Abstract
Congenital anomalies of the kidney and urinary tract (CAKUT) are the most common cause of chronic kidney disease in the first three decades of life, and in utero obstruction to urine flow is a frequent cause of secondary upper urinary tract malformations. Here, using whole-exome sequencing, we identified three different biallelic mutations in CHRNA3, which encodes the α3 subunit of the nicotinic acetylcholine receptor, in five affected individuals from three unrelated families with functional lower urinary tract obstruction and secondary CAKUT. Four individuals from two families have additional dysautonomic features, including impaired pupillary light reflexes. Functional studies in vitro demonstrated that the mutant nicotinic acetylcholine receptors were unable to generate current following stimulation with acetylcholine. Moreover, the truncating mutations p.Thr337Asnfs∗81 and p.Ser340∗ led to impaired plasma membrane localization of CHRNA3. Although the importance of acetylcholine signaling in normal bladder function has been recognized, we demonstrate for the first time that mutations in CHRNA3 can cause bladder dysfunction, urinary tract malformations, and dysautonomia. These data point to a pathophysiologic sequence by which monogenic mutations in genes that regulate bladder innervation may secondarily cause CAKUT.
Keywords: genetics, CAKUT, neurogenic bladder, kidney, dysautonomia
Main Text
Congenital anomalies of the kidney and urinary tract (CAKUT) represent up to 20%–30% of all prenatally detected anomalies and are the most common cause of chronic kidney disease in the first three decades of life.1, 2, 3 The discovery of more than 40 monogenic causes of CAKUT in humans has led to the understanding that urogenital malformations often arise from defects in the signaling pathways that regulate nephrogenesis.4, 5, 6 In addition, animal studies have demonstrated that intrauterine obstruction to urine flow can secondarily lead to CAKUT, although the genetic etiologies and molecular pathogenesis of these processes are not well understood.7
Nicotinic acetylcholine receptors (nAChR) are heteropentameric ligand-gated ion channels that are widely expressed in the nervous system and in certain non-neuronal tissues, such as the bladder urothelium.8,9 Interestingly, mice lacking Chrna3, the gene encoding the α3 nAChR subunit, develop a prominent genitourinary phenotype, with reduced bladder contractility, megacystis, and recurrent urinary tract infections.10 The α3 nAChR subunit mediates fast synaptic transmission in the parasympathetic, sympathetic, and enteric ganglia and plays a critical role in modulating normal bladder function.11
To date, only one gene involved in neuronal synaptic transmission, CHRM3 (MIM: 118494), has been implicated in lower urinary tract obstruction in humans.12 Here, we describe the discovery of biallelic mutations in CHRNA3 in three families with CAKUT and additional extra-renal dysautonomic features.
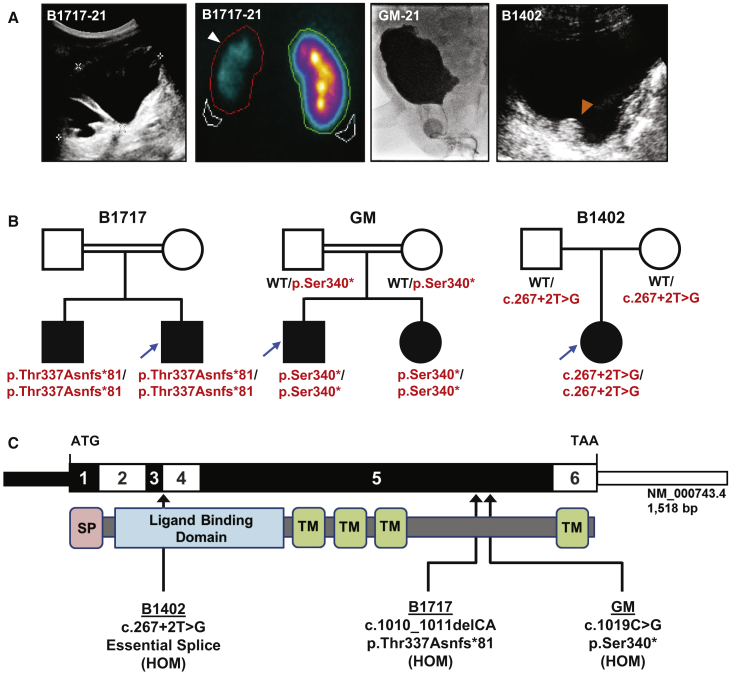
Approval for human subject research was obtained from the Institutional Review Board at the respective institutions, and samples were obtained after written informed consent. The index case subject, B1717-21, is a young man who was born to consanguineous parents of Arabic descent and who presented in childhood with recurrent urinary tract infections. Renal ultrasound demonstrated bilateral hydronephrosis, a thickened bladder wall, and a large post-void residual (Figure 1A). Voiding cysturethrogram (VCUG) revealed bilateral grade 5 vesicoureteral reflux (VUR) without posterior urethral valves (not shown), and the affected individual was given a diagnosis of non-neurogenic neurogenic bladder. He developed progressive renal insufficiency, and by 19 years of age, a DMSA scan demonstrated a small, atrophic left kidney with 10% residual function (Figure 1A). He also presented to the ophthalmologist in adolescence for difficulty seeing in bright light and was found to have bilateral mydriasis with impaired pupillary constriction. Moreover, orthostatic hypotension was diagnosed on routine physical examination (Table 1). The proband’s brother, B1717-22, was also noted to have an impaired pupillary light reflex. He additionally has a history of recurrent urinary tract infections, although renal ultrasound revealed normal-appearing kidneys and bladder (not shown).
