Abstract
Background
In 2013, the Pan American Health Organization established a multi-site, multi-country network to evaluate influenza vaccine effectiveness (VE). We pooled data from five consecutive seasons in five countries to conduct an analysis of southern hemisphere VE against laboratory-confirmed influenza hospitalizations in young children and older adults.
Methods
We used a test-negative design to estimate VE against laboratory-confirmed influenza in hospitalized young children (aged 6─24 months) and older adults (aged ≥60 years) in Argentina, Brazil, Chile, Colombia, and Paraguay. Following country-specific influenza surveillance protocol, hospitalized persons with severe acute respiratory infections (SARI) at 48 sentinel hospitals (March 2013–December 2017) were tested for influenza virus infection by rRT-PCR. VE was estimated for young children and older adults using logistic random effects models accounting for cluster (country), adjusting for sex, age (months for children, and age-in-year categories for adults), calendar year, country, preexisting conditions, month of illness onset and prior vaccination as an effect modifier for the analysis in adults.
Results
We included 8426 SARI cases (2389 children and 6037 adults) in the VE analyses. Among young children, VE against SARI hospitalization associated with any influenza virus was 43% (95%CI: 33%, 51%) for children who received two doses, but was 20% (95%CI: −16%, 45%) and not statistically significant for those who received one dose in a given season. Among older adults, overall VE against SARI hospitalization associated with any influenza virus was 41% (95%CI: 28%, 52%), 45% (95%CI: 34%, 53%) against A(H3N2), 40% (95%CI: 18%, 56%) against A(H1N1)pdm09, and 20% (95%CI: −40%, 54%) against influenza B viruses.
Conclusions
Our results suggest that over the five-year study period, influenza vaccination programs in five South American countries prevented more than one-third of laboratory confirmed influenza-associated hospitalizations in young children receiving the recommended two doses and vaccinated older adults.
Keywords: Influenza vaccine effectiveness, Children, Adults, Southern hemisphere, Latin America, Severe acute respiratory infections, Test-negative case-control design, Hospitalizations
1. Introduction
Since 2012, countries of Latin America and the Caribbean (LAC) have explored ways to generate regional evidence for the effectiveness of southern hemisphere influenza vaccines in preventing influenza-associated hospitalizations among persons targeted for vaccination in order to inform policy decisions regarding continued investments in influenza vaccines [1], [2]. For vaccine-naïve children, national vaccination schedules in LAC follow the WHÓs influenza vaccination recommendation to administer two doses of influenza vaccine [3]; nevertheless, reaching high coverage for the second dose remains a challenge. Gaps in the evidence remain about vaccine effectiveness (VE) against rRT-PCR–confirmed influenza hospitalizations especially among young children, a major vaccination target group in LAC. To address this gap in knowledge, 13 countries in Latin America collaborated through the REVELAC-i (Spanish acronym for Network for the Evaluation of influenza Vaccine Effectiveness in Latin America and the Caribbean) network [4] to estimate influenza VE among hospitalized young children by comparing VE among those vaccine-naïve who received two doses and those who received only one dose versus none, and VE among hospitalized older adults, another target group for vaccination in the Americas [3]. Following the 2012–13 launch of REVELAC-i [1], [5], Argentina, Brazil, Chile, Colombia, and Paraguay have consistently contributed data for VE analyses. For this study we pooled data from 2013 to 2017 in these five South American countries to conduct an analysis of southern hemisphere VE against influenza hospitalization in young children and older adults.
2. Methods
2.1. Study design and population
We implemented a test-negative design that compares the odds of vaccination between influenza test–positive hospitalized cases and influenza test–negative hospitalized controls [6], [7], [8], [9], [10]. We focused our evaluation on the population of community-dwelling children aged 6–24 months, and older adults targeted for government-sponsored influenza vaccination, i.e., those aged ≥60 years in Brazil, Colombia, and Paraguay and those aged ≥65 years in Argentina and Chile. For this study, we combined older adults within their local targeted age ranges into a common group of older adults aged ≥60 years. We used a common protocol, and an online data collection platform as previously described [1], [5] to conduct the VE evaluation at 48 severe acute respiratory infection (SARI) sentinel surveillance hospitals participating in SARINet, the Pan American Health Organization (PAHO) hospital-based influenza surveillance network in the Americas. Sentinel sites were located in Argentina (n = 4), Brazil (n = 29), Chile (n = 6), Colombia (n = 7), and Paraguay (n = 2) and followed standard PAHO regional surveillance guidelines [2]. In all countries except Colombia, all identified SARI patients at the participant hospitals were tested. In Colombia, as per protocol, only a convenient sample of five specimens per week per hospital was tested [5], [11].
2.2. Study period
The VE evaluation start date for each country in any given year was the date of illness onset of the first SARI case with rRT-PCR–confirmed influenza that was at least two weeks after the start of the country’s annual national influenza vaccination campaign (typically April). The VE evaluation period ended two weeks after illness onset of the last SARI case with rRT-PCR–confirmed influenza.
2.3. Definitions and data collection
We extracted information from SARI surveillance databases, including age, sex, date of illness onset, date of respiratory specimen collection, diagnosed preexisting medical conditions, receipt of antiviral treatment, intensive care unit admission, death, influenza vaccination status, and vaccination dates in the current and prior season. Reference laboratories for influenza provided information about influenza type/subtype through rRT-PCR results [5]. A “case” was defined following the surveillance SARI case definition (acute respiratory infection with history of fever or measured fever of ≥38 °C and cough with onset within the last 10 days, requiring hospitalization) with rRT-PCR–confirmed infection with influenza A or B viruses. Controls were participants with SARI who tested negative for influenza by rRT-PCR.
