Abstract
BACKGROUND
The optimal timing of corticosteroid (CS) treatment in patients with primary central nervous system (CNS) lymphoma (PCNSL) remains controversial. While poor clinical presentation may justify early treatment with CS, this may ultimately result in reduced concentrations of chemotherapeutic agents via perturbations in the permeability of the blood-brain barrier.
OBJECTIVE
To investigate whether early CS exposure is associated with beneficial outcomes and/or reduced occurrence of adverse events as opposed to delayed/concomitant administration.
METHODS
Herein we performed a retrospective observational analysis using patients that were prospectively entered into a database. All patients whom were admitted to the University Hospital between 2009 and 2015 with newly diagnosed PCNSL were included within our study.
RESULTS
Our cohort included 50 consecutive patients diagnosed with PCNSL; of these, in 30 patients CS administration was initiated prior to chemotherapy (early), whilst in the remaining 20 patients CS administration was initiated concomitantly with their chemotherapeutic regimen (concomitant). Within the early vs concomitant CS administration groups, no significant differences were observed with regard to progression-free survival (PFS) (P = .81), overall survival (OS) (P = .75), or remission (P = .68; odds ratio 0.76 and confidence interval [95%] 0.22-2.71). Critically, the timing of CS initiation was not associated with either PFS (P = .81) or PFS (P = .75).
CONCLUSION
Early CS administration was not associated with a deterioration in response to chemotherapy, PFS, or OS. As such, administration of CS prior to initiation of chemotherapy is both reasonable and safe for patients with newly diagnosed PCNSL.
Keywords: Primary CNS lymphoma, Corticosteroids, Systemic therapy, Treatment response, Survival, Complete response
ABBREVIATIONS
- BBB
blood-brain barrier
- CI
confidence interval
- CNS
central nervous system
- CS
corticosteroid
- CSF
cerebrospinal fluid
- ECOG
Eastern Cooperative Oncology Group
- HIV
human immunodeficiency virus
- HR
hazard ratio
- KPS
Karnofsky performance scale
- NHL
non-Hodgkin lymphoma
- OR
odds ratio
- OS
overall survival
- PCNSL
primary central nervous system lymphoma
- PFS
progression-free survival
Primary central nervous system (CNS) lymphoma (PCNSL) is a rare variant of extranodal non-Hodgkin lymphoma (NHL) that involves the brain, leptomeninges, eyes, and/or spinal cord, without additional evidence suggestive of systemic disease.1 PCNSL accounts for approximately 3% of all newly diagnosed primary brain tumors and 3% of all cases of NHL.2
Historically, patients with PCNSL have been associated with a poorer prognosis compared to patients with aggressive systemic lymphomas.3 However, recent data have come to suggest improvement in outcomes for patients with lymphoma.4-7 While current clinical trials have focused primarily on the development and/or combination of chemotherapeutic agents,8 little attention has been paid to the administration of corticosteroids (CS) in PCNSL.
In contrast to the treatment paradigms employed for other intracranial lesions, wherein CSs are principally employed to reduce perilesional edema, CS employed in the treatment of PCNSL exhibit cytolytic effects on lymphoma cells.9,10 Such findings are also reflected in a number of multicenter prospective trials that have incorporated CS administration into chemotherapeutic cycles.11
Of note, CSs have been shown to impact several critical properties of the blood-brain barrier (BBB), including tight junction integrity.12 Consequently, CS administration not only influences brain hemostasis, but also the delivery/bioavailability of CNS-targeted therapeutics.12 Despite such findings, the literature lacks focus on the impact of treatment with CS prior to the initiation of chemotherapy in PCNSL. As such, we herein assess the association of patient outcomes with the timing of the initiation of CS administration (ie, prior to chemotherapy vs concomitant with chemotherapy).
METHODS
Patients
All patients with newly-diagnosed, histologically-confirmed PCNSL who presented at the corresponding author's institution between January 2009 and December 2015 were prospectively entered into a curated institutional database as per approval of the institutional ethics committee (reference # 04/09 SNO 01/08). Patient follow-up was sustained via outpatient hematology/oncology and neurosurgery departments. For the purposes of this study, additional follow-up was performed via a standardized phone call. Beyond baseline demographics, the patients’ Karnofsky performance scale (KPS) and Eastern Cooperative Oncology Group (ECOG) statuses were obtained at admission by an attending neurosurgeon. Moreover, histopathologic features, extra-CNS-manifestations, and the clinical course of each patient were recorded.
Within the indicated inclusion period, 62 patients were diagnosed with CNS lymphoma manifesting as an intracerebral lesion. We excluded patients with lymphoma manifestations outside of the CNS from our analysis (n = 8) and patients without documented CS treatment during their clinical course (n = 4). Fifty patients with newly diagnosed PCNSL remained, and were subsequently used to populate our retrospective cohort. All patients received dexamethasone as per the institutional standard. For the diagnostic procedure, stereotactic biopsy, written informed consent was obtained from the patient or the patient's legal representative. Clinical indications for or against perioperative CS treatment were determined by at least two experienced specialists in neurosurgery, based on clinical parameters and the expected clinical course of the patient.