Figure 1.
Identification of Biallelic CHRNA3 Mutations in Three Families with CAKUT
(A) Renal and bladder imaging for affected individuals. The two left panels depict a renal ultrasound and DMSA (dimercaptosuccinic acid) scan from individual B1717-21. The renal ultrasound demonstrates severe left-sided hydronephrosis with cortical thinning, and DMSA scan shows reduced cortical uptake of the left kidney (arrow head). VCUG from individual GM-21 and bladder ultrasound from individual B1402 both demonstrate thickened and irregular bladder walls. The echogenic circular irregularity on the bladder ultrasound for B1402 (orange arrowhead) is an artifact from a STING procedure that was done after recurrent vesicoureteral reflux developed following bilateral ureteral reimplantation.
(B) Pedigrees for the three affected families. In the pedigrees, squares represent males and circles represent females. Open symbols represent unaffected individuals, and filled symbols represent affected individuals. Consanguineous unions are depicted as double horizontal lines. Probands (individuals -21 of each family) are denoted by blue arrows. WT, wild type.
(C) Exon and protein domain structure of CHRNA3. The exon structure is depicted in the upper bar, with positions of the start codon (ATG) and stop codon (TAA) indicated. The lower bar depicts the protein structure of CHRNA3, with an N-terminal signal peptide (pink), a large extracellular ligand-binding domain (blue), and four transmembrane helices (green). The three different mutations detected in three families are mapped to the exon and protein structures.
Table 1.
Recessive Mutations Identified in CHRNA3 in Three Families with CAKUT
| Family | Ethnic Origin | Gender | Exon (Zygosity) | Nucleotide Change; Amino Acid Change (Segregation)a | gnomAD Allele Frequenciesb | Genitourinary Manifestations | Dysautonomic Manifestations | Otherc |
|---|---|---|---|---|---|---|---|---|
| B1402 | Macedonian | female | intron 3 (hom) | c.267+2T>G (essential splice); (m. het; p. het) | 0/1/246,220 | bilat. VUR, grade IV recurrent VUR post ureteral reimplantation CKD (stage 2) |
none | none |
| B1717-21 | Arabic | male | exon 5 (hom) | c.1010_1011delCA (p.Thr337Asnfs∗81); (ND) | NP | non-neurogenic neurogenic bladder bilat. VUR, grade V bilat. hydronephrosis atrophic left kidney CKD (stage 2) |
impaired pupillary light reflex orthostatic hypotension |
none |
| Β1717-22 | Arabic | male | exon 5 (hom) | c.1010_1011delCA (p.Thr337Asnfs∗81); (ND) | NP | recurrent UTIs | impaired pupillary light reflex | none |
| GM-21 | Pakistani | male | exon 5 (hom) | c.1019C>G (p.Ser340∗); (m. het; p. het) | 0/4/246,010 | non-neurogenic neurogenic bladder left hydronephrosis right cystic kidney hypospadias |
impaired pupillary light reflex flat CTG tracing in utero |
hypertelorism broad nasal root intellectual disability 2q31.1-32.3 duplication (de novo) |
| GM-22 | Pakistani | female | exon 5 (hom) | c.1019C>G (p.Ser340∗); (m. het; p. het) | 0/4/246,010 | voiding dysfunction recurrent UTIs |
impaired pupillary light reflex flat CTG tracing in utero |
GERD, failure to thrive |
Abbreviations: Bilat., bilateral; CKD, chronic kidney disease; CTG, cardiotocography; GERD, gastresophageal reflux; het, heterozygous; Hom, homozygous; m, maternal allele; ND, no data; NP, not present; p, paternal allele; UTI, urinary tract infection; VUR, vesicoureteral reflux.