Depending on the data sources available in each country, influenza vaccination status was documented by reviewing vaccination cards brought in by cases or family members during hospitalization (in Argentina, Colombia, and Paraguay); local Expanded Program on Immunization records (in Argentina, Brazil, Colombia, and Paraguay); or electronic immunization registries (in Chile and Colombia). We considered a person vaccinated if he/she received vaccination at least 14 days before illness onset [5]. We considered a child singly vaccinated if he/she received one dose of trivalent inactivated influenza virus vaccine (IIV3) in the current season and as twice vaccinated if he/she received two doses of vaccine in the current season [3], [12]. Southern Hemisphere formulation unadjuvanted IIV3 was available in all countries for the VE evaluation period. From the enrollment goals, we anticipated requiring a sample size of at least 138 influenza cases and 414 controls per age group and per year from the region to detect a hypothetical VE of 50% with 80% power, an alpha-type error of 5%, and an estimated vaccine coverage of 30% among the controls [5].
2.4. Exclusions
We excluded SARI cases with an illness onset ≤2 weeks after the start of the national influenza vaccination campaign in each country. We also excluded SARI cases with >10 days between illness onset and specimen collection to avoid including false negatives in the control group [2] and those for whom illness onset date was unavailable because of the potential risk of misclassification of case status. Additionally, patients with missing vaccination status or missing vaccination dates were excluded because of the potential risk of misclassification of exposure. Cases vaccinated after illness onset were classified as unvaccinated; those vaccinated <2 weeks before the onset of illness were excluded from the analysis.
2.5. Statistical analysis
For each group, young children and older adults, we compared demographic and clinical characteristics between cases and controls and by vaccination status and evaluated differences using the Chi-square test. We calculated the adjusted odds ratio of vaccination in cases versus controls, by type/subtype. We adjusted models for variables selected a priori: sex, age (age in months for children, and in subcategorized age groups 60–69, 70–79, 80+ years for older adults), calendar year, country, preexisting conditions (presence of at least one vs. none), and month of illness onset, and vaccination in the previous year was added as an effect modifier for older adults [9], [13]. Other variables including time between illness onset and specimen collection and ICU admission did not modify VE significantly (by 5%) and therefore were not included in the models. We built random effect models with a robust variance adjusted for cluster samples (countries) by the Huber-White method to incorporate heterogeneity [14]. We present adjusted VE (1- adjusted odds ratio of vaccination in cases vs. controls, multiplied by 100) for each model as well as their 95% confidence intervals. For young children, we evaluated single and two-dose vaccination VE for influenza seasons 2013–2017. We used unvaccinated children in any given season and prior years as the reference group for single and two-dose VE. We excluded those who received prior year vaccination because of indeterminate priming history. We performed all analyses using the R software (version 3.4.4).
2.6. Ethical considerations
The institutional review boards at the Pan American Health Organization, US-CDC, and each of the participant countries reviewed the protocol and considered it a vaccination effectiveness evaluation (non-intervention study). We did not collect personal identifiers.
3. Results
3.1. SARI case enrollment
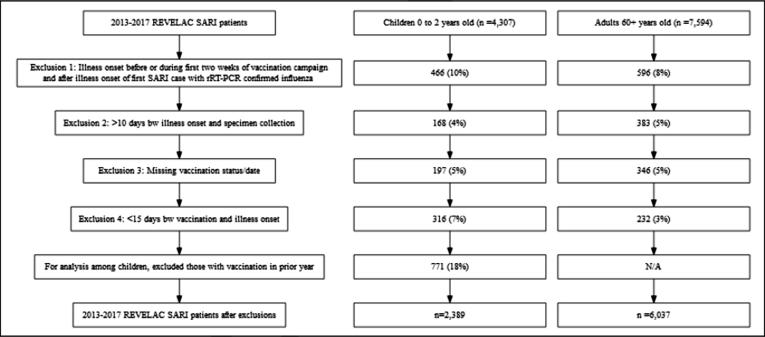
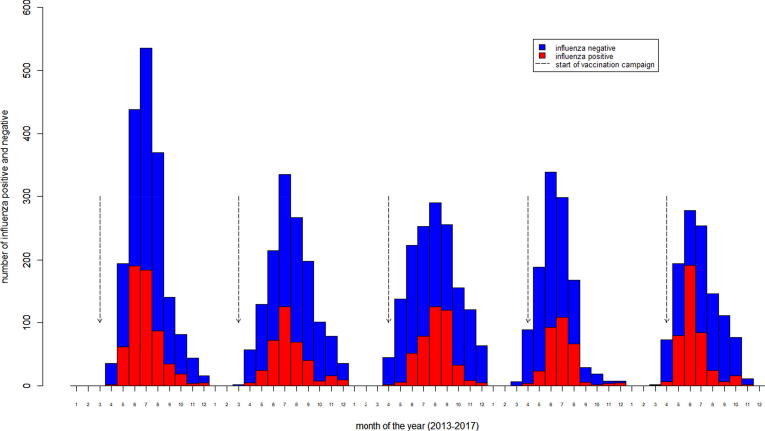
During April 2013 through December 2017, we identified 4037 SARI patients aged 6–24 months; of these, we excluded 466 (10%) enrolled outside the VE evaluation period, 168 (4%) who had >10 days between illness onset and specimen collection, 197 (5%) who had missing vaccination status, and 316 (7%) who had <2 weeks between vaccination and illness onset. We also excluded those children with vaccination in the prior year (n = 771). We identified 7594 SARI patients aged ≥60 years; of these, we excluded 596 (8%) enrolled outside the program evaluation period, 383 (5%) who had >10 days between illness onset and specimen collection, 346 (5%) who had missing vaccination status, and 232 (3%) who had <2 weeks between vaccination and illness onset (Fig. 1; more information on Supplementary Tables A1-2 and B1-2). The majority (93%) of the influenza cases had illness onset during May-September, with the peak of activity occurring during the two months of June and July when 56% of influenza cases were identified (Fig. 2, Fig. 3).
Fig. 1.
Selection of severe acute respiratory infections (SARI) patients included in influenza vaccine effectiveness analyses, REVELAC-i, five South America American countries, 2013–2017.
Fig. 2.
