Abstract
Emerging evidence suggests that adverse early life events can affect long-term health trajectories throughout life. Preterm birth, in particular, is a significant early life event that affects approximately 10% of live births. Worldwide, prematurity is the number one cause of death in children less than 5 years of age and has been shown to disrupt normal lung development with lasting effects into adult life. Along with impaired lung development, interventions used to support gas exchange and other sequelae of prematurity can lead to the development of bronchopulmonary dysplasia (BPD). BPD is a chronic respiratory disease of infancy characterized by alveolar simplification, small airways disease, and pulmonary vascular changes. Although many survivors of BPD improve with age, survivors of BPD often have chronic lung disease characterized by airflow obstruction and intermittent pulmonary exacerbations. Long-term lung function trajectories as measured by FEV1 can be lower in children and adults with a history BPD. In this review, we discuss the epidemiology and manifestations of BPD and its long-term consequences throughout childhood and into adulthood. Available evidence suggests that disrupted lung development, genetic susceptibility and subsequent environment and infectious events that occur in prenatal and postnatal life likely increase the predisposition of children with BPD to develop early onset chronic obstructive pulmonary disease (COPD).
The reviews of this paper are available via the supplemental material section.
Keywords: bronchopulmonary dysplasia, COPD, lung function
Introduction
Worldwide, preterm birth is the leading cause of death in children less than 5 years of age, with approximately 10.6% of live births categorized as preterm (<37 weeks gestation).1 While the majority of preterm births occur in late gestation (between 34 and 37 weeks gestation), these children are at higher risk for respiratory morbidity compared with term infants. These respiratory morbidities include higher rates of respiratory distress in the neonatal period and diagnosis of asthma in adulthood.2,3 However, few late-term premature infants go on to develop bronchopulmonary dysplasia (BPD).4 Indeed, it is the very early gestational age infants (⩽32 weeks gestation) who are at highest risk for mortality, and who are most likely to develop BPD.5–8 Due to advances in the treatment of preterm birth, many more very low birthweight preterm infants are surviving to hospital discharge, contributing to the increase in children at risk for BPD development and respiratory morbidities in later life.9,10
Survivors of prematurity are frequently burdened with a variety of complications, including respiratory disease. These respiratory complications can include pulmonary hypertension, asthma-like phenotypes, alveolar hypoplasia, obstructive sleep apnea, large airway malacia, and, most prominently, BPD, which can encompass one or more of these morbidities.11 In the United States alone, it is estimated that nearly 50,000 preterm infants develop chronic lung disease due to their prematurity annually.11 The disruption of alveolar growth associated with BPD may have lifelong consequences, including a possible link to early onset chronic obstructive pulmonary disease (COPD) in adult life. In this article, we will discuss the impact of prematurity on respiratory health, factors that contribute to the development of BPD, and the long-term consequences of BPD, particularly as they relate to COPD.
Respiratory health trajectories
A number of studies have demonstrated the existence of lung function trajectories where maximal lung function is achieved in early adulthood with a subsequent decline with aging.12 However, considerable heterogeneity exist in the maximal lung function achieved and the rate of decline.12 This heterogeneity in lung function trajectories is likely influenced in part by adverse prenatal and postnatal life events. Although the conducting airways are formed at the end of the pseudoglandular stage of fetal lung development, the lung is immature at birth with few alveoli.13 The majority of postnatal alveolar growth occurs between birth and 8 years of age, with most alveolar growth occurring by 3 years of age.14,15 Recently, however, studies have shown that some additional alveolar growth can occur through late adolescence and early adulthood.16 Factors in early life that adversely affect respiratory growth can lead to heterogeneity in achieving maximal lung function. These events, with often seemingly normal recoveries, have been demonstrated to impair adult lung function. For example, a history of radiologically diagnosed pneumonia within the first 3 years of life has been associated with lower lung function in young adults, compared with their counterparts without such a history.17 Lung function trajectories during life can be also be influenced by prenatal exposures, most notably exposure to tobacco smoke.18 Exposure to tobacco smoke in utero has been associated with increased wheezing in preschool children, and is a major risk factor for BPD development in preterm infants exposed to maternal smoking during pregnancy.19,20 Additionally, preclinical studies in mice confirm that disruption of lung growth during early postnatal life can adversely affect lung function in adult life.21–23 Taken together, these preclinical and clinical studies suggest that prenatal and postnatal insults and exposures occurring during critical periods of lung growth are likely to impair lung function in later life.
From these studies, it is possible to surmise that premature birth and the development of BPD can have long-term effects on respiratory health, lung function, and, potentially, the development of COPD. Supporting this link between early life events and adult respiratory outcomes, Barker and colleagues reported that men born with low birth weights between 1911 and 1930, were more likely to have lower adult lung function and death from COPD.24 In their report, the presence of lower respiratory tract infections was additive to the reduction of lung function in adult life. A recent literature review of 16 studies identified that preterm birth and low birth weight, in addition to in utero tobacco exposure, early childhood asthma, and pneumonia were factors that increased the likelihood of lung function impairment in late childhood.25 Other studies have also reported that preterm birth is associated with respiratory limitations on exercise studies in older children and adults,26–29 and several studies have reported lower lung function in preterm infants with and without BPD compared with age-matched controls born at term.30,31 Chest imaging of children with BPD has also demonstrated abnormalities of airflow obstruction. One study reported areas of hyperexpansion and hyperlucency in chest radiographic and computed tomography (CT) scans from children with a history of BPD.32 Unlike imaging in COPD, however, studies are lacking on lung CT imaging scores and correlation with lung function and clinical symptoms in people with BPD.33
The diagnosis of BPD and gestational age at birth has the greatest influence on later lung function in children with BPD.30,34 Balinotti and colleagues, reported that infants and toddlers with a history of prematurity and chronic lung disease had impaired alveolar growth compared with full-term age-matched participants.35 Another study reported that alveolar catch-up growth was achievable in older extremely low birth weight (ELBW) children (ages 10–14 years); however, the ELBW children had significantly lower FEV1 than the full-term controls.36 It is not entirely understood why people with a history of ELBW with and without BPD have airflow obstruction; however, it is known that preterm infants have fewer and larger alveoli,37 resulting in less lung surface area for gas exchange. It is plausible that fewer alveoli and alveolar attachments in the preterm lung causes less stenting open of small airways during a period of rapid postnatal lung growth, resulting in dysanaptic airway growth and fixed airflow obstruction, similar to airflow obstruction in COPD.38
Worldwide, COPD is a leading cause of death in adults, with 3.2 million people dying of COPD in 2015.39 Risk factors for developing COPD include cigarette smoking, indoor air pollution, diagnosis of asthma, and genetic risk factors. Similar to BPD, COPD is characterized by small airway disease and lower predicted forced expiratory volume in the first 1 s (FEV1 %).40 People with COPD commonly present with shortness of breath, chronic cough, and mucus production, with declines in FEV1 triggered by upper respiratory tract infections.41,42 Hallmarks of COPD progression, include an accelerated decline in FEV1 with age, radiographic evidence of emphysema, and end-stage lung disease. Disruption of normal lung development by preterm birth and other early life events,43–45 likely increases an individual’s risk of reaching a critical threshold of lung disease, and going on to develop COPD in early adult life.