Segregation is listed as (maternal allele, paternal allele) when available. If parental DNA was not available, segregation is listed as ND.
None of the identified CHRNA3 mutations have been reported homozygously in gnomAD, which includes exome or genome sequencing data from 141,456 unrelated individuals.
One affected individual was found to have additional genetic abnormalities that were thought to explain some of his extra-renal manifestations. GM-21 has a de novo 2q31.1–32.3 duplication which may explain his facial dysmorphisms and intellectual disability. This duplication was not shared by his sister, GM-22.
We applied whole-exome sequencing (WES) and homozygosity mapping to individual B1717-21.13,14 Mutation calling was performed in line with proposed guidelines by clinician-scientists who had knowledge of the clinical phenotypes and pedigree structure (Figure S1).15 We identified a homozygous truncating mutation (GenBank: NM_000743.4; c.1010_1011delCA [p.Thr337Asnfs∗81]) in exon 5 of the gene CHRNA3 (Cholinergic Receptor Nicotinic Alpha 3 Subunit), which encodes the α3 nAChR subunit. The same homozygous mutation was found in the proband’s affected older brother, B1717-22 (Figure 1B, Table 1).
Through the use of the on-line tool, GeneMatcher,16,17 we identified two siblings of Pakistani descent (GM-21 and GM-22) who also have biallelic mutations in CHRNA3 (c.1019C>G [p.Ser340∗]). GM-21 was diagnosed prenatally with hydronephrosis, and post-natal imaging revealed a dilated, cystic right kidney, left hydroureteronephrosis, and a thickened, trabeculated bladder wall (Figure 1A). He was diagnosed with non-neurogenic neurogenic bladder and was managed with clean intermittent catheterizations and subsequent vesicostomy. His younger sister, GM-22, had recurrent urinary tract infections, and VCUG demonstrated a large-capacity bladder with incomplete emptying (not shown). Ophthalmology examination for both children revealed constant miosis with pupils that did not dilate, and both siblings additionally had flat cardiotocography (CTG) tracings in utero. This was detected at 36 weeks gestational age in the older child, for which he underwent emergent cesarean section. A flat CTG tracing was noticed at 29 weeks gestational age for the younger sibling and persisted until she was delivered at full term.
We subsequently queried WES data in our cohort of 380 families with CAKUT and identified one additional affected individual with biallelic CHRNA3 mutations. In individual B1402, who has bilateral VUR and hydronephrosis, we detected a homozygous essential splice site mutation (c.267+2T>G). Interestingly, this individual underwent ureteral reimplantation as a child but developed recurrence of her VUR, for which she underwent a STING procedure (Figure 1A). We did not detect any biallelic mutations in CHRNA3 in a control cohort of 419 families with either nephrotic syndrome or nephronophthisis.
All three CHRNA3 mutations were confirmed via Sanger sequencing (Figure S3). A summary of the clinical characteristics of the affected individuals and the mutations identified is provided in Table 1, and a schematic of the CHRNA3 exon and protein structure with locations of the three mutations is depicted in Figure 1C. Both the p.Thr337Asnfs∗81 and p.Ser340∗ variants are predicted to lead to premature termination of the protein prior to the fourth transmembrane helix (Figure 1C). As RNA was not available from the individual with the c.267+2T>G splice mutation, we used in silico prediction tools to determine the splicing effect. Because the c.267+2T>G change occurs at an obligatory splice site, we predict that this will lead to skipping of exon 3 and an in-frame deletion of 15 amino acids in the protein’s extracellular ligand-binding domain. However, it should be noted that this may not recapitulate the splicing effect in vivo.
In the two families in which homozygosity mapping was available, the CHRNA3 mutations were all located within regions of homozygosity by descent (Figure S4). None of the three CHRNA3 variants were present homozygously in the large population database, gnomAD (Table 1). Exome data for each proband from the three families were also analyzed for mutations in known CAKUT genes5 and no causative variants were identified. However, individual GM-21 was noted to have a de novo 25.6 Mb duplication of chromosome 2q31.1–2q32.3 that was not shared by his affected sister. This is thought to contribute to his cognitive deficits, although further studies will be required for causality to be more definitively established.
