Abstract
Background
Pulmonary arterial hypertension (PAH) is a chronic disease that ultimately progresses to right-sided heart failure (HF) and death. Close monitoring of pulmonary artery pressure (PAP) and right ventricular (RV) function allows clinicians to appropriately guide therapy. However, the burden of commonly used methods to assess RV hemodynamics, such as right heart catheterization, precludes frequent monitoring. The CardioMEMS HF System (Abbott) is an ambulatory implantable hemodynamic monitor, previously only used in patients with New York Heart Association (NYHA) class III HF. In this study, we evaluate the feasibility and early safety of monitoring patients with PAH and right-sided HF using the CardioMEMS HF System.
Methods
The CardioMEMS HF sensors were implanted in 26 patients with PAH with NYHA class III or IV right-sided HF (51.3 ± 18.3 years of age, 92% women, 81% NYHA class III). PAH therapy was tracked using a minimum of weekly reviews of CardioMEMS HF daily hemodynamic measurements. Safety, functional response, and hemodynamic response were tracked up to 4 years with in-clinic follow-ups.
Results
The CardioMEMS HF System was safely used to monitor PAH therapy, with no device-related serious adverse events observed and a single preimplant serious adverse event. Significant PAP reduction and cardiac output elevation were observed as early as 1 month postimplant using trends of CardioMEMS HF data, coupled with significant NYHA class and quality of life improvements within 1 year.
Conclusions
The CardioMEMS HF System provided useful information to monitor PAH therapy, and demonstrated short- and long-term safety. Larger clinical trials are needed before its widespread use to guide therapy in patients with severe PAH with right-sided HF.
Key Words: CardioMEMS, hemodynamics, pulmonary hypertension, ride-sided heart failure
Abbreviations: 6MWD, 6-min walk distance; AGH, Allegheny General Hospital; AUC, area under the curve; CO, cardiac output; HF, heart failure; HR, heart rate; IHM, implantable hemodynamic monitor; NYHA, New York Heart Association; PAH, pulmonary arterial hypertension; PAP, pulmonary artery pressure; PAPd, diastolic PAP; PAPm, mean PAP; PAPs, systolic PAP; RHC, right heart catheterization; RV, right ventricular; SV, stroke volume; SVi, stroke volume index; SW, stroke work; TPR, total pulmonary resistance
Pulmonary arterial hypertension (PAH) is a progressive disease leading to right-sided heart failure (HF) and death,1 with a survival rate of 68% at 3 years.2, 3, 4 Goal-oriented treatment strategies must be continually adapted to each patient’s changing status. Although there are several accepted risk algorithms to predict outcome and guide management,5, 6, 7, 8, 9 all are limited by the need to assess patients face-to-face in the clinic. Therefore, prognostication is confined to a few touch points per year. In a rapidly progressive disease, the utility of increasing the number of touch points as efficiently and conveniently as possible is both logical and intuitive.
Close monitoring of right ventricular (RV) function is necessary to appropriately guide therapy and predict survival. Noninvasive imaging of the right ventricle is complicated by its unique contractile properties, resulting in limited accuracy. Invasive techniques such as right heart catheterization (RHC) are often more accurate, but the repeated procedures required to track disease progression are not feasible or risk free. Furthermore, both imaging and RHC approaches must be performed in clinic. Logistics aside, resting hemodynamics do not represent the total cardiovascular burden experienced in daily life. The development of ambulatory implantable hemodynamic monitors (IHMs) provides clinicians with remote access to daily cardiovascular measurements to monitor progression, guide therapy, and detect or prevent early decompensation.
The results of the CHAMPION (CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III Heart Failure Patients) trial10, 11 confirmed that incorporating data from a wireless IHM afforded better clinical outcomes.11, 12 In addition, IHMs have demonstrated safety and utility in reducing hospitalizations in group 2 pulmonary hypertension.13 In light of these results, we tested the initial safety and monitoring capabilities of the CardioMEMS HF System (Abbott) in monitoring patients with severe PAH and right-sided HF.
Methods
These results were combined from studies that were part of an ongoing National Institute of Health-funded contract (No. HHSN268201400008C) conducted at Allegheny General Hospital (AGH) and a separate investigator-initiated trial funded by Abbott at Duke University. Both studies were conducted after institutional review board approval (No. AHN ROC 5830) and written patient informed consent. Enrollment in both studies included patients with New York Heart Association (NYHA)/World Health Organization functional class III or IV PAH at time of consent and age ≥ 18 years with a hospitalization for right-sided HF within the year prior to consent. Exclusions were an active infection, recent pulmonary embolus, or allergy to aspirin or clopidogrel, if not treated with warfarin. Patients were requested to transmit data thrice weekly with a minimum of once weekly. Enrolled patients were implanted with a CardioMEMS sensor, monitored via a minimum of weekly reviews of daily pulmonary artery pressure (PAP)-based metrics, and followed for up to 4.2 years. Newly diagnosed patients were defined as those within 3 months of diagnostic catheterization. The primary end point of both studies (AGH and Duke University) was safety at 1 year postimplant. The secondary end point of the AGH study was the feasibility of monitoring IHM-derived hemodynamic responses over a course of therapy.
