Abstract
All-trans-retinoic acid (ATRA) is the standard of care for the management of acute promyelocytic leukemia (APL), but can be associated with differentiation syndrome (DS). Over a seven-year period, we sought to determine the impact of ATRA initiation time on the development of DS. ATRA administration time had no impact on DS occurrence (p = =0.13), APL risk (p = =0.28) or regimen received (p = =0.1). Patients with higher mean body mass index (BMI) were more likely to develop moderate or severe DS (p = =0.02). Early treatment of APL is essential and maybe strongly considered in patients with elevated BMI.
Keywords: Acute promyelocytic leukemia (APL), All-trans retinoic acid (ATRA), Differentiation syndrome
1. Introduction
Acute promyelocytic leukemia (APL) often presents with pancytopenia, disseminated intravascular coagulopathy and usually associated with a fusion of the promyelocytic leukemia (PML) gene on chromosome 15 with the retinoic acid receptor alpha (RARa) gene on chromosome 17 due to a chromosomal translocation t(15;17) [1]. All-trans retinoic acid (ATRA) is the standard of care for the initial emergent management of APL due to its ability to reverse the associated potentially life-threatening coagulopathy [2]. Early death rates (EDR), defined as death in the first 30 days from APL presentation are less than 10% in clinical trials but are 12–24% in registry studies [3,4]. ATRA plus arsenic trioxide (ATO) chemotherapy is highly effective and leads to cure in over 90% of cases, but differentiation (DS) or cytokine release syndrome is a potentially life-threatening complication [5]. The availability and early use of ATRA when APL is suspected may lead to a decrease in the EDR and is considered an important treatment quality measure. We performed a retrospective chart review to determine if timing of initial ATRA administration affects the development of DS symptoms and EDR.
The primary endpoint was to evaluate the rate of DS in patients with confirmed APL based on the time of ATRA administration relative to presentation and analyze rates and causes of early death. Secondary endpoints were evaluation of the time interval between presentation, prescribing and administration of ATRA over time for any patient who presented with a suspicion of APL.
2. Methods
2.1. Study design and patient population
We performed a retrospective chart review of all patients who received ATRA at our institution from May 2009 to October 2016. Patients were identified through an electronic medical system report. Patients who were at least 18 years old, with suspected or confirmed APL and who received at least one dose of ATRA for induction therapy were included. Patients were excluded if they received tretinoin for any indication other than suspected or confirmed APL, if they received ATRA prior to admission, if they received steroids prophylactically, or if they received ATRA for non-induction purposes, including relapsed or consolidative therapy.
Patients with confirmed APL had to fulfill the morphologic and cytogenetic criteria as per the French-American-British (FAB) classification [6]. Differentiation syndrome was defined by Frankel et al. and patients required to have any of the following the symptoms; dyspnea, unexplained fever, weight gain more than 5 kg, unexplained hypotension, acute renal failure, hyperbilirubinemia and radiological findings suggesting interstitial infiltrates and pleural or pericardial effusions [7]. Patients who experienced only 1 symptom of the mentioned above were not included and deemed indeterminate. Patients with 2–3 symptoms were classified as having moderate DS and patients who had at least 4 symptoms were classified as severe DS. Our institutional guidelines recommend initiation of dexamethasone 10 mg twice daily upon DS suspicion. Patients did not receive prophylactic therapy for DS, which coincides with our institutional practice. We retrospectively evaluated patients for 30 days from ATRA initiation for induction therapy. This time frame was chosen to assess for early death rate; defined as death within 30 days from ATRA initiation [8].
A total of 110 patients were screened who had an order for tretinoin. Twenty-nine patients were excluded due to the following reasons: received topical tretinoin (n = =4), APL diagnosis was confirmed prior to 2009 (n = =2), ATRA initiated at an outside facility (n = =16) or no ATRA doses were administered (n = =7). Eighty-one patients received at least one dose of ATRA and were included in the quality assurance analysis. Forty-nine patients had a confirmed APL diagnosis and received initial ATRA at Brigham and Women's Hospital/ Dana-Farber Cancer Institute inpatient hospitals, a large tertiary care academic cancer center. These patients were included for the analysis for the primary endpoint. The study was approved by the Dana-Farber/Harvard Cancer Center (DF/HCC) Institutional Review Board.