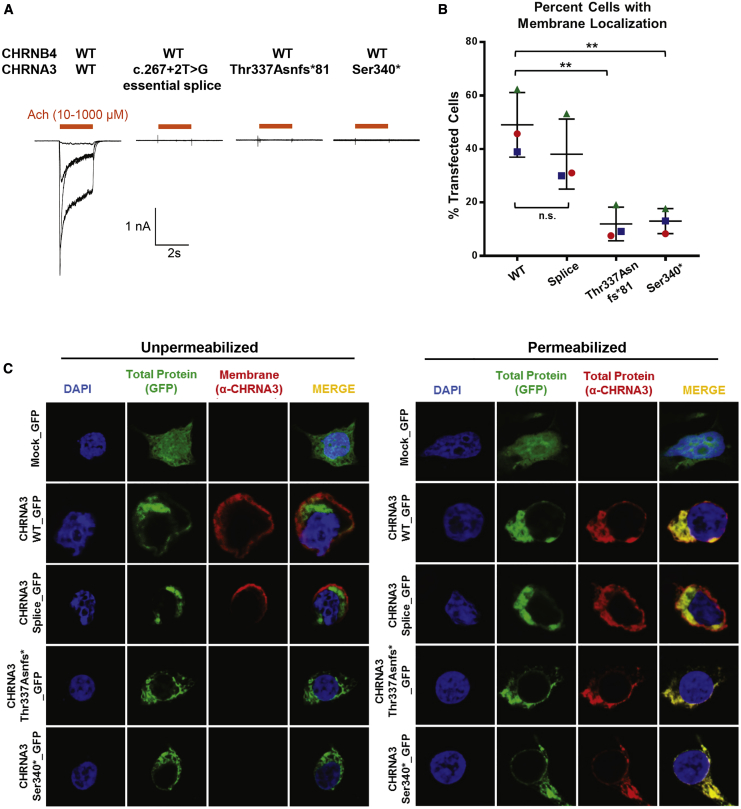
In order to examine whether the identified mutations in CHRNA3 affect receptor function, we performed electrophysiology and immunofluorescence studies in HEK293 cells. The α3 nAChR subunit is known to heteropentamerize with the β4 nAChR subunit,18 and we first aimed to determine whether the CHRNA3 mutations affect the ability of the α3β4 nAChR to induce current after stimulation with acetylcholine. Patch-clamping experiments were performed in HEK293 cells co-transfected with wild-type CHRNB4 cDNA and either wild-type or mutant CHRNA3 cDNA. Acetylcholine induced a dose-dependent inward current in cells overexpressing the wild-type α3β4 nAChR (Figure 2A). In contrast, no current was generated in cells expressing receptors composed of the splice site, p.Thr337Asnfs81∗, or p.Ser340∗ mutant α3 subunits (Figure 2A). These data demonstrate complete loss of function for the essential splice and two truncating variants.
Figure 2.
CHRNA3 Mutants Perturb α3β4 nAChR Function and Impair Plasma Membrane Trafficking
(A) Whole-cell currents obtained from HEK293 cells overexpressing both wild-type and the indicated mutant α3β4 nicotinic acetylcholine receptors (nAChRs). Wild-type α3β4 nAChR generates a strong current after incubation with acetylcholine (n = 7). In contrast, the essential splice (n = 4), p.Thr337Asnfs∗81 (n = 4), and p.Ser340∗ (n = 4) mutants were unable to generate any current.
(B) Membrane localization of α3 nAChR in HEK293 cells. Quantification of immunofluorescence images of HEK293 cells overexpressing either wild-type or mutant GFP-tagged CHRNA3. Graphs represent an average of three independent experiments. For each experiment, 100–150 transfected cells were evaluated for membrane staining. 49% of cells transfected with WT-CHRNA3 demonstrated membrane staining. In contrast, only 12%–13% of cells transfected with the p.Thr337Asnfs∗81 and p.Ser340∗ mutants demonstrate membrane staining, respectively. The error bars represent SEM for three experiments. ∗∗p value < 0.01 when compared to wild type; n.s. not significant when compared to wild type.
(C) Representative immunofluorescence images depicting the cellular localization of overexpressed GFP-tagged wild-type and mutant α3 nAChRs in HEK293 cells. Unpermeabilized (left) and permeabilized (right) cells are stained with an anti-CHRNA3 antibody raised against the protein’s extracellular N terminus. The green GFP signal demonstrates total transfected CHRNA3 protein, whereas in unpermeabilized cells, the red signal (anti-CHRNA3) depicts protein localized to the plasma membrane. Wild-type CHRNA3 and the splice site mutant both demonstrate membrane localization. In contrast, there is no signal from the anti-CHRNA3 antibody for the protein truncating p.Thr337Asnfs∗ and p.Ser340∗ mutants, suggesting impaired membrane localization. In permeabilized cells, both the GFP signal (green) and antibody staining (red) demonstrate cytoplasmic localization of the p.Thr337Asnfs∗ and p.Ser340∗ mutant proteins.
