Abstract
Objectives
Feline immunodeficiency virus (FIV) and feline leukaemia virus (FeLV) are retroviruses affecting cats worldwide. The objectives of the study were to estimate the prevalence of these retroviruses in domestic cats in Hungary and to characterise the phylogenetic relationships of FIV strains.
Methods
A total of 335 anticoagulated whole-blood samples obtained from both a healthy and ill cat population were examined for the presence of FIV and FeLV with two methods: ELISA and PCR. Statistical analysis was carried out to analyse the data obtained. Sequencing and phylogenetic analysis of partial polymerase (pol) gene sequences was performed to describe circulating FIV subtypes.
Results
Statistical analysis showed 11.8% and 9.9% true prevalence of FeLV and FIV, respectively, with ELISA. The apparent prevalence calculated from the PCR results were 17.3% for FeLV and 13.1% for FIV. Phylogenetic analysis of partial pol gene sequences obtained from 22 FIV strains showed that all observed Hungarian strains belonged to FIV subtype B. The strains were grouped into several monophyletic subgroups reflecting the geographic locations of the origin of the samples. The overall mean genetic similarity between the analysed strains was 98.2%.
Conclusions and relevance
We report the first thorough overview of the prevalence of FeLV and FIV in Hungary, which is relatively high, and give insight into the genetic diversity of Hungarian strains of FIV.
Keywords: Immunodeficiency virus, leukaemia virus, prevalence, phylogeny, Hungary
Introduction
Feline leukaemia virus (FeLV) is an enveloped single-stranded RNA virus belonging to the Retroviridae family, genus Gammaretrovirus, and it infects feline species. The predominant subgroup, designated FeLV-A, represents the transmissible form of the virus spread cat to cat in nature and from which the other subgroups, FeLV-B,1,2 FeLV-C and FeLV-T,3 arise de novo during the course of infection.4–6 The infection is common worldwide and is transmitted by secretions via the oronasal route, predominantly by prolonged contact, but also by direct inoculation.7
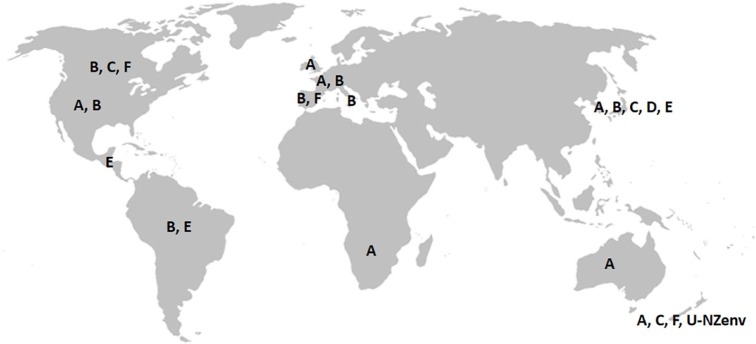
Feline immunodeficiency virus (FIV) is a member of the Lentivirus genus within the Retroviridae family and it infects species of the families Felidae and Hyaenidae.8 It causes an acquired immune deficiency syndrome (AIDS) in cats, resembling AIDS caused by HIV in humans. Transmission is usually by direct inoculation (eg, bite and scratch wounds). The strains are grouped into seven phylogenetic subtypes A–F and U-NZenv.9–19 Distribution of discovered subtypes is illustrated in Figure 1.11,19–25
Figure 1.
Worldwide distribution of feline immunodeficiency virus subtypes (map scheme: www.outline-world-map.com). Subtypes A and B are spread widely, subtypes C, D, E, F and U-NZenv are distributed only regionally
To test the infection status of cats, point-of-care ELISA tests are widely used, which detect antibodies against the p24 protein of FIV and the p27 antigen of FeLV. The most frequent test used to confirm ELISA results, or in case of false/non-interpretable results, is PCR.26
Studies report a relative low prevalence of these viruses worldwide. In North America, FeLV prevalence ranges between 2.3% and 7.5%, and is 2% in Australia, whereas in Europe it is slightly higher (3.6–15.6%). FIV prevalence levels are quite similar: 2.5–7.5% in North America, 15% in Australia and 3.2–8.3% in Europe.27–37
The aim of this study was to estimate the prevalence of these retrovirus infections in domestic cats in Hungary, to evaluate the main factors affecting the infection rate and to examine the phylogenetic relations of the FIV strains detected.