2.2. Outcome measures
We evaluated the following factors and their association with the development of DS; timing to ATRA administration from admission time, body mass index (BMI), platelet count, fibrinogen levels, INR, chemotherapy type, (including ATRA with arsenic compared to ATRA with any other chemotherapy), and APL risk stratification. APL risk stratification was defined according to Sanz et al. criteria with low-risk patients having a platelet count ≥ 40,000/μL and WBC < 10,000/μL, moderate risk having platelet count < 40,000μ/L and WBC < 10,000 μ/L and high-risk having platelet count < 40,000 μ/L and WBC ≥ 10,000 μ/L [9]. We gathered information on the time to onset of DS after ATRA administration. Death was attributed to DS when there was no alternative explanation.
For the primary endpoint, patients with confirmed APL were grouped based on time of administration of ATRA that was defined as time from admission to time of ATRA administration. Early ATRA administration was defined as ATRA administration within 24 h of admission or APL suspicion at the time of the hematology-oncology consult. Delayed ATRA administration was defined as ATRA administration beyond 24 h of admission. Patients who were transferred from Dana-Farber Cancer Institute clinic were considered to have their consult prior to admission to the Brigham and Women's Hospital.
For the secondary endpoint, we report a descriptive analysis on the time intervals between presentation, prescribing, and administration of ATRA over time as depicted in Fig. 1.
Fig. 1.
Timeline for patient admission, APL suspicion and ATRA order review.
2.3. Statistical methods
For the association between pre-ATRA treatment baseline characteristics and the development of DS, Wilcoxon rank sum test was used for continuous variables and Fisher's exact test was used for analysis of categorical variables.
3. Results
3.1. Patient demographics
For the primary endpoint, a total of 49 adult patients who received at least one dose of ATRA for a confirmed APL diagnosis, were identified during the study period. Baseline characteristics are shown in Table 1. ATRA dose was administered as 45 mg/m2/day in 2 divided doses and rounded to the nearest 10 mg capsule size. Median baseline INR was 1.3 between the 2 groups. The median age for patients who received early ARTA was 53 years and those who received it 24-hour post admission was 62 years. There were no differences in baseline characteristics for patients based on time to ATRA administration. Patients received concurrent arsenic trioxide or chemotherapy based on their APL risk classification, at the attending physician's discretion. As the treatment for APL has evolved over the course of this analysis, concurrent ATRA and arsenic was administered to 29 patients, ATRA and chemotherapy with daunorubicin and cytarabine to 15 patients, ATRA, arsenic and idarubicin to 3 patients, ATRA, arsenic and gemtuzumab ozogamicin to 2 patients. Eleven patients received hydroxyurea during therapy to reduce white blood cell counts. Thirty-five patients experienced some degree of differentiation syndrome. Twenty patients (40%) met criteria for moderate DS and were treated with dexamethasone 10 mg twice daily. Fifteen patients did not receive dexamethasone, at the discretion of the treating team, but they met the clinical definition of DS. Nine patients had 2–3 symptoms and 5 patients had at least 4 symptoms, of which 4 out of 5 were classified as low risk APL.
Table 1.
Baseline characteristics.
| ATRA < 24 h of presentation | ATRA ≥ 24 h of presentation | |||
|---|---|---|---|---|
| n | Percentage (%), Median (range) | n | Percentage (%), Median (range) | |
| Age (years) | 26 | 53 (21, 69) | 23 | 55 (22, 85) |
| Gender | ||||
| Male | 16 | 62 | 8 | 35 |
| Female | 10 | 38 | 15 | 65 |
| Weight (kg) | 26 | 96.5 (55.3, 159.7) | 23 | 75.2 (55, 180.7) |
| BMI (kg/m2) | 26 | 29.3 (21.7, 48.9) | 23 | 27.1 (18.5, 56.4) |
| Platelet (109/L) | 26 | 23.5 (0, 97) | 23 | 35 (5, 86) |
| Fibrinogen (mg/dL) | 26 | 173.5 (60, 404) | 23 | 167 (29, 548) |
| INR | 26 | 1.3 (1.0, 2.1) | 23 | 1.3 (1.1, 1.9) |
| APL risk | ||||
| Low | 8 | 31 | 6 | 26 |
| Intermediate | 12 | 46 | 11 | 48 |
| High | 6 | 23 | 6 | 26 |
3.2. Association of clinical characteristics and the development of DS
Thirty-five patients developed DS. Fourteen out of 35 (40%) were classified as intermediate APL risk, 9 out of 35 (26%) as high risk and 12 out of 35 (34%) as low risk. The incidence of DS between low, intermediate and high risk was not statistically significant (p = 0.28). Ten out of 14 (71%) were classified as intermediate risk, 2 out of 14 (14%) as high risk and 2 out of 14 (14%) as low risk. All the 3 patients who received ATRA, arsenic and idarubicin experienced at least 4 symptoms of DS. Most patients encountered 2–3 symptoms of DS; 12 patients who received ATRA and arsenic, 7 patients who received ATRA in addition to cytarabine and daunorubicin and 2 patients who received ATRA and gemtuzumab ozogamicin.