Epidemiology and manifestations of BPD
Although a number of respiratory morbidities occur at higher rates in preterm infants compared with their full-term counterparts, the lung disease most associated with extreme prematurity is bronchopulmonary dysplasia (BPD).4 Based on autopsy specimens, Northway and colleagues described key findings of BPD in 1967 as including alveolar emphysema, pulmonary arteriolar changes, and hypertrophy of airway smooth muscle and the right ventricle.46 The disease has evolved since that time, with the advent of surfactant administration in the late 1980s and incremental improvements in ventilatory technologies and strategies. BPD in the postsurfactant era is characterized by hypoplasia of the alveoli and simplification of the pulmonary vasculature. Despite these advances, the incidence of BPD has increased over the past two decades,8 due partially to increased survival of earlier gestational infants, but also in part to the absence of therapies that promote lung growth and attenuate inflammation and oxidative stress in the preterm lung.8,47,48
In clinical practice, manifestations of BPD may include elements of gas exchange abnormalities (chronic respiratory failure or insufficiency with hypercarbia or hypoxia) associated with alveolar disease and asthma-like phenotypes (wheezing or coughing) associated with small airway disease. Pulmonary hypertension (PH) may be present in infants and children with BPD, and is most often associated with pulmonary vascular disease; however, left ventricular (LV) dysfunction, intracardiac shunts, persistent patent ductus arteriosus, and pulmonary venous stenosis may contribute to the severity and development of PH. Severity of respiratory disease in children with BPD may range from mild tachypnea to the need for home mechanical ventilation. Based on a commonly used definition for BPD from a NHLBI committee,49 there may be as many as 48,000 preterm infants in the United States who develop BPD, of whom 12,000 may require home supplemental oxygen, and, an additional 200 home mechanical ventilation.11
Owing to catch-up lung growth within the first 2 years of life, the majority of infants with BPD who are discharged on supplemental oxygen are weaned to room air by 12 months of age, and the median age of those on home mechanical ventilation being weaned off is 25 months.50–55 However, infants with BPD still remain at high risk for rehospitalization, up to 50% within the first 2 years of life, usually secondary to respiratory infections.56 Diagnosis of BPD, severity of BPD during initial hospitalization and length of time requiring supplemental oxygen has been shown to be predictive of lung function abnormalities in school age children in several studies.57,58
Pathophysiology of BPD
BPD is a multifactorial disorder with a complex pathophysiology much like COPD. While preterm birth is the greatest risk factor for developing BPD, not every infant born prematurely will develop BPD, and, similarly, every smoker may not develop COPD. Risk factors for developing BPD in preterm infants include extremely low birth weight,4,59 small for gestational age,60,61 genetic/epigenetic factors, prenatal events such as exposure to maternal smoking,20 events occurring within the initial neonatal intensive care unit (NICU) admission, and exposures occurring after initial discharge into childhood.62 Exposures in the NICU that increase the likelihood of developing BPD include high oxygen concentrations, positive pressure ventilation, aspiration events, pneumonia, and sepsis.
Alveolar hypoplasia with paucity of pulmonary vessels is a predominant feature of the BPD lung, and is exacerbated by extreme prematurity and environmental factors.34 Murine studies have shown that blocking VEGF-VEGF receptor signaling leads to alveolar hypoplasia and impaired capillary growth.63,64 Another study reported downregulation of HIF and VEGF mRNA in lung of neonatal mice exposed to intermittent hypoxia and hyperoxia.65 These studies indicate that disruption of angiogenesis and subsequently of pulmonary vasculature are major factors in the pathophysiology and development of BPD in the preterm lung. Preclinical studies have also demonstrated that lung inflammation and oxidative stress interrupt lung growth and repair in the lungs of infants with BPD.66,67 Both clinical and preclinical investigations are ongoing on the efficacy of Vitamin A supplementation, use of donor human milk, supplementation with deferoxamine, and the paracrine effects of mesenchymal stem cells (MSCs) and exosomes to improve the inflammatory milieu of the neonatal lung and enhance lung growth.68,69 Recent studies have also identified changes in the airway microbiome associated with the development of BPD. In particular, decreased Lactobacilli has been associated with BPD progression.70 Although it is known that BPD is associated with alveolar hypoplasia, lung inflammation, and impaired angiogenesis, currently there are no therapies in clinical practice that can promote lung growth and improve injury repair in the neonatal lung. The contributions of genetics, epigenetics, prenatal, and postnatal factors to the pathophysiology of BPD will be addressed in further detail below.
Genetic factors in BPD and COPD
There is a heritability component in people who develop asthma and COPD.71 In BPD, classic twin studies have also suggested that genetic effects account for 53–82% of the variance in the development of BPD.72,73 However, no common polymorphisms have been identified between three genome-wide association studies of BPD patients,74–76 nor have loci been identified in a large copy number variant study.77 However, rare variant studies performed using exome sequencing suggest that populations with BPD may have an excess of rare variants in genes associated with host defense, extracellular matrix breakdown, collagen fibril organization, morphogenesis of embryonic epithelium, and regulation of Wnt signaling pathway.78,79
In contrast to BPD, recent genome-wide association studies (GWAS) using meta-analyses and improved imputation in COPD and asthma populations has increased the number of identified SNPs causally associated with lung function and predictive of COPD and asthma.80 It is possible that SNP variation associated with COPD has relevance to BPD and vice versa. In one example, alpha-1-antrypsin (A1AT) deficiency was identified and linked to early onset COPD in the 1960s. Deficient A1AT levels secondary to variants in the SERPINA1 gene have been found to account for about 1–3% of cases of COPD.71 A preclinical BPD study in baboons reported low levels of alpha-1-antitrypsin elastase inhibitory activity in airways of animals with severe but not mild BPD.81 However, whether a relationship exists between A1AT and BPD is not clearly known at this time.
In general, it is not known whether any specific asthma or COPD gene variants are more likely to be present in children with BPD, particularly severe BPD. Preclinical studies in mice support a relationship between certain gene variants and susceptibility to COPD-like phenotypes, particularly when augmented by exposure to tobacco smoke.82–84 In humans, gene variants in the HIP gene have been associated with an increased risk of COPD, depending on pack-years of smoking exposure.85 The HIP gene is in the Hedgehog signaling pathway, which plays a role in early lung development and injury responses. Taken together, human and preclinical studies indicate that certain genetic susceptibility factors may exist that increase the likelihood of chronic lung disease in individuals exposed to environmental or adverse insults such as tobacco smoke or preterm birth. Using meta-analyses and improved imputation of genome-wide association studies may help to identify more SNPs causally related to BPD phenotype and lung function. Identification of SNPs in BPD will help to identify critical alleles and provide mechanistic insights into disease severity and progression in BPD, and potentially have relevance for COPD.
Epigenetic and other factors in BPD
Epigenetic changes (DNA methylation) have been reported between the preterm and term human lung, but it is unclear whether these changes reflect cause or effect with regards to the development of BPD.86 Intriguingly, preterm infants who develop BPD have differential gene expression in the chromatin remodeling pathway on umbilical cord samples compared with their preterm counterparts who did not develop BPD.87 Sex differences in the development of BPD in preterm infants has been widely reported with males having a higher prevalence, which may be secondary to later surfactant production in males.88 Race/ethnicity may also influence respiratory symptoms in preschool children born prematurely. Although Black race has been noted to be associated with a lower risk of BPD,89 Wai and colleagues reported that maternal Black race was associated with an increased odds ratio of 2.9 of persistent wheeze in former extremely low birth weight preschool children.90 Telomere length has not been found to be associated with a history of BPD in blood samples from 10-year-old children or adolescent salivary samples, but was associated with abnormal airflow.91,92
Prenatal influences on BPD
Early life events affecting respiratory health can include factors that impact fetal development prior to birth. Prenatally, inflammatory stimuli may increase the risk for developing BPD after birth. These exposures may include Ureaplasma spp. infection and maternal smoking,20,93 although the role of maternal chorioamnionitis in the development of BPD is more controversial.94 Additionally, factors leading to reduced birth weight for a given gestational age also increase the risk for developing BPD, such as intrauterine growth restriction (IUGR) or multiple gestation.94
Neonatal influences on BPD
After birth, the NICU is a complex set of exposures for the developing lung. Preclinical studies have been used to identify exposures and mechanisms that can impair alveolar growth and injury repair in preterm infants.95–99 Unfortunately many of the therapies intended to aid these infants and improve survival can have unintended consequences for the lungs. The use of ventilators can lead to barotrauma or volutrauma,100 need for intubation and prolonged NICU stays can be a risk factor for lower respiratory infections,101,102 enteral feeds to prime the gut carries a risk of aspiration,103,104 and the relative hyperoxia of even room air compared with hypoxic in utero conditions can lead to free radical injury.105,106 Newer interventions, including avoidance of intubation and use of noninvasive ventilation and less invasive surfactant administration, are currently being implemented in an attempt to mitigate harm in extremely preterm infants.107,108
Early childhood influences on BPD
While the majority of alveolarization occurs within the first 2 years of life, evidence exists that ongoing alveolarization can occur in 10- to 14-year-old children with a history of prematurity, 14,36 thus suggesting that events even as late as early adolescence can affect long-term trajectories. Overall, it is likely that the additive effects of prematurity and early life respiratory insults/exposures disproportionately impact short- and long-term respiratory health and function in children diagnosed with BPD.
