Abstract
Objective
Survival differences in oral cancer between black and white patients have been reported, but the contributing factors, especially the role of stage, are incompletely understood. Furthermore, the outcomes for Hispanic and Asian patients have been scarcely examined.
Study Design
Retrospective, population-based national study.
Setting
Surveillance, Epidemiology, and End Results 18 Custom database (January 1, 2010, to December 31, 2014).
Subjects and Methods
In total, 7630 patients with primary squamous cell carcinoma in the oral cavity were classified as non-Hispanic white (white), non-Hispanic black (black), Hispanic, or Asian. Cox regression was used to obtain unadjusted and adjusted hazard ratios (HRs) of 5-year mortality for race/ethnicity with sequential adjustments for stage and other covariates. Logistic regression was used to examine the relationship between race/ethnicity and stage with adjusted odds ratios (aORs).
Results
The cohort consisted of 75.0% whites, 7.6% blacks, 9.1% Hispanics, and 8.3% Asians. Compared to whites, the unadjusted HR for all-cause mortality for blacks was 1.68 (P < .001), which attenuated to 1.15 (P = .039) after adjusting for stage and became insignificant after including insurance. The unadjusted HRs for all-cause mortality were not significant for Hispanics and Asians vs whites. Compared to whites, blacks and Hispanics were more likely to present at later stages (aORs of 2.63 and 1.42, P < .001, respectively).
Conclusion
The greater mortality for blacks vs whites was largely attributable to the higher prevalence of later stages at presentation and being uninsured among blacks. There was no statistically significant difference in mortality for Hispanics vs whites or Asians vs whites.
Keywords: oral cancer, survival rate, stage difference, SEER database, Hispanic, Asian, black race, white race
Oral cavity cancers account for nearly 25% of all head and neck cancers.1 More than 30,000 new cases of tongue and mouth cancers are diagnosed annually with over 5000 deaths.2 Cancers in the oral cavity can be diagnosed at local stages by visual or tactile examination of the mouth, but one-third are discovered with regional involvement.3 Stage at diagnosis is regarded as one of the most critical predictors of oral cancer survival, with the 5-year survival rate substantially greater for local stage disease (71.4%) than for distant stage disease (21.8%).1 In addition, treatments of advanced oral cancer may involve significant impairment of speech and swallow functions, affecting quality of life. Therefore, identifying the subgroup(s) of patients who are at a greater risk for delayed diagnosis is important for the development of improved screening strategies, educational efforts, and access to care.
Previous studies examining racial/ethnic disparities in head and neck cancer outcomes have reported that black patients have worse survival than white patients for cancers in the larynx, oral cavity, oropharynx, and salivary glands.4-15 It has also been reported that black patients with oral cancer presented at later stages than their white counterparts.7,15 Although late-stage disease has been postulated as one of the sources for the worse survival among black patients in oral cancer, the relative role of stage after accounting for demographics and other clinical factors has not been extensively examined. Furthermore, very few studies have addressed the outcome for Hispanic patients, and to our knowledge, no large-scale study has included Asian patients as a separate group.9 With the growing Hispanic and Asian populations in the United States, cancer registries from recent years will enable us to investigate their outcomes.16 Thus, the objective of the present study was to conduct a national-level study to examine the prevalence of various stages at diagnosis for 4 racial/ethnic groups, including non-Hispanic whites, non-Hispanic blacks, Hispanics, and Asians, and to examine whether the survival differences between the racial/ethnic groups can be explained by the stage differences after accounting for demographical and clinical factors.
Methods
This population-based study used data from the Surveillance, Epidemiology, and End Results (SEER) 18 Custom database. This cancer registry covers an estimated 27.8% of the US population, consisting of records from San Francisco–Oakland, California; Connecticut; metropolitan Detroit, Michigan; Hawaii; Iowa; New Mexico; Seattle (Puget Sound), Washington; Utah; metropolitan Atlanta, Georgia; San Jose–Monterey, California; Los Angeles, California; Alaska (Natives); rural Georgia; greater California; Kentucky; Louisiana; New Jersey; and greater Georgia. The study was exempt from the University of Southern California Institutional Review Board approval since SEER 18 is a publicly available database with no personal identifiers.
The study population included adult (≥18 years old) patients with primary squamous cell carcinoma (SCC) in the oral cavity site diagnosed between January 1, 2010, and December 31, 2014. Only primary, previously untreated SCCs were included. Squamous cell carcinoma was determined by the histological type codes 8070, 8071, 8072, 8073, 8074, 8075, 8076, and 8078, according to the third edition of the International Classification of Diseases for Oncology (ICD-O-3). Cases with missing or unknown values on overall stage and age were excluded from the analyses. The survival months flag variable was used to exclude the cases with missing or incomplete data on survival time, including unknown survival time, death reported by autopsy or death certificate only (no determination of diagnosis date), or no follow-up time recorded. The final cohort meeting the inclusion criteria consisted of 7630 patients.
The main outcome of interest was all-cause death. The main predictor of interest was race/ethnicity in 4 classifications, non-Hispanic white (white), non-Hispanic black (black), Hispanic, and Asian, defined using the SEER variables of “race recode” and “origin recode.” The overall stage at presentation (I, II, III, IV) was defined according to the seventh edition of the American Joint Committee on Cancer (AJCC) manual. Other covariates available for statistical adjustment included age, sex, marital status (not married [single/widowed/divorced/separated/unmarried], married, unknown), insurance status (insured, any Medicaid, uninsured, unknown), grade of the tumor (low [well or moderately differentiated], high [poorly differentiated or undifferentiated], unknown), subsite of the tumor (tongue, floor of mouth, other mouth), and treatment modalities (surgery alone, radiation alone, chemotherapy + radiation, surgery + radiation, triple therapy [surgery + radiation + chemotherapy], other combinations, no treatment/unknown). The subsite of the oral cavity site was classified using the following primary site ICD-O-3 codes: tongue (anterior tongue: C02.0-2.3, C02.8-2.9), floor of mouth (floor of mouth: C04.0-4.1, C04.8-4.9), and other mouth (lower, upper, and other gums: C03.0-3.1, C03.9, C06.2; hard palate: C05.0; other mouth: C05.8-5.9, C06.8-6.9; buccal mucosa: C06.0-6.1).
