Abstract
Reducing HIV among men who have sex with men (MSM) is a national goal, and early diagnosis, timely linkage to HIV medical care, and ongoing care and treatment are critical for improving health outcomes for MSM with HIV and preventing transmission to others. We assessed demographic, social, and economic factors associated with HIV antiretroviral treatment among HIV-infected MSM. Data are from the National HIV Behavioral Surveillance (NHBS) collected in 2014 among MSM. We estimated prevalence ratios and 95% confidence intervals using average marginal predictions from logistic regression. Overall, 89% of HIV-positive MSM reported currently taking antiretroviral therapy (ART). After controlling for other variables, we found that higher perceived community stigma and not having health insurance were significant risk factors for not taking ART. We also found that high socioeconomic status (SES) was associated with taking ART. Race/ethnicity was not significantly associated with taking ART in either the unadjusted or adjusted analyses. Findings suggest that to increase ART use for MSM with HIV, we need to move beyond individual-level approaches and move towards the development, dissemination, and evaluation of structural and policy interventions that respond to these important social and economic factors.
Keywords: MSM, National HIV Behavioral Surveillance, Antiretroviral Therapy, Stigma, Health Insurance, SES, Race/ethnicity
Introduction
Although gay, bisexual, and other men who have sex with men (collectively referred to as MSM) represent approximately 2% of the US population [1], in 2017, they accounted for 66.5% of new diagnoses of HIV infection in the US and six dependent areas; MSM who inject drugs account for an additional 3.2% [2]. In addition, incidence (i.e., new infections) of HIV infection decreased from 2008 to 2015 for all transmission categories except for MSM [3]. Reducing HIV among MSM is a national goal, and early diagnosis, timely linkage to HIV medical care, ongoing care and treatment, and achieving and maintaining viral suppression will contribute to improving the health outcomes for MSM with HIV and preventing transmission to others [4]. Previous research shows that antiretroviral therapy (ART) reduces the risk of sexual HIV transmission [5, 6]. More recent research shows that when people taking ART can achieve and maintain viral suppression, they have effectively no risk of sexually transmitting HIV [7–11]. A National HIV Behavioral Surveillance (NHBS) data trend analysis among self-reported HIV-positive MSM found that from 2008 to 2014, ART prevalence increased from 69 to 88% [12]. Although this trend is promising, National HIV Surveillance System (NHSS) data report that among MSM with diagnosed HIV infection at the end of 2015, only 57.9% were retained in continuous HIV medical care and only 63% had achieved viral suppression [13]. It is important to understand the reasons that some HIV-positive MSM are not taking the ART they need to become virally suppressed.
The purpose of this study is to learn more about the factors associated with HIV-positive MSM’s engagement in HIV medical care and treatment. We analyze 2014 NHBS data of HIV-positive MSM to further assess whether key social and economic factors, including socioeconomic status, homelessness, incarceration, health insurance, discrimination related to being gay/bisexual, and perceived community stigma related to having HIV were associated with taking ART for self-reported HIV-positive MSM in 20 US cities.
Methods
Design
For this cross-sectional study, we analyzed data from NHBS collected in 2014 among MSM.
Sample, Setting, and Data Collection
NHBS monitors HIV seroprevalence, risk behaviors, testing, treatment, and prevention in the USA among three populations: MSM, persons who inject drugs, and heterosexual adults at increased risk for HIV. Each population is surveyed every three calendar years and data for this analysis were collected in 2014 during the fourth MSM cycle of NHBS. Detailed sampling and data collection procedures have been previously published [14–16]. Data were collected from 20 metropolitan statistical areas (MSAs) with the highest HIV prevalence overall: Atlanta, GA; Baltimore, MD; Boston, MA; Chicago, IL; Dallas, TX; Denver, CO; Detroit, MI; Houston, TX; Los Angeles, CA; Miami, FL; New Orleans, LA; Nassau-Suffolk, NY; Newark, NJ; New York, NY; Philadelphia, PA; San Diego, CA; San Francisco, CA; San Juan, PR; Seattle, WA; and Washington, DC.
MSM were recruited for interviews and HIV testing using venue-based, time-location sampling methods. Through a formative assessment, staff identified venues frequented (e.g., bars, clubs, gyms, parks, street locations, or social organizations) where at least 50% of the patrons were MSM and determined days and times at each identified venue when MSM were most likely to be present. Sampling involved randomly selecting venues and day/time periods for recruitment events. During an event, a trained recruiter systematically approached men attending the venue to ask about screening for eligibility. Eligible men were those who were born male and self-identified as male, were aged 18 years or older, lived in the participating city, were able to complete the survey in English or Spanish, reported ever having oral or anal sex with another man, and had not previously participated in the NHBS-MSM survey during 2014. Data were collected by trained interviewers using a computer-assisted personal interview. All MSM who self-reported a prior HIV-positive test with a valid interview (as assessed by the interviewer’s confidence in the respondent’s answers) were included in this analysis.