Distribution of patients with influenza positive and negative severe acute respiratory infections per month of illness onset and start of influenza vaccination campaign, REVELAC-i, five South American countries, 2013–2017.
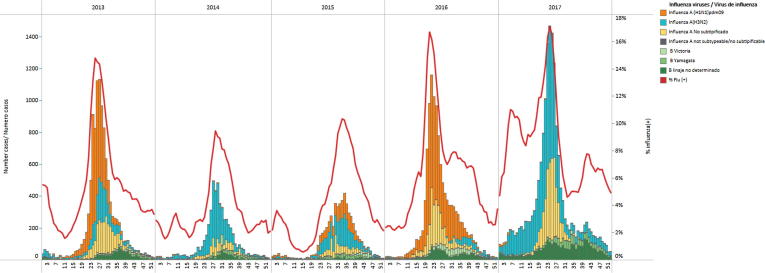
Fig. 3.
Influenza virus circulation in five South American countries, 2013–2017. *No SARI data available from Brazil, year 2016. Data Source: Influenza and Other Respiratory Virus Surveillance Systems in the Americas. Pan American Health Organization/World Health Organization.
3.2. Characteristics of SARI cases aged 6–24 months
Among children, 413 (17%) were influenza positive (cases) and 1976 (83%) were influenza negative (controls) (Table 1). Among cases, 122 (33%), 187 (50%), and 66 (18%) were identified as being infected with influenza A(H3N2), A(H1N1)pdm09, and B viruses, respectively. Compared to controls, cases were less likely to receive two doses of influenza vaccination in a given season. We did not observe significant differences in gender, chronic conditions, and severe hospitalization outcomes (e.g., ICU admission, death) between cases and controls; the proportion of cases sampled between day 6 and 10 from symptom onset was slightly higher than that among controls (22 vs 16%, p-value = 0.01).
Table 1.
Demographic and clinical characteristics of enrolled patients aged 6–24 months with influenza-associated severe acute respiratory infections (SARI), by case status and vaccination status, REVELAC-i, five South American countries, 2013–2017 (n = 2389).
| Variable | Cases n = 413 n (%) |
Controls n = 1976 n (%) |
p-value* | Vaccinated Two-dose n = 611 n (%) |
Vaccinated Single n = 445 n (%) |
Unvaccinated n = 1333 n (%) |
p-value* |
|---|---|---|---|---|---|---|---|
| Country | |||||||
| Argentina | 34 (8) | 205 (10) | <0.001 | 88 (14) | 2 (0.4) | 149 (11) | <0.001 |
| Brazil | 97 (23) | 255 (13) | 119 (19) | 107 (24) | 126 (9) | ||
| Chile | 141 (34) | 877 (44) | 309 (51) | 187 (42) | 522 (39) | ||
| Colombia | 38 (9) | 97 (5) | 74 (12) | 23 (5) | 38 (3) | ||
| Paraguay | 103 (25) | 542 (27) | 21 (3) | 126 (28) | 498 (37) | ||
| Year | |||||||
| 2013 | 165 (40) | 540 (27) | <0.001 | 201 (33) | 178 (40) | 326 (24) | <0.001 |
| 2014 | 47 (11) | 327 (17) | 156 (26) | 48 (11) | 170 (13) | ||
| 2015 | 83 (20) | 505 (26) | 121 (20) | 91 (20) | 376 (28) | ||
| 2016 | 89 (22) | 343 (17) | 81 (13) | 73 (16) | 278 (21) | ||
| 2017 | 29 (7) | 261 (13) | 52 (9) | 55 (12) | 183 (14) | ||
| Gender | |||||||
| Male | 225 (54) | 1071 (46) | 0.96 | 326 (53) | 239 (54) | 731 (55) | 0.80 |
| Female | 188 (46) | 905 (54) | 285 (47) | 206 (46) | 602 (45) | ||
| ≥one chronic condition**** | 100 (25) | 527 (27) | 0.46 | 167 (30) | 86 (20) | 374 (28) | <0.001 |
| Asthma | 6 (2) | 49 (3) | 0.32 | 15 (3) | 10 (2) | 30 (3) | 0.69 |
| Diabetes | 0 (0) | 3 (0.1) | 0.98 | 1 (0.1) | 1 (0.2) | 1 (0.1) | 0.70 |
| Respiratory disease | 14 (4) | 87 (5) | 0.51 | 37 (7) | 15 (4) | 49 (4) | 0.005 |
| Cardiovascular disease | 8 (2) | 37 (2) | 1.00 | 14 (3) | 6 (2) | 25 (2) | 0.44 |
| Liver disease | 2 (0.5) | 10 (0.5) | 1.00 | 1 (0.2) | 0 (0) | 11 (1) | 0.04 |
| Renal disease | 2 (0.5) | 11 (0.6) | 1.00 | 6 (1) | 3 (0.7) | 4 (0.3) | 0.11 |
| Immune disease | 2 (0.5) | 22 (1) | 0.38 | 8 (2) | 3 (0.7) | 13 (1) | 0.43 |
| Influenza vaccination status | |||||||
| Two-dose vaccination** | 89 (22) | 522 (26) | 0.05 | – | – | ||
| Single vaccination*** | 83 (20) | 362 (18) | 0.44 | – | – | ||
| Unvaccinated | 241 (58) | 1092 (55) | 0.27 | – | – | ||
| Positive influenza case | – | – | 89 (15) | 83 (19) | 241 (18) | 0.12 | |
| Days from illness onset to specimen collection | |||||||
| 0–2 | 169 (41) | 890 (45) | 0.006 | 277 (45) | 189 (42) | 593 (44) | 0.72 |
| 3–5 | 152 (37) | 774 (39) | 229 (37) | 173 (39) | 524 (39) | ||
| 6–10 | 92 (22) | 312 (16) | 105 (17) | 83 (19) | 216 (16) | ||
| Admitted to ICU | 47 (12) | 197 (10) | 0.40 | 51 (8) | 50 (12) | 143 (11) | 0.34 |
| Deceased | 8 (2) | 20 (1) | 0.17 | 4 (0.7) | 5 (1) | 19 (1) | 0.41 |
X2 test. **Received two vaccine doses in current season. ***Received one vaccine dose in current season. ****Percentages exclude missing data.
