Abstract
Background
Currently, there is no topical treatment available for any form of cutaneous leishmaniasis (CL) in most of the endemic areas. The aim of the current study was to develop a topical nano-liposomal Amphotericin B (AmB) for the treatment of CL.
Methodology/principal findings
Liposomes containing 0.1, 0.2 and 0.4% AmB (Lip-AmB) were formulated and characterized for the size, entrapment efficiency, long term stability, and skin penetration properties using Franz diffusion cells. Liposomes diameters were around 100 nm with no change during more than 20 months’ storage either at 4 °C or at room temperature. Franz diffusion cells studies showed that almost 4% of the applied formulations penetrated across the skin and the highest skin retention (73.92%) observed with Lip-AmB 0.4%. The median effective doses (ED50), the doses of AmB required to kill 50% of L. major amastigotes were 0.151, 0.151, and 0.0856 (μg/mL) in Lip-AmB 0.1, 0.2, 0.4%, respectively. Lip-AmB 0.4% caused 80% reduction in fluorescence intensity of GFP+ L. tropica infected macrophages at 5 μg/mL of AmB concentration. Topical Lip-AmB was applied twice a day for 4 weeks to the skin of BALB/c mice to treat lesions caused by L. major. Results showed the superiority of Lip-AmB 0.4% compared to Lip-AmB 0.2 and 0.1%. The parasite was completely cleared from the skin site of infection and spleens at week 8 and 12 post-infection in mice treated with Lip-AmB 0.4%. The results suggest that topical Lip-AmB 0.4% may be a useful tool in the treatment of CL and merits further investigation.
Keywords: Nano-liposomal amphotericin B, Cutaneous leishmaniasis, Topical treatment, Leishmania tropica, BALB/C mice
Graphical abstract
Highlights
-
•
Novel topical AmB liposome for treatment of cutaneous leishmaniasis was formulated.
-
•
The formulation inhibits L. major promastigotes and amastigotes growth in vitro.
-
•
The formulation inhibits L. tropica amastigotes growth in vitro.
-
•
The formulation is able to treat lesions caused by L. major in BALB/c mice.
-
•
Lip-AmB 0.4 was stable at room temperature for more than 20 months.
1. Introduction
Leishmaniasis annual incidence rate is estimated at around 0.9–1.2 million, the disease is a major public health problem in some endemic Countries, the most common form being cutaneous leishmaniasis (CL). Although CL is not a life-threatening disease, it causes a serious social stigma with life-long disfiguring scars. The majority of CL cases occurs in Afghanistan, Algeria, Brazil, Colombia, Iran, Peru, Saudi Arabia and Syria. Two types of cutaneous leishmaniasis are prevalent in Iran, zoonotic CL (ZCL) caused by L. major (about 80% of cases) and anthroponotic CL (ACL) caused by L. tropica (about 20% of the cases) (Aronson et al., 2017; Dowlati, 1996; Nadim and Aflatoonian, 1995).
Treatment of CL continues to the use of pentavalent antimonial derivatives which require multiple injections with numerous side effects and variable efficacy (Aronson et al., 2017; Fairlamb et al., 2016; Hadighi et al., 2006; Neves et al., 2011; Ponte-Sucre et al., 2017). Amphotericin B (AmB), a polyene anti-fungal antibiotic derived from Streptomyces nodosus, is the second-line leishmaniasis treatment which interferes with ergosterol in the cell membrane of Leishmania and induces leakage of intracellular components and eventual pathogen death. The clinical use of the classical form of AmB (Fungizone®) has been limited, due to chronic nephrotoxicity (Berman, 2015; Deray, 2002). Thus, nano-carriers were used to reduce the toxicity of AmB. Among various formulations including Amphotec®, Abelcet® and AmBisome® (Adler-Moore et al., 2016), the latter has shown more tolerance and less severe side effects when compared to Fungizone® (Berman, 2015; Wijnant et al., 2018). AmBisome®, the intravenous (i.v) liposomal form of AmB, is a FDA-approved medication for the treatment of visceral leishmaniasis (Gutiérrez et al., 2016; Hamill, 2013). Previous clinical studies have shown the efficacy of AmBisome® for the treatment of Leishmania tropica (Solomon et al., 2011) as well as other strains of some Leishmania species (Mushtaq et al., 2016; Wortmann et al., 2010).
Using AmBisome® for the treatment of CL may not be as successful as VL due to poor infrastructure and number of patients in the endemic areas (Bhattacharya and Ali, 2016; Knöpfel et al., 2018; Wortmann et al., 2010). Search for new treatment modalities for CL is needed (Butsch et al., 2016).
In the last decades, several conventional formulations for topical applications were introduced such as sodium nitrite, paromomycin alone or in combination with gentamicin; the latter being more successful in clinical trials (Ben et al., 2013; Butsch et al., 2016; Lecoeur et al., 2007). In a recent study, topical Miltefosine in different solvents had a limited effect on treatment of lesions induced by L. major in BALB/c mice (Van Bocxlaer et al., 2016).
A suitable topical formulation must be able to target the Leishmania parasites in the dermal layers of the skin. Thus, carriers are crucial in enhancing drug penetration into the skin and promoting drug release. In contrast to the conventional formulations, liposomes are generally retained longer at the site of administration, thus are able to modify the distribution of the associated drug (Croft and Yardley, 2002; Mauël, 1990). Liposomes have long been used for skin delivery of pharmaceuticals i.e. AmB, but no topical formulations of AmB are available for the treatment of CL. Liposomes in the proper formulation and size, pass through stratum corneum of the intact skin and reach the dermis where Leishmania parasite normally lodge and multiply. In previous studies using sub-micron size liposomes as vehicles for paromomycin and AmB, the formulations showed to be effective against some Leishmania species (Ahsan et al., 2002; Devi et al., 2011; El Maghraby et al., 2008; Huang et al., 2009; Manosroi et al., 2004; Mauël, 1990; Singodia et al., 2010).
The aim of this study is as follows (i) to develop topical liposomal formulation containing various concentrations of AmB, (ii) to characterize the liposomal size, zeta potential, stability, in vitro permeation characteristics using Franz diffusion cells, and (iii) to assess the effect of the formulation on L. major and L. tropica in vitro, and on L. major infection in BALB/c mice.
2. Materials and methods
2.1. Chemicals
Amphotericin B was purchased from ASENCE Pharma Private Limited (Vadodara, India). Alamar Blue was purchased from Biosource (International, Inc., USA) and all other chemicals were of reagent grade and used as received.
2.2. Preparation and characterization of liposomal AmB
Liposomes containing AmB (Synbiotics Limited, Vadodara, India) were prepared using phosphatidylcholine and cholesterol as previously reported (Wohlrab J, 2005).
The particle diameter of each sample was measured in triplicate by dynamic light scattering (Malvern, Nano-ZS, UK). The measurements were performed at 25 °C after the appropriate dilution with the buffer of the formulation. Zeta potential of the liposomes was measured using electrophoretic light scattering by a Malvern Zetasizer Nano ZS. The measurement was performed at 25 °C after appropriate dilution with MOPS buffer (10 mM, pH 7.4). All of the measurements were repeated at least three times.
2.3. Liposomal AmB formulations analysis
The concentration of AmB in liposomal formulations was determined using HPLC (Knauer, Germany) method, as previously described (Barratt and Bretagne, 2007). The method for AmB determination by HPLC was validated using LOD (limit of detection) and LOQ (limit of quantitation) of 0.001 μg/mL and 0.06 μg/mL, respectively in two separate experiments in triplicate (S4 Fig). The chromatographic analysis was performed on a reverse phase C18 column (4.6 × 250 mm, 5 μm particle size, Atlantis T3, waters, Ireland). The chromatographic separation was achieved in less than 12 min on a reverse-phase C column using an acetonitrile-acetic acid-water (52:4.3:43.7, v/v/v) mixture as mobile phase. The flow rate was 1 mL/min and the effluent was monitored at 406 nm. Lip-AmB was dissolved in dimethyl sulfoxide (DMSO) and diluted with ethanol to have a concentration of 8 μg/mL of AmB. The area under the curve of Lip-AmB preparations was compared to the one of AmB USP reference standard (8 μg/mL).
2.4. Encapsulation efficiency of AmB in liposomes
Liposomes containing AmB were diluted 1 to 10, and were transferred into a 15 mL Amicon Ultra centrifugal filter unit (with a cut off 100 kDa, Sigma, USA) and centrifuged at 4,000 × g for 30 min, then, the filtrate was analyzed for AmB using HPLC.
2.5. In vitro effect of Lip-AmB on L. major promastigote and amastigote growth
The effect of Lip-AmB formulations on L. major promastigotes (MRHO/IR/75/ER) and amastigotes was assessed and compared with Fungizone. Alamar Blue was used for promastigote growth (Biosource International, Inc., USA) and for the amastigote growth, the slides were stained using Giemsa and were checked microscopically and compared with the untreated control wells. The experiments were repeated at least twice in triplicate. The ED50 for each formulation was calculated using CalcuSyn software Version 2.1 (Biosoft, Cambridge, UK) (Iman et al., 2011).
