Abstract
Keloids can be treated in a number of ways, including by surgery. Multiple studies now show that while surgical monotherapy associates with extremely high rates of recurrence (50%–80%), postoperative radiotherapy can significantly reduce these recurrence rates. Ongoing improvements in radiation technology have further increased the safety and efficacy of this combination protocol. Of the various radiotherapies that have been used in this setting, electron beam (β-ray) irradiation is currently the best due to its excellent dose distribution and safety. The maximal biologically effective dose (BED) for keloids is 30 Gy (using an estimated α / β ratio of 10); increasing the dose has no further benefits and elevates side effects. Over the last two decades, we have modified and then fine-tuned our radiotherapy protocol for keloid excision wounds. Thus, our early protocol was used for all body sites and consisted of 15 Gy/3 fr/3 days. We then customised the radiotherapy protocol so that body sites that are highly prone to recurrence (e.g. the anterior chest) receive higher doses while low recurrence sites like the earlobe receive a much smaller dose. More recently, we tweaked this body site-customised protocol so that fewer fractions are employed. Therefore, we currently apply 18 Gy/3 fr/3 days to high-recurrence sites, 8 Gy/1 fr/1 day to earlobes and 15 Gy/2 fr/2 days to other body sites. These radiotherapy protocol changes were accompanied by the evolution of body site-customised surgical approaches. As a result of these developments, our overall keloid recurrence rate is now below 10%.
Keywords: Keloid, hypertrophic scar, pathological scar, radiotherapy, electron beam, superficial X-ray, soft X-ray, brachytherapy, radiation monotherapy
Lay Summary:

Keloids can be treated in a number of ways, including by surgery. Multiple studies now show that postoperative radiotherapy can significantly reduce these recurrence rates. Ongoing improvements in radiation technology have further increased the safety and efficacy of this combination protocol. Of the various radiotherapies that have been used in this setting, electron beam (β-ray) irradiation is currently the best due to its excellent dose distribution and safety. The maximal biologically effective dose (BED) for keloids is 30 Gy (using an estimated α / β ratio of 10); increasing the dose has no further benefits and elevates side effects. Over the last two decades, we have modified and then fine-tuned our radiotherapy protocol for keloid excision wounds. More recently, we tweaked a body site-customized protocol so that fewer fractions are employed. Therefore, we currently apply 18Gy/3fr/3days to high-recurrence sites, 8Gy/1fr/1day to earlobes, and 15Gy/2fr/2days to other body sites. These radiotherapy protocol changes were accompanied by the evolution of body site-customized surgical approaches. As a result of these developments, our overall keloid recurrence rate is now below 10%.
Introduction
Keloids frequently respond to conservative treatments such as steroid tape administration and steroid injections, but the treatment durations are often extremely long. A much faster approach is to extirpate the keloid. Initially, however, surgical monotherapy was strongly avoided and used in only extreme cases due to the witheringly high rates of recurrence,1 which were often much worse than the original lesion. This problem can be alleviated by the use of postoperative radiotherapy, which inhibits keloid recurrence by preventing new keloidogenic inflammation, possibly by inhibiting immune cell function and neovascularisation.2 Extensive evidence suggests that postoperative radiotherapy significantly reduces the risk of recurrence after keloid excision.3 This article will also demonstrate the tremendous impact of adjuvant postoperative radiotherapy on keloid recurrence rates.
In the past, keloids were treated with superficial4 or orthovoltage X-rays (photons).5 Today, a better modality is electron beam (β-ray) irradiation.6–8 The excellent safety and efficacy of this radiation technology probably reflects the fact that electron beams reach the reticular dermis more selectively than soft X-rays (the reticular dermis is where all the keloidogenic inflammatory cellular activities take place).2 Alternative approaches that have been used for keloids recently include high dose rate-superficial brachytherapy (HDR-SB)9 and other types of brachytherapy. Several studies show that these approaches are effective.10–12 With regard to the mould method,9 if the shape of the surgical scar permits, an HDR-SB applicator can be used to ensure the even and appropriate focus of the radiation onto the wound surface. Nevertheless, the electron beam remains superior to brachytherapy for skin lesions because its excellent dose distribution results in a lower risk of internal organ exposure.13,14 Thus, at present, the electron beam is the most effective and safest postoperative radiotherapy for keloids. However, it should be emphasised that despite the tremendous efficacy of postoperative radiotherapy in keloids, it is essential to maintain stringent safety standards. These considerations are discussed in detail towards the end of this article.
In relation to the postoperative radiation dose for keloids, it has been shown that completing radiation treatment with a biologically effective dose (BED) of at least 30 Gy one week after keloid resection improves the recurrence rate to 10% or less (compared to a rate of 50%–80% after surgery alone).15–17 When the BED with an estimated α / β ratio for keloids of 10 exceeds 30 Gy, the non-relapse rate plateaus. Therefore, it is not recommended to increase the dose beyond 30 Gy because of the limited added clinical benefit and possibility of more side effects.
