Abstract
The present investigation was carried out to establish an efficient and reproducible micropropagation protocol for the production of morphologically, genetically and chemically uniform plants of Curcuma zedoaria. Axillary bud explants of C. zedoaria were inoculated into MS basal medium supplemented with various combinations and concentrations of 6-benzyladenine (2.2–22.2 µM, BA), kinetin (2.3–23.2 µM, Kin), indole-3-acetic acid (2.9–11.4 µM, IAA), α-naphthalene acetic acid (2.7–10.2 µM, NAA) and adenine sulphate (33.9–203.6 µM, Ads). Almost 95% of rhizome buds sprouted on MS medium supplemented with 13.3 μM BA, 5.7 μM IAA and 63.9 μM Ads giving rise to an average of 12.89 ± 0.02 shoots within 6 weeks. However, the maximum number of roots (25.8 ± 0.07 roots per explant) was obtained on half strength MS medium supplemented with 7.4 µM of IBA after 4 weeks of inoculation. Morphological characteristics were similar in both conventionally propagated and micropropagated plants. Additionally, genetic homogeneity of in vitro plants was further confirmed through ISSR and flow cytometry analysis. A total of 27 ISSR primers were screened, out of which 13 ISSR primers generated 58 monomorphic and reproducible bands thereby confirming the genetic uniformity of obtained plants. The mean 2C DNA content of the mother plant (2.96 pg) was similar to that of in vitro derived plants (3.07 pg). Gas chromatography-mass spectrometry (GC–MS) analysis showed similarity in the qualitative profile of chemical constituents of essential oil and high-performance liquid chromatography analysis revealed no significant differences in curcumin content in the tissue culture regenerants and mother plants of C. zedoaria. Therefore, the present micropropagation protocol could be effectively employed to generate true to type plantlets of C. zedoaria.
Electronic supplementary material
The online version of this article (10.1007/s13205-019-2009-9) contains supplementary material, which is available to authorized users.
Keywords: Micropropagation, Curcuma zedoaria, Genetic fidelity, Essential oil, GC–MS, HPLC
Introduction
Curcuma zedoaria (Christm.) Roscoe. (Zingiberaceae) also known as white turmeric is a rhizomatous herb, widely cultivated throughout Asia (Lobo et al. 2009; Tariq et al. 2016). The rhizomes are used for treating stomach diseases, leucoderma, tuberculosis, toothache and menstruation (Lakshmi et al. 2011). Curcuma zedoaria possesses remarkable antioxidant, antifungal, antimicrobial and anti-inflammatory properties (Ayati et al. 2019; Lee et al. 2019; Lourembam et al. 2019). The medicinal properties of C. zedoaria can be attributed to the presence of diverse constituents such as terpenoids, flavonoids, and phenylpropanoids (Tariq et al. 2016). There is an ever increasing demand of C. zedoaria and its important phytochemicals like curcumin, in the international market. Curcumin is a bioactive phenylpropanoid present in Curcuma species and is extensively used in traditional medicine due to its outstanding anti-cancer, antioxidant, anti-inflammatory and antimalarial properties (Rahmani et al. 2018; Shakeri et al. 2019; Carolina Alves et al. 2019). Slow multiplication rate of rhizomes by the conventional propagation and high susceptibility of the plant to rhizome rot diseases are the major stumbling blocks in commercial cultivation of C. zedoaria (Bharalee et al. 2005; Stanly and Keng 2007). Additionally, continuous habitat loss and indiscriminating uprooting of rhizomes have enlisted the plant as critically threatened in the wild (Islam 2004; Anisuzzaman et al. 2008). Therefore, the present study was carried out to develop an efficient and reproducible micropropagation system for large-scale commercial production of C. zedoaria. Micropropagation is generally used for rapid and large scale production of several medicinal plants species including Curcuma species, such as Curcuma angustifolia (Jena et al. 2018), Curcuma caesia (Zuraida 2013), Curcuma longa (Panda et al. 2007) and Curcuma amada (Prakash et al. 2004). However, there is a possibility of somaclonal variation in the tissue culture regenerants due to the presence of genetic obstruction which can limit the broader utility of the plant tissue culture techniques (Salvi et al. 2001). For the commercial utilization of tissue culture technique, it is necessary to evaluate the genetic fidelity of in vitro regenerants. Among molecular markers, ISSR markers being polymorphic, reproducible and informative is widely used for estimating genetic stability of tissue culture-derived plants (Xing et al. 2010; Werner et al. 2015; Saha et al. 2016; Tripathi et al. 2018). Molecular markers along with flow cytometry technique have been successfully employed for the assessment of genetic uniformity of in vitro regenerants (Liu et al. 2011; Alatar et al. 2017; Konar et al. 2018). Flow cytometry analysis is commonly used for the estimation of genome size and ploidy changes in the in vitro regenerants (Rewers et al. 2012).
Few studies have been established on the in vitro propagation of C. zedoaria (Loc et al. 2005; Bharalee et al. 2005; Stanly and Keng 2007; Shahinozzaman et al. 2013), however they failed to evaluate the genetic and biochemical uniformity of tissue culture raised plants. Thus, the present work was carried out to establish an efficient method for rapid plant regeneration of genetically uniform C. zedoaria, which could be utilized for large scale propagule production. Further an attempt was made to verify the genetic fidelity of clonally raised plants by ISSR and flow cytometry analysis. Subsequently, validation of chemical uniformity among the regenerated plants and the mother plant of C. zedoaria were tested by assessing the essential oil profile and and curcumin content using GC–MS and HPLC analysis.
Materials and methods
Plant material and explant preparation
The axillary bud explants were obtained from the 2 years old plant of C. zedoaria growing in the greenhouse of Centre for Biotechnology, Siksha ‘O’ Anusandhan (Deemed to be University), Odisha, India (Fig. 1a). The collected explants were rinsed under running tap water followed by washing in liquid detergent (Extran, Merck, Mumbai, India). Thereafter, the explants were washed with sterile double distilled water followed by surface sterilization with mercury chloride (HgCl2, 0.1%) for 3–4 min. The sterilized axillary buds were washed with sterile water before inoculation.
Fig. 1.
Curcuma zedoaria (a) control plant (b) axillary bud as explant (c) multiple shoot induction within 6 weeks supplemented with 13.3 µM BA, 5.7 µM IAA and 63.9 µM Ads (d) well developed rooted plantlets were transferred to ex vitro conditions (e) acclimatized plantlets after 2 years in field
Shoot induction and plantlet formation
Axillary bud were maintained in a culture tube containing MS basal medium supplemented with 6-benzyladenine (2.2–22.2 µM, BA), kinetin (2.3–23.2 µM, Kin), indole-3-acetic acid (2.9–11.4 µM, IAA), α-naphthalene acetic acid (2.7–10.2 µM, NAA) and adenine sulphate (33.9–203.6 µM, Ads) either individually or in combination for shoot multiplication. The pH of the media was adjusted to 5.8 before gelling with 0.8% (w/v) agar. The culture tube was autoclaved at 121 °C for 20 min. Further, auxiliary buds were aseptically cultured on MS basal medium with different combinations and concentrations of cytokinins and auxins. The culture tubes were incubated at 25 ± 1 °C in a tissue culture room under a light dark cycle of 16:8 h and white fluorescent light with 50 µmol m2/s light intensity. MS basal medium without any plant growth hormones was taken as control.
Rooting and acclimatization of plantlets
For adventitious rooting, in vitro derived shoots (3–4 cm long) were removed from the culture vessels and were transferred into half strength MS medium supplemented with various concentrations of IAA and IBA alone. After 4 weeks of subculture in root induction medium, the rooted plants were taken out from the culture bottle and rinsed with autoclaved double distilled water. The plantlets were then shifted to small pots filled with autoclaved soil, sand and cow dung with an equal proportion of 1:1:1 in the growth chamber for acclimatization and maintained at 24–25 °C and 80–90% relative humidity. After 30 days of acclimatization, plantlets were successfully shifted to the ex vitro condition for maturity. Both micropropagated and field grown plants were compared for different morphological, biochemical and molecular characteristics. After 2 years, a comparative morphological study was carried out by randomly selecting 20 replicates each for field grown tissue culture plants and the mother plant of C. zedoaria.