2.6. Cell diffusion study
Cell diffusion experiment was performed based on the previous study (Bavarsad et al., 2012). Jacketed Franz diffusion cells were used, and two experiment were completed in triplicate at 37 °C. BALB/c mice full-thickness skin was mounted in the Franz cell, with the stratum corneum sides facing upward. The membranes were initially left in the Franz cells for 30 min to facilitate hydration. Subsequently, 0.25 g of liposomal AmB was deposited onto the membrane surface. A 250 μl aliquot of receiver solution (PBS, pH 7.4) was withdrawn from each receiver solution at 1-h interval and replaced with the same volume of blank PBS solution. Aliquots of the collected samples were analyzed for AmB content as explained above. The derived concentration values were corrected using the following equation:
| Mt(n) = Vr × Cn + Vs × ΣCm |
Where Mt(n) is the current cumulative mass of drug transport across the skin at time t, Cn is the current concentration in the receiver medium, ΣCm is the summed total of the previous measured concentrations, Vr is the volume of the receiver medium, and the Vs correspond to the volume of the sample removed for analysis.
Liposome retention into the skin was assessed by measuring AmB content of the remaining formulation on the surface of membrane.
2.7. The effect of Lip-AmB 0.4% on infected macrophages with GFP+L. tropica
To evaluate the effective Lip-AmB 0.4% formulation on GFP-expressing L. tropica amastigotes, macrophage B10 cells were incubated and infected with L. tropica parasite (parasite-per macrophage ratio 10 to 1) for 24 h, then the cells were washed and different concentrations of Lip-AmB 0.4% (0.325–20 μg/mL), empty liposome and USP-AmB (dissolved in DMSO) were added and incubated for 24 h, then the cells were detached and GFP expression was monitored using flow cytometry (Taheri et al., 2015). The experiment was done in duplicate.
2.8. Animals and parasites
Female BALB/c mice, 6–8 weeks old, were purchased from Pasteur Institute (Tehran, Iran). The animals were maintained in Animal House of Nanotechnology Research Center of Mashhad University of Medical Sciences and fed with tap water and standard laboratory diet (Khorassan Javane Co, Mashhad, Iran). The animals were housed in a colony room 12/12 h light/dark cycle at 21 °C with free access to water and food.
The virulence of Leishmania major (MRHO/IR/75/ER) and GFP+ L. tropica (MOHM/IR/09/Khamesipour-Mashhad) were maintained by passage in BALB/c mice. The amastigotes were isolated from spleens (L. major) and lymph nodes (L. tropica) of infected mice and cultured on NNN media and then subcultured in RPMI 1640 (Sigma) containing 10% v/v heat inactivated FCS, 2 mM glutamine, 100 U/mL of penicillin and 100 μg/mL of streptomycin sulfate (RPMI-FCS) at 25 ± 1 °C.
2.9. Serum and tissue distribution of AmB after topical application of Lip-AmB 0.4% in uninfected (healthy) mice
Female BALB/c mice (3 per group) were applied twice a day at a shaved surface area of 2 × 2 cm with 100 mg of liposomal AmB 0.4% for 30 days. The amount of applied formulation was measured based on weight, since the formulations were too viscose to measure based on the volume. The massage duration was approximately 10 s for each mouse. After 2 h of the last application at day 30, the mice were sacrificed, blood samples were taken by heart puncture, and then the kidneys, livers and spleens were removed and weighed. Serum and tissues samples were stored frozen at −80 °C until the day of assay for AmB by high-performance liquid chromatography (HPLC) method.
To extract the AmB from the tissue, the organs were transferred into tubes containing 1 mL methanol and zirconia beads. The samples were completely homogenized by bead beating (Bead Beater; Biospec, Bartlesville, OK) at 5,000 rpm. The homogenates were incubated for 1 h at room temperature. The samples were then vortexed, transferred to Eppendorf polypropylene centrifuge tubes, and centrifuged for 10 min at 14,000 rpm, then the supernatant was separated and injected into the HPLC system and assayed for AmB.
2.10. Pathological examinations of kidneys after topical application of lip-AmB 0.4%
After topical application of liposomal AmB 0.4% for 30 days twice a day as explained in previous section, one of the kidneys from each mouse was removed and fixed in 10% neutral-buffered formalin solution, and then was embedded in paraffin. Embedded kidneys were cut at 3 μm thickness and stained with hematoxylin and eosin (HE). The images were prepared at 40 × magnification. Histopathological analyses were performed at Qaem Hospital Pathology Laboratory, Mashhad university of Medical Sciences, Mashhad, Iran.
2.11. Stability studies of liposomal AmB formulation
Among different formulations, Lip-AmB 0.4% was the most effective to control the progress of the lesion and showed the lowest parasite burden in the spleen of infected BALB/c mice. So, the stability study was conducted on this formulation. The stability of Lip-AmB 0.4% was assessed at 4 °C and also at room temperature (25 °C) and the formulation was analyzed for the particle diameters, zeta potential, AmB concentration (HPLC), and biological activity on L. major promastigotes.
2.12. In vivo experiment
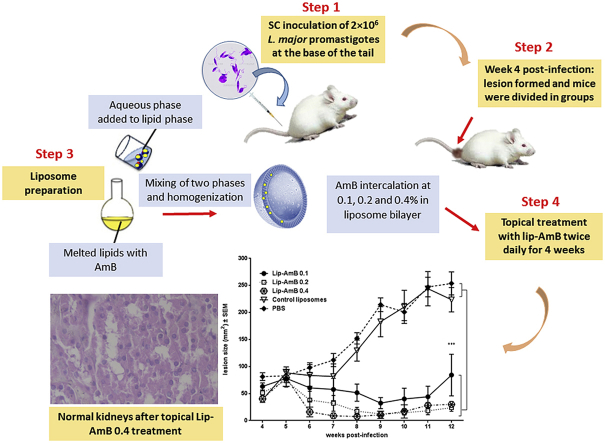
Fifty female 6–8 weeks BALB/c mice were inoculated subcutaneously (SC) at the base of the tail with 2 × 106 L. major promastigotes harvested at stationary-phase. Lesions were developed at week 4 post-infection, lesions were measured in two dimensions using calipers, and the animals were randomly divided into 5 groups of 10 mice/group including Lip-AmB 0.1, 0.2 or 0.4, empty liposomes and PBS. No significant differences (p>0.05) was seen in the lesion size among the different groups at initiation of the treatment. Lesions in the first three groups were treated topically with 50 mg liposomal AmB formulations twice a day for 4 weeks. The lesion size was measured weekly during the treatment period and for up to 12 weeks post-infections, in week 8, 3 mice per group were sacrificed for parasite burden.
One-way ANOVA statistical test was used to assess the significance of the differences among the various groups, p < 0.05 was considered as statistically significant.
At weeks 8 and 12 post-infection, spleen and lesion of 3 mice from each group were aseptically removed to assess the number of viable L. major parasites using limiting dilution assay. The spleen was homogenized in 1 mL RPMI-FCS with a sterile syringe piston. The lesion was transferred into the tubes containing 1 mL RPMI-FCS, and 1 g of zirconium beads. Samples were then completely homogenized for 20s by bead beater and diluted with the same media in 8 serial of 10-fold dilutions. The homogenates were transferred into flat-bottom 96-well microtiter plates containing solid layer of rabbit blood agar in triplicate and incubated at 25 ± 1 °C for 7 days. The positive (presence of motile parasite) and negative (absence of motile parasite) wells were detected using an inverted microscope (CETI, UK). The reported data is the calculated mean and standard error of mean of the last positive well multiplied by the dilution factor. Two separate experiments were done in triplicate.
2.13. Ethics statement
All procedures involving animals and the proposal were approved by the Institutional Ethical Committee and the Research Advisory Committee of Mashhad University of Medical Sciences, based on the Specific National Ethical Guidelines for Biomedical Research issued by the Research and Technology Deputy of the Ministry of Health and Medicinal Education (MOHME) of Iran issued in 2005. Animal experiments were carried out according to the Ethical Committee Acts of Mashhad University of Medical Sciences (Grant number 900050; October 27, 2011).
3. Results
3.1. Liposomes characterization
The final liposomes containing 0.1, 0.2 and 0.4% AmB were round-shaped (Figure in S1 Fig), yellow paste-like with average particle diameters of around 100 nm (Table 1). As indicated, no significant difference (p>0.05) was observed in the size of Lip-AmB compared to control liposomes, all liposomes were negatively charged and there were no significant differences in the surface charges of different formulations (Table 1).
Table 1.
Particle size, zeta potential, AmB concentrations, MICa (against L. major promastigotes) and ED50 (against L. major amastigotes in J774 A.1 macrophage) of topical liposomal AmB formulations.
| Liposome formulations | Z-average (nm) | Zeta potential (mv) ± SD | AmB concentration (mg/g) ± SD | MICa(μg/mL) | ED50(μg/mL) (lower and upper 95% limit) |
|---|---|---|---|---|---|
| Control empty liposomes | 106.3 ± 2.1 | −32.9 ± 4.59 | – | Inactive | Inactive |
| Lip-AmB 0.1% | 119.2 ± 14.4 | −32.7 ± 6.13 | 1.17 ± 0.0058 | 0.625 | 0.151 (0.0523–0.434) |
| Lip-AmB 0.2% | 116.7 ± 9.9 | −36.4 ± 7.98 | 2.36 ± 0.0058 | 0.625 | 0.151 (0.085–0.267) |
| Lip-AmB 0.4% | 113.5 ± 10.4 | −36.5 ± 4.2 | 4.11 ± 1.53 | 0.625 | 0.0856 (0.0147–0.5) |
| Fungizone | – | – | – | 0.625 | 0.063 (0.027–0.146) |
The values are means ± standard deviations (n = 3).