Procedures
Attending neuropathologists participated in every stereotactic biopsy to confirm that tissue was obtained from the pathological lesion of interest. Stereotactic trajectories were planned and performed by the attending neurosurgeon. The surgeon accounted for tumor location, contrast enhancement, peritumoral edema, central necrosis, and patient history. Stereotactic planning was performed as has been published.13 For all stereotactic biopsies, a stereotactic frame was utilized (Leksell Coordinate Frame G; Elekta Instruments, Stockholm, Sweden). The procedure was performed and/or supervised by 1 of 3 experienced neurosurgeons with clinical expertise in stereotactic procedures as previously described.14 All tumor specimens were evaluated using classic hematoxylin/eosin staining, and selected specimens were investigated using immunohistochemistry. All specimens were examined by at least 2 board-certified neuropathologists and at least 2 board-certified pathologists at the local referral center at the Senckenberg Institute for Surgical Pathology.
The number of biopsies, trajectories, and all results from histopathological and molecular analyses were prospectively entered into the abovementioned institutional database.
Outcomes
Data were collected in both primary neurosurgery and follow-up hematology/oncology centers (ie, where patients were transferred for management after their initial diagnosis of PCNSL). Obtained data were entered into the database by either the treating physician or study nurse. Outcome parameters were response, progression-free survival (PFS), and overall survival (OS). Progress was defined clinically or radiologically as per standard criteria.15
Statistical Analyses
We defined 2 cohorts for statistical analysis: patients with the first administration of CS prior to the initiation of chemotherapy patients were assigned to the early treatment group, whereas patients with the first administration of CS concomitant with their chemotherapeutic regimen were assigned to the concomitant treatment group.
The Mann–Whitney U-test was employed for nonparametric data. Binary parameters were analyzed using a χ2-test and binary logistic regression was employed for multivariable analysis. Sex, age, KPS at admission, ECOG at admission, marker expression, immunocompetency status, cerebrospinal fluid (CSF) manifestations, other CNS manifestations, systemic therapy, biopsy-related parameters, time/onset of CS treatment, and histology were considered independent variables. Dependent variables were defined as treatment response, PFS, and OS for the analysis of outcomes. OS was defined as the time between stereotactic biopsy for the definitive diagnosis of lymphoma and the date of death; PFS was defined as the time between stereotactic biopsy for the definitive diagnosis of lymphoma and the date of clinical or radiological progression. For both OS and PFS, subjects were censored at the time of their last clinical follow-up appointment.
Groups were compared using a log rank test and pointwise 95% confidence intervals (CIs). A multivariable Cox's proportional hazards regression backward stepwise model (likelihood ratio) was performed to find independent predictors for outcome parameters.
Results with P < .05 were considered to be statistically significant. To rule out potential confounding factors in the univariate analysis, we performed multivariable analysis to determine independent risk factors and included parameters identified in univariate with a P value of P < .1.
All calculations/analyses were performed with SPSS (Version 22, IBM, Armonk, New York).
RESULTS
Of the 50 patients included within our study, CS were administered prior to chemotherapy in 30 patients (early), whilst the remaining 20 patients received CS concomitantly with their chemotherapeutic regimen (concomitant).
Baseline Characteristics
All patients included within our analyses underwent stereotactic biopsy for the diagnosis of lymphoma; none of the patients underwent microsurgical resection of their lesions. Further analysis of the administration of CS revealed that 3 patients within the early CS group (10%) received steroids prior to biopsy (Table 1). Five patients (16.7%) within the early CS group and 2 patients (10%) within the concomitant CS group were in an immunocompromised state at the time of diagnosis (eg, human immunodeficiency virus (HIV) infection). The majority of the patients were diagnosed with a diffuse large B-cell lymphoma in both the early CS (n = 30/100%) and concomitant CS (n = 18/90%) cohorts. Only 2 patients (10%) within the concomitant CS treatment group were diagnosed with a T-cell rich B-cell lymphoma. Further, the majority of patients within both early CS (n = 17/56.7%) and concomitant CS (n = 12/60%) were treated with high-dose methotrexate (HD-MTX)-containing regimens and included in clinical trials whenever possible. Median time from administration of CS to initiation of systemic chemotherapy was 9.1 d (SD 4.4 d, range 1-18 d) in the early CS group (Figure, Supplemental Digital Content 1).
TABLE 1.
