Abstract
Air pollution poses a great environmental risk to health. Outdoor fine particulate matter (particulate matter with an aerodynamic diameter < 2.5 μm) exposure is the fifth leading risk factor for death in the world, accounting for 4.2 million deaths and > 103 million disability-adjusted life years lost according to the Global Burden of Disease Report. The World Health Organization attributes 3.8 million additional deaths to indoor air pollution. Air pollution can harm acutely, usually manifested by respiratory or cardiac symptoms, as well as chronically, potentially affecting every organ in the body. It can cause, complicate, or exacerbate many adverse health conditions. Tissue damage may result directly from pollutant toxicity because fine and ultrafine particles can gain access to organs, or indirectly through systemic inflammatory processes. Susceptibility is partly under genetic and epigenetic regulation. Although air pollution affects people of all regions, ages, and social groups, it is likely to cause greater illness in those with heavy exposure and greater susceptibility. Persons are more vulnerable to air pollution if they have other illnesses or less social support. Harmful effects occur on a continuum of dosage and even at levels below air quality standards previously considered to be safe.
Key Words: air pollution, mechanism of damage, noncommunicable diseases
Abbreviations: CO, carbon monoxide; NO2, nitrogen dioxide; O3, ozone; PM0.1, particulate matter with an aerodynamic diameter < 0.1 μm; PM2.5, particulate matter with an aerodynamic diameter < 2.5 μm; PM10, particulate matter with an aerodynamic diameter < 10 μm; SO2, sulfur dioxide
FOR RELATED ARTICLE, SEE PAGE 417
Introduction
Air pollution may be the greatest environmental risk to health in the world.1 According to the Global Burden of Disease estimates, one component of ambient (or outdoor air) pollution, fine particulate matter, or particulate matter with an aerodynamic diameter < 2.5 μm (PM2.5), is the fifth leading risk factor for death in the world, accounting for 4.2 million deaths (7.6% of total global deaths) and > 103 million disability-adjusted life years lost in 2015. Exposure to ambient ozone (O3) caused an additional 254,000 deaths,2 and estimates based on other statistical techniques set these numbers even higher.3 The World Health Organization reported that indoor air pollution from fires for cooking and heating accounted for 3.8 million deaths; this number ranged from 10% in low- and middle-income countries to 0.2% in high-income countries.4 In almost all cases, the greatest affliction of air pollution falls on the vulnerable population.
Air pollution may be associated with symptoms immediately upon exposure, such as coughing, tearing, difficulty breathing, and angina. It may also be associated with long-term harm that is more subtle. People are usually unaware of how long-term exposure affects their health or worsens their medical problems over time. Polluted air gains access to the body through the respiratory tract but has systemic effects that can damage many other organs.
The main purpose of these two papers is to review the available evidence to support the hypothesis that air pollution affects many organs beyond the lungs. The review omits infectious diseases and tobacco smoke exposure, and it generally does not distinguish between ambient and indoor (household) air pollution, although they can have very different compositions. Tobacco smoke could be considered a form of high-dose air pollution. There are similarities between tobacco smoke and air pollution in how they injure the body; in addition, their harmful health effects become increasingly similar as the toxicity and dosages of the inhaled materials become more alike. However, the lung disease resulting from exposure to indoor smoke has more bronchitic elements and fewer emphysematous elements than tobacco smoking.5 In Part 1 of this report, we review the mechanisms and multisystem health effects of air pollution in general and among vulnerable populations. In Part 2,6 we review the evidence for air pollution’s effects on individual organ systems.
What Air Pollution Is and How It Causes Illness
Air pollution is defined as any substance in the air that may harm humans, animals, vegetation, or materials.7 Pollutants come from various sources, and each can have differing characteristics depending on the composition, source, and conditions under which they were produced. Common gases include the sulfur oxides (mainly sulfur dioxide [SO2]), nitrogen oxides (mainly nitric oxide and nitrogen dioxide [NO2]), reactive hydrocarbons (often referred to as volatile organic compounds), and carbon monoxide (CO). They are released directly into the atmosphere, usually from industrial or transportation sources, and are called “primary pollutants.” Gaseous and particle pollutants can also form in the atmosphere, largely from the primary pollutants and are called “secondary pollutants.” For example, O3 is formed from nitrogen oxides and hydrocarbons in the atmosphere; sulfuric acid is produced from atmospheric sulfur; and ammonium nitrate aerosols are created from atmospheric nitrogen oxide gases.
The damage to human tissue by gases depends on their water solubility, concentration, ability to oxidize tissue, and the affected person’s susceptibility. SO2 is highly soluble in water and largely damages the upper airways and skin, whereas NO2 and O3 are less soluble and therefore can penetrate deeper into the lung. CO is highly soluble and nonirritating and readily passes into the bloodstream. Its toxicity mainly results from successfully competing with oxygen in binding to hemoglobin, which results in tissue hypoxia. Its effects are acute: a 2-day increase of mean CO levels of 1 mg/m3 was associated with a 1.2% increase in total deaths in a large European study.8 Nitric oxide also attaches to hemoglobin and other iron-containing proteins, but it generally acts only a short distance from its contact point because of its binding affinity.