Measures
Outcome
The outcome of this analysis was current ART. Current ART was measured by asking whether the participant was currently taking ART at the time of the interview (i.e., “Are you currently taking antiretroviral medicines to treat your HIV infection?”). The response options were “yes” or “no.”
Demographics
Participants reported one or more race categories (American Indian or Alaska Native, Asian, Black or African American, Native Hawaiian or other Pacific Islander, and White). Ethnicity was asked about separately and participants who reported Hispanic or Latino ethnicity were considered Hispanic or Latino, regardless of their reported race. Participants’ race/ethnicity was coded into four mutually exclusive categories (Hispanic/Latino, Black/African American, White, or other). The “other” race/ethnicity category included American Indian or Alaska Native, Asian, Native Hawaiian or other Pacific Islander, and multiple races. Age was calculated from the reported date of birth and categories used for this analysis were 18–29, 30–39, 40–49, and ≥ 50 years. Participants reported whether they were born in the USA (yes/no). Region of residence was analyzed using the four continental regions defined by the US Census Bureau (South, West, Midwest, Northeast). Puerto Rico was combined with the South due to small sample sizes.
Time since Diagnosis with HIV
Participants reported the date of their first HIV-positive test. The time since diagnosis was dichotomized as ≤ 3 years and > 3 years for this analysis. The 3-year time frame was selected because this was the most recent cutoff that provided sufficient sample size to conduct analysis for both groups, with a sample size just over 500 in the smaller group.
Discrimination Related to Being Gay or Bisexual
Participants reported whether they had experienced specific types of discrimination related to being gay or bisexual (i.e., “During the past 12 months, have any of the following things happened to you because someone knew or assumed you were attracted to men?”). The five questions asked whether they had: been called names/insulted; received poorer services than other people in restaurants, stores, other business, or agencies; been treated unfairly at work or school; been denied or given lower-quality healthcare; or been physically attacked or injured. Questions were adapted from a previous study [19]. Responses to questions were combined and coded as yes (experienced one or more of the five types of discrimination) or no (experienced none).
Perceived Community Stigma Related to Having HIV
Perceived community stigma was measured using four questions with five possible responses for each ranging from strongly agree to strongly disagree (i.e., “Please tell me how strongly you agree or disagree with each statement.”). Questions were adapted from two previous studies [20, 21]. The four questions asked whether most people in the respondent’s city would: discriminate against someone with HIV; support the rights of a person with HIV to live and work wherever they wanted to; not be friends with someone with HIV; and think that people who got HIV through sex or drug use have gotten what they deserve. Response scores were reverse coded as appropriate. A lower score represents less perceived community stigma and a higher score represents more perceived community stigma. For all respondents with data for at least three of the four questions, the average score was calculated (with possible scores ranging between 1 and 5). The Cronbach’s alpha for the four items was .72.
Socioeconomic Status, Homelessness, Health Insurance, and Incarceration History
Participants reported whether they had been homeless in the last year (yes/no), currently had health insurance (yes/no), and were ever incarcerated (yes/no). Similar to previous NHBS studies, we used a composite measure of socioeconomic status (SES) [17, 18]. SES was constructed as three categories based upon reported income and educational attainment. Low SES was defined as having an income < $25,000 or no high school diploma. Medium SES was defined as having an income $25,000–$49,999 or high school diploma (but not categorized as low SES based upon the criterion above). High SES was defined as having an income > $50,000 and a college degree.
Analyses
Descriptive statistics and models were conducted using weighted analyses to account for the complex sampling methodology used to recruit MSM. The weights accounted for sampling, non-response, and multiplicity (the increased probability of recruiting individuals who frequently visited venues in the sampling universe) [22, 23]. To assess the association between the independent variables and taking ART, univariable and multivariable logistic regressions were conducted. Unadjusted prevalence ratios (PR) and adjusted prevalence ratios (aPR) and 95% confidence intervals were estimated using average marginal predictions from regression. Analyses reflect complete case analysis, and approximately 1% of records were omitted due to missing data on independent or dependent variables. Although perceived community stigma related to having HIV was analyzed as a continuous variable in the logistic model, prevalence ratios were estimated at each point of the scale (as compared to the lowest possible score of 1). All analyses were conducted using SUDAAN 11 (RTI International, Research Triangle Park, NC).
Results
Sample Characteristics
This analysis included 1716 MSM who, in 2014, reported having a previous HIV-positive test (prior to applying weights). Characteristics of the weighted analyzed sample are summarized in Table 1. In the sample, 38% were Black/African American, 34% were White, and 21% were Hispanic/Latino. The mean age was 39.2 (SD = 1.7) years (categorical frequencies provided in Table 1). The majority (90%) were born in the USA, 46% lived in the South or Puerto Rico, and nearly half (49%) were low SES. Homelessness in the past year was reported by 12% and ever been incarcerated was reported by 33%. Most had health insurance (86%) and were diagnosed with HIV more than 3 years ago (69%). Thirty-eight percent reported experiencing discrimination related to being gay or bisexual in the past year. The average perceived community stigma related to having HIV was 2.70 (SD = 0.81) on a scale from 1 to 5, suggesting moderate stigma (not shown in the table). Overall, 89% of MSM reported currently taking ART (not shown in the table).