Patient Characteristics
| Early corticosteroids (n = 30) | Concomitant corticosteroids (n = 20) | ||
|---|---|---|---|
| Age (yr[IQR]) | 67.5 (53-78) | 69 (60-74) | P = .84 |
| Female sex (n[%]) | 12 (40%) | 10 (50%) | P = .49 |
| KPS (n[%]) | P = .56 | ||
| 100-70 | 22 (73.4%) | 17 (85%) | |
| 60-50 | 4 (13.3%) | 1 (5%) | |
| <50 | 4 (13.3%) | 2 (10%) | |
| ECOG (n[%]) | P = .49 | ||
| 0 | 9 (30.3%) | 7 (35%) | |
| 1 | 13 (45.5%) | 8 (40%) | |
| 2 | 1 (3.3%) | 3 (15%) | |
| 3 | 3 (10%) | 1 (5%) | |
| 4 | 4 (13.3%) | 1 (5%) | |
| Preoperative CS (n[%]) | 3 (10%) | 0 (0%) | P = .15 |
| Immunosuppression (n[%]) | 5 (16.7%) | 2 (10%) | P = .69 |
| CNS manifestation (n[%]) | P = .9 | ||
| CSF | 2 (46.7%) | 1 (40%) | |
| Other | 6 (13.3%) | 3 (40.0%) | |
| Histology (n[%]) | P = .15 | ||
| Diffuse large B cell lymphoma | 30 (100%) | 18 (90%) | |
| T cell rich B cell lymphoma | 0 (0%) | 2 (10%) | |
| Ki67 (n[%]) | P = .7 | ||
| 90% | 6 (20%) | 3 (15%) | |
| 80% | 7 (23.3%) | 7 (35%) | |
| 70% | 1 (3.3%) | 2 (10%) | |
| ≤60% | 16 (53.3%) | 8 (40%) | |
| Systemic therapy (n[%]) | P = .32 | ||
| MTX/radiotherapy | 4 (13.3%) | 0 (0%) | |
| Rituximab/MTX/procarbazine | 13 (43.3%) | 12 (60%) | |
| Other | 13 (43.3%) | 8 (40%) | |
| Response (n[%]) | P = .77 | ||
| Complete response | 14 (46.7%) | 11 (55%) | |
| Partial response | 4 (13.3%) | 3 (15%) | |
| Failed response | 12 (40%) | 6 (30%) |
CNS, central nervous system; CS, corticosteroid medication; CSF, cerebrospinal fluid; ECOG, Eastern Cooperative Oncology Group score; IQR, indicates interquartile range; KPS, Karnofsky performance scale
Data are given as means except for age which is presented as the median. Data comparisons were made with Mann–Whitney U-test or χ2-test, where applicable.
No significant differences in baseline or clinical parameters were observed between either of the treatment groups (Table 1). Specifically, no significant differences in prebiopsy CS medication, the number of immunocompromised patients, CSF, other CNS manifestations, or systemic therapy were observed. No significant differences regarding histology (Ki67 as a molecular marker) were observed (Table 1). No significant differences were observed with regard to intraoperative parameters or perioperative complications (Table, Supplemental Digital Content 2).
Biopsy-related Characteristics
Altogether, 6 patients (12%) displayed an intraoperative effusion of blood via the sampling cannula despite thorough planning of the stereotactic procedure (Table, Supplemental Digital Content 2). In all of these patients, postoperative signs of blood were demonstrated on postoperative CT (k = 1, P < .001, and data not shown). Two patients, both of whom were among the early CS treated patients (4%), displayed postoperative neurological deficits. No significant differences were observed regarding the above-named biopsy-related parameters when comparing both groups.
Effect of CS Administration Timing on Response
In the early CS group, 14 patients (46.7%) achieved complete response, compared to 11 patients within the concomitant CS treatment group (55%). No significant differences in response were observed when analyzing the influence of CS medication before the initiation of chemotherapy for PCNSL (P = .77; Table 1).
Those patients who attained a complete response had a significantly better clinical status upon presentation, reflected by the significant association of a poor ECOG status with a lack of complete response (odds ratio [OR] 0.08, 95% CI, 0.01-0.69, P = .007, and univariate, Table 2). Moreover, a trend was observed suggesting that a poor KPS upon presentation is associated with lack of complete response (OR 0.38, 95% CI, 0.1-1.4, and P = .1).
TABLE 2.
Univariate and Multivariate Analysis of Parameters Associated With Complete Response (Nagelkerke R2 = 0.242)
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age > 68 yr | 0.96 (0.27-3.36) | .95 | ||
| KPS < 90 | 0.38 (0.1-1.4) | .1 | .72 | |
| ECOG < 1 | 0.08 (0.01-0.69) | .007 | 0.08 (0.01-0.69) | .022 |
| Early CS | 0.76 (0.22-2.71) | .68 | ||
| Immunosuppression | 0.75 (0.09-5.9) | .79 | ||
| CSF manifestation | 1.7 (0.14-20.4) | .67 | ||
| Other CNS manifestation | 3.79 (0.38-37.3) | .23 | ||
| Intracerebral blood on CT | 1.78 (0.29-11) | .53 | ||
| New postop deficit | 0.81 (0.05-13.9) | .88 | ||
| T cell rich B cell lymphoma | 0.52 (0.39-0.71 | .19 | ||
| Ki 67 ≥ 80% | 0.78 (0.16-3.67) | .75 | ||
| Rituximab/MTX/procarbazine | 0.8 (0.1-6.3) | .82 | ||
CNS, central nervous system; CI, confidence interval; CS, corticosteroid medication; CSF, cerebrospinal fluid; ECOG, Eastern Cooperative Oncology Group score; OR, odds ratio
OR is displayed in significant outcome parameters/where applicable.
Data comparisons were made with χ2-test for univariate analysis and binary logistic regression with stepwise exclusion was used for multivariable analysis.
Critically, no association with the timing of initiation of CS treatment with regard to a complete response was observed (OR 0.76, 95% CI, 0.22-2.71; P = .68, and univariate).