Molecular analysis
The total genomic DNA was extracted from the fresh leaf of both mother plant as well as 20 in vitro propagated plants of Curcuma zedoaria independently using modified cetyltrimethyl ammonium bromide (CTAB) method as mentioned by Doyle and Doyle (1987). DNA was quantified at 260 nm and purity was checked using the ratio of 260/280 nm. PCR reactions were performed in a final volume of 25 µl comprising 25 ng of template DNA, 5 pM each primer, 10X reaction buffer containing 15 mM MgCl2 (Bangalore Genei), 200 µM of each dNTP (Bangalore Genei) and 3U/µl of Taq polymerase (Bangalore Genei). Amplification of genomic DNA was performed using thermal cycler (Applied Biosystems Veriti 96 Well Thermal Cycler) and programmed as follows: preliminary step at 94 °C for 5 min, followed by 40 cycles of 1 min denaturation at 94 °C, 1 min of annealing followed by 2 min of extension at 72 °C followed by 7 min of final extension at 72 °C. Amplified reaction products were resolved electrophoretically on 1.5% agarose gel stained with 0.5 µg/ml of ethidium bromide and visualized under UV light using a gel documentation system (BioRad, CA, USA).
Flow cytometry analysis
The nuclear DNA content of both micropropagated and conventionally propagated plants of Curcuma zedoaria was performed using a previously described method of Jena et al. (2018). Briefly, leaf samples of both the plant were homogenized and treated with Otto 1 buffer followed by the addition of propidium iodide (50 µM) and RNAse (100 µM). The reference standard was set as Glycine max. The nuclear suspension of treated samples was filtered and analyzed using flow cytometry.
Essential oil analysis
For GC–MS analysis of essential oil, rhizome and leaf of following plant material was selected: in vitro derived plants obtained from axillary buds and cultured on MS medium containing 13.3 μM BA, 5.7 μM IAA and 63.9 μM Ads and maintaining it for 1 year by sub culturing and then transferring to field for 2 years and conventionally propagated plants grown in field for 2 years. The essential oil was extracted by hydrodistillation according to the method of Guenther (1972), using a Clevenger-type apparatus. The extracted essential oils were dehydrated over anhydrous Na2SO4. GC–MS analysis was performed on Clarus 580 Gas Chromatograph (Perkin-Elmer, USA) equipped with an Elite-5 MS capillary column (30 m length, 0.25 mm internal diameter and 0.25 µm film thickness) and coupled with a SQ-8 MS detector. 0.1 µl volume of essential oil was injected. The oven temperature was programmed at 60 °C then increased to 220 °C at a rate of 3 °C per min and the final temperature was kept for 7 min at 220 °C. Helium at a flow rate of 1 ml/min was used as a carrier gas. The injector and transfer interface temperature was kept at 250 °C. Mass spectra were recorded at 70 electron volt. The source temperature was kept at 250 °C.
The identification of the compounds was based on comparison of the mass spectra (MS) data obtained for each compound with inbuilt spectral library (NIST 08). Additional confirmation was carried out by measuring the retention indices using straight chain alkane (C8–C20) series and comparing the obtained retention indices values with published literature (Adams 2007).
Sample preparation and HPLC analysis
In vitro regenerants obtained from axillary buds cultured on MS medium supplemented with 13.3 μM BA, 5.7 μM IAA and 63.9 μM Ads and maintaining it for 1 year by sub culturing and then transferring to field for 2 years and conventionally propagated plants grown in the field for 2 years were used for HPLC analysis. Rhizomes of both tissue culture raised plants and mother plants of C. zedoaria were powdered and subsequently extracted with methanol for 6 h using a soxhlet apparatus. The prepared extract was filtered, concentrated using rotary evaporator and stored in airtight container at 4 °C until analysis. The dried extract (0.5 g) was mixed in 50 ml of HPLC grade methanol. The stock solution of standard (curcumin, 0.2 mg/ml) was prepared by dissolving 1 mg of curcumin in 5 ml of methanol. All the solvent used in this study were analytical grade. Standard and sample were filtered through 0.22 micron filter membrane prior analysis.
A modular HPLC system (Shimadzu, Kyoto, Japan) consisting of two LC-20 AD pump, a Rheodyne 8125 injector, an SPD-20A diode array detector, a CBM-20A controller and CT0-20AC column oven was used for estimating curcumin content. The separation was carried out on a reverse phase Shimpak C18 column (Shimadzu, Kyoto, Japan, 250 × 4.6 mm ID, 5 µm). Separation was achieved in the gradient mode, using mobile phase of acetonitrile (A) and 0.1% formic acid (B) which were as follows: 0–7.0 min, 40–64% B; 7–10 min 64–90% B and 10–20 min 90% B. Elution was carried out at a column temperature of 35 °C, a flow rate of 1.0 ml/min and a run time of 20 min. The injection volume was 20 µl. The detection wavelength was set at 425 nm.
Experimental design and statistical analysis
The experiments were set according to completely randomized design and all the experiments (no. of shoots per explant, % of shoot initiation, no of roots per explant, % of root response) were performed in triplicates by taking twenty explants for each experiment. The results were expressed using mean ± SD and the significant difference between the mean were evaluated using analysis of variance (ANOVA) followed by Tukey’s HSD test at p < 0.05 using Minitab 17 statistical software (Minitab Inc, PA, USA).
Results
Plant multiplication
The axillary buds of the conventionally propagated plant of C. zedoaria were used as explants (Fig. 1b). The explants grown only on MS basal medium were considered as control as they did not exhibit any shoot induction. Therefore for optimum shoot induction and multiplication of shoot from inoculated explant, various experiments were performed with several concentration of auxins and cytokinins alone and in combinations. The shoot bud multiplication increased on increasing the concentration of BA from 2.2 to 13.3 µM. When the BA concentration increased beyond 13.3 µM, it did not show any positive effect on shoot multiplication of C. zedoaria. From the results it was observed that apart from BA, Kin, NAA and IBA did not showed much response of root induction (Table 1). Among the media concentration tried for BA, 13.3 µM was found to give maximum response of shoot induction (90%) producing 7.6 ± 0.31 shoots/explant. On incorporation of IAA (5.7 µM) and Ads (63.9 µM) in the BA (13.3 µM) containing culture medium shoot multiplication response increased to 95% producing maximum of 12.9 ± 0.37 shoots per explant (Fig. 1c). From the present findings, it was observed that higher concentration of BA and Ads with a lower concentration of IAA, resulted in better shoot multiplication response. However, less numbers of shoots were obtained on MS media fortified with 2.3 µM of Kin (1.8 shoots/explant).
Table 1.