Minimum inhibitory concentrations.
Control empty liposome containing DMSO with the same percent in Lip-AmB 0.4% was used. As presented in Table 1, control empty liposome was inactive against L. major promastigotes and L. major amastigotes in J774 A.1 macrophage.
After centrifugation of diluted liposomes in Amicon Ultra centrifugal filter unit (100 kDa, cut off), no AmB was detected in the filtrate; therefore, the entrapment efficiency of AmB in liposomes was 100%.
3.2. Effect of liposomal AmB on L. major promastigote in vitro
The effect of Lip-AmB formulations on L. major promastigote (MRHO/IR/75/ER) was assessed and compared with Fungizone using Alamar Blue. Minimum inhibitory concentration (MIC) of AmB in different Lip-AmB formulations against L. major promastigotes in culture was 0.625 μg/mL, based on AmB concentration. This value corresponds to 625, 312.5 and 156.26 μg based on the total amount of liposomes for Lip-AmB 0.1, 0.2 and 0.4%, respectively (Table 1). There was no significant difference in the minimum inhibitory concentration (MIC) of AmB in different Lip-AmB formulations (Table 1), Fungizone (Bristol-Myers Squibb Company, Princeton, NJ) was used as positive control. The MIC for Fungizone was also 0.625 μg/mL, there was no significant difference in the MIC of different formulations.
3.3. In vitro effect of liposomal AmB on L. major amastigotes
The ED50 which is the dose of AmB required to kill 50% of Leishmania parasites in vivo was determined from infected macrophages. The ED50 of Lip-AmB 0.4, 0.2 and 0.1% against L. major amastigote in macrophage are shown in Table 1. The ED50 of Lip-AmB 0.4% was two times lower than that of Lip-AmB 0.2% and 0.1%. The values in Table 1 are reported based on the amount of AmB in each formulation and are equivalent to 151, 75.5 and 21.4 μg of AmB based on the total amount of liposomes for Lip-AmB 0.1, 0.2 and 0.4%, respectively. The ED50 values of Fungizone® was lower compared to the liposomal AmB formulations (0.063 μg/mL versus 0.151 for Lip-AmB 0.1, 0.2 and 0.0856 for Lip-AmB 0.4), which was expected due to the rapid dissociation of AmB from micellar formulation.
3.4. Cell diffusion study
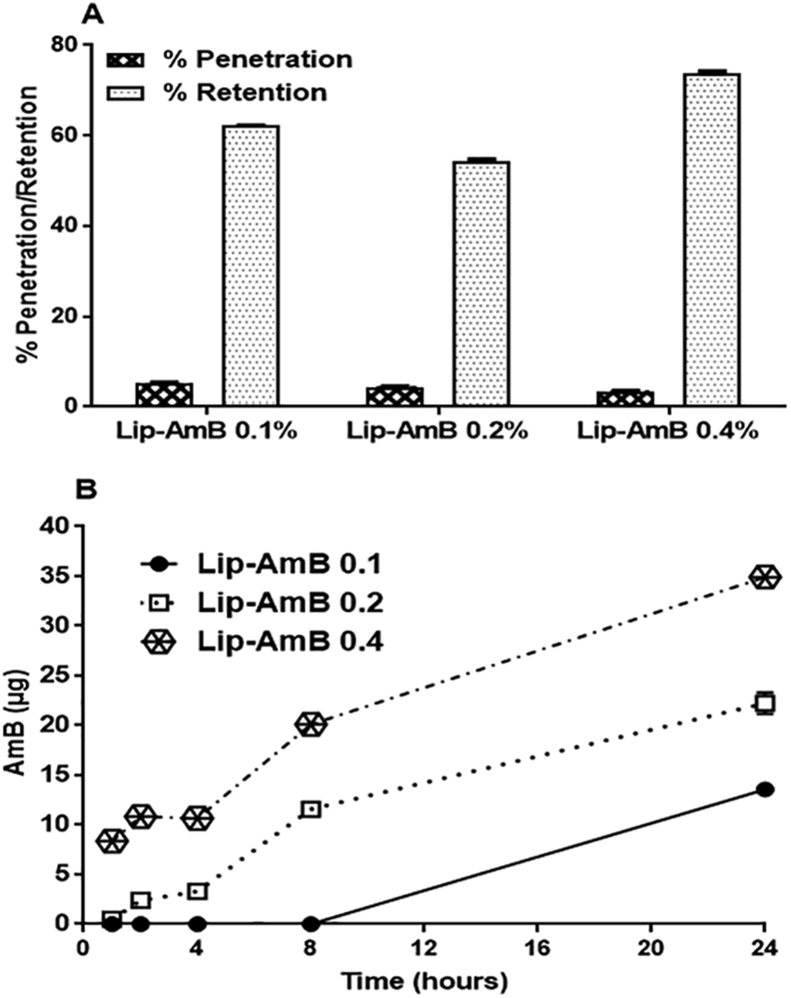
The delivery of AmB to and through the mouse skin was determined during a 24 h experiment using diffusion cells with PBS in the receptor compartment. The amount of AmB penetrated through the skin after application of Lip-AmB 0.1, 0.2 and 0.4% were 5.34%, 4.45% and 3.49%, respectively (Fig. 1A). The rate of permeation of liposomal AmB through the mouse skin was determined as equivalent to 13.35, 22.25 and 34.9 μg based on the amount of AmB (Fig. 1B). The proportions of AmB in different Lip-AmB formulations retained in the skin were 62.22%, 54.47% and 73.92% (155.55, 273.35 and 739.2 μg based on amount of AmB) for Lip-AmB 0.1, 0.2 and 0.4%, respectively, as shown in Fig. 1A. The results with PBS plus 5% DMSO or PBS plus 10% methanol in the receptor compartment at either 32 or 37 °C showed no significant difference compared to PBS (Figure in S5 Fig).
Fig. 1.
Percent of penetration and retention of AmB from Lip-AmB 0.1, 0.2 and 0.4% across the mouse skin after 24 h. Franz cell diffusion studies were carried out with jacketed Franz cells contain PBS as the receiver medium at 37 °C, and samples were drawn at 1-h intervals for up to 24 h (A). At the end of the experiment, the formulation that remained on the top of the mouse skin was collected and assayed for detection of the amount of AmB. The permeation rate of AmB in regard to time (B). The percent formulation retention was calculated by considering the percentage of the drug released and the remaining amount on the skin at the end of the 24 h experiment. Values are means ± standard deviations (n = 3).
3.5. The effect of Lip-AmB 0.4% on GFP+L. tropica infected macrophages
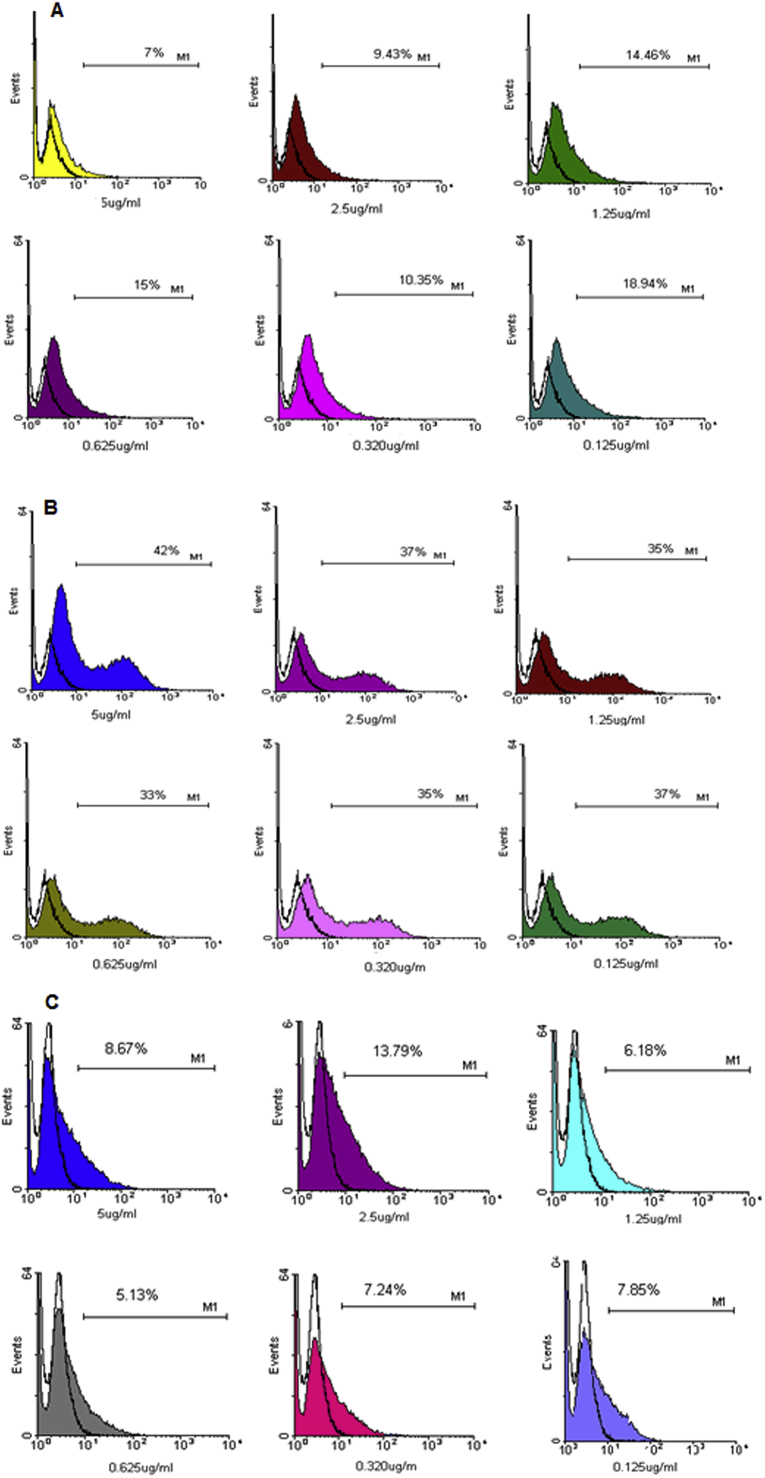
GFP expression in transgenic promastigotes indicated that the percentage of GFP+ L. tropica significantly increased after 24 h incubation with macrophages (31.2%) when compared to 48 and 72 h incubation time (25% and 17%, respectively) (Figure in S2 Fig). Fig. 2A shows GFP expression following the treatment of L. tropica infected B10 macrophages with Lip-AmB 0.4%. The percentage of GFP-expressing parasites in the presence of free liposomes was higher than that of other groups treated with different concentrations (Fig. 2B). GFP expression gradually diminished with increased drug concentration. The result of treatment with Lip-AmB 0.4% was comparable to that of USP AmB (80% reduction in parasite GFP fluorescence at 5 μg/mL AmB compared to empty liposomes, Fig. 2C). To assess the toxicity of Lip-AmB 0.4% on macrophages, GFP+ L. tropica infected macrophages were exposed to either control empty liposomes or Lip-AmB 0.4%; the resulting viability of the macrophages was the same in both treated groups (Figure in S3 Fig).