Furthermore, only ECOG status (OR 0.08, 95% CI, 0.01-0.69, and P = .022) was confirmed as an independent parameter associated with complete response via multivariable analysis (Nagelkerke R2 = 0.242). KPS status (P = .72) was not associated with the achievement of complete response.
Effect of CS Timing on PFS
No significant difference was observed in the PFS between the early and concomitant CS groups (Figure 1A). Progression did not occur earlier in the early CS group, with a median time of 348 d (95% CI, 75-620 d) in the early group and a median time of 553 d (95% CI, 256-850 d) in the concomitant group (P = .81; test for proportional hazard assumption P = .46). Multivariable analysis identified the postoperative detection of blood as an independent parameter associated with early progression (hazard ratio (HR) 3.5; 95% CI, 1.22-10; P = .04; Table 3, including univariable analysis).
FIGURE 1.
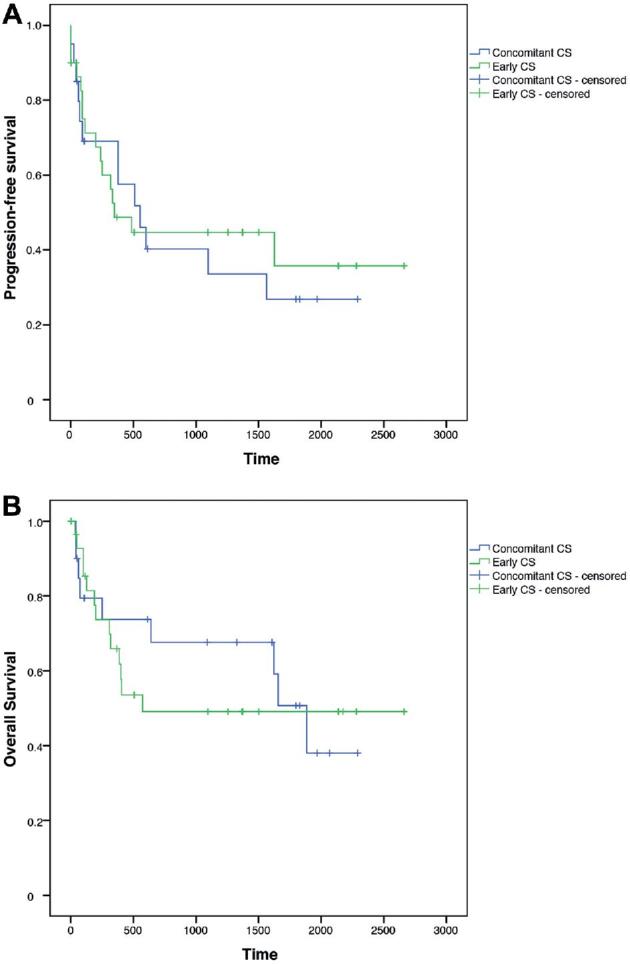
Kaplan–Meier estimates. A, Progression-free survival (logrank test χ2 = 0.058, P = .81). B, Overall survival (logrank test χ2 = 0.102, P = .75).
TABLE 3.
Univariate and Multivariate Analysis of Parameters Associated With Progression-Free Survival
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Median (95% CI) | P value | HR (95% CI) | P value | |
| Age | .42 | |||
| Age > 68 yr | 377 (133-621) | |||
| Age < 68 yr | 1564 (0-3685) | |||
| KPS | .96 | |||
| KPS < 90 | 377 (0-1865) | |||
| KPS ≥ 90 | 511 (227-795) | |||
| ECOG | .71 | |||
| ECOG < 1 | 333 (0-684) | |||
| ECOG ≥ 1 | 511 (209-813) | |||
| CS administration | .81 | |||
| Early | 348 (75-620) | |||
| Concomitant | 553 (256-850) | |||
| Immunosuppression | .67 | |||
| Present | 511 (226-796) | |||
| Absent | 1546 (0-3211) | |||
| CSF manifestation | .11 | |||
| Present | – | |||
| Absent | – | |||
| Other CNS manifestation | .29 | |||
| Present | 1097 (-) | |||
| Absent | 377 (149-605) | |||
| Intracerebral blood on CT | .004 | 3.5 (1.22-10.0) | .04 | |
| Present | 81 (58-104) | |||
| Absent | 553 (0-1390) | |||
| New postop deficit | .005 | .08 | ||
| Present | 0 (-) | |||
| Absent | 511 (260-762) | |||
| Histology | .19 | |||
| T cell rich BCL | 511 (213-808) | |||
| Diffuse large cell BCL | 70 (-) | |||
| Ki 67 | .19 | |||
| ≥80% | 377 (132-622) | |||
| <80% | – | |||
| Adjuvant therapy | .6 | |||
| Rituximab/MTX/procarbazine | 511 (203-819) | |||
| Other | 348 (-) | |||
CI, confidence interval CNS, central nervous system; CS, corticosteroid medication; CSF, cerebrospinal fluid; CT, computed tomography; ECOG, Eastern Cooperative Oncology Group score; HR, hazard ratio; KPS, Karnofsky performance scale
Data comparisons were made with Kaplan–Meier estimates for univariate analysis. Column median indicates median of parameter displayed. No median was calculated for “CSF manifestation” as all cases were censored. Cox-regression analysis with stepwise exclusion was used for multivariable analysis.