Effect of various plant growth regulators on in vitro shoot initiation and multiplication of Curcuma zedoaria
| MS media with growth regulators (µM) | % of shoot initiation | No of shoots per explant | ||||
|---|---|---|---|---|---|---|
| BA | Kin | IAA | NAA | Ads | ||
| 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0p | 0.0 ± 0.00v |
| 2.2 | – | – | – | – | 65e | 3.3 ± 0.11jkl |
| 4.4 | – | – | – | – | 50 h | 4.8 ± 0.11 g |
| 13.3 | – | – | – | – | 85b | 7.6 ± 0.31e |
| 22.2 | – | – | – | – | 70d | 4.3 ± 0.18ghi |
| – | 2.3 | – | – | – | 65e | 1.8 ± 0.06qrs |
| – | 4.6 | – | – | – | 45i | 2.9 ± 0.13klmn |
| – | 13.9 | – | – | – | 55 g | 4.7 ± 0.23gh |
| – | 23.2 | – | – | – | 50 h | 3.4 ± 0.18jk |
| – | – | 2.9 | – | – | 30 l | 1.9 ± 0.08qrs |
| – | – | 5.7 | – | – | 35 k | 2.3 ± 0.05nopq |
| – | – | 8.6 | – | – | 25 m | 1.1 ± 0.06tu |
| – | – | 11.4 | – | – | 20n | 0.8 ± 0.07tu |
| – | – | – | 2.7 | – | 20n | 1.1 ± 0.08tu |
| – | – | – | 5.4 | – | 25 m | 1.3 ± 0.04st |
| – | – | – | 8.1 | – | 20n | 0.8 ± 0.01tu |
| – | – | – | 10.2 | – | 15o | 0.6 ± 0.01uv |
| – | – | – | – | 33.9 | 50 h | 2.0 ± 0.12pqr |
| – | – | – | – | 63.9 | 65e | 4.1 ± 0.05hi |
| – | – | – | – | 135.7 | 45i | 2.1 ± 0.11opq |
| – | – | – | – | 203.6 | 40j | 1.0 ± 0.06tu |
| 13.3 | 2.3 | – | – | – | 65e | 1.8 ± 0.04qrs |
| 13.3 | 4.6 | – | – | – | 65e | 2.6 ± 0.09mnop |
| 13.3 | 13.9 | – | – | – | 55 g | 2.4 ± 0.06nopq |
| 13.3 | 23.2 | – | – | – | 50 h | 1.4 ± 0.08rst |
| 13.3 | – | 2.9 | – | – | 60f | 5.6 ± 0.22f |
| 13.3 | – | 5.7 | – | – | 85b | 9.8 ± 0.42c |
| 13.3 | – | 8.6 | – | – | 70d | 7.2 ± 0.26e |
| 13.3 | – | 11.4 | – | – | 60f | 6.1 ± 0.52f |
| 13.3 | – | – | 2.7 | – | 55 g | 2.2 ± 0.13opq |
| 13.3 | – | – | 5.4 | – | 50 h | 3.9 ± 0.22ij |
| 13.3 | – | – | 8.1 | – | 65e | 3.1 ± 0.15klm |
| 13.3 | – | – | 10.2 | – | 60f | 2.7 ± 0.12lmno |
| 13.3 | – | 5.7 | – | 33.9 | 85b | 9.6 ± 0.38c |
| 13.3 | – | 5.7 | – | 63.9 | 95a | 12.9 ± 0.47a |
| 13.3 | – | 5.7 | – | 135.7 | 85b | 10.5 ± 0.32b |
| 13.3 | – | 5.7 | – | 203.6 | 80c | 8.7 ± 0.21d |
The values represent the mean ± SD of three independent experiments. Twenty explants were raised for each experiment and the experiments were repeated thrice. Mean having different letter in a column were significantly according to Tukey’s HSD test at p < 0.05. MS media devoid of any plant growth regulators is considered as control
BA 6-benzyladenine, NAA α-naphthaleneacetic acid, IAA indole-3-acetic acid, Kin kinetin, Ads adenine sulphate
In vitro rooting and acclimatization of micropropagated plants
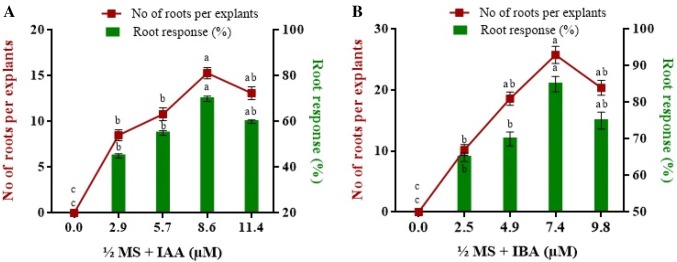
For adventitious rooting, microshoots (3–4 cm long) were sub cultured on half strength MS medium containing IAA or IBA at different concentrations. It was observed that when fully grown shoots were rooted on the half strength MS media supplemented with IBA (7.4 µM), maximum number of roots (25.8 ± 0.07 roots/explant) were observed whereas, no roots were observed in control media even after 4 weeks of inoculation (Fig. 2). Healthy plants having well-developed roots were removed from the culture bottle and successfully transferred to pots filled with soil, sand and cow dung (1:1:1) for hardening in a growth chamber (Fig. 1d). After 30 days, plantlets were later shifted to ex vitro condition and about 98% of the in vitro regenerants survived when transferred to field condition (Fig. 1e). After 2 years of field establishment of micro propagated plant of C. zedoaria, morphological parameters such as plant height, tiller number, leaf width and leaf length were assessed and compared with that of mother plant. No significant changes were observed in the morphological characters between the tissue culture raised and conventionally propagated plants of C. zedoaria, thus confirming that in vitro derived plants were morphologically similar to that of the mother plant (Table 2). These results can be attributed to the genetic stability of axillary derived and conventionally grown plants of C. zedoaria. Similar findings showing no differences in the morphological attributes were observed in meristem derived and conventionally propagated plants of Strawberry (Naing et al. 2019).
Fig. 2.
Effect of IAA and IBA at different concentrations on root response (%) and root number per explant after 4 weeks of inoculation. Bars represent SE. Significantly different treatment means are indicated by different letters (Tukey’s test, p < 0.05)
Table 2.
Comparison of different morphological characters between field grown mother plant and in vitro derived plants of Curcuma zedoaria
| Morphological parameters | Mother plant | In vitro derived plants |
|---|---|---|
| Plant height (cm) | 118.5 ± 3.4a | 123.2 ± 4.6a |
| Tiller number per plant | 2.9 ± 0.2a | 3.2 ± 0.3a |
| No. of leaves/clump | 10.1 ± 1.4a | 11.7 ± 1.2a |
| Leaf length (cm) | 45.2 ± 2.3a | 46.2 ± 1.9a |
| Leaf width (cm) | 11.13 ± 0.8a | 11.6 ± 0.5a |
| Rhizome diameter (cm) | 3.2 ± 0.3a | 3.5 ± 0.2a |
| Total rhizome weight (g) | 88.5 ± 3.1a | 92.7 ± 4.1a |
| Rhizome length (mm) | 42.1 ± 1.7a | 47.8 ± 1.8a |
The values represent the mean ± SD of 20 replicates. Mean having different letter in a row were significantly different according to Tukey’s HSD test at p < 0.05
Genetic homogeneity of regenerants
In order to assess the genetic uniformity, ISSR banding pattern of both tissue culture raised and conventionally propagated plants of Curcuma zedoaria was carried out. Out of 27 ISSR primers initially screened, 13 primers produced 58 well resolved and reproducible bands ranging from 350 to 2500 bp in size (Table 3). A total of 1218 bands produced monomorphic banding patterns across 20 micropropagated plants and mother plant thereby confirming generic uniformity among all plants analyzed (Fig. 3). The total number of bands amplified per primer ranged from 3 to 8 with a mean of 4.46 bands per primer. The representative gel pic of ISSR 24 and ISSR 12 are depicted in Fig. 3a, b, respectively. The gel photographs of remaining 11 primers are given as supplementary file (Figs S1–S3). The result obtained from the ISSR analysis confirmed absence of DNA polymorphism among tissue culture raised and conventionally propagated plant of C. zedoaria.
Table 3.
ISSR banding patterns of micropropagated and conventionally grown plants of Curcuma zedoaria
| No. | Primer code | Primer sequence (5´–3´) | Total no of bands amplified | No. of scorable bands | Range of amplicons (bp) |
|---|---|---|---|---|---|
| 1 | ISSR 7 | GACGACGACGACGAC | 84 | 4 | 600–2250 |
| 2 | ISSR 9 | GTGTGTGTGTGTGTGTA | 63 | 3 | 1000–2500 |
| 3 | ISSR 10 | GTGTGTGTGTGTGTGTT | 84 | 4 | 750–1900 |
| 4 | ISSR 11 | AGAGAGAGAGAGAGAGC | 63 | 3 | 650–1300 |
| 5 | ISSR 12 | AGAGAGAGAGAGAGAGG | 168 | 8 | 350–1800 |
| 6 | ISSR 14 | GAGAGAGAGAGAGAGAA | 84 | 4 | 450–1700 |
| 7 | ISSR 17 | CTCTCTCTCTCTCTCTG | 84 | 4 | 350–1600 |
| 8 | ISSR 18 | CACACACACACACACAT | 63 | 3 | 1000–2000 |
| 9 | ISSR 19 | CACACACACACACACAA | 63 | 3 | 800–1400 |
| 10 | ISSR 20 | GTGGTGGTGGTGGTG | 126 | 6 | 750–1800 |
| 11 | ISSR 22 | TGAGAGAGAGAGAGAGAGA | 126 | 6 | 500–1200 |
| 12 | ISSR 24 | GAGAGAGAGAGAGAGAGAT | 63 | 3 | 350–800 |
| 13 | ISSR 25 | GACAGACAGACAGACA | 147 | 7 | 900–1950 |
| Total | 1218 | 58 | 350–2500 | ||
Fig. 3.
ISSR profile of mother plant and micropropagated plants of Curcuma zedoaria with primers a ISSR 24 and b ISSR 12. Lane 1: mother plant, Lane 2–21: randomly selected 20 micropropagated plants
Genetic integrity of tissue culture regenerants with that of the mother plant of Curcuma zedoaria was further confirmed by measuring their 2C DNA content. In the present investigation, nuclear suspension of young leaves from tissue culture raised plants and mother plant of C. zedoaria were subjected to flow cytometry analysis. Flow cytometric histograms indicated two peaks: the first peak representing nuclei in the G1 phase of the cell cycle of C. zedoaria, while the second peak representing nuclei in the G1 phase of the internal standard (Glycine max) (Fig. 4). The mean 2C DNA content of tissue culture regenerants with that of the field grown mother plant of C. zedoaria was found to be 3.07 and 2.96 pg, respectively, thereby revealing low level of difference in their nuclear DNA content.