Of 2389 children, 611 (26%) and 445 (19%) received two and one dose of influenza vaccination, respectively. The remaining were classified as unvaccinated (n = 1333). Chronic conditions were more prevalent among those who received two doses of vaccine and among the unvaccinated. We did not observe significant differences in severe outcomes by vaccination status.
3.3. VE among young children
With those unvaccinated in current and prior season as the reference group, the adjusted vaccine effectiveness (VE) of two-doses of IIV3 against any influenza virus type/subtype was 43% (95%CI: 33%, 51%) (Table 2). Point estimates for VE of two-dose vaccination were 42% (95%CI: −9%, 69%) against A(H3N2), 48% (95%CI: 31%, 60%)against A(H1N1)pdm09, and 36% (95%CI: −34%, 69%) against influenza B SARI; only the VE for influenza A(H1N1)pdm09 was statistically significant. The VE of single-dose IIV3 vaccination (i.e., one dose among otherwise vaccine-naïve children) against any influenza was 20% (95%CI: −16%, 45%), and neither this effect nor virus-specific VE (-2% (95%CI: −24%, 16%) for A[H3N2], 35% (95%CI: −30%, 67%) for A[H1N1]pdm09, and 3% (95%CI: −110%, 55%) for B influenza) were statistically significant. Point estimates for VE of two-doses were higher than a single dose against all viruses, A(H3N2), A(H1N1)pdm09 and influenza B viruses, although confidence intervals overlapped.
Table 2.
Estimates of influenza vaccine effectiveness for children aged 6–24 months, single and two dose vaccination, REVELAC-i, five South American countries, 2013–2017.
| Cases |
Controls |
aVE % (95%CI) |
|||
|---|---|---|---|---|---|
| Vaccinated (%) | Unvaccinated (%) | Vaccinated (%) | Unvaccinated (%) | ||
| Any influenza virus | |||||
| Two-dose vaccination | 89 (27) | 241 (73) | 522 (32) | 1092 (68) | 43 (33, 51)† |
| Single vaccination | 83 (26) | 241 (74) | 362 (25) | 1092 (75) | 20 (-16, 45)† |
| Unvaccinated | Reference | ||||
| H3N2 | |||||
| Two-dose vaccination | 31 (32) | 66 (68) | 522 (32) | 1092 (68) | 42 (-9, 69)† |
| Single vaccination | 25 (27) | 66 (73) | 362 (22) | 1092 (75) | −2 (-24, 16)† |
| Unvaccinated | Reference | ||||
| H1N1p | |||||
| Two-dose vaccination | 39 (26) | 113 (74) | 522 (32) | 1092 (68) | 48 (31, 60)† |
| Single vaccination | 35 (24) | 113 (76) | 362 (22) | 1092 (75) | 35 (-30, 67)† |
| Unvaccinated | Reference | ||||
| B | |||||
| Two-dose vaccination | 16 (24) | 50 (76) | 522 (32) | 1092 (68) | 36 (-34, 69)† |
| Single vaccination | 20 (29) | 50 (71) | 362 (22) | 1092 (75) | 3 (-110, 55)† |
| Unvaccinated | Reference | ||||
Adjusted for sex, age (in months), month of illness onset, pre-existing conditions, year and country. Random effect model with a robust variance adjusted for cluster samples (country) by the Huber-White method.
3.4. Characteristics of SARI cases aged ≥60 years
Among older adults, 1524 (25%) were cases and 4513 (75%) were controls (Table 3). Among cases, 791 (52%), 431 (28%), and 219 (14%) were identified as being infected with influenza A(H3N2), A(H1N1)pdm09, and B viruses, respectively. Compared to controls, cases were less likely to receive influenza vaccination in a given season. We did not observe significant differences in having at least one chronic condition, and severe hospitalization outcomes (e.g., ICU admission, death) between cases and controls. Among 6037 adults, 2793 (46%) received influenza vaccination in a given season. Chronic conditions were more prevalent among those who received vaccination. In addition, having a positive diagnosis of influenza was more likely to occur among the unvaccinated persons. In-hospital death was also more likely among this group. We did not observe a significant difference in ICU admission by vaccination status.
Table 3.
Demographic and clinical characteristics of enrolled patients aged ≥60 years with severe acute respiratory infections (SARI) among influenza cases and controls and vaccination status, REVELAC-i, five South American countries, 2013–2017 (n = 6037).