Effect of CS Timing on OS
No significant differences in OS between the early and concomitant CS groups were observed (Figure 1B). OS was not significantly decreased in the early CS group, with a median of 573 d in the early group and a median of 1885 d in the concomitant CS group (95% CI, 1524-2246 d, P = .75; test for proportional hazard assumption P = .3). Multivariable analysis demonstrated that the occurrence of a newly developed postoperative deficit (HR 10.84; 95% CI, 2.24-52.5; P = .02) was an independent factor associated with reduced OS (Table 4, including univariable analysis).
TABLE 4.
Univariate and Multivariate Analysis of Parameters Associated With Overall Survival
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Median (95% CI) | P value | HR (95% CI) | P value | |
| Age | .33 | |||
| Age > 68 yr | 641 (0-1633) | |||
| Age < 68 yr | 1885 (-) | |||
| KPS | .44 | |||
| KPS < 90 | 1856 (-) | |||
| KPS ≥ 90 | 1801 (334-2909) | |||
| ECOG | .82 | |||
| ECOG < 1 | 1881 (-) | |||
| ECOG ≥ 1 | 1843 (234-3077) | |||
| CS administration | .75 | |||
| Early | 573 (-) | |||
| Concomitant | 1885 (1524-2246) | |||
| Immunosuppression | .7 | |||
| Present | 1885 (-) | |||
| Absent | 1656 (0-3345) | |||
| CSF manifestation | .17 | |||
| Present | – | |||
| Absent | – | |||
| Other CNS manifestation | .19 | |||
| Present | – | |||
| Absent | 1622 (232-3012) | |||
| Intracerebral blood on CT | .09 | .13 | ||
| Present | 199 (0-405) | |||
| Absent | 1885 (1432-2338) | |||
| New postop deficit | < .001 | 10.84 (2.24-52.5) | .02 | |
| Present | 34 (-) | |||
| Absent | 1885 (325-3445) | |||
| Histology | .22 | |||
| T cell rich BCL | – | |||
| Diffuse large cell BCL | – | |||
| Ki 67 | .293 | |||
| ≥80% | 1622 (60-3184) | |||
| <80% | – | |||
| Systemic therapy | .98 | |||
| Rituximab/MTX/procarbazine | 1885 (1428-2342) | |||
| Other | 401 (-) | |||
CI, confidence interval; CNS, central nervous system; CS, corticosteroid medication; CSF, cerebrospinal fluid; CT, computed tomography; ECOG, Eastern Cooperative Oncology Group score; HR, hazard ratio; KPS, Karnofsky performance scale
Data comparisons were made with Kaplan–Meier estimates for univariate analysis. Column median indicates median of parameter displayed. No median was calculated for “Other CNS manifestation and CSF manifestation” as all cases were censored. Cox-regression analysis with stepwise exclusion was used for multivariable analysis.
DISCUSSION
PCNSL accounts for 3% of all systemic lymphomas and for 3% of all primary brain tumors.16 Of note, PCNSL mainly affects elderly patients,16 and the optimal therapeutic regimen for PCNSL continues to be a matter of clinical debate.
Unfortunately, while standalone whole-brain irradiation results in complete remission in 50% of patients with PCNSL, more than 90% of these patients go on to develop a recurrent tumor.17 Therefore, standalone radiation therapy is not an ideal first treatment in those patients diagnosed with PCNSL.18 Meanwhile, several studies have demonstrated the value of combinational chemotherapeutic approaches with regard to PCNSL. For example, studies that combined HD-MTX with other chemotherapeutic agents demonstrated a higher response rate and a prolonged PFS compared to those studies that employed HD-MTX monotherapy.11,19-22 Due to the poor prognosis of PCNSL patients as compared to patients with aggressive systematic lymphomas, an expanded chemotherapeutic regimen has been evaluated.6,7 While the results are encouraging, the experience with high-dose chemotherapy supported by autologous stem cell transplantation is still limited to phase II trials.23
While these highly sophisticated therapeutic regimens have been a matter of intense study, there has been little attention towards evaluating the administration of CS. Interestingly, CS have demonstrated a highly cytolytic action on lymphoma cells in vitro.24 The mechanism involves DNA fragmentation and the induction of apoptosis in a p53-independent fashion,25-28 as has been extensively reviewed.29 Beyond the induction of apoptosis, other studies have come to suggest an induction of autophagy after treatment with CS.30,31 In line with the aforementioned, PCNSL patients display a highly-responsive clinical course upon administration of CS. Initial treatment with CS may produce rapid symptomatic improvement, coupled with a dramatic radiographic response in approximately 40% of patients22,32; such cytolytic activity may result in changes to tissue pathology, altering that which may be detected by a pathologist.33 As such, it is critical to refrain from CS administration in suspected PCNSL cases until a diagnostic biopsy has been performed.34-36
However, controversial recommendations regarding the administration of postdiagnostic CS in PCNSL continue to exist. Due to the cytotoxic and antiedematous effects described in detail above, CS are commonly used in the treatment of lymphomatous lesions within the CNS, and included in almost all chemotherapy protocols for lymphoid malignancies.11,29 It is prudent to note that the administration of CS may also impact the delivery of other chemotherapeutic agents, as steroids have been shown to impact several critical properties related to the permeability of the BBB, such as tight junction integrity. Consequently, administration of CS not only influences brain hemostasis, but also the delivery/bioavailability of CNS-targeted therapeutics.12
In an experimental model of glioma, CS treatment resulted in diminished delivery of the chemotherapeutic agent MTX (ie, the most commonly-used agent in PCNSL regimens) to both the tumor and brain.37 Hence, CS administration may in fact impact the administration of other agents via perturbations in BBB permeability when used as a systemic treatment in PCNSL. Therefore, we sought to analyze our cohort of patients with PCNSL for outcomes related to the timing of CS administration in their clinical course.
