Abstract
Recurrent miscarriage (RM) affects millions of couples globally, and half of them have no demonstrated etiology. Genome sequencing (GS) is an enhanced and novel cytogenetic tool to define the contribution of chromosomal abnormalities in human diseases. In this study we evaluated its utility in RM-affected couples. We performed low-pass GS retrospectively for 1,090 RM-affected couples, all of whom had routine chromosome analysis. A customized sequencing and interpretation pipeline was developed to identify chromosomal rearrangements and deletions/duplications with confirmation by fluorescence in situ hybridization, chromosomal microarray analysis, and PCR studies. Low-pass GS yielded results in 1,077 of 1,090 couples (98.8%) and detected 127 chromosomal abnormalities in 11.7% (126/1,077) of couples; both members of one couple were identified with inversions. Of the 126 couples, 39.7% (50/126) had received former diagnostic results by karyotyping characteristic of normal human male or female karyotypes. Low-pass GS revealed additional chromosomal abnormalities in 50 (4.0%) couples, including eight with balanced translocations and 42 inversions. Follow-up studies of these couples showed a higher miscarriage/fetal-anomaly rate of 5/10 (50%) compared to 21/93 (22.6%) in couples with normal GS, resulting in a relative risk of 2.2 (95% confidence interval, 1.1 to 4.6). In these couples, this protocol significantly increased the diagnostic yield of chromosomal abnormalities per couple (11.7%) in comparison to chromosome analysis (8.0%, chi-square test p = 0.000751). In summary, low-pass GS identified underlying chromosomal aberrations in 1 in 9 RM-affected couples, enabling identification of a subgroup of couples with increased risk of subsequent miscarriage who would benefit from a personalized intervention.
Keywords: recurrent miscarriage, chromosomal abnormality, cryptic structural rearrangements, low-pass genome sequencing, balanced translocation, inversion, copy number variants, preimplantation genetic testing, genetics complexity, chromothripsis and chomoplexy
Introduction
Recurrent miscarriage (RM) is defined by loss of two or more clinical pregnancies and affects 1%–2% of couples.1 RM is an important global health issue and carries an underappreciated psychological and financial burden for affected couples. Anatomic factors, antiphospholipid syndrome,1, 2 and endocrinological and chromosomal abnormalities2 are the most commonly recognized etiologies for RM. However, the etiology of RM in 40%–60% of couples remains idiopathic,1 resulting in costly testing and remaining a challenge to both counseling and treatment.
Chromosomal abnormalities are the major recognized genetic causes for any miscarriage, accounting for up to 60% of cases;3 a chromosome abnormality can be found in lymphocyte metaphases in approximately 2%–4% (1 in 50) of couples with RM by routine chromosome analysis,4, 5 which is significantly higher than that reported in the general population (∼0.3%).6 Couples in whom one partner has a balanced translocation or inversion may have an overall miscarriage rate as high as 49%7 resulting from unbalanced gametes. This depends on the specific chromosomes involved, the type and size of the rearrangements, and sex of the carrier.8 However, current recommendations for management from various professional societies differ largely (Table 1). Emerging studies have demonstrated that genome sequencing (GS)6, 9, 10 can be used to delineate breakpoints of balanced translocations/inversions and detect additional CNVs compared to chromosomal microarray analysis (CMA).6, 11, 12 We undertook investigation of the role of low-pass GS in deciphering the contribution of chromosome abnormalities in RM in a large cohort of 1,090 RM-affected couples.
Table 1.
Genetic Study Recommendations in Different Societies and Communities for Recurrent Miscarriage
| Professional Societies | Parental Karyotyping | Study on Products of Conception (POCs) |
|---|---|---|
| The American Society of Reproductive Medicine (ASRM)/the American College of Obstetricians & Gynecologists (ACOG) | recommended | not recommended |
| The European Society of Human Reproduction (ESHRE) | not routinely recommended | not routinely recommended |
| The National Institute for Health and Care Excellence (NICE)/the Royal College of Obstetricians & Gynaecologists (RCOG) | recommended only when testing of POC reports an unbalanced structural chromosomal abnormality | only recommended in the third and subsequent consecutive miscarriages |
Subjects and Methods
Subject Recruitment and Demographics
This study was approved by the Institutional Review Boards of Shandong University and The Chinese University of Hong Kong. RM-affected couples with two or more consecutive clinical pregnancy losses2, 13 were enrolled from RM clinics at these two universities. Couples diagnosed with an established cause (see Supplemental Subjects and Methods)4 other than chromosomal abnormalities2 were excluded. During the period from 2004 to 2015, a total of 1,090 consecutive idiopathic RM-affected couples were recruited with informed consent; demographic data are provided in Table S1.
Sample Collection and Preparation
At enrollment, a 5 mL peripheral blood sample was collected into a heparinized tube from both partners for diagnostic chromosome analysis. In addition, a 1 mL peripheral blood sample was collected into an EDTA tube for the collection of buffy coat, which was separated and stored at −80°C for future DNA extraction and genetic analysis. Beginning in 2013 when low-pass GS was developed,14, 15 genomic DNA extraction was performed with a DNeasy Blood & Tissue Kit 250 (cat No./ID:69506, QIAGEN) and quality assessed by Qubit dsDNA HS Assay Kit (Invitrogen, Life Technologies) and gel electrophoresis.
Chromosome Analysis
Chromosome analysis was performed at both institutions following standard methods for preparing G-banded metaphases from cultured peripheral blood-derived lymphocytes. Results were reported at a resolution of 400–550 bands (Supplemental Subjects and Methods).
Low-Pass Genome Sequencing
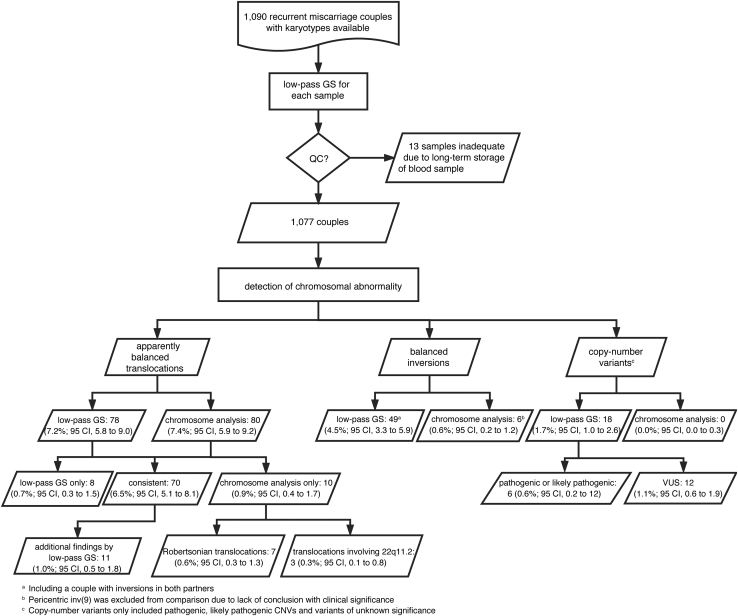
Low-pass GS was performed in batches of 96 samples and according to the workflow outlined in Figure 1 (Supplemental Subjects and Methods and Figures S1–S3). Analysis for each sample was performed without knowledge of any previous cytogenetic results.