Sensor Implant and Use
A CardioMEMS sensor was implanted in the patient’s pulmonary artery according to manufacturer instructions14 and calibrated with RHC PAP (AGH and Duke University) and thermal and/or indirect Fick cardiac output (CO) (AGH). The CardioMEMS-derived CO is calculated using a proprietary algorithm (Abbott) based on the PAP waveform, mean PAP (PAPm), heart rate (HR), and a reference CO measured at implant and was used only in the AGH cohort. This algorithm has been previously developed, refined, and retrospectively tested against clinical RHC data, demonstrating noninferiority to current, clinically used CO measurement methods.15, 16 After hospital discharge, each patient used a home electronics unit to transmit daily contiguous 18-s PAP waveforms. From these waveforms and CO assessments, the following daily parameters were made accessible on a dedicated website: systolic PAP (PAPs), diastolic PAP (PAPd), PAPm, HR, CO, RV stroke volume (SV) and stroke volume index (SVi), RV stroke work (SV) and stroke work index, RV efficiency (SV/PAPm), total pulmonary resistance (TPR), pulmonary vascular compliance (SV/[PAPs − PAPd]), and vascular elastance (PAPs/SV). The CardioMEMS readings available for standard clinical practice only include HR, PAPs, PAPd, and PAPm. All other parameters, including CO, were available for use only in the feasibility study at AGH.
Follow-Up of Cardiovascular Status
After the first 21 implants, follow-up visits at AGH were conducted peri-implant and at 1, 4, 7, 12, and 24 months postimplant. The following data were collected at each visit: Minnesota Living with Heart Failure Questionnaire to assess quality of life, NYHA/World Health Organization functional class, vital signs, physical examinations, 6-min walk distance (6MWD), and laboratory measurements. Safety was assessed peri-implant and at 1, 4, and 12 months. If a patient completed the 24-month protocol-related follow-up, they continued to be monitored by the device and seen according to standard of care practices. The final two implants at AGH were completed for safety data collection only. At Duke University, only safety data at implant and up to 1 year, in addition to vital statistics at last follow-up, were transferred and merged with the AGH data.
Medication Adjustment
At their discretion, investigators at AGH used trends in PAP-based metrics to guide additions, deletions, or upward and/or downward dose adjustments in PAH-specific medications. The main goal of this pilot study was to test the feasibility of monitoring therapy, not guiding therapy per se. No set standardization or protocol was used to improve these parameters. However, if used, we asked investigators to aim for an improvement in SVi, a reduction in TPR or elastance, and improved RV efficiency when possible.
Statistical Analysis
All statistical analyses were performed using SAS version 9.4 (SAS Institute), MedCalc Statistical Software 16.8 (MedCalc), and MATLAB R2015b (The MathWorks, Inc). Changes in hemodynamics were displayed over time using two approaches: raw value and area under the curve (AUC). Raw values for each patient were averaged across the three daily measurements surrounding each follow-up time point. Plots show mean ± SE, with mean ± SD reported in the text. Changes in hemodynamic metrics, relative to baseline, were evaluated using a mixed model with repeated measures.
AUC methodology was used to visualize the cumulative changes in unit-days relative to baseline. Baseline values were computed as the mean of the measurements during the first week after implant, excluding any reading in first 6 h after implant. For each parameter value, the AUC between each day and the corresponding baseline was computed for each patient by simple numerical integration, and displayed as mean ± SE. Linear interpolation was used to account for missed daily readings. Significance tests for differences in the AUC were computed by paired t test with equal variance, with unpaired t tests used to compare among management cohorts (Supplemental Data).
NYHA functional class and quality of life are displayed in plots as mean ± SE. Changes relative to baseline were evaluated in patients with at least 1-month follow-up using a mixed model with repeated measures. For natriuretic peptides, the percentage of patients achieving a B-type natriuretic peptide < 340 pg/mL or N-terminal pro B-type natriuretic peptide < 1,100 pg/mL17 is displayed, and P values were calculated from logistic regression with repeated measures. Differences of all aforementioned parameters at each time point, relative to baseline, were considered statistically significant for P < .05.
Correlations between pre-6MWD hemodynamic parameters (SVi, compliance, TPR, and elastance) and 6MWD were evaluated using the square of the Pearson correlation coefficient.
Results
Patient Population
Patient disposition among AGH and Duke University is noted in e-Figure 1. Of the 33 patients who provided study consent, six failed study screening (four were deemed too ill, one experienced recurrent gastrointestinal bleeding, and one had no prior HF hospitalization) and one experienced a serious periprocedural complication prior to attempt at device implantation. The remaining 26 patients were implanted with CardioMEMS sensors (51.3 ± 18.3 years of age, 92% women, 81% NYHA class III). Twenty-three patients were implanted at AGH, and three were implanted at Duke University. One patient withdrew consent at 4 months, and the rest were followed for up to 2.5 ± 1.4 years. Demographic data for all implanted patients at time of consent are provided in Table 1. The mean time between consent and implantation was 15.3 ± 26 days to ensure clinical stability in treatment-naïve patients posthospital discharge. The mean ± SD of daily transmission compliance was 65.5% ± 27%, and the median daily transmission compliance was 67.3% (25th-75th percentile, 37.9-91.4). The mean ± SD of weekly transmission compliance was 94.9% ± 12%, and the median weekly transmission compliance was 98.2% (25th-75th percentile, 95.8-100.0).