To examine the association between race/ethnicity and stage, we first performed univariate logistic regression with stage (advanced stages III/IV vs nonadvanced stages I/II) as the dependent variable and race/ethnicity (whites as the reference group) as the independent variable and obtained the odds ratio (OR). We then performed multivariable logistic regression analyses with adjustments for age, sex, marital status, insurance status, grade of the tumor, and subsite of the tumor and obtained the adjusted odds ratio (aOR).
The overall survival time was calculated in months from diagnosis to the earliest date of death from any cause, last contact, or administrative end of study (December 31, 2014), with censoring at the latter 2 events. We obtained Kaplan-Meier estimates of 1-year, 3-year, and 5-year overall survival rates for each race/ethnicity, as well as Kaplan-Meier curves by race/ethnicity for each stage. Comparisons of Kaplan-Meier survival estimates across race/ethnicity were performed using the log-rank test. To understand the association between race/ethnicity and mortality, we employed Cox proportional hazards regression models for 5-year all-cause and cause-specific mortality. We obtained unadjusted hazard ratios (HRs) for blacks, Hispanics, and Asians vs whites (reference group) (model 1) and then adjusted HRs (aHRs) with progressive adjustments for stage (model 2), stage and insurance (model 3), and stage, insurance, and other variables, including age, sex, marital status, grade of the tumor, subsite of the tumor, and treatment modalities (model 4). In cause-specific mortality analyses, deaths due to non-cancer-related deaths were treated as additional censoring events. P values of ≤.05 were deemed statistically significant. All statistical analyses were performed using SPSS Statistics version 25 (SPSS, Inc, an IBM Company, Chicago, Illinois).
Results
Table 1 presents the demographical and clinical characteristics of the cohort by race/ethnicity, which comprised 75.0% whites, 7.6% blacks, 9.1% Hispanics, and 8.3% Asians. The proportion of males appeared to be similar across race/ethnicity (P = .519). Marital and insurance statuses, however, differed across the groups; in particular, blacks had the highest rate of being not married (62.7%) and the lowest rate of being insured (57.0%) (P < .001). The prevalence of the stages at presentation also differed greatly by race/ethnicity ( Table 1 and Figure 1 ). More than half of blacks presented at stage IV (61.5%) compared to less than half of Hispanics (40.2%), whites (34.7%), and Asians (33.7%); in contrast, only 13.8% of blacks presented at stage I compared to 36.6% of whites, 34.7% of Asians, and 26.1% of Hispanics (P < .001). Compared to the other racial/ethnic groups, blacks were treated less frequently with surgery alone (24.9%) but more commonly with chemotherapy + radiation (14.7%) and triple therapy (23.3%) (P < .001).
Table 1.
Demographical and Clinical Characteristics by Race/Ethnicity.a
| Characteristic | Overall (N = 7630) | White (n = 5725; 75.0%) | Black (n = 579; 7.6%) | Hispanic (n = 694; 9.1%) | Asian (n = 632; 8.3%) | P Valueb |
|---|---|---|---|---|---|---|
| Stage | ||||||
| I | 2573 (33.7) | 2093 (36.6) | 80 (13.8) | 181 (26.1) | 219 (34.7) | <.001 |
| II | 1168 (15.3) | 890 (15.5) | 65 (11.2) | 107 (15.4) | 106 (16.8) | |
| III | 1055 (13.8) | 756 (13.2) | 78 (13.5) | 127 (18.3) | 94 (14.9) | |
| IV | 2834 (37.1) | 1986 (34.7) | 356 (61.5) | 279 (40.2) | 213 (33.7) | |
| Age, mean ± SD, y | 62.7 ± 13.8 | 63.3 ± 13.8 | 60.2 ± 11.7 | 60.2 ± 14.7 | 61.8 ± 14.5 | <.001 |
| Male | 4596 (60.2) | 3439 (60.1) | 365 (63.0) | 411 (59.2) | 381 (60.3) | .519 |
| Marital status | <.001 | |||||
| Not married | 3295 (43.2) | 2457 (42.9) | 363 (62.7) | 288 (41.5) | 187 (29.6) | |
| Married | 3786 (49.6) | 2855 (49.9) | 177 (30.6) | 351 (50.6) | 403 (63.8) | |
| Unknown | 549 (7.2) | 413 (7.2) | 39 (6.7) | 55 (7.9) | 42 (6.6) | |
| Insurance status | <.001 | |||||
| Insured | 5900 (77.3) | 4651 (81.2) | 330 (57.0) | 447 (64.4) | 472 (74.7) | |
| Any Medicaid | 1151 (15.1) | 674 (11.8) | 186 (32.1) | 178 (25.6) | 113 (17.9) | |
| Uninsured | 370 (4.8) | 238 (4.2) | 51 (8.8) | 50 (7.2) | 31 (4.9) | |
| Unknown | 209 (2.7) | 162 (2.8) | 12 (2.1) | 19 (2.7) | 16 (2.5) | |
| Grade of the tumor | .005 | |||||
| Low | 5723 (75.0) | 4346 (75.9) | 401 (69.3) | 501 (72.2) | 475 (75.2) | |
| High | 1294 (17.0) | 931 (16.3) | 116 (20.0) | 140 (20.2) | 107 (16.9) | |
| Unknown | 613 (8.0) | 448 (7.8) | 62 (10.7) | 53 (7.6) | 50 (7.9) | |
| Subsite of the tumor | <.001 | |||||
| Tongue | 4084 (53.5) | 3086 (53.9) | 231 (39.9) | 393 (56.6) | 374 (59.2) | |
| Floor of mouth | 1281 (16.8) | 978 (17.1) | 171 (29.5) | 93 (13.4) | 39 (6.2) | |
| Other mouth | 2265 (29.7) | 1661 (29.0) | 177 (30.6) | 208 (30.0) | 219 (34.7) | |
| Treatment modalities | <.001 | |||||
| Surgery alone | 3548 (46.5) | 2835 (49.6) | 144 (24.9) | 278 (40.1) | 291 (46.0) | |
| Radiation alone | 251 (3.3) | 194 (3.4) | 31 (5.4) | 15 (2.2) | 11 (1.7) | |
| Chemotherapy + XRT | 572 (7.5) | 372 (6.5) | 85 (14.7) | 67 (9.7) | 48 (7.6) | |
| Surgery + XRT | 1417 (18.6) | 1053 (18.4) | 101 (17.4) | 134 (19.3) | 129 (20.4) | |
| Triple therapy | 1280 (16.8) | 891 (15.6) | 135 (23.3) | 140 (20.2) | 114 (18.0) | |
| Other combinations | 174 (2.3) | 107 (1.9) | 31 (5.4) | 24 (3.5) | 12 (1.9) | |
| No treatment/unknown | 388 (5.1) | 273 (4.8) | 52 (9.0) | 36 (5.2) | 27 (4.3) |
Abbreviations: SD, standard deviation; XRT, radiation therapy.