Materials and methods
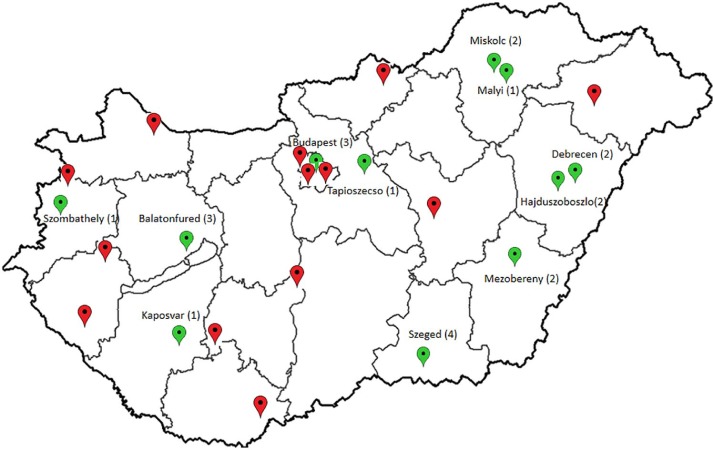
A total of 335 randomly selected, client-owned domestic cats, presented in 24 clinics, over a period of 3 years (2016–2018) were tested from all over Hungary (Figure 2). No free-roaming or sheltered cats were included in the survey. Age, sex, patient history, vaccination status and the household status of cats were registered. General physical examination was performed in each case and EDTA-anticoagulated blood samples were drawn as a part of the routine diagnostic or screening process. A Witness FeLV-FIV ELISA test (Zoetis) was performed immediately, according to the manufacturer’s instructions. The rest of the blood samples were frozen and sent to the Department of Pathology, at the University of Veterinary Medicine, Budapest, and they were stored at −80°C until further examination.
Figure 2.
Origin of the samples collected in this study (map scheme: www.terkepek.net). On the map of Hungary, the 24 locations from where the blood samples were taken are indicated (red and green pinpoints). Cities highlighted with green pinpoints are the locations of partially sequenced FIV strains. Beside the name of city, number in brackets indicate how many sequences were obtained from that location
Serology testing
The point-of-care tests used in this study detect the presence of FeLV p27 antigen (92.9% sensitivity and 96.5% specificity) and FIV antibodies against p24 antigen (93.8% sensitivity and 93.4% specificity).38,39 Sensitivity and specificity values were given according to data provided by Zoetis (used in the statistical analysis), although slightly different values can be found in some field studies (eg, 89.0%/95.5% for FeLV and 94.7%/100% for FIV).40 One drop (0.05 ml) of EDTA-anticoagulated whole blood was used as per the manufacturer’s instructions (Zoetis).
PCR
From the stored whole-blood specimens, nucleic acid extraction was carried out in a QIAcube instrument (Qiagen) with the use of QIAmp cador Pathogen Mini Kit (Qiagen), according to the manufacturer’s instructions. Nucleic acid was eluted in 60 μl RNase-free distilled water (Qiagen). The preparation of endpoint PCR for FIV, TopTaq Master Mix Kit (Qiagen) was used in accordance with manufacturer’s instructions: 25 μl master mix, 0.5 μl forward primer (40 μM), 0.5 μl reverse primer (40 μM), 5 μl CoralLoad Concentrate, 18 μl RNase-free water and 1 μl template DNA, and were mixed together for each sample. The hot-start PCR amplification with the given protocol was carried out as follows: 95°C for 15 mins; 95°C for 45 s, 60°C for 45 s and 72°C for 1 min (40 cycles); and 72°C for 15 mins. The FIV primers used in the study amplify early reverse transcription products (process of reverse transcription RNA to DNA); both bind in the long terminal repeat (LTR) region, which enables the detection of low-dose and/or recent infections (primer sensitivity was 102–103 cell-associated virus).41,42 For the detection of FeLV, a One Step RT-PCR Kit (Qiagen) was used. A master mix containing 5.7 μl RNase-free water, 2 μl 5× buffer, 0.4 μl dNTP, 0.4 μl enzyme mix, 0.1 μl RNase inhibitor, 0.2 μl forward primer (40 μM) and 0.2 μl reverse primer (40 μM) were added into tubes for each sample (0.5 μl each). The amplification was 50°C for 30 mins and 94°C for 3 mins (reverse transcription and initial denaturation); 95°C for 15 s, 60°C for 1 min and 72°C for 1 min (45 cycles); and 72°C for 10 mins. FeLV primers were obtained from a publication.43 The 163 base pair (bp) (FIV) and 150 bp (FeLV) long amplicons were visualised by electrophoresis in 1.5% agarose gel.