In 1988, our hospital started to routinely treat keloids that were refractory to conservative therapies with surgical removal followed by electron-beam radiotherapy. In the subsequent years, our surgical and postoperative radiotherapy protocols have undergone periodic modification as a result of our experience, the scientific literature and our statistical analyses of the treatment outcomes, particularly the keloid recurrence rates. In this article, we will first discuss how we modified the postoperative radiotherapy protocol until we found safe irradiation modalities that strongly limited the recurrence of keloids on specific body sites. This modification started being implemented in all excised keloid cases in 2003. More recently, in 2013, we further modified our radiotherapy protocol slightly to alleviate the demand on the hospital radiation unit. Thus, the history of our radiotherapy protocol can be divided into three periods, namely, 1988–2002, 2003–2012 and 2013 onwards. In this article, we will also discuss how we modified the surgical protocols for keloids until we arrived at detailed protocols for specific body sites that, in association with our radiotherapy improvements, have significantly reduced the recurrence rates. These surgical protocols evolved over time but started to be used routinely in all cases by 2008. This article will present the effect of these radiotherapy and surgical protocol changes on keloid recurrence rates. It should be noted that these data only involved moderately sized keloids that we could excise completely in one operation. Large keloids that could not be extirpated in a single operation and minor keloids that were treated with conservative therapies were not included. It should also be noted that recurrence in these data was defined as any elevation with increased stiffness, with or without redness: even if the changes were tiny and the patient was still satisfied with the result, we considered these changes as indicating a recurrence. In all cases presented here, the treatment outcome was judged after a follow-up duration of at least 18 months.
Changes over time in the postoperative radiotherapy protocol of Nippon Medical School
Early standard radiotherapy protocol (1988–2003): 15 Gy on all body sites
Between 1988 and 2002, we surgically excised the keloids, closed the wounds primarily with three-layer sutures (i.e. sutures in the subcutaneous, dermal, and superficial layers; the subcutaneous and dermal sutures were absorbable) and then treated the resulting wounds with postoperative radiotherapy composed of a total electron-beam irradiation dose of 15 Gy. This dose was applied regardless of body site. The protocol involved administering 15 Gy with an 4MeV electron beam with a linear accelerator over three consecutive or semi-consecutive days (5 Gy per day) starting the day after the operation: thus, it is designated the 15 Gy/3 fr/3 days protocol.
Our analysis of the 147 consecutive cases with keloids on various body sites that were treated in 1988–2000 with 15 Gy/3 fr/3 days showed an overall recurrence rate of 32.7%. The anterior chest wall, scapular and suprapubic cases exhibited the highest recurrence rates (mean = 41.1%; range = 36.4%–43.1%). The lower limb, ear and neck cases had lower recurrence rates (9.1%–16.7%) (Table 1).6
Table 1.
Recurrence rates of consecutive excised keloids on various body sites after irradiation with our early standard radiotherapy protocol (total dose 15 Gy) in 1988–2000.
| Keloid site | Radiotherapy protocol | No. keloids | No. recurring (%) |
|---|---|---|---|
| Ear | 15 Gy/3 fr/3 days | 14 | 2 (14.3) |
| Neck | 15 Gy/3 fr/3 days | 12 | 2 (16.7) |
| Upper limb | 15 Gy/3 fr/3 days | 15 | 4 (26.7) |
| Lower limb | 15 Gy/3 fr/3 days | 11 | 1 (9.1) |
| High-recurrence areas | 15 Gy/3 fr/3 days | 95 | 39 (41.1) |
| Anterior chest wall | 51 | 22 (43.1) | |
| Scapular region | 33 | 13 (39.4) | |
| Suprapubic region | 11 | 4 (36.4) | |
| Total | 147 | 48 (32.7) |
fr, fractions; Gy, Gray.
We then increased this cohort to 249 cases by adding another 102 consecutive keloid cases that were treated over the next two years (2001–2002) with 15 Gy/3 fr/3 days. In the analysis shown in Table 2, we divided the ear keloids into those on the earlobe and those on the auricle not involving the earlobe. The overall recurrence rate was 29.3% and, again, the anterior chest wall, scapular and suprapubic cases had the highest recurrence rates (mean = 38.3%; range = 36.4%–39.0%). The auricles also had a very high recurrence rate (38.5%) whereas the earlobe did not (5.7%) (Table 2).
Table 2.
Recurrence rates of consecutive excised keloids on various body sites after irradiation with our early standard radiotherapy protocol (total dose 15 Gy) in 1988–2002.
| Keloid site | Radiotherapy protocol | No. keloids | No. recurring (%) |
|---|---|---|---|
| Earlobe | 15 Gy/3 fr/3 days | 35 | 2 (5.7) |
| Auricle excluding earlobe | 15 Gy/3 fr/3 days | 13 | 5 (38.5) |
| High-recurrence areas | 15 Gy/3 fr/3 days | 149 | 57 (38.3) |
| Anterior chest wall | 82 | 32 (39.0) | |
| Scapular region | 45 | 17 (37.8) | |
| Suprapubic region | 22 | 8 (36.4) | |
| Others | 15 Gy/3 fr/3 days | 52 | 9 (17.3) |
| Total | 249 | 73 (29.3) |
fr, fractions; Gy, Gray.
