Abstract
Supraventricular tachycardia (SVT) is one of the most common conditions requiring emergency cardiac care in neonates. Atrioventricular reentrant tachycardia utilizing an atrioventricular bypass tract is the most common form of SVT presenting in the neonatal period. There is high likelihood for spontaneous resolution for most of the common arrhythmia substrates in infancy. Pharmacological agents remain as the primary therapy for neonates.
Keywords: Supraventricular tachycardia, Neonate
1. Introduction
Supraventricular tachycardia (SVT) is one of the most common conditions requiring emergency cardiac care in neonates. SVT is defined as any tachycardia which requires participation of at least one supraventricular structure above the bifurcation of the His bundle (HB) for perpetuation, including the atrial myocardium, the atrioventricular node, the proximal HB, the coronary sinus, the pulmonary veins, the vena cavae, or abnormal atrioventricular connections other than the HB (i.e., bypass tracts) [1]. The incidence of SVT has been estimated at 1 in every 250–1000 pediatric patients [[2], [3], [4], [5], [6]].
A population-based study in infants from England estimated the incidence of clinically significant sustained arrhythmias to occur in 24 per 100,000 live births (1:4000), of which two-thirds was atrioventricular re-entry tachycardia (AVRT) with an incidence of 16.3 per 100,000 [7]. A recent population-based study from a national birth cohort database in Taiwan of children born between 2000 and 2008 with complete postnatal medical data, estimated the cumulative incidence of SVT <1 month as 0.06 per 1000 patient-years overall and 0.05 per 1000 patient-years in those without major congenital heart disease (CHD). More than one-fourth (28%) of the patients were diagnosed with SVT by the age of 1 year, of whom, one-fourth (23%) were diagnosed during the neonatal period. Coexisting CHD was noted in 25% in this study, with the 6 leading diagnoses being atrial septal defect (37.8%), ventricular septal defect (19.5%), patent ductus arteriosus (7.6%), tetralogy of Fallot (7.6%), pulmonary stenosis (5.8%), and Ebstein anomaly (3.6%) [8].
2. Postnatal outcomes of fetal SVT
In a retrospective multicenter cohort study of 103 subjects diagnosed with fetal SVT by Hinkle et al., refractory SVT was found in 37% with this group being more likely to be delivered prematurely. Refractory SVT did not increase the risk of postnatal SVT. Postnatal SVT was seen in 61%. A strong correlation was found between postnatal SVT and later gestational age at fetal SVT diagnosis. Subjects with refractory SVT or hydrops did not have a higher risk of postnatal SVT [9].
3. Electrophysiological mechanisms of arrhythmias
For the purpose of this discussion, a brief description of the three mechanisms implicated in arrhythmias are listed below. The clinical presentation including onset, stability and termination often helps in deduction of these arrhythmia mechanisms and thereby help in their acute management.
3.1 Reentry: Reentrant tachycardia, also called reciprocating tachycardia is a continuous repetitive propagation of the activation wave in a circular path, returning to its site of origin to reactivate that site.
3.2 Automaticity: or spontaneous impulse initiation, is the ability of cardiac cells to depolarize spontaneously, and initiate a propagated action potential (AP) in the absence of external electrical stimulation. Enhanced normal automaticity refers to the accelerated generation of an AP by normal pacemaker tissue. Abnormal automaticity occurs in the cardiac cells only when there are major abnormalities in their transmembrane potentials such as reduced maximum diastolic potential, spontaneous upward drift of the phase 4 diastolic potential or a combination of the two. Both normal and abnormal pacemaker activity can be accelerated by drugs, various forms of cardiac disease, reduction in extracellular potassium, or alterations of autonomic nervous system tone.
3.3 Triggered activity: is impulse initiation in cardiac fibers caused by oscillations in membrane potential called afterdepolarizations that occur consequent to a preceding impulse or series of impulses. Afterdepolarizations can occur early during the repolarization phase of the AP or late, after completion of the repolarization phase.
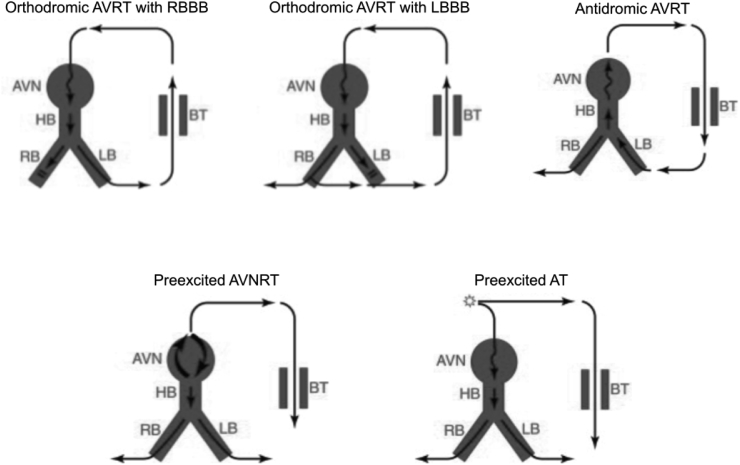
Table 1 lists the clinical features that suggest each of the above basic arrhythmia mechanisms. The entire set of clinical tachyarrhythmias can be categorized by considering the above three mechanisms arranged according to supraventricular arrhythmia substrate. Table 2 lists common clinical SVTs categorized based on arrhythmia substrate and mechanism. Fig. 1 provides schematic illustration of common reentrant SVTs presenting typically with narrow QRS-complex and Fig. 2 illustrates those presenting as wide QRS-complex tachycardias.
Table 1.
Clinical features suggesting arrhythmia mechanism.
| Reentrant tachycardia | Automatic tachycardia | Triggered arrhythmia |
|---|---|---|
|
|
|
Table 2.