Statistical analysis
Statistical calculations were performed in an R environment (R Core Team, Vienna, Austria) and a logistical regression model was used to detect the relationship between the examined variants and retroviral infection status.44 The Epi.prev function was used to calculate prevalences.45 Cohen’s kappa (κ) was counted to show possible cross-compliance between the ELISA and PCR methods.46
Phylogenetic analysis
In FIV-positive cases, partial proviral pol genes were amplified with the previously described protocol of Adams et al.47 PCR products were subjected to gel electrophoresis, amplicons of 576 bp length were cut out manually and purified by a Qiagen Gel Extraction Kit (Qiagen). Bidirectional Sanger sequencing reaction was performed with the corresponding primers and the capillary electrophoresis was made by a commercial provider (Microsynth). In two cases (I/14/16 and II/7/8) repeated sequencing did not result in clean sequences; thus, these PCR products were cloned into a pJET1.2 blunt vector and subsequently transformed into Escherichia coli, with the help of a CloneJET PCR Cloning Kit (ThermoFisher Scientific).
After proofreading of the obtained sequences, they were assembled using the E-INS-i method of the online software MAFFT version 7,48 and aligned against available FIV genomes downloaded from GenBank that represent the overall diversity of the virus. Maximum likelihood analyses were carried out and the trees were visualised and edited with MEGA749 using the Tamura-Nei model with 1000 bootstrap replicates and gamma distribution.50 Pairwise genetic analyses were conducted using the Kimura 2-parameter model in MEGA7.51
Results
Altogether, samples from 335 domestic cats were included in the study. All cats had owners – no stray or shelter animals were included. The mean age of cats in this study was 4.9 years (range 5 months to 18 years). A total of 155 females (46.3%) and 180 males (53.7%) were included in the research. Of the 155 females, 90 (58.1%) were intact and 65 (41.9%) were spayed; of the 180 males, 119 (66.1%) were intact and 61 (33.9%) were castrated. Seventy-seven cats were kept strictly indoors (23.0%) and 258 had outdoor access (77.0%). A total of 136 cats were found to be clinically healthy during the physical examination. We observed an overall low prevalence of vaccination: only 98 (29.3%) were immunised at some point of their life-span, usually with a combined vaccine, and among these cats, only 39 (11.6%) were vaccinated against FeLV (however, two of them proved to be FeLV-infected).
Of the 335 samples, 44 (13.1%) and 51 (15.2%) were positive for FeLV and FIV, respectively, with the Witness ELISA test kit. Using subsequent PCR tests, 58 (17.3%) were positive for FeLV and 47 (14.0%) for FIV. FeLV–FIV coinfection was observed in 10 (0.03%) cats. In some cases, only the ELISA test or the PCR test was positive. In the case of FIV, 11/51 ELISA tests and 4/47 PCR test results were positive, when the parallel assay was negative. In the case of FeLV, 4/44 ELISA results and 14/58 PCR results were single-positive.
Total apparent prevalence, counted from data obtained with the Witness test, was 14.03 (95% confidence interval [CI] 10.72–18.16) in the case of FeLV and 15.22 (95% CI 11.77–19.46) in the case of FIV. True prevalence was also calculated with the previously estimated sensitivity and specificity of the Witness test: 11.78 (95% CI 8.08–16.4) for FeLV and 9.89 (95% CI 5.93–14.75) for FIV.
Apparent prevalences calculated from the PCR results were 17.31 (95% CI 13.64–21.73) and 13.13 (95% CI 9.93–17.17) for FeLV and FIV, respectively. Cross-compliance was analysed by calculating Cohen’s κ coefficient. This coefficient was 0.786 (95% CI 0.68–0.87) for FeLV (P < 0.05) and 0.816 (95% CI 0.72–0.9) for FIV (P < 0.05). Both results indicate a significant coherence between the Witness ELISA and PCR results.