PM is usually classified by its size or aerodynamic diameter; PM10 denotes particles < 10 μm in diameter; PM2.5 particles are < 2.5 μm in diameter; and PM0.1 particles are < 0.1 μm in diameter. All PM2.5 and PM0.1 are included in PM10. Therefore, adverse effects attributed to PM10 could be caused by smaller particles. The term “coarse particles” is used to refer to particulates between PM10 and PM2.5 in size. In contrast to large particles that can be visible as dust or haze with appropriate lighting, small particles are invisible. Large particles may affect mucous membranes and the upper airways, causing cough and tearing. Fine particles (PM2.5) easily find their way into lung alveoli, and ultrafine particles (PM0.1) pass through the alveolar-capillary membrane, are readily picked up by cells, and carried via the bloodstream to expose virtually all cells in the body. Smaller particles, therefore, have greater systemic toxicity (Table 1).
Table 1.
How Different Types of Air Pollution Damage Tissue
| Pollutant | Injury Determinants | Tissue Affected |
|---|---|---|
| Sulfur dioxide | Highly soluble | Upper airway and skin damage |
| Nitrogen dioxide Ozone Carbon monoxide |
Less soluble (nitrogen dioxide and ozone are irritating) | Deeper lung penetration Bronchial and bronchiolar injury Carbon monoxide: tissue hypoxia |
| Particulate matter (PM10, PM2.5, PM0.1) | Size, structure, and composition determine toxicity | Large particles: mucous membranes, upper airways Small particles: bronchioles and alveoli Ultrafine particles: systemic tissue reactions |
PM0.1 = particulate matter with an aerodynamic diameter < 0.1 μm; PM2.5 = particulate matter with an aerodynamic diameter < 2.5 μm; PM10 = particulate matter with an aerodynamic diameter < 10 μm.
Beyond its size, the harm caused by PM relates to its structure and composition. For example, particles that are highly acidic are more noxious. Toxic components may lie on the particle’s surface and be responsible for the tissue damage on contact. Toxic “hitchhikers,” elements such as arsenic, lead, or cadmium, or compounds such as sulfuric acid or polycyclic aromatic hydrocarbons, can be picked up during the combustion process and be carried deep into the lung on the surface of the ultrafine particles. This scenario is most relevant to particles resulting from fossil fuel combustion, especially coal combustion, which contains many heavy metal constituents and high levels of sulfur. If similar-sized particles do not contain as many toxic add-ons, they generally cause less harm.9 PM, however, can also interact with airborne allergens as hapten carriers to trigger or even induce allergic asthma reactions in sensitized subjects.10
In addition to encroaching on an organ and causing direct harm, exposure to pollutants, including toxic metals, organic compounds, and gases, can cause inflammation with systemic effects. The inflammation, usually in the lung, causes oxidative stress. Oxidative stress entails lipid peroxidation, depletion of antioxidants, and activation of pro-inflammatory signaling. The pro-inflammatory signaling sets off a cascade of events that may affect distant organs. The greater the surface area of ultrafine particles, the greater the ability to produce oxidative stress.11 Increases in particulate exposure are associated with elevated C-reactive protein, fibrinogen, circulating blood leukocytes and platelets, and plasma viscosity.12 Leukocytes, adhesion proteins, clotting proteins, and an array of cytokines and inflammatory mediators tax the endothelium, which may lose its modulating function.13 Repeated insults from pollution can contribute to vascular conditions, such as atherosclerosis, and can have a wide range of effects on metabolism. Ultrafine particles that go directly into different organs also can be responsible for inflammation in that organ.14
In addition, the lung faces the damaging effects of filtering PM and accumulation of “soot” in the lungs if the clearance mechanisms cannot handle the load. The sheer volume of PM may overwhelm macrophage function and the lymphatic system, leaving deposits of material centered around the terminal bronchioles and early-generation respiratory bronchioles (Fig 1).15 The particulate burden may lead to chronic focal inflammation and fibrosis, and could predispose to “scar” lung carcinoma.16 The efficiency of particulate clearance is a factor in how pollution affects the body.
Figure 1.

Anthracotic lung. Inhaled particulates are usually cleared through the respiratory mucociliary apparatus and scavenged by alveolar macrophages. Particles can move into the interlobular septal lymphatics and be cleared by the lymphatic system, but if these mechanisms are overwhelmed, particulates may clog lymphatics and be deposited in the lung interstitium. Ultrafine particles gain entrance to mobile cells and can be transported to all parts of the body. Although this anthracotic lung is characteristic of smokers and workers in dusty occupations, anthracotic deposits are often found in urban dwellers from air pollution.