First body site-customised radiotherapy protocol (2003–2012)
The 15 Gy/3 fr/3 days protocol data (Tables 1 and 2) made us realise that the body sites that exhibited high recurrence should be treated with higher radiation doses. The good response of the earlobes to 15 Gy/3 fr/3 days also made us think that this low-recurrence site could respond effectively to a lower dose. Thus, in 2003, we started to treat the anterior chest wall, scapular region and suprapubic region with 20 Gy/4 fr/4 days and the earlobe with 10 Gy/2 fr/2 days. We continued to treat auricles without earlobe involvement and other sites with 15 Gy/3 fr/3 days.
An analysis of the 121 consecutive cases between 2003 and 2004 showed that this body site-customised approach halved the overall recurrence rate from 29.3% in 1988–2002 to 14.0%. This was largely because this protocol more than halved the recurrence rates in the high-recurrence sites from 38.3% to 17.2%. The auricles still had high recurrence rates (27.3%) but no recurrences were seen in the 28 earlobes (Table 3).7
Table 3.
Recurrence rates of consecutive excised keloids on various body sites after irradiation with our body site-customised radiotherapy protocol in 2002–2004.
| Keloid site | Radiotherapy protocol | No. keloids | No. recurring (%) |
|---|---|---|---|
| Earlobe | 10 Gy/2 fr/2 days | 28 | 0 (0) |
| Auricle excluding earlobe | 15 Gy/3 fr/3 days | 11 | 3 (27.3) |
| High-recurrence areas | 20 Gy/4 fr/4 days | 58 | 10 (17.2) |
| Anterior chest wall | 35 | 5 (14.3) | |
| Scapular region | 13 | 3 (23.1) | |
| Suprapubic region | 10 | 2 (20.0) | |
| Others | 15 Gy/3 fr/3 days | 24 | 4 (16.7) |
| Total | 121 | 17 (14.0) |
fr, fractions; Gy, Gray.
When this cohort was increased to 317 by adding another 196 cases that were treated with the same new protocol between 2005–2007, the overall recurrence rate was 12.3% and the high-recurrence sites exhibited even lower recurrence rates (13.0%). The earlobes had a low recurrence rate of 6.9% but the auricles continued to exhibit extensive recurrence (21.4%) (Table 4).
Table 4.
Recurrence rates of consecutive excised keloids on various body sites after irradiation with our body site-customised radiotherapy protocol in 2003–2007.
| Keloid site | Radiotherapy protocol | No. keloids | No. recurring (%) |
|---|---|---|---|
| Earlobe | 10 Gy/2 fr/2 days | 101 | 7 (6.9) |
| Auricle excluding earlobe | 15 Gy/3 fr/3 days | 14 | 3 (21.4) |
| High-recurrence areas | 20 Gy/4 fr/4 days | 131 | 17 (13.0) |
| Anterior chest wall | 59 | 8 (13.6) | |
| Scapular region | 44 | 5 (11.4) | |
| Suprapubic region | 28 | 4 (14.3) | |
| Others | 15 Gy/3 fr/3 days | 71 | 12 (16.9) |
| Total | 317 | 39 (12.3) |
fr, fractions; Gy, Gray.
Body site-customised surgical protocol, which was used together with the first body site-customised radiotherapy protocol (2008–2012)
While we were optimising the radiation dose, we simultaneously sought to further reduce the keloid recurrence rates by improving the surgical methods. Up until 2008, we had used a wide variety of surgical approaches to excise keloids and we closed the wounds with sutures in three layers, namely, the subcutaneous, dermal and superficial layers. Absorbable sutures were used for the subcutaneous and dermal sutures. The surgical modality that was used depended on the surgeon’s judgement and preferences. In 2008, however, we decided to define and routinely adopt standard institutional surgical approaches for keloids on specific body sites.
Thus, the sites that were not highly prone to recurrence were treated very differently to the sites characterised by high recurrence. All primary earlobe keloids, which associated with low recurrence, were treated by wedge excision and simple superficial sutures with 6-0 polypropylene (Proline®; Ethicon, Inc., Somerville, NJ, USA).18 Table 5 shows the outcomes of primary earlobe keloids that arose specifically from ear-piercing holes. The routine use of this surgical procedure (followed by 10 Gy/2 fr/2 days radiation) associated with an earlobe recurrence rate of 4.6%. This is slightly lower than the 6.9% recurrence associated with the same radiotherapy protocol but varying surgical modalities (although it should be noted that the earlobe keloid cases described in Tables 2–4 included primary keloids that arose from causes other than piercing).
Table 5.