Following discharge from the NICU, it is probable that certain environmental exposures may lead to slower resolution or incomplete resolution of alveolar disease (and a reduction in adult lung function), potentially through increased inflammation.109 For preterm infants and young children with BPD, the most significant source of inflammation is respiratory tract infections, which are also the most significant source of respiratory morbidity in infancy and early childhood.110,111 Premature infants have been reported to have significant respiratory morbidities from even the common cold virus: rhinovirus.112,113 The mechanism for common respiratory viruses during early postnatal life resulting in severe lower respiratory tract illnesses for infants and young children with BPD may be the immature immune system associated with preterm birth.114,115 In addition to the immaturity of the immune system, some therapies employed to aid with respiratory disease can also worsen immune function. Preclinical studies have reported that neonatal hyperoxia can alter the lung’s immune responses in adult mice both at baseline and when challenged with influenza or bleomycin.116–119 In addition, exposure to respiratory virus and the subsequent host inflammatory response may influence trajectory of lung function in children with BPD and induce airway reactivity in susceptible individuals. Associations have been reported with early life wheezing from rhinovirus and development of asthma.120 It is plausible that exposure to rhinovirus and other respiratory viruses during infancy and early childhood could contribute to the airflow obstruction present in children and adults born preterm, especially those with BPD. Even within the BPD population, certain infants may be at higher risk for respiratory infections; two studies found that infants and children (<3 years old) with BPD who attended daycare had more frequent respiratory symptoms and lower respiratory tract infections and greater acute healthcare usage than those who did not attend.121,122 Overall, the evidence would suggest that early infections in combination with an underdeveloped preterm lung may contribute to lasting abnormalities in adult lung function, but it is not clear whether these potent effects continue into later adult life with development of COPD.
In addition to respiratory infections, there are other exposures that can induce inflammatory changes in the developing lung, which include secondhand smoke, recurrent aspiration, air pollution, or animal exposure.17,123–125 Higher hair nicotine levels in preschool children with moderate to severe BPD were associated with more hospitalizations and greater activity limitations compared with children with lower or undetectable hair nicotine levels.126 Another study found that maternal smoking was a strong predictor of reduced airflow and poorer respiratory function in young adults with BPD compared with controls.127 Although the link between air pollution and BPD has not been studied, it is plausible to assume that air pollution may be a risk factor for decline in lung function in young adults with BPD, as a large study from the United Kingdom found that ambient air pollution was associated with higher prevalence of COPD.128 Other exposures, including early exposures to cats and dogs, may influence long-term Th1 and Th2 inflammatory responses to environmental and infectious pulmonary insults in children with BPD.
In addition to inflammatory stimuli, nutritional factors may also affect the developing lung. Preterm infants with BPD are at increased risk for growth failure,129 and animal studies suggest that malnutrition can negatively impact alveolarization.130,131 However, limited spirometric data from children who were malnourished in early childhood [reduced FEV1 and forced vital capacity (FVC) with preserved FEV1/FVC ratios] have led to the hypothesis of ‘lung-sparing growth’, where lung development is spared compared with other organs.129,132 Several factors may also mitigate the effect of BPD on respiratory morbidities. For instance, breast milk administration beyond 6 months of age was associated with a reduced likelihood of acute and chronic respiratory morbidities compared with BPD infants who did not receive breast milk beyond 6 months of age, and adherence to prescribed respiratory medications was also associated with better respiratory outcomes in children with BPD.133,134 Based on these studies and extrapolation from non-BPD studies, there appears to be modifiable risk factors that can positively or negatively affect respiratory symptoms and lung function in children with BPD, which, in turn, may influence the development of COPD in this population (Table 1).
Table 1.
Risk factors across development that can positively or negatively affect respiratory symptoms and lung function in children with BPD.
| Developmental stages and conditions
that may influence adult lung function |
||||
|---|---|---|---|---|
| Fetal life | Infancy | Childhood | Adolescence | |
| Gestational | ||||
| Intrauterine growth restriction | x | x | ||
| Fetal exposure to maternal smoking | x | x | x | x |
| Genetic conditions and mutations | x | x | x | x |
| Infancy | ||||
| Prematurity | x | x | x | |
| Bronchopulmonary dysplasia | x | x | x | |
| Infant wheezing | x | x | x | |
| Pneumonia | x | x | x | |
| Secondhand smoke | x | x | x | |
| Genetic conditions and mutations | x | x | x | |
| Nutrition | x | x | x | |
| Childhood | ||||
| Asthma | x | x | ||
| Secondhand smoke/air pollution | x | x | ||
| Allergic diseases | x | x | ||
| COPD susceptibility genes | x | x | ||
| Adolescence | ||||
| Primary smoking | x | |||
| COPD susceptibility genes | x | |||
| Secondhand smoke/air pollution | x | |||
BPD, bronchopulmonary dysplasia; COPD, chronic obstructive pulmonary disease.
Respiratory phenotypes in older children with BPD
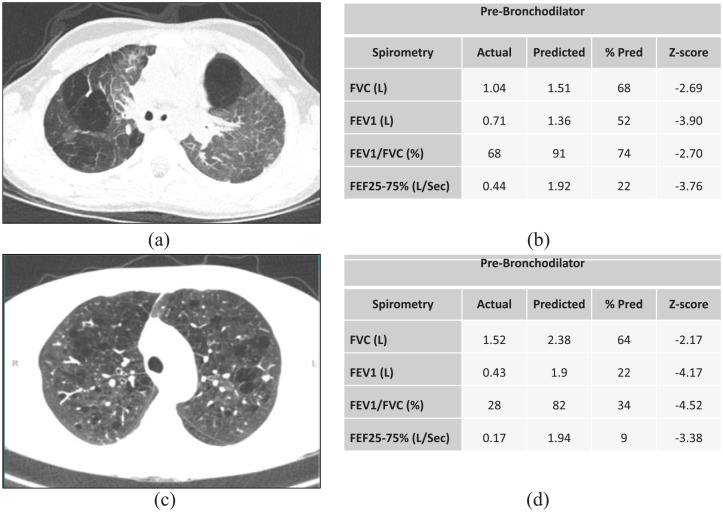
In children with BPD, obstructive lung disease (asthma-like phenotypes) may persist into adulthood,135,136 which is frequently demonstrated through lower spirometric values.44 While preterm survivors with BPD have been shown to have significantly lower z-scores for FEV1 compared with preterm survivors without BPD,137 extreme prematurity alone can result in reduced airflow in young adults compared with term controls.26 Respiratory phenotypes in older children are not limited to obstructive lung disease. Although not as common, restrictive lung disease can also be present in children with BPD.138 A 2016 study that included young adults with BPD, a history of prematurity without BPD, and full-term control subjects, observed a higher likelihood of fixed airway obstruction and higher HRCT scan severity scores in the group with BPD compared with the other two groups, in addition to lower spirometric values.139 In the same study, both groups of preterm infants had a greater reduction in gas transfer (KCO % predicted) and exercise study impairment compared with full-term controls. Chronic lung injury may also impair airway clearance in people with a history of BPD. Two studies that included school age children and young adults with a history of BPD reported that both age groups had impaired lung clearance indexes compared with age-matched controls.139,140 Reported respiratory symptoms are also more frequent in adults with a previous history of BPD. For example, Caskey and colleagues studied 25 young adult survivors of BPD, and found that they were significantly more likely to wake up coughing, or be breathless with wheezing, compared with controls. Certain school-age children with BPD may be at higher risk for respiratory morbidities in adult life, including those with abnormal lung function and parenchymal changes on lung imaging. The structural and functional changes in the BPD lung can resemble that of an individual with COPD (Figure 1). Risk stratification that includes prenatal and NICU history, postinitial hospitalization respiratory symptoms, environmental exposures, acute health care usage, lung imaging, and longitudinal lung function trajectories may be useful in identifying children with BPD at higher risk for early onset COPD.
