Abstract
Objectives
Drugs for Neglected Diseases initiative (DNDi) has identified three chemical lead series, the nitroimidazoles, benzoxaboroles and aminopyrazoles, as innovative treatments for visceral leishmaniasis. The leads discovered using phenotypic screening, were optimised following disease- and compound-specific criteria. Several leads of each series were progressed and preclinical drug candidates have been nominated. Here we evaluate the efficacy of the lead compounds of each of these three chemical classes in in vitro and in vivo models of cutaneous leishmaniasis.
Methods
The in vitro activity of fifty-five compounds was evaluated against the intracellular amastigotes of L. major, L. aethiopica, L. amazonensis, L. panamensis, L. mexicana and L. tropica. The drugs demonstrating potent activity (EC50 < 5 μM) against at least 4 of 6 species were subsequently evaluated in vivo in different L. major – BALB/c mouse models using a 5 or 10-day treatment with either the oral or topical formulations. Efficacy was expressed as lesion size (measured daily using callipers), parasite load (by quantitative PCR – DNA) and bioluminescence signal reduction relative to the untreated controls.
Results
The selected drug compounds (3 nitroimidazoles, 1 benzoxaborole and 3 aminopyrazoles) showed consistent and potent activity across a range of Leishmania species that are known to cause CL with EC50 values ranging from 0.29 to 18.3 μM. In all cases, this potent in vitro antileishmanial activity translated into high levels of efficacy with a linear dose-response against murine CL. When administered at 50 mg/kg/day, DNDI-0690 (nitroimidazole), DNDI-1047 (aminopyrazole) and DNDI-6148 (benzoxaborole) all resulted in a significant lesion size reduction (no visible nodule) and an approximate 2-log-fold reduction of the parasite load as measured by qPCR compared to the untreated control.
Conclusions
The lead compounds DNDI-0690, DNDI-1047 and DNDI-6148 showed excellent activity across a range of Leishmania species in vitro and against L. major in mice. These compounds offer novel potential drugs for the treatment of CL.
Keywords: Cutaneous leishmaniasis, Drug discovery, Aminopyrazole, Benzoxaborole, Nitroimidazole
Graphical abstract
1. Introduction
The leishmaniases are a complex of diseases caused by Leishmania parasites with divergent disease manifestations, classified predominantly as visceral (VL) and cutaneous leishmaniasis (CL). There are over 15 species of Leishmania that cause different forms of CL, ranging from self-healing localised CL to chronic and disseminated CL, as well as mucosal leishmaniasis (MCL). As parasite transmission occurs via bites of the female sandfly, CL is often associated with skin lesions on exposed and visible areas of the body including the face. The disfiguration caused by this disease can result in psychological damage and stigma especially in women and children (Bennis et al., 2017; Kassi et al., 2008). However, as a non-fatal disease, CL remains one of the most neglected of neglected diseases in terms of drug discovery and development efforts.
Drugs and treatments used to cure CL today show many limitations (Aronson et al., 2017; Croft and Olliaro, 2011), which may be reflected in the absence of treatment-seeking behaviour of CL patients. All current treatments involve re-purposed drugs with a relatively high molecular weight and high polarity (except miltefosine) which results in poor oral bioavailability and limited room for optimisation of drug delivery through the skin (Bos and Meinardi, 2000; Hadgraft and Pugh, 1998; Lipinski et al., 1997). There is a clear medical need for safe, effective and short-course treatment.
For successful treatment, a potent antileishmanial drug needs to reach the target site in the skin following either oral or topical drug administration to ensure effective treatment. The route of choice of drug administration is governed by both drug properties (Lipinski et al., 1997; Naik et al., 2000) and therapeutic concerns, and is an important factor in drug delivery.
Over the past decade, the Drugs for Neglected Disease Initiative (DNDi), a public private partnership that focusses on drug development for infectious diseases including neglected tropical diseases, has identified three highly potent anti-leishmanial chemical classes, the benzoxaboroles, the aminopyrazoles and the nitroimidazoles, with lead compounds for both VL and human African trypanosomiasis (HAT) (Jacobs et al., 2011a; Mowbray et al., 2015; Thompson et al., 2018). Compounds from these series have shown (i) potent antileishmanial activity in vitro, (ii) favourable pharmacokinetic profiles to ensure bioavailability upon oral drug administration and (iii) high levels of activity against murine visceral leishmaniasis (Van den Kerkhof et al., 2018).
Nitroimidazoles are a class of anti-microbials that show broad spectrum activity against protozoans, mycobacteria and anaerobic bacteria. For example, metronidazole, a 5-nitroimidazole, is available in oral and vaginal dosage forms to treat Trichomonas and bacterial infections, and more specifically the 2-nitroimidazole, benznidazole, is the front-line drug treatment for Chagas disease. Structural modifications have led to the discovery of two drugs, pretomanid (also known as PA-824, a 5-nitroimidazopyran) and delamanid (OPC-67683, a 6-nitro-2,3-dihydroimidazooxazole), for the treatment of multidrug-resistant tuberculosis caused by the intracellular Mycobacterium tuberculosis (Fairlamb and Patterson, 2018). Benzoxaboroles are bicyclic heterocycles in which the nitrogen of a benzo[c]isoxazole has been replaced by boron. Over the past decade, benzoxaboroles have been associated with a variety of antimicrobial properties that led to the initiation of several drug development programs. Tavaborole, another benzoxaborole, was approved by the FDA in 2014 for the treatment of onychomycosis (Elewski and Tosti, 2014). Anacor Pharmaceuticals (now Pfizer), SCYNEXIS and DNDi investigated a wide range of antitrypanosomal benzoxaboroles analogues as part of a lead optimisation program against HAT that resulted in the identification of acoziborole that is currently in Phase IIb/III trials (b; Nare et al., 2010). AN13762 is currently in preclinical development for malaria (Zhang et al., 2017) and DNDI-6148 is awaiting phase I clinical trials for VL. The antileishmanial activity of the aminopyrazoles was discovered through the high-throughput screening of a Pfizer small molecule diversity collection (Mowbray et al., 2015). In collaboration with Takeda Pharmaceutical Company Ltd, DNDi further optimised several potent aminopyrazole compounds for VL (Mowbray et al., 2015). Whilst DNDI-5561 was selected as the preclinical candidate, there are other promising candidates strengthening the pipeline of this class of compounds.
Here we report the evaluation of lead compounds of each of these three chemical classes aiming at the identification of a potential drug candidate to treat CL. It is important to note that the drug delivery targets for VL and CL are different - the liver, spleen and bone marrow, and the skin, respectively.
2. Material and methods
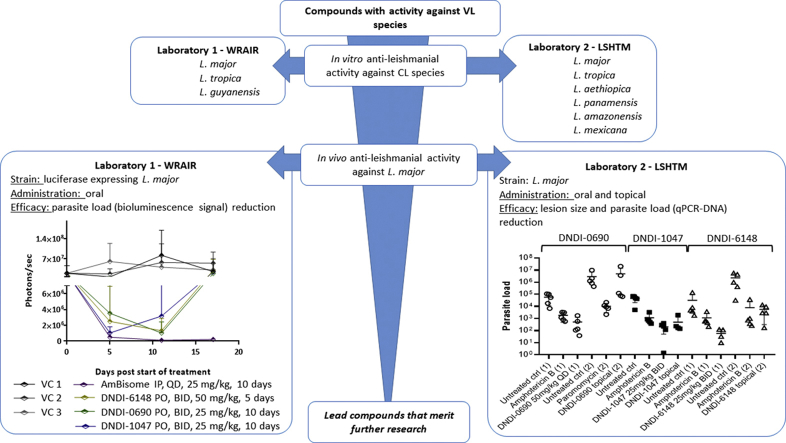
In vitro and in vivo studies were conducted by two independent research groups at the London School of Hygiene & Tropical Medicine (LSHTM) and the Walter Reed Army Institute of Research (WRAIR) and methodologies are summarised in Table 1.
Table 1.