Figure 1.
Flowchart of Low-Pass GS in Detection of Chromosomal Abnormalities in 1,090 Recurrent Miscarriage-Affected Couples
Detailed methods and results are described in the main text and the diagnostic rate of chromosomal abnormalities was calculated based on the number of couples.
Library Construction and Sequencing
For each sample, 1.5 μg genomic DNA was sheared with the HydroShear device (GeneMachines, Digilab) into fragments ranging from 3 to 8 kb.14 A modified mate-pair library construction approach was applied with ∼140 million paired-end read-pairs (∼26-bp) generated from a DNA nanoarray-sequencing platform16 (Complete Genomics; Supplemental Subjects and Methods and Figure S2).
Data Analysis, Interpretation, and Validation
Paired-end reads were aligned to the NCBI human reference genome (hg19).17 For each sample passing data QC (genome-wide standard deviation of copy-ratios < 0.15, Figure S4),15 detection of chromosomal rearrangement and CNVs was performed in parallel14, 15 (Supplemental Subjects and Methods and Figure S5) and reported integrally. Chromosomal translocations and inversions (100-kb cutoff14) were then validated with polymerase chain reaction (PCR) and Sanger sequencing (Figures 2 and S6).9, 14 Fluorescence in situ hybridization (FISH) was performed with resubmitted fresh samples. Structural variants were compared to the Database of Genomic Variants, and genes (RefSeq) disrupted at rearrangement breakpoints were curated using Human Phenotype Ontology and Online Mendelian Inheritance in Man (OMIM).
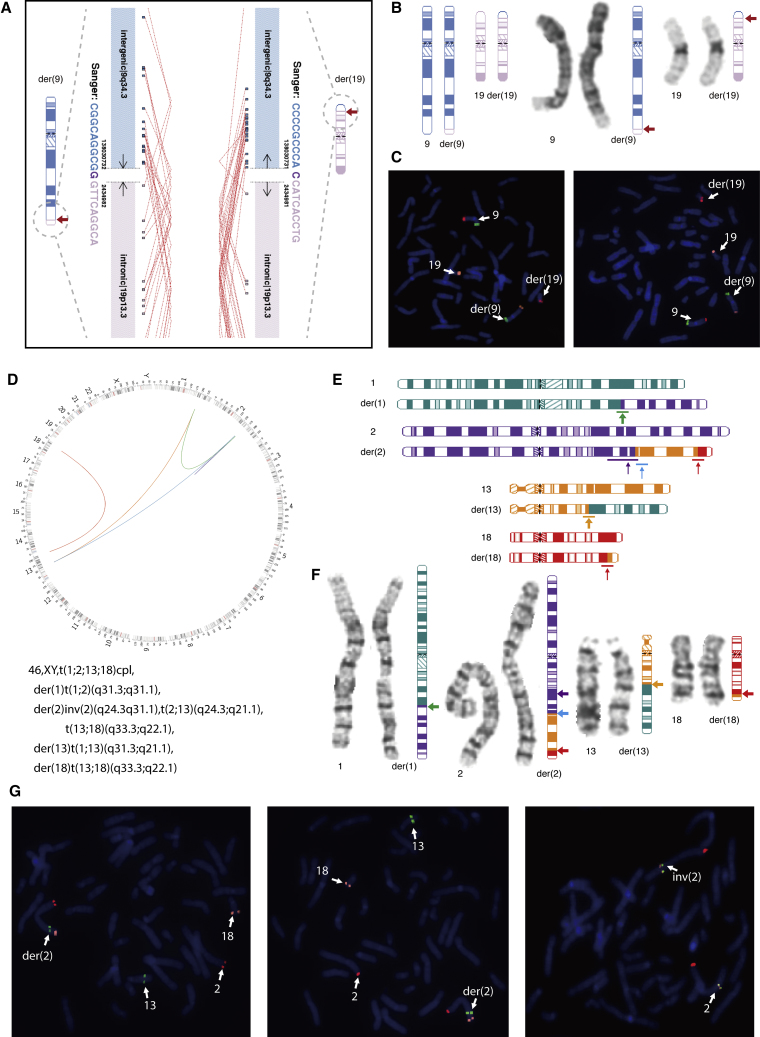
Figure 2.
Cryptic Balanced Translocations Detected by Low-Pass Genome Sequencing (GS).
(A) Low-pass GS revealed a balanced translocation 46,XX,t(9;19)(q34.3;p13.3) in subject SD-RSA_11189. Genomic coordinates of breakpoints are indicated next to each derivative chromosome, while the genomic orientation of each chromosomal segment is shown with black arrows. Genic and intergenic regions (RefSeq) of the breakpoints are labeled in each derivative chromosome, and corresponding ideograms for the der(9) and der(19) are depicted. Sequence pairs were independently aligned to the human genome and each chimeric pair of reads is indicated with a red dotted line. Two independent sets of chimeric read pairs support the composition of the two derivative chromosomes. Sanger sequencing results are shown in the same color as the corresponding chromosome, while micro-homology is shown in dark purple in each derivative chromosome.
(B) Ideograms of the balanced translocation are shown on the left (note the banding and size similarities between normal and derivative chromosomes), while the G-banded chromosome is to the right with the corresponding ideogram of the derivative chromosomes for reference. Breakpoint regions are indicated with red arrows.
(C) FISH validation of the translocation is shown. Derivative chromosomes and normal chromosomes are labeled and indicated by arrows. BAC probes RP11-31F19 at 9p24.3 (green), RP11-100C15 at 9q34.3 (red), and RP11-75H6 at 19p13.3 (orange) were used.