Clinical supraventricular tachycardias categorized based on arrhythmia substrate and mechanism.
| Reentrant | Automatic | Triggered | |
|---|---|---|---|
| Atrium |
|
|
Some focal atrial tachycardia |
| AV junction | AV nodal reentrant tachycardia | Junctional ectopic tachycardia (congenital and postoperative) | |
| Accessory pathways | Orthodromic atrioventricular reentrant tachycardia utilizing concealed and manifest (WPW) AV accessory pathway, Antidromic reentrant tachycardia utilizing manifest (WPW) AV accessory pathway and atypical accessory pathways (“Mahaim tachycardias”), Preexcited atrial fibrillation |
AV, Atrioventricular; WPW, Wolff-Parkinson-White ventricular preexcitation pattern.
Fig. 1.
Common reentrant supraventricular tachycardias presenting as narrow QRS-complex tachycardia. The direction of the arrows in each case indicates the direction of propagation within reentrant circuits. The slow pathways within the circuit are represented by ‘wavy’ arrows. The lower panel illustrates the presence (“manifest” with ventricular preexcitation) and absence (“concealed” without ventricular preexcitation) of antegrade conduction via atrioventricular bypass tract in sinus rhythm which in turn is a substrate for orthrodromic atrioventricular reentrant tachycardia. Note that the ventricle is not primarily a part of the reentrant circuit in atrial flutter, atrial fibrillation and atrioventricular nodal reentrant tachycardia. AVN, Atrioventricular node; AV BT, Atrioventricular bypass tract; HB, His bundle.
Fig. 2.
Common reentrant supraventricular tachycardias presenting as wide QRS-complex tachycardia. The direction of the arrows in each case indicates the direction of propagation within reentrant circuits. The slow pathways within the circuit are represented by ‘wavy’ arrows. Preexcited SVT refers to supraventricular tachycardias where atrioventricular accessory pathways capable of antegrade conduction act only as bystanders and not part of the primary arrhythmia substrate. AVN, Atrioventricular node; AVRT, Atrioventricular reentrant tachycardia; AVNRT, Atrioventricular nodal reentrant tachycardia; BT, Bypass tract; HB, His bundle; LB, Left bundle branch; LBBB, Left bundle branch block; RB, Right bundle branch; RBBB, Right bundle branch block; SVT, supraventricular tachycardia.
4. Embryologic basis of neonatal supraventricular tachyarrhythmias
Jongbloed et al., provided a thorough and extensive review focusing on embryonic development of the cardiac conduction system in relation to clinical arrhythmias, as well as on specific cardiac conduction abnormalities that are observed in patients with congenital heart disease [10]. A detailed review of this subject is beyond the scope of this paper. In a mature heart, the atrioventricular (AV) conduction axis comprises the only myocardial continuity between the atria and ventricles, the remainder being separated by a layer of fibrous tissue at the AV junction i.e., the annulus fibrosus. The development of the fibrous structures of the heart that includes the annulus fibrosus, extends into post-natal life. Though not all accessory pathways show the same cellular morphology, pathological maturation of the annulus fibrosus has developmentally been related to deficient contributions of epicardial derived cells and lack of expression of periostin seen in deletions of Alk3, which in turn could result in broad accessory AV connections.
5. Analysis of tachycardia mechanism on surface electrocardiogram
In the acute clinical situation of SVT, the immediate focus should be on the overall picture and stabilizing the hemodynamic status of the patient. However, once this is accomplished, it is useful to be able to narrow the differential diagnosis of the tachycardia mechanism based on the surface electrocardiogram (ECG). A 12-lead ECG with rhythm strips is preferable to a single channel/lead rhythm strip only. In general, all the available channels of ECG should be reviewed from the beginning to the end of the recording. Fig. 3 provides a useful algorithm towards reaching a differential diagnosis for tachyarrhythmia based on the ECG features. This algorithm relies on the ECG characteristics including QRS width, AV (atrio-ventricular) ratio, regularity of rate and RP interval (measured from onset of QRS complex to onset of subsequent P wave on ECG). The initial factor to determine is whether the QRS width is “narrow” or “wide”. In young children, this decision is not always easy, as there are different ranges acceptable as “normal” for different pediatric age groups. The determination as “narrow QRS width” should be used only if the QRS duration falls within the normal range for age in all 12 ECG leads. Though the prime consideration in wide QRS-complex tachycardias (WCT) must always be VT, this may also be observed in some SVT, if ventricular activation is altered by bundle branch block or preexcitation via a bypass tract. For acute management purposes, one should assume VT unless there is convincing data to prove otherwise. Another practical issue that is often encountered while assessing AV ratio and RP interval is the absence of clearly discernible or low-amplitude P waves. The P wave in these instances is often seen as “pseudo-ST segment depression”, particularly if it follows immediately after QRS complex, which points to a “very short RP interval”. P waves could also be seen at times as altering the morphology of the T-wave. If visible, P wave axis may help in determining the origin of atrial activity. Sinus tachycardia is one of the commonest types of ‘long RP narrow QRS-complex tachycardia’ with a P-wave axis between 0 and 90° (positive in leads I and aVF). Lastly, any deviation in AV ratio from 1:1 even for few beats is often helpful in narrowing the differential diagnosis. AVRT and AVNRT in children are typically 1:1 in A:V ratio. More P waves than R waves would imply atrial tachyarrhythmia such as an ectopic atrial tachycardia or atrial flutter.
Fig. 3.
Algorithm for analysis of tachyarrhythmia on surface electrocardiogram. A.Flutter, Atrial flutter; A.Fib, Atrial fibrillation; AT, Atrial tachycardia; Atyp.AVNRT, Atypical AVNRT; AV, atrioventricular; BBB, Bundle branch block; AVRT, Atrioventricular reentrant tachycardia; AVNRT, Atrioventricular nodal reentrant tachycardia; IART, Intra-atrial reentrant tachycardia; JET, Junctional ectopic tachycardia; MAT, Multifocal atrial tachycardia; Orthodrom. AVRT, Orthodromic atrioventricular reentrant tachycardia; PJRT, Permanent Junctional Reciprocating Tachycardia; RP interval, measured from onset of QRS complex to onset of subsequent P wave on ECG; SVT, Supraventricular tachycardia; VT, Ventricular tachycardia; WPW, Wolff-Parkinson-White syndrome.