Figure 1.
Lung function and imaging from an 8-year-old child with a history of severe BPD and an adult with COPD. (a) Emphysematous changes on a chest CT from child with BPD; (b) Spirometry showing mixed restrictive and obstructive lung disease from child with BPD; (c) Emphysematous changes on chest CT from adult with COPD; and (d) Spirometry showing severe obstructive lung disease from adult with COPD.
BPD, bronchopulmonary dysplasia; COPD, chronic obstructive pulmonary disease; CT, computed tomography.
Link between BPD and COPD
COPD is the fourth leading cause of death worldwide, and accounts for over 3 million deaths a year.141 It is well known that environmental and genetic causes contribute to disease development and phenotype expression in COPD. Less is known, however, regarding the impact of early life events that contribute to COPD development in adult life. Premature delivery and BPD can alter the trajectory of normal lung growth during childhood. Indeed, a longitudinal study of a cohort of extremely low birth weight children born in Japan found that these children exhibited a significant decline in lung function between the ages of 8 and 12 years in both FEV1 % predicted and the FEV1/FVC ratio.40 In a meta-analysis of 59 studies that included lung function on preterm and term subjects, Kotecha and colleagues found that the preterm subjects with a diagnosis of BPD had a 18.95% decrease in FEV1 compared with term controls.44 Lower lung function in children with BPD may be associated with lower lung function in adult life, increasing the likelihood of COPD. This is supported by several longitudinal studies that found a correlation between childhood lung function and lung function trajectory in adult life.18,142 Bui and colleagues found that lowest quartile of FEV1/FVC ratio at 7 years of age was associated with asthma-COPD overlap syndrome and COPD at 45 years of age.143
Similar to asthma, the BPD lung is characterized by functional abnormalities in FEV1, FEV1/FVC ratio, and air trapping. Although mild-to-moderate persistent asthma in childhood has been linked to COPD,142 the underlying pathophysiology for functional changes in the BPD lung likely differs from that of asthma. For instance, children with allergic asthma commonly have elevated levels of fractional exhaled nitric oxide (FENO), indicative of eosinophilic inflammation, whereas preterm children (with or without BPD) have been shown to have normal FENO values.144 It is also believed that the BPD lung is characterized by impaired alveolar development leading to obstructive lung disease, in part through dysanaptic airway growth and airway inflammation.38 In addition, children with BPD who are hospitalized for treatment of common respiratory viruses early in life have been shown to have worse lung function in later childhood.145,146 Whether this is cause and effect or due to more severe BPD at the onset is unclear, but, nevertheless, it may contribute in an additive way to worse lung function in adult life and a greater propensity to develop COPD. Further studies are also needed to determine if variability in BPD lung phenotype is influenced by differential DNA injury repair and inflammatory responses to infectious and environmental insults that may increase the likelihood of COPD in adult life.
Lastly, it is unknown whether impaired alveolar growth, abnormal lung injury repair, and oxidative stress during early life can predispose the lung to premature aging, although this is currently being explored.147 Reduced recruitment of endothelial progenitor cells from the bone marrow has been reported in neonatal mice exposed to hyperoxia.148 However, although oxidative stress is associated with premature aging there is currently no evidence to indicate that BPD is associated with accelerated aging of the lung.149
Conclusions and gaps in knowledge
Taken together, disrupted lung development, genetic susceptibility and subsequent environment and infectious events that occur during critical periods of postnatal lung development likely increase the predisposition of children with BPD to develop early onset COPD. With advances in neonatal care there will continue to be a greater number of extremely low birth-weight infants surviving to hospital discharge. Understanding variability in BPD outcomes that can occur in different geographic areas and from different NICUs is needed to identify optimal treatments.150 As such, there is a need to identify those individuals born premature who are at greatest risk for developing long-term lung pathology due to their BPD, and those who will go on to develop COPD. As with COPD stratification, lung CT imaging scores to determine correlation with lung function and clinical symptoms in people with BPD may be useful in BPD. Studies that examine the link between upper respiratory tract viral exacerbations in later life and correlation with lung trajectories in people born preterm with BPD may also help identify subsets of children at highest risk for developing COPD. The role of early nutritional interventions and weight on long-term respiratory outcomes in preterm infants with BPD should be investigated along with guidelines for long-term follow up of preterm individuals with BPD to monitor lung function values and treatments of these individuals after they leave the NICU, ideally through establishment of prospective birth cohorts. Ultimately, large prospective observational studies in children with BPD are needed to more clearly understand and identify risk factors associated with COPD development in adult life.
Supplemental Material
Supplemental material, Author_Response for Bronchopulmonary dysplasia: what are its links to COPD? by Sharon A. McGrath-Morrow and Joseph M. Collaco in Therapeutic Advances in Respiratory Disease
Supplemental Material
Supplemental material, Reviewer_1_v.1_1 for Bronchopulmonary dysplasia: what are its links to COPD? by Sharon A. McGrath-Morrow and Joseph M. Collaco in Therapeutic Advances in Respiratory Disease
Supplemental Material
Supplemental material, Reviewer_1_v.2 for Bronchopulmonary dysplasia: what are its links to COPD? by Sharon A. McGrath-Morrow and Joseph M. Collaco in Therapeutic Advances in Respiratory Disease
Supplemental Material
Supplemental material, Reviewer_2_v.1 for Bronchopulmonary dysplasia: what are its links to COPD? by Sharon A. McGrath-Morrow and Joseph M. Collaco in Therapeutic Advances in Respiratory Disease
Supplemental Material
Supplemental material, Supplementary_table_1_for_figure_1_10-21-2019 for Bronchopulmonary dysplasia: what are its links to COPD? by Sharon A. McGrath-Morrow and Joseph M. Collaco in Therapeutic Advances in Respiratory Disease
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and publication of this article: Funded in part through R01 HL114800 to SAM-M
Conflict of interest statement: The authors declare that there is no conflict of interest.
Disclosures: All authors disclose that they have no financial interests in the subject of this manuscript.
ORCID iD: Sharon A. McGrath-Morrow  https://orcid.org/0000-0002-1576-5394
https://orcid.org/0000-0002-1576-5394
Supplemental material: The reviews of this paper are available via the supplemental material section.
Contributor Information
Sharon A. McGrath-Morrow, Eudowood Division of Pediatric Respiratory Sciences, David M. Rubenstein Building, Suite 3075B, 200 North Wolfe Street, Baltimore, MD, 21287-2533, USA.