Comparison of the In vitro and in vivo assay design at LSHTM and WRAIR.
| LSHTM | WRAIR | |
|---|---|---|
| In vitro assay | ||
| Host cells | peritoneal macrophages from CD-1 mice | RAW 264.7 |
| Leishmania strain |
L. major (MHOM/SA/85/JISH118) L. mexicana (MNYC/BZ/62/M379) L. amazonensis (L. amazonensis: DsRed2) L. aethiopica (MHOM/ET/84/KH) L. panamensis (MHOM/PA/67/BOYNTON) |
L. major (MHOM/IL/SU73/WR779) L. guyanensis (MHOM/GY/06/PAB-3985-WR-2853/A Chan) L. tropica (MHOM/SU/74/K-27 WR-2995) |
| Assay medium | RPMI-1640 + 10% HiFBS | DMEM + 10% HiFBS |
| Assay format | 16 –well Lab Tek slide | 384-well plate |
| Drug start concentration | 10 μM – 1:3 dilutions | 10 μg/ml – 1:2 dilutions |
| Drug incubation time | 72 h | 96 h |
| Assay temperature | 34 °C from infection onwards | 37 °C from infection onwards |
| Drug solutions | 100% DMSO – in assay: < 1% DMSO | 100% DMSO – in assay: 0.2% DMSO |
| Control drugs | Amphotericin B (Fungizone) and miltefosine | Amphotericin B |
| Read out |
Microscopic counting Ratio of infected cells upon drug treatment vs untreated controls |
Bioluminescent signal Total parasite counting (signal based) |
| In vivo assay | ||
| Leishmania strain | L. major (MHOM/SA/85/JISH118) | L. major (NIH173 (MHOM/IR/-/173) |
| Mouse strain | Female BALB/c mice – 6–8 weeks old (Charles River) | Female BALB/c mice – 6 weeks old (Charles River) |
| Group size | 5 | 5–6 |
| Infection | Low passage (p < 5) stationary phase promastigotes | Stationary phase promastigotes |
| Inoculum size | 200 μl containing 4 × 107 promastigotes | 100 μl containing 1 × 107 promastigotes |
| Place of infection | Rump above the tail | Rump above the tail |
| Treatment initiation | Average nodule diameter of 3–4 mm | Average nodule surface of 20 mm2 |
| Drug efficacy assessment | Reduction vs untreated or vehicle control of:
|
Reduction vs untreated or vehicle control of:
|
| Study duration | 11 days after the first dose administration | Until relapse occurs |
| Positive control | AmBisome®, IV, 25 mg/kg QAD, 10 days Paromomycin, IP, 50 mg/kg QD, 10 days |
AmBisome®, IP, 25 mg/kg QD, 10 days |
2.1. Drugs and drug formulations
LSHTM: Miltefosine was donated by Paladin Labs Inc and amphotericin B deoxycholate (Fungizone, E.R. Squibb & Sons, UK) was purchased from John Bell & Croyden Ltd. (London, UK). Both drugs were prepared to a stock concentration of 20 mM in sterile PBS (0.9% NaOH, pH 7.4; Sigma Aldrich, UK) and sterile water respectively and stored at −20 °C until required.
DNDi (Geneva, Switzerland) provided the experimental compounds, which were prepared as a stock solution of 20 mM in dimethyl sulfoxide (DMSO, Sigma Aldrich, UK), sonicated (CamLab, Cambridge, UK) for 15 min and stored 4 °C.
WRAIR: For the in vitro antileishmanial activity evaluation, the experimental compounds and solubilized amphotericin B (Fungizone, Sigma Aldrich, USA) were prepared at a stock solution of 11.5 mM in DMSO and stored at −20 °C.
2.2. Parasite strains and animals
2.2.1. Parasite strains in vitro assays
LSHTM: L. major (MHOM/SA/85/JISH118), L. mexicana (MNYC/BZ/62/M379), L. amazonensis (L. amazonensis: DsRed2), L. aethiopica (MHOM/ET/84/KH) and L. panamensis (MHOM/PA/67/BOYNTON) amastigotes were isolated from mouse skin lesions. They were allowed to transform to promastigotes and were maintained in Schneider's insect medium (Sigma Aldrich, UK) (for L. major, L. tropica and L. mexicana) or M199 medium (Sigma-Aldrich, UK) (for L. amazonensis, L. aethiopica and L. panamensis) supplemented with 10% HiFBS at 26 °C. L. tropica (MHOM/AF/2015/HTD7) was isolated from a skin biopsy of a CL patient that was inoculated into Novy-Nicolle-McNeil medium at the London Hospital of Tropical Diseases. Upon observation of parasite growth, the parasites were transferred to LSHTM, London, where the promastigotes were gradually adapted to Schneider's insect medium supplemented with 10% of HiFBS (Gibco, UK). Low passage number promastigotes (typically below passage number 3) were used for this experiment.
WRAIR: L. major (MHOM/IL/SU73/WR779), L. guyanensis (MHOM/GY/06/PAB-3985-WR-2853/A Chan), and L. tropica (MHOM/SU/74/K-27 WR-2995) were maintained in Schneider's insect medium (Lonza BioWhitaker, USA) supplemented with 20% heat inactivated fetal bovine serum (Corning, USA) at 22 °C. All parasite lines were transfected with a luciferase-expressing construct as described in (Lecoeur et al., 2007).
2.2.2. Parasite strains for in vivo assays
LSHTM: All Leishmania strains were regularly passaged through mice to maintain virulence. Late stationary phase promastigote cultures were counted with a Neubauer hemocytometer using light microscopy (x40 magnification), centrifuged at 900× g for 10 min at 4 °C and re-suspended in RPMI medium without HiFBS to 2 × 108 promastigotes per mL. Mice were injected subcutaneously on the rump above the tail with 200 μL of the promastigote suspension.
WRAIR: L. major promastigotes (NIH173 [MHOM/IR/−/173]) were harvested from infected BALB/c mouse footpads and were cultured in Schneider's medium (Lonza Life Sciences, Walkersville, MD) supplemented with 20% hiFBS. Cultures were maintained in T75 tissue culture flasks (Corning Life Sciences, Manassas, VA) at 22 °C. Promastigotes for infection were harvested from the culture by spinning at 872×g for 20 min. The medium was removed, and the resulting pellet was suspended in 1 × PBS. Two additional spins at 872×g were conducted in PBS. After the second spin, a low volume of PBS was added, and stationary-phase promastigotes were counted and suspended at 1 × 108 parasites/mL. Animals were infected at the base of the tail with 100 μL of parasite culture containing 1 × 107 L. major luciferase-expressing stationary-phase promastigotes.
2.2.3. Animals and ethical statements
LSHTM: BALB/c mice (age 6–8 weeks) were purchased from Charles River (Margate, UK), whereas female CD-1 mice (age 7–8 weeks) were obtained in-house (London School of Hygiene & Tropical Medicine). The mice were housed in a controlled environment of 55% relative humidity and 26 °C and provided with tap water and a standard laboratory diet. They were left to acclimatise for 5 days prior to the beginning of research studies.
All in vivo experiments were carried out under license (X20014A54) at the London School of Hygiene and Tropical Medicine (LSHTM) after discussion with the veterinarian and according to UK Home Office regulations.
WRAIR: Female BALB/c mice aged 6 weeks were purchased from Charles River Laboratories (Wilmington, MA). The mice were left to acclimatise for 7 days prior to the beginning of research studies. All mice were assigned a study number with an individual ear tag. All animals were quarantined for stabilization for 7 days prior to infection. Mice were housed in a designated room with food and water supplied ad libitum and a 12:12 light:dark cycle.
The animal protocol for this study was approved by the Walter Reed Army Institute of Research, Institutional Animal Care and Use Committee (Protocol number 16-ET-33) in accordance with national and Department of Defence guidelines. Research was conducted in an AAALACi accredited facility in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals and adheres to principles stated in the Guide for the Care and Use of Laboratory Animals, NRC Publication, 2011 edition.
2.3. In vitro antileishmanial activity
LSHTM: Peritoneal macrophages were isolated from CD-1 mice 24 h after intraperitoneal starch induction. The macrophages were washed and re-suspended in RPMI-1640 with 10% heat-inactivated fetal bovine serum (HiFBS) at a density of 4 × 105 macrophages per ml. Of this suspension, 100 μL was added to each well of a 16-well Lab Tek slide and left to adhere for at 37 °C in the presence of 5% CO2. After 24 h, 100 μL of stationary phase promastigotes of six different strains were counted, re-suspended in RPMI-1640 supplemented with 10% HiFBS and added in a ratio of 3:1 (L. major and L. mexicana), 5:1 (L. tropica, L. panamensis and L. amazonensis) and 7:1 (L. aethiopica) parasites to macrophages. The slides were left overnight at 34 °C in a 5% CO2/95% air mixture.
Prior to adding drugs, the infection rate was evaluated. Briefly, the 24 h control slide was fixed, stained with Giemsa and evaluated microscopically. In a minimum of four wells, 100 macrophages were evaluated for the presence or absence of amastigotes. If the result, expressed as percentage infection, was higher than 75% the experiment was continued. Stock solutions of the drugs in dimethylsulfoxide were prepared to a final concentration of 20 mM and sonicated for 15 min. The cultures were washed to remove extracellular promastigotes and 100 μl of the drug solution in RPMI-1640 supplemented with 10% HiFBS was added over a range of 30, 10, 3 and 1 μM in quadruplicate for each concentration. Prior to adding the drug solutions to the infected macrophage cultures, the solubility of the test compound in RPMI-1640 with 10% HiFBS was evaluated using an inverted light microscope (x200). The presence of particles was evaluated as an indicator of solubility.
Amphotericin B deoxycholate (Fungizone®) and miltefosine were included as positive control drugs. After 72 h of incubation at 34 °C in a 5% CO2/95% air mixture, all slides were methanol-fixed and Giemsa-strained. The percentage inhibition was determined microscopically (x400 magnification) as mentioned above. The EC50 and EC90 were calculated by non-linear sigmoidal curve fitting (variable slope) using Prism Software (GraphPad, Surrey, UK).