5.1. Ventricular tachycardia versus aberrantly conducted supraventricular tachycardia
WCT should be presumed to be VT in the absence of evidence to the contrary. This guards against inappropriate and potentially dangerous therapy. In general, most WCTs can be classified as having one of two patterns: a right bundle branch block (RBBB)-like pattern (QRS polarity is predominantly positive in leads V1 and V2) or a left bundle branch block (LBBB)-like pattern (QRS polarity is predominantly negative in leads V1 and V2). This initial determination is useful in evaluating other features on the ECG, which would help in distinguishing criteria favoring VT. The finding of ventriculo-atrial (VA) dissociation is one of the most useful practical criteria for the diagnosis of VT. However, retrograde VA conduction, whether 1 to 1 or 2 to 1, or Wenckebach VA conduction occurs in up to 50% of all VT. A multitude of ECG criteria have been published for differentiating VT from SVT with BBB as well preexcited SVT. However, they are beyond the scope of this review [[11], [12], [13], [14], [15]].
6. Specific supraventricular arrhythmia substrates
A study based on Pediatric Electrophysiology Registry found AVRT involving atrioventricular bypass tracts, decreasing in proportion with increasing age; conversely, atrioventricular nodal reentrant tachycardia (AVNRT) increasing with increasing age. No significant gender differences were present at younger ages (<12 years). Similar results have been found in older studies [16,17].
6.1. Atrial flutter
The incidence of atrial flutter (AFL) is ∼2.1 per 100,000 live births, with about 36% associated with structural heart disease [7]. In a series on perinatal atrial flutter from Lisowski et al., typical counterclockwise AFL was common, with synchronized electrical cardioversion successful in converting majority of the patients, and no recurrences occurring beyond the neonatal period [18]. Once AFL is converted to sinus rhythm, one may elect to wait and observe if flutter recurs. Alternatively, one may elect to treat with a class III agent such as Sotalol for six months to a year, although it appears that recurrence of AF is exceedingly rare beyond the neonatal period [19,20].
6.2. Ectopic/focal atrial tachycardia
The incidence of focal atrial tachycardia (FAT) has been estimated to be around 1.1 per 100,000 live births [7]. Ectopic atrial tachycardia (EAT) resulting from abnormal automaticity is characterized electrophysiologically such that: 1) the P wave of the first beat is identical to that of subsequent beats; 2) the cycle length of the first few beats progressively shorten; 3) atrioventricular block can occur in the presence of continuing SVT; and 4) tachycardia typically cannot be induced or terminated with standard pacing protocols. In the single center experience from Salerno et al., younger children (<3 years) presented as irregular heart rate, tachycardia, feeding difficulties, ventricular dysfunction, and cardiomyopathy. About 23% were associated with congenital heart disease. They respond to antiarrhythmic therapy and have a high incidence of EAT resolution, thus warranting a trial of antiarrhythmic therapy [21]. In Multifocal atrial tachycardia (MAT), also known as chaotic atrial rhythm, defined by three or more distinct P-waveforms, irregular P–P intervals, isoelectric baseline between P-waves and rapid rate on an electrocardiogram, is a rare tachyarrhythmia in infants. The majority of children with MAT in series from Bradley et al., were healthy infants under one year of age; a few exhibiting mild to life-threatening cardiorespiratory disease. Less often (50%), MAT accompanies structural heart disease. Mild ventricular dysfunction may be observed in the presence of MAT, but symptoms are few and resolution is generally complete. Response to antiarrhythmic agents is mixed with Amiodarone offering superior control of rhythm compared to other agents, and electrical cardioversion is of no avail [22].
6.3. Permanent junctional reciprocating tachycardia (PJRT)
PJRT is rare form of reentrant SVT with low likelihood of spontaneous resolution. The arrhythmia substrate is an accessory pathway with slow, decremental retrograde conduction that is commonly located in the posteroseptal region of the atrioventricular (AV) junction. The PJRT diagnosis is based on electrocardiographic (ECG) criteria [23]: (1) narrow QRS tachycardia, (2) negative P waves in inferior leads, (3) PR interval to RP interval ratio < 1, (4) 1:1 AV relation during tachycardia with no evidence of functional AV block, and (5) absent delta waves in the PR segment during sinus rhythm. In the largest series of PJRT from Kang et al., involving 198 patients, PJRT cases were observed in a unimodal distribution peaking in infancy, 57% were diagnosed in infancy and 27% presenting as fetal tachycardia. In the majority of affected patients, PJRT is incessant and the risk of tachycardia-induced cardiomyopathy is significant. Medications remain as the primary therapy for infants and small children while catheter ablation for PJRT should be considered more often as the initial treatment for older children and adolescents [24]. Fig. 4 shows 12-lead ECG in a neonate presenting with PJRT.
Fig. 4.
Three-day-old neonate presenting with permanent junctional reciprocating tachycardia (PJRT). Initial two beats are sinus beats followed by induction of PJRT. Note long-RP narrow QRS complex supraventricular tachycardia with inverted P waves in inferior frontal leads (II, III, aVF).
6.4. Congenital junctional ectopic tachycardia (JET)
Congenital JET is a rare tachyarrhythmia presenting in infancy that is associated with a high rate of morbidity and mortality. The mechanism of the tachycardia is thought to be abnormal automaticity arising from the region of the atrio-ventricular junction. In the largest series of JET from Collins et al., involving 99 patients, patients presenting at age ≤6 months were more likely to have incessant JET and faster JET rates. In most patients, the QRS complex is narrow during tachycardia. The relationship between ventricular and atrial contractions during tachycardia was ventricular-atrial dissociated in 56%, with 1:1 ventricular/atrial relationship in 32%. The median JET rate on presentation was 209 beats/min (136–320 beats/min) and tended to be higher in younger patients. Death occurred in 4% of patients, all of whom presented with JET at age ≤6 months. Most patients (75%) were doing well on no current therapy for JET during median follow-up of 4.5 years (range 0.03–28 years) [25].
Fig. 5 illustrates the analysis of ECG during tachycardia in four patients based on this algorithmic approach.
Fig. 5.