Recurrence rates of consecutive excised keloids on various body sites after treatment with our body site-customised surgical protocol plus irradiation with our body site-customised radiotherapy protocol in 2008–2012.
| Keloid site | Radiotherapy protocol | No. keloids | No. recurring (%) |
|---|---|---|---|
| Earlobe | 10 Gy/2 fr/2 days | 108 | 5 (4.6) |
| Core excision and simple suture | |||
| Auricle excluding earlobe | 15 Gy/3 fr/3 days | 32 | 3 (9.4) |
| Wedge excision and simple suture | |||
| High-recurrence areas | 20 Gy/4 fr/4 days | 145 | 21 (14.5) |
| Anterior chest wall | 72 | 10 (13.9) | |
| Tension reduction | |||
| Fascial/subcutaneous | |||
| Sutures and z-plasty | |||
| Scapular region | 38 | 5 (13.2) | |
| Tension reduction | |||
| Fascial/subcutaneous | |||
| Sutures and z-plasty | |||
| Suprapubic region | 35 | 6 (17.1) | |
| Tension reduction | |||
| Fascial/subcutaneous | |||
| Sutures and z-plasty | |||
| Others | 15 Gy/3 fr/3 days | 42 | 7 (16.7) |
| Tension reduction | |||
| Fascial/subcutaneous | |||
| Sutures and z-plasty | |||
| Total | 327 | 36 (11.0) | |
fr, fractions; Gy, Gray.
Auricle keloids that did not involve the earlobe were now routinely treated by core excision: wedge excision is not suitable for these cases because it can cause auricle deformity.19 Excision was followed with simple sutures and the radiotherapy protocol continued to be 15 Gy/3 fr/3 days. Close adherence to this protocol markedly reduced the auricle recurrence rate from 21.4% in 2002–2007 to 9.4% (Table 5).
The keloids on body sites that exhibit high recurrence rates (e.g. the anterior chest wall, scapula and suprapubic region) were all routinely treated with excision, subcutaneous/fascial tensile reduction sutures and z-plasties.3,20 The subcutaneous/fascial tensile reduction suture approach involves removing the keloid completely together with a minimal normal skin margin and the fatty tissues below the keloid (this removes all tissues above the deep fascia of the muscles), undermining the wound edges below the deep fascia, and then suturing the deep fascia with 0 polydioxanone sutures (PDS®II; Ethicon, Inc., Somerville, NJ, USA). Thereafter, the fibrous membrane in the fatty tissues (the superficial fascia) is sutured using 2-0 and 3-0 PDS®II. This suturing protocol smoothly elevates the wound edges and causes them to approximate each other. This minimises tension on the dermis. After suturing the deep and superficial fasciae, we designed z-plasties. The sides of each triangular flap were 7–10 mm long and the pitch between each z-plasty was 2–4 cm, depending on the total wound length. In our experience, this pitch yields the most satisfactory results (personal observations). After confirming that the triangular flaps were fully elevated and could be easily transposed with each other, dermal sutures with 4-0 PDS®II were started and followed by superficial sutures with 6-0 polypropylene.
Table 5 shows that the latter approach did not markedly alter the recurrence rates of keloids on the anterior chest wall, scapula, and suprapubic region: the mean recurrence rate was 14.5% compared to 13.0% in 2002–2007. This reflects the fact that in the preceding 2002–2007 period, we often treated keloids on high-recurrence areas with the same surgical protocol described above. Thus, the drop in recurrence observed for the high-recurrence body sites in the preceding period reflected: (1) more frequent adoption of the surgical methods that are now used routinely; and (2) the improved radiotherapy protocol.
The most recent radiotherapy protocol (2013–present)
The increasing number of patients who required keloid treatment in our institution led to high demands on the radiology unit. To alleviate this and to further improve the safety of the treatment, we slightly altered the radiotherapy protocols in 2013 so that fewer fractions would be used (Table 6). Thus, high-recurrence sites are now treated with 18 Gy/3 fr/3 days, low-recurrence sites (i.e. the earlobe) are now treated with 8 Gy/1 fr/1 day, and the other body sites are treated with 15 Gy/2 fr/2 days. These changes in the radiotherapy protocol had no adverse effects on recurrence rates. Indeed, these protocols (together with the routinely adopted changes in the surgical protocol described in the preceding section) associated with an excellent total recurrence rate of 9.3% (Table 6). Examples of severe earlobe-piercing, lower jaw and anterior chest keloids that were effectively treated with this new approach are shown in Figures 1–3, respectively.
Table 6.