Fig. 4.
Histogram showing 2C nuclear DNA content of a conventionally propagated and b micropropagated plant of Curcuma zedoaria with respect to standard (Glycine max)
Evaluation of biochemical fidelity
The biochemical stability of in vitro regenerants was estimated by measuring essential oil chemical constituent and curcumin content. The essential oil yield obtained from the leaf of mother plant and micropropagated plants of C. zedoaria was 0.25 and 0.30% (v/w), respectively, whereas that of rhizome mother plant and tissue culture derived plants was 0.40 and 0.45% (v/w), respectively. GC–MS analysis was performed to identify the compositional profile in leaf and rhizome oil of C. zedoaria (Tables 4, 5). A total of 49 components corresponding to 91.6 and 93.42% of the total oils were identified in leaf oil of mother plants and tissue culture regenerated plants of C. zedoaria, respectively. Likewise, a total of 57 compounds accounting for 93.65 and 95.94% of total essential oil were identified in the rhizome of the mother plant and tissue culture regenerated plants, respectively. 1, 8-cineole, γ-eudesmol acetate, curzerenone and camphor were common to both leaf oil of mother plant and tissue culture regenerated plants of C. zedoaria. Similarly, major constituent like curzerenone, γ-eudesmol acetate, germacrone, α-zingiberene and camphor were found to be common in rhizome oils of field grown and in vitro propagated plants of C. zedoaria. Leaf oils of both field grown mother plants and in vitro generated plant of C. zedoaria were dominated by 1, 8-cineole which comprises about 10.33% of peak area. Curzerenone was reported to be a predominant component of rhizome oil comprising about 22.94% of the total peak area.
Table 4.
Chemical constituents identified in rhizome oil of conventionally propagated and micropropagated plants of Curcuma zedoaria
| No. | Compounds | RIa | RIb | Peak area % | |
|---|---|---|---|---|---|
| Conventionally propagated | Micropropagated | ||||
| 1 | α-Thujene | 929 | 924 | 0.20 ± 0.01a | 0.21 ± 0.01a |
| 2 | α-Pinene | 932 | 932 | 0.44 ± 0.02a | 0.46 ± 0.01a |
| 3 | α-Fenchene | 945 | 945 | 0.62 ± 0.03a | 0.65 ± 0.02a |
| 4 | Camphene | 949 | 946 | 1.49 ± 0.08a | 1.51 ± 0.06a |
| 5 | Sabinene | 969 | 969 | 0.13 ± 0.01a | 0.13 ± 0.01a |
| 6 | β-Pinene | 977 | 974 | 0.95 ± 0.03a | 0.97 ± 0.02a |
| 7 | Myrcene | 985 | 988 | 0.34 ± 0.01a | 0.35 ± 0.01a |
| 8 | Limonene | 1026 | 1024 | 0.56 ± 0.01a | 0.59 ± 0.01b |
| 9 | 1,8-Cineole | 1031 | 1026 | 3.23 ± 0.05a | 3.26 ± 0.03a |
| 10 | Terpinolene | 1087 | 1086 | 0.13 ± 0.01a | 0.14 ± 0.01a |
| 11 | Cis-Thujone | 1099 | 1101 | 0.11 ± 0.01a | 0.11 ± 0.01a |
| 12 | Camphor | 1145 | 1141 | 3.99 ± 0.08a | 4.03 ± 0.05a |
| 13 | Camphene hydrate | 1161 | 1145 | 1.56 ± 0.06a | 1.59 ± 0.04a |
| 14 | Borneol | 1168 | 1165 | 0.66 ± 0.11a | 0.70 ± 0.08b |
| 15 | Terpinen-4-ol | 1176 | 1174 | 0.22 ± 0.01a | 0.23 ± 0.01a |
| 16 | α-Terpineol | 1191 | 1186 | 0.66 ± 0.09a | 0.69 ± 0.06a |
| 17 | γ-Terpineol | 1215 | 1199 | 0.15 ± 0.01a | 0.16 ± 0.01a |
| 18 | Nerol | 1228 | 1227 | 0.14 ± 0.01a | 0.18 ± 0.01a |
| 19 | Azulene | 1282 | 1298 | 3.00 ± 0.06a | 3.06 ± 0.04a |
| 20 | δ-Elemene | 1330 | 1335 | 1.00 ± 0.02a | 1.05 ± 0.01b |
| 21 | α-Ylangene | 1376 | 1373 | 0.14 ± 0.01a | 0.15 ± 0.01a |
| 22 | β-Elemene | 1384 | 1389 | 3.04 ± 0.06a | 3.22 ± 0.05b |
| 23 | (E)-Caryophyllene | 1416 | 1417 | 0.53 ± 0.03a | 0.56 ± 0.01a |
| 24 | γ-Elemene | 1423 | 1434 | 0.25 ± 0.02a | 0.26 ± 0.01a |
| 25 | (Z)-β-Farnesene | 1442 | 1440 | 0.12 ± 0.01a | 0.15 ± 0.01a |
| 26 | α-Humulene | 1448 | 1452 | 0.20 ± 0.01a | 0.24 ± 0.01b |
| 27 | ar-Curcumene | 1477 | 1479 | 0.32 ± 0.02a | 0.33 ± 0.02a |
| 28 | Germacrene D | 1484 | 1484 | 2.46 ± 0.04a | 2.51 ± 0.02a |
| 29 | α-Zingiberene | 1491 | 1493 | 5.75 ± 0.13a | 5.78 ± 0.08a |
| 30 | Curzerene | 1499 | 1499 | 1.45 ± 0.15a | 1.49 ± 0.11a |
| 31 | γ-Cadinene | 1514 | 1513 | 0.31 ± 0.01a | 0.32 ± 0.01a |
| 32 | β-Curcumene | 1518 | 1514 | 0.12 ± 0.01a | 0.15 ± 0.01b |
| 33 | β-Sesquiphellandrene | 1520 | 1521 | 0.19 ± 0.01a | 0.21 ± 0.01a |
| 34 | Elemol | 1551 | 1548 | 2.22 ± 0.05a | 2.27 ± 0.03a |
| 35 | Germacrene B | 1555 | 1559 | 0.13 ± 0.01a | 0.16 ± 0.01b |
| 36 | Caryophyllene oxide | 1581 | 1582 | 0.46 ± 0.02a | 0.51 ± 0.01a |
| 37 | ar-Turmerol | 1587 | 1582 | 0.34 ± 0.01a | 0.34 ± 0.01a |
| 38 | Curzerenone | 1597 | 1605 | 22.94 ± 0.61a | 23.15 ± 0.57a |
| 39 | Humulene epoxide II | 1613 | 1608 | 0.69 ± 0.11a | 0.73 ± 0.08a |
| 40 | 10-epi-γ-Eudesmol | 1620 | 1622 | 0.24 ± 0.01a | 0.27 ± 0.01a |
| 41 | 1-epi-Cubenol | 1630 | 1627 | 0.27 ± 0.01a | 0.31 ± 0.01b |
| 42 | epi-α-Cadinol | 1634 | 1638 | 0.38 ± 0.01a | 0.41 ± 0.01b |
| 43 | β-Eudesmol | 1650 | 1649 | 1.12 ± 0.02a | 1.17 ± 0.01a |
| 44 | α-Cadinol | 1660 | 1652 | 0.36 ± 0.01a | 0.40 ± 0.01b |
| 45 | ar-Turmerone | 1668 | 1668 | 0.31 ± 0.02a | 0.33 ± 0.01a |
| 46 | Germacrone | 1690 | 1693 | 6.67 ± 0.17a | 6.74 ± 0.14a |
| 47 | (Z)-epi-β-Santalol | 1704 | 1702 | 0.71 ± 0.04a | 0.77 ± 0.03b |
| 48 | 2E,6Z-Farnesol | 1712 | 1715 | 0.55 ± 0.03a | 0.61 ± 0.02b |
| 49 | γ-(Z)-Curcumen-12-ol | 1730 | 1728 | 0.28 ± 0.01a | 0.32 ± 0.01a |
| 50 | (E)-β-Santalol | 1737 | 1738 | 3.08 ± 0.06a | 3.11 ± 0.03a |
| 51 | (2E,6E)-Farnesal | 1743 | 1740 | 0.35 ± 0.01a | 0.37 ± 0.01a |
| 52 | Xanthorrhizol | 1752 | 1751 | 0.97 ± 0.02a | 1.19 ± 0.01b |
| 53 | γ-Curcumen-15-al | 1767 | 1766 | 1.55 ± 0.02a | 1.61 ± 0.01a |
| 54 | γ- Eudesmol acetate | 1782 | 1783 | 14.62 ± 0.35a | 14.72 ± 0.31a |
| 55 | β-Eudesmol acetate | 1792 | 1792 | 0.19 ± 0.01a | 0.19 ± 0.01a |
| 56 | Eudesm-7(11)-en-4-ol, acetate | 1840 | 1839 | 0.39 ± 0.02a | 0.44 ± 0.01b |
| 57 | (Z,Z)-Farnesyl acetone | 1859 | 1860 | 0.37 ± 0.02a | 0.39 ± 0.01a |
Data are represented as mean ± SD (n = 3). Mean having different letter in a column were significantly different according to Tukey’s HSD test at p < 0.05. Rhizome essential oils are derived from in vitro grown plants of Curcuma zedoaria on MS medium with 13.3 µM BA, 5.7 µM IAA and 63.9 µM Ads
aRelative retention indices calculated against homologous n-alkane series (C8–C20) on the Elite-5 MS column
bRelative retention indices from literature (Adams 2007)
Table 5.