Joseph M. Collaco, Department of Pediatrics, Eudowood Division of Respiratory Sciences, Johns Hopkins University School of Medicine, Baltimore, MD, USA
References
- 1. Chawanpaiboon S, Vogel JP, Moller AB, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health 2019; 7: e37–e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hibbard JU, Wilkins I, Sun L, et al. Respiratory morbidity in late preterm births. JAMA 2010; 304: 419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kajantie E, Strang-Karlsson S, Evensen KAI, et al. Adult outcomes of being born late preterm or early term - what do we know? Semin Fetal Neonatal Med 2019; 24: 66–83. [DOI] [PubMed] [Google Scholar]
- 4. Stoll BJ, Hansen NI, Bell EF, et al. Neonatal outcomes of extremely preterm infants from the NICHD neonatal research network. Pediatrics 2010; 126: 443–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yannekis G, Passarella M, Lorch S. Differential effects of delivery hospital on mortality and morbidity in minority premature and low birth weight neonates. J Perinatol. Epub ahead of print 24 June 2019. DOI: 10.1038/s41372-019-0423-9. [DOI] [PubMed] [Google Scholar]
- 6. Petrova A, Mehta R, Anwar M, et al. Impact of race and ethnicity on the outcome of preterm infants below 32 weeks gestation. J Perinatol 2003; 23: 404–408. [DOI] [PubMed] [Google Scholar]
- 7. Ancel PY, Goffinet F, Kuhn P, et al. Survival and morbidity of preterm children born at 22 through 34 weeks’ gestation in France in 2011: results of the EPIPAGE-2 cohort study. JAMA Pediatr 2015; 169: 230–238. [DOI] [PubMed] [Google Scholar]
- 8. Stoll BJ, Hansen NI, Bell EF, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA 2015; 314: 1039–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Martin JA, Hamilton BE, Osterman MJK, et al. Births: final data for 2017. Natl Vital Stat Rep 2018; 67: 1–50. [PubMed] [Google Scholar]
- 10. Glass HC, Costarino AT, Stayer SA, et al. Outcomes for extremely premature infants. Anesth Analg 2015; 120: 1337–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Collaco JM, McGrath-Morrow SA. Respiratory phenotypes for preterm infants, children, and adults: bronchopulmonary dysplasia and more. Ann Am Thorac Soc 2018; 15: 530–538. [DOI] [PubMed] [Google Scholar]
- 12. Krishnan JK, Martinez FJ. Lung function trajectories and chronic obstructive pulmonary disease: current understanding and knowledge gaps. Curr Opin Pulm Med 2018; 24: 124–129. [DOI] [PubMed] [Google Scholar]
- 13. Burri PH. Fetal and postnatal development of the lung. Annu Rev Physiol 1984; 46: 617–628. [DOI] [PubMed] [Google Scholar]
- 14. Thurlbeck WM. Postnatal human lung growth. Thorax 1982; 37: 564–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zeltner TB, Burri PH. The postnatal development and growth of the human lung. II. Morphology. Respir Physiol 1987; 67: 269–282. [DOI] [PubMed] [Google Scholar]
- 16. Narayanan M, Owers-Bradley J, Beardsmore CS, et al. Alveolarization continues during childhood and adolescence: new evidence from helium-3 magnetic resonance. Am J Respir Crit Care Med 2012; 185: 186–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chan JY, Stern DA, Guerra S, et al. Pneumonia in childhood and impaired lung function in adults: a longitudinal study. Pediatrics 2015; 135: 607–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Postma DS, Bush A, van den Berge M. Risk factors and early origins of chronic obstructive pulmonary disease. Lancet 2015; 385: 899–909. [DOI] [PubMed] [Google Scholar]
- 19. Duijts L, Jaddoe VW, van der Valk RJ, et al. Fetal exposure to maternal and paternal smoking and the risks of wheezing in preschool children: the generation R study. Chest 2012; 141: 876–885. [DOI] [PubMed] [Google Scholar]
- 20. Morrow LA, Wagner BD, Ingram DA, et al. Antenatal determinants of bronchopulmonary dysplasia and late respiratory disease in preterm infants. Am J Respir Crit Care Med 2017; 196: 364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yee M, Chess PR, McGrath-Morrow SA, et al. Neonatal oxygen adversely affects lung function in adult mice without altering surfactant composition or activity. Am J Physiol Lung Cell Mol Physiol 2009; 297: L641–L649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Berger J, Bhandari V. Animal models of bronchopulmonary dysplasia. The term mouse models. Am J Physiol Lung Cell Mol Physiol 2014; 307: L936–L947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McGrath-Morrow SA, Lauer T, Collaco JM, et al. Neonatal hyperoxia contributes additively to cigarette smoke-induced COPD changes in adult mice. Am J Respir Cell Mol Biol. Epub ahead of print 14 January 2011. DOI: 10.1165/rcmb.2010-0259OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barker DJ, Godfrey KM, Fall C, et al. Relation of birth weight and childhood respiratory infection to adult lung function and death from chronic obstructive airways disease. BMJ 1991; 303: 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Savran O, Ulrik CS. Early life insults as determinants of chronic obstructive pulmonary disease in adult life. Int J Chron Obstruct Pulmon Dis 2018; 13: 683–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Clemm HH, Vollsaeter M, Roksund OD, et al. Exercise capacity after extremely preterm birth. Development from adolescence to adulthood. Ann Am Thorac Soc 2014; 11: 537–545. [DOI] [PubMed] [Google Scholar]
- 27. Lovering AT, Elliott JE, Laurie SS, et al. Ventilatory and sensory responses in adult survivors of preterm birth and bronchopulmonary dysplasia with reduced exercise capacity. Ann Am Thorac Soc 2014; 11: 1528–1537. [DOI] [PubMed] [Google Scholar]
- 28. Vrijlandt EJ, Gerritsen J, Boezen HM, et al. Lung function and exercise capacity in young adults born prematurely. Am J Respir Crit Care Med 2006; 173: 890–896. [DOI] [PubMed] [Google Scholar]
- 29. MacLean JE, DeHaan K, Fuhr D, et al. Altered breathing mechanics and ventilatory response during exercise in children born extremely preterm. Thorax 2016; 71: 1012–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lombardi E, Fainardi V, Calogero C, et al. Lung function in a cohort of 5-year-old children born very preterm. Pediatr Pulmonol 2018; 53: 1633–1639. [DOI] [PubMed] [Google Scholar]
- 31. Sanchez-Solis M, Perez-Fernandez V, Bosch-Gimenez V, et al. Lung function gain in preterm infants with and without bronchopulmonary dysplasia. Pediatr Pulmonol 2016; 51: 936–942. [DOI] [PubMed] [Google Scholar]
- 32. Oppenheim C, Mamou-Mani T, Sayegh N, et al. Bronchopulmonary dysplasia: value of CT in identifying pulmonary sequelae. AJR Am J Roentgenol 1994; 163: 169–172. [DOI] [PubMed] [Google Scholar]
- 33. van Mastrigt E, Logie K, Ciet P, et al. Lung CT imaging in patients with bronchopulmonary dysplasia: a systematic review. Pediatr Pulmonol 2016; 51: 975–986. [DOI] [PubMed] [Google Scholar]
- 34. Thebaud B, Abman SH. Bronchopulmonary dysplasia: where have all the vessels gone? Roles of angiogenic growth factors in chronic lung disease. Am J Respir Crit Care Med 2007; 175: 978–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Balinotti JE, Chakr VC, Tiller C, et al. Growth of lung parenchyma in infants and toddlers with chronic lung disease of infancy. Am J Respir Crit Care Med 2010; 181: 1093–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Narayanan M, Beardsmore CS, Owers-Bradley J, et al. Catch-up alveolarization in ex-preterm children: evidence from (3)He magnetic resonance. Am J Respir Crit Care Med 2013; 187: 1104–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chang DV, Assaf SJ, Tiller CJ, et al. Membrane and capillary components of lung diffusion in infants with bronchopulmonary dysplasia. Am J Respir Crit Care Med 2016; 193: 767–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kennedy JD. Lung function outcome in children of premature birth. J Paediatr Child Health 1999; 35: 516–521. [DOI] [PubMed] [Google Scholar]
