, 95: 148–153. [DOI] [PubMed] [Google Scholar]
- 3. Quraishi, Nasir A, Rajabian, et al Reoperation rates in the surgical treatment of spinal metastases. Spine J, 2015, 15: S37–S43. [DOI] [PubMed] [Google Scholar]
- 4. Aebi M. Spinal metastasis in the elderly. Eur Spine J, 2003, 12: S202–S213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jacobs WB, Perrin RG. Evaluation and treatment of spinal metastases: an overview. Neurosurg Focus, 2001, 11: e10. [DOI] [PubMed] [Google Scholar]
- 6. Patil CG, Lad SP, Justin S, Maxwell B. National inpatient complications and outcomes after surgery for spinal metastasis from 1993‐2002. Cancer, 2010, 110: 625–630. [DOI] [PubMed] [Google Scholar]
- 7. Barzilai O, Laufer I, Yamada Y, et al Integrating evidence‐based medicine for treatment of spinal metastases into a decision framework: neurologic, oncologic, mechanicals stability, and systemic disease. J Clin Oncol, 2017, 35 : 2419–2427. 10.1200/JCO.2017.72.736. [DOI] [PubMed] [Google Scholar]
- 8. Zoccali C, Skoch J, Walter CM, Torabi M, Borgstrom M, Baaj AA. The Tokuhashi score: effectiveness and pitfalls. Eur Spine J, 2016, 25: 673–678. [DOI] [PubMed] [Google Scholar]
- 9. Wright E, Ricciardi F, Arts M, et al Metastatic spine tumor epidemiology: comparison of trends in surgery across two decades and three continents. World Neurosurg, 2018, 114: e809–e817. [DOI] [PubMed] [Google Scholar]
- 10. Sohn S, Kim J, Chung CK, et al Nationwide epidemiology and healthcare utilization of spine tumor patients in the adult Korean population, 2009–2012. Neurooncol Pract, 2015, 2: 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Horn SR, Dhillon ES, Poorman GW, et al Epidemiology and national trends in prevalence and surgical management of metastatic spinal disease. J Clin Neurosci, 2018, 53: 183–187. [DOI] [PubMed] [Google Scholar]
- 12. Zhang XJ, Wang Z, Guo Z, Li J, Wang H‐X. A epidemiological analysis of 640 cases of tumors and tumor‐like lesions of spine. Lin Chuang Zhong Liu Xue Za Zhi, 2012, 17: 543–548. [Google Scholar]
- 13. Zheng W, Wu J, Yang L‐L, et al Epidemiology and survival analysis in patients with spinal tumor after surgery. Zhong Guo Jiao Xing Wai Ke Za Zhi, 2011, 19: 1649–1653. [Google Scholar]
- 14. Middendorp JJV, Goss B, Urquhart S, et al Diagnosis and prognosis of traumatic spinal cord injury. Global Spine J, 2011, 1: 001–008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short‐Form McGill Pain Questionnaire (SF‐MPQ), Chronic Pain Grade Scale (CPGS), Short Form‐36 Bodily Pain Scale (SF‐36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res, 2011, 63: S240–S252. [DOI] [PubMed] [Google Scholar]
- 16. Quraishi NA, Esler C. Metastatic spinal cord compression. BMJ, 2011, 342: d2402. [DOI] [PubMed] [Google Scholar]
- 17. Tomita K, Kawahara N, Kobayashi T, et al Surgical strategy for spinal metastases. Spine, 2001, 27: 298–306. [DOI] [PubMed] [Google Scholar]
- 18. Bollen L, Ym VDL, Pondaag W, et al Prognostic factors associated with survival in patients with symptomatic spinal bone metastases: a retrospective cohort study of 1 043 patients. Neuro Oncol, 2014, 16: 991–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Masashi M, Hideyuki H, Hirofumi A, et al Prognostic factors and a scoring system for survival after radiotherapy for metastases to the spinal column: a review of 544 patients at Shizuoka Cancer Center Hospital. Cancer, 2010, 113: 2816–2822. [DOI] [PubMed] [Google Scholar]
- 20. Karhade AV, Thio QCBS, Ogink PT, et al Predicting 90‐day and 1‐year mortality in spinal metastatic disease: development and internal validation. Neurosurgery, 2019, 0: 1–11. [DOI] [PubMed] [Google Scholar]
- 21. Sohn S, Kim J, Chung CK, et al A nationwide epidemiological study of newly diagnosed spine metastasis in the adult korean population. Spine J, 2016, 16: 937–945. [DOI] [PubMed] [Google Scholar]
- 22. Torre LA, Freddie B, Siegel RL, et al Global cancer statistics, 2012. CA Cancer J Clin, 2015, 65: 87–108. [DOI] [PubMed] [Google Scholar]
- 23. Rougraff BT, Cudahy TJ. Evaluation of the patient with carcinoma of unknown origin metastatic to bone. Clin Orthop Relat Res, 2003, 415: S105–S109. [DOI] [PubMed] [Google Scholar]
- 24. Bartels RHMA, Ton F, Richard VDM, et al Development of a model with which to predict the life expectancy of patients with spinal epidural metastasis. Cancer, 2010, 110: 2042–2049. [DOI] [PubMed] [Google Scholar]
- 25. Lee BH, Park JO, Kim HS, Park YC, Lee HM, Moon SH. Perioperative complication and surgical outcome in patients with spine metastases: retrospective 200‐case series in a single institute. Clin Neurol Neurosurg, 2014, 122: 80–86. [DOI] [PubMed] [Google Scholar]
- 26. Soon Bum Y, Wonik C, Ung‐Kyu C. Analysis of prognostic factors relating to postoperative survival in spinal metastases. J Korean Neurosurg Soc, 2012, 51: 127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Arrigo RT, Paul K, Ivan C, et al Predictors of survival after surgical treatment of spinal metastasis. Neurosurgery, 2012, 68: 674. [DOI] [PubMed] [Google Scholar]
- 28. Ahmed AK, Goodwin CR, Heravi A, et al Predicting survival for metastatic spine disease: a comparison of nine scoring systems. Spine J, 2018, 18: 1804–1814. [DOI] [PubMed] [Google Scholar]
- 29. Sciubba DM, Gokaslan ZL. Diagnosis and management of metastatic spine disease. Surg Oncol, 2006, 15: 141–151. [DOI] [PubMed] [Google Scholar]