With logistic regression, a 2.85 (95% CI 1.19–8.49; P = 0.033) times higher chance of infection with FeLV and a 4.14 (95% CI 1.62–14.07; P = 0.0083) times higher chance of FIV was found in correlation with outdoor access, according to the Witness test results. Calculating the PCR results, a 2.51 (95% CI 1.156–6.29; P = 0.0306) and a 3.41 (95% CI 1.32–11.64; P = 0.0235) times higher possibility was found in correlation with FeLV and FIV infection, respectively.
No significant correlation was found with the animals’ age regarding FeLV infection (either with results of ELISA or PCR), but a 1.12 times higher chance (both with the Witness test and PCR [95% CI 1.04–1.19 (P = 0.0012) for ELISA] and [95% CI 1.04–1.19 (P = 0.0014) for PCR]) was observed in the case of FIV infection for every year older the cats were. We could not find significant correlation of infection status with sex (although males were 1.32 times more likely to have FIV and/or be FeLV-infected than females [95% CI 0.71–2.5, P = 0.3872]). The same was observed for neutering status: there was a 1.34 times higher risk for intact males to be infected (95% CI 0.57–3.42; P = 0.5187) and a 0.78 higher risk for intact females (95% CI 0.3–2.08; P = 0.609), but these data were not statistically significant.
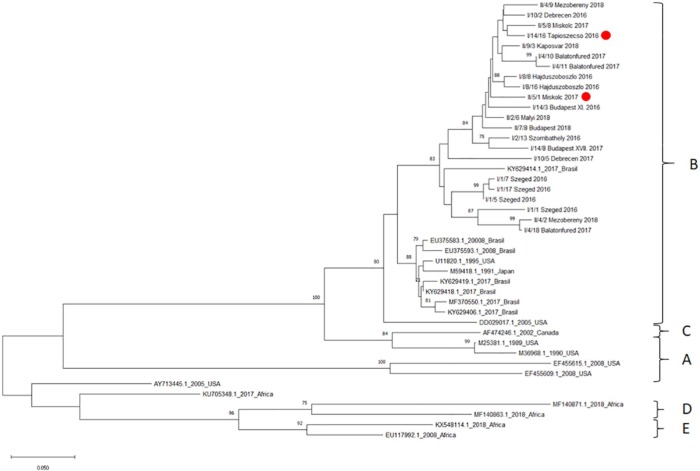
Altogether, we were able to sequence 22 partial pol genes out of the 47 FIV PCR-positive cases. We suspect that the rest of the samples contained insufficient amounts or poor quality nucleic acids. Multiple alignments and maximum likelihood tree reconstructions revealed that all of the strains were clustered into subtype B (Figure 3). Within clade B, the Hungarian sequences were located into a monophyletic group that also included a strain originating from Joinville, Brazil, 2017 (acc. number: KY629414.1). Within this ‘Hungarian’ cluster, the strains formed several subgroups of closely related sequences that mostly reflected the geographic location of their origin. The subgroups were supported by relatively high bootstrap values.
Figure 3.
Phylogenetic tree reconstructed from the partial pol gene sequences of Hungarian feline immunodeficiency virus strains and selected reference sequences from GenBank. Maximum likelihood bootstrap support values (⩾70) are shown as percentages above the branches. The two indoor cats are marked with red dots; all the others had outdoor access. Strains displayed on the phylogenetic tree are coded differently in the case of Hungarian strains, the code of sample and city of the cat’s origin are shown. In case of reference sequences obtained from GenBank, accession number, year of publication and country of collection are displayed
Pairwise genetic analyses revealed that the overall mean genetic similarity between the analysed strains was 98.2% (the lowest similarity was 88.1% and the highest was 99.8%). Only two strains originated from indoor cats and the majority of these cats were males (16 males, six females). The samples represent most of geographic regions of Hungary (Figure 2). GenBank accession numbers are MN401425–MN401446.