- 39. GBD 2015 Chronic Respiratory Disease Collaborators. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the global burden of disease study 2015. Lancet Respir Med 2017; 5: 691–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hirata K, Nishihara M, Kimura T, et al. Longitudinal impairment of lung function in school-age children with extremely low birth weights. Pediatr Pulmonol 2017; 52: 779–786. [DOI] [PubMed] [Google Scholar]
- 41. Duffy SP, Criner GJ. Chronic obstructive pulmonary disease: evaluation and management. Med Clin N Am 2019; 103: 453–461. [DOI] [PubMed] [Google Scholar]
- 42. Cen J, Ma H, Chen Z, et al. Monitoring peak expiratory flow could predict COPD exacerbations: a prospective observational study. Respir Med 2019; 148: 43–48. [DOI] [PubMed] [Google Scholar]
- 43. Stocks J, Hislop A, Sonnappa S. Early lung development: lifelong effect on respiratory health and disease. Lancet Respir Med 2013; 1: 728–742. [DOI] [PubMed] [Google Scholar]
- 44. Kotecha SJ, Edwards MO, Watkins WJ, et al. Effect of preterm birth on later FEV1: a systematic review and meta-analysis. Thorax 2013; 68: 760–766. [DOI] [PubMed] [Google Scholar]
- 45. Burrows B, Knudson RJ, Lebowitz MD. The relationship of childhood respiratory illness to adult obstructive airway disease. Am Rev Respir Dis 1977; 115: 751–760. [DOI] [PubMed] [Google Scholar]
- 46. Northway WH, Jr, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N Engl J Med 1967; 276: 357–368. [DOI] [PubMed] [Google Scholar]
- 47. Abman SH, Bancalari E, Jobe A. The evolution of bronchopulmonary dysplasia after 50 years. Am J Respir Crit Care Med 2017; 195: 421–424. [DOI] [PubMed] [Google Scholar]
- 48. Capasso L, Vento G, Loddo C, et al. Oxidative stress and bronchopulmonary dysplasia: evidences from microbiomics, metabolomics, and proteomics. Front Pediatr 2019; 7: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001; 163: 1723–1729. [DOI] [PubMed] [Google Scholar]
- 50. Yeh J, McGrath-Morrow SA, Collaco JM. Oxygen weaning after hospital discharge in children with bronchopulmonary dysplasia. Pediatr Pulmonol 2016; 51: 1206–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Silva DT, Hagan R, Sly PD. Home oxygen management of neonatal chronic lung disease in Western Australia. J Paediatr Child Health 1995; 31: 185–188. [DOI] [PubMed] [Google Scholar]
- 52. Bertrand P, Alvarez C, Fabres J, et al. Home oxygen therapy in children with chronic respiratory failure. Rev Med Chil 1998; 126: 284–292. [PubMed] [Google Scholar]
- 53. Saletti A, Stick S, Doherty D, et al. Home oxygen therapy after preterm birth in Western Australia. J Paediatr Child Health 2004; 40: 519–523. [DOI] [PubMed] [Google Scholar]
- 54. Norzila MZ, Azizi BH, Norrashidah AW, et al. Home oxygen therapy for children with chronic lung diseases. Med J Malaysia 2001; 56: 151–157. [PubMed] [Google Scholar]
- 55. Henningfeld JK, Maletta K, Ren B, et al. Liberation from home mechanical ventilation and decannulation in children. Pediatr Pulmonol 2016; 51: 838–849. [DOI] [PubMed] [Google Scholar]
- 56. Kinsella JP, Greenough A, Abman SH. Bronchopulmonary dysplasia. Lancet 2006; 367: 1421–1431. [DOI] [PubMed] [Google Scholar]
- 57. Landry JS, Chan T, Lands L, et al. Long-term impact of bronchopulmonary dysplasia on pulmonary function. Can Respir J 2011; 18: 265–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Vom HM, Prenzel F, Uhlig HH, et al. Pulmonary outcome in former preterm, very low birth weight children with bronchopulmonary dysplasia: a case-control follow-up at school age. J Pediatr 2014; 164: 40–45. [DOI] [PubMed] [Google Scholar]
- 59. Laughon MM, Langer JC, Bose CL, et al. Prediction of bronchopulmonary dysplasia by postnatal age in extremely premature infants. Am J Respir Crit Care Med 2011; 183: 1715–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Harkin P, Marttila R, Pokka T, et al. Survival analysis of a cohort of extremely preterm infants born in Finland during 2005–2013. J Matern Fetal Neonatal Med. Epub ahead of print 26 September 2019. DOI: 10.1080/14767058.2019.1668925. [DOI] [PubMed] [Google Scholar]
- 61. Rocha G, de Lima FF, Machado AP, et al. Small for gestational age very preterm infants present a higher risk of developing bronchopulmonary dysplasia. J Neonatal Perinatal Med. Epub ahead of print 25 June 2019. DOI: 10.3233/NPM-180129. [DOI] [PubMed] [Google Scholar]
- 62. Alvarez-Fuente M, Moreno L, Mitchell JA, et al. Preventing bronchopulmonary dysplasia: new tools for an old challenge. Pediatr Res 2019; 85: 432–441. [DOI] [PubMed] [Google Scholar]
- 63. Le Cras TD, Markham NE, Tuder RM, et al. Treatment of newborn rats with a VEGF receptor inhibitor causes pulmonary hypertension and abnormal lung structure. Am J Physiol Lung Cell Mol Physiol 2002; 283: L555–L562. [DOI] [PubMed] [Google Scholar]
- 64. McGrath-Morrow SA, Cho C, Cho C, et al. Vascular endothelial growth factor receptor 2 blockade disrupts postnatal lung development. Am J Respir Cell Mol Biol 2005; 32: 420–427. [DOI] [PubMed] [Google Scholar]
- 65. Elberson VD, Nielsen LC, Wang H, et al. Effects of intermittent hypoxia and hyperoxia on angiogenesis and lung development in newborn mice. J Neonatal Perinatal Med 2015; 8: 313–322. [DOI] [PubMed] [Google Scholar]
- 66. Savani RC. Modulators of inflammation in bronchopulmonary dysplasia. Semin Perinatol 2018; 42: 459–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lignelli E, Palumbo F, Myti D, et al. Recent advances in our understanding of the mechanisms of lung alveolarization and bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. Epub ahead of print 9 October 2019. DOI: 10.1152/ajplung.00369.2019. [DOI] [PubMed] [Google Scholar]
- 68. Lesage F, Thebaud B. Nanotherapies for micropreemies: stem cells and the secretome in bronchopulmonary dysplasia. Semin Perinatol 2018; 42: 453–458. [DOI] [PubMed] [Google Scholar]
- 69. Naeem A, Ahmed I, Silveyra P. Bronchopulmonary dysplasia: an update on experimental therapeutics. Eur Med J (Chelmsf) 2019; 4: 20–29. [PMC free article] [PubMed] [Google Scholar]
- 70. Pammi M, Lal CV, Wagner BD, et al. Airway microbiome and development of bronchopulmonary dysplasia in preterm infants: a systematic review. J Pediatr 2019; 204: 126–133.e122. [DOI] [PubMed] [Google Scholar]
- 71. Janciauskiene SM, Bals R, Koczulla R, et al. The discovery of alpha1-antitrypsin and its role in health and disease. Respir Med 2011; 105: 1129–1139. [DOI] [PubMed] [Google Scholar]
- 72. Bhandari V, Bizzarro MJ, Shetty A, et al. Familial and genetic susceptibility to major neonatal morbidities in preterm twins. Pediatrics 2006; 117: 1901–1906. [DOI] [PubMed] [Google Scholar]
- 73. Lavoie PM, Pham C, Jang KL. Heritability of bronchopulmonary dysplasia, defined according to the consensus statement of the national institutes of health. Pediatrics 2008; 122: 479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hadchouel A, Durrmeyer X, Bouzigon E, et al. Identification of SPOCK2 as a susceptibility gene for bronchopulmonary dysplasia. Am J Respir Crit Care Med 2011; 184: 1164–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wang H, St Julien KR, Stevenson DK, et al. A genome-wide association study (GWAS) for bronchopulmonary dysplasia. Pediatrics 2013; 132: 290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ambalavanan N, Cotten CM, Page GP, et al. Integrated genomic analyses in bronchopulmonary dysplasia. J Pediatr 2015; 166: 531–537. e513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hoffmann TJ, Shaw GM, Stevenson DK, et al. Copy number variation in bronchopulmonary dysplasia. Am J Med Genet A 2014; 164A: 2672–2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Carrera P, Di Resta C, Volonteri C, et al. Exome sequencing and pathway analysis for identification of genetic variability relevant for bronchopulmonary dysplasia (BPD) in preterm newborns: a pilot study. Clin Chim Acta 2015; 451: 39–45. [DOI] [PubMed] [Google Scholar]
- 79. Li J, Yu KH, Oehlert J, et al. Exome sequencing of neonatal blood spots and the identification of genes implicated in bronchopulmonary dysplasia. Am J Respir Crit Care Med 2015; 192: 589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hall R, Hall IP, Sayers I. Genetic risk factors for the development of pulmonary disease identified by genome-wide association. Respirology 2019; 24: 204–214. [DOI] [PubMed] [Google Scholar]
