1. Introduction
Electro-anatomical mapping (EAM) helps us to analyse the activation wave fronts arising from structures responsible for genesis of arrhythmias. With the ever growing subset of complex arrhythmias, it has become inevitable for the electrophysiologists to have an accurate anatomical representation of the substrate. The newer advances in 3D EAM mapping have made it possible to acquire precise geometry points for mapping and ablation.
EAM systems are broadly based on two technologies: 1. Abbott EnSite Nav X™, uses low level current applied through orthogonally placed electrodes. Mapping is then performed by these impedance based points. 2. Biosense Webster CARTO™, uses low level magnetic fields to localise anatomic points for geometry creation [1]. While impedance based catheters give the operator the edge in being open platform, the proficiency of magnet based technology in accurate delineation of geometry especially in high impedance areas is well known. The newer Abbott EnSite Precision™ technology with ‘magnet sensor enabled’ catheters which were recently launched in India gives us the best of both technologies. Infact, EnSite Precision™ scores over its predecessor EnSite Nav X Velocity™ in two novel aspects: AutoMap® and AutoMark®.
We describe a complex case of incessant left atrial tachycardia who underwent radiofrequency ablation using the latest Abbott EnSite Precision™ mapping system with ‘magnetic sensor-enabled (SE) catheters’.
2. Case description
A 38 year old female with drug refractory palpitation presented to us with incessant atrial tachyarrhythmia with a history of attempted radiofrequency ablation (RFA) elsewhere. The 12-lead ECG revealed a narrow complex tachycardia with a rate of 240 bpm (Fig. 1A). Intracardiac electrogram showed significant variation in tachycardia cycle length ranging from 390 ms to as long as 500 ms (Fig. 1B). Post-pacing analysis of the 12-lead ECG during the tachycardia revealed relatively narrow, +/- P waves in lead V1 with inferior axis and -in 1, aVL (Fig. 2). This was suggestible of a septal tachycardia. Tachycardia could only be induced by burst pacing during the EP study (Fig. 3). No other manoeuvres induced the tachycardia. Tachycardia could not be entrained from atrium or the ventricle. We narrowed our diagnosis to a focal atrial tachycardia as evidenced by ventriculo-atrial (VA) dissociation and variable VA linking (Fig. 4 A, B). Dense mapping was performed using 3-D electro-anatomical (EA) mapping system (EnSite Precision) and a 4mm tip, quadripolar Magnetic sensor-enabled™ Bi-Directional irrigation ablation catheter (FlexAbility™). The right and left atrial geometry was created using the impedance parameters as well as the magnetic-sensor parameters. There was no fluoroscopy used for the procedure other than for trans-septal access. The atrial tachycardia (AT) was localised to left atrial septum. Activation mapping revealed early atrial signals at the left atrial aspect of interatrial septum (Fig. 5, Fig. 6). Exit into the right atrium through the inter-atrial septum was evident. Radiofrequency energy (RF) lesions (30w, 43c, 17ml/sec) with a 4mm-tip open-irrigation catheter (FlexAbility™) with aid of Agilis™ sheath at sites of early signals resulted in termination of the AT (Fig. 7). Aggressive induction protocols did not result in further tachyarrhythmia. The patient had no recurrence at 5-month follow-up.
Fig. 1.
A: Shows a regular narrow QRS tachycardia (HR 240 bpm) with long RP interval. 1B - Intra cardiac electrogram demonstrating tachycardia with cycle length of 510 ms with 1:1 AV conduction.
Fig. 2.
Post-pacing analysis of the 12-lead ECG during the tachycardia revealed relatively narrow, +/- P waves in lead V1 with inferior axis and - in 1, aVL. This was suggestible of a septal tachycardia.
Fig. 3.
Induction of tachycardia was only possible by burst pacing.
Fig. 4.
A,B demonstrates VA dissociation and variable VA linking.