Overall, patients who experienced DS had significantly higher BMI (30.5 kg/m2 vs. 25.1 kg/m2; p = 0.02), regardless of time of ATRA administration (p = =0.13). There was a non-significant trend towards increased DS incidence with increasing BMI after adjusting for gender and diabetes (p = =0.05 and 0.11 respectively). Of the patients who received ATRA 24 h post admission (n = 23), 19 patients experienced DS as compared to 4 patients who did not. Thirty-three percent (n = 16) received early ATRA and experienced DS compared to 20% (n = 10). Although not statistically significant, patients who developed DS had a higher median baseline WBC (1. 28 × 109 vs. 1.04 × 109/mL; p = 0.26) (Table 2).
Table 2.
Association of clinical characteristics with the development of DS.
| Without DS | With DS | ||||
|---|---|---|---|---|---|
| n | Percentage (%), Median (IQR) | n | Percentage (%), Median (IQR) | p-value | |
| ATRA administration from presentation | 0.13 | ||||
| Early <24 h | 10 | 71 | 16 | 46 | |
| Late ≥ 24 h | 4 | 29 | 19 | 54 | |
| Weight (kg) | 14 | 72.2 (64.1, 80.3) | 35 | 98 (73, 119.3) | 0.009 |
| BMI (kg/m2) | 14 | 25.1 (22.6, 27.5) | 35 | 30.5 (26.1, 38.5) | 0.02 |
| WBC (109/L) | 14 | 1.04 (0.66, 1.77) | 35 | 1.28 (0.77, 13.09) | 0.26 |
| Platelet (109/L) | 14 | 22 (12, 36) | 35 | 35 (17, 56) | 0.18 |
| INR | 14 | 1.3 (1.2, 1.4) | 35 | 1.2 (1.2, 1.5) | 0.88 |
| APL Risk | 0.28 | ||||
| Low | 2 | 14 | 2 | 34 | |
| Intermediate | 9 | 64 | 14 | 40 | |
| High | 3 | 21 | 9 | 26 | |
| Treatment regimen | 0.1 | ||||
| ATRA/ATO | 12 | 86 | 20 | 57 | |
| ATRA/ anthracyclines | 2 | 14 | 15 | 43 | |
3.3. Association of time to ATRA administration and the development of DS
Of the patients who experienced DS, a higher number of patients who received ATRA more than 24-hours post admission experienced severe DS (n = 10 vs. 5; 67 vs. 33%). Although this was not statistically significant, there was a higher percentage of patients who developed severe DS and had received ATRA 24 h post admission (10 vs. 5 patients; p = 0.31) (Table 3). A total of 8 patients received ATRA 48 h after presentation (median 64 h; range 50–123 h). APL suspicion was low in 2 patients; one thought to have hairy cell leukemia and one presumed ALL based on their smear. Three patients diagnosed in 2010, 2012 and 2013 waited until a confirmed APL diagnosis was made to receive ATRA.
Table 3.
Association of timing of ATRA administration with the severity of DS.
| Moderate DS | Severe DS | ||||
|---|---|---|---|---|---|
| n | Percentage (%) | n | Percentage (%) | p-value | |
| ATRA administration from presentation | 0.31 | ||||
| Early < 24 h | 11 | 55 | 5 | 33 | |
| Late ≥ 24 h | 9 | 45 | 10 | 67 | |
We also looked at chemotherapy administered to our patients. Higher rates of DS were observed with ATRA plus arsenic therapy compared to ATRA plus chemotherapy but that was not statistically significant (57 vs. 43%; p = =0.1). Our patients did not receive steroids prophylactically.
3.4. Early deaths
For patients diagnosed with APL, there was one death within 30 days, which was due to an intracranial hemorrhage. Patient had presented with high risk features; baseline WBC and platelets were 56.87 × 109/L and 14 × 109/L respectively. Her WBC increased to 73.35 × 109/L after the first dose and peaked at 131 × 109/L following day 2 with ATRA therapy. She rapidly progressed and developed severe DS; she was febrile to 38.9 ᵒC on day 2, developed acute kidney injury with an increase in her serum creatinine from 1.39 mg/dL to 2.87 mg/dL on day 2 and was found to have pleural effusions and pulmonary infiltrates. Her baseline fibrinogen was 171, international normalized ratio (INR) was 2 and D-dimer that was greater than assay (>4000), reflecting a DIC picture. She passed a week after starting ATRA therapy.