The immune and inflammatory responses to air pollutants may be genetically regulated. Many important genes involve inflammation and variation in glutathione synthesis.17 Genetic variation in the glutathione pathway reportedly increases susceptibility to pollution-related lung function decrements in children.18 Variations in genes that control inflammatory mediators, which include Toll-like receptor 4, tumor necrosis factor-α, transforming growth factor-β, and many others, have been found to increase susceptibility to the respiratory effects of pollution.17 Air pollutants affect both the innate and adaptive immune systems. PM disturbs the balance of the Th1 and Th2 leukocyte populations, resulting in dominant Th2 leukocytes, which is a feature of asthma.19
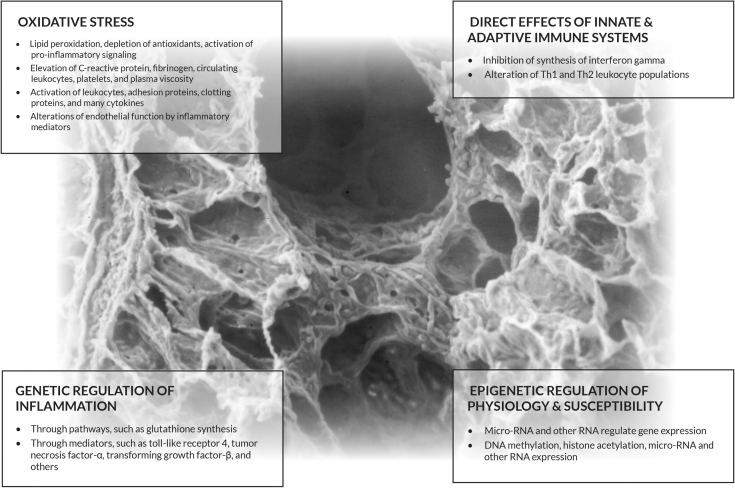
Epigenetics refers to potentially reversible modifications to DNA that control how genes are expressed, without altering the DNA sequence. Epigenetics mediate genetic and physiologic responses to air pollution and are, therefore, an important cause of susceptibility to pollution-related health effects.20 Changes in microRNA and other RNA species also regulate gene expression, often through signaling pathways. Air pollution exposure may affect these epigenetic processes.21 Cord blood samples from several birth cohort studies showed that prenatal NO2 exposure was associated with DNA methylation in several mitochondria-related genes, as well as in several genes involved in antioxidant defense pathways.22 Figure 2 illustrates the different ways in which air pollution can mediate tissue damage.
Figure 2.
Pollution damage by systemic inflammation. This scanning electron micrograph of the terminal and respiratory bronchioles are the sites where most material accumulates, making it the area of the lung most vulnerable to pollution. In addition, this figure depicts four ways that pollution can affect all organs through systemic inflammation. Ultrafine particles pass through the alveolar-capillary membrane, are endocytosed, and distributed throughout the body. They induce similar inflammatory reactions in other organs. (Copyright reserved Dean Schraufnagel.)
Air Pollution and Exercise
Physical exertion that results in increased ventilation and mouth breathing augments inhalation of air pollutants. In athletes who stress their ventilatory and cardiac reserves, pollution exposure has been found to decrease exercise performance. Maximal oxygen consumption and exercise duration are decreased with exposure to CO.23 Experimental exposure to O3 reduces the exercise capacity of athletes and leads to a transient decrease in spirometric function.24 Increased ambient PM10 concentrations are associated with reduced marathon performance in women.25 Despite these harmful effects, studies suggest that the health benefits of exercise outweigh the adverse effects of pollution exposure during exercise26 in all but the most polluted areas.27
Sleep
Sleep efficiency is decreased in most polluted areas, especially with increased exposure to NO2 and PM.28 Several studies show that air pollution is associated with increased sleep apnea symptoms, possibly because of upper airway inflammation from irritant pollutants and airborne allergens29 and household biomass smoke.30
Air pollution may affect sleep adversely in other ways. Traffic-related air pollution is highest near busy streets, which confounds sleep studies because the environment is more often noisy and illuminated. Pollution may also disturb sleep by exacerbating asthma, COPD, or other respiratory or chronic diseases. In addition, pollutants may lead to an inflammatory reaction in the CNS or directly interfere with neuronal function that may affect sleep.31
Children
Children are especially harmed by air pollution for both environmental and biologic reasons. Children breathe more air per unit body weight and, therefore, inhale more airborne toxicants than adults exposed to the same amount of air pollution. In many parts of the world where biomass is burned indoors for cooking and heating, small children are heavily exposed to indoor air pollution along with their mothers. Children all over the world generally spend more time outdoors and are more physically active than adults, which can result in greater exposure to outdoor air pollution.