Case 1: ECG characteristics: ‘Narrow QRS complex’, 1:1 AV ratio, short RP interval (∼80 ms), and stable cycle length consistent with orthodromic atrioventricular reentrant tachycardia. Case 2: Short runs of nonsustained tachycardia shown. ‘S’ represents sinus beats. ‘A’ represents tachycardia beats. ECG characteristics: ‘Narrow QRS complex tachycardia with 1:1 AV ratio and long RP interval. Differential diagnosis includes Atrial tachycardia and Permanent Junctional Reciprocating Tachycardia (a form of orthodromic atrioventricular tachycardia utilizing a slowly conducting decremental retrograde only AV bypass tract, often presenting as incessant tachycardia). Case 3: ECG characteristics: ‘Narrow QRS complex’, <1:1 AV ratio, variable cycle length consistent with junctional ectopic tachycardia. Case 4: Top panel shows narrow QRS complex tachycardia with >1:1 AV ratio recorded at 25 mm/s. Bottom panel shows the same tachycardia recorded at 50 mm/s. Adenosine administered accentuates AV block and makes the atrial flutter waves easily visible. AV, atrioventricular.
7. Management of neonatal SVT
7.1. Acute management
The effective treatment of any arrhythmia requires attention to overall cardiovascular status. Basic emergency support including cardiopulmonary resuscitation and securement of airway using endotracheal intubation, if clinically indicated should be initiated in a parallel fashion along with anti-arrhythmic interventions. Appropriate acute resuscitation adopting Pediatric Advanced Life Support (PALS) Tachycardia Algorithm [26,27] is recommended, the discussion of which is not the focus of this review. Attention must be given to correction of hypoxia, acidosis and electrolyte imbalance. Vagal maneuvers (e.g., ‘application of ice on face’) or intravenous Adenosine (0.1–0.3 mg/kg) is used for termination of hemodynamically stable acute episodes of reentrant SVT involving atrioventricular node. When using Adenosine it is important to ensure rapid push through an optimal intravenous access preferably close to the heart. Adenosine, though not capable of terminating atrial flutter (macro-reentrant atrial tachycardia), or ectopic atrial tachycardia, often helps to establish the diagnosis, when a simultaneous ECG is obtained during administration. Synchronized electrical cardioversion is used for acute conversion of hemodynamically unstable reentrant SVT as well as intra-atrial reentry such as atrial flutter/fibrillation. There is no role for DC cardioversion in patients who are terminable with vagal maneuvers or adenosine but keep recurring back to SVT. Such patients need drug therapy management to “maintain” sinus rhythm rather than just to convert to sinus rhythm, which has been shown to be achievable by other, less harmful ways.
Rate control through transesophageal overdrive pacing (TOP) is a safe and effective option to restore acceptable hemodynamics in infants with refractory supraventricular tachycardia and severe impairment of ventricular function. It presents the possibility of immediate heart rate control and offers time for myocardial recovery and safe implementation of antiarrhythmic drug therapy. Apart from focal atrial tachycardia or atrial reentrant tachycardias, junctional ectopic tachycardia represents the only narrow complex tachycardia that may not respond sufficiently to TOP [[28], [29], [30]].
Table 3 lists common options for acute management of SVT in neonate.
Table 3.
Acute interventions to control supraventricular tachycardia in neonates.
| Treatment | Dosing | Comments |
|---|---|---|
| DC shock | ORT or AVNRT 0.25 J/kg A.flutter 0.5 J/kg A.fib 1–2 J/kg All of above double for each subsequent attempt |
|
| Adenosine | 0.1–0.3 mg/kg rapid IV bolus. Preferably administered via a venous access close to heart |
|
| Amiodarone | Loading 5 mg/kg over 20–60 min (infusion rate not exceeding 0.25 mg/kg/min); may repeat twice up to maximum total dose of 15 mg/kg during. Followed by initial: 10 mg/kg/day, increase incrementally as clinically needed range: 10–20 mg/kg/day. There is variability in the preferred administration rates and acute loading dosage of Amiodarone based on institutional preference. | Follow QTc interval on serial ECG. Baseline labs including complete blood count, thyroid function tests and liver enzymes is recommended when considering Amiodarone. |
| Esmolol | 0.5 mg/kg rapid IV bolus followed by 50–200 mcg/kg/min | Watch for exacerbation of bronchospasm in patients with asthma |
| Procainamide | 7–15 mg/kg IV over 15–30 min followed by 20–60 mcg/kg/min | Particularly useful in SVT involving AV bypass tracts and stable wide complex tachycardia. Dosing adjustment and serum drug concentrations recommended in hepatic/renal impairment. Drug levels also required in those receiving higher maintenance doses for >24 h. |
| Transesophageal Overdrive Pacing | A 4- to 7-French bi- to quadripolar intracardiac/5- to 10-French esophageal electrode catheter is introduced through the mouth/nose to the estimated depth (distance from the tip of the nose to about 2–5 cm above the xiphoid) in the esophagus. At that point, a transoesophageal stimulator is attached. After determination of the tachycardia cycle length, pacing is established at a cycle length at least 20–30 ms shorter than the spontaneous tachycardia cycle length. | A standard temporary external pacemaker might not provide sufficient impulse duration (10–15 ms) to capture the atrium with the lowest possible, yet effective amplitude tolerated by the patient. |
A.fib, atrial fibrillation; A.flutter, atrial flutter; ORT, orthodromic atrioventricular reentrant tachycardia; AV, atrioventricular; AVNRT, Atrioventricular nodal reentrant tachycardia; IART, intra-atrial reentrant tachycardia; IV, intravenous; WPW, Wolff-Parkinson-White syndrome.
7.2. Maintenance pharmacological therapy for control of SVT
The object of prophylactic drug therapy is to prevent SVT while anticipating spontaneous resolution of the SVT substrate. Optimal medical management with respect to agent and duration of treatment of SVT in infants has not been well studied. The tachycardia can typically be managed conservatively with anti-arrhythmic medication [[31], [32], [33]], with the knowledge that 60–90% of patients who present with WPW or AVRT in infancy, and 20–50% with less common tachycardias, will not have a recurrence of tachycardia after medication is discontinued at six to 12 months of age [32,34,35]. Beta-blockers, particularly Propranolol, have become established as the most common first-line agent for maintenance therapy for SVT in infants. The standard practice has been to continue treatment for 6 months to a year, following which medications are typically weaned off. Table 4 lists the common first-line medications used in the treatment of SVT.