Fig. 2.
GFP expression of macrophage transfected with L. tropica in the presence of different concentrations of Lip-AmB 0.4% (A), free liposome (B) and USP AmB (C). L. tropica GFP+ infected Macrophages B10 cells were treated with different concentrations of lip-AmB formulation, empty liposome and USP-AmB and GFP expression was monitored using flow cytometry.
3.6. Serum and tissue distribution of AmB after topical application of Lip-AmB 0.4% in uninfected (healthy) mice
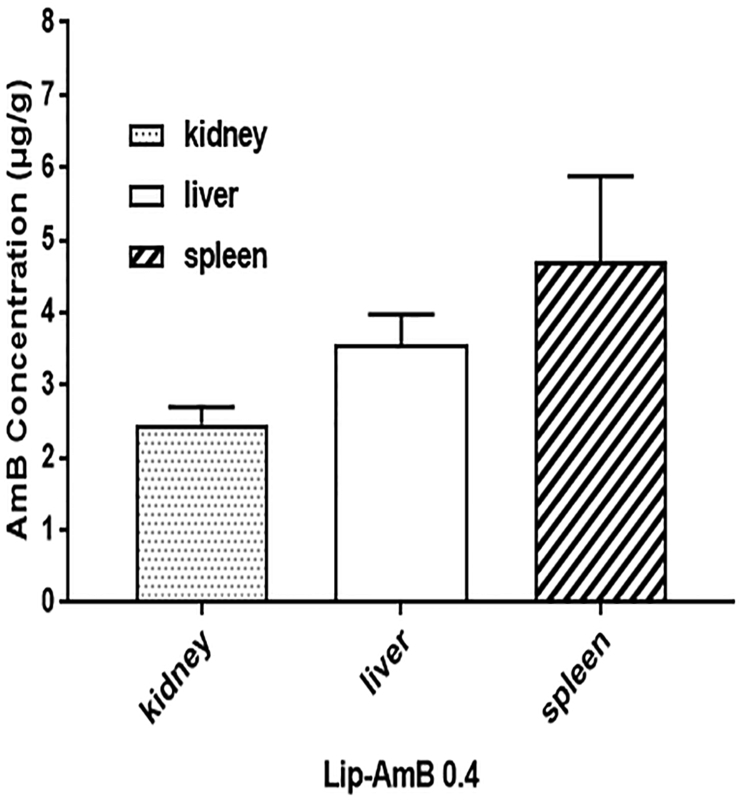
The results indicated that following topical application of liposomal AmB 0.4% for one month, although the serum concentration of AmB was 0.367 ± 0.009 μg/mL, accumulation in the kidney, spleen and liver was noticeable. The highest concentration of AmB was achieved in the spleen of uninfected animals (Fig. 3).
Fig. 3.
Tissue AmB concentrationAmB. Female BALB/c mice (3 per group) were applied with 100 mg of liposomal AmB 0.4% for 30 days, twice a day on the shaved surface area of 2 × 2 cm of the skin. At day 30, 2 h post last application, the mice were sacrificed, and then the kidneys, livers and spleens were removed and assayed for AmB using high-performance liquid chromatography (HPLC) method.
3.7. Pathological examinations of kidneys after topical application of lip-AmB 0.4%
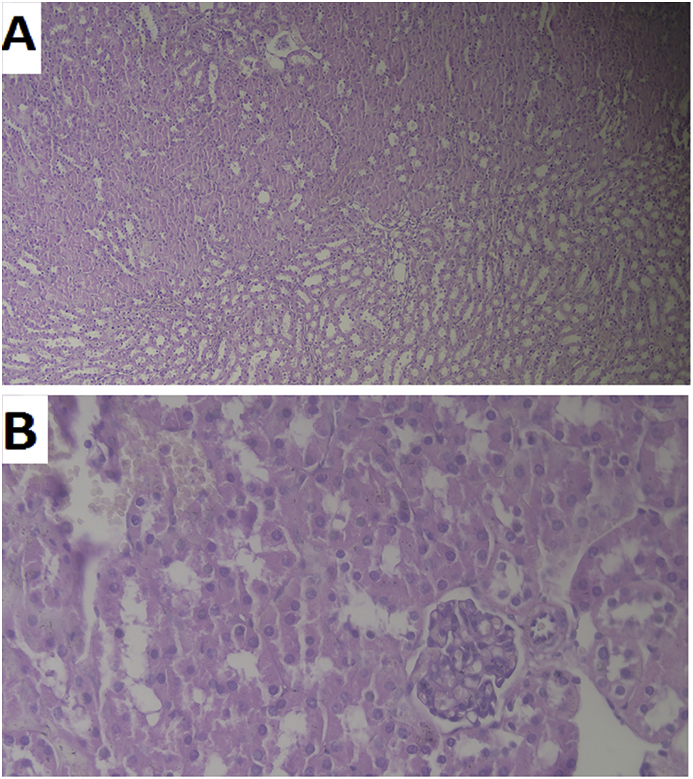
The renal toxicity as the main toxicity of AmB was evaluated by pathological examination of kidneys after topical application of Lip-AmB 0.4% twice a day for 30 days. The kidneys were normal and no adverse effects were found in the examined kidneys (Fig. 4).
Fig. 4.
Changes in kidneys pathology. The renal toxicity evaluation by pathological examination of kidneys after topical application of liposomal AmB 0.4% twice a day for 30 days. One of the kidneys from each mouse was removed and fixed in 10% neutral-buffered formalin solution, and then was embedded in paraffin. Embedded kidneys were cut at 3 μm thickness and stained with hematoxylin and eosin (HE). The images were prepared at A) 10 × and B) 40 × magnification. The kidneys were normal and no adverse effect was found in the examined kidneys.
3.8. Stability of liposomes
The stability of Lip-AmB 0.4% was determined at both 4 °C and room temperature (25 °C). Further, the formulation was analyzed for any changes in particle diameters, zeta potential, AmB concentration (HPLC), and the biological activity on L. major promastigotes. Results showed that the concentration of AmB remained unchanged in Lip-AmB 0.4% during storage at either 4 or 25 °C up to 20 months (Table 2). Furthermore, no significant changes were observed in the liposome size and zeta potential for up to 20 months when Lip-AmB 0.4% was stored at 4 or 25 °C. The MIC of AmB in Lip-AmB 0.4% against L. major promastigote in culture was 0.625 μg/mL and remained unchanged when stored at either temperature condition for up to 20 months.
Table 2.