Table 1.
Characteristics of HIV-positive men who have sex with men, National HIV Behavioral Surveillance, 20 US cities, 2014 (N = 1716)
| Number | % | |
|---|---|---|
| Demographics | ||
| Race/ethnicity | ||
| Black/African American | 659 | 38.4 |
| Hispanic/Latino a | 355 | 20.7 |
| White | 582 | 33.9 |
| Other b | 120 | 7.0 |
| Age (years) | ||
| 18–29 | 455 | 26.5 |
| 30–39 | 432 | 25.2 |
| 40–49 | 450 | 26.2 |
| 50+ | 379 | 22.1 |
| Born in the USA | ||
| Yes | 1545 | 90.0 |
| No | 171 | 10.0 |
| Region of residence | ||
| South and Puerto Rico | 796 | 46.4 |
| West | 465 | 27.1 |
| Midwest | 168 | 9.8 |
| Northeast | 287 | 16.7 |
| Social characteristics | ||
| Socioeconomic status c | ||
| Low | 834 | 48.6 |
| Medium | 612 | 35.7 |
| High | 270 | 15.7 |
| Homeless, past year | ||
| Yes | 202 | 11.8 |
| No | 1514 | 88.2 |
| Incarcerated, ever | ||
| Yes | 557 | 32.5 |
| No | 1159 | 67.5 |
| Health insurance | ||
| Yes | 1470 | 85.7 |
| No | 245 | 14.3 |
| Years since HIV diagnosis | ||
| ≤ 3 years | 518 | 30.6 |
| > 3 years | 1177 | 69.4 |
| Discrimination related to being gay/bisexuald | ||
| Yes | 657 | 38.3 |
| No | 1058 | 61.7 |
Frequencies are based upon weighted counts to account for sampling, non-response, and multiplicity. Some totals may not add up due to the exclusion of observations with missing data
aHispanic/Latino based upon ethnicity reported and may include individuals of any race
bOther race/ethnicity includes American Indian or Alaska Native, Asian, Native Hawaiian or Other Pacific Islander, and multiple races
cLow SES was defined as having an income < $25,000 or no high school diploma. Medium SES was defined as having an income $25,000–$49,999 or high school diploma (but not categorized as low SES based upon the criterion above). High SES was defined as having an income > $50,000 and a college degree
dExperiencing one or more of five measured types of discrimination related to being gay or bisexual
Factors Associated with Taking ART
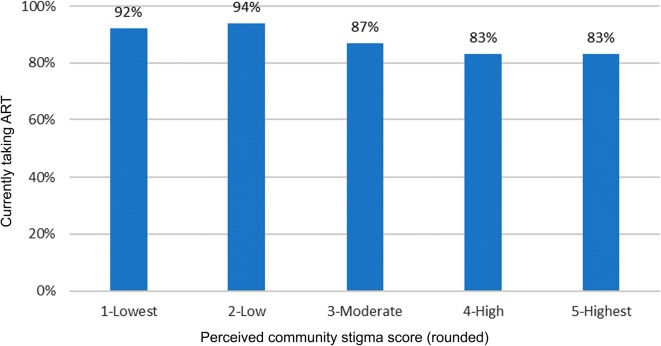
Descriptive statistics for taking ART by covariates and the results of analyses are presented in Table 2. In the unadjusted models, a higher prevalence of taking ART was associated with older age, residence in the West, Midwest, or Northeast regions (as compared to the South and Puerto Rico), and high SES (as compared to low). A lower prevalence of taking ART was associated with homelessness in the past year, ever incarcerated, not having health insurance, being diagnosed for within 3 years, and higher perceived community stigma related to having HIV. In the adjusted analysis, taking ART was significantly lower for those reporting higher perceived community stigma related to having HIV and those reporting not currently having health insurance. Also, taking ART was significantly higher for those with high SES (as compared to low). The prevalence of taking ART was significantly lower for MSM reporting higher perceived community stigma related to having HIV. When estimating prevalence ratios to compare “low,” “moderate,” “high,” and “highest” stigma scores to the lowest score, they ranged from adjusted prevalence ratio (aPR) = 0.97 (95% CI 0.96–0.99) for the lowest non-referent stigma score (“low”) to aPR = 0.84, 95% (CI 0.71–0.98) for the highest stigma score (“highest”). Figure 1 shows the percentage of MSM taking ART stratified by perceived community stigma score, rounded to the nearest integer. The prevalence of taking ART was significantly lower (aPR = 0.85, 95% CI 0.76–0.96) among MSM without health insurance compared to those with health insurance. Finally, compared to those with low SES, the prevalence of taking ART was significantly higher among those with high SES (aPR = 1.08, 95% CI 1.01–1.16).