Table 4.
Common First-Line Medications in the Treatment of SVT in Neonates (Oral therapy).
| Medication | Class | Dosing | Comments |
|---|---|---|---|
| Propranolol | β-blocker | 2–5 mg/kg/day in divided doses every 6–8 h | Bronchoconstriction may occur in patients with chronic lung disease; risk of hypoglycemia |
| Digoxin | Cardiac glycoside | 8–10 μg/kg/d given in divided doses every 12 h | Arrhythmia with toxic effects; avoided in WPW syndrome, as it may enhance antegrade conduction via accessory pathway by slowing AV nodal conduction |
In a study published by Wong et al., in 2006 based on a survey of pediatric cardiologists in North America to assess the practice pattern in management of infant SVT, the most commonly used drugs for acute management in the infant without preexcitation were Digoxin (42%), Procainamide (21%), Esmolol (13%), Propranolol (10%), and Amiodarone (8%). In the infant with preexcitation, Propranolol (34%), Procainamide (23%), Esmolol (17%), Amiodarone (11%), and Digoxin (6%) were used. The most commonly used drugs in the chronic setting were Digoxin (52%), Propranolol (33%), Amiodarone (4%), and Sotalol (3%). In the infant with preexcitation, Propranolol (70%), Amiodarone (6%), Digoxin (6%), Atenolol (6%), and Flecainide (5%) were used. Medication choices were influenced by additional electrophysiology training and the presence of preexcitation, with Digoxin being used less in the setting of preexcitation [36].
Multiple studies have been published assessing the practice variation in management of neonatal and infant SVT using Pediatric Health Information System database, an inpatient administrative database in the United States. In a study by Guerrier et al. of SVT hospitalizations from 2003 to 2013 including a total of 851 patients (60% male, 44% neonates) hospitalized at 43 hospitals, monotherapy was employed in 534 (86%) patients. Propranolol, Digoxin, and amiodarone were the most frequently utilized agents while Flecainide and Procainamide were the least frequently utilized antiarrhythmic medications. The majority of infants with SVT were treated with a small number of antiarrhythmic medications during index hospitalization. Although hospital-to-hospital variation in antiarrhythmic choice exists, there appears to be no difference in readmission [37]. Seslar et al., found similar frequency of usage of anti-arrhythmic drugs in their study [38]. In another study by Bolin et al., involving patients admitted at ≤2 days of age with structurally normal hearts and treated with an antiarrhythmic medication, Digoxin use steadily decreased to 23% of antiarrhythmic medication administrations over the study period from 2004 to 2015, whereas Propranolol increased to 77% [39].
The Study of Antiarrhythmic Medications in Infancy (SAMIS) [33], a randomized, double-blind, multicenter study of infants with SVT, excluding Wolff-Parkinson-White, comparing digoxin with propranolol, showed no significant difference in SVT recurrence in infants treated with Digoxin versus Propranolol (6-month recurrence-free status was 79% for Digoxin and 67% for Propranolol). Moreover, there were no first recurrences in either group between 6 and 12 months, suggesting the current standard practice may be treating infants longer than required. In a study by Barton et al., involving 287 patients with infantile SVT, high-dose Propranolol at a total daily dose of 3.6 ± 1.0 mg/kg/day was successful in controlling SVT throughout the inpatient stay in 67.3% of patients. This study identified the presence of congenital heart disease and Wolff-Parkinson-White pattern as significant factors for lack of a successful result with inpatient propranolol monotherapy. Of 190 patients discharged on Propranolol monotherapy, 87.7% were recurrence free during follow-up [40].
A retrospective review by Price et al. involving 10 patients, showed the combination of Flecainide and Sotalol to be safe and effective in control of refractory SVT for those who failed at least two antiarrhythmic agents. Efficacy was achieved in all patients with no proarrhythmia [41].
For neonatal atrial flutter patients, chronic therapy is limited to those in whom flutter recurs after the initial cardioversion. It can be hard to control and require the use of combination therapy with drugs such as Digoxin, Propranolol, Amiodarone, Flecainide and Sotalol.
Ectopic atrial tachycardia is an important arrhythmia since it can be hard to control. Furthermore, it has a tendency to be incessant, and, because of this, can result in cardiac dilation and dysfunction (so called tachycardia-induced cardiomyopathy). Being an automatic focus tachycardia, it does not usually respond to electrical cardioversion. Often hard to control, it may require the use of combination therapy with drugs such as Digoxin, Propranolol, Amiodarone, Flecainide and Sotalol. Treatment is usually continued for 2–3 years and spontaneous resolution of the focus can be expected in that time frame [21].
In the largest series of patients with PJRT by Kang et al., β-blockers were the most common choice. There was clinical benefit for 64% of all regimens, including complete suppression of PJRT in 23%. Among medication regimens, a combination of Amiodarone and a β-blocker achieved clinical benefit in 100%. Used individually, Amiodarone and β-blockers achieved clinical benefit in 75% and 55% cases, respectively. Monotherapy with Flecainide or Propafenone and combination therapy with Digoxin and Propafenone were each beneficial in >70% of the cases [24].
For congenital JET, many patients required >2 medications, either sequentially or in combination, to eventually see a salutary drug effect on JET rate. The largest series from Collins et al., supported the use of Amiodarone for treatment of JET. Many patients required amiodarone in combination with a second agent [25]. A recent report from Dieks et al., showed that adjunctive treatment with Ivabradine resulted in effective and safe rhythm/heart rate control in patients with congenital JET [42].
Table 5 lists common second line agents for maintenance anti-arrhythmic therapy.
Table 5.