The most recent (2013–2017) therapeutic outcomes of keloids that were treated with the body-site customised surgical protocol followed by our slightly modified body site-customised radiotherapy protocol.
| Keloid site | Radiotherapy protocol | No. keloids | No. recurring (%) |
|---|---|---|---|
| Earlobe | 8 Gy/1 fr/1 day | 75 | 5 (6.7) |
| Core excision and simple suture | |||
| Auricle excluding earlobe | 15 Gy/2 fr/2 days | 44 | 2 (4.5) |
| Wedge excision and simple suture | |||
| High-recurrence areas | 18 Gy/3 fr/3 days | 221 | 23 (10.4) |
| Anterior chest wall | 141 | 15 (10.6) | |
| Tension reduction | |||
| Fascial/subcutaneous | |||
| Sutures and z-plasty | |||
| Scapular region | 56 | 4 (7.1) | |
| Tension reduction | |||
| Fascial/subcutaneous | |||
| Sutures and z-plasty | |||
| Suprapubic region | 24 | 4 (16.7) | |
| Tension reduction | |||
| Fascial/subcutaneous | |||
| Sutures and z-plasty | |||
| Others | 15 Gy/2 fr/2 days | 154 | 16 (10.4) |
| Tension reduction | |||
| Fascial/subcutaneous | |||
| Sutures and z-plasty | |||
| Total | 494 | 46 (9.3) | |
fr, fractions; Gy, Gray.
Figure 1.
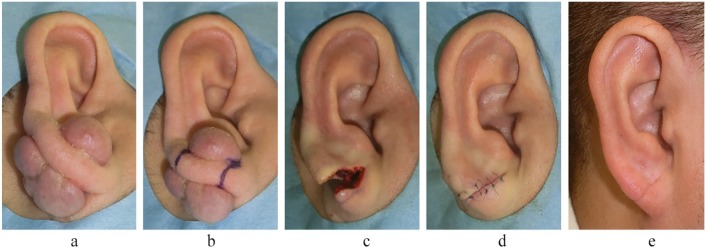
A case of severe right earlobe keloids in a 35-year-old man. The keloids were caused by piercing and were removed by wedge excision and sutured primarily. Postoperative 8 Gy/1 fr/1 day radiotherapy was performed. Recurrence was not observed in the 18 months after the operation. (a) Preoperative view. (b) Design of the wedge excision. (c) Immediately after wedge excision. (d) Immediately after surgery. (e) Eighteen months after the operation.
Figure 2.
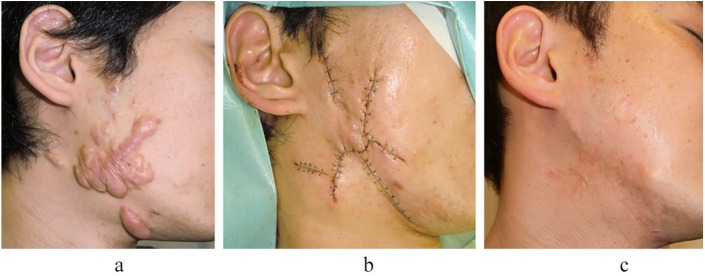
A case of severe lower jaw keloids in a 33-year-old man. The keloids were generated from acne and were treated by surgical excision followed by 15 Gy/2 fr/2 days radiotherapy. Recurrence was not observed in the three years after the operation. (a) Preoperative view. (b) Immediately after the operation. (c) Three years after the operation.
Figure 3.
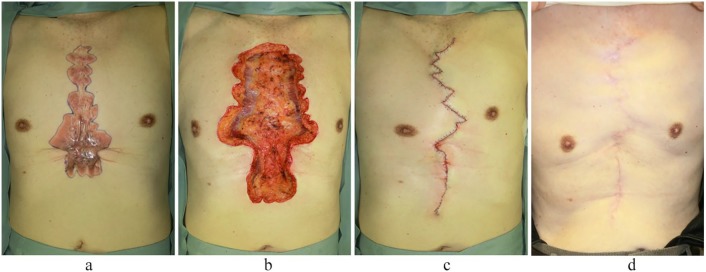
A case of severe anterior chest and upper abdominal keloids in a 78-year-old man. The keloids developed after cardiac surgery and were treated by surgical excision followed by 18 Gy/3 fr/3 days radiotherapy. Recurrence was not observed in the two years after the operation. (a) Preoperative view. (b) Immediately after keloidectomy. (c) Immediately after the operation. (d) Two years after the operation.
It should be noted that the current surgical and radiotherapy protocols are routinely accompanied by continuous long-term fixation with silicone tape and close postoperative follow-up: all patients are followed up for > 24 months via visits to the outpatient clinic at three months, six months and every 3–6 postoperative months thereafter. If even a tiny part of the postoperative scar exhibits inflammation, as indicated by stiffness with or without redness, the silicone tape is replaced with steroid plaster (Eclar® plaster; Hisamitsu Pharmaceutical Co., Inc., Tokyo, Japan).21 The patient is asked to change the plaster every day. If the stiffness has disappeared at the next visit 3–6 months later, the steroid plaster is stopped and replaced with heparinoid ointment (Hirudoid® Soft Ointment; Maruho Co., Inc., Osaka, Japan) to keep the scar surface moist. Figure 4 shows an example of severe groin keloids that were effectively treated with surgery, postoperative radiation and heparinoid ointment alone. However, if the steroid plaster does not eliminate all of the stiffness in six months, the lesion is considered to have recurred. In such cases, periodic steroid injection is added to the steroid plaster treatment. The injection is delivered with a 30-G needle and consists of 5–10 mg of triamcinolone acetonide (Kenacort®; Bristol-Myers Squibb K.K., Tokyo, Japan) that is diluted with 1% lidocaine to generate a solution of 2–3 mL.