Because the two truncating mutations (p.Thr337Asnfs∗81 and p.Ser340∗) lead to premature termination of the CHRNA3 protein prior to the fourth transmembrane helix, we hypothesized that this would disrupt membrane trafficking of the mutant receptors. We expressed GFP-tagged wild-type and mutant CHRNA3 cDNA in HEK293 cells and labeled unpermeabilized cells with an antibody to the extracellular N terminus of the α3 nAChR subunit, which is expected to bind only those proteins that are inserted into the plasma membrane (Figures 2B and 2C). We detected membrane localization of the wild-type α3 nAChR subunit in 49% of transfected cells. In contrast, only 12%–13% of transfected cells demonstrated membrane staining for the p.Thr337Asnfs∗81 and p.Ser340∗ mutants, suggesting impaired membrane trafficking (Figure 2B). There is a trend toward reduced membrane localization for the essential splice site variant, but this did not reach statistical significance. Representative immunofluorescence images are depicted in Figure 2C. Permeabilized cells, in which both extracellular and intracellular labeling is established, were utilized as controls.
We here discovered by whole-exome sequencing three different homozygous loss-of-function mutations in CHRNA3 in three families with CAKUT. We demonstrate that all three mutations attenuate the ability of the α3β4 nAChR to generate current after stimulation with acetylcholine. Additionally, the two truncating mutations, p.Thr337Asnfs∗ and p.Ser340∗, impair receptor trafficking to the plasma membrane.
Micturition requires coordinated stimulation of the urinary bladder and urethral sphincters by the parasympathetic, sympathetic, and somatic nervous systems (Figure S5).11 CHRNA3 mediates fast synaptic transmission in the autonomic ganglia, and we predict that loss of CHRNA3 may result in discoordinated detrusor and urethral function. CHRNA3 is also expressed in the bladder urothelium and therefore may play additional roles in regulating bladder contraction beyond its known function in the autonomic ganglia.9 Of interest, all three families in our cohort developed secondary upper urinary tract malformations, such as hydronephrosis and renal cysts, consistent with the notion that obstruction to urinary flow in utero can lead to abnormalities in renal development.7,19,20 Indeed, individual B1402 underwent bilateral ureteral reimplantation, only to develop recurrent VUR and worsening hydronephrosis, likely because her underlying bladder dysfunction had not been recognized. We propose that disruption of CHRNA3 can result in a pathophysiological sequence by which impaired neuronal innervation leads to bladder dysfunction, functional lower urinary tract obstruction, and subsequent upper urinary tract anomalies.
In addition to their renal manifestations, families B1717 and GM, in whom truncating CHRNA3 mutations were found, have dysautonomic features, most notably an impaired pupillary light reflex. Compellingly, autoantibodies to the α3 nAChR subunit have been described to cause an autoimmune autonomic ganglionopathy in humans.21 These individuals develop profound autonomic failure, with symptoms overlapping those found in families with truncating CHRNA3 mutations, including bladder dysfunction, impaired pupillary light reflexes, and orthostatic hypotension.21,22 Notably, individual B1402 does not have dysautonomic manifestations. This may be due to subtle findings that are not clinically manifest, or it may be the case that hypomorphic mutations lead to a milder phenotype. Identification of additional affected individuals with CHRNA3 mutations may provide further insight into genotype-phenotype correlations.
The genitourinary and ocular phenotypes seen in families B1717 and GM are strikingly similar to that of the Chrna3−/− mice, which have megacystis, recurrent urinary tract infections, and persistent mydriasis.10 Bladder strips from these animals fail to contract in response to nicotine, and neurons from the superior cervical ganglia do not generate current in response to acetylcholine, consistent with the notion that loss of CHRNA3 results in impaired fast synaptic transmission within the autonomic ganglia.10 It is interesting to note that there is variable expressivity among affected individuals with CHRNA3 mutations. The two siblings in family B1717, who harbor the same CHRNA3 p.Thr337Asnfs∗87 truncating mutation, for example, exhibit a range of renal phenotypes, from only recurrent urinary tract infections to severe hydronephrosis, vesicoureteral reflux, and chronic kidney disease. This supports the notion that the renal disease manifesting in these individuals is the result of a pathophysiological sequence whereby impairment of bladder contraction results in secondary upper urinary tract malformations. The degree of renal impairment is likely a result of stochastic changes that occur in utero, and such variable expressivity is often seen in a variety of other monogenic diseases that cause CAKUT.4,23
To date, few monogenic causes of bladder dysfunction have been described in humans, including mutations in the genes CHRM3 (MIM: 118494), ACTG2 (MIM: 102545), ACTA2 (MIM: 102620), MYH11 (MIM: 160745), MYLK (MIM: 600922), HPSE2 (MIM: 613469), and LRIG2 (MIM: 608869).12,24, 25, 26, 27, 28, 29, 30 These genes have been implicated in the regulation of smooth muscle actin contraction, neuronal patterning, and synaptic neuronal transmission (Figure S5), and mutations cause syndromes such as megacystis-microcolon-intestinal hypoperistalsis syndrome (MMIHS [MIM: 155310, 613834]) or urofacial syndrome (MIM: 236730, 615112). Interestingly, individuals with mutations in CHRM3, which encodes a muscarinic acetylcholine receptor, also present with persistent mydriasis.12 The findings in our study provide additional evidence that disruption of the neural pathways regulating bladder function can be important genetic causes of both CAKUT and autonomic dysfunction in humans.