(D) Low-pass GS revealed rearrangements involving four different chromosomes in sample SD-RSA_20749. Circos diagram shows complex rearrangements involving chromosomes 1, 2, 13, and 18. Next-generation cytogenetic nomenclature is shown below. Green line indicates t(1;2)(q31.3;q24.3) in der(1), while orange line shows t(1;13)(q31.3;q21.1) in der(13). In der(2), inv(2)(q24.3q31.1) is shown by a purple line, while t(2;13)(q24.3;q21.1) is indicated by a blue line. The red line denotes the balanced translocation t(13;18)(q33.3;q22.1) in both der(2) and der(18).
(E) Ideograms of the chromosomal rearrangements. For der(2), the inv(2)(q24.3q31.1) is shown by a purple arrow, and translocations between chromosomes 2 and 13 and between chromosomes 13 and 18 are shown with arrows in blue and red, respectively. The colored arrows correspond to the chromosome of origin in (D).
(F) High-resolution chromosome analysis shows a three-way chromosome translocation, t(1;2;13)(q31;q24;q21), but cannot readily identify the inv(2)(q24.3q31.1) and translocation between chromosomes 13 and 18. The corresponding ideograms of the exact composition of each derivative chromosome are shown with arrows indicating breakpoint regions. The color of the arrows represents the chromosome of origin shown in (D) and in (E).
(G) FISH confirms the additional rearrangements in der(2) identified by low-pass GS. In the left and middle images, BAC probes RP11-664N22 at 2p25.3 (red), RP11-43P9 at 13q22.2 (green), and RP11-7H17 at 18q23 (orange) were used to validate rearrangements of chromosomes 2, 13, and 18. In the right image, BAC probes RP11-664N22 at 2p25.3 (red), RP11-11N16 at 2q24.3 (orange), and RP11-608P21 at 2q31.1 (green) were used to validate the inv(2)(q24.3q31.1).
Recombination rate based on deCODE, Genethon, or Marshfield maps (UCSC Genome Browsers), a dataset of the recombination rates for each chromosome with 1-Mb bin size, was used for inversion (>1-Mb) interpretation with cutoffs of recombination hotspot bin/region in males and females reported18 (Supplemental Subjects and Methods).
Classification of CNVs followed guidelines from the American College of Medical Genetics and Genomics (ACMG, Supplemental Subjects and Methods)12, 15, 19, 20 and pathogenic, likely pathogenic CNVs, or variants of uncertain significance (VUS) were validated (Figure S7) using a customized 8x60K Fetal DNA Chip according to the manufacturers’ protocols with analysis using CytoGenomics (Agilent Technologies).15
Results
We recruited 1,090 couples with RM, representing a total number of 2,180 subjects. GS yielded results in all but 13 samples (2,167/2,180 subjects, 99.4%). The 13 failed samples (representing 13 couples) were collected between 2004 and 2006, and the quality of their DNA was insufficient for data analysis due to degradation presumed from long-term storage (Figure S4). All of these 13 couples had normal karyotyping results. Among the remaining 1,077 couples (98.8%), 127 balanced structural chromosomal abnormalities were detected in 126 couples (11.7%), including 78 subjects with balanced translocations and 48 with inversions (Table 2 and Figure 1). All these 127 events (Tables 3 and S2–S5) were selected for validation by PCR and Sanger sequencing (Figure S6), and all results were confirmed. In addition, FISH conducted in three case subjects with resubmitted peripheral bloods and all were confirmed (Figures 2C, 2G, and S8C). The rate of 11.7% by GS represents a 16.7-fold increase compared to the rate defined by GS in participants in the 1000 Genomes Project (0.7%, ∼1 in 146),21 who were not known to have chromosomal rearrangements and presumed to represent a normal control population.
Table 2.
Comparison of Detection Yields in 1,077 RM-Affected Couples between Low-Pass GS and G-Banded Chromosome Analysis
| Chromosomal Abnormalities# | G-Banded Chromosome Analysisa | Low-Pass GSa | Additional Diagnosis in Couples by Low-Pass GSa | |
|---|---|---|---|---|
| Apparently balanced translocation | 80b (7.4%, 95 CI, 5.9 to 9.2) | 78 (7.2%, 95 CI, 5.8 to 9.0) | 8 (0.7%, 95 CI, 0.3 to 1.5) | |
| Inversions | 6 (0.6%, 95 CI, 0.2 to 1.2) | 48 (4.5%, 95 C.I., 3.4 to 5.9) | 42 (3.9%, 95 CI, 2.9 to 5.2) | |
| CNVs |
Pathogenic or likely pathogenic CNVs | 0 (0%, 95 CI, 0 to 0.3) | 6 (0.6%, 95 CI, 0.2 to 1.2) | 6 (0.6%, 95 CI, 0.2 to 1.2) |
| VUS | 0 (0%, 95 CI, 0 to 0.3) | 12 (1.1%, 95 CI, 0.6 to 1.9) | 12 (1.1%, 95 CI, 0.6 to 1.9) | |
| Overall chromosomal structural variantsc | 86b (8%, 95 CI, 6.4 to 9.8) | 126 (11.7%, 95 CI, 9.9 to 13.8) | 50 (4.6%, 95 CI, 3.5 to 6.1) | |
Calculation was performance based on couples
Including ten samples with balanced translocations that were not detected by low-pass GS (Table S2)
Only translocations and inversions were calculated
Table 3.
Eight Translocations Only Reported by Low-Pass GS
| Sample IDa | Karyotype | Low-Pass GS |
Length of Translocated Segments |
Suspected Reasons of Missed Detection by Chromosome Analysis | |
|---|---|---|---|---|---|
| ChrA (Mb) | ChrB (Mb) | ||||
| SD-RSA_10835Y | 46,XX | 46,XX,t(12;21)(p11.21;q21.1) | 31.6 | 30.2 | similar banding patterns |
| SD-RSA_10949 | 46,XX | 46,XX,t(2;17)(q37.3;p11.2) | 3.2 | 16.8 | involving G light band |
| SD-RSA_10970N | 46,XX | 46,XX,t(7;17)(p22.2;p13.1) | 4.3 | 8.5 | cryptic translocation |
| SD-RSA_11189Y | 46,XX | 46,XX,t(9;19)(q34.3;p13.3) | 2.4 | 3.1 | cryptic translocation |
| SD-RSA_11359Y | 46,XX | 46,XX,t(5;14)(p13.2;q24.2) | 38.1 | 35.7 | similar banding patterns |
| SD-RSA_20504 | 46,XY | 46,XY,t(20;21)(p12.1;q22.11) | 13.5 | 13.7 | similar banding patterns |
| SD-RSA_21394 | 46,XY | 46,XY,t(2;21),der(2)t(2;21)(p24.3;q21.3),der(21)t(2;21)(p24.3;q21.3), inv(21)(q21.1q21.3) | 13.2 | 20.9 | involving G light band |
| SD-RSA_22134N | 46,XY | 46,XY,t(1;19)(p36.22;q13.2) | 16.1 | 12.6 | similar banding patterns |
Y (Yes) or N (No) refer to couple with miscarriage(s) or fetal structural abnormalities identified in subsequent pregnancy following cytogenetic testing with normal reports
Balanced Translocations
Among 78 subjects (78 couples; 7.2%) with balanced translocations identified by GS, 70 rearrangements were also identified by the original karyotyping, while eight were detected by GS alone (Tables 2 and 3). However, GS failed to detect ten translocations (0.9%) including seven Robertsonian translocations and three translocations with one breakpoint located in 22q11.2 (Table S2).