Table 1.
Patient Demographics
| Characteristic | Monitored by CardioMems (N = 26) | Newly Diagnosed (n = 10) | Previously Diagnosed (n = 16) |
|---|---|---|---|
| Age at PAH diagnosis, y | 51.3 ± 18.3 | 55.7 ± 21.7 | 48.6 ± 15.9 |
| Age at study consent, y | 55 ± 18.3 | 55.8 ± 21.7 | 54.6 ± 16.6 |
| Time to implant, d | 15 ± 26.2 | 13.6 ± 27.3 | 16.1 ± 26.5 |
| Female | 24 (92) | 9 (90) | 15 (94) |
| Race | |||
| White | 23 (88) | 8 (80) | 15 (94) |
| Black | 3 (12) | 2 (20) | 1 (6) |
| WHO group 1 PAH subgroup | |||
| Idiopathic | 13 (50) | 6 (60) | 7 (43.8) |
| Heritable | 2 (7.7) | 2 (20) | 0 (0) |
| Drug- and toxin-induced | 3 (11.5) | 0 (0) | 3 (18.8) |
| Associated with CTD | 8 (30.8) | 2 (20) | 6 (37.5) |
| Scleroderma | 6 (23.1) | 1 (18.8) | 5 (31.3) |
| Nonscleroderma | 2 (7.7) | 1 (10) | 1 (6.3) |
| BMI, kg/m2 | 33.9 ± 9.9 | 33.9 ± 11.1 | 33.8 ± 9.5 |
| Heart rate, beats/min | 78.8 ± 13.9 | 74.6 ± 16.9 | 81.4 ± 11.4 |
| 6MWD, m | 266.8 ± 153.8 | 207.1 ± 141.1 | 300.5 ± 154.5 |
| NYHA class | |||
| III | 22 (84.6) | 7 (70) | 15 (93.8) |
| IV | 4 (15.4) | 3 (30) | 1 (6.3) |
| Creatinine, mg/dL | 0.89 ± 0.24 | 0.78 ± 0.14 | 0.95 ± 0.27 |
| REVEAL Registry risk score | 10 ± 1.5 | 10 ± 1.2 | 10 ± 1.8 |
| Baseline BP prior to implant | |||
| Fick cardiac index, L/(min-m2) | 2.5 ± 0.8 | 2 ± 0.6 | 2.8 ± 0.9 |
| Systemic systolic BP, mm Hg | 117.7 ± 17.6 | 118.5 ± 14.3 | 117.1 ± 19.8 |
| Systemic BP, mm Hg | 68.1 ± 10.6 | 70.8 ± 10 | 66.4 ± 10.9 |
| Systolic PAP, mm Hg | 90 ± 18.7 | 90.2 ± 24.3 | 89.8 ± 14 |
| Diastolic PAP, mm Hg | 34.8 ± 8.1 | 37 ± 7.1 | 33.1 ± 8.7 |
| Mean PAP, mm Hg | 56.6 ± 10.5 | 57.3 ± 13.6 | 56.1 ± 8 |
| PA wedge pressure, mm Hg | 10.9 ± 3.6 | 9.3 ± 3.2 | 12.2 ± 3.5 |
| Right atrial pressure, mm Hg | 10.3 ± 4.3 | 10.5 ± 4.8 | 10.1 ± 4.1 |
| Pulmonary vascular resistance, Wood units | 917.1 ± 511.5 | 1162.3 ± 589.3 | 712.8 ± 339.3 |
| PDE5i | 18 (69) | 6 (60) | 12 (75) |
| ERAs | 14 (54) | 3 (30) | 11 (69) |
| Riociguat | 3 (12) | 1 (10) | 2 (13) |
| Parenteral prostacyclins | 18 (69) | 6 (60) | 12 (75) |
| Two of listed drugs | 7 (27) | 4 (40) | 3 (19) |
| Three of listed drugs | 16 (62) | 5 (50) | 11 (69) |
Values are No. (%) or mean ± SD. 6MWD = 6-min walk distance; CTD = connective tissue disease; ERA = endothelin antagonist; NYHA = New York Heart Association; PAH = pulmonary arterial hypertension; PAP = pulmonary arterial pressure; PDE5i = phosphodiesterase type 5 inhibitor; WHO = World Health Organization.
Short- and Long-Term Safety
Of the 27 attempted implant procedures, there was only one periprocedural complication, a microperforation of the pulmonary artery during the predeployment angiogram, prior to any attempt at deploying the CardioMEMS sensor. The patient was withdrawn from the study and ultimately died from this complication. At the time of data lock (October 29, 2018), of the 26 patients implanted and monitored for safety and/or hemodynamic trends, three patients have been monitored > 4 years, six patients have been monitored between 3 and 4 years, six patients have been monitored between 2 and 3 years, four patients have been monitored between 1 and 2 years (two deaths; AGH), and seven patients have been monitored for < 1 year (2 deaths; 1 AGH, 1 Duke University; 1 consent withdrawn AGH) (e-Fig 1).