Values are presented as number (%) unless otherwise indicated.
For continuous variable(s), P values were based on the results of the analysis of variance test. For categorical variables, P values were based on the results from the Pearson χ2 test.
Figure 1.
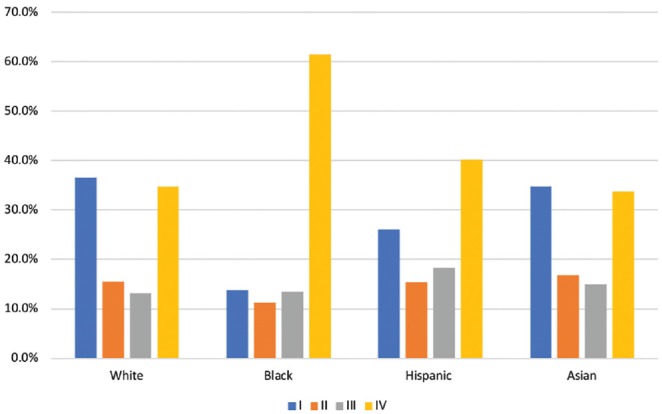
Distribution of stages at presentation by race/ethnicity.
Table 2 presents the association of race/ethnicity and advanced stages (III and IV) at presentation. Compared to whites, unadjusted ORs for advanced stages at presentation were 3.26 (P < .001) for blacks, 1.53 (P < .001) for Hispanics, and 1.03 (P = .745) for Asians. In the fully adjusted model, black race and Hispanic ethnicity remained significantly associated with advanced stages at presentation (P < .001). Other statistically significant factors in the model included male sex (P < .001), marital statuses such as married (P < .001) and unknown (P < .001), insurance statuses such as Medicaid (P < .001) and uninsured (P = .001), high-grade tumor (P < .001), and subsites such as floor of mouth (P < .001) and other mouth (P < .001).
Table 2.
Association of Race/Ethnicity and Advanced Stages in Univariate and Multivariable Logistic Regressions.
| Characteristic | OR | 95% CI | P Value | aOR | 95% CI | P Value |
|---|---|---|---|---|---|---|
| Race/ethnicity | ||||||
| White | 1 | 1 | ||||
| Black | 3.26 | 2.68-3.96 | <.001 | 2.63 | 2.14-3.22 | <.001 |
| Hispanic | 1.53 | 1.31-1.80 | <.001 | 1.42 | 1.20-1.68 | <.001 |
| Asian | 1.03 | 0.87-1.21 | .745 | 1.01 | 0.85-1.20 | .938 |
| Age (per 1 year older) | 1.00 | 0.99-1.00 | .111 | |||
| Sex | ||||||
| Female | 1 | |||||
| Male | 1.39 | 1.26-1.54 | <.001 | |||
| Marital status | ||||||
| Not married | 1 | |||||
| Married | 0.74 | 0.67-0.82 | <.001 | |||
| Unknown | 0.63 | 0.51-0.77 | <.001 | |||
| Insurance status | ||||||
| Insured | 1 | |||||
| Any Medicaid | 1.71 | 1.48-1.97 | <.001 | |||
| Uninsured | 1.48 | 1.18-1.86 | .001 | |||
| Unknown | 0.84 | 0.62-1.14 | .273 | |||
| Grade of the tumor | ||||||
| Low | 1 | |||||
| High | 2.35 | 2.06-2.68 | <.001 | |||
| Unknown | 1.18 | 0.99-1.41 | .060 | |||
| Subsite of the tumor | ||||||
| Tongue | 1 | |||||
| Floor of mouth | 1.34 | 1.17-1.53 | <.001 | |||
| Other mouth | 2.23 | 1.99-2.49 | <.001 | |||
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; OR, odds ratio.
Over the mean follow-up time of 20.7 months, there were 2417 (31.7%) deaths. Figure 2 presents the Kaplan-Meier survival curves by race/ethnicity with more detailed estimates of the overall survival rates in Table 3 . The Kaplan-Meier 5-year overall survival rate for blacks (30.8%) was substantially lower than for whites (53.6%), Hispanics (53.8%), and Asians (57.9%). Using the log-rank test, blacks had significantly worse overall survival compared to any of the other racial/ethnic groups (P < .001). In the unadjusted Cox model ( Table 4 , model 1), as expected, blacks had significantly higher mortality than whites (HR, 1.68; P < .001), while Hispanics and Asians did not have statistically significant differences in mortality compared to whites (P = .105 and .405, respectively).
Figure 2.