Chemical constituents identified in leaf oil of conventionally propagated and micropropagated plants of Curcuma zedoaria
| No. | Compounds | RIa | RIb | Peak area % | |
|---|---|---|---|---|---|
| Conventionally propagated | Micropropagated | ||||
| 1 | Tricyclene | 921 | 921 | 0.18 ± 0.01a | 0.22 ± 0.01a |
| 2 | α-Pinene | 932 | 932 | 1.42 ± 0.03a | 1.46 ± 0.02a |
| 3 | Camphene | 949 | 946 | 4.33 ± 0.09a | 4.38 ± 0.07a |
| 4 | Sabinene | 969 | 969 | 0.32 ± 0.02a | 0.33 ± 0.01a |
| 5 | β-Pinene | 977 | 974 | 2.24 ± 0.14a | 2.29 ± 0.12a |
| 6 | Myrcene | 985 | 988 | 0.54 ± 0.05a | 0.55 ± 0.03a |
| 7 | Limonene | 1026 | 1024 | 1.77 ± 0.07a | 1.81 ± 0.06a |
| 8 | 1,8-Cineole | 1031 | 1026 | 10.33 ± 0.37a | 10.38 ± 0.31a |
| 9 | Cis-Thujone | 1099 | 1101 | 1.30 ± 0.11a | 1.34 ± 0.08a |
| 10 | Camphor | 1145 | 1141 | 6.88 ± 0.48a | 6.92 ± 0.42a |
| 11 | Camphene hydrate | 1161 | 1145 | 2.24 ± 0.12a | 2.27 ± 0.09a |
| 12 | Borneol | 1168 | 1165 | 1.37 ± 0.14a | 1.39 ± 0.11a |
| 13 | α-Terpineol | 1191 | 1186 | 0.45 ± 0.03a | 0.47 ± 0.01a |
| 14 | Azulene | 1282 | 1298 | 0.85 ± 0.09a | 0.88 ± 0.06a |
| 15 | δ-Elemene | 1330 | 1335 | 0.27 ± 0.01a | 0.34 ± 0.01b |
| 16 | α-Ylangene | 1376 | 1373 | 0.21 ± 0.01a | 0.23 ± 0.01a |
| 17 | β-Elemene | 1384 | 1389 | 2.99 ± 0.08a | 3.11 ± 0.06b |
| 18 | (E)-Caryophyllene | 1416 | 1417 | 2.34 ± 0.06a | 2.38 ± 0.04a |
| 19 | γ-Elemene | 1423 | 1434 | 0.12 ± 0.01a | 0.12 ± 0.01a |
| 20 | β-Gurjunene | 1426 | 1431 | 0.14 ± 0.01a | 0.15 ± 0.01a |
| 21 | α-Humulene | 1448 | 1452 | 5.04 ± 0.12a | 5.12 ± 0.09a |
| 22 | cis-Muurola-4(14),5-diene | 1468 | 1465 | 0.13 ± 0.01a | 0.14 ± 0.01a |
| 23 | 10-epi-β-Acoradiene | 1474 | 1474 | 1.27 ± 0.08a | 1.31 ± 0.05a |
| 24 | ar-Curcumene | 1477 | 1479 | 0.18 ± 0.01a | 0.20 ± 0.01b |
| 25 | α-Zingiberene | 1491 | 1493 | 2.84 ± 0.07a | 2.86 ± 0.03a |
| 26 | Curzerene | 1499 | 1495 | 1.61 ± 0.04a | 1.63 ± 0.02a |
| 27 | γ-Cadinene | 1514 | 1513 | 0.20 ± 0.01a | 0.27 ± 0.01b |
| 28 | Elemol | 1551 | 1548 | 1.81 ± 0.05a | 1.83 ± 0.03a |
| 29 | (E)-Nerolidol | 1584 | 1561 | 0.36 ± 0.01a | 0.37 ± 0.01a |
| 30 | Germacrene D-4-ol | 1573 | 1574 | 0.58 ± 0.02a | 0.61 ± 0.01a |
| 31 | Caryophyllene oxide | 1581 | 1582 | 0.39 ± 0.01a | 0.41 ± 0.01a |
| 32 | Curzerenone | 1592 | 1605 | 7.57 ± 0.19a | 7.62 ± 0.16a |
| 33 | Humulene epoxide II | 1613 | 1608 | 0.88 ± 0.01a | 0.93 ± 0.01b |
| 34 | 10-epi-γ-Eudesmol | 1620 | 1622 | 0.87 ± 0.03a | 0.89 ± 0.02a |
| 35 | 1-epi-Cubenol | 1630 | 1627 | 0.11 ± 0.01a | 0.17 ± 0.01b |
| 36 | epi-α-Cadinol | 1634 | 1638 | 0.27 ± 0.01a | 0.31 ± 0.01b |
| 37 | β-Eudesmol | 1650 | 1649 | 0.79 ± 0.09a | 0.82 ± 0.05a |
| 38 | α-Bisabolol oxide B | 1655 | 1656 | 0.16 ± 0.01a | 0.17 ± 0.01a |
| 39 | ar-Turmerone | 1668 | 1668 | 0.12 ± 0.01a | 0.13 ± 0.01a |
| 40 | epi-α-Bisabolol | 1683 | 1683 | 4.15 ± 0.11a | 4.18 ± 0.08a |
| 41 | Eudesm-7(11)-en-4-ol | 1699 | 1700 | 0.13 ± 0.01a | 0.13 ± 0.01a |
| 42 | (Z)-epi-β-Santalol | 1704 | 1702 | 0.18 ± 0.02a | 0.22 ± 0.01b |
| 43 | 2E,6Z-Farnesol | 1712 | 1715 | 0.87 ± 0.09a | 0.93 ± 0.07b |
| 44 | γ-(Z)-Curcumen-12-ol | 1730 | 1728 | 0.72 ± 0.02a | 0.80 ± 0.01b |
| 45 | (E)-β-Santalol | 1737 | 1738 | 1.85 ± 0.17a | 1.88 ± 0.12a |
| 46 | Xanthorrhizol | 1752 | 1751 | 0.77 ± 0.01a | 0.79 ± 0.01a |
| 47 | γ-Curcumen-15-al | 1767 | 1766 | 2.69 ± 0.07a | 2.73 ± 0.03a |
| 48 | (Z)-α-Santalol acetate | 1777 | 1777 | 5.45 ± 0.14a | 5.51 ± 0.11a |
| 49 | γ-Eudesmol acetate | 1782 | 1783 | 9.32 ± 0.25a | 9.44 ± 0.23a |
Data are represented as mean ± SD (n = 3). Mean having different letter in a column were significantly different according to Tukey’s HSD test at p < 0.05. Leaf essential oils are derived from in vitro grown plants of Curcuma zedoaria on MS medium with 13.3 µM BA, 5.7 µM IAA and 63.9 µM Ads
aRelative retention indices calculated against homologous n-alkane series (C8–C20) on the Elite-5 MS column
bRelative retention indices from literature (Adams 2007)
Quantification of curcumin in the methanol extracts of conventionally propagated and tissue culture derived plants of C. zedoaria were performed using HPLC analysis. From the results, it was observed that there was no significant variation in the curcumin content in the rhizome of conventionally propagated and in vitro derived plants. The content of curcumin in the rhizome methanol extract of micropropagated plants and conventionally propagated mother plant were 135.1 µg/100 mg and 122.4 µg/100 mg dry weight (DW), respectively. Thus, indicating that the plant derived by the present micropropagation protocol could maintain the consistency of curcumin content in C. zedoaria without any variation.