There were no postprocedural complications, no changes in peripheral oxygen saturations postimplant, and no device-related safety issues to date at 1, 4, and 12 months for 26, 26, and 19 implanted patients that passed that time period, respectively. In terms of long-term safety beyond implant, no device-related serious adverse events occurred. However, 43 serious adverse events occurred, resulting in 40 all-cause hospitalizations and three deaths out of the hospital (e-Table 1). Of these hospitalizations, 28 were in four patients, including 13 in one patient. There were eight HF hospitalizations, six in two patients, including four in the same patient. Mean time to first hospitalization was 220 ± 169 days. Common adverse events are noted in e-Table 2, none of which are related to device or device-specific anticoagulation and are typical of this ill patient population. There were four deaths in the postimplant period, two within the first year and two within the second year, and none were related to the device (e-Fig 1, Supplemental Safety Results). There were four bleeding episodes in two patients, one requiring 1 unit of packed RBCs for hemorrhoid bleed, all of which were deemed unrelated to the device. One patient required two device recalibrations using echocardiography at month 5 and 20.
Hemodynamic Response
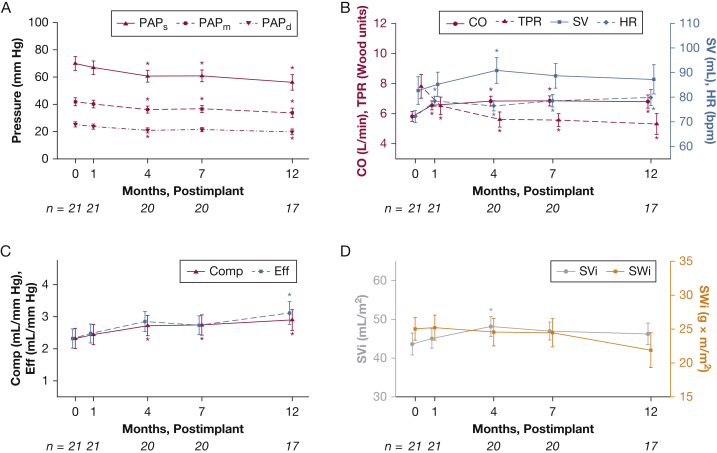
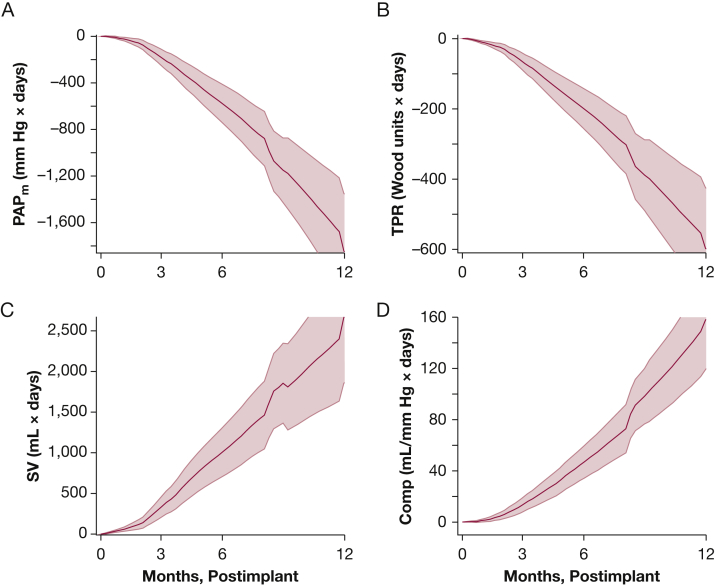
The hemodynamic profile in the first 12 months after implant for 21 patients at AGH with > 1-month follow-up is summarized in Figure 1. Direct CardioMEMS measurements include PAPs, PAPm, PAPd (Fig 1A), and HR, and derived measurements are shown in the remaining panels (CO, RV SV, compliance, SVi, RV SW, TPR, and efficiency). Significant reductions in PAP (PAPm, 42 ± 13 to 34 ± 14 mm Hg) and elevations in CO (5.8 ± 1.5 to 6.8 ± 1.8 L/min) were well visualized over 1 year of CardioMEMS-monitored therapy. Notable elevations in RV SV, vascular compliance, SVi, and RV efficiency were also observed, as well as reductions in RV SW and TPR. The AUC analyses in all patients are shown in Figure 2. RV SV, TPR, and compliance all exhibited significant changes at 12 months relative to baseline (P < .05). In addition, in patients that were highly managed (nine or more medication changes) within the first 4 months (most with serial changes in parenteral prostacyclins, based on knowledge of hemodynamics), early hemodynamic changes were visualized and captured quite well using the monitor (e-Appendix 1, e-Figs 2-4). Therefore, home monitoring and capturing significant changes in hemodynamic responses to changes in drug therapy over time are certainly feasible.
Figure 1.