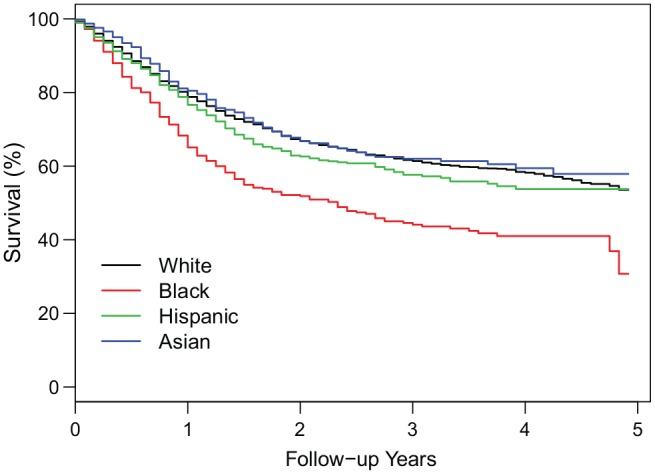
Kaplan-Meier estimates of overall survival by race/ethnicity.
Table 3.
Estimated Overall Survival Rates and Mean Survival Times by Race/Ethnicity.
| Overall Survival Rate, % |
Log-Rank Test P Valuea |
||||||
|---|---|---|---|---|---|---|---|
| Characteristic | 1 Year | 3 Years | 5 Years | Overall | Multiple Comparisons | ||
| White | 78.8 | 61.4 | 53.6 | <.001 | — | — | — |
| Black | 65.1 | 44.1 | 30.8 | <.001b | — | — | |
| Hispanic | 76.6 | 57.6 | 53.8 | .102b | <.001c | — | |
| Asian | 80.5 | 62.0 | 57.9 | .404b | <.001c | .067d | |
P values for multiple comparisons of the log-rank test were based on the Bonferroni technique using an adjusted α = 0.008.
P values compared to white.
P values compared to black.
P value compared to Hispanic.
Table 4.
Hazard Ratios for 5-Year All-Cause Death by Race/Ethnicity.a
| Characteristic | Model 1 (Unadjusted) |
Model 2 |
Model 3 |
Model 4 |
||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | aHR (95% CI) | P Value | aHR (95% CI) | P Value | aHR (95% CI) | P Value | |
| Race/ethnicity (white as reference) | ||||||||
| Black | 1.68 (1.47-1.91) | <.001 | 1.15 (1.01-1.31) | .039 | 1.08 (0.94-1.23) | .269 | 1.00 (0.87-1.14) | .970 |
| Hispanic | 1.12 (0.98-1.29) | .105 | 0.98 (0.85-1.12) | .756 | 0.94 (0.81-1.08) | .345 | 1.00 (0.87-1.15) | .963 |
| Asian | 0.94 (0.80-1.09) | .405 | 0.91 (0.78-1.06) | .216 | 0.89 (0.76-1.03) | .122 | 0.89 (0.76-1.04) | .139 |
| Stage (I as reference) | ||||||||
| II | 2.38 (2.03-2.80) | <.001 | 2.35 (2.00-2.76) | <.001 | 2.06 (1.75-2.43) | <.001 | ||
| III | 3.74 (3.21-4.35) | <.001 | 3.65 (3.13-4.25) | <.001 | 3.38 (2.86-3.99) | <.001 | ||
| IV | 6.50 (5.73-7.37) | <.001 | 6.30 (5.55-7.15) | <.001 | 5.45 (4.68-6.34) | <.001 | ||
| Insurance status (insured as reference) | ||||||||
| Any Medicaid | 1.35 (1.22-1.50) | <.001 | 1.42 (1.28-1.58) | <.001 | ||||
| Uninsured | 1.17 (0.98-1.39) | .084 | 1.37 (1.14-1.64) | .001 | ||||
| Unknown | 1.03 (0.79-1.34) | .818 | 1.13 (0.86-1.48) | .379 | ||||
| Age (per 1 year older) | 1.03 (1.03-1.03) | <.001 | ||||||
| Male (vs female) | 1.07 (0.98-1.17) | .133 | ||||||
| Marital status (not married as reference) | ||||||||
| Married | 0.89 (0.82-0.97) | .009 | ||||||
| Unknown | 0.82 (0.69-0.98) | .026 | ||||||
| Grade of the tumor (low as reference) | ||||||||
| High | 1.24 (1.12-1.37) | <.001 | ||||||
| Unknown | 0.88 (0.77-1.02) | .081 | ||||||
| Subsite of the tumor (tongue as reference) | ||||||||
| Floor of mouth | 1.04 (0.94-1.17) | .442 | ||||||
| Other mouth | 0.87 (0.79-0.96) | .004 | ||||||
| Treatment modalities (surgery alone as reference) | ||||||||
| Radiation alone | 2.61 (2.18-3.12) | <.001 | ||||||
| Chemo + XRT | 1.51 (1.30-1.76) | <.001 | ||||||
| Surgery + XRT | 0.71 (0.62-0.82) | <.001 | ||||||
| Triple therapy | 0.94 (0.81-1.08) | .345 | ||||||
| Other combinations | 2.49 (2.03-3.07) | <.001 | ||||||
| No treatment/unknown | 5.03 (4.31-5.86) | <.001 | ||||||
Abbreviations: aHR, adjusted hazard ratio; CI, confidence interval; HR, hazard ratio; SD, standard deviation; XRT, radiation therapy.
Model 1: unadjusted. Model 2: adjusted for stage. Model 3: adjusted for stage and insurance. Model 4: adjusted for stage, insurance, age, sex, marital status, grade of the tumor, subsite of the tumor, and treatment modalities.