- 81. Karaaslan C, Hirakawa H, Yasumatsu R, et al. Elastase inhibitory activity of airway alpha1-antitrypsin is protected by treatment with a catalytic antioxidant in a baboon model of severe bronchopulmonary dysplasia. Pediatr Res 2011; 70: 363–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Borel F, Sun H, Zieger M, et al. Editing out five Serpina1 paralogs to create a mouse model of genetic emphysema. Proc Natl Acad Sci U S A 2018; 115: 2788–2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Warburton D, Gauldie J, Bellusci S, et al. Lung development and susceptibility to chronic obstructive pulmonary disease. Proc Am Thorac Soc 2006; 3: 668–672. [DOI] [PubMed] [Google Scholar]
- 84. Farkas L, Farkas D, Warburton D, et al. Cigarette smoke exposure aggravates air space enlargement and alveolar cell apoptosis in Smad3 knockout mice. Am J Physiol Lung Cell Mol Physiol 2011; 301: L391–L401. [DOI] [PubMed] [Google Scholar]
- 85. Van Durme YM, Eijgelsheim M, Joos GF, et al. Hedgehog-interacting protein is a COPD susceptibility gene: the Rotterdam study. Eur Respir J 2010; 36: 89–95. [DOI] [PubMed] [Google Scholar]
- 86. Cuna A, Halloran B, Faye-Petersen O, et al. Alterations in gene expression and DNA methylation during murine and human lung alveolar septation. Am J Respir Cell Mol Biol 2015; 53: 60–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Cohen J, Van Marter LJ, Sun Y, et al. Perturbation of gene expression of the chromatin remodeling pathway in premature newborns at risk for bronchopulmonary dysplasia. Genome Biol 2007; 8: R210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ali Z, Schmidt P, Dodd J, et al. Bronchopulmonary dysplasia: a review. Arch Gynecol Obstet 2013; 288: 325–333. [DOI] [PubMed] [Google Scholar]
- 89. Ryan RM, Feng R, Bazacliu C, et al. Black race is associated with a lower risk of bronchopulmonary dysplasia. J Pediatr 2019; 207: 130–135.e132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wai KC, Hibbs AM, Steurer MA, et al. Maternal black race and persistent wheezing illness in former extremely low gestational age newborns: secondary analysis of a randomized trial. J Pediatr 2018; 198: 201–208.e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Hadchouel A, Marchand-Martin L, Franco-Montoya ML, et al. Salivary telomere length and lung function in adolescents born very preterm: a prospective multicenter study. PLoS One 2015; 10: e0136123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Henckel E, Svenson U, Nordlund B, et al. Telomere length was similar in school-age children with bronchopulmonary dysplasia and allergic asthma. Acta Paediatr 2018; 107: 1395–1401. [DOI] [PubMed] [Google Scholar]
- 93. Kallapur SG, Kramer BW, Jobe AH. Ureaplasma and BPD. Semin Perinatol 2013; 37: 94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kalikkot Thekkeveedu R, Guaman MC, Shivanna B. Bronchopulmonary dysplasia: a review of pathogenesis and pathophysiology. Respir Med 2017; 132: 170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. McGrath SA. Induction of p21WAF/CIP1 during hyperoxia. Am J Respir Cell Mol Biol 1998; 18: 179–187. [DOI] [PubMed] [Google Scholar]
- 96. Warner BB, Stuart LA, Papes RA, et al. Functional and pathological effects of prolonged hyperoxia in neonatal mice. Am J Physiol 1998; 275: L110–L117. [DOI] [PubMed] [Google Scholar]
- 97. Velten M, Heyob KM, Rogers LK, et al. Deficits in lung alveolarization and function after systemic maternal inflammation and neonatal hyperoxia exposure. J Appl Physiol (1985) 2010; 108: 1347–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Saadoon A, Ambalavanan N, Zinn K, et al. Effect of prenatal versus postnatal vitamin D deficiency on pulmonary structure and function in mice. Am J Respir Cell Mol Biol 2017; 56: 383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Mobius MA, Thebaud B. Bronchopulmonary dysplasia: where have all the stem cells gone?: origin and (potential) function of resident lung stem cells. Chest 2017; 152: 1043–1052. [DOI] [PubMed] [Google Scholar]
- 100. Rossor TE, Hunt KA, Shetty S, et al. Neurally adjusted ventilatory assist compared to other forms of triggered ventilation for neonatal respiratory support. Cochrane Database Syst Rev 2017; 10: Cd012251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Pichler K, Assadian O, Berger A. Viral respiratory infections in the neonatal intensive care unit-a review. Front Microbiol 2018; 9: 2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Bennett NJ, Tabarani CM, Bartholoma NM, et al. Unrecognized viral respiratory tract infections in premature infants during their birth hospitalization: a prospective surveillance study in two neonatal intensive care units. J Pediatr 2012; 161: 814–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Capilouto GJ, Cunningham TJ, Giannone PJ, et al. A comparison of the nutritive sucking performance of full term and preterm neonates at hospital discharge: a prospective study. Early Hum Dev 2019; 134: 26–30. [DOI] [PubMed] [Google Scholar]
- 104. Gulati IK, Jadcherla SR. Gastroesophageal reflux disease in the neonatal intensive care unit infant: who needs to be treated and what approach is beneficial? Pediatr Clin North Am 2019; 66: 461–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Hadchouel A, Franco-Montoya ML, Delacourt C. Altered lung development in bronchopulmonary dysplasia. Birth Defects Res A Clin Mol Teratol 2014; 100: 158–167. [DOI] [PubMed] [Google Scholar]
- 106. Millan I, Pinero-Ramos JD, Lara I, et al. Oxidative stress in the newborn period: useful biomarkers in the clinical setting. Antioxidants (Basel) 2018; 7. pii: E193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Aldana-Aguirre JC, Pinto M, Featherstone RM, et al. Less invasive surfactant administration versus intubation for surfactant delivery in preterm infants with respiratory distress syndrome: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed 2017; 102: F17–F23. [DOI] [PubMed] [Google Scholar]
- 108. Isayama T, Iwami H, McDonald S, et al. Association of noninvasive ventilation strategies with mortality and bronchopulmonary dysplasia among preterm infants: a systematic review and meta-analysis. JAMA 2016; 316: 611–624. [DOI] [PubMed] [Google Scholar]
- 109. Martinez FD. Early-life origins of chronic obstructive pulmonary disease. N Engl J Med 2016; 375: 871–878. [DOI] [PubMed] [Google Scholar]
- 110. Stevenson DK, Verter J, Fanaroff AA, et al. Sex differences in outcomes of very low birthweight infants: the newborn male disadvantage. Arch Dis Child Fetal Neonatal Ed 2000; 83: F182–F185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Collaco JM, Aherrera AD, McGrath-Morrow SA. The influence of gender on respiratory outcomes in children with bronchopulmonary dysplasia during the first 3 years of life. Pediatr Pulmonol 2017; 52: 217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Drysdale SB, Alcazar M, Wilson T, et al. Respiratory outcome of prematurely born infants following human rhinovirus A and C infections. Eur J Pediatr 2014; 173: 913–919. [DOI] [PubMed] [Google Scholar]
- 113. Drysdale SB, Alcazar-Paris M, Wilson T, et al. Rhinovirus infection and healthcare utilisation in prematurely born infants. Eur Respir J 2013; 42: 1029–1036. [DOI] [PubMed] [Google Scholar]