Stability studies of liposomal AmB 0.4% stored at 4 °C and 25 °C.
| Temperatures | Months after liposome preparation | AmB Concentrations (mg/g) ± SD | Liposome Diameter (nm) | Zeta potential (mv) | MIC (μg/mL) for L. major promastigotes |
|---|---|---|---|---|---|
| 4 ° C | 0 | 4.11 ± 0.02 | 113.5 ± 10.4 | −36.5 ± 4.2 | 0.625 |
| 25° C | 4.11 ± 0.015 | 113.5 ± 10.4 | −36.5 ± 4.2 | 0.625 | |
| 4 ° C | 1 | 4.13 ± 0.01 | 89.0 ± 20.9 | −39.4 ± 6.0 | 0.625 |
| 25° C | 4.18 ± 0.05 | 102.5 ± 32.1 | −40.1 ± 5.4 | 0.625 | |
| 4 ° C | 3 | 4.21 ± 0.03 | 90.1 ± 33.3 | −39.9 ± 6.8 | 0.625 |
| 25° C | 4.14 ± 0.01 | 106.5 ± 9.1 | −41.8 ± 5.2 | 0.625 | |
| 4 ° C | 6 | 4.58 ± 0.04 | 107.2 ± 0.7 | −41.3 ± 6.9 | 0.625 |
| 25° C | 4.84 ± 0.03 | 118.6 ± 8.7 | −41.8 ± 5.9 | 0.625 | |
| 4 ° C | 12 | 4.08 ± 0.04 | 85.4 ± 10.6 | −41.1 ± 5.8 | 0.625 |
| 25° C | 4.06 ± 0.09 | 120.8 ± 7.2 | −40.3 ± 5.3 | 0.625 | |
| 4 ° C | 20 | 4.00 ± 0.05 | 91.1 ± 6.4 | −41.8 ± 5.7 | 0.625 |
| 25° C | 4.05 ± 0.06 | 111.2 ± 0.7 | −42.7 ± 5.5 | 0.625 |
3.9. Anti-Leishmania activity in murine model of CL
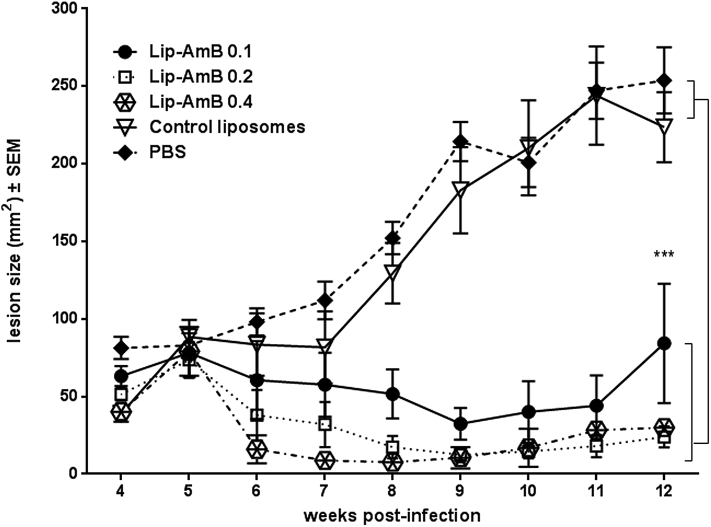
As shown in Fig. 5, there was no significant difference in the lesion size in different groups at initiation of treatment. All liposomes induced regression in the lesion size following treatment. Though the difference was not significant in the first 3 weeks following treatment, liposomal AmB showed a remarkable reduction in the size of lesion in treated animals from week 8 onward. By week 12 post-treatment, animals in the control groups receiving either empty liposomes or PBS, developed significantly larger lesions compared to those treated with liposomal formulations (p < 0.001). There was no significant difference in the lesion size among different groups receiving liposomal AmB; the efficacy of Lip-AmB 0.4% on the lesion size was more pronounced compared to other concentrations (Lip-AmB 0.1 and 0.2%) (Fig. 5).
Fig. 5.
Activity of topical liposomal AmB on the course of L. major lesion in BALB/c mice. Female BALB/c mice were SC inoculated at the base of the tail with stationary-phase L. major promastigotes (2 × 106). At week 4 post-infection, lesions were measured and topically treated with 50 mg liposomal AmB formulations twice a day for 4 weeks. The lesion size was monitored by weekly measurement for 12 weeks. Values represent means ± standard errors of the means. The results for the Lip-AmB 0.1, 0.2 and 0.4-treated group of mice were significantly different from the control groups (mice receiving PBS or empty liposomes) (***, P<0.001).
3.10. Quantitative parasite burden
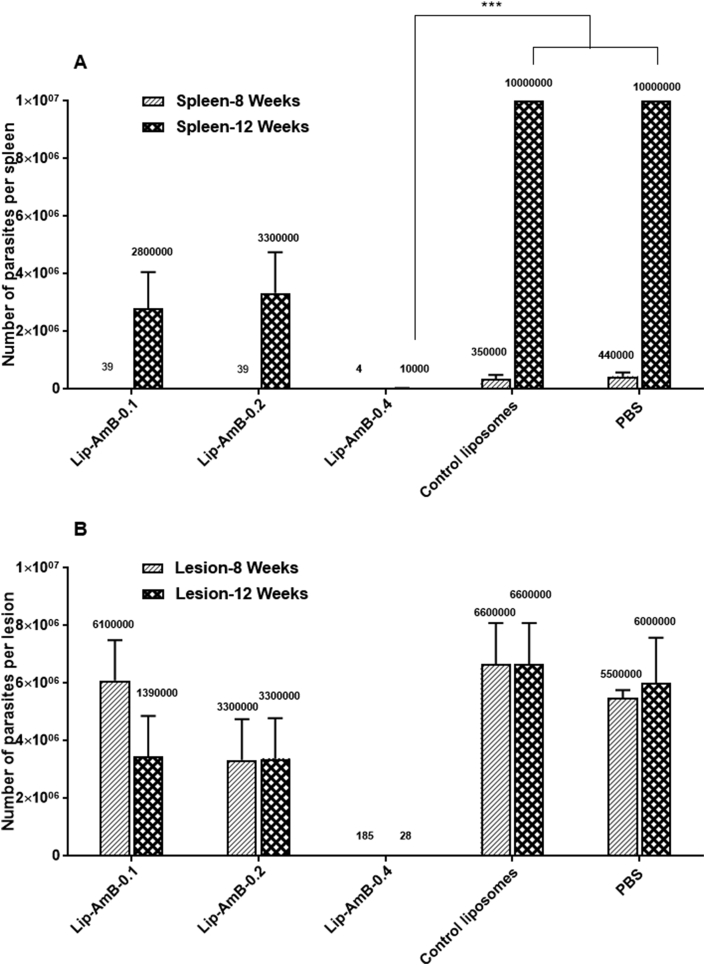
The number of viable L. major parasites were assessed in the lesions and spleens of different groups of mice at week 8 and 12 post-infection using limiting dilution assay. At week 8 post-infection, splenic parasite count was significantly (p<0.001) lower in mice treated with Lip-AmB 0.1, 0.2 and 0.4% compared to the PBS or empty liposomes (Fig. 6A). At week 12, the splenic parasites load increased in different treatment groups, except in those mice that received Lip-AmB 0.4%. The count of splenic parasites was significantly lower in Lip-AmB 0.4% (p<0.001) compared to the groups receiving PBS or empty liposomes. A similar trend was observed in the count of parasites in the lesions. Among the different Lip-AmB concentrations, the lowest number of parasites were observed in animals treated with Lip-AmB 0.4%. The count of parasites in the lesions of Lip-AmB 0.4% treated animals was significantly (p<0.001) lower when compared to all other groups at weeks 8 and 12 post-infection (Fig. 6B).
Fig. 6.
Splenic and lesion parasite burden in BALB/c mice treated with liposomal AmB formulations. Spleens and infected lesions were aseptically removed and homogenized in 1 mL RPMI-FCS at weeks 8 and 12 after infection. The homogenates were diluted and placed in 96-well microtiter plates containing solid layer of rabbit blood agar in triplicate and incubated at 25 ± 1 °C for 7 days. The motile promastigotes were checked using an inverted microscope (CETI, UK). The splenic parasite load in group of mice treated with Lip-AmB 0.4% was significantly lower than groups of mice treated with PBS or empty liposomes at week 12 post infection (***, p<0.001) (Fig. 6A). Load of parasite in the lesion of mice treated with Lip-AmB 0.4% was significantly lower than all other groups at day 8 and 12 post infection (***, p<0.001) (Fig. 6B).
4. Discussion
Treatment of CL is dependent on the use of pentavalent antimonite derivatives which are painful, need multiple injections and are accompanied by side effects (Aronson et al., 2017; Croft et al., 2006; Dowlati, 1996). Several second line treatments were used to treat CL, including liposomal AmB which is expensive and impractical to use in endemic foci (Wortmann et al., 2010). AmBisome®, a non-pyrogenic lyophilized liposomal product for intravenous administration is very effective in the treatment of visceral leishmaniasis (Kala-azar); however inefficacious in cutaneous form due to poor delivery of AmB at the lesion site upon i.v. administration as well as toxicity (Iman et al., 2017).
Considering the status of CL treatment in endemic areas, the search for new treatments is well justified. Toxic effects can be greatly reduced following topical application since it requires lower drug dosages which reduces toxic effects and offers easier administration (Garnier and Croft, 2002). Over the past decades, conventional dosage forms were used with variable efficacies and toxicities (Ben et al., 1995, 2013; Frankenburg et al., 1998; Garnier and Croft, 2002; Garnier et al., 2007; Soto et al., 1995). Currently, only topical formulation of paromomycin is marketed for the treatment of CL by TEVA as Leshcutan. AmB topical preparation in white paraffin was ineffective for the treatment of cutaneous Leishmania major lesions in mice due to the high molecular weight and hydrophobic nature of the drug molecule, hindering its penetration into the dermal layer (El-On et al., 1984).