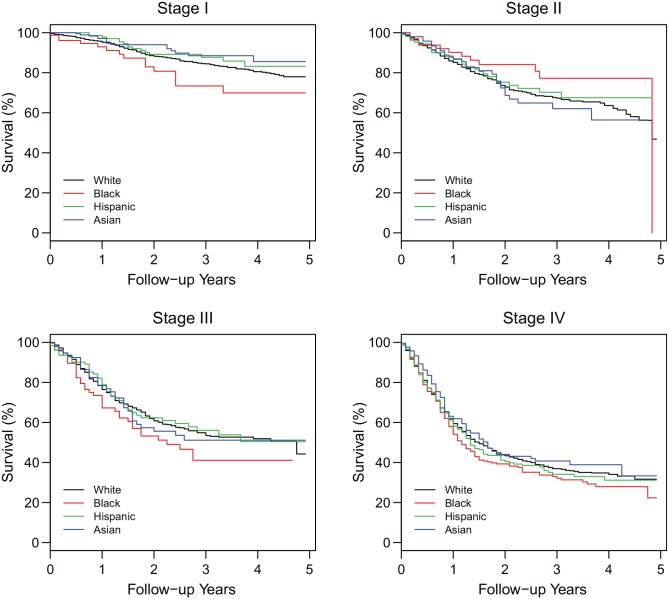
Figure 3 presents the Kaplan-Meier survival curves by race/ethnicity for each stage. Using the log-rank test, for each of the stages II to IV, the survival curves were not much different across race/ethnicity (P > .04), but for stage I, blacks had worse survival than whites (P = .043), Hispanics (P = .020), and Asians (P = .005), suggesting that stage was an important but not the only factor explaining the crude racial/ethnic survival differences seen in Figure 2 . When we performed Cox regression models, after adjusting for stage ( Table 4 , model 2), as expected, blacks still had a 15% significantly higher mortality risk than whites (aHR, 1.15; P = .039). After adjusting for both stage and insurance ( Table 4 , model 3), there was no longer a significant difference in mortality between blacks and whites (P = .269). Finally, in the fully adjusted model ( Table 4 , model 4), there remained no significant difference in mortality between blacks and whites (P = .970), while stage, insurance, age, marital status, grade of the tumor, subsite of the tumor, and treatment modalities were significant predictors of mortality.
Figure 3.
Kaplan-Meier estimates of overall survival by race/ethnicity in each stage.
Table 5 presents the hazard ratios for 5-year cause-specific mortality by race/ethnicity in the unadjusted and adjusted Cox regression models. Similar to the pattern seen with all-cause mortality, compared to whites, the unadjusted HR for blacks was 1.78 (P < .001), which attenuated to 1.17 (P = .031) after adjusting for stage. After adjusting for both stage and insurance, the HR for blacks vs whites became insignificant (P = .201). In the fully adjusted model, there was again no statistically significant difference in mortality across race/ethnicity, while stage and insurance remained significant contributors to mortality.
Table 5.
Hazard Ratios for 5-Year Cause-Specific Death by Race/Ethnicity.a
| Characteristic | Model 1 (Unadjusted) |
Model 2 |
Model 3 |
Model 4 |
||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | aHR (95% CI) | P Value | aHR (95% CI) | P Value | aHR (95% CI) | P Value | |
| Race/ethnicity (white as reference) | ||||||||
| Black | 1.78 (1.54-2.06) | <.001 | 1.17 (1.02-1.35) | .031 | 1.10 (0.95-1.27) | .201 | 1.01 (0.87-1.17) | .916 |
| Hispanic | 1.21 (1.04-1.41) | .012 | 1.04 (0.90-1.21) | .589 | 1.00 (0.86-1.16) | .975 | 1.06 (0.91-1.23) | .462 |
| Asian | 1.02 (0.86-1.20) | .835 | 0.98 (0.83-1.16) | .830 | 0.96 (0.81-1.13) | .623 | 0.96 (0.81-1.14) | .654 |
| Stage (I as reference) | ||||||||
| II | 2.75 (2.25-3.37) | <.001 | 2.72 (2.22-3.33) | <.001 | 2.33 (1.90-2.87) | <.001 | ||
| III | 5.27 (4.38-6.33) | <.001 | 5.14 (4.27-6.18) | <.001 | 4.51 (3.69-5.51) | <.001 | ||
| IV | 9.40 (8.02-11.02) | <.001 | 9.11 (7.76-10.69) | <.001 | 7.38 (6.12-8.89) | <.001 | ||
| Insurance status (insured as reference) | ||||||||
| Any Medicaid | 1.32 (1.18-1.48) | <.001 | 1.36 (1.21-1.54) | <.001 | ||||
| Uninsured | 1.20 (0.99-1.45) | .060 | 1.34 (1.10-1.63) | .003 | ||||
| Unknown | 0.86 (0.62-1.19) | .359 | 0.94 (0.68-1.32) | .725 | ||||
| Age (per 1 year older) | 1.03 (1.02-1.03) | <.001 | ||||||
| Male (vs female) | 1.01 (0.92-1.11) | .829 | ||||||
| Marital status (not married as reference) | ||||||||
| Married | 0.95 (0.86-1.04) | .250 | ||||||
| Unknown | 0.82 (0.67-1.00) | .052 | ||||||
| Grade of the tumor (low as reference) | ||||||||
| High | 1.19 (1.06-1.33) | .002 | ||||||
| Unknown | 0.83 (0.71-0.98) | .026 | ||||||
| Subsite of the tumor (tongue as reference) | ||||||||
| Floor of mouth | 1.01 (0.89-1.14) | .873 | ||||||
| Other mouth | 0.85 (0.77-0.95) | .003 | ||||||
| Treatment modalities (surgery alone as reference) | ||||||||
| Radiation alone | 3.10 (2.54-3.79) | <.001 | ||||||
| Chemo + XRT | 1.75 (1.47-2.07) | <.001 | ||||||
| Surgery + XRT | 0.77 (0.66-0.90) | .001 | ||||||
| Triple therapy | 1.05 (0.90-1.23) | .529 | ||||||
| Other combinations | 2.82 (2.24-3.54) | <.001 | ||||||
| No treatment/unknown | 5.88 (4.95-6.99) | <.001 | ||||||
Abbreviations: aHR, adjusted hazard ratio; CI, confidence interval; HR, hazard ratio; SD, standard deviation; XRT, radiation therapy.
Model 1: unadjusted. Model 2: adjusted for stage. Model 3: adjusted for stage and insurance. Model 4: adjusted for stage, insurance, age, sex, marital status, grade of the tumor, subsite of the tumor, and treatment modalities.