WRAIR: RAW 264.7 macrophages (ATCC, USA) were maintained in Dulbecco's modified eagle's medium (DMEM) (ATCC, USA) supplemented with 10% HiFBS (Corning, USA) at 37 °C in an incubator supplied with 5% CO2. Macrophages were harvested from culture, assessed for viability using trypan blue, and re-suspended at 2 × 105 cells/mL. The resulting suspension was dispensed at 50 μL/well (10,000 macrophages/well) in 384-well white plates. After incubating for 24 h at 37 °C and 5% CO2, media was removed from the wells and replaced with 50 μL of promastigote culture suspended in DMEM supplemented with 10% HiFBS (macrophage to promastigote ratio was 1:10 for L. major, and 1:40 for L. guyanensis and L. tropica). Promastigotes were left to invade the macrophages for 24 h at 37 °C and 5% CO2. Each well was washed three times in DMEM media supplemented with 10% HiFBS to remove any extracellular promastigotes and 77 μL of drug solution in DMEM supplemented with 10% HiFBS was added over an initial testing range of 23000 to 10 nM (2-fold serial dilutions across 12 wells) in quadruplicate for each concentration. Amphotericin B was included as a positive control and tested from 2160 to 1 nM in octuplicate for each parasite strain. After 96 h of incubation at 37 °C and 5% CO2, Xenolight D-luciferin Potassium Salt (Perkin Elmer, USA) was added to each well at a final concentration of 150 μg/mL and incubated for an additional 30 min at 37 °C and 5% CO2. Each plate was read for luminescence activity using an Infinite M200 plate reader (Tecan Inc., USA). EC50s were calculated for each drug using GraphPad Prism (GraphPad, USA) using the nonlinear regression (sigmoidal dose-response/variable slope) equation (Khraiwesh et al., 2016).
2.4. In vitro cytotoxicity (LSHTM)
KB cells were maintained in RPMI-1640 medium supplemented with L-glutamine and 10% HiFBS. This human-derived cell line was left in an incubator at 37 °C and 5% CO2 and passaged to new medium once a week (1/10 ratio). To assess cytotoxicity, the cells were counted and seeded in a 96-well plate at a concentration of 40,000 cells per well.
After a 24h incubation, the drug solutions were prepared by diluting the stock solution (20 mM in DMSO) in RPMI-1640 with 10% HiFBS. The top concentration (200 μM) was subsequently five-fold diluted across the plate. Podophyllotoxin was included as positive control drug (5 μM highest test concentration). Untreated controls and blanks, containing only medium were also included. Each test compound was tested in triplicate. Plates were incubated for a further 72 h at 37 °C and 5% CO2.
After incubation, the wells were assessed microscopically and 20 μl Alamar Blue® was added to each well. The plates were incubated for a further 2–4 h before reading at EX/EM 560/585 (cut-off 570) in a Spectramax™ M3 Plate reader. The EC50 value was calculated by non-linear sigmoidal curve fitting (variable slope) using Prism Software (GraphPad, Surrey, UK).
2.5. Evaluation of physicochemical properties of drugs (LSHTM)
Physicochemical properties of the test compounds (partition coefficient (log D), H-bond donors and acceptors and molecular weight) were calculated using ChemDraw 3D 16.0 software (PerkinElmer, Waltham, UK).
2.6. In vivo antileishmanial activity
2.6.1. Drug formulations
LSHTM: To maximise skin permeation for topical applications, saturated solutions in propylene glycol-ethanol (PG-EtOH 1:1) were prepared by adding an excess of drug compound to a glass vial together with 1 mL of PG-EtOH (1:1) and a magnetic stirrer. The vial covered with aluminium foil was left at 34 °C for 24 h. An aliquot of this suspension was transferred to a vial and centrifuged for 15 min at 18,407× g and 34 °C after which the supernatant was transferred to a clean vial and stored at 4 °C until drug administration. For DNDI-VL-2098, a topical solution of 0.25 mg/ml in PG-EtOH (1:1) was prepared. Drug concentrations and dosing frequency (for oral treatments) were determined based on efficacy observed against VL (Van den Kerkhof et al., 2018).
In preparation for the oral formulations, appropriate amounts of drugs were weighed and transferred to a clean glass vial. On the day of dosing, the exact volume of vehicle (Table 4) and glass beads were added to the vial before thoroughly mixing. The suspension was sonicated for 15 min prior to usage. Liposomal amphotericin B (AmBisome®, Gilead, UK) was prepared according to the manufacturer's instructions to a stock solution of 4 mg/ml that was consequently diluted with 5% sterile dextrose (aq) to a 2.5 mg/ml solution ready for use.
Table 4.
In vivo study design including dose regimen and formulation details.
| Group | Active compound | Vehicle | Administration route | Treatment regimen |
|---|---|---|---|---|
| Untreated control | N/A | N/A | N/A | N/A |
| Liposomal amphotericin B (AmBisome®) | Amphotericin B | Dextrose 5% | Intraveneous | 25 mg/kg (2.5 mg/ml) QAD, 5 doses |
| Paromomycin | Paromomycin sulfate | PBS | Intraperitoneal | 50 mg/kg (5 mg/ml) QD, 10 days |
| Vehicle control topical | N/A | PG-EtOH (1:1) | Topical | 50 μl BID, 10 days |
| Experimental topical formulation 1 | Aminopyrazoles Benzoxaboroles Nitroimidazoles |
PG-EtOH (1:1) | Topical | 50 μl of saturated drug solution BID, 10 days |
| Experimental oral formulation 1 | Nitroimidazoles: DNDI-0690 DNDI-VL-2098 |
Polyethylene glycol 400 10% of Tween 80-EtOH (7:3) in ddH2O |
Oral | 6.25 mg/kg (0.625 mg/ml), 12.5 mg/kg (1.25 mg/ml), 25 mg/kg (2.5 mg/ml) or 50 mg/kg (5.0 mg/ml) QD, 10 days |
| Experimental oral formulation 2 | Aminopyrazoles | 1% methylcellulose (w/v, 4000cps)/5% Tween 80/ddH2O | Oral | 3.125 mg/kg (0.3125 mg/ml), 6.25 mg/kg (0.625 mg/ml), 12.5 mg/kg (1.25 mg/ml), 25 mg/kg (2.5 mg/ml) or 50 mg/kg (5.0 mg/ml) BID, 5 or 10 days |
| Experimental oral formulation 3 | Benzoxaboroles | 2% ethanol, NaOH (1M), 5% dextrose | Oral | 12.5 mg/kg (1.25 mg/ml), 25 mg/kg (2.5 mg/ml) or 50 mg/kg (5.0 mg/ml) BID or QD, 5 or 10 days |
WRAIR: Nitroimidazole (DNDI-0690) was formulated in polyethylene glycol 400 (PEG400). Benzoxaborole (DNDI-6148) was formulated in 2% ethanol (EtOH), 1N sodium hydroxide (NaOH) (0.96 equiv), 5% dextrose (aq). Aminopyrazole (DNDI-1047) was formulated in 1% w/v methylcellulose (4000cps)/5% Tween 80/ddH2O. When needed, drugs were ground using a ProScientific 300D homogenizer and the particle size was measured using a Horiba LA-950V2 particle size analyser.
2.6.2. Murine CL model
LSHTM: The rump of female BALB/c mice were shaved using electric clippers (iClipper P6). Twenty-four hours later, stationary-phase L. major promastigotes were counted and re-suspended to a concentration of 2 × 108 per ml in Schneider's insect medium. Each mouse was injected subcutaneously on the rump with 200 μl of this parasite suspension. Approximately 10 days after infection a measurable nodule developed. The nodule diameter was calculated by averaging the lesion diameters measured in 2 dimensions on a daily basis using digital callipers (Jencons Scientific Ltd., UK). When the nodule attained a diameter of approximately 4 mm, the mice were re-grouped in groups of five to ensure similar nodule sizes in each group (one-way ANOVA, p > 0.05) and drug treatment was started. Each experimental compound was administered both orally and topically. As a positive control either liposomal amphotericin B (25 mg/kg/QAD; iv) or paromomycin sulfate (50 mg/kg/day; ip) was included as well as a topical vehicle control to assess the impact of the vehicle alone.
The treatment efficacy was evaluated i) daily, by measuring the lesion size diameter and plotting as a function of time and ii) at the end of the experiment by quantification of the parasite load in the CL nodule by quantitative PCR (18S DNA target) at day 11 (one day after the last dose was administered) as described in detail by Van Bocxlaer et al. (2018).