Table 2.
Prevalence of taking ART among men who have sex with men, National HIV Behavioral Surveillance, 20 US cities, 2014 (N = 1716)
| Weighted % (n/N) | PR (95% CI) | aPR (95% CI) | |
|---|---|---|---|
| Demographics | |||
| Race/Ethnicity | |||
| Black/African American | 85.6% (548/658) | 0.93 (0.87, 1.00) | NA |
| Hispanic/Latinoa | 91.9% (315/355) | 1.00 (0.94, 1.06) | NA |
| White | 91.7% (535/581) | Referent | NA |
| Otherb | 82.1% (101/120) | 0.90 (0.78, 1.03) | NA |
| Age (years) | |||
| 18–29 | 82.4% (354/454) | Referent | NA |
| 30–39 | 85.7% (371/432) | 1.04 (0.94, 1.15) | NA |
| 40–49 | 93.4% (417/450) | 1.13 (1.05, 1.22)* | NA |
| 50+ | 93.6% (357/378) | 1.14 (1.05, 1.24)* | NA |
| Born in the USA | |||
| Yes | 88.4% (1347/1543) | Referent | NA |
| No | 92.0% (152/171) | 1.04 (0.98, 1.11) | NA |
| Region of Residence | |||
| South and Puerto Rico | 84.9% (678/794) | Referent | NA |
| West | 91.9% (426/465) | 1.08 (1.02, 1.15)* | NA |
| Midwest | 93.4% (148/168) | 1.10 (1.02, 1.18)* | NA |
| Northeast | 92.3% (247/287) | 1.09 (1.02, 1.16)* | NA |
| Social characteristics | |||
| Socioeconomic statusc | |||
| Low | 85.6% (699/832) | Referent | NA |
| Medium | 88.8% (542/612) | 1.04 (0.98, 1.10) | NA |
| High | 96.9% (258/270) | 1.13 (1.07, 1.19)* | 1.08 (1.01, 1.16)* |
| Homeless, past year | |||
| Yes | 76.7% (159/201) | 0.85 (0.75, 0.97)* | NA |
| No | 90.2% (1340/1513) | Referent | Referent |
| Incarcerated, ever | |||
| Yes | 83.8% (457/556) | 0.92 (0.86, 0.99)* | NA |
| No | 91.1% (1042/1158) | Referent | NA |
| Health insurance | |||
| Yes | 91.7% (1327/1469) | Referent | Referent |
| No | 71.7% (172/244) | 0.78 (0.67, 0.91)* | 0.85 (0.76, 0.96)* |
| Years since HIV diagnosis | |||
| ≤ 3 years | 83.6% (412/518) | 0.92 (0.86, 0.98)* | NA |
| > 3 years | 91.1% (1069/1176) | Referent | NA |
| Discrimination related to being gay/bisexuald | |||
| Yes | 86.7% (556/656) | 0.96 (0.90, 1.03) | NA |
| No | 90.0% (942/1057) | Referent | NA |
| Perceived community stigma related to living with HIVe | |||
| Lowest | NA | Referent | Referent |
| Low | NA | 0.97 (0.96, 0.98)* | 0.97 (0.96, 0.99)* |
| Moderate | NA | 0.92 (0.89, 0.95)* | 0.94 (0.90, 0.98)* |
| High | NA | 0.86 (0.78, 0.93)* | 0.89 (0.82, 0.97)* |
| Highest | NA | 0.76 (0.63, 0.92)* | 0.84 (0.71, 0.98)* |
Frequencies are based upon weighted counts to account for sampling, non-response, and multiplicity
*p < .05
aHispanic/Latino based upon ethnicity reported and may include individuals of any race
bOther race/ethnicity includes American Indian or Alaska Native, Asian, Native Hawaiian or Other Pacific Islander, and multiple races
cLow SES was defined as having an income < $25,000 or no high school diploma. Medium SES was defined as having an income $25,000–$49,999 or high school diploma (but not categorized as low SES based upon the criterion above). High SES was defined as having an income > $50,000 and a college degree
dExperiencing one or more of five measured types of discrimination related to being gay or bisexual
eVariable was analyzed as a continuous variable. To provide results in a way that could be interpreted, aPRs were estimated from the model for four categories of stigma as compared to the lowest category for presentation of results
Fig. 1.
Percentage of HIV-positive men who have sex with men currently taking ART by perceived community stigma related to having HIV
Discussion
For persons with HIV infection, taking and adhering to ART leads to sustained viral suppression, positive health outcomes, and effectively no risk of sexual transmission to others when viral suppression is achieved and maintained [5–11]. We found that among self-reported HIV-positive MSM recruited from 20 major cities with high HIV prevalence, 89% were currently taking ART. Our study explored which factors were associated with taking ART among MSM after controlling for several variables. We found that not having health insurance and higher perceived community stigma related to having HIV were significant risk factors for not taking ART. We also found that having a high SES, compared to a low SES, was associated with taking ART. Interestingly, race/ethnicity was not significantly associated with taking ART in either the unadjusted or adjusted analyses. Although the prevalence of taking ART was 6.1 percentage points lower among Black MSM than White MSM in the descriptive analysis, the difference was not statistically significant. In an analysis of 2008 NHBS data, the prevalence of taking ART was 13 percentage points lower among Black MSM than White MSM [12]. This suggests that progress is being made in reducing long-standing racial disparities in HIV treatment among MSM.