Discussion
Our results show a relatively high rate of infection of FeLV in owned domestic cats in Hungary vs the most recent data from surrounding (or more distant) countries (with the exclusion of prevalence data including free-roaming or shelter cats). The prevalence rate of FIV does not show such a difference.20 Data regarding vaccination status showed that there is a strong need for improving in the vaccination prevalence in Hungarian cat populations. Two of the immunised cats had FeLV-positive results, both determined by ELISA and PCR, but their vaccinations were not regular, which could be an answer to possible unprotected periods of time and the ability to become infected.52 Moreover, 77.0% of cats had outdoor access and most of them were not neutered. Previous publications and our statistics show a higher occurence of retro-viral infection in intact (usually male) outdoor cats.53
Another possible explanation for the higher observed prevalence data could be the weak positive predictive value of every diagnostic test that does not have 100% specificity in the case of low prevalence rates in a population (‘false positive paradox’).
In relation to the discordant results between ELISA and PCR in some cases, where only one test method was positive, there can be various explanations. In the case of FIV, the Witness test detects antibodies, whereas the PCR amplifies and detects part of the LTR region of the virus. In the early stages of infection there are no detectable antibodies (most cats produce antibodies within 8 weeks of exposure);54 moreover, there might be extremely low levels of antibodies in the terminal phase of the disease, causing a false-negative ELISA result, whereas the PCR result will be positive as a result of increased viral load in the circulation.37 However, a positive ELISA and negative PCR result can suggest extremely low levels of circulating proviral DNA/viral RNA (which can be the case in the asymptomatic phase of FIV infection) or viral genetic diversity (where nucleotide sequence dissimilarity can result in failure of primer binding).37 The presence and interference of maternal antibodies with the ELISA can be excluded in our cases, as the youngest animals included in the study (5–6 months of age) were FIV negative. Maternal antibodies can be present in kittens up to 6 months of age.55 In the case of FeLV, the Witness test detects the p27 antigen, whereas the PCR amplifies and detects the U3 part of the LTR region. A negative ELISA and positive PCR result can also suggest an early phase of infection or illness in a regressive stage, when small amounts of proviral DNA/viral RNA are already present in the circulation, but the amount of the antigen is still below detectable levels.
Regarding the phylogenetic analysis of the FIV sequences, all the sequenced samples belonged to subtype B, and they form a unique cluster of strains that includes Hungarian sequences and also a Brazilian one. The monophyletic pattern suggests a common ancestor for all the Hungarian (and the single Brazilian) sequences analysed in the study. Based on our data, it can be speculated that there was a single event of virus introduction into the country and the genetic diversity observed is the result of divergent local evolution of the strains.
It must be highlighted that our 22 sequences are insufficient to give a complete picture of the circulating FIV strains in Hungary, but it can be suggested that mainly subtype B strains are present in Hungary. This result is largely in harmony with data published from surrounding countries, where mostly subtypes A and B were discovered.20
Conclusions
This is the first report of a thorough investigation on retroviral prevalence and phylogenetic analyses of FIV strains in Hungary, and suggests that FIV subtype B is the most prevalent in this country. This data set and prospective phylogenetic analyses of further FIV strains can give useful information for vaccine developers as the only existing FIV vaccine, which is not available in Hungary (Fel-O-Vax FIV; Boehringer Ingelheim), contains inactivated whole virus subtypes A and D, which will not confer a sufficient protective effect against subtype B.21,56
Acknowledgments
We wish to thank all the veterinarians who provided blood samples and the background data of the cats, and also Zoetis Hungary, with special thanks to Zsolt Varga for providing the Witness ELISA tests. Kitti Schönhardt, molecular biologist of the Department of Pathology, University of Veterinary Medicine, Budapest, was generous with her time helping with the PCR procedures.
Footnotes
Accepted: 8 November 2019
Conflict of interest: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This research was supported by the 17896-4/2018/FEKUTSTRAT grant of the Hungarian Ministry of Human Capacities. Gyula Balka was supported by the János Bolyai Scholarship of the Hungarian Academy of Sciences.
Ethical approval: This work involved the use of non-experimental animals only (owned or unowned), and followed internationally recognised high standards (‘best practice’) of individual veterinary clinical patient care. Ethical approval from a committee was not necessarily required.
Informed consent: Informed consent (either verbal or written) was obtained from the owner or legal custodian of all animal(s) described in this work for the procedure(s) undertaken. No animals or humans are identifiable within this publication, and therefore additional informed consent for publication was not required.