Our findings may point to important therapeutic implications. Current management for children with lower urinary obstruction involves surgical intervention to relieve anatomic obstruction and subsequent medical management of the sequelae from chronic kidney disease.31 However, surgical techniques alone may not be successful for individuals in whom mutations in CHRNA3 are identified, as was the case for individual B1402. The neuronal pathways regulating bladder contraction additionally provide tractable therapeutic targets that may be amenable to pharmacological intervention. In addition, early prenatal genetic diagnoses might eventually allow for pharmacological interventions in utero, which could prevent the development of renal dysgenesis. Further identification of novel genetic causes of urinary tract obstruction will provide additional strategies toward precision medicine.
Declaration of Interests
F.H. is a cofounder and S.A.C. member and holds stocks in Goldfinch-Bio. All other authors declare that they have no competing financial interests.
Acknowledgments
We thank the affected individuals and their families for their contributions to this study. We also would like to thank Michael Wangler, Arthur Beaudet, Reza Bekheirnia, William Newman, and Fowzan Alkuraya for helpful discussion. This research was supported by grants from the National Institutes of Health to F.H. (DK0668306). N.M. is supported by funding from the National Institutes of Health (grant T32-DK007726). F.K. is supported by funding from the Biomedical Education Program. D.M.C. is funded by the Health Research Board, Ireland (HPF-206-674), the International Pediatric Research Foundation Early Investigators’ Exchange Program, and the Amgen Irish Nephrology Society Specialist Registrar Bursary. M.N. is supported by a grant from the Japan Society for the Promotion of Science. V.K. is supported by the Deutsche Forschungsgemeinschaft (403877094). A.J.M. is supported by funding from the National Institutes of Health (grant T32-DK007726), the 2017 Harvard Stem Cell Institute Fellowship Grant, and the 2018 Jared J. Grantham Research Fellowship from the American Society of Nephrology Ben J. Lipps Research Fellowship Program. C.-H.W.W. is supported by funding from the National Institutes of Health (grant T32-GM007748). R.S.L. is supported by funding from the National Institutes of Health (DK096238). S.E. is a Wellcome Senior Investigator. F.H. is also supported by the Begg Family Foundation. We also thank the Yale Center for Mendelian Genomics for whole-exome sequencing analysis (U54HG006504).
Published: November 7, 2019
Footnotes
Supplemental Data can be found online at https://doi.org/10.1016/j.ajhg.2019.10.004.
Web Resources
1000 Genomes Project, https://www.internationalgenome.org/1000-genomes-browsers
Clustal Omega, https://www.ebi.ac.uk/Tools/msa/clustalo/
Exome Aggregation Consortium (ExAC), http://exac.broadinstitute.org
Genome Aggregation Database (gnomAD), http://gnomad.broadinstitute.org
MutationTaster, http://www.mutationtaster.org
NHLBI Exome Sequencing Project (ESP), https://evs.gs.washington.edu/EVS
Online Mendelian Inheritance in Man (OMIM), https://www.omim.org
PolyPhen2, http://genetics.bwh.harvard.edu/pph2
Sorting Intolerant From Tolerant (SIFT), http://sift.jcvi.org
UCSC Genome Browser, https://genome.ucsc.edu
Supplemental Data
References
- 1.Ingelfinger J.R., Kalantar-Zadeh K., Schaefer F., World Kidney Day Steering Committee World Kidney Day 2016: Averting the legacy of kidney disease-focus on childhood. Pediatr. Nephrol. 2016;31:343–348. doi: 10.1007/s00467-015-3255-7. [DOI] [PubMed] [Google Scholar]
- 2.Calderon-Margalit R., Golan E., Twig G., Leiba A., Tzur D., Afek A., Skorecki K., Vivante A. History of Childhood Kidney Disease and Risk of Adult End-Stage Renal Disease. N. Engl. J. Med. 2018;378:428–438. doi: 10.1056/NEJMoa1700993. [DOI] [PubMed] [Google Scholar]
- 3.Queisser-Luft A., Stolz G., Wiesel A., Schlaefer K., Spranger J. Malformations in newborn: results based on 30,940 infants and fetuses from the Mainz congenital birth defect monitoring system (1990-1998) Arch. Gynecol. Obstet. 2002;266:163–167. doi: 10.1007/s00404-001-0265-4. [DOI] [PubMed] [Google Scholar]