Common Second-Line Medications in the Treatment of SVT in Neonates (Oral therapy)*.
| Medication | Class | Dosing | Comments |
|---|---|---|---|
| Sotalol | Class III | Initial: 2 mg/kg/day divided every 8 h; if needed, increase dosage gradually by 1–2 mg/kg/day increments; allow at least 3 days between increments. Proposed target dose: 4 mg/kg/day divided every 8 h (up to 6 mg/kg/day in 1 month-old) | Monitor QTc interval on ECG; monitor for pro-arrhythmia, worsening of ventricular systolic dysfunction |
| Flecainide | Class IC | Initial: 2 mg/kg/day divided every 12 h; titrate to clinical response. Usual dose: 3–6 mg/kg/day | Monitor QRS duration and QTc interval on ECG; obtain plasma trough concentrations once steady state has been achieved (>5 doses after initiation or change); check interactions with other drugs; avoid concurrent administration with milk or milk-based formula feedings; Amiodarone may increase the serum concentration of Flecainide (decrease Flecainide dose by 50% in the presence of Amiodarone) |
| Propafenone | Class IC | Initial: 7–10 mg/kg/day divided every 8 h; increased by 20%–30% up to a dose of 18–20 mg/kg/day if indicated | Monitor QRS duration and QTc interval on ECG; check interactions with other drugs |
| Amiodarone | Class III | Oral loading: 10–20 mg/kg/day in 2 divided doses for 7–10 days; then reduce to 5–10 mg/kg/day once daily and continue for 2–7 months | Monitor QTc interval on ECG; monitor for pro-arrhythmia; dosage adjustment is necessary in substantial hepatic impairment; follow thyroid function tests, metabolic profile including serum glucose, electrolytes, triglycerides, liver function test, ophthalmologic exams; check interactions with other anti-arrhythmic drugs |
*The above are suggested doses. Please refer to appropriate references and drug formulary for alternative dosing.
7.3. Catheter ablation
Despite the success of medical therapy in most infants, catheter ablation may be necessary in small children when medical therapy is ineffective. The PACES/HRS expert consensus statement [43] on the use of catheter ablation in children and patients with congenital heart disease lists the following as class I indications for ablation in infants and small children:
-
•
Documented SVT, recurrent or persistent, when medical therapy is either not effective or associated with intolerable adverse effects.
-
•
WPW pattern following resuscitated cardiac arrest.
-
•
WPW pattern with syncope when there are predictors of high risk for cardiac arrest
-
•
Persistent or recurrent idiopathic JET, or congenital JET associated with ventricular dysfunction, when medical therapy is either not effective or associated with intolerable adverse effects. When ablation of JET is being performed, cryotherapy is the preferred first choice due to the high risk of AV block. RF energy should be used with extreme caution, after a detailed discussion with the family or patient concerning the high risk of AV block and the potential need for permanent pacing.
-
•
Ventricular ectopy or tachycardia with ventricular dysfunction, when medical therapy is either not effective or associated with intolerable adverse effects.
-
•
Recurrent or persistent SVT related to accessory AV connections or twin AV nodes in patients with CHD when medical therapy is either not effective or associated with intolerable adverse effects.
-
•
Ablation is effective for recurrent symptomatic atrial tachycardia occurring outside the early postoperative phase (less than three to six months) in patients with CHD, when medical therapy is either not effective or associated with intolerable adverse effects.
-
•
Pediatric cardiovascular surgical support should be available in-house during ablation procedures for smaller patients.
Most of the reported experience regarding ablation of cardiac arrhythmias in small children have involved the use of radiofrequency (RF) energy; with some using cryoablation as their first choice. Most practitioners consider the smallest infants (less than 5 kg) to be at higher risk from catheter ablation and use a higher threshold for transitioning from medical-to catheter-based therapy. However, there are no data other than anecdotal reports to support differences in outcomes or complications between these smallest patients and those up to approximately 15 kg. The risk of complications such as AV block, cardiac perforation, pericardial effusion, coronary injury and death should be considered and therefore, this option should only be considered in specialized centers by electrophysiologists with significant pediatric ablation experience.
8. Conclusions
SVT is one of the most common conditions requiring emergency cardiac care in neonates. AVRT utilizing an atrioventricular bypass tract is the most common form of SVT in neonates. There is high likelihood for spontaneous resolution of the most common arrhythmia substrates in infancy. Pharmacological agents remain the primary therapy for neonates, while, in rare cases refractory to multiple drugs singly and in combination, catheter ablation could be considered after careful consideration of risk.
Declarations of interest
None.
Funding
None.
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society.
Contributor Information
Chandra Srinivasan, Email: Chandra.Srinivasan@uth.tmc.edu.
Seshadri Balaji, Email: balajis@ohsu.edu.