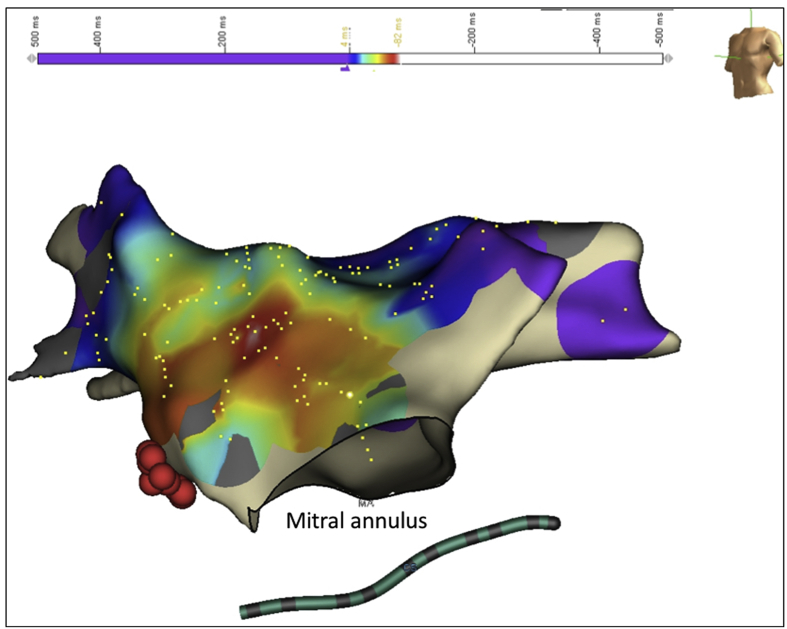
Fig. 5.
Shows 3 D Electro-anatomical activation Map in LAO view with HIS signals (red) and Mitral valve annulus. Activation mapping revealed early atrial signals at the left atrial aspect of interatrial septum.
Fig. 6.
Shows 3 D Electro-anatomical Map (AP and LAO view) with earliest activation at left atrial aspect of inter atrial septum. Ablation at this site was performed with a 4mm-tip open-irrigation FlexAbility™ catheter.
Fig. 7.
Shows Intra cardiac electrogram during termination of tachycardia during RFA.
3. Discussion
We have demonstrated a successful ‘near-zero’ fluoroscopic ablation of incessant left atrial tachycardia using ‘magnetic sensor-enabled’ EnSite Precision™ system. A dense 3D EAM did not show any evidence of a ‘micro-rentry circuit’. Moreover there was no scar within the atria. Hence the mechanism of the tachycardia was likely ‘focal automaticity’ from the left atrial septum.
Three-dimensional electro-anatomical mapping systems in India, have been predominantly CARTO™ based or EnSite Precision™ technology. Although launched way back in January 2017 in Western world, this Ensite Precision™ Technology with ‘magnet sensor enabled (SE) catheters’ have ushered in Indian markets only recently in month of November 2018.
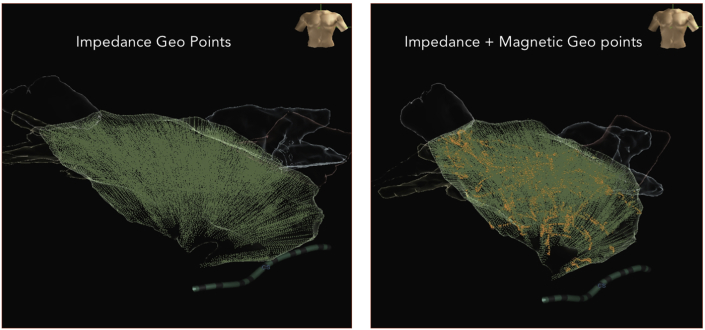
In our case, the magnetic sensor enabled EnSite Precision™ system was extremely helpful in creating an accurate geometry of both the atria. The differences between the geometry created by the impedance-only system and the hybrid system were obvious especially with respect to the anatomy of the pulmonary veins. Model creation by impedance based catheters are less accurate in areas of higher impedance like pulmonary veins, SVC, IVC or CS OS and pericardial space due to distortion of anatomical data as a result of stretching of geo-points (Fig. 8). These short-comings are taken care by magnet SE catheters. The new ‘Hybrid Magneto–Impedance’ based EA mapping utilises the strength of both impedance and magnetic technology to localise points and create an accurate anatomical map [[2], [3], [4]].