| Variable | Cases n = 1524 n (%) |
Controls n = 4513 n (%) |
p-value* | Vaccinated n = 2793 n (%) |
Unvaccinated n = 3244 n (%) |
p-value* |
|---|---|---|---|---|---|---|
| Age in years | ||||||
| 60–70 | 463 (30) | 1217 (27) | 0.03 | 680 (24) | 1000 (31) | <0.001 |
| 71–80 | 494 (32) | 1556 (34) | 1006 (36) | 1044 (32) | ||
| ≥80 | 567 (37) | 1740 (39) | 1107 (40) | 1200 (37) | ||
| Country | ||||||
| Argentina | 110 (7) | 303 (7) | <0.001 | 162 (6) | 251 (8) | <0.001 |
| Brazil | 272 (18) | 725 (16) | 646 (23) | 351 (11) | ||
| Chile | 849 (56) | 2901 (64) | 1834 (66) | 1916 (59) | ||
| Colombia | 88 (6) | 221 (5) | 49 (2) | 260 (8) | ||
| Paraguay | 205 (13) | 363 (8) | 102 (4) | 466 (14) | ||
| Year | ||||||
| 2013 | 385 (25) | 1166 (26) | <0.001 | 807 (29) | 744 (23) | <0.001 |
| 2014 | 270 (18) | 931 (21) | 616 (22) | 585 (18) | ||
| 2015 | 316 (21) | 903 (20) | 509 (18) | 710 (22) | ||
| 2016 | 194 (13) | 705 (16) | 364 (13) | 535 (16) | ||
| 2017 | 359 (24) | 808 (18) | 497 (18) | 670 (21) | ||
| Gender | ||||||
| Male | 635 (42) | 2090 (46) | 0.002 | 1317 (47) | 1408 (43) | 0.004 |
| Female | 889 (58) | 2423 (54) | 1476 (53) | 1836 (57) | ||
| ≥one chronic condition | 1189 (80) | 3555 (80) | 0.69 | 2247 (83) | 2497 (78) | <0.001 |
| Asthma | 111 (11) | 283 (9) | 0.15 | 182 (10) | 212 (9) | 0.21 |
| Diabetes | 379 (26) | 1025 (23) | 0.05 | 676 (25) | 728 (23) | 0.13 |
| Respiratory disease | 282 (31) | 924 (34) | 0.04 | 601 (40) | 605 (29) | <0.001 |
| Cardiovascular disease | 279 (32) | 864 (32) | 0.73 | 558 (35) | 585 (30) | <0.001 |
| Liver disease | 24 (2) | 78 (2) | 0.82 | 37 (1) | 65 (2) | 0.04 |
| Renal disease | 134 (10) | 433 (11) | 0.43 | 287 (12) | 280 (9) | 0.007 |
| Obesity | 87 (7) | 264 (7) | 0.99 | 162 (7) | 189 (7) | 0.99 |
| Immune disease | 65 (5) | 235 (7) | 0.16 | 123 (6) | 177 (7) | 0.19 |
| Annual influenza vaccination** | 568 (37) | 2225 (49) | <0.001 | – | – | |
| Positive influenza case | – | – | 568 (20) | 956 (29) | <0.001 | |
| Days from illness onset to specimen collection | ||||||
| 0–2 | 619 (41) | 1977 (44) | 0.08 | 1237 (44) | 1359 (42) | 0.02 |
| 3–5 | 615 (40) | 1697 (38) | 1075 (38) | 1237 (38) | ||
| 6–10 | 290 (19) | 839 (19) | 481 (17) | 648 (20) | ||
| Admitted to ICU | 282 (19) | 852 (20) | 0.85 | 529 (19) | 605 (20) | 0.58 |
| Deceased | 192 (13) | 557 (13) | 0.86 | 291 (11) | 458 (15) | <0.001 |
X2 test.
Received influenza vaccination in concurrent season, regardless prior season.
3.5. VE among older adults
Among older adults, the VE of current season IIV3 against all influenza viruses was 41% (95% CI: 28, 52). By subtype, VE was 45% (95%CI: 34%, 53%) against A(H3N2), 40% (95%CI: 18%, 56%) against A(H1N1)pdm09, and 20% (95%CI: −40%, 54%) against influenza B SARI; only the VE for B virus was not significant (Table 4).
Table 4.
Estimates of influenza vaccine effectiveness for adults ≥60 years, REVELAC-i, five South American countries, 2013–2017.
| Cases |
Controls |
aVE † % (95%CI) |
|||
|---|---|---|---|---|---|
| Vaccinated (%) | Unvaccinated (%) | Vaccinated (%) | Unvaccinated (%) | ||
| Any influenza virus | 568 (37) | 956 (63) | 2225 (49) | 2288 (51) | 41 (28, 52) |
| H3N2 | 307 (39) | 484 (61) | 2225 (49) | 2288 (51) | 45 (34, 53) |
| H1N1p | 148 (34) | 283 (66) | 2225 (49) | 2288 (51) | 40 (18, 56) |
| B | 100 (46) | 119 (54) | 2225 (49) | 2288 (51) | 20 (-40, 54) |
| Unvaccinated | Reference | ||||
Adjusted for prior vaccination (interaction term), sex, age (in subcategorized age group −60–69, 70–79, 80+ years- for adults 60+), month of illness onset, pre-existing conditions, year and country. Random effect model with a robust variance adjusted for cluster samples (country) by the Huber-White method.
4. Discussion
In our multi-country 5-year pooled analysis, young children who received two doses of IIV3 in the same influenza season were 43% less likely to be hospitalized with influenza-associated SARI compared to young children who received no influenza vaccine that season or in the previous season. Vaccination with IIV3 also reduced the likelihood of SARI-influenza hospitalization among older adults by 41% compared to older adults not receiving the influenza vaccine that season.
This VE evaluation suggests that young vaccine-naïve children in South America benefited the most when receiving two doses of influenza vaccine. Our VE findings were consistent with those published by Segaloff et al [15] in a study in Israel in children under eight years of age looking at influenza vaccine effectiveness between fully versus partially vaccinated children. In their analysis covering season 2015–16 to 2017–18, they found an overall VE of 54% (95%CI 39, 68) against any influenza virus, among children fully vaccinated; whereas VE among those partially vaccinated was 26% (95%CI −3, 47). Our VE estimate against influenza B virus was also consistent with their VE estimate of 42% (95%CI −3, 47) and 4% (95%CI −45, 38) among those fully and partially vaccinated, respectively. Segaloff et al, however, do not present estimates by subtype but per season. Although some of their estimates per season and per influenza virus type are significant, they may differ from what we obtained due to sample size. We observe, however, a similar trend of higher VE estimates among those fully vaccinated as compared to those partially vaccinated. In addition, their age group included those aged 6 months to 8 years, whereas our analysis focused on those aged 6 months to 2 years and it is thought that younger children may not achieve the same level of protective response as older children due to their immature immune system [16]. Although there are limited VE data for children aged <2 years, our finding is in agreement with previous studies conducted in multiple countries in a variety of populations that support two-dose influenza vaccination among vaccine-naïve children [17], [18], [19], [20], [21], [22], [23], [24], [25]. In addition, full two-dose vaccination may also improve the protective benefit of vaccination in subsequent seasons [18]. Immunization programs could assess local barriers to the timely uptake of a second dose of influenza vaccine among vaccine-naïve children and design strategies to improve its uptake.