MSM who believed that people in their city would discriminate against them because they had HIV were less likely to take ART. These men may have been discouraged from accessing HIV medical care for fear that they would be treated poorly because they had HIV. This finding is consistent with other studies that have shown that among persons with HIV, high levels of HIV-related stigma are associated with lower levels of adherence to ART and lower access to HIV medical care and medical care in general [24–28]. For MSM, HIV-related stigma may be compounded by stigma related to being gay or bisexual—a stigma commonly experienced by MSM. One study found that 41% of MSM reported experiencing verbal harassment, discrimination, or physical assault related to being a sexual minority over the past year [29].
We found that the prevalence of taking ART was lower among MSM who did not have health insurance compared to MSM with health insurance. Persons who are uninsured are substantially less likely to have a usual source of health care or recent health care visit than persons who have health insurance. Not only does not having health insurance limit access to ART, it also limits access to other types of care that would help prevent illness, control acute episodes, or manage chronic conditions (including HIV) to avoid making them worse [30]. Having health insurance is also a predictor of whether persons at high risk for HIV, including MSM, use pre-exposure prophylaxis (PrEP) to prevent HIV infection prior to exposure. One study found that insured patients were four times more likely to use PrEP services compared to the uninsured [31]. Lack of access to health care coupled with fear of being stigmatized by the healthcare system may lead to poor health-related outcomes for MSM with HIV.
Prado et al. argue that to effectively respond to the HIV epidemic at a population level and reduce HIV health disparities that exist among ethnic minority youth, there must be an emphasis placed on macro-level interventions (e.g., structural or policy interventions) [32]. For example, to respond to stigma and protect people with HIV from violence, retaliation, and discrimination, national HIV goals support strengthening proactive enforcement of civil rights laws; ensuring that federal and state criminal laws reflect current evidence-based public health approaches regarding HIV transmission and prevention; ensuring that messages about anti-stigma civil rights and health information privacy rights are incorporated into federal documents, programs, and educational campaigns; mobilizing communities to educate people and reduce HIV-related stigma; and promoting public leadership of people with HIV [4]. Increasing public awareness of “Treatment as Prevention”—that is that people with HIV who take HIV medicine as prescribed and keep an undetectable viral load (or stay virally suppressed) have effectively no risk of transmitting HIV to their HIV-negative sexual partners—may also help reduce HIV-related stigma for MSM [33].
Another potential approach for reducing the impact that perceived community stigma related to having HIV has on ART use is to promote medical home models similar to those used by Ryan White HIV/AIDS (RWHAP) program facilities. RWHAP facilities offer comprehensive, patient-centered care where care, case management, and support services are integrated and patient-provider relationships are emphasized [34]. One study found that persons with HIV who attended a non-RWHAP-funded facility reported higher HIV-related discrimination compared to those who attended a RWHAP funded facility [35].
To help MSM who lack health insurance, trained health navigators (also known as peer navigators or patient navigators) can work with clients and service providers to connect MSM to timely and essential HIV-related medical and social services. There is evidence to suggest that HIV navigation services may lead to clients obtaining health insurance and improved health outcomes [36].
There are several limitations to the findings in this report. First, NHBS is not a national representative sample and results may not be generalizable to all cities. Weighted results are generalizable to all venue-attending HIV-positive MSM in participating cities. HIV-positive venue-attending MSM may be different from HIV-positive MSM who do not attend venues in unknown but important ways. For example, MSM who are not well enough to attend social venues are unlikely to be included in our sample. Second, because we relied on self-reported data, social desirability may have resulted in the overestimation of ART use. Third, we were only able to look at individual-level factors at the patient level. Factors at the systems, structural, or provider levels were not available for this analysis. For example, beyond examining region, we did not conduct a multi-level analysis to allow for the consideration of characteristics of MSAs as structural predictors. Finally, the cut-off point for defining recent diagnosis was not empirically derived; rather, it was selected to allow for sufficient sample size to conduct analysis for this variable.
In summary, our findings suggest that to increase access to and use of ART among MSM with HIV, we need to continue to develop, disseminate, and evaluate interventions that will respond to these macro-level social and economic factors that prevent access to care and optimal health outcomes.