WRAIR: The lesion cure model was conducted as described in Caridha et al. (2017a). The day before infection, the dorsolumbar regions (base of the tail) of the mice were shaved and hair was removed using NAIR™ to prevent quick hair re-growth. The shaved areas treated with NAIR were then washed with clean water 2–3 times and dried using clean gauze. On the day of infection each mouse was infected intra-dermally (ID) with 100 μL parasite culture containing 1 × 107 luciferase-expressing L. major stationary phase promastigotes. Starting from the third week post infection, the lesion induration diameters (length = D1 and width = D2) were measured using a calliper instrument (Fisher Scientific, USA) with 0.1 mm sensitivity. Length and width measurements were taken to account for asymmetrical lesions. Lesion size area was then calculated using the πR1*R2 formula (where R1 = D1/2 and R2 = D2/2). Lesions were measured at a 10-day ( ±2 days) interval until the end of the study. Treatment was initiated approximately 3–4 weeks post infections, when lesions progressed to an average size of approximately 20 mm2. Cohorts of five or six (respectively for study I and II) BALB/c mice were assigned to all treatment groups such that the mean lesion sizes for all groups were not statistically different from each other. The experimental endpoint for the murine cure model is lesion cure (100% re epithelialization or lesion size 0 × 0).
To measure the bioluminescence signal, luciferin (D-Luciferin potassium salt, Xenogen, CA and Gold Biotechnology, St. Louis, MO), the luciferase substrate, was inoculated intraperitoneally (IP) into BALB/c mice and glutathione at a concentration of 200 mg/kg, 18 min before bioluminescence analysis. Animals were anaesthetized in a 2.5% isoflurane atmosphere (MWI Veterinary Supply, Harrisburg, PA) for 7 min and maintained in the imaging chamber for analysis. Emitted photons were collected by auto acquisition with a charge couple device (CCD) camera (PerkinElmer IVIS Spectrum In vivo Imaging System) using the medium resolution (medium binning) mode. Analysis was performed after defining a region of interest (ROI) that delimited the surface of the affected area. Total photon emission from the base of the tail infected area was quantified with Living Image software (Xenogen Corporation, Almeda, CA), and results were expressed in numbers of photons/sec.
Experimental design: Two separate studies were conducted with the purpose of determining the efficacy of three compounds (nitroimidazole (DNDI-0690), benzoxaborole (DNDI-6148) and aminopyrazole (DNDI-1047)) in the BALB/c mouse/L. major lesion cure model.
In the first study, 10-day treatments of nitroimidazole (DNDI-0690) and aminopyrazole (DNDI-1047), as well as 5-day treatments of the benzoxaborole (DNDI-6148) compound, were administered orally (PO), twice a day (BID). Three vehicle control groups (2% EtOH, 5% dextrose (aq); PEG 400; and 0.5% w/v methylcellulose and 5% v/v Tween 80/ddH2O), were given once a day (QD), PO for 10 consecutive days, except for 2% ETOH, 5% dextrose (aq) which was instead chosen to be given BID.
In the second study, the benzoxaborole (DNDI-6148) compound and the vehicle control group (2% ETOH, 5% dextrose (aq)) were administered PO, BID, for 10 consecutive days. In addition, in both studies, the positive control AmBisome® was administered intraperitoneally (IP), QD for 10 consecutive days.
2.7. Statistical analyses
LSHTM: A one-way ANOVA with the Tukey post hoc test (p < 0.05, SPSSv23, IBM, Portsmouth, UK) was performed to indicate the statistical differences between average lesion diameters, surface area, and parasite loads of the group at the end of treatment. Further, repeated-measures ANOVA (Dunnett's Multi Comparison Test) (p < 0.05) allowed the determination of whether the progression of lesion size in the experimental groups was statistically different from the included controls.
WRAIR: Statistical analysis was performed using the GraphPad Prism 7.04 software package (GraphPad Software, Inc., USA). One–way ANOVA with Dunnet's Multi Comparison Test and an unpaired t-test with Welch's correction were used to compare mean lesion size and bioluminescence signal differences between group means. A p-value < 0.05 was considered statistically significant.
3. Results
3.1. In vitro antileishmanial activity
LSHTM: All experimental compounds consistently (within 10-fold range) showed potent activity against amastigotes in primary murine peritoneal macrophages for both New World (L. mexicana, L. panamensis and L. amazonensis) and Old World (L. major, L. tropica and L. aethiopica) species with EC50 values ranging from 0.22 to 24.61 μM (Table 2). Aminopyrazoles DNDI-1047, -1044 and −8012 were the only compounds to demonstrate a nanomolar range activity against all Leishmania species in a similar range to amphotericin B. The Leishmania parasites are typically less susceptible to miltefosine, the other control drug, indicated by EC50 values ranging from 9 to 36 μM (Escobar et al., 2002; Van Bocxlaer et al., 2018). Together with the above mentioned aminopyrazoles, the benzoxaborole and the nitroimidazoles demonstrated consistently high activity (EC50 < 5 μM) against an Old and a New World strain and occasionally lower levels of activity (5 μM < EC50 < 25 μM) against one or two specific strains.
Table 2.
Susceptibility of a range of Leishmania species that cause CL against the experimental compounds (EC50 in μM (95% confidence interval), n is experiment number) [LSHTM data].
| Compound ID | n | L. major | L. tropica | L. aethiopica | L. mexicana | L. panamensis | L. amazonensis | Cytotoxicity | |
|---|---|---|---|---|---|---|---|---|---|
| Reference drugs | Amphotericin B | 1 | 0.07 (0.06–0.09) | 0.07 (0.07–0.08) | 0.11 (0.10–0.13) | 0.78 (1.6–0.20) | 0.07 (0.07–0.08) | ||
| 2 | 0.03 (0.03–0.03) | 0.34 (0.25–0.46) | 0.08 (0.06–0.19) | 0.13 (0.09–0.20) | |||||
| Miltefosine | 1 | 28.89 | 35.20 | 36.10 (27.95–46.64) | 11.21 (7.57–16.60) | 21.33 (18.91–24.06) | |||
| 2 | 30.02 | 29.52 | 9.22 (7.35–11.56) | 14.95 (12.08–18.49) | |||||
| Nitroimidazoles | DNDI-0690 | 1 | 4.56 (2.73–7.62) | 1.41 (1.29–1.53) | 24.61 | 1.91 (1.60–2.31) | 0.77 | <1.11 | >200 |
| 2 | 7.94 (4.31–14.64) | 2.38 (1.91–2.97) | < 1.11 | <1.11 | |||||
| DNDI-VL-2098 | 1 | 0.83 (0.62–1.12) | 1.34 (1.17–1.54) | 6.67 (5.75–7.73) | >200 | ||||
| DNDI-8219 | 1 | 3.24 (1.09–9.69) | 1.33 (0.95–1.85) | 3.17 (2.84–3.53) | 1.17 (0.82–1.68) | 0.34 (0.31–0.37) | 4.68 | >200 | |
| 2 | 1.44 (1.30–1.60) | 1.80 (1.66–1.96) | <1.11 | 4.68 (3.57–6.12) | |||||
| Benzoxaboroles | DNDI-6148 | 1 | 2.10 (1.70–2.60) | 7.25 (6.00–8.77) | 12.35 (8.51–17.92) | 2.36 (1.79–3.12) | 18.26 (9.58–34.80) | 2.04 | 180.70 |
| 2 | 1.20 (0.61–2.33) | 6.54 (4.17–10.25) | <1.11 | <1.11 | |||||
| Aminopyrazoles | DNDI-1044 | 1 | 0.63 (0.56–0.72) | 0.83 (0.78–0.89) | 0.29 (0.28–0.32) | <0.33 | <0.33 | <1.11 | 48.89 |
| 2 | <1.11 | <1.11 | <1.11 | <1.11 | |||||
| DNDI-1047 | 1 | 0.24 (0.22–0.26) | 0.34 (0.31–0.37) | <0.33 | <0.33 | <0.33 | <1.11 | >200 | |
| 2 | <1.11 | <1.11 | <1.11 | <1.11 | |||||
| DNDI-8012 | 1 | 0.62 (0.58–0.67) | 0.71 (0.61–0.84) | 0.39 (0.38–0.41) | <0.33 | <0.33 | <1.11 | >200 | |
| 2 | <1.11 | <1.11 | <1.11 | <1.11 |
WRAIR: Experimental results are shown in Table 3. As with the in vitro testing conducted against peritoneal macrophages, all experimental compounds consistently (within 10-fold) demonstrated nM range activity against both Old World (L. major and L. tropica) and New World (L. guyanensis) species when tested in the assay using amastigotes in a macrophage cell line assay. All tested drugs demonstrated in vitro efficacies similar to amphotericin B.
Table 3.
Susceptibility of a range of Leishmania species in the luciferase amastigote macrophage assay that cause CL against the experimental compounds (EC50 in μM (95% confidence interval), n is number of experiments) [WRAIR data].
| Compound ID | n | L. major | L. guyanensis | L. tropica | |
|---|---|---|---|---|---|
| Reference Drug | Amphotericin B | 2 | 0.03 (0.004–0.014) | 0.03 (0.003–0.013) | 0.01 (0.001–0.002) |
| Nitroimidazoles | DNDI-0690 | 2 | 0.34 (0.27–0.39) | 0.85 (0.18–2.61) | 2.36 (1.69–2.18) |
| DNDI-8219 | 2 | 0.03 (0.02–0.03) | 0.38 (0.05–2.14) | 1.20 (1.63–2.24) | |
| Benzoxaboroles | DNDI-6148 | 2 | 0.07 (0.05–0.11) | 0.05 (0.05–0.06) | 0.24 (0.16–0.31) |
| Aminopyrazoles | DNDI-1044 | 2 | 0.02 (0.02–0.03) | 0.03 (0.02–0.03) | 0.05 (0.02–0.05) |
| DNDI-1047 | 2 | 0.01 (0.01–0.02) | 0.02 (0.01–0.03) | 0.06 (0.03–0.10) | |
| DNDI-8012 | 2 | 0.02 (0.01–0.03) | 0.03 (0.03–0.04) | 0.07 (0.04–0.08) |
3.2. Cytotoxicity
Cytotoxicity assessment of the compounds demonstrated at least 10-fold selectivity between Leishmania species and host cells for all test compounds. The EC50 values of the test compounds when incubated with KB cells were above the highest test concentration (200 μM), except for DNDI-1044 (aminopyrazole) and DNDI-6148 (benzoxaborole) with EC50 values of 48.89 μM and 180.70 μM, respectively.