In our cohort, the majority of patients (n = 30) received CS prior to further chemotherapy for the treatment of their PCNSL. Clinical indications related to the administration of CS were apparently not based upon the clinical status at presentation, as no significant differences were observed with regard to KPS and ECOG between early and concomitant groups. Both treatment groups included patients with a state of immunosuppression resulting from HIV or posttransplant lymphoproliferative disease. The relatively high number of patients with immunosuppression due to HIV infection may be attributed with the authors’ institution, a primary center for HIV/infectious diseases in Germany.38 Other clinical and pathological parameters such as distribution of sex, age, and histopathologic diagnosis are in line with those reported within the literature.39
When looking at complete response, no significant differences were observed between those patients who received early CS and those patients who received concomitant CS. Of the analyzed factors, only ECOG was associated with complete response in multivariable analysis. This finding is partly in line with the literature, where the performance status has been shown to be associated with survival.19,20,40 However, age was not among the prognosticators associated with complete response in our population. This might be attributed to the combined analysis of immunocompromised and immunocompetent patients, who are significantly younger upon development of PCNSL.
The early administration of CS was not associated with a change in PFS or OS in our analysis. The detection of intracerebral blood on a postoperative CT scan, as an indicator for an intraoperative injury to intracerebral/intratumoral vessels, was the only parameter associated with PFS in our analysis. Moreover, the development of a new postoperative deficit was the only parameter in our analysis associated with OS. Both findings speak to the importance of thorough planning and careful execution of surgical biopsies, thereby avoiding the development of complications/deficits. As observed within the literature, other parameters such as meningeal dissemination were not associated with survival.41
While in general, treatment for PCNSL is carried out in oncology/neurooncology departments, the diagnosis of PCNSL is acquired with a neurosurgical biopsy. According to the data presented within this study, patients may be treated with CS postbiopsy as clinically warranted. However, future studies further assessing potential interactions of tumor cells with the immune system may be impeded by early CS administration prior to the initiation of chemotherapy for PCNSL.
Limitations
While this is the first series focused on the timing of CS administration in patients with PCNSL postbiopsy, it is nonetheless important to note that the study has several limitations. One limitation is the retrospective nature of the study and the lack of randomization with regard to early and concomitant CS groups. Furthermore, there are no standard clinical indications for initiation of CS in the early group, thereby introducing the possibility of selection bias. Finally, while the major strength of this study is the considerably large patient population relative to the limited incidence/prevalence of this neoplasm, the sample size of 50 patients may limit the power of our analyses.
CONCLUSION
To our knowledge, this is the first study focused on the timing of CS administration after biopsy derived sample acquisition in patients with PCNSL. Our data indicate that the early administration of CS in PCNSL is not associated with changes in achieving complete response, PFS, and/or OS. While our data substantiate the results and conclusions drawn from previous studies, discounting differentiation between early and concomitant administration of CS, future clinical trials investigating the induction of cell death and ultimately outcomes in PCNSL should take the onset of CS medication into account due to its broad clinical and biological implications.
Disclosures
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Supplementary Material
Acknowledgments
The authors thank Marina Heibel and Anne Sicking for assistance with the preparation of Figures and Tables. The authors further thank Eva Herrmann, PhD, Director of the Institute for Biostatistics and Mathematical Modelling for continuous support regarding questions on statistical aspects of the manuscript.
Notes
This manuscript has been orally presented in parts on the annual meeting of the German Society for Neurosurgery (DGNC), in Frankfurt, Germany, June 12-15, 2016.
Supplemental Digital Content 1. Figure. Distribution of patients undergoing early CS administration. Early CS administration was defined as initiation prior to systemic therapy, concomitant CS administration was defined as initiation of CS concomitant with systemic therapy. In total, 30 patients underwent early CS treatment and 20 patients underwent concomitant CS treatment.
Supplemental Digital Content 2. Table. Surgical procedure-related characteristics. Data are given as means except for no. of samples that is presented as the median. Data comparisons were made with Mann–Whitney U-test or χ2-test, where applicable. OR is displayed in significant outcome parameters and where applicable. CT, computed tomography; CS, corticosteroid medication; IQR indicates Interquartile range.