Acknowledgements
The authors would like to thank all NHBS participants in 2014, as well as members of the NHBS Study Group: Atlanta, GA: Pascale Wortley, Jeff Todd, Kimi Sato; Baltimore, MD: Colin Flynn, Danielle German; Boston, MA: Dawn Fukuda, Rose Doherty, Chris Wittke; Chicago, IL: Nikhil Prachand, Nanette Benbow, Antonio D. Jimenez; Dallas, TX: Jonathon Poe, Shane Sheu, Alicia Novoa; Denver, CO: Alia Al-Tayyib, Melanie Mattson; Detroit, MI: Vivian Griffin, Emily Higgins, Kathryn Macomber; Houston, TX: Salma Khuwaja, Hafeez Rehman, Paige Padgett; Los Angeles, CA: Ekow Kwa Sey, Yingbo Ma; Miami, FL: Marlene LaLota, John-Mark Schacht, David Forrest; Nassau-Suffolk, NY: Bridget Anderson, Amber Sinclair, Lou Smith; New Orleans, LA: William T. Robinson, Narquis Barak, Meagan C. Zarwell; New York City, NY: Alan Neaigus, Kathleen H. Reilly; Newark, NJ: Barbara Bolden, Afework Wogayehu, Henry Godette; Philadelphia, PA: Kathleen A. Brady, Mark Shpaner, Jennifer Shinefeld; San Diego, CA: Lissa Bayang, Veronica Tovar-Moore; San Francisco, CA: H. Fisher Raymond, Theresa Ick; San Juan, PR: Sandra Miranda De León, Yadira Rolón-Colón; Seattle, WA: Tom Jaenicke, Hanne Thiede, Richard Burt; Washington, DC: Jenevieve Opoku, Irene Kuo; CDC: Winston Abara, Alexandra Balaji, Dita Broz, Laura Cooley, Melissa Cribbin, Paul Denning, Teresa Finlayson, Kathy Hageman, Kristen Hess, Brooke Hoots, Wade Ivy, Rashunda Lewis, Stacey Mason, Gabriela Paz-Bailey, Kathryn Salo, Catlainn Sionean, Amanda Smith, Justin Smith, Michael Spiller, Cyprian Wejnert, Mingjing Xia.
Funding information
Financial suppport for NHBS was provided by the US Centers for Disease Control and Prevention (CDC) through a cooperative agreement with state and local health departments.
Compliance with Ethical Standards
Statement about Research Involving Human Participants and Informed Consent
NHBS activities were approved by local institutional review boards for each of the 20 MSAs. The project underwent a CDC review and approval process and was determined to be research in which CDC was not directly engaged and, therefore, did not require review by CDC IRB. All participants were explicitly assured during the recruitment process of the anonymous nature of the survey and the HIV testing. All participants provided oral informed consent to participate in the interview and be tested for HIV. Oral consent was documented electronically on the survey instrument by interviewers for all participants and on hard copy as required by local IRBs. Data collection was anonymous, so written consent was not possible and participant’s names or other personal identifiers were not linked to any NHBS instruments. All consent procedures, including oral consent, were approved by local IRBs.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Purcell DW, Johnson CH, Lansky A, et al. Estimating the population size of men who have sex with men in the United States to obtain HIV and syphilis rates. Open AIDS J. 2012;6:98–107. doi: 10.2174/1874613601206010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. HIV surveillance report. Vol 29. Atlanta, GA: US Department of Health and Human Services, CDC; 2017. November 2018. http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Accessed 13 July 2019
- 3.Singh Sonia, Song Ruiguang, Johnson Anna Satcher, McCray Eugene, Hall H. Irene. HIV Incidence, Prevalence, and Undiagnosed Infections in U.S. Men Who Have Sex With Men. Annals of Internal Medicine. 2018;168(10):685. doi: 10.7326/M17-2082. [DOI] [PubMed] [Google Scholar]
- 4.Office of National AIDS Policy. National HIV/AIDS strategy for the United States: updated to 2020. Washington, DC: Office of National AIDS Policy; 2015. https://obamawhitehouse.archives.gov/sites/default/files/docs/national_hiv_aids_strategy_update_2020.p. Accessed 13 July 2019
- 5.Cohen MS, Chen YQ, McCauley M, et al. HPTN 052 Study Team. Prevention of HIV- 1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. https://www.nejm.org/doi/10.1056/NEJMoa1105243?url_ver=Z39.88 2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dwww.ncbi.nlm.nih.gov. Accessed 13 July 2019 [DOI] [PMC free article] [PubMed]