Children are biologically more susceptible to pollution because their bodies are still not mature. Lung and immune system development occurs over the entire prenatal period, beginning with embryogenesis and continuing for many years after birth. Infants are born with only about 20% of the alveoli that they will eventually make once they have reached adulthood. Exposures to air pollutants during the prenatal period and during childhood can have harmful and irreversible effects on the lung and other organ systems.
Postnatal exposures to air pollutants, including PM, O3, and NO2, have been associated with increased infant mortality, even in developed countries such as the United States. The strongest associations have been with postneonatal respiratory mortality,32, 33, 34 which in part may be related to respiratory infections that have links to pollution.35 Air pollution may be a “second hit” in newborns who are susceptible to infection because of their immature immune systems. Postnatal diesel pollution exposure also has been found to attenuate the lung’s immune response to respiratory infection and to augment the inflammatory response, which likely results in a worse course of illness.36
Air pollution has also been found to affect growth trajectories of the lung and its function during childhood, which can affect the level of respiratory health achieved in adulthood. PM2.5 exposure reportedly impairs prenatal and postnatal development of the tracheobronchial tree.37 Many studies have found that higher exposure to PM and traffic are linked to worse lung function in childhood, and slower child lung function growth,38, 39, 40 which, in turn, may limit lung function in adulthood.41 Long-term exposure to pollution during childhood, especially traffic-related pollution, has been associated with the risk of developing childhood asthma42, 43 and is another example of how pollution exposure during childhood affects organ development and risk of subsequent chronic disease.
Maternal-Fetal Health and Reproductive Health
Exposure to air pollution during pregnancy is associated with adverse pregnancy outcomes and reduced fetal growth. A review of > 13,000 pregnancies in Scotland found that exposure to higher levels of PM2.5, PM10, and NO2 were associated with lower infant head size during pregnancy and at birth.44 Another study across all trimesters of pregnancy reported that the risk of intrauterine growth restriction was increased among women exposed to higher levels of CO, NO2, and PM2.5.45 A meta-analysis that included nearly 3 million births across 14 centers from nine developed countries found that, after adjusting for socioeconomic status, maternal exposure to particulate air pollution was associated with a higher risk of low birth weight infants.46
Although many studies measure air pollution exposures over the entire course of pregnancy, it is believed that exposure in the first trimester of pregnancy poses a greater risk than subsequent exposures. A study of nearly 30,000 term single births in Japan found that exposure to pollutants over the course of the entire pregnancy was not associated with fetal growth restriction. However, when they examined exposures in the first trimester, O3 exposure was associated with higher odds of small for gestational age and low birth weight infants.47 Another study of > 5,000 mother-child pairs of the Boston Birth Cohort found that women who were exposed to the highest levels of PM were more likely to have intrauterine inflammation48 compared with those exposed to the lowest levels. The risk was highest for exposures measured in the first trimester of pregnancy.
Air pollution increases the risk of preterm birth and low birth weight independently and additively to other known risk factors, such as lower socioeconomic status, diabetes, hypertension, and smoking.49 Women who are exposed to higher levels of traffic-related air pollution during pregnancy may be at increased risk of preeclampsia, which may be one mechanism explaining the association with preterm birth.50 Also, increased exposure to O3 and PM2.5 within 5 h of delivery has been linked to higher risk of premature rupture of membranes, which predisposes women to preterm delivery.51 These adverse effects on pregnancy and birth outcomes have been observed even at relatively low levels of air pollution exposure. They are especially concerning because preterm birth, low birth weight, and small for gestational age infants are at increased risk of a variety of health problems, including reduced lung growth and cognitive problems, that can persist for their lifetimes. Conversely, reducing air pollution has prompt benefits. When measures were taken to effectively reduce air pollution during the 2008 Beijing Olympic Games, there was an improvement in infant birth weight in association with the reduction in NO2, a marker of traffic-related air pollution.52
Fertility
Several studies have found that air pollution is associated with reduced fertility rates and increased risk of miscarriage. A Mongolian study found a dose-dependent relationship between the monthly average SO2, NO2, CO, PM10, and PM2.5 levels during pregnancy and risk of spontaneous abortions.53 A few studies have shown or suggested that semen or sperm quality is decreased in areas of high pollution.54, 55
Vulnerable Populations
Although air pollution affects people of all regions, ages, and social and economic groups, it is more likely to cause ill health and death in certain individuals. Exposures to air pollution and other environmental factors and biological susceptibility are the most important factors determining response. People living in Africa, Asia, and the Middle East on average breathe higher levels of pollutants than those in other parts of the world1 and, therefore, sustain a greater health burden.