REFERENCES
- 1. Louis DN, Perry A, Reifenberger G, et al.. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 2. Villano JL, Koshy M, Shaikh H, Dolecek TA, McCarthy BJ. Age, gender and racial differences in incidence and survival in primary CNS lymphoma. Br J Cancer. 2011;105(9):1414–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Norden AD, Drappatz J, Wen PY, Claus EB. Survival among patients with primary central nervous system lymphoma, 1973–2004. J Neurooncol. 2011;101(3):487–493. [DOI] [PubMed] [Google Scholar]
- 4. Illerhaus G, Muller F, Feuerhake F, Schafer AO, Ostertag C, Finke J. High-dose chemotherapy and autologous stem-cell transplantation without consolidating radiotherapy as first-line treatment for primary lymphoma of the central nervous system. Haematologica. 2008;93(1):147–148. [DOI] [PubMed] [Google Scholar]
- 5. Bromberg JE, Doorduijn JK, Illerhaus G, et al.. Central nervous system recurrence of systemic lymphoma in the era of stem cell transplantation - an International Primary Central Nervous System Lymphoma Study Group project. Haematologica. 2013;98(5):808–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Illerhaus G, Marks R, Ihorst G, et al.. High-dose chemotherapy with autologous stem-cell transplantation and hyperfractionated radiotherapy as first-line treatment of primary CNS lymphoma. J Clin Oncol. 2006;24(24):3865–3870. [DOI] [PubMed] [Google Scholar]
- 7. Kasenda B, Schorb E, Fritsch K, Finke J, Illerhaus G. Prognosis after high-dose chemotherapy followed by autologous stem-cell transplantation as first-line treatment in primary CNS lymphoma—a long-term follow-up study. Ann Oncol. 2012;23(10):2670–2675. [DOI] [PubMed] [Google Scholar]
- 8. Schorb E, Finke J, Ferreri AJ, et al.. High-dose chemotherapy and autologous stem cell transplant compared with conventional chemotherapy for consolidation in newly diagnosed primary CNS lymphoma—a randomized phase III trial (MATRix). BMC Cancer. 2016;16(1):282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Roth P, Korfel A, Martus P, Weller M. Pathogenesis and management of primary CNS lymphoma. Expert Rev Anticancer Ther. 2012;12(5):623–633. [DOI] [PubMed] [Google Scholar]
- 10. Heckmann JG, Bockhorn J, Stolte M, Druschky A, Neundorfer B. An instructive false diagnosis: Steroid-induced complete remission of a CNS tumor–probably lymphoma. Neurosurg Rev. 1998;21(1):48–51. [DOI] [PubMed] [Google Scholar]
- 11. Thiel E, Korfel A, Martus P, et al.. High-dose methotrexate with or without whole brain radiotherapy for primary CNS lymphoma (G-PCNSL-SG-1): a phase 3, randomised, non-inferiority trial. Lancet Oncol. 2010;11(11):1036–1047. [DOI] [PubMed] [Google Scholar]
- 12. Witt KA, Sandoval KE. Steroids and the blood-brain barrier: therapeutic implications. Adv Pharmacol. 2014;71:361–390. [DOI] [PubMed] [Google Scholar]
- 13. Weise LM, Harter PN, Eibach S, et al.. Confounding factors in diagnostics of MGMT promoter methylation status in glioblastomas in stereotactic biopsies. Stereotact Funct Neurosurg. 2014;92(3):129–139. [DOI] [PubMed] [Google Scholar]
- 14. Gessler F, Baumgarten P, Bernstock JD, et al.. Assessment of molecular markers demonstrates concordance between samples acquired via stereotactic biopsy and open craniotomy in both anaplastic astrocytomas and glioblastomas. J Neurooncol. 2017;133(2): 399–407. [DOI] [PubMed] [Google Scholar]
- 15. Abrey LE, Batchelor TT, Ferreri AJ, et al.. Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol. 2005;23(22):5034–5043. [DOI] [PubMed] [Google Scholar]
- 16. Olson JE, Janney CA, Rao RD, et al.. The continuing increase in the incidence of primary central nervous system non-Hodgkin lymphoma. Cancer. 2002;95(7):1504–1510. [DOI] [PubMed] [Google Scholar]
- 17. Nelson DF. Radiotherapy in the treatment of primary central nervous system lymphoma (PCNSL). J Neurooncol. 1999;43(3):241–247. [DOI] [PubMed] [Google Scholar]
- 18. Weller M. The vanishing role of whole brain radiotherapy for primary central nervous system lymphoma. Neuro Oncol. 2014;16(8):1035–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ferreri AJ, Reni M, Foppoli M, et al.. High-dose cytarabine plus high-dose methotrexate versus high-dose methotrexate alone in patients with primary CNS lymphoma: a randomised phase 2 trial. Lancet. 2009;374(9700):1512–1520. [DOI] [PubMed] [Google Scholar]
- 20. Ferreri AJ, Reni M, Pasini F, et al.. A multicenter study of treatment of primary CNS lymphoma. Neurology. 2002;58(10):1513–1520. [DOI] [PubMed] [Google Scholar]