Among the eight translocations additionally identified by GS, two translocations (Figures 2A–2C and S8) involved a reciprocal exchange between segments below the typical level of resolution limits of karyotyping (e.g., the translocated segments in female subject SD-RSA_11189 were only 2.4 Mb and 3.1 Mb in size; Figure 2A). Contributing to the absence of detection of the remaining six case subjects by the original chromosome analysis was the similar size and banding pattern of the reciprocally exchanged segments, such as in SD-RSA_11359 (Figure S9D).
Although 70 balanced translocations were reported by previous cytogenetic analysis, low-pass GS showed its increased precision in revision of the breakpoints by at least one sub-band in 42 case subjects (60.0%) (Tables S2 and S3) and in the discovery of additional findings of complex chromosomal rearrangements in 11 case subjects (15.7%, Table S4). For example, in subject SD-RSA_20749 (Figures 2D–2G), chromosome analysis indicated an apparently balanced three-way translocation involving chromosomes 1, 2, and 13; low-pass GS revealed a complex rearrangement involving four chromosomes, in which the derivative chromosome 2 harbored an inversion at the breakpoint of the t(2;13) and a translocated segment containing material from both chromosomes 13 and 18. Further, GS identified chromothripsis22 or chromoplexy-like events23 in four subjects (Table S4 and Figure S10).
Inversions
GS also elucidated 49 inversions of size ranging from 114.7 kb to 94.5 Mb (Tables 2 and S5 and Figure S6), and in one couple, both partners had the same inv(21) (Supplemental Subjects and Methods and Figures S11 and S12). Overall, GS provided an incidence of inversions of 4.5% per couple (48/1,077) or 2.3% per individual (49/2,154), which is significantly higher than that reported in the 1000 Genomes Project21 (4/1,166 or 0.3%; chi-square testing p < 0.0001).
Among the 49 inversions (in 48 couples), 15 were found to have at least one breakpoint in a recombination hot-spot bin/region (Supplemental Subjects and Methods and Table S5),18 supporting an increased risk for producing duplication-deletion offspring if a cross-over were to occur in the inversion loop during gametogenesis. In addition to these 15 inversions, 4 inversions were found to disrupt an OMIM disease-causing gene (Table S5). Of note, only six inversions were detected by metaphase chromosome analysis with a size ranging from 14.3 to 94.6 Mb (Figures S13A and S13B).
Copy Number Variants
In total, 2,124 copy-number losses and 4,623 gains were reported by low-pass GS, indicative of 2.0 deletions and 4.2 duplications per couple (Figure S14). Based on ACMG guidelines, we further defined 6 pathogenic or likely pathogenic CNVs and 12 VUS (Tables 2 and S6) ranging from 85.9 kb to 8.1 Mb. Two were located in chromosome X and 16 were in autosomes. All these 18 CNVs, classified as pathogenic, likely pathogenic, or VUS (Tables S6), were selected for validation with CMA, and all were confirmed (Figure S7). A recombination event of the aberrant chromosome segregated to the embryo/fetus would be the mechanism underlining pathogenicity. A female carrier (SD-RSA_10358) was identified with an 8.1-Mb deletion in Xq22.3q23 associated with X-linked Alport syndrome (MIM: 301050), resulting in increased morbidity and mortality in a male fetus. Of note, none was detectable by the original cytogenetic analysis. The extent of the contribution of the detected CNVs to RM remains to be established but our data provide further evidence of the increased detection and precision of GS. In summary, low-pass GS reported a total number of 145 genomic variants, including 78 translocations, 49 inversions (Tables 2 and S2–S5), and 18 CNV classified as pathogenic, likely pathogenic, or VUS (Table S6) in this study, and all were validated.
Secondary Follow-up and Outcome
Fifty couples had additional diagnoses by low-pass GS but with normal karyotypes (8 couples with balanced translocations and 42 with inversions). Ten of these couples subsequently had a spontaneous conception, and five of these couples (50.0%) reported miscarriage or fetal structural anomalies (Tables 3 and S5). The frequency was even more remarkable when compared with 93 RM-affected couples who had both normal GS and karyotype in our study cohort with follow-up pregnancy; among them only 21 (22.6%) couples experienced the same poor outcomes, thus resulting in an odds ratio (OR) of 3.4 (95% confidence interval: 1.1 to 4.6).
Among the 126 couples with an abnormal chromosomal diagnosis by GS, 26 “carrier” couples (maternal age: 28.5 ± 4.4) sought in vitro fertilization (IVF) and preimplantation genetic testing (for aneuploidy [PGT-A] and structural rearrangement [PGT-SR]; Supplemental Subjects and Methods24) after reproductive counseling. In comparison, IVF and PGT-A were also pursued by 68 RM-affected couples with normal GS and karyotype results (68/975; maternal age: 32.1 ± 4.7). Livebirth and miscarriage rates of the first cycle pregnancy were calculated for the two groups. Among the carrier group, 19 clinical pregnancies (18/26, 69.2%) were achieved resulting in 1 miscarriage (1/18, 5.6%) and 18 livebirths (17/26, 65.4%, Table S7). In contrast, in the non-carrier group, 52 clinical pregnancies (52/68, 76.5%) were observed with an outcome of 17 miscarriages (17/52, 32.7%) and 35 livebirths (35/68, 51.5%). This could suggest that intervention by means of IVF and PGT in couples with a chromosomal diagnosis by GS can result in a significantly lower miscarriage rate (OR: 0.1, 95% CI, 0.0 to 0.9, absolute risk reduction 27.2%, Fisher exact test p = 0.0283) in their subsequent first IVF cycle.