Among older adults, we found that our overall VE estimate using pooled data from 2013 to 2017 was consistent with the adult inpatient VE estimates from New Zealand for the winter seasons of 2012–2015 [26]. Our VE estimates for A(H1N1)pdm09, H3N2, and B viruses in inpatients were also similar to the pooled estimates of VE by Rondy et al. among inpatients in Europe (VE of 33% (95%CI: 21, 45) against H3N2, 37% (95%CI: 30, 44) against A(H1N1)pdm09 and 31% (95%CI: 11, 51) against B viruses) [27]. We included vaccination in the prior year as an effect modifier in our models to account for the potential residual immunological effect of prior influenza vaccination, thus reducing bias that could modify influenza VE [13], [28]. We further analyzed VE by prior vaccination status (Supplementary Table C) and although point estimates followed trends that have been described in previous studies -lower VE estimates against H3N2 and B viruses among those who received vaccination in previous and current season as compared to those who received vaccination in the current season only [28], [29]; the confidence intervals overlapped. The effect of repeated vaccination remains a subject of investigation that might be best addressed by including more comprehensive data or through more rigorous study designs [28].
The REVELAC-i Network offers a unique opportunity in LAC to address important questions regarding influenza vaccination and adds to the global literature on influenza vaccine effectiveness with laboratory confirmation of hospitalized cases and verified vaccination status in the southern hemisphere. One strength of the REVELAC-i network is its design and common protocol to estimate VE [1], [5]. In the past decade, the test-negative design has become the most popular approach to effectively measure influenza VE based on surveillance networks because of its practicality, low cost, and validity when detecting laboratory-confirmed influenza illnesses using RT-PCR [9], [27], [30]. Through this approach, we were able to pool data from five countries across multiple seasons. All participating countries implemented multi-site data collection using the standard PAHO regional surveillance guidelines that recommended active case-finding and systematic specimen collection from all SARI cases, which allowed for methodological consistency across the countries [2]. Another strength of our data set was the vaccination status ascertainment, using documented vaccination history only, to minimize recall biases. REVELAC-i documents vaccination status and history from vaccination cards kept by patients and their families, medical records, and registries. Although our network lacks precise estimates of the number of vaccination records we have missed, Jackson’s simulation suggests this would bias our VE estimates toward the null, meaning we likely underestimated true VE [31].
There were several limitations to this analysis. First, our study was based on an existing sentinel surveillance system which was not designed to generate VE estimates. We tried to homogenize case inclusion and specimen collection in participating sites by conducting trainings and implementing standard operating procedures but acknowledge that underreporting of SARI cases may have occurred, which could have led to selection bias if the underreporting was not equally distributed across all groups. It is noteworthy that patients seeking healthcare at sentinel sites may not represent the country population, therefore, we only aimed for internal validity of our analyses. Second, despite the network’s advantage of being able to pool data across countries, we were not able to achieve sample sizes for the analyses by subtype, much less by year and subtype. Furthermore, our sample size calculations did not account for model adjustment or cluster design (by hospital). In addition, sample size was insufficient for young children because of the smaller age range targeted for vaccination in the countries included in this analysis and an insufficient number of pediatric hospitals among sentinel sites. Third, we ultimately excluded approximately 20% of our original data due to data failing to meet inclusion criteria or missing data. Working with individual countries to increase data quality and sample size would benefit further analyses. Fourth, despite our best effort to adjust by season and country differences, it is likely that by pooling the data across seasons and sites we did not account for all inherent characteristics of unique influenza seasons and country-specific characteristics including recruitment heterogeneity and adjusting for hospital cluster. However, we observed similar trends of results by season (across sites) and by site (across seasons) (data not shown). Fifth, by adding an interaction term to the VE analysis in adults we may have reduced confounding by repeated vaccination [13]. However, we did not take into consideration the unique effects of the interactions of different strains in the vaccine and those that circulated every year on the risk of influenza infection. Finally, SARI cases included in the analysis were not individually linked to antigenic and genetic characterizations of identified influenza viruses, including B lineages. The REVELAC-i network is currently working to improve the integration of VE and virus characterization data to assess VE by genetic group.
This monitoring system provided multi-season regional evidence for the benefits of influenza vaccination in South America that substantiates the value of national investments in vaccination of children and older adults. Such investments are currently in place in 56% and 67% of Latin American and Caribbean countries, respectively [32]. To further advance national investments in influenza vaccination, VE estimates can be combined with local influenza burden of disease and cost of illness data to estimate the impact of vaccination on averted medically-attended influenza illnesses and associated costs. In addition, by increasing vaccination coverage among target groups, countries can further reduce influenza-related disease burden and medical costs associated with hospitalizations.
Funding details
This work was supported by a grant from the U.S. Centers for Disease Control and Prevention (CDC) through cooperative agreements with the Pan American Health Organization/World Health Organization.