Several strategies have been proposed to enhance topical absorption including the use of penetration enhancers (Garnier and Croft, 2002). For example, commercial AmB formulations including Amphocil (AmB and cholesteryl sulfate complex), and ABPLC (AmB and phospholipid complex) enhanced skin penetration in the presence of ethanol following topical application (Frankenburg et al., 1998; Zvulunov et al., 2003). Ethanol is a well-known penetration enhancer as it damages the skin by extracting the skin lipids and thus making it more permeable.
The use of drug carrier system could further enhance drug penetration through the skin. Lipid nanocarriers constitute a plausible alternative due to the penetration potential of individual phospholipid molecule into the lipid layers of stratum corneum (Pierre and Dos, 2011). Liposomes could enhance skin penetration as well as drug targeting to the macrophages (Manosroi et al., 2004; Schwendener et al., 1984; Singodia et al., 2010). We have previously developed topical liposomal forms of paromomycin (Jaafari et al., 2009), glucantime (Kalat et al., 2014), miltefosine (Kavian et al., 2019) and AmB, among which the latter significantly improved skin lesion size in some Leishmania strains-infected mice (Jaafari, 2015).
In the current study, we used fusion as an efficient, simple, reproducible and easily scalable method yielding homogeneous liposomes with high encapsulation efficiencies (Jaafari et al., 2009; Wohlrab J and Jahn, 2005). Further, liposomes devoid of organic solvents, had viscose consistency to be directly applied on the skin. SPC at a high concentration of 15% was used as the main phospholipid with its choline head group efficiently hydrating the skin and inducing strong penetration (Bhatia et al., 2004; Ghyczy et al., 1994). The low phase transition temperature of SPC (around −30 °C) also provided a fluid flexible bilayer (Wohlrab J and Jahn, 2005). Other ingredients including propylene glycol, DMSO as well as oleic acid not only assist with dissolving the lipid components (SPC and cholesterol) and AmB, but also facilitate penetration properties of the vesicles (Malajczuk et al., 2013; Murakami et al., 1998; Srisuk et al., 2012). Cholesterol included in the formulations intends to stabilize AmB in the liposome bilayer through hydrophobic interaction (Adler-Moore and Proffitt, 2008; Iman et al., 2011). Vitamin E was used to prevent SPC oxidation and PP and MP were added as microbial preservatives.
Several concentrations of AmB have shown results with Lip-AmB 0.4% significantly more effective in treating L. major lesions and eliminating parasites from the lesions and the spleens of the infected mice. Lip-AmB with concentrations of 0.1 and 0.2% were less effective, leaving the parasitic load in the lesions and spleens at higher levels. It was shown that there is a correlation between AmB concentration in the lesion and parasite load reduction at the infection site (Wijnant et al., 2018). Here, the AmB concentration in the skin was not measured however, the in vitro permeation assay showed the highest AmB accumulation in the skin with Lip-AmB 0.4% (73.92 percent), confirming a correlation between AmB retention in the skin and anti-Leishmania activity. The results indicated that serum concentration of AmB was negligible in the uninfected animals, while splenic drug accumulation was observed following Lip-AmB 0.4% topical application. Thus, it is conceivable that both parasites removal at the site of infection by liposomes, and gradual accumulation of free AmB in the spleen of the animals might involve in the reduction of parasites in the spleen of infected animals.
Following systemic administration, the small size of liposomes promotes the extravasation in the inflamed lesions on the skin and thus facilitates macrophage targeting residence in the skin (Griffin et al., 2017; Wijnant et al., 2018). We have previously compared the serum and organ distribution of DSHemsPC liposomes (liposomal AmB, 10 mg/kg) following iv injection to that of AmBisome in healthy BALB/c mice. Results showed AmB serum concentration of 36, 9.8, 2.2, 1.9 μg/mL at 1, 6, 12, and 24 h, respectively, while liver, spleen and kidney concentrations were 36.8, 13.8, 1 μg/g, respectively 24 h following injection (Iman et al., 2017). The results were comparable to that of AmBisome and both treatments reduced AmB accumulation in the kidneys of mice, a finding which supports the reduced nephrotoxicity of AmB associated with liposomes. In the current study, concentration of AmB in kidney, liver and spleen after twice daily topical application of Lip-AmB 0.4% for one month was around 2.5, 3.5 and 5.0 μg/g, respectively (mice were sacrificed 2 h after the last application). The comparison shows that topical application of Lip-AmB 0.4% produce low level of AmB in kidney and low to moderate concentration in liver and spleen which is the tissue target for the for Leishmania infection.
The high molecular weight and amphoteric nature of AmB hampers its cutaneous penetration (Perez et al., 2016), while liposomes could promote penetration due to the high flexibility of the formulation, similarity to the natural bilayer structure and interaction of the individual phospholipids with lipid layers of stratum corneum (Doppalapudi et al., 2017; Mohamed-Ahmed et al., 2013; Pierre and Dos, 2011). Following topical application, small size liposomes (100 nm) pass through the SC pores and reach the epidermis and dermal layer of the skin. In the dermis, the infected macrophages phagocytose the Lip-AmB which are then hydrolyzed by the acidic lysosomal enzymes, releasing AmB where Leishmania parasites live and multiply (Huang et al., 2009; Schwendener et al., 1984). The in vitro study indicated that Lip-AmB 0.4% could target the parasites inside infected macrophages, as shown by the level of GFP expression in transgenic parasites.
Similar to AmBisome®, the encapsulation efficiency of AmB in Lip-AmB 0.4% was around 100%. The lower transition temperature of the formulation compared to AmBisome® (around 55 °C) made it flexible for topical application. A further advantage is that the formulation is patient-compliant with minimal adverse effects compared to injectable treatment options. The results showed that AmB in this rational pharmaceutical design eradicated L. major parasites when applied directly at the site of infection. Pharmacokinetic of liposomes facilitated the drug accumulation within the lesion which was further improved by the inflammatory state of the infected skin. The choice of a safe manufacturing process is a major concern in the scale up production of the formulation. Using the fusion method, different features of Lip-AmB 0.4%, including AmB concentrations, particle diameters, zeta potentials and biological activities remained unchanged during the 20-month storage period at 4 °C and at 25 °C. The irritancy Draize test in the skin and eyes of rabbits and phase I safety study in human have been completed by DNDi support (Eskandari et al., 2018, 2019). The efficacy trial of the formulation is underway with (under EMRO WHO support) and ZCL (Eskandari et al., 2018, 2019).
The main limitations of this work include a single treatment regimen (50 mg Lip-AmB twice a day for 4 weeks) as well as a lack of skin and plasma pharmacokinetic. The 28-day treatment might be far from ideal for CL patients and there is a need for more convenient treatment to assure compliance and complete healing of the lesion. Further, it should be mention that the response might be variable against different Leishmania species. Thus, future studies are required to investigate the relationship between the skin pharmacokinetic and therapeutic efficacy of topical AmB liposomes to expedite achievement of an optimal clinical dose regimen. There is a need to explore the impact of topical formulation on the host immune response (Varikuti et al., 2017), since there is a possibility that topical liposomal AmB 0.4% induces cellular immune responses which may count for the lower number of splenic parasites observed in this study.
5. Conclusions/significance
Lip-AmB 0.4% liposome is a stable, concentrated and viscous topical product with a high AmB content and high penetration properties. It facilitates AmB permeation through the skin layers and showed to be effective against L. major in vitro and in vivo. The results suggest that topical Lip-AmB 0.4% may be a useful tool in the treatment of CL and merits further investigation.
Conflicts of interest
There is no conflict of interest.
Funding source
Drugs for Neglected Diseases initiative (DNDi, Research Collaborative Agreement made on 26 September 2011).
Acknowledgments
/The financial support of Drugs for Neglected Diseases initiative (DNDi, Research Collaborative Agreement made on 26 September 2011) is greatly appreciated. The results of this study presented in a meeting which was held in DNDi, Geneva, Switzerland, on 16th July 2012.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijpddr.2019.09.004.
Contributor Information
Mahmoud Reza Jaafari, Email: jafarimr@mums.ac.ir.