Discussion
In this study, low-pass GS enables identification of RM-related chromosomal abnormalities at higher diagnostic yield and resolution independent of routine chromosome analysis. Low-pass GS had a diagnostic yield of 11.7% (126/1,077) in couples with RM. Previous large-scale studies on RM-affected couples found balanced chromosomal abnormalities (inversions and reciprocal and Robertsonian translocations) in 4.4% of 11,708 couples with at least two miscarriages25 and balanced translocations in 3.8% of 10,216 couples.26 In comparison to chromosome analysis, low-pass GS significantly increased the diagnostic yield of chromosomal abnormalities (chi-square test p = 0.000751) and established a diagnosis in an additional 50 (4.6%) couples with prior normal chromosome analyses.
Balanced translocations are the most common genetic abnormality identified among RM-affected couples (61.9%, 78/126), confirming previous findings by karyotype.27, 28 Among these translocations, affected chromosomes and the sizes of exchanged segments varied (Tables 3, S3, and S4). We were able to define and discover those cryptic chromosomal rearrangements by low-pass GS due to its agnostic advantage to size and banding pattern of chromosomal segments. In addition, among the 70 cases detected by both low-pass GS and karyotyping, revision of rearranged chromosome bands was made in 60.0% (42/70) of subjects by at least a sub-band with implications for interpretation of the underlying molecular mechanism of the RM. Most importantly, in 15.7% (11/70) of cases, additional findings identified by low-pass GS revealed more complex rearrangements than evident from chromosome analysis alone. Breakpoints and chromosomes involved are unique for each couple, thus eliminating empirical evidence of the segregation patterns in gametes or pregnancies,8 compared to the known segregation patterns for the Robertsonian translocations involving acrocentric chromosomes (i.e., 13, 14, 15, 21, and 22).29 Specifically, it has been observed that offspring of a parent with such a complex rearrangement may inherit either a subset or the full set of the chromothripsis-like events from the presumably healthy parent but acquired de novo rearrangements leading to copy-number changes resulting in miscarriages, or severe congenital disorders.30 Therefore, refined information of the chromosomal abnormalities by GS provides carrier couples with individualized estimated miscarriage risk and actionable plan with respect to future pregnancies.30
Inversions were the second most common type of chromosomal abnormality (38.1%, 48/126). Only six of these inversion cases were reported by routine karyotyping (Figures 1 and S13) due to the limited resolution and similar banding patterns. Five consecutive miscarriages were reported in the non-consanguineous couple in which both partners carried the same 1.2-Mb inv(21)(q22.11q22.12) located within a recombination hotspot region,18 supporting that the inv(21) could be associated with RM and worthy of further study.
Low-pass GS revealed a failure to detect 10 (0.9%) translocations with one breakpoint in an extensive length of repetitive sequence (Table S2), recognized for Robertsonian translocations31 and the recurrent t(11;22)(q23;q11.2),32 which has been known to be the limitation by current molecular technologies.14 With our current protocol, low-pass GS may be viewed most appropriately as a complementary tool to conventional karyotyping. Employing both methods, the incidence of balanced translocations and inversions was further increased to 12.6% (136/1,077), revealing the underlying chromosomal aberrations in 1 in 8 couples with RM.
Modifications to the protocol were employed to increase the DNA fragment size to 40 kb, and the results show that further optimization of low-pass GS can improve detection of such translocations mediated by low-copy repeats (Figure S15). For the Robertsonian translocations, although the breakpoint junction regions have been mapped to p11.2 in the acrocentric chromosomes, there is still more than 100 kb of sequence located in the junction region without complete delineation due to the presence of repetitive elements or satellite DNAs.33 With development of third-generation sequencing methods to provide access to longer DNA sequences (i.e., 100 kb), further modifications would be possible to optimize low-pass GS for their detection. Nonetheless, with the current protocol, an alternative approach is to count copy numbers of rDNA clusters (Figures S16), so as to overcome the limitation of their direct detection by the current protocol. In addition, further improvement of the current protocol, such as increasing read-depth for comprehensive detection of genetic and genomic abnormalities34, 35, 36, 37 including single-nucleotide variants, is warranted in the near future. Nonetheless, with a comparable cost, similar turn-around-time, and using a standardized and reproducible protocol as presented herein (Table S8), it is clear that low-pass GS can enable greater detection and provide higher precision than a combination of chromosomal analysis and CMA.
Recurrent miscarriage represents a management challenge because of the uncertainty of the outcome of subsequent pregnancies. Described general interventions such as the use of aspirin or heparin38 and progesterone39 failed to improve live births in clinical trials. Identifying RM-affected couples with a chromosomal structural rearrangement, particularly for the rearrangements cryptic to karyotype analysis, has direct clinical relevance for genetic counseling and individualized treatment interventions. IVF and PGT have made possible a significant reduction of miscarriage or birth with untoward outcomes for such RM-affected couples.
IVF with PGT-A is a controversial intervention offered to RM-affected couples around the world, and a survey of 386 clinics in 70 countries found that RM is the indication in 31% of PGT cycles.40 However, the largest study of PGT to date, analyzing 46,439 day-3 and day-5 embryos,28 found no increased number of aneuploidies when the testing indication was only RM. This supports the rationale that RM couples with normal karyotypes will not receive any benefit from PGT-A unless in the setting of advanced maternal age.41 In addition, the study also demonstrated that carrier couples of translocations, apart from segregation of unbalanced gametes,42, 43 have an increased risk of having an embryo with non-mosaic aneuploidy due to meiotic (OR = 2.2, 95 CI, 1.8 to 3.4 in day 3 and OR = 1.4, 95 CI, 1.1 to 2.4 in day 5) but not mitotic errors.28 However, studies show that for embryos with mosaic aneuploidies, a self-correction mechanism such as trisomy rescue might exist, resulting in a livebirth.44, 45 Such a mechanism is not applicable to embryos with non-mosaic aneuploidy25, 46 or with unbalanced translocations, which can lead to impaired trophoblastic differentiation causing implantation failure or miscarriage.47 Therefore, detection of parental chromosomal abnormalities is an invaluable tool of diagnostic importance in the management of RM-affected couples.
It has been suggested that IVF and PGT-A/SR would improve the pregnancy outcome for the carrier couples by significantly reducing the miscarriage rate.42, 43 This possibility is further confirmed by our secondary preliminary analysis: 26 couples with diagnosed chromosomal abnormalities who pursued pregnancy by PGT achieved a low miscarriage rate (5.0%) with an absolute reduction of 27.2% by PGT compared to 68 couples with normal GS and PGT-A. Although increased maternal age in the non-carrier group was observed, we noted the reduction in RM after intervention with IVF and PGT was greater in “carrier” couples compared to “non-carrier” couples. The effect of increased maternal age in increased rate of aneuploidies was minimized by excluding embryos with aneuploidies through IVF and PGT-A. Because the sample size in this aspect of our study is limited, a larger follow-up sample size in our future investigations is warranted to substantiate the observation. Furthermore, future clinical trials should assess the primary goal of decreasing miscarriage rate and comparing the usage of PGT-A/SR in these couples with chromosomal rearrangements as defined by GS.