Figure 4.
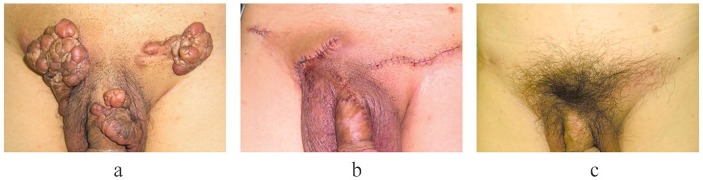
A case of severe abdomen, groin and penile keloids in a 62-year-old man. The keloids developed after folliculitis developed on the lower abdomen, groin and penis. They were treated by surgical excision and 15 Gy/3 fr/3 days radiotherapy. After radiotherapy, heparinoid ointment was used to keep the scar surface moist. Recurrence and side effects were not observed in the 18 months after the operation. (a) Preoperative view. (b) Immediately after the operation. (c) Eighteen months after the operation.
Safety of radiotherapy
In 2007, Preston et al. reported that when people aged 18–64 years are exposed to 1 Gy of whole body irradiation, 670 of 10,000 people (6.7%) will develop skin cancer.22 The general mortality rate of skin cancer is 1/500 patients. Therefore, 1/7500 people exposed to 1 Gy whole-body irradiation will die from skin cancer (6.7% × 1/500 = 0.0134%). In our current postoperative radiotherapy protocol for earlobe keloids, 0.05% of the skin of the whole body is irradiated with 8 Gy. Thus, one in almost 4000 patients treated with this protocol will be expected to develop skin cancer (incidence = 6.7 × 8 × 0.05/100 = 0.0268%). The mortality rate associated with this secondary carcinogenesis will be one in 2,000,000 patients (0.0268/500 = 0.000055%). We believe that this risk is clinically acceptable if informed consent is obtained from the patients after the benefits and side effects of this type of treatment are explained to them.
Nevertheless, safety regimens should be stringently followed. In particular, because the thyroid, mammary glands and gonads are very sensitive to radiation and are close to the skin, these areas should be shielded. Moreover, since the cells of infants and children aged < 20 years are still actively proliferating, they are also very radiosensitive and should not undergo irradiation.14 As a result, keloids on the thyroid and mammary glands and near the gonads, and keloids on growing children, should be treated without radiation. Indeed, in Japan, paediatric keloids are treated by steroid tape alone until the patient reaches the age of about 20 years.2,3,21
We sometimes employ radiation monotherapy to treat older patients or patients with severe huge keloids.3 Since these patients receive a higher total radiation dose than the postoperative radiation cases, it is essential to obtain informed consent and apply the radiation carefully. However, the risks of primary radiation therapy should be weighed against its remarkable benefits: it immediately alleviates subjective symptoms such as pain and itching and causes the scar colour and thickness to improve progressively over the next year.
Non-malignancy-related side effects of our keloid radiotherapy protocol
Radiotherapy also associates with a number of benign side effects, including telangiectasia, hyperpigmentation and hypopigmentation. The incidences of these radiotherapy-induced sides effects are very small in the Japanese population: for example, a recent randomised controlled trial on the side effects of radiotherapy after breast-conserving surgery in 312 Japanese breast cancer patients showed that the frequencies of telangiectasia, hyperpigmentation and hypopigmentation were 0.3%, 0.7% and 0%, respectively.23 It should be noted that the radiotherapy dose used was 42.5 Gy/6 fr delivered with 4–6 MV X-ray and that this was boosted in 20% of patients with 10.64 Gy/4 fr delivered by 6–13 MeV electron beam. Thus, the radiation dose of this treatment was substantially higher that that used in our keloid radiotherapy protocol. Despite this, the incidence of benign side effects was extremely low. This is borne out by our own clinical experiences: these side effects are vanishingly rare in our patients. The transitions in our radiation protocol also did not influence these incidences.
With regard to telangiectasia, it arises when the connective tissues that physically support the blood vessels shrink, thereby causing the vessels to expand. In our clinical experience, it is most likely to occur in keloid patients who were first treated with steroids. It rarely occurs in patients where surgery and postoperative radiation therapy were the first treatment.
With regard to hyperpigmentation, it is the result of radiation dermatitis. In our clinical experience, radiation dermatitis seems to reflect individual skin sensitivity and constitution and is thus difficult to predict. Steroid ointment/cream should be started when radiation dermatitis arises.
While hypopigmentation is very rare in Japanese patients, this may reflect their intermediate levels of pigmentation. It may be a more serious problem for populations with darker skin.