References
- 1.Lau E.W. Infraatrial supraventricular tachycardias: mechanisms, diagnosis, and management. Pacing Clin Electrophysiol. 2008;31:490–498. doi: 10.1111/j.1540-8159.2008.01020.x. [DOI] [PubMed] [Google Scholar]
- 2.Geggel R.L. Conditions leading to pediatric cardiology consultation in a tertiary academic hospital. Pediatrics. 2004;114:e409–e417. doi: 10.1542/peds.2003-0898-L. [DOI] [PubMed] [Google Scholar]
- 3.Garson A., Gillette P.C., McNamara D.G. Supraventricular tachycardia in children: clinical features, response to treatment, and long-term follow-up in 217 patients. J Pediatr. 1981;98:875–882. doi: 10.1016/s0022-3476(81)80578-1. [DOI] [PubMed] [Google Scholar]
- 4.Lundberg A. Paroxysmal tachycardia in infancy. A clinical and experimental study. Acta Paediatr. 1963;52:192–195. doi: 10.1111/j.1651-2227.1963.tb03766.x. [DOI] [PubMed] [Google Scholar]
- 5.Nadas A.S., Daeschner C.W., Roth A., Blumenthal S.L. Paroxysmal tachycardia in infants and children; study of 41 cases. Pediatrics. 1952;9:167–181. [PubMed] [Google Scholar]
- 6.Weindling S.N., Saul J.P., Walsh E.P. Efficacy and risks of medical therapy for supraventricular tachycardia in neonates and infants. Am Heart J. 1996;131:66–72. doi: 10.1016/s0002-8703(96)90052-6. [DOI] [PubMed] [Google Scholar]
- 7.Turner C.J., Wren C. The epidemiology of arrhythmia in infants: a population-based study. J Paediatr Child Health. 2013;49:278–281. doi: 10.1111/jpc.12155. [DOI] [PubMed] [Google Scholar]
- 8.Wu M.-H., Chen H.-C., Kao F.-Y., Huang S.-K. Postnatal cumulative incidence of supraventricular tachycardia in a general pediatric population: a national birth cohort database study. Heart Rhythm. 2016;13 doi: 10.1016/j.hrthm.2016.06.006. 2070–5. [DOI] [PubMed] [Google Scholar]
- 9.Hinkle K.A., Peyvandi S., Stiver C., Killen S.A.S., Weng H.-Y., Etheridge S.P. Postnatal outcomes of fetal supraventricular tachycardia: a multicenter study. Pediatr Cardiol. 2017;38:1317–1323. doi: 10.1007/s00246-017-1662-1. [DOI] [PubMed] [Google Scholar]
- 10.Jongbloed M.R.M., Vicente Steijn R., Hahurij N.D., Kelder T.P., Schalij M.J., Gittenberger-de Groot A.C. Normal and abnormal development of the cardiac conduction system; implications for conduction and rhythm disorders in the child and adult. Differentiation. 2012;84:131–148. doi: 10.1016/j.diff.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Wellens H.J., Bär F.W., Lie K.I. The value of the electrocardiogram in the differential diagnosis of a tachycardia with a widened QRS complex. Am J Med. 1978;64:27–33. doi: 10.1016/0002-9343(78)90176-6. [DOI] [PubMed] [Google Scholar]
- 12.Wellens H.J.J., Bär F.W., Vanagt E.J., Brugada P., Farré J. Springer Netherlands; Dordrecht: 1981. The differentiation between ventricular tachycardia and supraventricular tachycardia with aberrant conduction: the value of the 12-lead electrocardiogram. What's new electrocardiogr; pp. 184–199. [Google Scholar]
- 13.Brugada P., Brugada J., Mont L., Smeets J., Andries E.W. A new approach to the differential diagnosis of a regular tachycardia with a wide QRS complex. Circulation. 1991;83:1649–1659. doi: 10.1161/01.cir.83.5.1649. [DOI] [PubMed] [Google Scholar]
- 14.Vereckei A., Duray G., Szenasi G., Altemose G.T., Miller J.M. Application of a new algorithm in the differential diagnosis of wide QRS complex tachycardia. Eur Heart J. 2006;28:589–600. doi: 10.1093/eurheartj/ehl473. [DOI] [PubMed] [Google Scholar]
- 15.Vereckei A., Duray G., Szénási G., Altemose G.T., Miller J.M. New algorithm using only lead aVR for differential diagnosis of wide QRS complex tachycardia. Heart Rhythm. 2008;5:89–98. doi: 10.1016/j.hrthm.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 16.Ko J.K., Deal B.J., Strasburger J.F., Benson D.W. Supraventricular tachycardia mechanisms and their age distribution in pediatric patients. Am J Cardiol. 1992;69:1028–1032. doi: 10.1016/0002-9149(92)90858-v. [DOI] [PubMed] [Google Scholar]
- 17.Anand R.G., Rosenthal G.L., Van Hare G.F., Snyder C.S. Is the mechanism of supraventricular tachycardia in pediatrics influenced by age, gender or ethnicity? Congenit Heart Dis. 2009;4:464–468. doi: 10.1111/j.1747-0803.2009.00336.x. [DOI] [PubMed] [Google Scholar]
- 18.Lisowski L.A., Verheijen P.M., Benatar A.A., Soyeur D.J., Stoutenbeek P., Brenner J.I. Atrial flutter in the perinatal age group: diagnosis, management and outcome. J Am Coll Cardiol. 2000;35:771–777. doi: 10.1016/s0735-1097(99)00589-6. [DOI] [PubMed] [Google Scholar]
- 19.Dunnigan A., Benson W., Benditt D.G. Atrial flutter in infancy: diagnosis, clinical features, and treatment. Pediatrics. 1985;75:725–729. [PubMed] [Google Scholar]
- 20.Mendelsohn A., Dick M., Serwer G.A. Natural history of isolated atrial flutter in infancy. J Pediatr. 1991;119:386–391. doi: 10.1016/s0022-3476(05)82050-5. [DOI] [PubMed] [Google Scholar]
- 21.Salerno J.C., Kertesz N.J., Friedman R.A., Fenrich A.L. Clinical course of atrial ectopic tachycardia is age-dependent: results and treatment in children <3 or ≥3 years of age. J Am Coll Cardiol. 2004;43:438–444. doi: 10.1016/j.jacc.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 22.Bradley D.J., Fischbach P.S., Law I.H., Serwer G.A., Dick M. The clinical course of multifocal atrial tachycardia in infants and children. J Am Coll Cardiol. 2001;38:401–408. doi: 10.1016/s0735-1097(01)01390-0. [DOI] [PubMed] [Google Scholar]
- 23.Critelli G., Gallagher J.J., Monda V., Coltorti F., Scherillo M., Rossi L. Anatomic and electrophysiologic substrate of the permanent form of junctional reciprocating tachycardia. J Am Coll Cardiol. 1984;4:601–610. doi: 10.1016/s0735-1097(84)80108-4. [DOI] [PubMed] [Google Scholar]