Discussions
In the present study, it was found that in vitro shoot induction and proliferation was dependent upon the concentration and combination of auxins and cytokinins. Present results differs in responses from the earlier report of C. zedoaria where BAP (1 mg/l) and NAA (0.5 mg/l) gave a maximum of 4.5 ± 0.15 shoots and 8.9 ± 0.09 roots per explant in media containing 0.5 mg/l IAA (Bharalee et al. 2005). The present study showed significantly high shoots of 12.89 ± 0.02 per culture vessel in Murashige and Skoog medium fortified BA (13.3 µM), IAA (5.7 µM) and Ads (63.9 µM) after 6 weeks of inoculation. The rate of shoot multiplication was found to be influenced by the concentration and combination of auxins and cytokinins rather than cytokinins alone (Jena et al. 2018; Tripathi et al. 2018). Moreover, the supplementation of Ads beyond 63.9 µM in the culture medium did not showed any positive response on shoot proliferation. Adenine in the form of Ads can stimulate cell growth and also help in the shoot multiplication (Siwach and Gill 2011; Ahmad et al. 2018). However, the stimulative effect of Ads in shoot proliferation has also been documented in different plant species such as Acacia chundra (Rout et al. 2008), Ficus religiosa (Siwach and Gill 2011), Curcuma angustifolia (Jena et al. 2018). The synergistic effects of cytokinins-auxins concentration for shoot multiplication were also documented by several authors (Mohanty et al. 2013; Moharana et al. 2017; Khatri et al. 2019).
In this study, full strength MS medium was used for the shoot multiplication of C. zedoaria whereas half strength MS media was found to be best for in vitro root enhancement. After 6 weeks, shoot plantlets were transferred from the culture tube and sub-cultured in half strength MS medium with various combination of IAA and IBA. Half strength MS basal medium with 7.4 µM IBA gave the best results for adventitious roots formation. The preferred rooting media for in vitro regenerants was selected as half strength MS over full strength MS due to the close resemblance of half strength osmotic strength with natural soil conditions. Another probable reason for best rooting response might be the low concentration of nutrients in half strength MS which in turn stimulates rooting (Tripathi et al. 2018). Similar observation with half strength MS as more efficient was reported in several plant species (Tripathi and Kumari 2010; Valizadeh and Valizadeh 2011). Among the different auxins tried, IBA was found to be most efficient for in vitro rooting. In general, IBA has been attributed to in vitro rooting in various plant species including Mentha arvensis Linn. (Faisal et al. 2014), Prunus armeniaca L. (Ozdemir and Gur 2018), Ceropegia candelabrum L. (Beena et al. 2003) and Centella asiatica L. (Kumar 2017).
Plant regeneration and successful propagation of genetically stable plantlets are the most important factor for successful in vitro conservation method. No significant changes in morphological characters were observed between tissue culture raised and conventionally propagated plants of C. zedoaria. Assessment based on morphological characters has some drawbacks as they are prone to environmental conditions and do not necessarily reflect the genetic makeup of the plant (Behera et al. 2018) whereas, DNA based molecular markers are not influenced by any environmental factors. No genetic variations among micropropagated and mother plants of C. zedoaria were observed using ISSR primers thereby confirming that in vitro regenerants are true-to-type clones. Similarly, the ISSR marker based stability assessment of micropropagated plants have been successfully used in various medicinal plant species including Curcuma angustifolia (Jena et al. 2018), Kaempferia galanga (Mohanty et al. 2011), Cornus alba L. (Ilczuk and Jacygrad 2016) and Puya berteroniana (Viehmannova et al. 2016). Further genetic stability of micropropagated plants was also analyzed by flow cytometry to check the level of genomic variations. Flow cytometry techniques have been frequently used to estimate the nuclear DNA content, genome size and ploidy uniformity of in vitro regenerants (Escobedo-GraciaMedrano et al. 2014; Faisal et al. 2014; Shilpha et al. 2014). The nuclear DNA content of micropropagated and field grown mother plants of C. zedoaria revealed a relatively low level of difference in the 2C DNA content. Another study by Islam (2004) have reported 2C DNA content of C. zedoaria collected from Bangladesh to be 3.321 pg. Similarly, flow cytometry has been extensively used for genetic uniformity of in vitro regenerants such as Quercus suber L. (Loureiro et al. 2005), Silene bolanthoides Quézel, Contandr. and Pamukç. (Çördük et al. 2018) and Curcuma angustifolia (Jena et al. 2018).
The essential oil composition of in vitro C. zedoaria rhizome and leaf was fairly stable and similar to that of the corresponding field-grown plants. The slight increase observed in the composition of minor constituents such as limonene, borneol, δ-elemene, β-elemene, 1-epi-cubenol, epi-α-cadinol, (Z)-epi-β-santalol, 2E, 6Z-farnesol, etc. in in vitro propagated plants could be the result of stress response to in vitro conditions. Our findings are in agreement with the report of Purkayastha et al. (2006), who reported curzerenone as the major component in the rhizome essential of C. zedoaria. Contrary to our results, Syamsir et al. (2017) reported camphor, zerumbone, curzerenone and isovelleral as the predominant components in C. zedoaria essential collected from Malaysia, whereas camphor, zerumbone, α-cuparenol and 1,8-cineole were the major constituents in C. zedoaria accession from Indonesia. Previous studies have investigated curzerenone to exhibit various pharmacological properties such as antioxidant, anti-inflammatory and analgesic activities (Dakebo et al. 2002; Makabe et al. 2006). The result of our study also supports the finding of the Rahman et al. (2014) who reported eucalyptol followed by α-caryophyllene, 1-octen-3-ol, β-elemene and caryophyllene oxide as the major constituents in the C. zedoaria leaf essential oil. The antioxidant and antimicrobial properties of 1,8-cineole have been previously reported by several authors (Juergens et al. 2003; Rašković et al. 2014; Ray et al. 2018). Quantity and quality of essential oil is affected by various abiotic and biotic factors like temperature, rainfall, humidity, plant nutrition, genetic variation, application of fertilizers, stress during maturity and geographical location (Sangwan et al. 2001; Rahimmalek et al. 2013; Raut and Karuppayil 2014).
HPLC chromatogram of the rhizome extract of field grown mother plant and randomly selected micropropagated plants of C. zedoaria showed similarity in the curcumin content. Similar studies showing consistency in the content of bioactive compounds of micropropagated plants with the conventionally propagated plants were previously reported in Decalepis salicifolia (Bedd. ex Hook. f.) (Ahmad et al. 2018), Melastoma malabatricum Linn. (Ghimire et al. 2016), Curcuma angustifolia (Jena et al. 2018) and Stevia rebaudiana Bert. (Lata et al. 2013).
Conclusions
In the present work, an effective protocol for rapid clonal propagation of C. zedoaria was established using axillary buds as explant. Genetic fidelity of the micropropagated plant was established using ISSR and flow cytometry analysis. The biochemical uniformity of the tissue culture derived plants was proved using GC–MS and HPLC analysis. Thus, the present micropropagation protocol may be useful for the large scale commercial propagation of true-to-type Curcuma zedoaria for stable supply of the drug to the market.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are grateful to Dr. S.C. Si, Dean, Centre for Biotechnology and Dr. M.R. Nayak, President, Siksha ‘O’ Anusandhan (Deemed to be University) for providing facilities and encouragement throughout.
Author contributions
SJ and AR conceived the idea. SJ, AR, AS and SS performed the research. SJ, BD and BK conducted the experiments. SJ and AR analyzed the results and conducted the statistical analysis. SJ wrote the manuscript. AR and SN revised the manuscript. All authors read and approved the final version of the manuscript.
Compliance with ethical standards
Conflict of interest
All authors declare that they have no conflict of interest.