Disclaimer
The findings and conclusions in this publication are those of the authors and do not necessarily represent the views of the United States Centers for Disease Control and Prevention (CDC) and the Pan American Health Organization (PAHO). The content of this article has not been previously presented.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank the national epidemiological and virological influenza surveillance and immunization teams in participating countries including among others, Julián Antman, Teresa Varela (Ministry of Health, Argentina); Erica Tatiane, Ernesto Issac Montenegro Renoiner, Ana Carolina de Lacerda Sousa, Daiana Araujo da Silva (Epidemiology Department, Ministry of Health Brazil), Jeanine Rocha Woycicki (Ministry of Health, Brazil); Leticia Garay Martins (Rio Grande do Sul, Brazil), Janaina Almeida, (Minas Gerais, Brazil), Patricia Marques Ferreira (São Paulo, Brazil), Laurina Tanabe (Paraná, Brazil), Eduardo Marques Macario (Santa Catarina, Brazil), Instituto Adolfo Lutz, National Influenza Center (NIC), Fundação Oswaldo Cruz (FIOCRUZ)–NIC, Brazil; Sonia Arza, Agueda Cabello, José Sánchez (Ministry of Health, Paraguay); Marcela Avendaño Vigueras and Pamela Burgos (Ministry of Health, Chile), Olga López, (Hospital Iquique, Chile), Miriam Blanco (Hospital G. Fricke, Chile), Tamara Tarride (Hospital S.J.D.Dios, Chile), Alberto Fica (Hospital Militar, Chile), Claudia Aguayo (Hospital G.G. Benavente, Chile), Tania Campos (Hospital Temuco, Chile), Carolina Nuñez (Hospital P. Montt); Antonio Mendez (PAHO consultant), Juliana Leite and Angel Rodriguez (PAHO influenza team, Washington); Dr Cuauhtémoc Ruiz-Matus and the PAHO immunization focal points for their support in the implementation of the REVELAC-i network: Mirta Magariños (Argentina), Samia Abdul Samad (Brazil), Viviana Calderón (Colombia) and Fabiana Michel (Paraguay). We thank John Fitzsimmons and Daniel Rodriguez (PAHO’s Revolving Fund) for providing information on vaccine procurement. We thank the US CDC for their technical and financial support; Sara Mirza and Victor Veguilla for their management of the cooperative agreement; Xiyan Xu, and her team at the Influenza Division’s Virology, Surveillance and Diagnosis branch for supporting genetic sequencing; and Alicia Fry and Jerome Tokars for their technical guidance. We thank Alain Levêque, Olivier Vandenberg, Tessa Goetghebuer, Yves Van Laethem at the Université libre de Bruxelles for their critical review of this work. Finally, we thank the I-MOVE team: Alain Moren, Marta Valenciano, Marc Rondy, Camelia Savulescu and Esther Kissling for their technical feedback and valuable exchanges.
Contributor Information
Carmen Sofia Arriola, Email: wus3@cdc.gov.
Nathalie El Omeiri, Email: elomeirin@paho.org.
the REVELAC-I network participants:
Cecilia Gonzalez, Sergio Loayza, Natalia Vergara, Patricia Bustos, Winston Andrade, Carla Magda S. Domingues, Ernesto Issac Montenegro Renoiner, Érica Tatiane da Silva, Swamy Lima Palmeira, Daiana Araujo da Silva, Ana Carolina de Lacerda Sousa, Marilda Mendonça Siqueira, Cynthia Vazquez, Silvia Battaglia, Carla Vizzotti, Elsa Baumeister, Carlos Giovacchini, Nathalia Katz, Oscar Pacheco, Juliana Barbosa, Diana Malo, Paola Pulido, Diego Garcia, and Consuelo Pinzón
References
- 1.El Omeiri N., Azziz-Baumgartner E., Clara W., Guzman-Saborio G. BMC Public Health; 2015. Pilot to evaluate the feasibility of measuring seasonal influenza vaccine effectiveness using surveillance platforms in Central-America, 2012; p. 673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Operational guidelines for sentinel severe acute respiratory infection (SARI) surveillance; 2014 [cited 2018 May 25]; Available from: http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=270&gid=31162&lang=en.
- 3.World Health Organization. Background Paper on Influenza Vaccines and Immunization. SAGE Working Group. 2012 [cited 2018 May 25]. Available from: http://www.who.int/immunization/sage/meetings/2012/april/1_Background_Paper_Mar26_v13_cleaned.pdf.
- 4.Pan American Health Organization. Red para la Evaluación de la Efectividad de la Vacuna en Latino América y el Caribe – influenza, (REVELAC-i). [cited 2018 May 25]; Available from: http://www.paho.org/revelac-i/.
- 5.El Omeiri N, Azziz-Baumgartner E, Thompson MG, R.E.-i.n. participants, et al., 2017. Seasonal influenza vaccine effectiveness against laboratory-confirmed influenza hospitalizations - Latin America, 2013. Vaccine; 2017. [DOI] [PMC free article] [PubMed]
- 6.De Serres G., Skowronski D.M., Wu X.W., Ambrose C.S. The test-negative design: validity, accuracy and precision of vaccine efficacy estimates compared to the gold standard of randomised placebo-controlled clinical trials. Euro Surveill. 2013;18(37) doi: 10.2807/1560-7917.es2013.18.37.20585. [DOI] [PubMed] [Google Scholar]
- 7.Foppa I.M., Haber M., Ferdinands J.M., Shay D.K. The case test-negative design for studies of the effectiveness of influenza vaccine. Vaccine. 2013;31(30):3104–3109. doi: 10.1016/j.vaccine.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 8.Jackson M.L., Nelson J.C. The test-negative design for estimating influenza vaccine effectiveness. Vaccine. 2013;31(17):2165–2168. doi: 10.1016/j.vaccine.2013.02.053. [DOI] [PubMed] [Google Scholar]
- 9.Sullivan S.G., Feng S., Cowling B.J. Potential of the test-negative design for measuring influenza vaccine effectiveness: a systematic review. Expert Rev Vaccines. 2014;13(12):1571–1591. doi: 10.1586/14760584.2014.966695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valenciano M., Kissling E., Ciancio B.C., Moren A. Study designs for timely estimation of influenza vaccine effectiveness using European sentinel practitioner networks. Vaccine. 2010;28(46):7381–7388. doi: 10.1016/j.vaccine.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 11.Sotomayor V., Fasce R.A., Vergara N., De la Fuente F. Estimating the burden of influenza-associated hospitalizations and deaths in Chile during 2012–2014. Influenza Other Respir Viruses. 2018;12(1):138–145. doi: 10.1111/irv.12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. Vaccines against influenza. WHO position paper November 20 2012 [cited 2018 May 25]; Available from: http://www.who.int/immunization/position_papers/PP_influenza_november2012_summary.pdf.