Both extrinsic and intrinsic factors determine vulnerability to adverse health effects from exposures to air pollution. The most important is the level of exposure. People of low social and economic status often have greater exposures to air pollution because they live in areas of greater traffic density and near point sources of pollution such as power plants and industrial facilities. Other extrinsic neighborhood factors that contribute to vulnerability include poor housing, the lack of stores to purchase healthy food (eg, fruits and vegetables that contain antioxidants), violent crime, segregation, lack of green space, and poor access to health care.56 Poorer people are also more likely to work in “dirty” jobs with occupational exposures to vapors, dusts, gases, and fumes.57 Intrinsic factors that increase vulnerability to air pollution include age (very young and very old), preexisting disease, pregnancy, genetic and epigenetic variation, smoking, and obesity.58, 59 The concept of cumulative risk combines both extrinsic and intrinsic factors when attempting to assess the vulnerability of an individual or a population to the ill effects of air pollution.
When factors are combined, the effects can be additive or multiplicative. For example, preexisting cardiopulmonary diseases and diabetes increase susceptibility to the effects of particulate air pollution.59 Psychosocial stress interacts with exposure to traffic-related air pollution to increase the risk of new-onset asthma in children.60, 61 It may also enhance the effects of particulate pollution on BP.62 The association between air pollution and cancer risk has been shown to be greater in neighborhoods with higher levels of ethnic minority segregation, an indicator that may capture the cumulative impact of multiple adverse social and psychosocial exposures.63
Impoverished individuals, especially ethnic minorities, are more likely to live in segregated neighborhoods that are near sources of pollution and busy roadways. Consequently, these individuals are more often exposed to higher concentrations of outdoor air pollutants than persons with higher economic status.59 They are also likely to have greater cumulative health risks from other detrimental neighborhood factors. In low-income countries, women, small children, and rural residents are likely to be exposed to higher concentration of household air pollutants during cooking and heating activities.64
Vulnerability is made worse by health inequality and environmental injustice.65 Proponents of environmental justice argue that investigators and regulatory agencies should evaluate the cumulative impacts of environmental and social stressors in research studies and regulatory policies. Pollutant and source-specific assessments of potential health risks of air pollution do not inherently reflect the multiple environmental and social stressors faced by vulnerable communities that can interact to harm health. Reducing vulnerability across a population calls for reducing poverty, segregation, and health-damaging neighborhood environmental factors as well as reducing the ambient levels of pollutants. Strategies to achieve health equality for vulnerable communities require societal commitment of resources as well as the promulgation of air quality control measures.66
Acknowledgments
Financial/nonfinancial disclosures: None declared.
Footnotes
FUNDING/SUPPORT: The contribution by G. D. T. was supported in part by a National Institute of Environmental Health Sciences Center grant [Grant E500260] to the New York University School of Medicine.
References
- 1.World Health Organization . World Health Organization; Geneva, Switzerland: 2016. Ambient Air Pollution. [Google Scholar]
- 2.Cohen A.J., Brauer M., Burnett R. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet. 2017;389(10082):1907–1918. doi: 10.1016/S0140-6736(17)30505-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burnett R., Chen H., Szyszkowicz M. Global estimates of mortality associated with long-term exposure to outdoor fine particulate matter. Proc Natl Acad Sci U S A. 2018;115(38):9592–9597. doi: 10.1073/pnas.1803222115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization . World Health Organization; Geneva, Switzerland: 2018. Global Health Observatory (GHO) Data, Mortality From Household Air Pollution. [Google Scholar]