- 4.van der Ven A.T., Vivante A., Hildebrandt F. Novel Insights into the Pathogenesis of Monogenic Congenital Anomalies of the Kidney and Urinary Tract. J. Am. Soc. Nephrol. 2018;29:36–50. doi: 10.1681/ASN.2017050561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Ven A.T., Connaughton D.M., Ityel H., Mann N., Nakayama M., Chen J., Vivante A., Hwang D.Y., Schulz J., Braun D.A. Whole-Exome Sequencing Identifies Causative Mutations in Families with Congenital Anomalies of the Kidney and Urinary Tract. J. Am. Soc. Nephrol. 2018;29:2348–2361. doi: 10.1681/ASN.2017121265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verbitsky M., Westland R., Perez A., Kiryluk K., Liu Q., Krithivasan P., Mitrotti A., Fasel D.A., Batourina E., Sampson M.G. The copy number variation landscape of congenital anomalies of the kidney and urinary tract. Nat. Genet. 2019;51:117–127. doi: 10.1038/s41588-018-0281-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chevalier R.L., Thornhill B.A., Forbes M.S., Kiley S.C. Mechanisms of renal injury and progression of renal disease in congenital obstructive nephropathy. Pediatr. Nephrol. 2010;25:687–697. doi: 10.1007/s00467-009-1316-5. [DOI] [PubMed] [Google Scholar]
- 8.Albuquerque E.X., Pereira E.F., Alkondon M., Rogers S.W. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol. Rev. 2009;89:73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beckel J.M., Kanai A., Lee S.J., de Groat W.C., Birder L.A. Expression of functional nicotinic acetylcholine receptors in rat urinary bladder epithelial cells. Am. J. Physiol. Renal Physiol. 2006;290:F103–F110. doi: 10.1152/ajprenal.00098.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu W., Gelber S., Orr-Urtreger A., Armstrong D., Lewis R.A., Ou C.N., Patrick J., Role L., De Biasi M., Beaudet A.L. Megacystis, mydriasis, and ion channel defect in mice lacking the alpha3 neuronal nicotinic acetylcholine receptor. Proc. Natl. Acad. Sci. USA. 1999;96:5746–5751. doi: 10.1073/pnas.96.10.5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fowler C.J., Griffiths D., de Groat W.C. The neural control of micturition. Nat. Rev. Neurosci. 2008;9:453–466. doi: 10.1038/nrn2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weber S., Thiele H., Mir S., Toliat M.R., Sozeri B., Reutter H., Draaken M., Ludwig M., Altmüller J., Frommolt P. Muscarinic Acetylcholine Receptor M3 Mutation Causes Urinary Bladder Disease and a Prune-Belly-like Syndrome. Am. J. Hum. Genet. 2011;89:668–674. doi: 10.1016/j.ajhg.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braun D.A., Lovric S., Schapiro D., Schneider R., Marquez J., Asif M., Hussain M.S., Daga A., Widmeier E., Rao J. Mutations in multiple components of the nuclear pore complex cause nephrotic syndrome. J. Clin. Invest. 2018;128:4313–4328. doi: 10.1172/JCI98688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hildebrandt F., Heeringa S.F., Rüschendorf F., Attanasio M., Nürnberg G., Becker C., Seelow D., Huebner N., Chernin G., Vlangos C.N. A systematic approach to mapping recessive disease genes in individuals from outbred populations. PLoS Genet. 2009;5:e1000353. doi: 10.1371/journal.pgen.1000353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacArthur D.G., Manolio T.A., Dimmock D.P., Rehm H.L., Shendure J., Abecasis G.R., Adams D.R., Altman R.B., Antonarakis S.E., Ashley E.A. Guidelines for investigating causality of sequence variants in human disease. Nature. 2014;508:469–476. doi: 10.1038/nature13127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sobreira N., Schiettecatte F., Boehm C., Valle D., Hamosh A. New tools for Mendelian disease gene identification: PhenoDB variant analysis module; and GeneMatcher, a web-based tool for linking investigators with an interest in the same gene. Hum. Mutat. 2015;36:425–431. doi: 10.1002/humu.22769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sobreira N., Schiettecatte F., Valle D., Hamosh A. GeneMatcher: a matching tool for connecting investigators with an interest in the same gene. Hum. Mutat. 2015;36:928–930. doi: 10.1002/humu.22844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skok V.I. Nicotinic acetylcholine receptors in autonomic ganglia. Auton. Neurosci. 2002;97:1–11. doi: 10.1016/s1566-0702(01)00386-1. [DOI] [PubMed] [Google Scholar]