Ali Khamesipour, Email: ali.khamesipour@gmail.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Adler-Moore J., Gangneux J., Pappas P. Comparison between liposomal formulations of amphotericin B. Med. Mycol. 2016;54:223–231. doi: 10.1093/mmy/myv111. [DOI] [PubMed] [Google Scholar]
- Adler-Moore J., Proffitt R. Amphotericin B lipid preparations: what are the differences? Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2008;14:25–36. doi: 10.1111/j.1469-0691.2008.01979.x. [DOI] [PubMed] [Google Scholar]
- Ahsan F., Rivas I.P., Khan M.A., Suárez A.I.T. Targeting to macrophages: role of physicochemical properties of particulate carriers—liposomes and microspheres—on the phagocytosis by macrophages. J. Control. Release. 2002;79:29–40. doi: 10.1016/s0168-3659(01)00549-1. [DOI] [PubMed] [Google Scholar]
- Aronson N., Herwaldt B., Libman M., Pearson R., Lopez-Velez R., Weina P., Carvalho E., Ephros M., Jeronimo S., Magill A. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the infectious diseases society of America (idsa) and the american society of tropical medicine and hygiene (astmh) Am. J. Trop. Med. Hyg. 2017;96:24–45. doi: 10.4269/ajtmh.16-84256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barratt G., Bretagne S. Optimizing efficacy of Amphotericin B through nanomodification. Int. J. Nanomed. 2007;2:301–313. [PMC free article] [PubMed] [Google Scholar]
- Bavarsad N., Fazly B.B., Khamesipour A., Jaafari M. Colloidal, in vitro and in vivo anti-leishmanial properties of transfersomes containing paromomycin sulfate in susceptible BALB/c mice. Acta Trop. 2012;124:33–41. doi: 10.1016/j.actatropica.2012.06.004. [DOI] [PubMed] [Google Scholar]
- Ben A.S., Ben N.M., Guedri E., Zaatour A., Ben N.A., Bettaieb J., Gharbi A., Belhadj N.H., Boukthir A., Chlif S. Topical paromomycin with or without gentamicin for cutaneous leishmaniasis. N. Engl. J. Med. 2013;368:524–532. doi: 10.1056/NEJMoa1202657. [DOI] [PubMed] [Google Scholar]
- Ben A.S., Zakraoui H., Zaatour A., Ftaiti A., Zaafouri B., Garraoui A., Olliaro P., Dellagi K., Ben R.I. A randomized, placebo-controlled trial in Tunisia treating cutaneous leishmaniasis with paromomycin ointment. Am. J. Trop. Med. Hyg. 1995;53:162–166. doi: 10.4269/ajtmh.1995.53.162. [DOI] [PubMed] [Google Scholar]
- Berman J. Amphotericin B formulations and other drugs for visceral leishmaniasis. Am. J. Trop. Med. Hyg. 2015;92:471–473. doi: 10.4269/ajtmh.14-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia A., Kumar R., Katare O.P. Tamoxifen in topical liposomes: development, characterization and in-vitro evaluation. J. Pharm. Pharm. Sci. 2004;7:252–259. [PubMed] [Google Scholar]
- Bhattacharya P., Ali N. Treatment of visceral leishmaniasis: anomalous pricing and distribution of AmBisome and emergence of an indigenous liposomal amphotericin B, fungisome. J. Parasit. Dis.: Off. Organ. Indian. Soc. Parasitol. 2016;40:1094–1095. doi: 10.1007/s12639-014-0607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butsch F., Lorenz B., Goldinger A. Topical treatment with a two-component gel releasing nitric oxide cures C57BL/6 mice from cutaneous leishmaniasis caused by Leishmania major. Exp. Dermatol. 2016;25:914–916. doi: 10.1111/exd.13098. [DOI] [PubMed] [Google Scholar]
- Croft S.L., Sundar S., Fairlamb A.H. Drug resistance in leishmaniasis. Clin. Microbiol. Rev. 2006;19:111–126. doi: 10.1128/CMR.19.1.111-126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft S.L., Yardley V. Chemotherapy of leishmaniasis. Curr. Pharmaceut. Des. 2002;8:319–342. doi: 10.2174/1381612023396258. [DOI] [PubMed] [Google Scholar]
- Deray G. Amphotericin B nephrotoxicity. J. Antimicrob. Chemother. 2002;49:37–41. doi: 10.1093/jac/49.suppl_1.37. [DOI] [PubMed] [Google Scholar]
- Devi M., Kumar M.S., Mahadevan N. Amphotericin-B loaded vesicular systems for the treatment of topical fungal infection. Int J Rec Adv Pharm Res. 2011;4:37–46. [Google Scholar]
- Doppalapudi S., Jain A., Chopra D., Khan W. Psoralen loaded liposomal nanocarriers for improved skin penetration and efficacy of topical PUVA in psoriasis. Eur. J. Pharm. Sci.: Off. J. Eur. Fed. Pharm. Sci. 2017;96:515–529. doi: 10.1016/j.ejps.2016.10.025. [DOI] [PubMed] [Google Scholar]
- Dowlati Y. Cutaneous leishmaniasis: clinical aspect. Clin. Dermatol. 1996;14:425–431. doi: 10.1016/0738-081x(96)00058-2. [DOI] [PubMed] [Google Scholar]
- El-On J., Jacobs G., Witztum E., Greenblatt C. Development of topical treatment for cutaneous leishmaniasis caused by Leishmania major in experimental animals. Antimicrob. Agents Chemother. 1984;26:745–751. doi: 10.1128/aac.26.5.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Maghraby G.M., Barry B.W., Williams A.C. Liposomes and skin: from drug delivery to model membranes. Eur. J. Pharm. Sci. 2008;34:203–222. doi: 10.1016/j.ejps.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Eskandari S.E., Firooz A., Nassiri-Kashani M., Jaafari M.R., Javadi A., Miramin-Mohammadi A., Valian-Keshavarz H., Khamesipour A. Safety evaluation of nano-liposomal formulation of amphotericin b (sinaampholeish) in animal model as a candidate for treatment of cutaneous leishmaniasis. J. Arthropod-Borne Dis. 2018;12:269–275. [PMC free article] [PubMed] [Google Scholar]
- Eskandari s.e., firooz a., nassiri-kashani m., jaafari m.r., javadi a., mohammadi a.m., khamesipour a. Safety evaluation of topical application of nano-liposomal form of amphotericin b (sinaampholeish) on healthy volunteers: phase i clinical trial. Iran. J. Parasitol. 2019;14:197–203. [PMC free article] [PubMed] [Google Scholar]
- Fairlamb A., Gow N., Matthews K., Waters A. Drug resistance in eukaryotic microorganisms. Nat. Microbiol. 2016;1 doi: 10.1038/nmicrobiol.2016.92. 16092-16092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankenburg S., Glick D., Klaus S., Barenholz Y. Efficacious topical treatment for murine cutaneous leishmaniasis with ethanolic formulations of amphotericin B. Antimicrob. Agents Chemother. 1998;42:3092–3096. doi: 10.1128/aac.42.12.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier T., Croft S. Topical treatment for cutaneous leishmaniasis. Curr. Opin. Investig. Drugs. 2002;3:538–544. London, England: 2000. [PubMed] [Google Scholar]
- Garnier T., Mäntylä A., Järvinen T., Lawrence M.J., Brown M.B., Croft S.L. Topical buparvaquone formulations for the treatment of cutaneous leishmaniasis. J. Pharm. Pharmacol. 2007;59:41–49. doi: 10.1211/jpp.59.1.0006. [DOI] [PubMed] [Google Scholar]
- Ghyczy M., Gareiss J., Kovats T. Liposomes from vegetable phosphatidylcholine: their production and effects on the skin. Cosmet. Toilet. 1994;109:75–80. [Google Scholar]
- Griffin J., Wang G., Smith W., Vu V., Scheinman R., Stitch D., Moldovan R., Moghimi S., Simberg D. Revealing dynamics of accumulation of systemically injected liposomes in the skin by intravital microscopy. ACS Nano. 2017;11:11584–11593. doi: 10.1021/acsnano.7b06524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez V., Seabra A., Reguera R., Khandare J., Calderón M. New approaches from nanomedicine for treating leishmaniasis. Chem. Soc. Rev. 2016;45:152–168. doi: 10.1039/c5cs00674k. [DOI] [PubMed] [Google Scholar]
- Hadighi R., Mohebali M., Boucher P., Hajjaran H., Khamesipour A., Ouellette M. Unresponsiveness to Glucantime treatment in Iranian cutaneous leishmaniasis due to drug-resistant Leishmania tropica parasites. PLoS Med. 2006;3 doi: 10.1371/journal.pmed.0030162. e162-e162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill R. Amphotericin B formulations: a comparative review of efficacy and toxicity. Drugs. 2013;73:919–934. doi: 10.1007/s40265-013-0069-4. [DOI] [PubMed] [Google Scholar]
- Huang Z., Jaafari M.R., Szoka F.C. Disterolphospholipids: nonexchangeable lipids and their application to liposomal drug delivery. Angew. Chem. 2009;121:4210–4213. doi: 10.1002/anie.200900111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iman M., Huang Z., Alavizadeh S.H., Szoka F.C., Jaafari M.R. Biodistribution and in vivo antileishmanial activity of 1, 2-Distigmasterylhemisuccinoyl-sn-Glycero-3-Phosphocholine liposome-intercalated amphotericin B. Antimicrob. Agents Chemother. 2017;61 doi: 10.1128/AAC.02525-16. e02525-02516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iman M., Huang Z., Szoka F.C., Jr., Jaafari M.R. Characterization of the colloidal properties, in vitro antifungal activity, antileishmanial activity and toxicity in mice of a distigmasterylhemisuccinoyl-glycero-phosphocholine liposome-intercalated amphotericin B. Int. J. Pharm. 2011;408:163–172. doi: 10.1016/j.ijpharm.2011.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaafari M., Bavarsad N., Bazzaz B., Samiei A., Soroush D., Ghorbani S., Heravi M., Khamesipour A. Effect of topical liposomes containing paromomycin sulfate in the course of Leishmania major infection in susceptible BALB/c mice. Antimicrob. Agents Chemother. 2009;53:2259–2265. doi: 10.1128/AAC.01319-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaafari M.R.,K.A. 2015. Topical Liposomal Compositions for Delivering Hydrophobic Drugs and Methods Preparing Same. [Google Scholar]