ORCID iD: Anna Szilasi  https://orcid.org/0000-0002-1634-6730
https://orcid.org/0000-0002-1634-6730
Reinhard Ertl  https://orcid.org/0000-0001-7485-3661
https://orcid.org/0000-0001-7485-3661
References
- 1. Sugai J, Eiden M, Anderson MM, et al. Identification of envelope determinants of feline leukemia virus subgroup B that permit infection and gene transfer to cells expressing human pit1 or pit2. J Virol 2001; 75: 6841–6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Faix PH, Feldman SA, Overbaugh J, et al. Host range and receptor binding properties of vectors bearing feline leukemia virus subgroup B envelopes can be modulated by envelope sequences outside of the receptor binding domain. J Virol 2002; 76: 12369–12375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chiu ES, Hoover EA, Vandewoude S. A retrospective examination of feline leukemia subgroup characterization: viral interference assays to deep sequencing. Viruses 2018; 10. DOI: 10.3390/v10010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Neil JC, Fulton R, Rigby M, et al. Feline leukaemia virus: generation of pathogenic and oncogenic variants. Curr Top Microbiol Immunol 1991; 171: 67–93. [DOI] [PubMed] [Google Scholar]
- 5. Roy-Burman P. Endogenous env elements: partners in generation of pathogenic feline leukemia viruses. Virus Genes 1995; 11: 147–161. [DOI] [PubMed] [Google Scholar]
- 6. Coelho FM, Bomfim MR, de Andrade Caxito F, et al. Naturally occurring feline leukemia virus subgroup A and B infections in urban domestic cats. Gen Virol 2008; 89: 2799–2805. [DOI] [PubMed] [Google Scholar]
- 7. Addie DD, Dennis JM, Toth S, et al. Long-term impact on a closed household of pet cats of natural infection with feline coronavirus, feline leukaemia virus and feline immunodeficiency virus. Vet Rec 2000; 146: 419–424. [DOI] [PubMed] [Google Scholar]
- 8. Perharić M, Biđin M, Starešina V, et al. Phylogenetic characterisation of feline immunodeficiency virus in naturally infected cats in Croatia indicates additional heterogeneity of subtype B in Europe. Arch Virol 2016; 161: 2567–2573. [DOI] [PubMed] [Google Scholar]
- 9. Olmsted RA, Langley R, Roelke ME, et al. Worldwide prevalence of lentivirus infection in wild feline species: epidemiologic and phylogenetic aspects. J Virol 1992; 66: 6008–6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Greene W, Meers J, Chdwick B, et al. Nucleotide sequences of Australian isolates os the feline immunodeficiency virus: comparision with other feline lentiviruses. Arch Virol 1993; 132: 369–379. [DOI] [PubMed] [Google Scholar]
- 11. Sodora DL, Shpaer EG, Kitchell BE, et al. Identification of three feline immunodeficiency virus (FIV) env gene subtypes and comparison of the FIV and human immunodeficiency virus type 1 evolutionary patterns. J Virol 1994; 68: 2230–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kakinuma S, Motokawa K, Hohdatsu T, et al. Nucleotide sequence of feline immunodeficiency virus: classification of Japanese isolates into two subtypes which are distinct from non-Japanese subtypes. J Virol 1995; 69: 3639–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pecoraro MR, Tomonaga K, Miyazawa T, et al. Genetic diversity of Argentine isolates of feline immunodeficiency virus. J Gen Virol 1996; 77: 2031–2035. [DOI] [PubMed] [Google Scholar]
- 14. Bachmann MH, Mathiason-Dubard C, Learn GH, et al. Genetic diversity of feline immunodeficiency virus: dual infection, recombination, and distinct evolutionary rates among envelope sequence clades. J Virol 1997; 71: 4241–4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pistello M, Cammarota G, Nicoletti E, et al. Analysis of the genetic diversity and phylogenetic relationship of Italian isolates of feline immunodeficiency virus indicates a high prevalence and heterogeneity of subtype B. J Gen Virol 1997; 78: 2247–2257. [DOI] [PubMed] [Google Scholar]
- 16. Uema M, Ikeda Y, Miyazawa T, et al. Feline immunodeficiency virus subtype C is prevalent in northern part of Taiwan. J Vet Med Sci 1999; 61: 197–199. [DOI] [PubMed] [Google Scholar]