- 24.Kang K.T., Potts J.E., Radbill A.E., La Page M.J., Papagiannis J., Garnreiter J.M. Permanent junctional reciprocating tachycardia in children: a multicenter experience. Heart Rhythm. 2014;11:1426–1432. doi: 10.1016/j.hrthm.2014.04.033. [DOI] [PubMed] [Google Scholar]
- 25.Collins K.K., van Hare G.F., Kertesz N.J., Law I.H., Bar-Cohen Y., Dubin A.M. Pediatric nonpost-operative junctional ectopic tachycardia medical management and interventional therapies. J Am Coll Cardiol. 2009;53:690–697. doi: 10.1016/j.jacc.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 26.Kleinman M.E., Chameides L., Schexnayder S.M., Samson R.A., Hazinski M.F., Atkins D.L. Part 14: pediatric advanced life support: 2010 American heart association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2010;122:S876–S908. doi: 10.1161/CIRCULATIONAHA.110.971101. [DOI] [PubMed] [Google Scholar]
- 27.de Caen A.R., Berg M.D., Chameides L., Gooden C.K., Hickey R.W., Scott H.F. Part 12: pediatric advanced life support: 2015 American heart association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132:S526–S542. doi: 10.1161/CIR.0000000000000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gallagher J.J., Smith W.M., Kerr C.R., Kasell J., Cook L., Reiter M. Esophageal pacing: a diagnostic and therapeutic tool. Circulation. 1982;65:336–341. doi: 10.1161/01.cir.65.2.336. [DOI] [PubMed] [Google Scholar]
- 29.Janoušek J. Diagnostic and therapeutic use of transesophageal atrial pacing in children. Int J Cardiol. 1989;25:7–14. doi: 10.1016/0167-5273(89)90155-1. [DOI] [PubMed] [Google Scholar]
- 30.Paech C., Janousek J., Wagner F., Gebauer R.A. Rate control by transoesophageal atrial overdrive pacing for refractory supraventricular tachycardia with severe ventricular dysfunction: a bridge to recovery. Pediatr Cardiol. 2017;38:228–233. doi: 10.1007/s00246-016-1503-7. [DOI] [PubMed] [Google Scholar]
- 31.Giardina A.C., Ehlers K.H., Engle M.A. Wolff-Parkinson-White syndrome in infants and children. A long-term follow-up study. Br Heart J. 1972;34:839–846. doi: 10.1136/hrt.34.8.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deal B.J., Keane J.F., Gillette P.C., Garson A. Wolff-Parkinson-White syndrome and supraventricular tachycardia during infancy: management and follow-up. J Am Coll Cardiol. 1985;5:130–135. doi: 10.1016/s0735-1097(85)80095-4. [DOI] [PubMed] [Google Scholar]
- 33.Sanatani S., Potts J.E., Reed J.H., Saul J.P., Stephenson E.A., Gibbs K.A. The study of antiarrhythmic medications in infancy (SAMIS): a multicenter, randomized controlled trial comparing the efficacy and safety of digoxin versus propranolol for prophylaxis of supraventricular tachycardia in infants. Circ Arrhythmia Electrophysiol. 2012;5:984–991. doi: 10.1161/CIRCEP.112.972620. [DOI] [PubMed] [Google Scholar]
- 34.Perry J.C., Garson A. Supraventricular tachycardia due to Wolff-Parkinson-White syndrome in children: early disappearance and late recurrence. J Am Coll Cardiol. 1990;16:1215–1220. doi: 10.1016/0735-1097(90)90555-4. [DOI] [PubMed] [Google Scholar]
- 35.Kugler J.D. Radiofrequency catheter ablation for supraventricular tachycardia. Should it be used in infants and small children? Circulation. 1994;90:639–641. doi: 10.1161/01.cir.90.1.639. [DOI] [PubMed] [Google Scholar]
- 36.Wong K.K., Potts J.E., Etheridge S.P., Sanatani S. Medications used to manage supraventricular tachycardia in the infant a North American survey. Pediatr Cardiol. 2006;27:199–203. doi: 10.1007/s00246-005-1126-x. [DOI] [PubMed] [Google Scholar]
- 37.Guerrier K., Shamszad P., Czosek R.J., Spar D.S., Knilans T.K., Anderson J.B. Variation in antiarrhythmic management of infants hospitalized with supraventricular tachycardia: a multi-institutional analysis. Pediatr Cardiol. 2016;37:946–952. doi: 10.1007/s00246-016-1375-x. [DOI] [PubMed] [Google Scholar]
- 38.Seslar S.P., Garrison M.M., Larison C., Salerno J.C. A multi-institutional analysis of inpatient treatment for supraventricular tachycardia in newborns and infants. Pediatr Cardiol. 2013;34:408–414. doi: 10.1007/s00246-012-0474-6. [DOI] [PubMed] [Google Scholar]
- 39.Bolin E.H., Lang S.M., Tang X., Collins R.T. Propranolol versus digoxin in the neonate for supraventricular tachycardia (from the pediatric Health information system) Am J Cardiol. 2017;119:1605–1610. doi: 10.1016/j.amjcard.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 40.Barton A.L., Moffett B.S., Valdes S.O., Miyake C., Kim J.J. Efficacy and safety of high-dose propranolol for the management of infant supraventricular tachyarrhythmias. J Pediatr. 2015;166:115–118. doi: 10.1016/j.jpeds.2014.08.067. [DOI] [PubMed] [Google Scholar]
- 41.Fenrich A.L., Friedman R.A., Kertesz N.J., Snyder C.S., Price J.F. Flecainide and sotalol: a new combination therapy for refractory supraventricular tachycardia in children <1 year of age. J Am Coll Cardiol. 2002;39:517–520. doi: 10.1016/s0735-1097(01)01773-9. [DOI] [PubMed] [Google Scholar]
- 42.Dieks J.-K., Klehs S., Müller M.J., Paul T., Krause U. Adjunctive ivabradine in combination with amiodarone: a novel therapy for pediatric congenital junctional ectopic tachycardia. Heart Rhythm. 2016;13:1297–1302. doi: 10.1016/j.hrthm.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 43.Philip Saul J., Kanter R.J., COMMITTEE W., Abrams D., Asirvatham S., Bar-Cohen Y. PACES/HRS expert consensus statement on the use of catheter ablation in children and patients with congenital heart disease: developed in partnership with the Pediatric and Congenital Electrophysiology Society (PACES) and the Heart Rhythm Society (HRS) E. Heart Rhythm. 2016;13:e251–e289. doi: 10.1016/j.hrthm.2016.02.009. [DOI] [PubMed] [Google Scholar]