- 5.Perez-Padilla R., Ramirez-Venegas A., Sansores-Martinez R. Clinical characteristics of patients with biomass smoke-associated COPD and chronic bronchitis, 2004-2014. Chronic Obstr Pulm Dis. 2014;1(1):23–32. doi: 10.15326/jcopdf.1.1.2013.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schraufnagel D.E., Balmes J.R., Cowl C.T. Air pollution and noncommunicable diseases: a review by the Forum of International Respiratory Societies’ Environmental Committee, Part 2: air pollution and organ systems. Chest. 2019;155(2):417–426. doi: 10.1016/j.chest.2018.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kampa M., Castanas E. Human health effects of air pollution. Environ Pollut. 2008;151(2):362–367. doi: 10.1016/j.envpol.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 8.Samoli E., Touloumi G., Schwartz J. Short-term effects of carbon monoxide on mortality: an analysis within the APHEA project. Environ Health Perspect. 2007;115(11):1578–1583. doi: 10.1289/ehp.10375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thurston G.D., Kipen H., Annesi-Maesano I. A joint ERS/ATS policy statement: what constitutes an adverse health effect of air pollution? An analytical framework. Eur Respir J. 2017;49(1) doi: 10.1183/13993003.00419-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baldacci S., Maio S., Cerrai S. Allergy and asthma: effects of the exposure to particulate matter and biological allergens. Respir Med. 2015;109(9):1089–1104. doi: 10.1016/j.rmed.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 11.Madl A.K., Plummer L.E., Carosino C., Pinkerton K.E. Nanoparticles, lung injury, and the role of oxidant stress. Annu Rev Physiol. 2014;76:447–465. doi: 10.1146/annurev-physiol-030212-183735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donaldson K., Mills N., MacNee W., Robinson S., Newby D. Role of inflammation in cardiopulmonary health effects of PM. Toxicol Appl Pharmacol. 2005;207(suppl 2):483–488. doi: 10.1016/j.taap.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 13.Tamagawa E., Bai N., Morimoto K. Particulate matter exposure induces persistent lung inflammation and endothelial dysfunction. Am J Physiol Lung Cell Mol Physiol. 2008;295(1):L79–L85. doi: 10.1152/ajplung.00048.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duffin R., Mills N.L., Donaldson K. Nanoparticles—a thoracic toxicology perspective. Yonsei Med J. 2007;48(4):561–572. doi: 10.3349/ymj.2007.48.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinkerton K.E., Green F.H., Saiki C. Distribution of particulate matter and tissue remodeling in the human lung. Environ Health Perspect. 2000;108(11):1063–1069. doi: 10.1289/ehp.001081063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oberdorster G. Lung particle overload: implications for occupational exposures to particles. Regul Toxicol Pharmacol. 1995;21(1):123–135. doi: 10.1006/rtph.1995.1017. [DOI] [PubMed] [Google Scholar]
- 17.Vawda S., Mansour R., Takeda A. Associations between inflammatory and immune response genes and adverse respiratory outcomes following exposure to outdoor air pollution: a HuGE systematic review. Am J Epidemiol. 2014;179(4):432–442. doi: 10.1093/aje/kwt269. [DOI] [PubMed] [Google Scholar]
- 18.Breton C.V., Salam M.T., Vora H., Gauderman W.J., Gilliland F.D. Genetic variation in the glutathione synthesis pathway, air pollution, and children's lung function growth. Am J Respir Crit Care Med. 2011;183(2):243–248. doi: 10.1164/rccm.201006-0849OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bezemer G.F., Bauer S.M., Oberdorster G. Activation of pulmonary dendritic cells and Th2-type inflammatory responses on instillation of engineered, environmental diesel emission source or ambient air pollutant particles in vivo. J Innate Immun. 2011;3(2):150–166. doi: 10.1159/000321725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Breton C.V., Yao J., Millstein J. Prenatal air pollution exposures, DNA methyl transferase genotypes, and associations with newborn LINE1 and Alu methylation and childhood blood pressure and carotid intima-media thickness in the Children's Health Study. Environ Health Perspect. 2016;124(12):1905–1912. doi: 10.1289/EHP181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jardim M.J. microRNAs: implications for air pollution research. Mutat Res. 2011;717(1-2):38–45. doi: 10.1016/j.mrfmmm.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 22.Gruzieva O., Xu C.J., Breton C.V. Epigenome-wide meta-analysis of methylation in children related to prenatal NO2 air pollution exposure. Environ Health Perspect. 2017;125(1):104–110. doi: 10.1289/EHP36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horvath S.M., Agnew J.W., Wagner J.A., Bedi J.F. Maximal aerobic capacity at several ambient concentrations of carbon monoxide at several altitudes. Res Rep Health Eff Inst. 1988;(21):1–21. [PubMed] [Google Scholar]
- 24.Gong H., Jr., Bradley P.W., Simmons M.S., Tashkin D.P. Impaired exercise performance and pulmonary function in elite cyclists during low-level ozone exposure in a hot environment. Am Rev Respir Dis. 1986;134(4):726–733. doi: 10.1164/arrd.1986.134.4.726. [DOI] [PubMed] [Google Scholar]
- 25.Marr L.C., Ely M.R. Effect of air pollution on marathon running performance. Med Sci Sports Exerc. 2010;42(3):585–591. doi: 10.1249/MSS.0b013e3181b84a85. [DOI] [PubMed] [Google Scholar]
- 26.Fisher J.E., Loft S., Ulrik C.S. Physical activity, air pollution, and the risk of asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2016;194(7):855–865. doi: 10.1164/rccm.201510-2036OC. [DOI] [PubMed] [Google Scholar]