Discussion
The present study examined racial/ethnic differences in survival and the role of stage in oral cavity cancer using nationally representative data. Compared to the other 3 racial/ethnic groups, blacks had a substantially greater prevalence of stage IV and a lower prevalence of stage I at presentation. Blacks also had significantly lower overall survival rates than whites. We found that the higher black mortality compared to whites was largely explained by the stage differences and also by the insurance differences, which was independent of the combined contribution of demographics and the other clinical factors considered. For Hispanics and Asians, there were no statistically significant differences in survival compared to whites.
Several earlier studies have examined racial/ethnic disparities in survival between black and white patients in head and neck cancers.4,6-10 Specifically in oral cancer, it has been reported that black patients had lower 5-year survival rates than their white counterparts, but only 1 study performed additional statistical analyses to control for relevant covariates.7,8 Furthermore, few studies in head and neck cancers have examined the Hispanic ethnicity and the Asian race, and to our knowledge, no study has formally addressed these 2 groups in an oral cancer population.5,7,11,12 From 2000 to 2010, the Hispanic and Asian populations have both increased by more than 40%.17,18 The proportion of Hispanics in our study population was drastically lower than the estimated proportion in the general US population (18.1%), while the proportions of whites and Asians in our study population were higher.16 These discrepancies could arise from the registry’s collection of data from certain geographic regions or the disproportionate burden of oral cancer among racial/ethnic groups. With the changing demographics pattern in the general US population,16 the present study represents a more recent analysis of the contemporary oral cancer population.
In head and neck cancers, the contributing factors to the worse survival for black patients, especially the role of stage, are not completely understood.5,15,19-21 Our results suggest that stage played a large role in the all-cause and cause-specific mortality differences between black and white patients with oral cancer. Molina et al5 found that controlling for stage led to only a modest reduction in the hazard ratio for all-cause mortality between black and white patients in a general head and neck cancer population. Arbes et al8 revealed similar findings in an oral cancer population. Both studies used the SEER summary staging categories of “local,”“regional,” and “distant,” instead of the TNM staging guidelines that provide finer stratification. Thus, the extent of the disease could vary within the broader stage categories (ie, blacks could have more advanced disease within each stage).8 In addition, differences in the sequence of covariate adjustments could be another reason for the differing findings; these 2 prior studies included certain patient variables in their baseline models before adding stage, whereas we included patient variables after adding stage to assess for the effect of stage alone. Nevertheless, in our final models, the effect of stage remained highly significant even after removing any possible confounding from age, suggesting that stage was an important factor for mortality independent of the other variables examined.
The present study found that blacks had a higher prevalence of presenting at later stages than the other racial/ethnic groups. After adjusting for differences in patient and tumor variables, we found that blacks were still more likely than their white counterparts to present at later stages. Additional factors that could be contributing to the later presentation of black patients include mistrust toward the health care system, lower level of public awareness, and lower rates of dental care services and oral screening examinations.22-25 To our knowledge, the effectiveness of an oral screening program implemented among a diverse racial/ethnic community has not been investigated. In addition, it is unclear if the lower rate of surgery treatment among blacks compared to the other racial/ethnic groups is a result of late presentation or other factors such as inappropriate treatment and patient preference.26
We found that the higher mortality for blacks vs whites was statistically significant in the unadjusted Cox regression model, which largely attenuated after adjusting for stage and became insignificant after including insurance. This finding implies that the greater all-cause mortality for blacks vs whites may be mostly attributed to the differences in stage and also to insurance. This is in contrast to a few previous studies that examined other types of head and neck cancers where the higher all-cause and cause-specific mortality for blacks persisted after controlling for patient, tumor, and treatment variables.5,6,27 We found that blacks were more likely to present at later stages and had a lower insurance rate than their white counterparts, which is consistent with previous reports.4,21,28 Gourin and Podolsky20 showed that black patients with insurance had better survival than uninsured black patients. The Affordable Care Act (ACA) mandate that was fully implemented in 2014 has resulted in a significant increase in the insurance rate for blacks.29,30 Thus, more equal access to health care may tend to equalize the outcomes between black and white patients with oral cancer.
The present study revealed that Hispanics were more likely to present at later stages but had no difference in mortality compared to whites. In other head and neck cancers, the reported staging and survival outcomes of Hispanics are mixed.4,5,27,31-33 These discrepancies may be explained by the inclusion of various head and neck cancers and different time periods of analyses, thus yielding distinct study populations. Our findings suggest that the pattern of mortality for Hispanics did not seem to be directly linked to their advanced disease. The overall lower rates of tobacco and alcohol use among Hispanics have been suggested to play a role in lowering the burden of aggressive disease.5,34 Other potential explanations for the favorable outcome of Hispanics include the heterogeneous composition and genetic complexity of this ethnicity group and the health selectivity of the Hispanic migrants to the United States (healthy individuals immigrate to the United States while the less healthy remain in or return to their places of origin).35-39 In addition, strong social support within the Hispanic population has been cited as a protective factor for patients with varying conditions.40-42 The precise cause of the Hispanics’ better-than-expected outcome despite advanced stages at presentation is likely multifactorial and warrants further investigations.
To our knowledge, this is the first study to specifically compare the survival outcome of Asians with other racial/ethnic groups with oral cancer. The burgeoning Asian population in the United States has presented the unique opportunity to use recent national data to investigate the outcome of this population group. While Asians appeared to have a lower mortality risk than whites ( Table 4 ), this difference was not statistically significant. When considering the Asian outcome in other types of head and neck cancers such as nasopharyngeal cancer, several studies have demonstrated that Asians had better overall survival compared to whites.31,43,44 In oral cancer, further studies that include larger Asian sample sizes are warranted to clarify their outcome.
The present findings should be interpreted with the limitations inherent to a large population database such as missing data or inaccurate recording of race/ethnicity and stage. SEER database also does not have data on individual-level socioeconomic status or risk factors such as tobacco and alcohol use, which would be useful to examine across race/ethnicity. Nevertheless, the strengths of our study include a nationally representative sample and the inclusion of racial/ethnic groups that have not been thoroughly examined before, including the Hispanic ethnicity and Asian race.