Customisation of our protocol with regard to racial differences
Our facility treats not only Asian patients but also patients with African and Caucasian ethnicity. The same protocol is used for all. In Asian, African and Caucasian countries, the keloid incidence ranges are 0–0.1%, < 0.1, and 5–10%.24–28 However, we do not think that African patients should be treated by high-dose radiation while Caucasian patients should be treated by low-dose radiation. This is because keloid is the result of chronic inflammation of the dermis2 and radiation therapy after extirpation of the keloid aims to suppress any new emergence of this inflammation. While racial differences in the initial strength of this inflammation have not, to our knowledge, been formally examined, we suspect that it will be the same between ethnicities in general. For this reason, we treat all ethnicities with the same protocol. Our general impression is that African and Caucasian patients respond as well to our protocol as Asians. However, the number of patients in each group is still too small for statistical analysis.
By contrast, the factor that has the biggest impact on keloid treatment is the presence of multiple keloids (as opposed to a single keloid). Multiple keloids associate with a higher rate of recurrence, including in the Japanese population. It should be noted that we excluded patients with multiple keloids from the cohorts described in this paper. Multiple keloids may be difficult to resolve in part because their complexity and/or large total keloidal surface area means they cannot be totally extirpated: thus, surgery leaves remaining keloids that continue to secrete proinflammatory cytokines that may promote new growth in the extirpated area.29 Another possibility is that patients with multiple keloids have a bodily constitution (e.g. genetic – possibly including racial genetics – and systemic features) that increases their susceptibility to keloidogenesis.2,3,29,30 Given the therapeutic challenges posed by multiple keloids, we recommend that large and/or strongly symptomatic keloids should be treated very early after they develop.
Conclusions
Our impression is that physicians in non-Caucasian societies often avoid actively treating keloids and, if they do treat these scars, they tend to prefer using steroid injections as the first-line therapy. However, we have found that combined surgery, radiotherapy and close follow-up with steroid tape/plaster therapy successfully manage keloids and hypertrophic scars. This approach is consequently increasingly being used, especially in Japan. Thus, there is now sufficient evidence on which to base a standard international algorithm for treating pathological scars. It is likely that this treatment algorithm will improve significantly as our knowledge of scar biology increases, higher quality clinical trials are performed and new agents are developed.
Footnotes
Declaration of Conflicting Interests: The authors declare that there is no conflict of interest.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Rei Ogawa  https://orcid.org/0000-0003-3658-555X
https://orcid.org/0000-0003-3658-555X
References
- 1. Siotos C, Uzosike AC, Hong H, et al. Keloid excision and adjuvant treatments: a network meta-analysis. Ann Plast Surg 2019; 83: 154–162. [DOI] [PubMed] [Google Scholar]
- 2. Ogawa R. Keloid and Hypertrophic Scars Are the Result of Chronic Inflammation in the Reticular Dermis. Int J Mol Sci 2017; 18: E606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ogawa R, Akaishi S, Kuribayashi S, et al. Keloids and Hypertrophic Scars Can Now Be Cured Completely: Recent Progress in Our Understanding of the Pathogenesis of Keloids and Hypertrophic Scars and the Most Promising Current Therapeutic Strategy. J Nippon Med Sch 2016; 83: 46–53. [DOI] [PubMed] [Google Scholar]
- 4. Enhamre A, Hammar H. Treatment of keloids with excision and postoperative X-ray irradiation. Dermatologica 1983; 167: 90–93. [DOI] [PubMed] [Google Scholar]
- 5. Ragoowansi R, Cornes PG, Glees JP, et al. Ear-lobe keloids: treatment by a protocol of surgical excision and immediate postoperative adjuvant radiotherapy. Br J Plast Surg 2001; 54: 504–508. [DOI] [PubMed] [Google Scholar]
- 6. Ogawa R, Mitsuhashi K, Hyakusoku H, et al. Postoperative electron-beam irradiation therapy for keloids and hypertrophic scars: retrospective study of 147 cases followed for more than 18 months. Plast Reconstr Surg 2003; 111: 547–553. [DOI] [PubMed] [Google Scholar]
- 7. Ogawa R, Miyashita T, Hyakusoku H, et al. Postoperative radiation protocol for keloids and hypertrophic scars: statistical analysis of 370 sites followed for over 18 months. Ann Plast Surg 2007; 59: 688–691. [DOI] [PubMed] [Google Scholar]
- 8. Bischof M, Krempien R, Debus J, et al. Postoperative electron beam radiotherapy for keloids: objective findings and patient satisfaction in self-assessment. Int J Dermatol 2007; 46: 971–975. [DOI] [PubMed] [Google Scholar]