References
- Adams RP. Identification of essential oil components by gas chromatography/mass spectroscopy. Carol Stream: Allured Publishing Corporation; 2007. [Google Scholar]
- Ahmad Z, Shahzad A, Sharma S, Parveen S. Ex vitro rescue, physiochemical evaluation, secondary metabolite production and assessment of genetic stability using DNA based molecular markers in regenerated plants of Decalepis salicifolia (Bedd. ex Hook. f.) Venter. Plant Cell Tissue Organ Cult. 2018;132:497–510. [Google Scholar]
- Alatar AA, Faisal M, Abdel-Salam EM, Canto T, Saquib Q, Javed SB, El-Sheikh MA, Al-Khedhairy AA. Efficient and reproducible in vitro regeneration of Solanum lycopersicum and assessment genetic uniformity using flow cytometry and SPAR methods. Saudi J Biol Sci. 2017;24:1430–1436. doi: 10.1016/j.sjbs.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisuzzaman M, Sharmin SA, Mondal SC, Sultana R, Khalekuzzaman M, Alam I, Alam MF. In vitro microrhizome induction in Curcuma zedoaria (Christm.) Roscoe-a conservation prioritized medicinal plant. J Biol Sci. 2008;8:1216–1220. [Google Scholar]
- Ayati Z, Ramezani M, Amiri MS, Moghadam AT, Rahimi H, Abdollahzade A, Emami SA, Sahebkar A. Ethnobotany, phytochemistry and traditional uses of Curcuma spp. and pharmacological profile of two important species (C. longa and C. zedoaria): a review. Curr Pharm Des. 2019;25:871–935. doi: 10.2174/1381612825666190402163940. [DOI] [PubMed] [Google Scholar]
- Beena MR, Martin KP, Kirti PB, Hariharan M. Rapid in vitro propagation of medicinally important Ceropegia candelabrum. Plant Cell Tissue Organ Cult. 2003;72:285–289. [Google Scholar]
- Behera S, Kamila PK, Rout KK, Barik DP, Panda PC, Naik SK. An efficient plant regeneration protocol of an industrially important plant, Hedychium coronarium J. Koenig and establishment of genetic and biochemical fidelity of the regenerants. Ind Crops Prod. 2018;126:58–68. [Google Scholar]
- Bharalee R, Das A, Kalita MC. In vitro clonal propagation of Curcuma caesia Roxb and Curcuma zedoaria Rosc from rhizome bud explants. J Plant Biochem Biot. 2005;14:61–63. [Google Scholar]
- Carolina Alves R, Perosa Fernandes R, Fonseca-Santos B, Damiani Victorelli F, Chorilli M. A critical review of the properties and analytical methods for the determination of curcumin in biological and pharmaceutical matrices. Crit Rev Anal Chem. 2019;49:138–149. doi: 10.1080/10408347.2018.1489216. [DOI] [PubMed] [Google Scholar]
- Çördük N, Yücel G, Akıncı N, Tuna M, Esen O. In vitro propagation of Silene bolanthoides Quézel, Contandr. and Pamukç. and assessment of genetic stability by flow cytometry. Arch Biol Sci. 2018;70:141–148. [Google Scholar]
- Dakebo A, Dagne E, Sterner O. Furanosesquiterpenes from Commiphora sphaerocarpa and related adulterant of true Myrrh. Fitoterapia. 2002;73:48–55. doi: 10.1016/s0367-326x(01)00367-7. [DOI] [PubMed] [Google Scholar]
- Doyle JJ, Doyle JL. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull. 1987;19:11–15. [Google Scholar]
- Escobedo-GraciaMedrano RM, Maldonado-Borges JI, Burgos-Tan MJ, Valadez-González N, Ku-Cauich JR. Using flow cytometry and cytological analyses to assess the genetic stability of somatic embryo-derived plantlets from embryogenic Musa acuminata Colla (AA) ssp. malaccensis cell suspension cultures. Plant Cell Tissue Organ Cult. 2014;116:175–185. [Google Scholar]
- Faisal M, Alatar AA, Hegazy AK, Alharbi SA, El-Sheikh M, Okla MK. Thidiazuron induced in vitro multiplication of Mentha arvensis and evaluation of genetic stability by flow cytometry and molecular markers. Ind Crop Prod. 2014;62:100–106. [Google Scholar]
- Ghimire BK, Seong ES, Nguyen TX, Yu CY, Kim SH, Chung IM. In vitro regeneration of Melastoma malabatricum Linn. through organogenesis and assessment of clonal and biochemical fidelity using RAPD and HPLC. Plant Cell Tissue Organ Cult. 2016;124:517–529. [Google Scholar]
- Guenther E. The production of essential oils. In: Robert E, editor. The essential oils. New York: Krieger; 1972. pp. 361–391. [Google Scholar]
- Ilczuk A, Jacygrad E. In vitro propagation and assessment of genetic stability of acclimated plantlets of Cornus alba L. using RAPD and ISSR markers. Vitro Cell Dev Biol Plant. 2016;52:379–390. doi: 10.1007/s11627-016-9781-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MA (2004) Genetic diversity of the genus Curcuma in Bangladesh and further biotechnological approaches for in vitro regeneration and long-term conservation of C. longa germplasm. Ph.D. thesis. Univ. of Hannover, Germany
- Jena S, Ray A, Sahoo A, Sahoo S, Kar B, Panda PC, Nayak S. High-frequency clonal propagation of Curcuma angustifolia ensuring genetic fidelity of micropropagated plants. Plant Cell Tissue Organ Cult. 2018;135:473–486. [Google Scholar]
- Juergens UR, Dethlefsen U, Steinkamp G, Gillissen A, Repges R, Vetter H. Anti-inflammatory activity of 1,8-cineole (eucalyptol) in bronchial asthma: a double-blind placebo-controlled trial. Resp Med. 2003;97:250–256. doi: 10.1053/rmed.2003.1432. [DOI] [PubMed] [Google Scholar]
- Khatri P, Rana JS, Sindhu A, Jamdagni P. Effect of additives on enhanced in vitro shoot multiplication and their functional group identification of Chlorophytum borivilianum Sant. Et Fernand. SN Appl Sci. 2019;1:1105. [Google Scholar]
- Konar S, Karmakar J, Ray A, Adhikari S, Bandyopadhyay TK. Regeneration of plantlets through somatic embryogenesis from root derived calli of Hibiscus sabdariffa L.(Roselle) and assessment of genetic stability by flow cytometry and ISSR analysis. PloS one. 2018;13:e0202324. doi: 10.1371/journal.pone.0202324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar MS. Rapid in vitro multiplication of Centella asiatica (L.) Urban through multiple shoots from leaf explants. Eur J Biotechnol Biosci. 2017;5:41–47. [Google Scholar]
- Lakshmi S, Padmaja G, Remani P. Antitumour effects of isocurcumenol isolated from Curcuma zedoaria rhizomes on human and murine cancer cells. Int J Med Chem. 2011;2011:253962. doi: 10.1155/2011/253962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lata H, Chandra S, Techen N, Wang YH, Khan IA. Molecular analysis of genetic fidelity in micropropagated plants of Stevia rebaudiana Bert. using ISSR marker. Am J Plant Sci. 2013;4:964. [Google Scholar]
- Lee TK, Trinh TA, Lee SR, Kim S, So HM, Moon E, Hwang GS, Kang KS, Kim JH, Yamabe N, Kim KH. Bioactivity-based analysis and chemical characterization of anti-inflammatory compounds from Curcuma zedoaria rhizomes using LPS-stimulated RAW264. 7 cells. Bioorg Chem. 2019;82:26–32. doi: 10.1016/j.bioorg.2018.09.027. [DOI] [PubMed] [Google Scholar]
- Liu LS, Li R, Zhao Y, Wen CL, Ren S, Guo YD. High efficiency regeneration and genetic stability analysis of somatic clones of Gynura bicolor DC. Afr J Biotechnol. 2011;10:10380–10386. [Google Scholar]
- Lobo R, Prabhu KS, Shirwaikar A, Shirwaikar A. Curcuma zedoaria Rosc. (white turmeric): a review of its chemical, pharmacological and ethnomedicinal properties. J Pharm Pharmacol. 2009;6:13–21. doi: 10.1211/jpp/61.01.0003. [DOI] [PubMed] [Google Scholar]
- Loc NH, Duc DT, Kwon TH, Yang MS. Micropropagation of zedoary (Curcuma zedoaria Roscoe)—a valuable medicinal plant. Plant Cell Tissue Organ Cult. 2005;81:119–122. [Google Scholar]
- Loureiro J, Pinto G, Lopes T, Doležel J, Santos C. Assessment of ploidy stability of the somatic embryogenesis process in Quercus suber L. using flow cytometry. Planta. 2005;221:815–822. doi: 10.1007/s00425-005-1492-x. [DOI] [PubMed] [Google Scholar]