Overall, our study supports that GS has an increased resolution and detection rate. Comparing karyotype as the gold standard versus GS, GS has a sensitivity of 90.57% (95% CI, 83.33–95.38), specificity of 100% (95% CI, 99.82–100.00), with positive predictive value (PPV) of 100%, negative predictive value (NPV) of 99.52% and 99.54% accuracy. The increased diagnostic yield of GS with demonstrably improved management in RM-affected couples challenges the current concerns raised by various medical societies (Table 1) and provides the basis of a rational recommendation for couples and clinicians. Given the increased diagnostic yield, enhanced precision and comprehensive identification of chromosomal abnormalities, our results call for the reconsideration of the relevance of chromosomal rearrangements in the pathogenesis of RM and support a paradigm shift for applying low-pass GS in routine clinical use to expand the scope of genetic diagnosis for chromosomal rearrangements in RM-affected couples.
Declaration of Interests
F.X., J. Yuan, L.Y., J.X., L.C., Q.L., X.Z., J.L., A.C., W.Z., T.M., W.-J.W., Y.J., K.K., H.Y., and H.J. are current employees of BGI-Shenzhen. Y.J. is also a current employee of Complete Genomics and has stock in this company. The other authors declare no conflict of interest.
Acknowledgments
We especially thank Terence T. Lao, Fernando Scaglia, and Feng Zhang for their contributions during manuscript preparation. This project is supported by the National Natural Science Foundation of China (81490743, 81370715, and 81300075), the National Key Research and Development Program of China (2016YFC1000202), the Health and Medical Research Fund (04152666), the General Research Fund (14115418), and the National Institute of General Medical Sciences (GM061354). C.C.M. is supported by the NIH/NIGMS (P01 GM061354) and the NIHR Manchester Biomedical Research Centre.
Published: October 31, 2019
Footnotes
Supplemental Data can be found online at https://doi.org/10.1016/j.ajhg.2019.10.003.
Contributor Information
Kwong Wai Choy, Email: richardchoy@cuhk.edu.hk.
Zi-Jiang Chen, Email: chenzijiang@hotmail.com.
Web Resources
Database of Genomic Variants, http://dgv.tcag.ca/dgv/app/home
Human Phenotype Ontology, http://human-phenotype-ontology.github.io/
Online Mendelian Inheritance in Man (OMIM), https://www.omim.org
Supplemental Data
References
- 1.Ford H.B., Schust D.J. Recurrent pregnancy loss: etiology, diagnosis, and therapy. Rev. Obstet. Gynecol. 2009;2:76–83. [PMC free article] [PubMed] [Google Scholar]
- 2.Practice Committee of the American Society for Reproductive Medicine Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Fertil. Steril. 2012;98:1103–1111. doi: 10.1016/j.fertnstert.2012.06.048. [DOI] [PubMed] [Google Scholar]
- 3.Levy B., Sigurjonsson S., Pettersen B., Maisenbacher M.K., Hall M.P., Demko Z., Lathi R.B., Tao R., Aggarwal V., Rabinowitz M. Genomic imbalance in products of conception: single-nucleotide polymorphism chromosomal microarray analysis. Obstet. Gynecol. 2014;124:202–209. doi: 10.1097/AOG.0000000000000325. [DOI] [PubMed] [Google Scholar]
- 4.Popescu F., Jaslow C.R., Kutteh W.H. Recurrent pregnancy loss evaluation combined with 24-chromosome microarray of miscarriage tissue provides a probable or definite cause of pregnancy loss in over 90% of patients. Hum. Reprod. 2018;33:579–587. doi: 10.1093/humrep/dey021. [DOI] [PubMed] [Google Scholar]
- 5.Fryns J.P., Van Buggenhout G. Structural chromosome rearrangements in couples with recurrent fetal wastage. Eur. J. Obstet. Gynecol. Reprod. Biol. 1998;81:171–176. doi: 10.1016/s0301-2115(98)00185-7. [DOI] [PubMed] [Google Scholar]
- 6.Redin C., Brand H., Collins R.L., Kammin T., Mitchell E., Hodge J.C., Hanscom C., Pillalamarri V., Seabra C.M., Abbott M.A. The genomic landscape of balanced cytogenetic abnormalities associated with human congenital anomalies. Nat. Genet. 2017;49:36–45. doi: 10.1038/ng.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franssen M.T., Musters A.M., van der Veen F., Repping S., Leschot N.J., Bossuyt P.M., Goddijn M., Korevaar J.C. Reproductive outcome after PGD in couples with recurrent miscarriage carrying a structural chromosome abnormality: a systematic review. Hum. Reprod. Update. 2011;17:467–475. doi: 10.1093/humupd/dmr011. [DOI] [PubMed] [Google Scholar]
- 8.Morel F., Douet-Guilbert N., Le Bris M.J., Herry A., Amice V., Amice J., De Braekeleer M. Meiotic segregation of translocations during male gametogenesis. Int. J. Androl. 2004;27:200–212. doi: 10.1111/j.1365-2605.2004.00490.x. [DOI] [PubMed] [Google Scholar]
- 9.Talkowski M.E., Ordulu Z., Pillalamarri V., Benson C.B., Blumenthal I., Connolly S., Hanscom C., Hussain N., Pereira S., Picker J. Clinical diagnosis by whole-genome sequencing of a prenatal sample. N. Engl. J. Med. 2012;367:2226–2232. doi: 10.1056/NEJMoa1208594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nilsson D., Pettersson M., Gustavsson P., Förster A., Hofmeister W., Wincent J., Zachariadis V., Anderlid B.M., Nordgren A., Mäkitie O. Whole-genome sequencing of cytogenetically balanced chromosome translocations identifies potentially pathological gene disruptions and highlights the importance of microhomology in the mechanism of formation. Hum. Mutat. 2017;38:180–192. doi: 10.1002/humu.23146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiang C., Jacobsen J.C., Ernst C., Hanscom C., Heilbut A., Blumenthal I., Mills R.E., Kirby A., Lindgren A.M., Rudiger S.R. Complex reorganization and predominant non-homologous repair following chromosomal breakage in karyotypically balanced germline rearrangements and transgenic integration. Nat. Genet. 