3.5. Time to ATRA administration
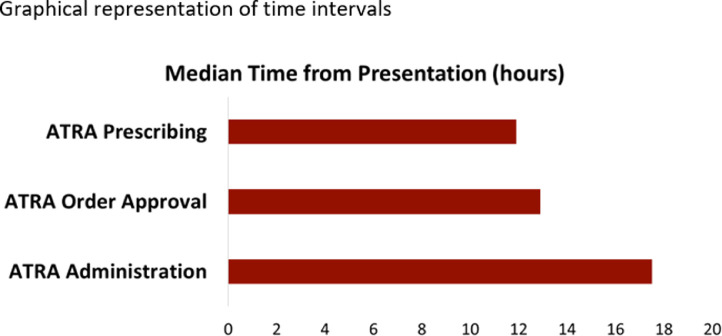
For our secondary endpoint, we conducted a descriptive analysis to examine our current practice and median time from presentation to examination by medical team to administration of ATRA. Eighty-one patients were seen by a hematology-oncology fellow or attending for a suspicion of APL and received a dose of ATRA. Sixty-eight percent (n = =55) were seen by a hematology physician within 24-hours of presentation. The rest were seen within 1 to 3 days. This was likely due to a low suspicion of APL, upon admission. For all patients, the median times from presentation to ATRA prescribing, ATRA order approval and first ATRA dose administration were 11.9 h, 12.9 h and 17.5 h respectively, also shown in Fig. 2. Eighteen patients (22%) received their first dose in the emergency room. Of note, patients transferred from an outside hospital are admitted straight to a bed on the floor.
Fig. 2.
Graphical representation of time intervals.
4. Discussion
ATRA induces the differentiation of leukemic promyelocytes and is the standard of care for the management of APL [10,11]. The accumulation of differentiated blasts may lead to cellular migration, endothelial activation, release of interleukins accountable for tissue damage and eventually leading to DS [12]. Initiation of ATRA reverses the coagulopathy and helps reduce rates of life-threatening bleeding [13]. In this retrospective study, we analyzed the rates of DS in association with the timing of ATRA administration. In our retrospective analysis, we did not find early ATRA administration to be associated with lower rates of developing moderate or severe DS. We did observe a trend that patients who received ATRA 24 h post admission were more likely to develop severe DS. However, further studies with larger sample sizes are warranted to confirm this hypothesis. DS was not affected by baseline white blood cell counts, platelets, fibrinogen levels or their respective APL risk stratification group. In contrast, patients with high body mass indexes were associated with higher rates of DS. This finding reiterates previous findings by our group and Breccia M. et al. [14,15]. The latter goes on to suggest that altered drug metabolism in this patient population may be responsible for higher DS outcomes. They also describe that increased levels of active circulating leptin, insulin or IGF1 may lead to higher proliferative effects in the leukemic patient and may be a potential cause [16], [17], [18], [19]. Another plausible explanation could be due to the pharmacological properties of ATRA. ATRA, a metabolite of vitamin A, is a lipophilic molecule and may have a higher tendency to be stored in fat tissue resulting in a continuous and slow release of ATRA over time and continuous blast differentiation [20]. Furthermore, at our institution we do not adjust ATRA dosing based on dose adjusted ideal body weight posing a potential high dose being administered to overweight and obese patients.
Our rates of DS were not affected by whether patients received ATRA plus chemotherapy compared to ATRA plus arsenic. Patients who were assessed for DS did not receive prophylactic steroids. Lo-Coco F et al. observed similar rates of DS between the groups who received ATRA plus arsenic and those who received ATRA plus chemotherapy [21]. However, patients of each group received prophylactic prednisone, which may have confounded their DS rates.
We also carried out a quality improvement analysis. We collected data on time of admission and time when patients were attended to by a hematology/oncology specialist, time to ordering, approving and administering of ATRA for all patients who had a suspicion of APL diagnosis. More than half of our patients were seen by an oncologist within 24 h. Eighteen patients received a dose in the emergency room, highlighting the proactivity of our APL program. Other patients received their first dose on the floor as they transferred from an outside hospital. This is an improvement from the median of at least 2 days reported by Altman JK et al. in 2013 [22]. This demonstrates advancements over time in education about APL and the general safety of administering ATRA prior to a confirmed diagnosis via bone marrow morphology results. In addition, we believe that there has been a progressive efficiency in utilizing technology to enhance medical practice, time to emergency ATRA order entry, pharmacy approval, delivery and administration.