- Lourembam RM, Yadav AS, Kundu GC, Mazumder PB. Curcuma zedoaria (christm.) roscoe inhibits proliferation of MDA-MB231 cells via caspase-cascade apoptosis. Orient Pharm Exp Med. 2019;19:235–241. [Google Scholar]
- Makabe H, Maru N, Kuwabara A, Kamo T, Hirota M. Anti-inflammatory sesquiterpenes from Curcuma zedoaria. Nat Prod Res. 2006;20:680–685. doi: 10.1080/14786410500462900. [DOI] [PubMed] [Google Scholar]
- Mohanty S, Parida R, Singh S, Joshi RK, Subudhi E, Nayak S. Biochemical and molecular profiling of micropropagated and conventionally grown Kaempferia galanga. Plant Cell Tissue Organ Cult. 2011;106:39–46. [Google Scholar]
- Mohanty P, Behera S, Swain SS, Barik DP, Naik SK. Micropropagation of Hedychium coronarium J. Koenig through rhizome bud. Physiol Mol Biol Plants. 2013;19:605–610. doi: 10.1007/s12298-013-0199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moharana A, Das A, Subudhi E, Naik SK, Barik DP. High frequency shoot proliferation from cotyledonary node of Lawsonia inermis L. and validation of their molecular finger printing. J Crop Sci Biotechnol. 2017;20:405–416. [Google Scholar]
- Naing AH, Kim SH, Chung MY, Park SK, Kim CK. In vitro propagation method for production of morphologically and genetically stable plants of different strawberry cultivars. Plant Methods. 2019;15:36. doi: 10.1186/s13007-019-0421-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdemir FA, Gur N. In vitro propagation of Cataloglu Apricot (Prunus armeniaca L.) cultivar using apical node as explant. Prog Nutr. 2018;20:176–181. [Google Scholar]
- Panda MK, Mohanty S, Subudhi E, Acharya L, Nayak S. Assessment of genetic stability of micropropagated plants of Curcuma longa L. by cytophotometry and RAPD analyses. Int J Integr Biol. 2007;1:189–195. [Google Scholar]
- Prakash S, Elangomathavan R, Seshadri S, Kathiravan K, Ignacimuthu S. Efficient regeneration of Curcuma amada Roxb. plantlets from rhizome and leaf sheath explants. Plant Cell Tissue Organ Cult. 2004;78:159–165. [Google Scholar]
- Purkayastha J, Nath SC, Klinkby N. Essential oil of the rhizome of Curcuma zedoaria (Christm.) Rose. native to Northeast India. J Essent Oil Res. 2006;18:154–155. [Google Scholar]
- Rahimmalek M, Mirzakhani M, Pirbalouti AG. Essential oil variation among 21 wild myrtle (Myrtus communis L.) populations from different geographical regions in Iran. Ind Crops Prod. 2013;51:328–333. [Google Scholar]
- Rahman A, Afroz M, Islam R, Islam KD, Hossain MA, Na M. In vitro antioxidant potential of the essential oil and leaf extracts of Curcuma zedoaria Rosc. J Appl Pharm Sci. 2014;4:107–111. [Google Scholar]
- Rahmani AH, Alsahli MA, Aly SM, Khan MA, Aldebasi YH. Role of curcumin in disease prevention and treatment. Adv Biomed Res. 2018;7:38. doi: 10.4103/abr.abr_147_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rašković A, Milanović I, Pavlović N, Ćebović T, Vukmirović S, Mikov M. Antioxidant activity of rosemary (Rosmarinus officinalis L.) essential oil and its hepatoprotective potential. BMC Complement Altern Med. 2014;14:225. doi: 10.1186/1472-6882-14-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raut JS, Karuppayil SM. A status review on the medicinal properties of essential oils. Ind Crops Prod. 2014;62:250–264. [Google Scholar]
- Ray A, Jena S, Dash B, Kar B, Halder T, Chatterjee T, Ghosh B, Panda PC, Nayak S, Mahapatra N. Chemical diversity, antioxidant and antimicrobial activities of the essential oils from Indian populations of Hedychium coronarium Koen. Ind Crop Prod. 2018;28:353–362. [Google Scholar]
- Rewers M, Kisiala A, Drouin J, Sliwinska E, Cholewa E. In vitro-regenerated wetland sedge Eriophorum vaginatum L. is genetically stable. Acta Physiol Plant. 2012;34:2197–2206. [Google Scholar]
- Rout GR, Senapati SK, Aparajeta S. Micropropagation of Acacia chundra (Roxb.) DC. Hort Sci. 2008;35:22–26. [Google Scholar]
- Saha S, Adhikari S, Dey T, Ghosh P. RAPD and ISSR based evaluation of genetic stability of micropropagated plantlets of Morus alba L. variety S-1. Meta gene. 2016;7:7–15. doi: 10.1016/j.mgene.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi ND, George L, Eapen S. Plant regeneration from leaf base callus of turmeric and random amplified polymorphic DNA analysis of regenerated plants. Plant Cell Tissue Organ Cult. 2001;66:113–119. [Google Scholar]
- Sangwan NS, Farooqi AHA, Shabih F, Sangwan RS. Regulation of essential oil production in plants. Plant Growth Reg. 2001;34:3–21. [Google Scholar]
- Shahinozzaman M, Faruq MO, Kalam Azad MA, Amin MN. Studies on in vitro propagation of an important medicinal plant-Curcuma zedoaria roscoe using rhizome explants. Persian Gulf crop Prot. 2013;2:1–6. [Google Scholar]
- Shakeri A, Panahi Y, Johnston TP, Sahebkar A. Biological properties of metal complexes of curcumin. BioFactors. 2019;45:304–317. doi: 10.1002/biof.1504. [DOI] [PubMed] [Google Scholar]
- Shilpha J, Silambarasan T, Largia MJV, Ramesh M. Improved in vitro propagation, solasodine accumulation and assessment of clonal fidelity in regenerants of Solanum trilobatum L. by flow cytometry and SPAR methods. Plant Cell Tissue Organ Cult. 2014;117:125–129. [Google Scholar]
- Siwach P, Gill AR. Enhanced shoot multiplication in Ficus religiosa L. in the presence of adenine sulphate, glutamine and phloroglucinol. Physiol Mol Biol Plant. 2011;17:271–280. doi: 10.1007/s12298-011-0074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanly C, Keng CL. Micropropagation of Curcuma zedoaria roscoe and Zingiber zerumbet smith. Biotechnology. 2007;6:555–560. [Google Scholar]
- Syamsir DR, Sivasothy Y, Hazni H, Abdul Malek SN, Nagoor NH, Ibrahim H, Awang K. Chemical constituents and evaluation of cytotoxic activities of Curcuma zedoaria (Christm.) roscoe oils from malaysia and indonesia. J Essent Oil Bear Plant. 2017;20:972–982. [Google Scholar]
- Tariq S, Imran M, Mushtaq Z, Asghar N. Phytopreventive antihypercholesterolmic and antilipidemic perspectives of zedoary (Curcuma Zedoaria Roscoe.) herbal tea. Lipids Health Dis. 2016;15:39. doi: 10.1186/s12944-016-0210-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi M, Kumari N. Micropropagation of a tropical fruit tree Spondias mangifera Willd. through direct organogenesis. Acta Physiol Plant. 2010;32:1011–1015. [Google Scholar]
- Tripathi D, Rai KK, Rai SK, Rai SP. Tripathi D, Rai KK, Rai SK, Rai SP (2018) An improved thin cell layer culture system for efficient clonal propagation and in vitro withanolide production in a medicinal plant Withania coagulans Dunal. Ind Crop Prod. 2018;119:172–182. [Google Scholar]
- Valizadeh J, Valizadeh M. Development of efficient micropropagation protocol for Withania coagulans (Stocks) Dunal. Afr J Biotechnol. 2011;10:7611–7616. [Google Scholar]
- Viehmannova I, Cepkova PH, Vitamvas J, Streblova P, Kisilova J. Micropropagation of a giant ornamental bromeliad Puya berteroniana through adventitious shoots and assessment of their genetic stability through ISSR primers and flow cytometry. Plant Cell Tissue Organ Cult. 2016;125:293–302. [Google Scholar]
- Werner ET, Soares TCB, Gontijo ABPL, Neto JS, Amaral JAT. Genetic stability of micropropagated plants of Crambe abyssinica Hochst using ISSR markers. Genet Mol Res. 2015;14:16450–16460. doi: 10.4238/2015.December.9.16. [DOI] [PubMed] [Google Scholar]
- Xing Y, Yu Y, Luo X, Zhang JN, Zhao B, Guo YD. High efficiency organogenesis and analysis of genetic stability of the regenerants in Solanum melongena. Biol Plant. 2010;54:231–236. [Google Scholar]
- Zuraida AR. Improved in vitro propagation of Curcuma caesia, a valuable medicinal plant. J Trop Agric and Food Sci. 2013;41:273–281. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.