2012;44 doi: 10.1038/ng.2202. 390–397, S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang H., Dong Z., Zhang R., Chau M.H.K., Yang Z., Tsang K.Y.C., Wong H.K., Gui B., Meng Z., Xiao K. Low-pass genome sequencing versus chromosomal microarray analysis: implementation in prenatal diagnosis. Genet. Med. 2019 doi: 10.1038/s41436-019-0634-7. Published online August 26, 2019. 10.1038/s41436-019-0634-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Practice Committee of American Society for Reproductive Medicine Definitions of infertility and recurrent pregnancy loss: a committee opinion. Fertil. Steril. 2013;99:63. doi: 10.1016/j.fertnstert.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 14.Dong Z., Jiang L., Yang C., Hu H., Wang X., Chen H., Choy K.W., Hu H., Dong Y., Hu B. A robust approach for blind detection of balanced chromosomal rearrangements with whole-genome low-coverage sequencing. Hum. Mutat. 2014;35:625–636. doi: 10.1002/humu.22541. [DOI] [PubMed] [Google Scholar]
- 15.Dong Z., Zhang J., Hu P., Chen H., Xu J., Tian Q., Meng L., Ye Y., Wang J., Zhang M. Low-pass whole-genome sequencing in clinical cytogenetics: a validated approach. Genet. Med. 2016;18:940–948. doi: 10.1038/gim.2015.199. [DOI] [PubMed] [Google Scholar]
- 16.Drmanac R., Sparks A.B., Callow M.J., Halpern A.L., Burns N.L., Kermani B.G., Carnevali P., Nazarenko I., Nilsen G.B., Yeung G. Human genome sequencing using unchained base reads on self-assembling DNA nanoarrays. Science. 2010;327:78–81. doi: 10.1126/science.1181498. [DOI] [PubMed] [Google Scholar]
- 17.Carnevali P., Baccash J., Halpern A.L., Nazarenko I., Nilsen G.B., Pant K.P., Ebert J.C., Brownley A., Morenzoni M., Karpinchyk V. Computational techniques for human genome resequencing using mated gapped reads. J. Comput. Biol. 2012;19:279–292. doi: 10.1089/cmb.2011.0201. [DOI] [PubMed] [Google Scholar]
- 18.Bhatt S.S., Manvelyan M., Moradkhani K., Hunstig F., Mrasek K., Puechberty J., Lefort G., Sarda P., Weise A., Liehr T., Pellestor F. Inverted segment size and the presence of recombination hot spot clusters matter in sperm segregation analysis. Cytogenet. Genome Res. 2014;142:145–149. doi: 10.1159/000356142. [DOI] [PubMed] [Google Scholar]
- 19.Kearney H.M., Thorland E.C., Brown K.K., Quintero-Rivera F., South S.T., Working Group of the American College of Medical Genetics Laboratory Quality Assurance Committee American College of Medical Genetics standards and guidelines for interpretation and reporting of postnatal constitutional copy number variants. Genet. Med. 2011;13:680–685. doi: 10.1097/GIM.0b013e3182217a3a. [DOI] [PubMed] [Google Scholar]
- 20.South S.T., Lee C., Lamb A.N., Higgins A.W., Kearney H.M., Working Group for the American College of Medical Genetics and Genomics Laboratory Quality Assurance Committee ACMG Standards and Guidelines for constitutional cytogenomic microarray analysis, including postnatal and prenatal applications: revision 2013. Genet. Med. 2013;15:901–909. doi: 10.1038/gim.2013.129. [DOI] [PubMed] [Google Scholar]
- 21.Dong Z., Wang H., Chen H., Jiang H., Yuan J., Yang Z., Wang W.J., Xu F., Guo X., Cao Y. Identification of balanced chromosomal rearrangements previously unknown among participants in the 1000 Genomes Project: implications for interpretation of structural variation in genomes and the future of clinical cytogenetics. Genet. Med. 2018;20:697–7079. doi: 10.1038/gim.2017.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu P., Erez A., Nagamani S.C., Dhar S.U., Kołodziejska K.E., Dharmadhikari A.V., Cooper M.L., Wiszniewska J., Zhang F., Withers M.A. Chromosome catastrophes involve replication mechanisms generating complex genomic rearrangements. Cell. 2011;146:889–903. doi: 10.1016/j.cell.2011.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zepeda-Mendoza C.J., Morton C.C. The Iceberg under Water: Unexplored Complexity of Chromoanagenesis in Congenital Disorders. Am. J. Hum. Genet. 2019;104:565–577. doi: 10.1016/j.ajhg.2019.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang L., Wei D., Zhu Y., Jiang W., Xia M., Li J., Yan J., Chen Z.J. Interaction of acrocentric chromosome involved in translocation and sex of the carrier influences the proportion of alternate segregation in autosomal reciprocal translocations. Hum. Reprod. 2019;34:380–387. doi: 10.1093/humrep/dey367. [DOI] [PubMed] [Google Scholar]
- 25.De Braekeleer M., Dao T.N. Cytogenetic studies in couples experiencing repeated pregnancy losses. Hum. Reprod. 1990;5:519–528. doi: 10.1093/oxfordjournals.humrep.a137135. [DOI] [PubMed] [Google Scholar]
- 26.Barber J.C., Cockwell A.E., Grant E., Williams S., Dunn R., Ogilvie C.M. Is karyotyping couples experiencing recurrent miscarriage worth the cost? BJOG. 2010;117:885–888. doi: 10.1111/j.1471-0528.2010.02566.x. [DOI] [PubMed] [Google Scholar]
- 27.Pujol A., Benet J., Staessen C., Van Assche E., Campillo M., Egozcue J., Navarro J. The importance of aneuploidy screening in reciprocal translocation carriers. Reproduction. 2006;131:1025–1035. doi: 10.1530/rep.1.01063. [DOI] [PubMed] [Google Scholar]
- 28.McCoy R.C., Demko Z.P., Ryan A., Banjevic M., Hill M., Sigurjonsson S., Rabinowitz M., Petrov D.A. Evidence of Selection against Complex Mitotic-Origin Aneuploidy during Preimplantation Development. PLoS Genet. 2015;11:e1005601. doi: 10.1371/journal.pgen.1005601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keymolen K., Van Berkel K., Vorsselmans A., Staessen C., Liebaers I. Pregnancy outcome in carriers of Robertsonian translocations. Am. J. Med. Genet. A. 2011;155A:2381–2385. doi: 10.1002/ajmg.a.33941. [DOI] [PubMed] [Google Scholar]