- 27.Tainio M., de Nazelle A.J., Gotschi T. Can air pollution negate the health benefits of cycling and walking? Prev Med. 2016;87:233–236. doi: 10.1016/j.ypmed.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fang S.C., Schwartz J., Yang M., Yaggi H.K., Bliwise D.L., Araujo A.B. Traffic-related air pollution and sleep in the Boston Area Community Health Survey. J Expo Sci Environ Epidemiol. 2015;25(5):451–456. doi: 10.1038/jes.2014.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zanobetti A., Redline S., Schwartz J. Associations of PM10 with sleep and sleep-disordered breathing in adults from seven US urban areas. Am J Respir Crit Care Med. 2010;182(6):819–825. doi: 10.1164/rccm.200912-1797OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castaneda J.L., Kheirandish-Gozal L., Gozal D., Accinelli R.A. Pampa Cangallo Instituto de Investigaciones de la Altura Research G. Effect of reductions in biomass fuel exposure on symptoms of sleep apnea in children living in the peruvian andes: a preliminary field study. Pediatr Pulmonol. 2013;48(10):996–999. doi: 10.1002/ppul.22720. [DOI] [PubMed] [Google Scholar]
- 31.Martinez-Lazcano J.C., Gonzalez-Guevara E., del Carmen Rubio M. The effects of ozone exposure and associated injury mechanisms on the central nervous system. Rev Neurosci. 2013;24(3):337–352. doi: 10.1515/revneuro-2012-0084. [DOI] [PubMed] [Google Scholar]
- 32.Loomis D., Castillejos M., Gold D.R., McDonnell W., Borja-Aburto V.H. Air pollution and infant mortality in Mexico City. Epidemiology. 1999;10(2):118–123. [PubMed] [Google Scholar]
- 33.Woodruff T.J., Darrow L.A., Parker J.D. Air pollution and postneonatal infant mortality in the United States, 1999-2002. Environ Health Perspect. 2008;116(1):110–115. doi: 10.1289/ehp.10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saldiva P.H., Lichtenfels A.J., Paiva P.S. Association between air pollution and mortality due to respiratory diseases in children in Sao Paulo, Brazil: a preliminary report. Environ Res. 1994;65(2):218–225. doi: 10.1006/enrs.1994.1033. [DOI] [PubMed] [Google Scholar]
- 35.Rice M.B., Rifas-Shiman S.L., Oken E. Exposure to traffic and early life respiratory infection: a cohort study. Pediatr Pulmonol. 2015;50(3):252–259. doi: 10.1002/ppul.23029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harrod K.S., Jaramillo R.J., Rosenberger C.L. Increased susceptibility to RSV infection by exposure to inhaled diesel engine emissions. Am J Respir Cell Mol Biol. 2003;28(4):451–463. doi: 10.1165/rcmb.2002-0100OC. [DOI] [PubMed] [Google Scholar]
- 37.Mauad T., Rivero D.H., de Oliveira R.C. Chronic exposure to ambient levels of urban particles affects mouse lung development. Am J Respir Crit Care Med. 2008;178(7):721–728. doi: 10.1164/rccm.200803-436OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rice M.B., Rifas-Shiman S.L., Litonjua A.A. Lifetime exposure to ambient pollution and lung function in children. Am J Respir Crit Care Med. 2016;193(8):881–888. doi: 10.1164/rccm.201506-1058OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gauderman W.J., Urman R., Avol E. Association of improved air quality with lung development in children. N Engl J Med. 2015;372(10):905–913. doi: 10.1056/NEJMoa1414123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schultz E.S., Hallberg J., Bellander T. Early-life exposure to traffic-related air pollution and lung function in adolescence. Am J Respir Crit Care Med. 2016;193(2):171–177. doi: 10.1164/rccm.201505-0928OC. [DOI] [PubMed] [Google Scholar]
- 41.Gauderman W.J., Avol E., Gilliland F. The effect of air pollution on lung development from 10 to 18 years of age. N Engl J Med. 2004;351(11):1057–1067. doi: 10.1056/NEJMoa040610. [DOI] [PubMed] [Google Scholar]
- 42.McConnell R., Islam T., Shankardass K. Childhood incident asthma and traffic-related air pollution at home and school. Environ Health Perspect. 2010;118(7):1021–1026. doi: 10.1289/ehp.0901232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carlsten C., Dybuncio A., Becker A., Chan-Yeung M., Brauer M. Traffic-related air pollution and incident asthma in a high-risk birth cohort. Occup Environ Med. 2011;68(4):291–295. doi: 10.1136/oem.2010.055152. [DOI] [PubMed] [Google Scholar]
- 44.Clemens T., Turner S., Dibben C. Maternal exposure to ambient air pollution and fetal growth in North-East Scotland: a population-based study using routine ultrasound scans. Environ Int. 2017;107:216–226. doi: 10.1016/j.envint.2017.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu S., Krewski D., Shi Y., Chen Y., Burnett R.T. Association between maternal exposure to ambient air pollutants during pregnancy and fetal growth restriction. J Expo Sci Environ Epidemiol. 2007;17(5):426–432. doi: 10.1038/sj.jes.7500503. [DOI] [PubMed] [Google Scholar]
- 46.Darrow L.A., Klein M., Flanders W.D., Mulholland J.A., Tolbert P.E., Strickland M.J. Air pollution and acute respiratory infections among children 0-4 years of age: an 18-year time-series study. Am J Epidemiol. 2014;180(10):968–977. doi: 10.1093/aje/kwu234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Michikawa T., Morokuma S., Fukushima K., Kato K., Nitta H., Yamazaki S. Maternal exposure to air pollutants during the first trimester and foetal growth in Japanese term infants. Environ Pollut. 2017;230:387–393. doi: 10.1016/j.envpol.2017.06.069. [DOI] [PubMed] [Google Scholar]