- 17. Reggeti F, Bienzle D. Feline immunodeficiency virus subtypes A, B and C and intersubtype recombinants in Ontario, Canada. J Gen Virol 2004; 85: 1843–1852. [DOI] [PubMed] [Google Scholar]
- 18. Weaver EA, Collisson EW, Slater M, et al. Phylogenetic analyses of Texas isolates indicate an evolving subtype of the clade B feline immunodeficiency viruses. J Virol 2004; 78: 2158–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hayward JJ, Rodrigo AG. Recombination in feline immunodeficiency virus from feral and companion domestic cats. Virol J 2008; 5. DOI: 10.1186/1743-422X-5-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Steinrigl A, Klein D. Phylogenetic analysis of feline immunodeficiency virus in Central Europe: a prerequisite for vaccination and molecular diagnostic. J Gen Virol 2004; 84: 1301–1307. [DOI] [PubMed] [Google Scholar]
- 21. Kann RKC, Kyaw-Tanner MT, Seddon JM, et al. Molecular subtyping of feline immunodeficiency virus from domestic cats in Australia. Aust Vet J 2006; 84: 112–116. [DOI] [PubMed] [Google Scholar]
- 22. Beczkowski PM, Hughes J, Biek R, et al. Rapid evolution of the env gene leader sequence in cats naturally infected with feline immunodeficiency virus. J Gen Virol 2015; 96: 893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cano-Ortiz L, Junqueira DM, Comerlato J, et al. Phylodynamics of the Brazilian feline immunodeficiency virus, infection, genetics and evolution. Infect Genet Evol 2017; 55: 166–171. [DOI] [PubMed] [Google Scholar]
- 24. Duarte A, Tavares L. Phylogenetic analysis of Portuguese feline immunodeficiency virus sequences reveals high genetic diversity. Vet Microbiol 2006; 114: 25–33. [DOI] [PubMed] [Google Scholar]
- 25. Weaver EA. A detailed phylogenetic analysis of FIV in the United States. PLoS One 2010; 5: e12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Levy J, Crawford C, Hartmann K, et al. 2008 American Association of Feline Practitioners’ feline retrovirus management guidelines. J Feline Med Surg 2008; 10: 300–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Arjona A, Escolar E, Soto I, et al. Seroepidemiological survey of infection by feline leukemia virus and immunodeficiency virus in Madrid and correlation with some clinical aspects. J Clin Microbiol 2000; 38: 3448–3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yilmaz H, Ilgaz A, Harbour DA. Prevalence of FIV and FeLV infections in cats in Istanbul. J Feline Med Surg 2000; 2: 69–70. [DOI] [PubMed] [Google Scholar]
- 29. Luria BJ, Levy JK, Lapinn MR. Prevalence of infectious diseases in feral cats in Northern Florida. J Feline Med Surg 2004; 6: 287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bandecchi P, Dell’Omodarme M, Magi M, et al. Feline leukaemia virus (FeLV) and feline immunodeficiency virus infections in cats in the Pisa district of Tuscany, and attempts to control FeLV infection in a colony of domestic cats by vaccination. Vet Rec 2006; 16: 555–557. [DOI] [PubMed] [Google Scholar]
- 31. Levy JK, Scott HM, Lachtara JL, et al. Seroprevalence of feline leukemia virus and feline immunodeficiency virus infection among cats in North America and risk factors for seropositivity. J Am Vet Med Assoc 2006; 3: 371–376. [DOI] [PubMed] [Google Scholar]
- 32. Gleich SE, Krieger S, Hartmann K. Prevalence of feline immunodeficiency virus and feline leukaemia virus among client-owned cats and risk factors for infection in Germany. J Feline Med Surg 2009; 11: 985–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Little S, Sears W, Lachtara J, et al. Seroprevalence of feline leukemia virus and feline immunodeficiency virus infection among cats in Canada. Can Vet J 2009; 50: 644–648. [PMC free article] [PubMed] [Google Scholar]
- 34. Ravi M, Wobeser GA, Taylor SM, et al. Naturally acquired feline immunodeficiency virus (FIV) infection in cats from western Canada: prevalence, disease associations, and survival analysis. Can Vet J 2010; 51: 271–276. [PMC free article] [PubMed] [Google Scholar]