- 30.de Pagter M.S., van Roosmalen M.J., Baas A.F., Renkens I., Duran K.J., van Binsbergen E., Tavakoli-Yaraki M., Hochstenbach R., van der Veken L.T., Cuppen E., Kloosterman W.P. Chromothripsis in healthy individuals affects multiple protein-coding genes and can result in severe congenital abnormalities in offspring. Am. J. Hum. Genet. 2015;96:651–656. doi: 10.1016/j.ajhg.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han J.Y., Choo K.H., Shaffer L.G. Molecular cytogenetic characterization of 17 rob(13q14q) Robertsonian translocations by FISH, narrowing the region containing the breakpoints. Am. J. Hum. Genet. 1994;55:960–967. [PMC free article] [PubMed] [Google Scholar]
- 32.Kurahashi H., Inagaki H., Ohye T., Kogo H., Tsutsumi M., Kato T., Tong M., Emanuel B.S. The constitutional t(11;22): implications for a novel mechanism responsible for gross chromosomal rearrangements. Clin. Genet. 2010;78:299–309. doi: 10.1111/j.1399-0004.2010.01445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jarmuz-Szymczak M., Janiszewska J., Szyfter K., Shaffer L.G. Narrowing the localization of the region breakpoint in most frequent Robertsonian translocations. Chromosome Res. 2014;22:517–532. doi: 10.1007/s10577-014-9439-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ellard S., Kivuva E., Turnpenny P., Stals K., Johnson M., Xie W., Caswell R., Lango Allen H. An exome sequencing strategy to diagnose lethal autosomal recessive disorders. Eur. J. Hum. Genet. 2015;23:401–404. doi: 10.1038/ejhg.2014.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qiao Y., Wen J., Tang F., Martell S., Shomer N., Leung P.C., Stephenson M.D., Rajcan-Separovic E. Whole exome sequencing in recurrent early pregnancy loss. Mol. Hum. Reprod. 2016;22:364–372. doi: 10.1093/molehr/gaw008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petrovski S., Aggarwal V., Giordano J.L., Stosic M., Wou K., Bier L., Spiegel E., Brennan K., Stong N., Jobanputra V. Whole-exome sequencing in the evaluation of fetal structural anomalies: a prospective cohort study. Lancet. 2019;393:758–767. doi: 10.1016/S0140-6736(18)32042-7. [DOI] [PubMed] [Google Scholar]
- 37.Choy K.W., Wang H., Shi M., Chen J., Yang Z., Zhang R., Yan H., Wang Y., Chen S., Chau M.H.K. Prenatal Diagnosis of Fetuses With Increased Nuchal Translucency by Genome Sequencing Analysis. Front. Genet. 2019;10:761. doi: 10.3389/fgene.2019.00761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaandorp S.P., Goddijn M., van der Post J.A., Hutten B.A., Verhoeve H.R., Hamulyák K., Mol B.W., Folkeringa N., Nahuis M., Papatsonis D.N. Aspirin plus heparin or aspirin alone in women with recurrent miscarriage. N. Engl. J. Med. 2010;362:1586–1596. doi: 10.1056/NEJMoa1000641. [DOI] [PubMed] [Google Scholar]
- 39.Coomarasamy A., Williams H., Truchanowicz E., Seed P.T., Small R., Quenby S., Gupta P., Dawood F., Koot Y.E., Bender Atik R. A Randomized Trial of Progesterone in Women with Recurrent Miscarriages. N. Engl. J. Med. 2015;373:2141–2148. doi: 10.1056/NEJMoa1504927. [DOI] [PubMed] [Google Scholar]
- 40.Weissman A., Shoham G., Shoham Z., Fishel S., Leong M., Yaron Y. Preimplantation genetic screening: results of a worldwide web-based survey. Reprod. Biomed. Online. 2017;35:693–700. doi: 10.1016/j.rbmo.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 41.Rubio C., Bellver J., Rodrigo L., Castillón G., Guillén A., Vidal C., Giles J., Ferrando M., Cabanillas S., Remohí J. In vitro fertilization with preimplantation genetic diagnosis for aneuploidies in advanced maternal age: a randomized, controlled study. Fertil. Steril. 2017;107:1122–1129. doi: 10.1016/j.fertnstert.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 42.Cai Y., Ding M., Lin F., Diao Z., Zhang N., Sun H., Zhou J. Evaluation of preimplantation genetic testing based on next-generation sequencing for balanced reciprocal translocation carriers. Reprod. Biomed. Online. 2019;38:669–675. doi: 10.1016/j.rbmo.2018.12.043. [DOI] [PubMed] [Google Scholar]
- 43.Lin S.B., Xie Y.J., Chen Z., Zhou Y., Wu J.Z., Zhang Z.Q., Shi S.S., Chen B.J., Fang Q. Improved assay performance of single nucleotide polymorphism array over conventional karyotyping in analyzing products of conception. J. Chin. Med. Assoc. 2015;78:408–413. doi: 10.1016/j.jcma.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 44.Greco E., Minasi M.G., Fiorentino F. Healthy Babies after Intrauterine Transfer of Mosaic Aneuploid Blastocysts. N. Engl. J. Med. 2015;373:2089–2090. doi: 10.1056/NEJMc1500421. [DOI] [PubMed] [Google Scholar]
- 45.Victor A.R., Tyndall J.C., Brake A.J., Lepkowsky L.T., Murphy A.E., Griffin D.K., McCoy R.C., Barnes F.L., Zouves C.G., Viotti M. One hundred mosaic embryos transferred prospectively in a single clinic: exploring when and why they result in healthy pregnancies. Fertil. Steril. 2019;111:280–293. doi: 10.1016/j.fertnstert.2018.10.019. [DOI] [PubMed] [Google Scholar]
- 46.Grati F.R., Gallazzi G., Branca L., Maggi F., Simoni G., Yaron Y. An evidence-based scoring system for prioritizing mosaic aneuploid embryos following preimplantation genetic screening. Reprod. Biomed. Online. 2018;36:442–449. doi: 10.1016/j.rbmo.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 47.Shpiz A., Kalma Y., Frumkin T., Telias M., Carmon A., Amit A., Ben-Yosef D. Human embryonic stem cells carrying an unbalanced translocation demonstrate impaired differentiation into trophoblasts: an in vitro model of human implantation failure. Mol. Hum. Reprod. 2015;21:271–280. doi: 10.1093/molehr/gau104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.