- 48.Nachman R.M., Mao G., Zhang X. Intrauterine Inflammation and maternal exposure to ambient PM2.5 during preconception and specific periods of pregnancy: the Boston Birth Cohort. Environ Health Perspect. 2016;124(10):1608–1615. doi: 10.1289/EHP243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yorifuji T., Naruse H., Kashima S. Residential proximity to major roads and adverse birth outcomes: a hospital-based study. Environ Health. 2013;12(1):34. doi: 10.1186/1476-069X-12-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu J., Ren C., Delfino R.J., Chung J., Wilhelm M., Ritz B. Association between local traffic-generated air pollution and preeclampsia and preterm delivery in the south coast air basin of California. Environ Health Perspect. 2009;117(11):1773–1779. doi: 10.1289/ehp.0800334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wallace M.E., Grantz K.L., Liu D., Zhu Y., Kim S.S., Mendola P. Exposure to ambient air pollution and premature rupture of membranes. Am J Epidemiol. 2016;183(12):1114–1121. doi: 10.1093/aje/kwv284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang C., Nichols C., Liu Y. Ambient air pollution and adverse birth outcomes: a natural experiment study. Popul Health Metr. 2015;13:17. doi: 10.1186/s12963-015-0050-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Enkhmaa D., Warburton N., Javzandulam B. Seasonal ambient air pollution correlates strongly with spontaneous abortion in Mongolia. BMC Pregnancy Childbirth. 2014;14:146. doi: 10.1186/1471-2393-14-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lafuente R., Garcia-Blaquez N., Jacquemin B., Checa M.A. Outdoor air pollution and sperm quality. Fertil Steril. 2016;106(4):880–896. doi: 10.1016/j.fertnstert.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 55.Zhou N., Cui Z., Yang S. Air pollution and decreased semen quality: a comparative study of Chongqing urban and rural areas. Environ Pollut. 2014;187:145–152. doi: 10.1016/j.envpol.2013.12.030. [DOI] [PubMed] [Google Scholar]
- 56.Sacks J.D., Stanek L.W., Luben T.J. Particulate matter-induced health effects: who is susceptible? Environ Health Perspect. 2011;119(4):446–454. doi: 10.1289/ehp.1002255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Matteis S., Heederik D., Burdorf A. Current and new challenges in occupational lung diseases. Eur Respir Rev. 2017;26(146) doi: 10.1183/16000617.0080-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sood A., Petersen H., Blanchette C.M. Wood smoke exposure and gene promoter methylation are associated with increased risk for COPD in smokers. Am J Respir Crit Care Med. 2010;182(9):1098–1104. doi: 10.1164/rccm.201002-0222OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li W., Dorans K.S., Wilker E.H. Residential proximity to major roadways, fine particulate matter, and adiposity: the Framingham Heart Study. Obesity (Silver Spring) 2016;24(12):2593–2599. doi: 10.1002/oby.21630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shankardass K., McConnell R., Jerrett M., Milam J., Richardson J., Berhane K. Parental stress increases the effect of traffic-related air pollution on childhood asthma incidence. Proc Natl Acad Sci U S A. 2009;106(30):12406–12411. doi: 10.1073/pnas.0812910106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Clougherty J.E., Levy J.I., Kubzansky L.D. Synergistic effects of traffic-related air pollution and exposure to violence on urban asthma etiology. Environ Health Perspect. 2007;115(8):1140–1146. doi: 10.1289/ehp.9863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hicken M.T., Adar S.D., Hajat A. Air pollution, cardiovascular outcomes, and social disadvantage: the Multi-ethnic Study of Atherosclerosis. Epidemiology. 2016;27(1):42–50. doi: 10.1097/EDE.0000000000000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morello-Frosch R., Jesdale B.M. Separate and unequal: residential segregation and estimated cancer risks associated with ambient air toxics in US metropolitan areas. Environ Health Perspect. 2006;114(3):386–393. doi: 10.1289/ehp.8500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gordon S.B., Bruce N.G., Grigg J., Hibberd P.L., Kurmi O.P., Lam K.B. Respiratory risks from household air pollution in low and middle income countries. Lancet Respir Med. 2014;2(10):823–860. doi: 10.1016/S2213-2600(14)70168-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Samet J.M., Gruskin S. Forum of International Respiratory Societies working group collaboration. Air pollution, health, and human rights. Lancet Respir Med. 2015;3(2):98–100. doi: 10.1016/S2213-2600(14)70145-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Samet J.M. Some current challenges in research on air pollution and health. Salud Publica Mex. 2014;56(4):379–385. doi: 10.21149/spm.v56i4.7358. [DOI] [PubMed] [Google Scholar]