- 6.Lundgren JD, Babiker AG, Gordin F, et al. INSIGHT START Study Group. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373:795–807. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4569751/. Accessed 13 July 2019 [DOI] [PMC free article] [PubMed]
- 7.Cohen MS, Chen YQ, McCauley M, et al. Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med. 2016;375:830–839. doi: 10.1056/NEJMoa1600693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodger Alison J., Cambiano Valentina, Bruun Tina, Vernazza Pietro, Collins Simon, van Lunzen Jan, Corbelli Giulio Maria, Estrada Vicente, Geretti Anna Maria, Beloukas Apostolos, Asboe David, Viciana Pompeyo, Gutiérrez Félix, Clotet Bonaventura, Pradier Christian, Gerstoft Jan, Weber Rainer, Westling Katarina, Wandeler Gilles, Prins Jan M., Rieger Armin, Stoeckle Marcel, Kümmerle Tim, Bini Teresa, Ammassari Adriana, Gilson Richard, Krznaric Ivanka, Ristola Matti, Zangerle Robert, Handberg Pia, Antela Antonio, Allan Sris, Phillips Andrew N., Lundgren Jens. Sexual Activity Without Condoms and Risk of HIV Transmission in Serodifferent Couples When the HIV-Positive Partner Is Using Suppressive Antiretroviral Therapy. JAMA. 2016;316(2):171. doi: 10.1001/jama.2016.5148. [DOI] [PubMed] [Google Scholar]
- 9.Bavinton Benjamin R, Pinto Angie N, Phanuphak Nittaya, Grinsztejn Beatriz, Prestage Garrett P, Zablotska-Manos Iryna B, Jin Fengyi, Fairley Christopher K, Moore Richard, Roth Norman, Bloch Mark, Pell Catherine, McNulty Anna M, Baker David, Hoy Jennifer, Tee Ban Kiem, Templeton David J, Cooper David A, Emery Sean, Kelleher Anthony, Grulich Andrew E, Grulich Andrew E, Zablotska-Manos Iryna B, Prestage Garrett P, Jin Fengyi, Bavinton Benjamin R, Grinsztejn Beatriz, Phanuphak Nittaya, Cooper David A, Kelleher Anthony, Emery Sean, Fairley Christopher K, Wilson David, Koelsch Kersten K, Triffitt Kathy, Doong Nicolas, Baker David, Bloch Mark, Templeton David J, McNulty Anna, Pell Catherine, Hoy Jennifer, Tee Ban Kiem, Moore Richard, Roth Norman, Orth David, Pinto Angie N. Viral suppression and HIV transmission in serodiscordant male couples: an international, prospective, observational, cohort study. The Lancet HIV. 2018;5(8):e438–e447. doi: 10.1016/S2352-3018(18)30132-2. [DOI] [PubMed] [Google Scholar]
- 10.Rodger AJ. Risk of HIV transmission through condomless sex in MSM couples with suppressive ART: The PARTNER2 Study extended results in gay men. Presented at the 22nd International AIDS Conference; July 23–27, 2018; Amsterdam, the Netherlands.
- 11.Centers for Disease Control.HIV treatment as prevention; 2018. Retrieved from www.cdc.gov/hiv/risk/art/index.html. Accessed 13 July 2019
- 12.Hoots Brooke E, Finlayson Teresa J, Wejnert Cyprian, Paz-Bailey Gabriela. Updated Data on Linkage to Human Immunodeficiency Virus Care and Antiretroviral Treatment Among Men Who Have Sex With Men—20 Cities, United States. The Journal of Infectious Diseases. 2017;216(7):808–812. doi: 10.1093/infdis/jix007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 dependent areas, 2016. HIV Surveillance Supplemental Report 2018;23(No. 4). http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Published June 2018.
- 14.Finlayson TJ, Le B, Smith A, et al. HIV risk, prevention, and testing behaviors among men who have sex with men—National HIV Behavioral Surveillance System, 21 U.S. cities, United States, 2008. MMWR Morb Mortal Wkly Rep. 2011;60:1–34. https://www.cdc.gov/mmwr/preview/mmwrhtml/ss6014a1.htm. Accessed 13 July 2019 [PubMed]
- 15.MacKellar Duncan A., Gallagher Kathleen M., Finlayson Teresa, Sanchez Travis, Lansky Amy, Sullivan Patrick S. Surveillance of HIV Risk and Prevention Behaviors of Men Who Have Sex with Men—A National Application of Venue-Based, Time-Space Sampling. Public Health Reports. 2007;122(1_suppl):39–47. doi: 10.1177/00333549071220S107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. National HIV Behavioral Surveillance System Round 4: Model Surveillance Protocol. December 11, 2015. https://www.cdc.gov/hiv/pdf/statistics/systems/nhbs/nhbs_round4modelsurveillanceprotocol.pdf.