- 21. Juergens A, Pels H, Rogowski S, et al.. Long-term survival with favorable cognitive outcome after chemotherapy in primary central nervous system lymphoma. Ann Neurol. 2010;67(2):182–189. [DOI] [PubMed] [Google Scholar]
- 22. Rubenstein JL, Gupta NK, Mannis GN, Lamarre AK, Treseler P. How I treat CNS lymphomas. Blood. 2013;122(14):2318–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ferreri AJ, Illerhaus G. The role of autologous stem cell transplantation in primary central nervous system lymphoma. Blood. 2016;127(13):1642–1649. [DOI] [PubMed] [Google Scholar]
- 24. Burton AF, Storr JM, Dunn WL. Cytolytic action of corticosteroids on thymus and lymphoma cells in vitro. Can J Biochem. 1967;45(2):289–297. [DOI] [PubMed] [Google Scholar]
- 25. Lowe SW, Schmitt EM, Smith SW, Osborne BA, Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993;362(6423):847–849. [DOI] [PubMed] [Google Scholar]
- 26. Smith KG, Strasser A, Vaux DL. CrmA expression in T lymphocytes of transgenic mice inhibits CD95 (Fas/APO-1)-transduced apoptosis, but does not cause lymphadenopathy or autoimmune disease. EMBO J. 1996;15(19):5167–5176. [PMC free article] [PubMed] [Google Scholar]
- 27. Helmberg A, Auphan N, Caelles C, Karin M. Glucocorticoid-induced apoptosis of human leukemic cells is caused by the repressive function of the glucocorticoid receptor. EMBO J. 1995;14(3):452–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ayroldi E, Zollo O, Bastianelli A, et al.. GILZ mediates the antiproliferative activity of glucocorticoids by negative regulation of Ras signaling. J Clin Invest. 2007;117(6):1605–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Roth P, Wick W, Weller M. Steroids in neurooncology: actions, indications, side-effects. Curr Opin Neurol. 2010;23(6):597–602. [DOI] [PubMed] [Google Scholar]
- 30. Grander D, Kharaziha P, Laane E, Pokrovskaja K, Panaretakis T. Autophagy as the main means of cytotoxicity by glucocorticoids in hematological malignancies. Autophagy. 2009;5(8):1198–1200. [DOI] [PubMed] [Google Scholar]
- 31. Laane E, Tamm KP, Buentke E, et al.. Cell death induced by dexamethasone in lymphoid leukemia is mediated through initiation of autophagy. Cell Death Differ. 2009;16(7):1018–1029. [DOI] [PubMed] [Google Scholar]
- 32. Bromberg JE, Siemers MD, Taphoorn MJ. Is a "vanishing tumor" always a lymphoma? Neurology. 2002;59(5):762–764. [DOI] [PubMed] [Google Scholar]
- 33. Porter AB, Giannini C, Kaufmann T, et al.. Primary central nervous system lymphoma can be histologically diagnosed after previous corticosteroid use: a pilot study to determine whether corticosteroids prevent the diagnosis of primary central nervous system lymphoma. Ann Neurol. 2008;63(5):662–667. [DOI] [PubMed] [Google Scholar]
- 34. Pirotte B, Levivier M, Goldman S, Brucher JM, Brotchi J, Hildebrand J. Glucocorticoid-induced long-term remission in primary cerebral lymphoma: case report and review of the literature. J Neurooncol. 1997;32(1):63–69. [DOI] [PubMed] [Google Scholar]
- 35. Geppert M, Ostertag CB, Seitz G, Kiessling M. Glucocorticoid therapy obscures the diagnosis of cerebral lymphoma. Acta Neuropathol. 1990;80(6):629–634. [DOI] [PubMed] [Google Scholar]
- 36. Choi YL, Suh YL, Kim D, Ko YH, Sung CO, Lee JI. Malignant lymphoma of the central nervous system: difficult histologic diagnosis after glucocorticoid therapy prior to biopsy. Clin Neuropathol. 2006;25(1):29–36. [PubMed] [Google Scholar]
- 37. Neuwelt EA, Barnett PA, Bigner DD, Frenkel EP. Effects of adrenal cortical steroids and osmotic blood-brain barrier opening on methotrexate delivery to gliomas in the rodent: the factor of the blood-brain barrier. Proc Natl Acad Sci. 1982;79(14):4420–4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cote TR, Manns A, Hardy CR, Yellin FJ, Hartge P. Epidemiology of brain lymphoma among people with or without acquired immunodeficiency syndrome. AIDS/Cancer Study Group. J Natl Cancer Inst. 1996;88(10):675–679. [DOI] [PubMed] [Google Scholar]
- 39. Schabet M. Epidemiology of primary CNS lymphoma. J Neurooncol. 1999;43(3):199–201. [DOI] [PubMed] [Google Scholar]
- 40. Hollender A, Kvaloy S, Nome O, Skovlund E, Lote K, Holte H. Central nervous system involvement following diagnosis of non-Hodgkin's lymphoma: a risk model. Ann Oncol. 2002;13(7):1099–1107. [DOI] [PubMed] [Google Scholar]
- 41. Korfel A, Weller M, Martus P, et al.. Prognostic impact of meningeal dissemination in primary CNS lymphoma (PCNSL): experience from the G-PCNSL-SG1 trial. Ann Oncol. 2012;23(9):2374–2380. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