Hemodynamic response measured by CardioMEMS for patients at Allegheny General Hospital. Plots show mean ± SE. Asterisks show statistically significant differences (P < .05) from 0-mo baseline. Number of patients at each time point is shown below the x-axes. bpm = beats/min; CO = cardiac output; Comp = compliance; Eff = right ventricular efficiency; HR = heart rate; PAPd = diastolic pulmonary artery pressure; PAPm = mean PAP pulmonary artery pressure; PAPs = systolic PAP pulmonary artery pressure; SV = right ventricular stroke volume; SVi = stroke volume index; SWi = stroke work index; TPR = total pulmonary resistance.
Figure 2.
Cumulative hemodynamic response illustrated by the area under the curve analyses across 365 d for patients at Allegheny General Hospital. PAPm, TPR, SV, and compliance are shown as mean (solid line) ± SE (shaded envelope) of the cumulative change over time. All P < .05 at 12 mo, relative to baseline. See Figure 1 legend for expansion of abbreviations.
Clinical Outcomes
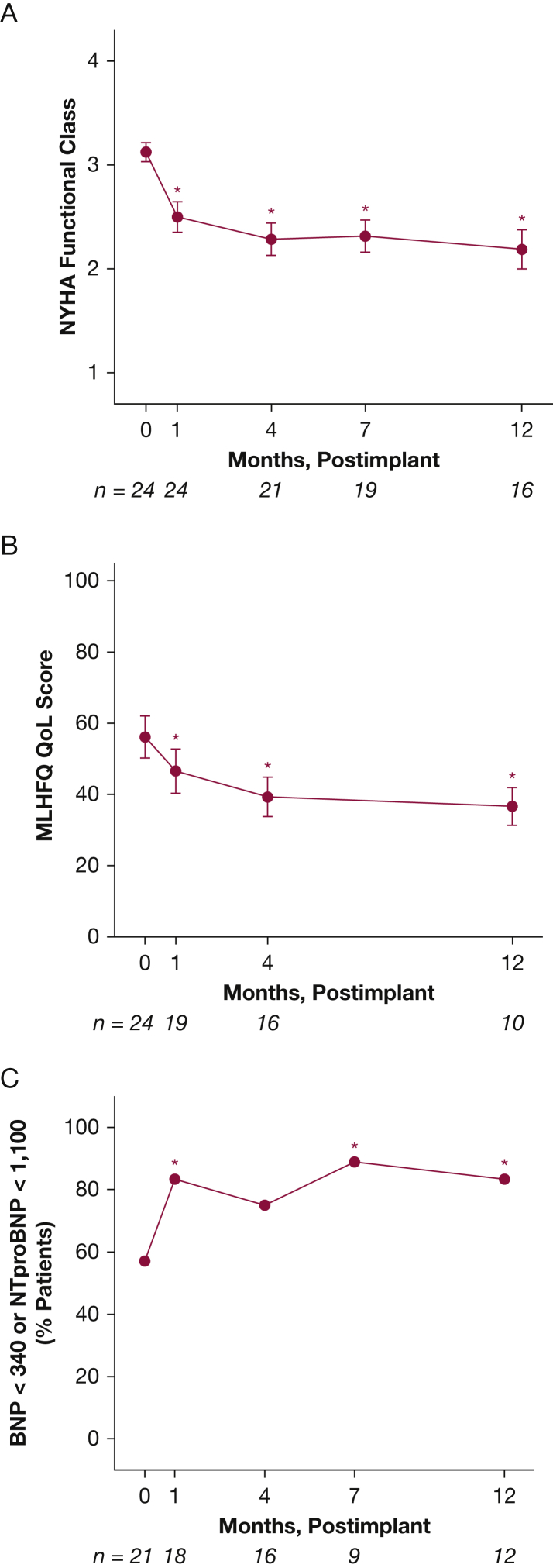
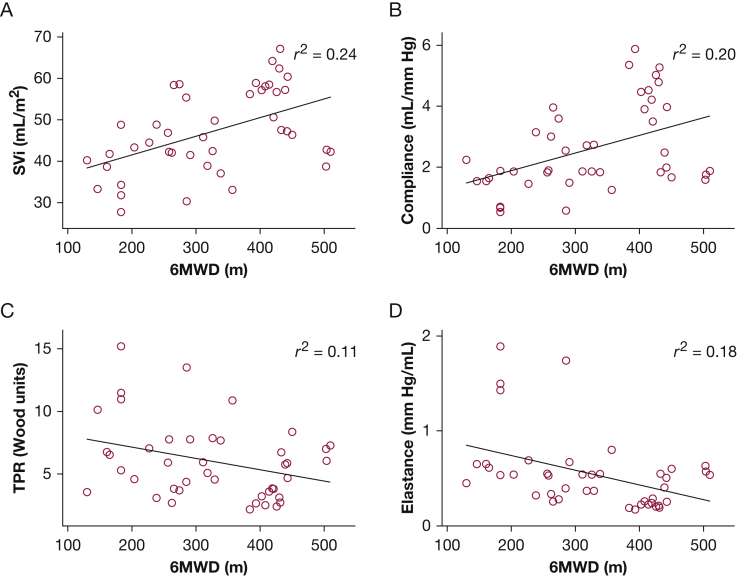
Changes in NYHA class, natriuretic peptides, and of life parameters for the implanted cohort with at least 1-month follow-up postimplant (n = 24) are shown in Figure 3. These changes were analyzed to demonstrate potential alternative efficacy end points that could be used in future trials of the device in PAH and to visualize the parallel between patterned hemodynamic changes obtained through home monitoring and other outcomes assessed in clinic. They are not meant to imply superiority of a hemodynamically guided approach over current clinically based goal-directed algorithms. As shown in Figure 3A, an overall improvement in NYHA functional class from baseline (P < .001), natriuretic peptides (P < .01), and Minnesota Living with Heart Failure Questionnaire Quality of Life score (P < .001) was observed over the course of monitoring, and mirrored the hemodynamic changes illustrated in Figures 1 and 2. In addition, 6MWD, a conventional marker of outcome, correlated with IHM-determined hemodynamics. As shown in Figure 4, for the 15 patients who completed the walk tests with prewalk hemodynamics recorded, 6MWD correlated positively with SVi (r2 = 0.24, P < .01) and compliance (r2 = 0.20, P < .01), and inversely with TPR (r2 = 0.11, P < .05) and elastance (r2 = 0.18, P < .01).