Fig. 8.
Shows distortion of anatomy demonstrated by stretching of geo points in impedance-based mapping as opposed to accurate anatomic delineation in hybrid technology.
Impedance system has the advantage of being open platform– that is they allow choice of multiple catheters from different manufacturers thus providing better flexibility to the operator. In contrast, the ‘Magnet SE’ catheters give us the benefit of supreme navigation accuracy of less than 1mm with good spatial resolution. Although SE catheters have the disadvantage of being time locked, these catheters after 18 hours can be used for impedance mapping. One of the major drawback is that they significantly increase the cost of the procedure by around 25% [1,2].
Abbott Precision™ is a much more improved version of its predecessor EnSite NavX Velocity™. It has a big advantage of being a hybrid magneto impedance based 3 D mapping system. It brings to the table, the strength of both the technologies at the same time taking care of their short comings. Other than these major hardware improvements, EnSite Precision™ gives the operator an enhanced mapping experience. EnSite™ AutoMap®, inherent in the EnSite Precision™ mapping system, is one of the intelligent automation tools available at operators disposal. AutoMap® helps acquiring points in areas of interest and automatically rejects points outside clinical morphology including catheter induced ectopy. Certain other AutoMap® programmable features like continuous mapping filters help to acquire geometry points swiftly with supreme accuracies. Catheter stability filter which help to map points only when catheter position is stable, the density filter which minimises acquisition points when catheter is stagnant and the cycle length filter which help to acquire points with a consistent cycle length are few of the many AutoMap® features which make experience with Precision™ phenomenal. Another major software upgradation in Precision™ mapping system is the AutoMark® feature which accurately and automatically highlights the area of lesion ablation and colour codes it to pre user-defined ablation catheter indices like force time integral or lesion index values. Abbott AutoMap® and AutoMark® are the two stand out software updates in Precision™ mapping system which redefines operator accuracy especially during complex arrhythmia mapping. Of note, integration with delayed enhanced MRI imaging makes it possible to visualise scar anatomy more accurately. Zero-fluoroscopic ablations of even left atrial arrhythmias have taken preponderance owing to the increased risks of radiation exposure amongst cardiac electrophysiologists. Keeping in mind the improved geometry with use of SE catheter, the use of which may further facilitate a zero-fluoroscopic approach for arrhythmia ablation [3,4].
To the best of our knowledge, there is no literature comparing the efficacy of the commonly used electro-anatomical mapping systems. In this hindsight, a technology that makes use of both magnetic and impedance mapping for electro-anatomical mapping is welcome. Also, we have reported the first Indian experience of use of these catheters and its utility in clinical practice.
4. Conclusion
Abbott EnSite Precision™ 3-D EAM, which incorporates hybrid ‘magneto-impedance’ based parameters for model creation can aid in better delineation of geometry, which is very crucial in complex radio frequency ablation as demonstrated in our case. Having these newer technologies may definitely give the electrophysiologist an added advantage of better model creation and substrate mapping, however, their added cost especially in Indian settings must be justified.
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society.
References
- 1.Skála T., Táborský M. Electromechanical mapping in electrophysiology and beyond. Cor Vasa. 2015 Dec 1;57(6):470–482. [Google Scholar]
- 2.Bhakta D., Miller J.M. Principles of electroanatomic mapping. Indian Pacing Electrophysiol J. 2008 Jan;8(1):32–50. [PMC free article] [PubMed] [Google Scholar]
- 3.Ptaszek L.M., Moon B., Rozen G., Mahapatra S., Mansour M. Novel automated point collection software facilitates rapid, high-density electroanatomic mapping with multiple catheter types. J Cardiovasc Electrophysiol. 2018;29(1):186–195. doi: 10.1111/jce.13368. [DOI] [PubMed] [Google Scholar]
- 4.Heist E.K., Danik S., Chalhoub F., Koci F., Barrett C., Perna F. Human and animal feasibility study of investigational 3D geometry acquisition software. Heart Rhythm. 2011;8 [Google Scholar]