- 17.Sionean C, Le BC, Hageman K. HIV risk, prevention, and testing behaviors among heterosexuals at increased risk for HIV infection— National HIV Behavioral Surveillance System, 21 U.S. Cities, 2010. MMWR Surveill Summ. 2014;63(SS14):1–39. https://www.cdc.gov/mmwr/preview/mmwrhtml/ss6314a1.htm. Accessed 13 July 2019 [PubMed]
- 18.DiNenno EA, Oster AM, Sionean C, Denning P, Lansky A. Piloting a system for behavioral surveillance among heterosexuals at increased risk of HIV in the United States. Open AIDS J. 2012;6(1):169–176. doi: 10.2174/1874613601206010169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams D, Yu Y, Jackson JS, Anderson NB. Racial differences in physical and mental health: socio-economic status, stress, and discrimination. J Health Psychol. 1997;2(3):335–351. doi: 10.1177/135910539700200305. [DOI] [PubMed] [Google Scholar]
- 20.Herek G, Capitanio JP, Widaman KF. HIV-related stigma and knowledge in the United States: prevalence and trends, 1991–1999. Am J Public Health. 2002;92:371–377. doi: 10.2105/AJPH.92.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalichman SC, Simbayi LC, Jooste S, et al. Development of a brief scale to measure AIDS-related stigma in South Africa. AIDS Behav. 2005;9(2):135–143. doi: 10.1007/s10461-005-3895-x. [DOI] [PubMed] [Google Scholar]
- 22.Iachan R, Finlayson T, Kyle T, Wejnert C, Paz-Bailey G. Weighting for venue-based sampling: the MSM3 study. Joint Statistical Meeting: August 3, 2013; Montreal, Canada.
- 23.Karon John M., Wejnert Cyprian. Statistical Methods for the Analysis of Time–Location Sampling Data. Journal of Urban Health. 2012;89(3):565–586. doi: 10.1007/s11524-012-9676-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vanable Peter A., Carey Michael P., Blair Donald C., Littlewood Rae A. Impact of HIV-Related Stigma on Health Behaviors and Psychological Adjustment Among HIV-Positive Men and Women. AIDS and Behavior. 2006;10(5):473–482. doi: 10.1007/s10461-006-9099-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sweeney Shannon M., Vanable Peter A. The Association of HIV-Related Stigma to HIV Medication Adherence: A Systematic Review and Synthesis of the Literature. AIDS and Behavior. 2015;20(1):29–50. doi: 10.1007/s10461-015-1164-1. [DOI] [PubMed] [Google Scholar]
- 26.Kinsler JJ, Wong MD, Sayles JN, Davis C, Cunningham WE. The effect of perceived stigma from a health care provider on access to care among low-income HIV-positive population. AIDS Patient Care STDs. 2007;21(8):584–92. https://www.liebertpub.com/doi/abs/10.1089/apc.2006.0202?url_ver=Z39.88- 2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed. Accessed 13 July 2019 [DOI] [PubMed]
- 27.Sayles JN, Wong MD, Kinsler J, Martins D, Cunningham WE. The association of stigma with self-reported access to medical care and antiretroviral therapy adherence in persons living with HIV/AIDS. J Gen Intern Med. 2009;24(10):1101–8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2762503/. Accessed 13 July 2019 [DOI] [PMC free article] [PubMed]
- 28.Rice Whitney S., Crockett Kaylee B., Mugavero Michael J., Raper James L., Atkins Ghislaine C., Turan Bulent. Association Between Internalized HIV-Related Stigma and HIV Care Visit Adherence. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2017;76(5):482–487. doi: 10.1097/QAI.0000000000001543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balaji AB, Bowles KE, Hess KL, Smith JC, Paz-Bailey G. Association between enacted stigma and HIV-related risk behavior among MSM, National Behavioral Surveillance System, 2011. AIDS Behav. 2017;21:227–37. https://link.springer.com/article/10.1007/s10461-016-1599-z. Accessed 13 July 2019 [DOI] [PubMed]
- 30.NCHS. Health insurance and access to care. 2017. https://www.cdc.gov/nchs/data/factsheets/factsheet_health_insurance_and_access_to_care.pdf/. Accessed 13 Jul 2019.
- 31.Patel RR, Mena L, Nunn A, et al. Impact of insurance coverage on utilization of pre-exposure prophylaxis for HIV prevention. PLoS One. 2017;12(5):e0178737. doi: 10.1371/journal.pone.0178737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prado G. Lightfoot m, Brown, CH. Macro-level approaches to HIV prevention among ethnic minority youth. Am Psychol. 2013;68(4J):286–99. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3771582/pdf/nihms492151.pdf. Accessed 13 July 2019 [DOI] [PMC free article] [PubMed]
- 33.CDC. HIV Treatment as prevention. https://www.cdc.gov/hiv/risk/art/index.html. Accessed 13 Jul 2019.
- 34.Beane SN, Culyba RJ, DeMayo M, Armstrong W. Exploring the medical home in Ryan White HIV care settings: a pilot study. J Assoc Nurse AIDS C. 2014;25(3):191–202. doi: 10.1016/j.jana.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baugher Amy R., Beer Linda, Fagan Jennifer L., Mattson Christine L., Shouse R. Luke. Discrimination in healthcare settings among adults with recent HIV diagnoses. AIDS Care. 2018;31(9):1077–1082. doi: 10.1080/09540121.2018.1545988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bradford Judith B., Coleman Sharon, Cunningham William. HIV System Navigation: An Emerging Model to Improve HIV Care Access. AIDS Patient Care and STDs. 2007;21(s1):S-49-S-58. doi: 10.1089/apc.2007.9987. [DOI] [PubMed] [Google Scholar]