3.3. Physicochemical property evaluation
All tested candidates have physicochemical properties (Table S1) consistent or approaching values recommended as suitable to allow passive skin permeation except for the partition (for non-ionisable molecules) or distribution (for ionisable molecules) coefficient and number of H-bond acceptors in the case of DNDI-0690 and DNDI-6148. With a distribution coefficient (log D) of 1.92 (pH7.4, DNDi unpublished data) the benzoxaborole (DNDI-6148) is more hydrophilic than the lipophilic nitroimidazole (DNDI-0690 – log D (pH7.4) = 2.45, DNDi unpublished data) and aminopyrazole (DNDI-1047 – log D (pH7.4) = 3.68, DNDi unpublished data). All three are within or only just borderline outside the ideal skin permeant range (1 < log D < 3, (Hadgraft and Pugh, 1998)) and it was, therefore, decided to further evaluate their therapeutic potential against experimental CL.
3.4. Drug activity against murine CL
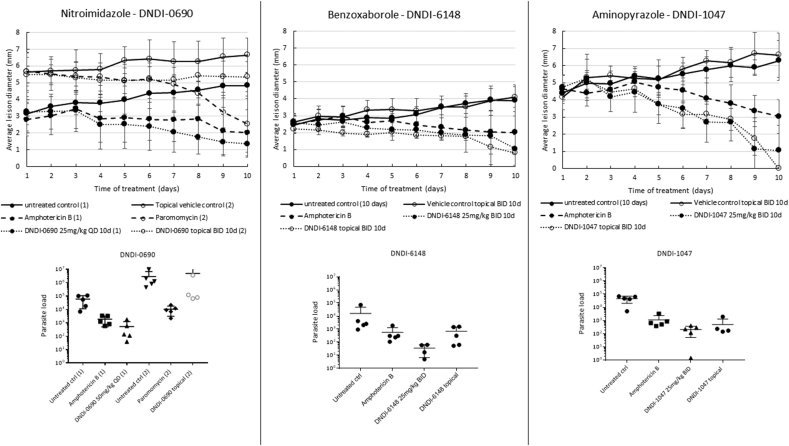
LSHTM: The antileishmanial activities for each drug and dose regimen, expressed as both parasite load and lesion size reduction compared to the appropriate control groups, are shown in Table 5 and illustrated in Fig. 1. Even though different regimens were evaluated, it is clear that DNDI-0690, -6148 and −1047 were able to significantly reduce the lesion size and parasite load in mice upon oral administration. At an equivalent dose of 25 mg/kg/day (i.e. 12.5 mg/kg/day BID or 25 mg/kg/day QD), DNDI-1047 was most effective in reducing the lesion size at the end of treatment, followed by DNDI-0690 and DNDI-6148 with 100%, 86.4% and 66.7% reduction, respectively. The reduction of parasite loads in the skin follows a similar trend, suggesting an appropriate distribution of the compounds to the location of the parasite. A linear correlation between the administered dose and the lesion size and parasite reduction was observed for DNDI-0690, DNDI-1047 and DNDI-6148 (Table 5).
Table 5.
The efficacy as mean % reduction of parasite load and lesion size of the lead nitroimidazoles, benzoxaboroles and aminopyrazoles upon treatment with different dosing regimens (n = 5).
| Compound | Administration route | Dose (mg/kg) | Application frequency | Duration (days) | % reduction |
||
|---|---|---|---|---|---|---|---|
| Lesion size | Parasite load | ||||||
| Reference | Liposomal amphotericin B | IV | 25 | QAD | 10 | 59.1 | 95.14 |
| Nitroimidazole | DNDI-0690 | Oral | 6.25 | QD | 10 | 72.1 | 50.19 |
| Oral | 12.5 | QD | 10 | 74.3 | 81.24 | ||
| Oral | 25 | QD | 10 | 86.4 | 84.18 | ||
| Oral |
50 |
QD |
10 |
100.0 |
94.89 |
||
| Topical | Sat sol | BID | 10 | 23.6 | 50.74 | ||
| DNDI-VL-2098 | Oral | 12.5 | QD | 10 | 45.0 | 62.68 | |
| Oral |
25 |
QD |
10 |
95.4 |
98.50 |
||
| Topical | 0.25 mg/ml | BID | 10 | 23.5 | 49.40 | ||
| Benzoxaborole | DNDI-6148 | Oral | 12.5 | BID | 10 | 66.7 | 92.35 |
| Oral | 25 | BID | 10 | 85.4 | 98.49 | ||
| Oral | 50 | BID | 5 | 43.2 | 100.00 | ||
| Oral | 50 | QD | 10 | 100.0 | 97.73 | ||
| Oral | 50 | BID | 10 | 100.0 | 99.56 | ||
| Oral | 50 | BID | 10 | 75.7 | 100.00 | ||
| Oral |
50 |
BID |
10 |
91.9 |
98.41 |
||
| Topical | Sat sol | BID | 10 | 71.4 | 71.49 | ||
| Topical | Sat sol | BID | 10 | 91.3 | 99.68 | ||
| Aminopyrazole | DNDI-1044 | Oral | 25 | BID | 10 | 89.2 | 99.99 |
| Oral |
25 |
BID |
10 |
100.0 |
99.96 |
||
| Topical | Sat sol | BID | 10 | 100.0 | 99.59 | ||
| DNDI-1047 | Oral | 3.125 | BID | 10 | 17.0 | 64.32 | |
| Oral | 6.25 | BID | 10 | 56.7 | 94.65 | ||
| Oral | 12.5 | BID | 10 | 100.0 | 99.96 | ||
| Oral | 12.5 | BID | 10 | 100.0 | 97.17 | ||
| Oral | 25 | BID | 10 | 100.0 | 99.63 | ||
| Oral | 25 | BID | 10 | 83.2 | 99.41 | ||
| Oral |
50 |
BID |
5 |
66.1 |
97.97 |
||
| Topical | Sat sol | BID | 10 | 100.0 | 98.22 | ||
| DNDI-8012 | Oral | 25 | BID | 10 | 92.0 | 99.96 | |
| Oral |
25 |
BID |
10 |
86.8 |
99.38 |
||
| Topical | Sat sol | BID | 10 | 93.8 | 100.00 | ||
Fig. 1.
Efficacy of the nitroimidazole (DNDI-0690), benzoxaborole (DNDI-6148) and aminopyrazole (DNDI-1047) in the L. major-BALB/c model of CL. Mice received 25 mg/kg of liposomal amphotericin B (IV) every other day or 50 mg/kg of paromomycin sulfate (IP) once daily or 50 mg/kg of DNDI-0690 once daily (oral) or 25 mg/kg of DNDI-1047 or DNDI-6148 (oral) twice daily. For topical treatment, 50 μl of a saturated solution in PG-EtOH was applied twice daily. All treatments were continued for 10 days. During treatment, lesion size was measured daily (a). The average lesion diameter represents the mean (n = 5). On day 11, lesion skin samples were collected and parasite load (b) was quantified. Each marker represents 1 parasite load. One-way ANOVA for parasite load and repeated measures for lesion size followed by Tukey's multiple comparison tests was used to analyse differences between untreated controls and experimental groups. A p-value < 0.05 was considered statistically significant.
When comparing the efficacy of the different drug compounds applied as saturated solution in a propylene glycol-ethanol (1:1, v:v) upon topical administration, the benzoxaborole (DNDI-6148) and the aminopyrazoles (DNDI-1044, -1047 and −8012) were found to reduce both the parasite load and the lesion size, suggesting permeation of the compound into the dermis. The nitroimidazoles, DNDI-0690 and DNDI-VL-2098, were the only compounds unable to significantly reduce the lesion size (23.6% and 23.5% reduction compared to the topical vehicle only control group, respectively). All treatments were well-tolerated and no overt signs of toxicity were observed in the mice.