- 35. Ortega-Pacheco A, Aguilar-Caballero AJ, Colin-Flores RF, et al. Seroprevalence of feline leukemia virus, feline immunodeficiency virus and heartworm infection among owned cats in tropical Mexico. J Feline Med Surg 2014; 16: 460–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Firth CL, Möstl K. A survey of feline leukaemia virus antigenaemia among cats in eastern Austria: a retrospective analysis of serum samples routinely tested between 1996 and 2011. J Feline Med Surg 2015; 1: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Westman ME, Malikband R, Norrisa JM. Diagnosing feline immunodeficiency virus (FIV) and feline leukaemiavirus (FeLV) infection: an update for clinicians. Aust Vet J 2019; 97: 47–55. [DOI] [PubMed] [Google Scholar]
- 38. Zoetis Inc., Data on file, Study Report No. D880Z-US-14-013. [Google Scholar]
- 39. Zoetis Inc., Data on file, Study Report No. D880Z-US-14-014. [Google Scholar]
- 40. Levy JK, Crawford PC, Tucker SJ. Performance of 4 point-of-care screening tests for feline leukemia virus and feline immunodeficiency virus. J Vet Intern Med 2017; 31: 521–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sutton S. Detection of feline immunodeficiency virus DNA products in lytic versus latent infection, including early reverse transcription, intermediate reverse transcription, and late circle junctions. Senior Honors thesis, Ohio State University, 2007. [Google Scholar]
- 42. Joshi A, Vahlenkamp TW, Garg H, et al. Preferential replication of FIV in activated CD4+CD25+T cells independent of cellular proliferation. J Virol 2004; 321: 307–322. [DOI] [PubMed] [Google Scholar]
- 43. Tandon R, Cattori V, Gomes-Keller MA, et al. Quantitation of feline leukaemia virus viral and proviral loads by TaqMan® real-time polymerase chain reaction. J Virol Methods 2005; 130: 124–132. [DOI] [PubMed] [Google Scholar]
- 44. R Core Team. A language and environment for statistical computing. https://www.R-project.org/ (2016, accessed November 20, 2019).
- 45. Sullivan KM, Dean A, Soe MM. OpenEpi: a web-based epidemiologic and statistical calculator for public health. Public Health Rep 2009; 124: 471–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dinya E, Solymosi N. Biometria a klinikumban. 1st ed Budapest: Medicina, 2016. [Google Scholar]
- 47. Adams H, van Vuuren M, Kania S, et al. Sensitivity and specificity of a nested polymerase chain reaction for detection of lentivirus infection in lions (Panthera leo). J Zoo Wildl Med 2010; 41: 608–615. [DOI] [PubMed] [Google Scholar]
- 48. Katoh K, Toh H. Recent developments in the MAFFT multiple sequence alignment program. Brief Bioinform 2008; 9: 286–298. [DOI] [PubMed] [Google Scholar]
- 49. Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 2016; 33: 1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tamura K, Stecher G, Peterson D, et al. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 2013; 12: 2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kimura M. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 1980; 16: 111–120. [DOI] [PubMed] [Google Scholar]
- 52. Hofmann-Lehmann R, Levy LS, Willett BJ. Comparing the efficacy of FeLV vaccines, comment on: Stuke, K. et al. Efficacy of an inactivated FeLV vaccine compared to a recombinant FeLV vaccine in minimum age cats following virulent FeLV challenge. Vaccine 2015; 33: 2737–2738. [DOI] [PubMed] [Google Scholar]
- 53. Goldkamp CE, Levy JK, Edinboro CH, et al. Seroprevalences of feline leukemia virus and feline immunodeficiency virus in cats with abscesses or bite wounds and rate of veterinarian compliance with current guidelines for retrovirus testing. J Am Vet Med Assoc 2008; 232: 1152. [DOI] [PubMed] [Google Scholar]
- 54. Sykes J, Greene C. Feline immunodeficiency virus. In: Sellon RK, Hartmann K. (eds). Infectious diseases of the dog and cat. 4th ed. St Louis, MO: Elsevier Saunders, 2012, p 142. [Google Scholar]
- 55. MacDonald K, Levy JK, Tucker SJ, et al. Effects of passive transfer of immunity on results of diagnostic tests for antibodies against feline immunodeficiency virus in kittens born to vaccinated queens. J Am Vet Med Assoc 2004; 225: 1554–1557. [DOI] [PubMed] [Google Scholar]
- 56. Yamamoto JK, Torres BA, Pu R. Development of the dual-subtype feline immunodeficiency virus vaccine. AIDScience 2002; 2. [DOI] [PubMed] [Google Scholar]