Conclusion
This national-level study revealed racial/ethnic differences in survival and the role of stage in oral cavity cancer. Specifically, we found that blacks had a higher prevalence of presenting at later stages and a lower prevalence of being insured than whites. The higher black mortality compared to whites was largely attributed to the differences in stage and almost entirely eliminated after including insurance differences. For Hispanics and Asians, there were no statistically significant differences in survival compared to whites.
Author Contributions
Alison J. Yu, initial study idea concept and design, data acquisition from SEER, data analysis and interpretation, statistical analysis using SPSS, manuscript preparation, critical revision, manuscript editing and review, approval of manuscript and its integrity; Janet S. Choi, initial study idea concept and design, data analysis and interpretation, critical revision, manuscript editing and review, approval of manuscript and its integrity; Mark S. Swanson, initial study idea concept and design, data analysis and interpretation, manuscript preparation, critical revision, manuscript editing and review, approval of manuscript and its integrity; Niels C. Kokot, initial study idea concept and design, data interpretation, manuscript preparation, critical revision, manuscript editing and review, approval of manuscript and its integrity; Tamara N. Brown, initial study idea concept and design, data interpretation, manuscript preparation, critical revision, manuscript editing and review, approval of manuscript and its integrity; Guofen Yan, initial study idea concept and design, data acquisition from SEER, data analysis and interpretation, statistical analysis using R, critical revision, approval of manuscript and its integrity; Uttam K. Sinha, initial study idea concept and design, data analysis and interpretation, critical revision, manuscript editing and review, approval of manuscript and its integrity.
Disclosures
Competing interests: None.
Sponsorships: None.
Funding source: Alison J. Yu received fellowship support from the Keck School of Medicine of the University of Southern California Dean’s Research Scholars Program, during which the design and conduct, data analysis, and writing of the manuscript were conducted.
Acknowledgments
A.J.Y. graciously acknowledges fellowship support from the USC Keck School of Medicine Dean’s Research Scholars Program. The authors would like to thank the SEER staff for their assistance in providing the SEER database.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
This article was presented at the AAO-HNSF 2018 Annual Meeting & OTO Experience; October 7-10, 2018; Atlanta, Georgia.
References
- 1. Carvalho AL, Nishimoto IN, Califano JA, Kowalski LP. Trends in incidence and prognosis for head and neck cancer in the United States: a site-specific analysis of the SEER database. Int J Cancer. 2005;114:806-816. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7-30. [DOI] [PubMed] [Google Scholar]
- 3. Weatherspoon DJ, Chattopadhyay A, Boroumand S, Garcia I. Oral cavity and oropharyngeal cancer incidence trends and disparities in the United States: 2000-2010. Cancer Epidemiol. 2015;39:497-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shavers VL, Harlan LC, Winn D, Davis WW. Racial/ethnic patterns of care for cancers of the oral cavity, pharynx, larynx, sinuses, and salivary glands. Cancer Metastasis Rev. 2003;22:25-38. [DOI] [PubMed] [Google Scholar]
- 5. Molina MA, Cheung MC, Perez EA, et al. African American and poor patients have a dramatically worse prognosis for head and neck cancer: an examination of 20,915 patients. Cancer. 2008;113:2797-2806. [DOI] [PubMed] [Google Scholar]
- 6. Nichols AC, Bhattacharyya N. Racial differences in stage and survival in head and neck squamous cell carcinoma. Laryngoscope. 2007;117:770-775. [DOI] [PubMed] [Google Scholar]
- 7. Shiboski CH, Schmidt BL, Jordan RC. Racial disparity in stage at diagnosis and survival among adults with oral cancer in the US. Community Dent Oral Epidemiol. 2007;35:233-240. [DOI] [PubMed] [Google Scholar]
- 8. Arbes SJ, Olshan AF, Caplan DJ, Schoenbach VJ, Slade GD, Symons MJ. Factors contributing to the poorer survival of black Americans diagnosed with oral cancer (United States). Cancer Causes Control. 1999;10:513-523. [DOI] [PubMed] [Google Scholar]
- 9. Moore RJ, Doherty DA, Do KA, Chamberlain RM, Khuri FR. Racial disparity in survival of patients with squamous cell carcinoma of the oral cavity and pharynx. Ethn Health. 2001;6:165-177. [DOI] [PubMed] [Google Scholar]
- 10. Osazuwa-Peters N, Massa ST, Christopher KM, Walker RJ, Varvares MA. Race and sex disparities in long-term survival of oral and oropharyngeal cancer in the United States. J Cancer Res Clin Oncol. 2016;142:521-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Choi SH, Terrell JE, Fowler KE, et al. Socioeconomic and other demographic disparities predicting survival among head and neck cancer patients. PLoS One. 2016;11:e0149886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schrank TP, Han Y, Weiss H, Resto VA. Case-matching analysis of head and neck squamous cell carcinoma in racial and ethnic minorities in the United States—possible role for human papillomavirus in survival disparities. Head Neck. 2011;33:45-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goodwin WJ, Thomas GR, Parker DF, et al. Unequal burden of head and neck cancer in the United States. Head Neck. 2008;30:358-371. [DOI] [PubMed] [Google Scholar]
- 14. Chen LM, Li G, Reitzel LR, et al. Matched-pair analysis of race or ethnicity in outcomes of head and neck cancer patients receiving similar multidisciplinary care. Cancer Prev Res (Phila). 2009;2:782-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ragin CC, Langevin SM, Marzouk M, Grandis J, Taioli E. Determinants of head and neck cancer survival by race. Head Neck. 2011;33:1092-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. US Census Bureau. National Population by Characteristics: 2010-2017. https://www.census.gov/data/datasets/2017/demo/popest/nation-detail.html. Accessed August 22, 2018.
- 17. Ennis S, Rios-Vargas M, Albert N. The Hispanic population: 2010. https://www.census.gov/prod/cen2010/briefs/c2010br-04.pdf. Accessed January 4, 2019.