- 13.Foppa I.M., Ferdinands J.M., Chung J., Flannery B. Vaccination history as a confounder of studies of influenza vaccine effectiveness. Vaccine. 2019;1(11) doi: 10.1016/j.jvacx.2019.100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abadie A, Athey S, Imbens GW, Wooldridge J. When should you adjust standard errors for clustering? NBER Working paper; 2017 [cited 2019 May 13].
- 15.Segaloff H.E., Leventer-Roberts M., Riesel D., Malosh R.E. Influenza vaccine effectiveness against hospitalization in fully and partially vaccinated children in Israel; 2015–16, 2016–17, and 2017–18. Clin Infect Dis. 2019 doi: 10.1093/cid/ciz125. [DOI] [PubMed] [Google Scholar]
- 16.Webster R.G., Monto A.S., Braciale T.J., Lamb R.A. 2nd ed. John Wiley & Sons; 2013. Textbook of Influenza. [Google Scholar]
- 17.Englund J.A., Walter E.B., Fairchok M.P., Monto A.S. A comparison of 2 influenza vaccine schedules in 6- to 23-month-old children. Pediatrics. 2005;115(4):1039–1047. doi: 10.1542/peds.2004-2373. [DOI] [PubMed] [Google Scholar]
- 18.Thompson M.G., Clippard J., Petrie J.G., Jackson M.L. Influenza vaccine effectiveness for fully and partially vaccinated children 6 months to 8 years old during 2011–2012 and 2012–2013: the importance of two priming doses. Pediatr Infect Dis J. 2016;35(3):299–308. doi: 10.1097/INF.0000000000001006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neuzil K.M., Englund J.A. Influenza vaccine for young children: two doses are better than one. J Pediatr. 2006;149(6):737–738. doi: 10.1016/j.jpeds.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Neuzil K.M., Jackson L.A., Nelson J., Klimov A. Immunogenicity and reactogenicity of 1 versus 2 doses of trivalent inactivated influenza vaccine in vaccine-naive 5-8-year-old children. J Infect Dis. 2006;194(8):1032–1039. doi: 10.1086/507309. [DOI] [PubMed] [Google Scholar]
- 21.Fu C., Xu J., Lin J., Wang M. Concurrent and cross-season protection of inactivated influenza vaccine against A(H1N1)pdm09 illness among young children: 2012–2013 case-control evaluation of influenza vaccine effectiveness. Vaccine. 2015;33(25):2917–2921. doi: 10.1016/j.vaccine.2015.04.063. [DOI] [PubMed] [Google Scholar]
- 22.Eisenberg K.W., Szilagyi P.G., Fairbrother G., Griffin M.R. Vaccine effectiveness against laboratory-confirmed influenza in children 6 to 59 months of age during the 2003–2004 and 2004–2005 influenza seasons. Pediatrics. 2008;122(5):911–919. doi: 10.1542/peds.2007-3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szilagyi P.G., Fairbrother G., Griffin M.R., Hornung R.W. Influenza vaccine effectiveness among children 6 to 59 months of age during 2 influenza seasons: a case-cohort study. Arch Pediatr Adolesc Med. 2008;162(10):943–951. doi: 10.1001/archpedi.162.10.943. [DOI] [PubMed] [Google Scholar]
- 24.Cochran L.W., Black S., Klein N.P., Dekker C.L. Vaccine effectiveness against laboratory-confirmed influenza in infants: a matched case control study. Hum Vaccin. 2010;(9):6. doi: 10.4161/hv.6.9.12470. [DOI] [PubMed] [Google Scholar]
- 25.Belongia E.A., Simpson M.D., King J.P., Sundaram M.E. Variable influenza vaccine effectiveness by subtype: a systematic review and meta-analysis of test-negative design studies. Lancet Infect Dis. 2016;16(8):942–951. doi: 10.1016/S1473-3099(16)00129-8. [DOI] [PubMed] [Google Scholar]
- 26.Thompson M.G., Pierse N., Sue Huang Q., Prasad N. Influenza vaccine effectiveness in preventing influenza-associated intensive care admissions and attenuating severe disease among adults in New Zealand 2012–2015. Vaccine. 2018;36(39):5916–5925. doi: 10.1016/j.vaccine.2018.07.028. [DOI] [PubMed] [Google Scholar]
- 27.Rondy M., El Omeiri N., Thompson M.G., Leveque A. Effectiveness of influenza vaccines in preventing severe influenza illness among adults: a systematic review and meta-analysis of test-negative design case-control studies. J Infect. 2017;75(5):381–394. doi: 10.1016/j.jinf.2017.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Belongia E.A., Skowronski D.M., McLean H.Q., Chambers C. Repeated annual influenza vaccination and vaccine effectiveness: review of evidence. Expert Rev Vaccines. 2017;16(7):1–14. doi: 10.1080/14760584.2017.1334554. [DOI] [PubMed] [Google Scholar]
- 29.Ramsay L.C., Buchan S.A., Stirling R.G., Cowling B.J. The impact of repeated vaccination on influenza vaccine effectiveness: a systematic review and meta-analysis. BMC Med. 2019;17(1):9. doi: 10.1186/s12916-018-1239-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sullivan S.G., Tchetgen Tchetgen E.J., Cowling B.J. Theoretical basis of the test-negative study design for assessment of influenza vaccine effectiveness. Am J Epidemiol. 2016;184(5):345–353. doi: 10.1093/aje/kww064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jackson M.L. Use of self-reported vaccination status can bias vaccine effectiveness estimates from test-negative studies. Vaccine. 2019;1 doi: 10.1016/j.jvacx.2018.100003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pan American Health Organization. Immunization in the Americas. 2018 Summary; 2018 [cited 2019 May 22]; 12]. Available from: https://www.paho.org/hq/index.php?option=com_docman&view=download&category_slug=brochures-1581&alias=42190-immunization-in-the-americas-2018-summary&Itemid=270&lang=en.