- Kalat S.M., Khamesipour A., Bavarsad N., Fallah M., Khashayarmanesh Z., Feizi E., Neghabi K., Abbasi A., Jaafari M. Use of topical liposomes containing meglumine antimoniate (Glucantime) for the treatment of L. major lesion in BALB/c mice. Exp. Parasitol. 2014;143:5–10. doi: 10.1016/j.exppara.2014.04.013. [DOI] [PubMed] [Google Scholar]
- Kavian Z., Alavizadeh S., Golmohamadzadeh S., Badiee A., Khamesipour A., Jaafari M. Development of topical liposomes containing miltefosine for the treatment of Leishmania major infection in susceptible BALB/c mice. Acta Trop. 2019;196:142–149. doi: 10.1016/j.actatropica.2019.05.018. [DOI] [PubMed] [Google Scholar]
- Knöpfel N., Noguera-Morel L., Azorin D., Sanz F., Torrelo A., Hernández-Martín A. Cutaneous Leishmania tropica in children: report of three imported cases successfully treated with liposomal amphotericin B. J. Eur. Acad. Dermatol. Venereol. 2018;32:e8–e10. doi: 10.1111/jdv.14434. [DOI] [PubMed] [Google Scholar]
- Lecoeur H., Buffet P., Morizot G., Goyard S., Guigon G., Milon G., Lang T. Optimization of topical therapy for Leishmania major localized cutaneous leishmaniasis using a reliable C57BL/6 Model. PLoS Neglected Trop. Dis. 2007;1 doi: 10.1371/journal.pntd.0000034. e34-e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malajczuk C., Hughes Z., Mancera R. Molecular dynamics simulations of the interactions of DMSO, mono-and polyhydroxylated cryosolvents with a hydrated phospholipid bilayer. Biochim. Biophys. Acta. 2013;1828:2041–2055. doi: 10.1016/j.bbamem.2013.05.010. [DOI] [PubMed] [Google Scholar]
- Manosroi A., Kongkaneramit L., Manosroi J. Stability and transdermal absorption of topical amphotericin B liposome formulations. Int. J. Pharm. 2004;270:279–286. doi: 10.1016/j.ijpharm.2003.10.031. [DOI] [PubMed] [Google Scholar]
- Mauël J. Macrophage‐parasite interactions in leishmania infections. J. Leukoc. Biol. 1990;47:187–193. doi: 10.1002/jlb.47.2.187. [DOI] [PubMed] [Google Scholar]
- Mohamed-Ahmed A.H., Seifert K., Yardley V., Burrell-Saward H., Brocchini S., Croft S.L. Antileishmanial activity, uptake, and biodistribution of an amphotericin B and poly (α-Glutamic Acid) complex. Antimicrob. Agents Chemother. 2013;57:4608–4614. doi: 10.1128/AAC.02343-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami T., Yoshioka M., Yumoto R., Higashi Y., Shigeki S., Ikuta Y., Yata N. Topical delivery of keloid therapeutic drug, tranilast, by combined use of oleic acid and propylene glycol as a penetration enhancer: evaluation by skin microdialysis in rats. J. Pharm. Pharmacol. 1998;50:49–54. doi: 10.1111/j.2042-7158.1998.tb03304.x. [DOI] [PubMed] [Google Scholar]
- Mushtaq S., Dogra D., Dogra N. Clinical Response with intralesional Amphotericin B in the treatment of old world cutaneous leishmaniasis: a preliminary report. Dermatol. Ther. 2016;29:398–405. doi: 10.1111/dth.12377. [DOI] [PubMed] [Google Scholar]
- Nadim A., Aflatoonian M. Anthroponotic cutaneous leishmaniasis in the city of Bam, southeast Iran. Iran. J. Public Health. 1995;24:15–24. [Google Scholar]
- Neves L., Talhari A., Gadelha E., Silva R.J., Guerra J., Ferreira L., Talhari S. A randomized clinical trial comparing meglumine antimoniate, pentamidine and amphotericin B for the treatment of cutaneous leishmaniasis by Leishmania guyanensis. An. Bras. Dermatol. 2011;86:1092–1101. doi: 10.1590/s0365-05962011000600005. [DOI] [PubMed] [Google Scholar]
- Perez A., Altube M., Schilrreff P., Apezteguia G., Celes F., Zacchino S., Romero E., Morilla M. Topical amphotericin B in ultradeformable liposomes: formulation, skin penetration study, antifungal and antileishmanial activity in vitro. Colloids Surfaces B Biointerfaces. 2016;139:190–198. doi: 10.1016/j.colsurfb.2015.12.003. [DOI] [PubMed] [Google Scholar]
- Pierre M., Dos I.S.M.C. Liposomal systems as drug delivery vehicles for dermal and transdermal applications. Arch. Dermatol. Res. 2011;303:607–621. doi: 10.1007/s00403-011-1166-4. [DOI] [PubMed] [Google Scholar]
- Ponte-Sucre A., Gamarro F., Dujardin J., Barrett M., López-Vélez R., García-Hernández R., Pountain A., Mwenechanya R., Papadopoulou B. Drug resistance and treatment failure in leishmaniasis: a 21st century challenge. PLoS Neglected Trop. Dis. 2017;11 doi: 10.1371/journal.pntd.0006052. e0006052-e0006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwendener R., Lagocki P., Rahman Y. The effects of charge and size on the interaction of unilamellar liposomes with macrophages. Biochim. Biophys. Acta. 1984;772:93–101. doi: 10.1016/0005-2736(84)90521-2. [DOI] [PubMed] [Google Scholar]
- Singodia D., Gupta G., Verma A., Singh V., Shukla P., Misra P., Sundar S., Dube A., Mishra P. Development and performance evaluation of amphotericin B transfersomes against resistant and sensitive clinical isolates of visceral leishmaniasis. J. Biomed. Nanotechnol. 2010;6:293–302. doi: 10.1166/jbn.2010.1121. [DOI] [PubMed] [Google Scholar]
- Solomon M., Pavlotsky F., Leshem E., Ephros M., Trau H., Schwartz E. Liposomal amphotericin B treatment of cutaneous leishmaniasis due to Leishmania tropica. J. Eur. Acad. Dermatol. Venereol. 2011;25:973–977. doi: 10.1111/j.1468-3083.2010.03908.x. [DOI] [PubMed] [Google Scholar]
- Soto J., Hernandez N., Mejia H., Grogl M., Berman J. Successful treatment of New World cutaneous leishmaniasis with a combination of topical paromomycin/methylbenzethonium chloride and injectable meglumine antimonate. Clin. Infect. Dis. 1995;20:47–51. doi: 10.1093/clinids/20.1.47. [DOI] [PubMed] [Google Scholar]
- Srisuk P., Thongnopnua P., Raktanonchai U., Kanokpanont S. Physico-chemical characteristics of methotrexate-entrapped oleic acid-containing deformable liposomes for in vitro transepidermal delivery targeting psoriasis treatment. Int. J. Pharm. 2012;427:426–434. doi: 10.1016/j.ijpharm.2012.01.045. [DOI] [PubMed] [Google Scholar]
- Taheri T., Seyed N., Rafati S. DNA integration in leishmania genome: an application for vaccine development and drug screening. Methods Mol. Biol. 2015;1403:603–622. doi: 10.1007/978-1-4939-3387-7_34. [DOI] [PubMed] [Google Scholar]
- Van Bocxlaer K., Yardley V., Murdan S., Croft S.L. Topical formulations of miltefosine for cutaneous leishmaniasis in a BALB/c mouse model. J. Pharm. Pharmacol. 2016;68:862–872. doi: 10.1111/jphp.12548. [DOI] [PubMed] [Google Scholar]
- Varikuti S., Oghumu S., Saljoughian N., Pioso M., Sedmak B., Khamesipour A., Satoskar A. Topical treatment with nanoliposomal Amphotericin B reduces early lesion growth but fails to induce cure in an experimental model of cutaneous leishmaniasis caused by Leishmania mexicana. Acta Trop. 2017;173:102–108. doi: 10.1016/j.actatropica.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijnant G., Van K.B., Yardley V., Harris A., Alavijeh M., Silva-Pedrosa R., Antunes S., Mauricio I., Murdan S., Croft S. Comparative efficacy, toxicity and biodistribution of the liposomal amphotericin B formulations Fungisome® and AmBisome® in murine cutaneous leishmaniasis. Int. J. Parasitol. Drug Resist. 2018;8:223–228. doi: 10.1016/j.ijpddr.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlrab J N.R., Jahn K. 2005. Pharmaceutical Formulation Comprising Cyclosporin and Use Thereof. [Google Scholar]
- Wortmann G., Zapor M., Ressner R., Fraser S., Hartzell J., Pierson J., Weintrob A., Magill A. Lipsosomal amphotericin B for treatment of cutaneous leishmaniasis. Am. J. Trop. Med. Hyg. 2010;83:1028–1033. doi: 10.4269/ajtmh.2010.10-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvulunov A., Cagnano E., Frankenburg S., Barenholz Y., Vardy D. Topical treatment of persistent cutaneous leishmaniasis with ethanolic lipid amphotericin B. Pediatr. Infect. Dis. J. 2003;22:567–569. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.