- 114. Miller EK, Bugna J, Libster R, et al. Human rhinoviruses in severe respiratory disease in very low birth weight infants. Pediatrics 2012; 129: e60–e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Townsi N, Laing IA, Hall GL, et al. The impact of respiratory viruses on lung health after preterm birth. Eur Clin Respir J 2018; 5: 1487214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Kumar VHS, Wang H, Nielsen L. Adaptive immune responses are altered in adult mice following neonatal hyperoxia. Physiol Rep 2018; 6: e13577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. O’Reilly MA, Marr SH, Yee M, et al. Neonatal hyperoxia enhances the inflammatory response in adult mice infected with influenza A virus. Am J Respir Crit Care Med 2008; 177: 1103–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Buczynski BW, Yee M, Martin KC, et al. Neonatal hyperoxia alters the host response to influenza A virus infection in adult mice through multiple pathways. Am J Physiol Lung Cell Mol Physiol 2013; 305: L282–L290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Yee M, Buczynski BW, Lawrence BP, et al. Neonatal hyperoxia increases sensitivity of adult mice to bleomycin-induced lung fibrosis. Am J Respir Cell Mol Biol 2013; 48: 258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Han M, Rajput C, Hershenson MB. Rhinovirus attributes that contribute to asthma development. Immunol Allergy Clin North Am 2019; 39: 345–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Hagen EW, Sadek-Badawi M, Palta M. Daycare attendance and risk for respiratory morbidity among young very low birth weight children. Pediatr Pulmonol 2009; 44: 1093–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. McGrath-Morrow S, Lee G, Stewart B, et al. Daycare attendance increases the risk of respiratory morbidity in infants and children with chronic lung disease of prematurity. Pediatrics 2010; 126: 632–637. [DOI] [PubMed] [Google Scholar]
- 123. McGrath-Morrow SA, Lauer T, Collaco JM, et al. Neonatal hyperoxia contributes additively to cigarette smoke-induced chronic obstructive pulmonary disease changes in adult mice. Am J Respir Cell Mol Biol 2011; 45: 610–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Lefton-Greif MA, McGrath-Morrow SA. Deglutition and respiration: development, coordination, and practical implications. Semin Speech Lang 2007; 28: 166–179. [DOI] [PubMed] [Google Scholar]
- 125. Usemann J, Decrue F, Korten I, et al. Exposure to moderate air pollution and associations with lung function at school-age: a birth cohort study. Environ Int 2019; 126: 682–689. [DOI] [PubMed] [Google Scholar]
- 126. Collaco JM, Aherrera AD, Breysse PN, et al. Hair nicotine levels in children with bronchopulmonary dysplasia. Pediatrics 2015; 135: e678–e686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Saarenpaa HK, Tikanmaki M, Sipola-Leppanen M, et al. Lung function in very low birth weight adults. Pediatrics 2015; 136: 642–650. [DOI] [PubMed] [Google Scholar]
- 128. Doiron D, de Hoogh K, Probst-Hensch N, et al. Air pollution, lung function and COPD: results from the population-based UK biobank study. Eur Respir J 2019; 54. pii: 1802140. [DOI] [PubMed] [Google Scholar]
- 129. Arigliani M, Spinelli AM, Liguoro I, et al. Nutrition and lung growth. Nutrients 2018; 10: 919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Mataloun MM, Leone CR, Mascaretti RS, et al. Effect of postnatal malnutrition on hyperoxia-induced newborn lung development. Braz J Med Biol Res 2009; 42: 606–613. [DOI] [PubMed] [Google Scholar]
- 131. Mataloun MM, Rebello CM, Mascaretti RS, et al. Pulmonary responses to nutritional restriction and hyperoxia in premature rabbits. J Pediatr (Rio J) 2006; 82: 179–185. [DOI] [PubMed] [Google Scholar]
- 132. Korten I, Usemann J, Latzin P. “Lung sparing growth”: is the lung not affected by malnutrition? Eur Respir J 2017; 49. pii: 1700295. [DOI] [PubMed] [Google Scholar]
- 133. Kim LY, McGrath-Morrow SA, Collaco JM. Impact of breast milk on respiratory outcomes in infants with bronchopulmonary dysplasia. Pediatr Pulmonol 2019; 54: 313–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Collaco JM, Kole AJ, Riekert KA, et al. Respiratory medication adherence in chronic lung disease of prematurity. Pediatr Pulmonol 2012; 47: 283–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Been JV, Lugtenberg MJ, Smets E, et al. Preterm birth and childhood wheezing disorders: a systematic review and meta-analysis. PLoS Med 2014; 11: e1001596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Jaakkola JJ, Ahmed P, Ieromnimon A, et al. Preterm delivery and asthma: a systematic review and meta-analysis. J Allergy Clin Immunol 2006; 118: 823–830. [DOI] [PubMed] [Google Scholar]
- 137. Gibson AM, Reddington C, McBride L, et al. Lung function in adult survivors of very low birth weight, with and without bronchopulmonary dysplasia. Pediatr Pulmonol 2015; 50: 987–994. [DOI] [PubMed] [Google Scholar]
- 138. Thunqvist P, Tufvesson E, Bjermer L, et al. Lung function after extremely preterm birth- a population-based cohort study (EXPRESS). Pediatr Pulmonol 2018; 53: 64–72. [DOI] [PubMed] [Google Scholar]
- 139. Caskey S, Gough A, Rowan S, et al. Structural and functional lung impairment in adult survivors of bronchopulmonary dysplasia. Ann Am Thorac Soc 2016; 13: 1262–1270. [DOI] [PubMed] [Google Scholar]
- 140. Sorensen JK, Buchvald F, Berg AK, et al. Ventilation inhomogeneity and NO and CO diffusing capacity in ex-premature school children. Respir Med 2018; 140: 94–100. [DOI] [PubMed] [Google Scholar]
- 141. Huang X, Mu X, Deng L, et al. The etiologic origins for chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2019; 14: 1139–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. McGeachie MJ, Yates KP, Zhou X, et al. Patterns of growth and decline in lung function in persistent childhood asthma. N Engl J Med 2016; 374: 1842–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Bui DS, Burgess JA, Lowe AJ, et al. Childhood lung function predicts adult chronic obstructive pulmonary disease and asthma-chronic obstructive pulmonary disease overlap syndrome. Am J Respir Crit Care Med 2017; 196: 39–46. [DOI] [PubMed] [Google Scholar]
- 144. Course CW, Kotecha S, Kotecha SJ. Fractional exhaled nitric oxide in preterm-born subjects: a systematic review and meta-analysis. Pediatr Pulmonol 2019; 54: 595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Greenough A. Long term respiratory outcomes of very premature birth (<32 weeks). Semin Fetal Neonatal Med 2012; 17: 73–76. [DOI] [PubMed] [Google Scholar]
- 146. Greenough A, Alexander J, Boit P, et al. School age outcome of hospitalisation with respiratory syncytial virus infection of prematurely born infants. Thorax 2009; 64: 490–495. [DOI] [PubMed] [Google Scholar]
- 147. Meiners S, Hilgendorff A. Early injury of the neonatal lung contributes to premature lung aging: a hypothesis. Mol Cell Pediatr 2016; 3: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Balasubramaniam V, Mervis CF, Maxey AM, et al. Hyperoxia reduces bone marrow, circulating, and lung endothelial progenitor cells in the developing lung: implications for the pathogenesis of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 2007; 292: L1073–L1084. [DOI] [PubMed] [Google Scholar]
- 149. Marseglia L, D’Angelo G, Manti S, et al. Oxidative stress-mediated aging during the fetal and perinatal periods. Oxid Med Cell Longev 2014; 2014: 358375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Adams M, Bassler D, Bucher HU, et al. Variability of very low birth weight infant outcome and practice in Swiss and US neonatal units. Pediatrics 2018; 141. pii: e20173436. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Author_Response for Bronchopulmonary dysplasia: what are its links to COPD? by Sharon A. McGrath-Morrow and Joseph M. Collaco in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.1_1 for Bronchopulmonary dysplasia: what are its links to COPD? by Sharon A. McGrath-Morrow and Joseph M. Collaco in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.2 for Bronchopulmonary dysplasia: what are its links to COPD? by Sharon A. McGrath-Morrow and Joseph M. Collaco in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.1 for Bronchopulmonary dysplasia: what are its links to COPD? by Sharon A. McGrath-Morrow and Joseph M. Collaco in Therapeutic Advances in Respiratory Disease
Supplemental material, Supplementary_table_1_for_figure_1_10-21-2019 for Bronchopulmonary dysplasia: what are its links to COPD? by Sharon A. McGrath-Morrow and Joseph M. Collaco in Therapeutic Advances in Respiratory Disease