We observed an impressive decline in the rates of early death. We had one death in a 25-year old patient who was otherwise healthy, due to intracranial hemorrhage. This is unlike the findings that were published in both clinical and registry trials as well as a higher rate of death in patients older than 60 years [23,24]. This could be justified by the recent time frame of patients being assessed as well as improved electronic medical record system, increased education and awareness of APL diagnosis and signs and symptoms of DS. Rahme et al. published retrospective data in 2014, showing a decline in EDR [25,26]. Larger sample size and a multi-center trial would need to be designed to confirm our findings.
There were several limitations to our study. This was a single center retrospective study. A major limitation was the small sample size that precludes a definitive statistical analysis. Per our institutional practice, patients with early signs and symptoms of hypotension or fever were initiated on dexamethasone therapy for presumed DS. However, we were not able to capture the time from diagnosis to hospital transfer. This is a limitation of this study as we may not have captured time from presentation to transfer.
In conclusion, the timing of ATRA administration did not significantly affect the rates of developing overt clinical DS. At our institution, patients who present with disseminated intravascular coagulation or have suspected APL via peripheral smear will receive ATRA immediately in the emergency room as they wait bed assignment. In addition, health care team members are very cognizant of the symptoms of DS and monitor patients closely. Finally, we observed a much lower EDR compared to previous studies, demonstrating the advancement in the assessment, diagnosis and treatment of APL over time.
Authors contribution
M.N. was involved in the conception and design of the study, acquisition of data, analysis and interpretation of data, as well as writing of the manuscript.
D.J.D. and R.M.S. were involved in design of the study as well as writing of the manuscript.
J.N. was involved in the acquisition of data.
L.W was involved in the analysis and interpretation of data.
A.M.M. was involved in the conception and design of the study, analysis and interpretation of data, as well as writing of the manuscript.
All authors revised and gave final approval of the manuscript
Funding
None
Declaration of Competing Interest
The authors do not have any pertinent commercial relationships.
References
- 1.Grignani Francesco. The acute promyelocytic leukemia-specific PML-RARα fusion protein inhibits differentiation and promotes survival of myeloid precursor cells. Cell. 1993;74(3):423–431. doi: 10.1016/0092-8674(93)80044-f. [DOI] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network. “NCCN guidelines: acute myeloid leukemia. version 1.2015.ˮ (2016). [DOI] [PubMed]
- 3.Lehmann S. Continuing high early death rate in acute promyelocytic leukemia: a population-based report from the Swedish adult acute leukemia registry. Leukemia. 2011;25.7:1128–1134. doi: 10.1038/leu.2011.78. [DOI] [PubMed] [Google Scholar]
- 4.Lehmann S. Early death rates remain high in high-risk APL: update from the Swedish acute leukemia registry 1997–2013. Leukemia. 2017;31.6:1457. doi: 10.1038/leu.2017.71. [DOI] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network. “NCCN guidelines: acute myeloid leukemia. version 1.2015.ˮ (2016). [DOI] [PubMed]
- 6.Bennett J.M. Proposed revised criteria for the classification of acute myeloid leukemia: a report of the French-American-British cooperative group. Ann. Intern. Med. 1985;103.4:620–625. doi: 10.7326/0003-4819-103-4-620. [DOI] [PubMed] [Google Scholar]
- 7.Frankel S.R. The retinoic acid syndrome in acute promyelocytic leukemia. Ann. Intern. Med. 1992;117.4:292–296. doi: 10.7326/0003-4819-117-4-292. [DOI] [PubMed] [Google Scholar]
- 8.Tallman M.S. All-trans-retinoic acid in acute promyelocytic leukemia. N. Engl. J. Med. 1997;337.15:1021–1028. doi: 10.1056/NEJM199710093371501. [DOI] [PubMed] [Google Scholar]