Figure 3.
NYHA functional class (A), quality of life (B), and natriuretic peptide levels (C) for patients at Allegheny General Hospital. Error bars indicate SE. Number of patients at each time point is shown below the x-axes. Asterisks show statistically significant differences in NYHA class and quality of life from baseline (month 0). BNP = B-type natriuretic peptide; MLHFQ QoL = Minnesota Living with Heart Failure Questionnaire Quality of Life score; NTproBNP = N-terminal pro B-type natriuretic peptide; NYHA = New York Heart Association.
Figure 4.
Correlations between 6MWD and prewalk SVi, compliance, TPR, and elastance. 6MWD = 6-min walk distance. See Figure 1 legend for expansion of other abbreviations.
Examples of Right HF Presentations
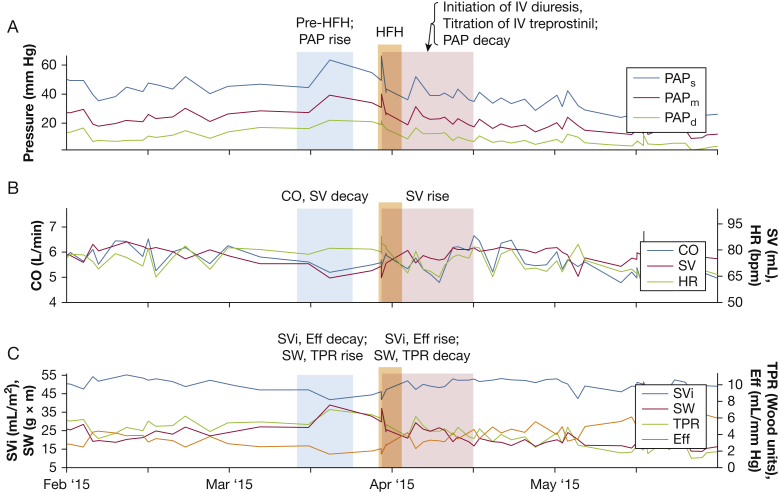
IHM monitoring may also be helpful to identify early clinical worsening. As examples of this, Figures 5 and 6 demonstrate two hemodynamic manifestations of right HF identified by the IHM. Figure 5 demonstrates acute RV failure precipitated by patient noncompliance with PAH medications. Tadalafil and ambrisentan were stopped (Fig 5, blue shading), resulting in an abrupt rise in PAP and a drop in CO, RV SV, and efficiency, with concomitant rise in RV SW and TPR. The patient was hospitalized (Fig 5, orange shading) for IV diuresis and reinstitution of medications with an improvement in all parameters (Fig 5, red shading). Note that the jump in PAP because of patient noncompliance was evident weeks before the subsequent hospitalization. Figure 5 also demonstrates the variety of alternative RV metrics that can be obtained and used clinically on an ongoing basis, including SV, SVi, and SW.
Figure 5.
Example of acute right ventricular (RV) failure because of medication noncompliance. Patient medication noncompliance (blue shading) resulted in rise in deteriorating hemodynamics and heart failure hospitalization (orange shading). IV diuresis and reinstitution of medications (red shading) resulted in an improvement in all parameters. HFH = heart failure hospitalization; PAP = pulmonary artery pressure; SW = RV stroke work. See Figure 1 legend for expansion of other abbreviations.