- 9. Kuribayashi S, Miyashita T, Ozawa Y, et al. Post-keloidectomy irradiation using high-dose-rate superficial brachytherapy. J Radiat Res 2011; 52: 365–368. [DOI] [PubMed] [Google Scholar]
- 10. Guix B, Henríquez I, Andrés A, et al. Treatment of keloids by high-dose-rate brachytherapy: A seven-year study. Int J Radiat Oncol Biol Phys 2001; 50: 167–172. [DOI] [PubMed] [Google Scholar]
- 11. Garg MK, Weiss P, Sharma AK, et al. Adjuvant high dose rate brachytherapy (Ir-192) in the management of keloids which have recurred after surgical excision and external radiation. Radiother Oncol 2004; 73: 233–236. [DOI] [PubMed] [Google Scholar]
- 12. Goutos I, Ogawa R. Brachytherapy in the adjuvant management of keloid scars: literature review. Scars Burn Heal 2017; 3: 2059513117735483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kaiser A, Eley JG, Onyeuku NE, et al. Proton Therapy Delivery and Its Clinical Application in Select Solid Tumor Malignancies. J Vis Exp 2019; (144). [DOI] [PubMed] [Google Scholar]
- 14. Ogawa R, Yoshitatsu S, Yoshida K, et al. Is radiation therapy for keloids acceptable? The risk of radiation-induced carcinogenesis. Plast Reconstr Surg 2009; 124: 1196–1201. [DOI] [PubMed] [Google Scholar]
- 15. Veen RE, Kal HB. Postoperative high-dose-rate brachytherapy in the prevention of keloids. Int J Radiat Oncol Biol Phys 2007; 69: 1205–1208. [DOI] [PubMed] [Google Scholar]
- 16. Kal HB, Veen RE. Biologically effective doses of postoperative radiotherapy in the prevention of keloids. Dose-effect relationship. Strahlenther Onkol 2005; 181: 717–723. [DOI] [PubMed] [Google Scholar]
- 17. Kal HB, Veen RE, Jürgenliemk-Schulz IM. Dose-effect relationships for recurrence of keloid and pterygium after surgery and radiotherapy. Int J Radiat Oncol Biol Phys 2009; 74: 245–251. [DOI] [PubMed] [Google Scholar]
- 18. Ogawa R, Huang C, Akaishi S, et al. Analysis of surgical treatments for earlobe keloids: analysis of 174 lesions in 145 patients. Plast Reconstr Surg 2013; 132: 818e–825e. [DOI] [PubMed] [Google Scholar]
- 19. Ogawa R, Akaishi S, Dohi T, et al. Analysis of the surgical treatments of 63 keloids on the cartilaginous part of the auricle: effectiveness of the core excision method. Plast Reconstr Surg 2015; 135: 868–875. [DOI] [PubMed] [Google Scholar]
- 20. Arima J, Dohi T, Kuribayashi S, et al. Z-plasty and Postoperative Radiotherapy for Anterior Chest Wall Keloids: An Analysis of 141 Patients. Plast Reconstr Surg Glob Open 2019; 7: e2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goutos I, Ogawa R. Steroid tape: A promising adjunct to scar management. Scars Burn Heal 2017; 3: 2059513117690937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Preston DL, Ron E, Tokuoka S, et al. Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiat Res 2007; 168: 1–64. [DOI] [PubMed] [Google Scholar]
- 23. Nozaki M, Kagami Y, Shibata T, et al. ; Radiation Therapy Study Group, Japan Clinical Oncology Group. A primary analysis of a multicenter, prospective, single-arm, confirmatory trial of hypofractionated whole breast irradiation after breast-conserving surgery in Japan: JCOG0906. Jpn J Clin Oncol 2019; 49: 57–62. [DOI] [PubMed] [Google Scholar]
- 24. Kelly AP. Keloids. Dermatol Clin 1988; 6: 413–424. [PubMed] [Google Scholar]
- 25. Louw L. Keloids in rural black South Africans. Part 1: general overview and essential fatty acid hypotheses for keloid formation and prevention. Prostaglandins Leukot Essent Fatty Acids 2000; 63: 237–245. [DOI] [PubMed] [Google Scholar]
- 26. Jovic G, Corlew DS, Bowman KG. Plastic and reconstructive surgery in Zambia: epidemiology of 16 years of practice. World J Surg 2012; 36: 241–246. [DOI] [PubMed] [Google Scholar]
- 27. Kiprono SK, Chaula BM, Masenga JE, et al. Epidemiology of keloids in normally pigmented Africans and African people with albinism: population-based cross-sectional survey. Br J Dermatol 2015; 173: 852–854. [DOI] [PubMed] [Google Scholar]
- 28. Ogawa R, Akaishi S, Hyakusoku H. Importance of Epidemiologic Investigation on Keloids. Scar Management 2009; 3: 62–64 (in Japanese). [Google Scholar]
- 29. Quong WL, Kozai Y, Ogawa R. A Case of Keloids Complicated by Castleman’s Disease: Interleukin-6 as a Keloid Risk Factor. Plast Reconstr Surg Glob Open 2017; 5: e1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ogawa R, Akaishi S. Endothelial dysfunction may play a key role in keloid and hypertrophic scar pathogenesis - Keloids and hypertrophic scars may be vascular disorders. Med Hypotheses 2016; 96: 51–60. [DOI] [PubMed] [Google Scholar]
How to cite this article
- Ogawa R, Tosa M, Dohi T, Akaishi S, Kuribayashi S. Surgical excision and postoperative radiotherapy for keloids. Scars, Burns & Healing, Volume 5, 2019. DOI: 10.1177/2059513118891113. [DOI] [PMC free article] [PubMed] [Google Scholar]