- 9.Sanz M.A., Montesinos P. How we prevent and treat differentiation syndrome in patients with acute promyelocytic leukemia. Blood. 2014;123.18:2777–2782. doi: 10.1182/blood-2013-10-512640. [DOI] [PubMed] [Google Scholar]
- 10.Platzbecker U. Improved outcomes with retinoic acid and arsenic trioxide compared with retinoic acid and chemotherapy in non-high-risk acute promyelocytic leukemia: final results of the randomized Italian-German APL0406 trial. J. Clinic. Oncol. 2017;35.6:605–612. doi: 10.1200/JCO.2016.67.1982. [DOI] [PubMed] [Google Scholar]
- 11.Menell J.S. Annexin II and bleeding in acute promyelocytic leukemia. N. Engl. J. Med. 1999;340.13:994–1004. doi: 10.1056/NEJM199904013401303. [DOI] [PubMed] [Google Scholar]
- 12.Montesinos P. Differentiation syndrome in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and anthracycline chemotherapy: characteristics, outcome, and prognostic factors. Blood. 2009;113.4:775–783. doi: 10.1182/blood-2008-07-168617. [DOI] [PubMed] [Google Scholar]
- 13.Sanz M.A. Management of acute promyelocytic leukemia: recommendations from an expert panel on behalf of the European Leukemianet. Blood. 2009;113.9:1875–1891. doi: 10.1182/blood-2008-04-150250. [DOI] [PubMed] [Google Scholar]
- 14.Breccia M. Increased BMI correlates with higher risk of disease relapse and differentiation syndrome in patients with acute promyelocytic leukemia treated with the AIDA protocols. Blood. 2012;119.1:49–54. doi: 10.1182/blood-2011-07-369595. [DOI] [PubMed] [Google Scholar]
- 15.Leblebjian H. Predictive factors for all-trans retinoic acid related differentiation syndrome in acute promyelocytic leukemia. Blood. 2010;116.21 doi: 10.1016/j.leukres.2013.04.011. 1052-1052. [DOI] [PubMed] [Google Scholar]
- 16.Konopleva M. Expression and function of leptin receptor isoforms in myeloid leukemia and myelodysplastic syndromes: proliferative and anti-apoptotic activities. Blood. 1999;93.5:1668–1676. [PubMed] [Google Scholar]
- 17.Tabe Y. PML-RARα is associated with leptin-receptor induction: the role of mesenchymal stem cell–derived adipocytes in APL cell survival. Blood. 2004;103.5:1815–1822. doi: 10.1182/blood-2003-03-0802. [DOI] [PubMed] [Google Scholar]
- 18.Percik R., Stumvoll M. Obesity and cancer. Exp. Clinic. Endocrinol. Diabetes. 2009;117.10:563–566. doi: 10.1055/s-0029-1241870. [DOI] [PubMed] [Google Scholar]
- 19.Breccia M. Increased BMI correlates with higher risk of disease relapse and differentiation syndrome in patients with acute promyelocytic leukemia treated with the AIDA protocols. Blood. 2012;119.1:49–54. doi: 10.1182/blood-2011-07-369595. [DOI] [PubMed] [Google Scholar]
- 20.Berry D.C., Noy N. All-trans-retinoic acid represses obesity and insulin resistance by activating both peroxisome proliferation-activated receptor β/δ and retinoic acid receptor. Mol. Cell. Biol. 2009;29.12:3286–3296. doi: 10.1128/MCB.01742-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lo-Coco F. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N. Engl. J. Med. 2013;369.2:111–121. doi: 10.1056/NEJMoa1300874. [DOI] [PubMed] [Google Scholar]
- 22.Altman J.K. Administration of ATRA to newly diagnosed patients with acute promyelocytic leukemia is delayed contributing to early hemorrhagic death. Leuk. Res. 2013;37.9:1004–1009. doi: 10.1016/j.leukres.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 23.Jillella, Anand, et al. “Decreasing early deaths in acute promyelocytic leukemia (APL) by using a simplified treatment algorithm and establishing a network with academic and community centers in USA.ˮ (2015): 3779–3779.
- 24.Lehmann S. Early death rates remain high in high-risk APL: update from the Swedish acute leukemia registry 1997–2013. Leukemia. 2017;31.6:1457. doi: 10.1038/leu.2017.71. [DOI] [PubMed] [Google Scholar]
- 25.Rahmé R. Early death in acute promyelocytic leukemia (APL) in French centers: a multicenter study in 399 patients. Leukemia. 2014;28.12:2422. doi: 10.1038/leu.2014.240. [DOI] [PubMed] [Google Scholar]
- 26.Lehmann S. Early death rates remain high in high-risk APL: update from the Swedish acute leukemia registry 1997–2013. Leukemia. 2017;31.6:1457. doi: 10.1038/leu.2017.71. [DOI] [PubMed] [Google Scholar]