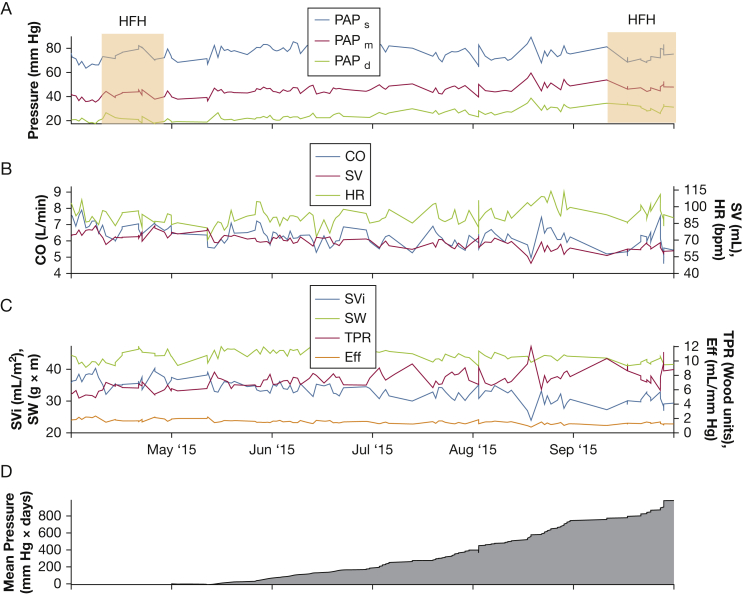
Figure 6.
Example of gradual right ventricular failure. Gradual decrease in SV and CO (B) occurred despite consistent SW (C). The cumulative mean PAP elevation starting after the first heart failure hospitalization is shown in panel D. See Figure 1 and 5 legends for expansion of abbreviations.
Figure 6 demonstrates a gradual depiction of RV failure between April and October of 2015, visualized best in the gray AUC PAPm tracing (Fig 6D). Note the gradual decline in RV SV, despite the same RV SW. This represents an energetically unfavorable situation, culminating in a prolonged hospitalization for HF in mid-September 2015. For comparison, e-Figure 5 demonstrates a non-HF hospitalization, in which no significant changes in hemodynamic metrics were observed. Therefore, it appears feasible that hemodynamics derived from the IHM may identify patients at risk of clinical worsening prior to a hospitalization.
Discussion
PAH remains a uniformly progressive disease, despite advances in therapeutic options. Therefore, there is a pressing need to closely monitor improvements in both survival and quality of life. In this feasibility study in the PAH population, we explored the possible utility of the CardioMEMS HF System to monitor changes in hemodynamics over the course of therapy in 26 patients to determine if this modality could be used in the future as a guide to direct medical therapy. In addition, and importantly, we wanted to provide early insight into its short- and long-term safety.
Short-term safety appeared acceptable with no complications because of actual sensor implantation, including no pulmonary thrombosis, infections, dysrhythmias, or hemorrhages. The one periprocedural complication, although not related to the actual implantation of the device, underscores the need for caution with this or any interventional procedure that involves the pulmonary arteries. Unfortunately, microperforation is a rare complication of any procedure that involves the pulmonary circulation, including both the RHC and pulmonary angiogram procedure, with an incidence ranging from 0.01% to 0.47%.18, 19, 20, 21 In the CHAMPION trial, there was only one case of pulmonary artery injury in 575 implant attempts.10, 11, 22 In the postmarketing surveillance of this device, of the approximately 5,500 implants of this device worldwide, there was a 0.5% incidence of pulmonary artery injury and/or hemoptysis, mostly related to excessive guidewire manipulations.23 This particular patient had complex pulmonary arterial anatomy manifested by collateral vessel formation on angiography, likely related to the etiology of their PAH, which was D transposition of the great vessels, including both prior Blalock-Taussig shunts and subsequent Jatene arterial switch procedure. Therefore, it is important to remember that in patients with PAH, it may be more important to choose appropriate anatomy and exclude those with excessive tortuosity or collateral vessel formation to minimize manipulations while in the pulmonary artery. In the current study, we amended our protocol to exclude complex congenital heart disease and vessel anatomy until further experience with the preimplant procedure is gained in this population. In terms of safety beyond implant, in the cumulative 52 patient-years, there were no device-related serious adverse events. Although there were several bleeding episodes, none were thought to be related to the anticoagulation or antiplatelet therapy required for the device.
Serial monitoring of hemodynamics over time, as demonstrated in Figure 1, is feasible and may provide added value compared with a single rest set of measurements made in the catheterization laboratory because these latter measurements do not fully capture disease severity and subclinical disease patterns. One can envision that this or other IHMs could be used as a treatment guide for clinicians treating PAH because frequent virtual touch points with the patient may provide a logistical advantage over the current risk assessment algorithms6, 7, 8, 9, 10 and serial imaging studies, which require face-to-face clinic visits and testing to guide therapeutic decision-making. Certainly, these frequent virtual interactions when coupled with existing prognostication tools could provide for more frequent and informed risk assessments and potentially detect clinical deterioration earlier with the potential to drive better outcomes. The usefulness of this or other IHM-guided treatment strategies as a complementary method or in place of other contemporary risk assessment strategies will need to be established in the setting of future randomized trials before this or other devices can be used routinely in the clinical environment.