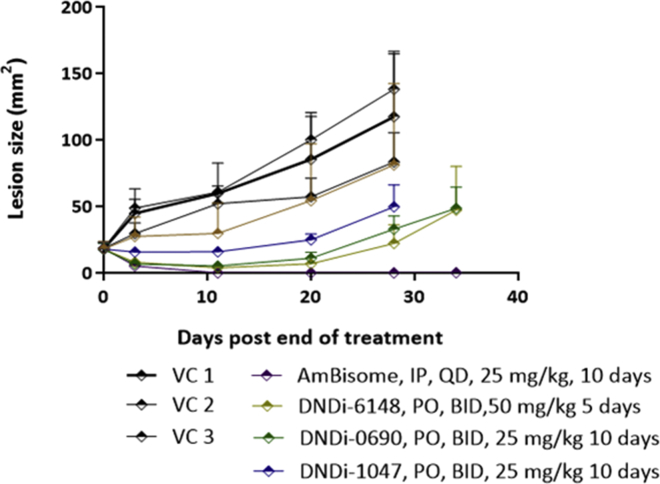
WRAIR: Experimental results from the first in vivo study are shown in Fig. 2. In this study, the lesion sizes in all study groups were measured on days 3, 11, 20, 28 and 34 days post end of treatment.
Fig. 2.
Nitroimidazole (DNDI-0690), benzoxaborole (DNDI-6148), and aminopyrazole (DNDI-1047) efficacy in the lesion cure model in BALB/c mice infected with luciferase-expressing L. major parasites. Mice received 50 mg/kg DNDI-6148, 25 mg/kg DNDI-0690, and 25 mg/kg DNDI-1047. All drugs were given PO, BID, for 10 consecutive days except for DNDI-6148 which was given for 5 consecutive days. The positive control group was treated with 25 mg/kg AmBisome® which was given IP, QD for 10 consecutive days. Three vehicle control groups VC 1, VC 2, and VC 3 consisted of 2% ETOH, 5% dextrose (aq); PEG 400; and 0.5% w/v methylcellulose, 5% v/v Tween 80/ddH2O respectively, which were the solvents used to dissolve DNDI-6148, DNDI-0690, and DNDI-1047 respectively. All vehicle control groups were treated PO, QD for 10 consecutive days, except for the VC1 which was randomly chosen to be administered BID. The average lesion size represents the mean ± standard error for each time point. One-way ANOVA was used to analyse differences between the positive, negative, and experimental groups. A p-value < 0.05 was considered statistically significant (*: p < 0.05).
After treatment ended, the lesion sizes in the benzoxaborole (DNDI-6148) and nitroimidazole (DNDi-0690) treated groups started decreasing at a similar rate to that of the AmBisome® treated group. On day 11 post end of treatment, 5/5 BALB/c and 1/5 BALB/c mice were clear of any detectable infection in the AmBisome® and benzoxaborole treated groups respectively, despite the fact that benzoxaborole treated animals received only 5 days’ worth of treatment compared to the 10 day treatments for all the other groups. Overall, when compared to their respective vehicle control groups, lesion sizes on day 11 post end of treatment were significantly reduced by 93.2%, 89.8%, 73.8% and 100% respectively for the benzoxaborole (DNDI-6148), nitroimidazole (DNDi-0690), aminopyrazole (DNDi-1047) and AmBisome® groups. Lesion sizes in the three vehicle control (VC) groups were not statistically different from each other. After day 11 post end of treatment, with the exception of the AmBisome® treated group, lesion sizes continued to increase as shown in Fig. 2.
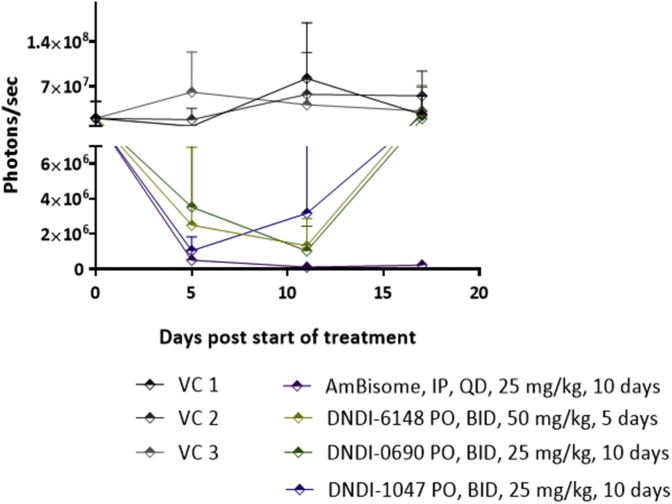
Bioluminescence signal progression in all treatment groups is shown in Fig. 3. On the day post end of treatment all groups including the AmBisome®, nitroimidazole, aminopyrazole and the benzoxaborole displayed a statistically significant reduction of parasite load when compared with their respective VC. The bioluminescence signal exponentially increased in all groups except for the AmBisome® treated group either immediately (DNDI-1047 and DNDI-0690) or a few days (DNDI-6148) after treatment ended. Both readouts (i.e. lesion size and in vivo bioluminescence) seem, therefore, to correlate well to post-treatment outcome of the monitored treated groups.
Fig. 3.
Nitroimidazole (DNDI-0690), benzoxaborole (DNDI-6148), and aminopyrazole (DNDI-1047) significantly reduce the bioluminescence signal (parasite load) at the infection site in BALB/c mice infected with luciferase-expressing L. major parasites. Mice received 50 mg/kg DNDI-6148, 25 mg/kg DNDI-0690, and 25 mg/kg DNDI-1047. All drugs were given PO, BID, for 10 consecutive days except for DNDI-6148 which was given for 5 consecutive days. The positive control group was treated with 25 mg/kg AmBisome® which was given IP, QD for 10 consecutive days. Vehicle control groups (VC 1, VC 2, and VC 3) consisted of 2% ETOH, 5% dextrose (aq); PEG 400; and 0.5% w/v methylcellulose, 5% v/v Tween 80/ddH2O respectively, which were the solvents used to dissolve DNDI-6148, DNDI-0690, and DNDI-1047 respectively. All vehicle control groups were treated PO, QD for 10 consecutive days, except for the VC1, which was chosen to be administered BID instead of QD. Each point represents mean ± standard error for the bioluminescence signal. One-way ANOVA was used to analyse differences between the positive, negative, and experimental groups. A p-value < 0.05 was considered statistically significant (*: p < 0.05).
On day 28 post end of treatment all vehicle control groups, as well as the aminopyrazole (DNDi-1047) group, were euthanized due to large lesion sizes. Animal groups treated with either the benzoxaborole (DNDI-6148) or the nitroimidazole (DNDi-0690) were monitored until day 34 post end of treatment, on which day they were euthanized due to rapidly increasing lesion sizes. On this day one BALB/c mouse belonging to the AmBisome® group had relapsed.
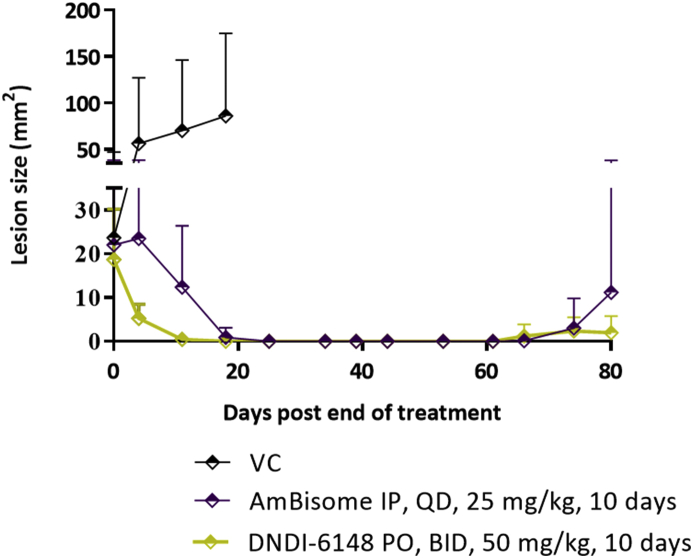
Experimental results from the second lesion cure study are shown in Fig. 4. Lesion sizes were measured on days 4, 11, 18, 25, 34, 39, 44, 53, 61, 66, 74 and 80 post end of treatment. After treatment ended, the lesion sizes in the benzoxaborole (DNDI-6148) treated group started decreasing at a much faster rate compared to the AmBisome treated group. On day 11 post end of treatment 2/6 and 3/6 BALB/c mice belonging to the AmBisome® and benzoxaborole (DNDI-6148) treated groups respectively were cured (no visible lesion) and did not present any sign of detectable parasite load as measured by bioluminescence. The overall lesion sizes were reduced by 78.8% and 99.3% respectively compared to the average VC lesion size. On day 18 post end of treatment 5/6 and 6/6 BALB/c mice belonging to the AmBisome® and benzoxaborole (DNDI-6148) treated groups respectively were cured and did not show any sign of detectable parasite load. On day 25 post end of treatments all animals of both treatment groups showed no visible lesions whereas the VC group was euthanized because of big lesion sizes.
Fig. 4.