- 19.Chevalier R.L., Forbes M.S., Thornhill B.A. Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney Int. 2009;75:1145–1152. doi: 10.1038/ki.2009.86. [DOI] [PubMed] [Google Scholar]
- 20.Becknell B., Carpenter A.R., Allen J.L., Wilhide M.E., Ingraham S.E., Hains D.S., McHugh K.M. Molecular basis of renal adaptation in a murine model of congenital obstructive nephropathy. PLoS ONE. 2013;8:e72762. doi: 10.1371/journal.pone.0072762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vernino S., Sandroni P., Singer W., Low P.A. Invited Article: Autonomic ganglia: target and novel therapeutic tool. Neurology. 2008;70:1926–1932. doi: 10.1212/01.wnl.0000312280.44805.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vernino S., Low P.A., Fealey R.D., Stewart J.D., Farrugia G., Lennon V.A. Autoantibodies to ganglionic acetylcholine receptors in autoimmune autonomic neuropathies. N. Engl. J. Med. 2000;343:847–855. doi: 10.1056/NEJM200009213431204. [DOI] [PubMed] [Google Scholar]
- 23.Vivante A., Hildebrandt F. Exploring the genetic basis of early-onset chronic kidney disease. Nat. Rev. Nephrol. 2016;12:133–146. doi: 10.1038/nrneph.2015.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wangler M.F., Gonzaga-Jauregui C., Gambin T., Penney S., Moss T., Chopra A., Probst F.J., Xia F., Yang Y., Werlin S., Baylor-Hopkins Center for Mendelian Genomics Heterozygous de novo and inherited mutations in the smooth muscle actin (ACTG2) gene underlie megacystis-microcolon-intestinal hypoperistalsis syndrome. PLoS Genet. 2014;10:e1004258. doi: 10.1371/journal.pgen.1004258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milewicz D.M., Østergaard J.R., Ala-Kokko L.M., Khan N., Grange D.K., Mendoza-Londono R., Bradley T.J., Olney A.H., Adès L., Maher J.F. De novo ACTA2 mutation causes a novel syndrome of multisystemic smooth muscle dysfunction. Am. J. Med. Genet. A. 2010;152A:2437–2443. doi: 10.1002/ajmg.a.33657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daly S.B., Urquhart J.E., Hilton E., McKenzie E.A., Kammerer R.A., Lewis M., Kerr B., Stuart H., Donnai D., Long D.A. Mutations in HPSE2 cause urofacial syndrome. Am. J. Hum. Genet. 2010;86:963–969. doi: 10.1016/j.ajhg.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stuart H.M., Roberts N.A., Burgu B., Daly S.B., Urquhart J.E., Bhaskar S., Dickerson J.E., Mermerkaya M., Silay M.S., Lewis M.A. LRIG2 mutations cause urofacial syndrome. Am. J. Hum. Genet. 2013;92:259–264. doi: 10.1016/j.ajhg.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts N.A., Hilton E.N., Lopes F.M., Singh S., Randles M.J., Gardiner N.J., Chopra K., Coletta R., Bajwa Z., Hall R.J. Lrig2 and Hpse2, mutated in urofacial syndrome, pattern nerves in the urinary bladder. Kidney Int. 2019;95:1138–1152. doi: 10.1016/j.kint.2018.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Halim D., Brosens E., Muller F., Wangler M.F., Beaudet A.L., Lupski J.R., Akdemir Z.H.C., Doukas M., Stoop H.J., de Graaf B.M. Loss-of-Function Variants in MYLK Cause Recessive Megacystis Microcolon Intestinal Hypoperistalsis Syndrome. Am. J. Hum. Genet. 2017;101:123–129. doi: 10.1016/j.ajhg.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gauthier J., Ouled Amar Bencheikh B., Hamdan F.F., Harrison S.M., Baker L.A., Couture F., Thiffault I., Ouazzani R., Samuels M.E., Mitchell G.A. A homozygous loss-of-function variant in MYH11 in a case with megacystis-microcolon-intestinal hypoperistalsis syndrome. Eur. J. Hum. Genet. 2015;23:1266–1268. doi: 10.1038/ejhg.2014.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chevalier R.L. Congenital urinary tract obstruction: the long view. Adv. Chronic Kidney Dis. 2015;22:312–319. doi: 10.1053/j.ackd.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.