- 18. Hoeffel E, Rastogi S, Kim MO, Shahid H. The Asian population: 2010. https://www.census.gov/prod/cen2010/briefs/c2010br-11.pdf. Accessed January 4, 2019.
- 19. Daraei P, Moore CE. Racial disparity among the head and neck cancer population. J Cancer Educ. 2015;30:546-551. [DOI] [PubMed] [Google Scholar]
- 20. Gourin CG, Podolsky RH. Racial disparities in patients with head and neck squamous cell carcinoma. Laryngoscope. 2006;116:1093-1106. [DOI] [PubMed] [Google Scholar]
- 21. Osazuwa-Peters N, Christopher KM, Hussaini AS, Behera A, Walker RJ, Varvares MA. Predictors of stage at presentation and outcomes of head and neck cancers in a university hospital setting. Head Neck. 2016;38:E1826-E1832. [DOI] [PubMed] [Google Scholar]
- 22. Gómez I, Warnakulasuriya S, Varela-Centelles PI, et al. Is early diagnosis of oral cancer a feasible objective? Who is to blame for diagnostic delay? Oral Dis. 2010;16:333-342. [DOI] [PubMed] [Google Scholar]
- 23. Kravietz A, Angara P, Le M, Sargi Z. Disparities in screening for head and neck cancer: evidence from the NHANES, 2011-2014. Otolaryngol Head Neck Surg. 2018;159:683-691. [DOI] [PubMed] [Google Scholar]
- 24. Gourin CG, Kaboli KC, Blume EJ, Nance MA, Koch WM. Characteristics of participants in a free oral, head and neck cancer screening program. Laryngoscope. 2009;119:679-682. [DOI] [PubMed] [Google Scholar]
- 25. Osazuwa-Peters N, Adjei Boakye E, Hussaini AS, et al. Characteristics and predictors of oral cancer knowledge in a predominantly African American community. PLoS One. 2017;12:e0177787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Parhar HS, Anderson DW, Janjua AS, Durham JS, Prisman E. Patient choice of nonsurgical treatment contributes to disparities in head and neck squamous cell carcinoma. Otolaryngol Head Neck Surg. 2018;158:1057-1064. [DOI] [PubMed] [Google Scholar]
- 27. Russell JL, Chen NW, Ortiz SJ, Schrank TP, Kuo YF, Resto VA. Racial and ethnic disparities in salivary gland cancer survival. JAMA Otolaryngol Head Neck Surg. 2014;140:504-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kwok J, Langevin SM, Argiris A, Grandis JR, Gooding WE, Taioli E. The impact of health insurance status on the survival of patients with head and neck cancer. Cancer. 2010;116:476-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen J, Vargas-Bustamante A, Mortensen K, Ortega AN. Racial and ethnic disparities in health care access and utilization under the Affordable Care Act. Med Care. 2016;54:140-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sealy-Jefferson S, Vickers J, Elam A, Wilson MR. Racial and ethnic health disparities and the Affordable Care Act: a status update. J Racial Ethn Health Disparities. 2015;2:583-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Patel VJ, Chen NW, Resto VA. Racial and ethnic disparities in nasopharyngeal cancer survival in the United States. Otolaryngol Head Neck Surg. 2017;156:122-131. [DOI] [PubMed] [Google Scholar]
- 32. Smith SP, Russell JL, Chen NW, Kuo YF, Resto VA. Sinonasal carcinoma: racial and ethnic disparities in survival—a review of 4714 patients. Otolaryngol Head Neck Surg. 2015;153:551-560. [DOI] [PubMed] [Google Scholar]
- 33. Parasher AK, Abramowitz M, Weed D, Franzmann E, Hu J, Lally B. Ethnicity and clinical outcomes in head and neck cancer: an analysis of the SEER database. J Racial Ethnic Health Disparities. 2014;1:267-274. [Google Scholar]
- 34. Velasco-Mondragon E, Jimenez A, Palladino-Davis AG, Davis D, Escamilla-Cejudo JA. Hispanic health in the USA: a scoping review of the literature. Public Health Rev. 2016;37:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Palloni A, Morenoff JD. Interpreting the paradoxical in the Hispanic paradox: demographic and epidemiologic approaches. Ann N Y Acad Sci. 2001;954:140-174. [DOI] [PubMed] [Google Scholar]
- 36. Palloni A, Arias E. Paradox lost: explaining the Hispanic adult mortality advantage. Demography. 2004;41:385-415. [DOI] [PubMed] [Google Scholar]
- 37. Franzini L, Ribble JC, Keddie AM. Understanding the Hispanic paradox. Ethn Dis. 2001;11:496-518. [PubMed] [Google Scholar]
- 38. Abraído-Lanza AF, Dohrenwend BP, Ng-Mak DS, Turner JB. The Latino mortality paradox: a test of the “salmon bias” and healthy migrant hypotheses. Am J Public Health. 1999;89:1543-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. González Burchard E, Borrell LN, Choudhry S, et al. Latino populations: a unique opportunity for the study of race, genetics, and social environment in epidemiological research. Am J Public Health. 2005;95:2161-2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Comas-Díaz L. Latino healing: the integration of ethnic psychology into psychotherapy. Psychotherapy (Chic). 2006;43:436-453. [DOI] [PubMed] [Google Scholar]
- 41. Bell CN, Thorpe RJ, Laveist TA. Race/ethnicity and hypertension: the role of social support. Am J Hypertens. 2010;23:534-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Brooks AT, Andrade RE, Middleton KR, Wallen GR. Social support: a key variable for health promotion and chronic disease management in Hispanic patients with rheumatic diseases. Clin Med Insights Arthritis Musculoskelet Disord. 2014;7:21-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bhattacharyya N. The impact of race on survival in nasopharyngeal carcinoma: a matched analysis. Am J Otolaryngol. 2004;25:94-97. [DOI] [PubMed] [Google Scholar]
- 44. Sun LM, Li CI, Huang EY, Vaughan TL. Survival differences by race in nasopharyngeal carcinoma. Am J Epidemiol. 2007;165:271-278. [DOI] [PubMed] [Google Scholar]