Benzoxaborole (DNDI-6148) efficacy in the lesion cure model in BALB/c mice infected with luciferase-expressing L. major parasites. Mice received 50 mg/kg DNDI-6148 PO, BID, for 10 consecutive days. The positive control group received 25 mg/kg AmBisome® IP, QD for 10 consecutive days. The vehicle control group was treated PO, BID with 2% ETOH, 5% dextrose (aq). DNDI-6148 was formulated in 2% ETOH, 1N NaOH (0.96 equiv), 5% dextrose (aq). The average lesion size represents the mean + standard error for each time point. An unpaired t-test with Welch's correction were used to compare mean lesion size and bioluminescence signal differences between group means. A p-value < 0.05 was considered statistically significant (*: p < 0.05).
On day 46 post end of treatment, the bioluminescence signal was assessed again with the purpose of determining possible relapses of the L. major infection at the inoculation site and evaluating the parasite load in both treatment groups. Parasites were visible in 2/5 and 4/6 BALB/c mice that belonged to the benzoxaborole (DNDI-6148) and AmBisome® treated groups respectively (Fig. S1). The overall parasite load evaluated by the intensity of the bioluminescence signal in the AmBisome® group was 32% higher than the parasite load in the benzoxaborole (DNDI-6148) treated group.
On day 61 post end of treatment, 3/6 and 0/5 BALB/c mice belonging to the AmBisome® and benzoxaborole (DNDI-6148) treated groups respectively had developed papules and onn day 74 post end of treatment, this had increased to 4/6 and 1/5 mice, respectively. On day 80, which was the last day of the study, 4/6 and 1/5 mice belonging to the AmBisome® and benzoxaborole treated groups had developed lesions with average sizes of 11.2 mm2 and 1.98 mm2 respectively; these values were statistically different from each other.
An unpaired t-test with Welch's correction, which was used to compare bioluminescence signal differences between group means on day 46 post end of treatment, showed no statistically significant difference between the two treated groups at this time point. Despite that, there seems to be a good correlation between the reappearance (relapse) of the L. major infections at the former lesion site and the formation of lesions at the same site within 1–2 weeks. The lack of statistical significance in the overall parasite load difference for the AmBisome® and the benzoxaborole (DNDI-6148) treated groups was probably due to the high variability in the bioluminescence signal emitted from animals belonging to the same treatment group.
In previous studies we have shown that, the bioluminescence signal (which reflects the parasite load) emitted by the infection sites in BALB/c mice infected with luciferase-expressing L. major parasites reaches a plateau and/or begins to diminish at approximately 35 days post infection. This is probably due to signal attenuation associated with the appearance of dark crust on lesions and not to an intrinsic decrease of the in vivo signal (Caridha et al., 2017b). For this reason, bioluminescence signal data observed for each animal was collected and followed as a means of evaluating the parasite load at the infection site but was not used as a main experimental endpoint in this study.
4. Conclusion
One of the major drug discovery challenges for CL is to find a drug that has i) a potent activity against the many different causative species and ii) the properties to ensure therapeutic drug exposure in the skin. To address this first need, both New World and Old World CL species were included in an in vitro drug susceptibility evaluation panel. All drug candidates tested demonstrated marked to outstanding levels of potency against Old and New World species regardless of the Institute and/or evaluation assay used. When tested in the in vitro peritoneal assay (LSHTM), the aminopyrazoles showed the most potent antileishmanial activity of the evaluated compounds with nanomolar-range EC50 values similar to amphotericin B. At WRAIR, all compounds demonstrated in vitro efficacies with nanomolar-range EC50 values similar to amphotericin B against both Old World (L. major and L. tropica) and New World (L. guyanensis) species.
The currently available drugs (apart from miltefosine), amphotericin B, paromomycin, and the pentavalent antimonials are high MW polar molecules, which accounts for their poor oral bioavailability and their need to be injected or infused. It is therefore an important part of the approach by DNDi and others to focus on bioavailability early on in the drug discovery process, resulting in the design of molecules with the drug-like properties needed for them to reach the required target sites (C E Mowbray, 2018), including their physicochemical properties (C. E. Mowbray et al., 2015; Mukkavilli et al., 2014; Thompson et al., 2017; Van Bocxlaer et al., 2018). This approach was clearly justified by the significant efficacy demonstrated by the potent lead compounds when delivered orally or topically (except as explained below for the nitroimidazoles) in mouse models of infection. In two independent mouse models of CL infection, compounds of all three classes demonstrated antileishmanial activity following oral administation indicated by significant – and in some cases complete - lesion size reduction, which correlated with a reduction in parasite load determined by both quantitative PCR and bioluminescent signal in the experimental treatment groups when compared to untreated and/or vehicle controls. The efficacy against experimental CL was both dose- and treatment duration-dependent in the different models used at LSHTM and WRAIR respectively.
When applied to the skin topically as a saturated solution, the benzoxaborole (DNDI-6148) and the aminopyrazoles (DNDI-1047, DNDI-1044 and DNDI-8012) were able to significantly reduce lesion size and some even reduced the parasite load in the skin. Given the complex architecture of the skin, stricter thresholds of physicochemical parameters are imposed for topical drug delivery (Choy and Prausnitz, 2011). This was apparent for the poorly soluble nitroimidazole DNDI-VL-2098, which was unable to reduce the lesion size when applied locally to the skin. This could be explained by the lower diffusive driving force of the DNDI-VL-2098 topical formulation that only contained 0.25 mg/ml of DNDI-VL-2098 (diffusive driving force < 1), whereas the other molecules were applied as a saturated solution (diffusive driving force of 1). The second nitroimidazole DNDI-0690 is also poorly soluble probably contributing to the limited activity when administered topically. CL pathology resulting from parasites residing in the skin may also influence topical drug delivery, for example due to the induced hydrophilic environment in the dermis (Van Bocxlaer, Yardley, Murdan and Croft, 2016); CL pathology has been shown to have an impact upon systemic drug delivery (Wijnant et al., 2018a,b).
Only a handful of known antileishmanial compounds (AmBisome® and paromomycin being two of these) have established efficacy and can cure lesions in these stringent models of rodent CL. The superior activity demonstrated by all three lead compounds, in two independent laboratories (see Table 1 for comparison of methods), is a strong testimony of the high antileishmanial efficacy of these three lead compounds (Caridha et al., 2017a).
CL affects poor people, often living in rural and remote areas that only seek treatment approximately 1–6 months after the first symptoms (Ruoti et al., 2013), when the disease has already progressed to a stage where scars can no longer be avoided. It is hence of great importance to select drugs that fit the target product profile which involves a i) safe ii) short course iii) patient-friendly iv) oral and/or topical treatment with v) stability in tropical climates in order to encourage early treatment-seeking behaviour. DNDI-0690, DNDI-6148 and DNDI-5561 have all been nominated as preclinical candidates for VL and the first two of these are already scheduled to progress into clinical development in the near future (Croft et al., 2017). Even though the target tissue for CL, the skin, is different to that for VL, where the drugs should reach therapeutic concentrations in the liver and spleen, the results reported here show that these compounds also represent promising classes for the therapy of CL.
Conflicts of interest
The authors have no conflicts of interest.
Disclaimer
Material has been reviewed by the Walter Reed Army Institute of Research. There is no objection to its presentation and/or publication. The opinions or assertions contained herein are the private views of the author, and are not to be construed as official, or as reflecting true views of the Department of the Army or the Department of Defense.
Research was conducted under an approved animal use protocol in an AAALACi accredited facility in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals and adheres to principles stated in the Guide for the Care and Use of Laboratory Animals, NRC Publication, 2011 edition.
Acknowledgements
DNDi received financial support for this work from the following donors: Federal Ministry of Education and Research through KfW, Germany; Dutch Ministry of Foreign Affairs; World Health Organization – Special Programme for Research and Training in Tropical Diseases (WHO-TDR); and for its overall mission from UK AID, UK; Médecins Sans Frontières and the Swiss Agency for Development and Cooperation, Switzerland.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijpddr.2019.02.002.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
References
- Aronson N., Herwaldt B.L., Libman M., Pearson R., Lopez-Velez R., Weina P. Diagnosis and Treatment of Leishmaniasis: Clinical Practice Guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH) Am. J. Trop. Med. Hyg. 2017;96(1):24–45. doi: 10.4269/ajtmh.16-84256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennis I., Belaid L., De Brouwere V., Filali H., Sahibi H., Boelaert M. "The mosquitoes that destroy your face". Social impact of Cutaneous Leishmaniasis in South-eastern Morocco, A qualitative study. PLoS One. 2017;12(12) doi: 10.1371/journal.pone.0189906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos J.D., Meinardi M.M. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp. Dermatol. 2000;9(3):165–169. doi: 10.1034/j.1600-0625.2000.009003165.x. [DOI] [PubMed] [Google Scholar]
- Caridha D., Parriot S., Hudson T.H., Lang T., Ngundam F., Leed S. Use of Optical Imaging Technology in the Validation of a New, Rapid, Cost-Effective Drug Screen as Part of a Tiered In Vivo Screening Paradigm for Development of Drugs To Treat Cutaneous Leishmaniasis. Antimicrob. Agents Chemother. 2017;61(4) doi: 10.1128/AAC.02048-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caridha D., Parriot S., Hudson T.H., Lang T., Ngundam F., Leed S. Use of Optical Imaging Technology in the Validation of a New, Rapid, Cost Effective Drug Screen as Part of a Tiered In vivo Screening Paradigm for Development of Drugs to Treat Cutaneous Leishmaniasis. Antimicrob. Agents Chemother. 2017 doi: 10.1128/AAC.02048-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy Y.B., Prausnitz M.R. The rule of five for non-oral routes of drug delivery: ophthalmic, inhalation and transdermal. Pharm. Res. 2011;28(5):943–948. doi: 10.1007/s11095-010-0292-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft S.L., Olliaro P. Leishmaniasis chemotherapy-challenges and opportunities. Clin. Microbiol. Infect. 2011;17(10):1478–1483. doi: 10.1111/j.1469-0691.2011.03630.x. [DOI] [PubMed] [Google Scholar]
- Croft Simon L., Chatelain Eric, Barrett Michael P. Antileishmanial and antitrypanosomal drug identification. Emerg. Topics Life Sci. 2017;1(6):613–620. doi: 10.1042/ETLS20170103. [DOI] [PubMed] [Google Scholar]
- Elewski B.E., Tosti A. Tavaborole for the treatment of onychomycosis. Expert Opin. Pharmacother. 2014;15(10):1439–1448. doi: 10.1517/14656566.2014.921158. [DOI] [PubMed] [Google Scholar]
- Escobar P., Matu S., Marques C., Croft S.L. Sensitivities of Leishmania species to hexadecylphosphocholine (miltefosine), ET-18-OCH(3) (edelfosine) and amphotericin B. Acta Trop. 2002;81(2):151–157. doi: 10.1016/s0001-706x(01)00197-8. [DOI] [PubMed] [Google Scholar]
- Fairlamb A.H., Patterson S. Current and Future Prospects of Nitro-compounds as Drugs for Trypanosomiasis and Leishmaniasis. Curr. Med. Chem. 2018 doi: 10.2174/0929867325666180426164352. [DOI] [PubMed] [Google Scholar]
- Hadgraft J., Pugh W.J. The selection and design of topical and transdermal agents: a review. J. Invest. Dermatol. Symp. Proc. 1998;3(2):131–135. doi: 10.1038/jidsymp.1998.27. [DOI] [PubMed] [Google Scholar]
- Jacobs R.T., Plattner J.J., Keenan M. Boron-based drugs as antiprotozoals. Curr. Opin. Infect. Dis. 2011;24(6):586–592. doi: 10.1097/QCO.0b013e32834c630e. [DOI] [PubMed] [Google Scholar]
- Jacobs R.T., Plattner J.J., Nare B., Wring S.A., Chen D., Freund Y. Benzoxaboroles: a new class of potential drugs for human African trypanosomiasis. Future Med. Chem. 2011;3(10):1259–1278. doi: 10.4155/fmc.11.80. [DOI] [PubMed] [Google Scholar]
- Kassi M., Afghan A., Rehman R., Kasi P.M. Marring leishmaniasis: the stigmatization and the impact of cutaneous leishmaniasis in Pakistan and Afghanistan. PLoS Neglected Trop. Dis. 2008;2(10):1–3. doi: 10.1371/journal.pntd.0000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khraiwesh M., Leed S., Roncal N., Johnson J., Sciotti R., Smith P. Antileishmanial Activity of Compounds Derived from the Medicines for Malaria Venture Open Access Box Against Intracellular Leishmania major Amastigotes. Am. J. Trop. Med. Hyg. 2016;94(2):340–347. doi: 10.4269/ajtmh.15-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecoeur H., Buffet P., Morizot G., Goyard S., Guigon G., Milon G., Lang T. Optimization of topical therapy for Leishmania major localized cutaneous leishmaniasis using a reliable C57BL/6 Model. PLoS Neglected Trop. Dis. 2007;1(2):e34. doi: 10.1371/journal.pntd.0000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinski Christopher A., Lombardo Franco, Dominy Beryl W., Feeney Paul J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997;23(1):3–25. doi: 10.1016/S0169-409X(96)00423-1. [DOI] [PubMed] [Google Scholar]
- Mowbray C.E. Anti-leishmanial drug discovery: past, present and future perspectives. In: Rivas L., Gil C., editors. Drug Discovery for Leishmaniasis. The Royal Society of Chemistry; Croyden, UK: 2018. pp. 24–36. [Google Scholar]
- Mowbray C.E., Braillard S., Speed W., Glossop P.A., Whitlock G.A., Gibson K.R. Novel Amino-pyrazole Ureas with Potent In Vitro and In Vivo Antileishmanial Activity. J. Med. Chem. 2015;58(24):9615–9624. doi: 10.1021/acs.jmedchem.5b01456. [DOI] [PubMed] [Google Scholar]
- Mukkavilli R., Pinjari J., Patel B., Sengottuvelan S., Mondal S., Gadekar A. In vitro metabolism, disposition, preclinical pharmacokinetics and prediction of human pharmacokinetics of DNDI-VL-2098, a potential oral treatment for Visceral Leishmaniasis. Eur. J. Pharm. Sci. 2014;65:147–155. doi: 10.1016/j.ejps.2014.09.006. [DOI] [PubMed] [Google Scholar]
- Naik A., Kalia Y.N., Guy R.H. Transdermal drug delivery: overcoming the skin's barrier function. Pharmaceut. Sci. Technol. Today. 2000;3(9):318–326. doi: 10.1016/s1461-5347(00)00295-9. [DOI] [PubMed] [Google Scholar]
- Nare B., Wring S., Bacchi C., Beaudet B., Bowling T., Brun R. Discovery of novel orally bioavailable oxaborole 6-carboxamides that demonstrate cure in a murine model of late-stage central nervous system african trypanosomiasis. Antimicrob. Agents Chemother. 2010;54(10):4379–4388. doi: 10.1128/AAC.00498-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoti M., Oddone R., Lampert N., Orue E., Miles M.A., Alexander N. Mucocutaneous leishmaniasis: knowledge, attitudes, and practices among paraguayan communities, patients, and health professionals. J. Trop. Med. 2013:538629. doi: 10.1155/2013/538629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson A.M., O'Connor P.D., Marshall A.J., Blaser A., Yardley V., Maes L. Development of (6 R)-2-Nitro-6-[4-(trifluoromethoxy)phenoxy]-6,7-dihydro-5 H-imidazo[2,1- b][1,3]oxazine (DNDI-8219): A New Lead for Visceral Leishmaniasis. J. Med. Chem. 2018;61(6):2329–2352. doi: 10.1021/acs.jmedchem.7b01581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson A.M., O'Connor P.D., Marshall A.J., Yardley V., Maes L., Gupta S. 7-Substituted 2-Nitro-5,6-dihydroimidazo[2,1-b][1,3]oxazines: Novel Antitubercular Agents Lead to a New Preclinical Candidate for Visceral Leishmaniasis. J. Med. Chem. 2017;60(10):4212–4233. doi: 10.1021/acs.jmedchem.7b00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bocxlaer K., Gaukel E., Hauser D., Park S.H., Schock S., Yardley V. Topical Treatment for Cutaneous Leishmaniasis: Dermato-Pharmacokinetic Lead Optimization of Benzoxaboroles. Antimicrob. Agents Chemother. 2018;62(5) doi: 10.1128/AAC.02419-17. e02419-02417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bocxlaer K., Yardley V., Murdan S., Croft S.L. Drug permeation and barrier damage in Leishmania-infected mouse skin. J. Antimicrob. Chemother. 2016;71(6):1578–1585. doi: 10.1093/jac/dkw012. [DOI] [PubMed] [Google Scholar]
- Van den Kerkhof M., Mabille D., Chatelain E., Mowbray C.E., Braillard S., Hendrickx S. In vitro and in vivo pharmacodynamics of three novel antileishmanial lead series. Int. J. Parasitol. Drugs Drug. Resist. 2018;8(1):81–86. doi: 10.1016/j.ijpddr.2018.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijnant G.J., Van Bocxlaer K., Yardley V., Harris A., Alavijeh M., Silva-Pedrosa R. Comparative efficacy, toxicity and biodistribution of the liposomal amphotericin B formulations Fungisome((R)) and AmBisome((R)) in murine cutaneous leishmaniasis. Int. J. Parasitol. Drugs Drug. Resist. 2018;8(2):223–228. doi: 10.1016/j.ijpddr.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijnant G.J., Van Bocxlaer K., Yardley V., Harris A., Murdan S., Croft S.L. Relation between Skin Pharmacokinetics and Efficacy in AmBisome Treatment of Murine Cutaneous Leishmaniasis. Antimicrob. Agents Chemother. 2018;62(3) doi: 10.1128/AAC.02009-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.K., Plattner J.J., Easom E.E., Jacobs R.T., Guo D., Freund Y.R. Benzoxaborole Antimalarial Agents. Part 5. Lead Optimization of Novel Amide Pyrazinyloxy Benzoxaboroles and Identification of a Preclinical Candidate. J. Med. Chem. 2017;60(13):5889–5908. doi: 10.1021/acs.jmedchem.7b00621. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.