In this small PAH cohort, using daily CardioMEMS measurements, we were able to visualize favorable changes in hemodynamics, particularly those patients treated aggressively with parenteral prostacyclins, including a decrease in TPR and an increase in SVi, vascular compliance, and cardiac efficiency. Therefore, using these and other parameters to evaluate, hemodynamic stability may be a distinct possibility and could perhaps help clinicians more effectively titrate parenteral prostacyclins. With this in mind, we noted improvements in NYHA functional class, natriuretic peptide levels, and quality of life, which tracked changes in serial hemodynamic measurements and also demonstrated that hemodynamic parameters derived from the IHM correlated with 6MWD. These correlations suggest that IHM-derived hemodynamic changes could serve as early markers of therapeutic responses that can be measured daily and conveniently in the patient’s home. In addition, these hemodynamic changes after further study and validation may eventually be used as part of a home monitoring algorithm to adjunctively guide therapy in patients with PAH to identify early decompensation or noncompliance, optimize medication dosing, and increase patient satisfaction by minimizing frequent visits to the office.24, 25
Although publications suggest that hemodynamic data may not be necessary in risk prognostication,8 the same group demonstrated the value of serially determined estimations in SVi.26 In addition, current assessments of hemodynamic measurements and their relative importance in PAH are based on infrequent recordings and generated in the artificial conditions of the cardiac catheterization laboratory. Therefore, the importance of detecting frequent serial changes in hemodynamics as afforded by IHMs should not be prematurely discounted, but considered as a new avenue to explore in the area of risk prediction.
In summary, the use of an IHM in patients with PAH was observed to be feasible in monitoring hemodynamic changes and safe, if appropriate patient selection and anatomy is mandated and standardized. The additional benefits of directing individual clinical practice using IHM data will need to be established in an appropriate clinical trial in which hemodynamic-guided changes are compared with contemporary measures of guiding therapy with respect to clinical outcomes. One could envision that long-term monitoring of moderate- and high-risk patients using IHM data coupled with data from advanced imaging studies (eg, MRI), contemporary risk scoring systems,6, 8, 9 or home-based algorithms akin to telemedicine approaches could strongly facilitate the application of goal-directed therapy while eliminating the cost, inconvenience, and risk for repeated trips to the catheterization laboratory. Additionally, the changes in observed hemodynamic parameters could serve as important independent or composite components of clinical trial proceedings and testing new therapeutics. These advantages need to be weighed against the personnel costs and time to monitor patients, but in left HF, IHMs have resulted in an economic advantage.11
Limitations
This was an exploratory, nonrandomized, uncontrolled feasibility trial with a limited number of patients, which importantly lacked a comparison with current risk strategies and therefore should not be construed to represent a fully viable treatment strategy in PAH (supplemental data). Importantly, because there was no control group, we do not have evidence from this study to demonstrate superiority of frequent hemodynamic analysis over traditional monitoring strategies. Indeed, the CardioMEMS device is not yet an approved modality for treatment guidance in PAH; however, CardioMEMS CO has been internally validated against repeat invasive measurements of CO. Although the correlations between the 6MWD and hemodynamic parameters were marginal (r2 value), they were still significant; therefore we can still draw important conclusions about how changes in hemodynamic values are associated with changes in walk distance. This will likely require additional patient data to build on these significant associations. Finally, further investigation is required before routine availability of CO-derived hemodynamic data (RV SV, compliance, SVi, RV SW, TPR, and efficiency) may be incorporated into routine clinical use with other standard, currently clinically available parameters. As such, a phase 3, randomized trial using the full spectrum of IHM-derived data is currently being designed to further test and validate both the safety and utility of a device based over a risk-based strategy to guide medical decision-making in PAH. Clinicians should wait for the results of this trial before pursuing utilization of this device in the clinical arena.
Acknowledgments
Author contributions: R. L. B. had full access to all the data in the study and takes responsibility for the integrity of the data and accuracy of results. R. L. B., M. D., and R. B. contributed to conception and design. R. L. B., M. D., R. B., J. W., N. B., G. G., and R. A. contributed to analysis and interpretation. R. L. B, M. D., D. L., R. B., J. W., N. B., G. G., R. A., M. K. K., A. R., S. M., P. C.-J., S. A., V. F., S. R., and K. S. P. drafted the manuscript for important intellectual content.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: R. L. B.’s institution has received monies for sponsor-initiated multicenter clinical trial work. K. S. P. and S. R.’s institution has received monies for investigator-initiated clinical trial work. N. B. and G. G. are employees of Abbott. S. A. is a speaker for Bayer and a consultant for Bayer and Actelion. V. F. has participated in advisory board review for United Therapeutics. M. K. K. has participated on the medical advisory board for Bayer. A. R. has been on the speaker’s bureau for Bayer, has been a consultant for United Therapeutics, and has been a primary investigator for clinical trials with United Therapeutics, Actelion, Liquidia, and Bellerophon. S. M. has participated in speaking activities for Actelion. R. A. is an employee and stockholder of Abbott. J. W. is a former employee of Abbott. None declared (M. D., D. L., P. C.-J., R. B.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or in the preparation of the manuscript.
Additional information: The e-Appendix, e-Figures, and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: The work performed at Allegheny General Hospital was supported by a National Heart, Lung, and Blood Institute VITA Contract [Contract HHSN268201400008C], and the work performed at Duke Clinical Research Institute was funded by an investigator-initiated award from Abbott.
Supplementary Data
References
- 1.Galiè N., Humbert M., Vachiery J.-L. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Respir J. 2015;46(6):903–975. doi: 10.1183/13993003.01032-2015. [DOI] [PubMed] [Google Scholar]
- 2.Benza R.L., Miller D.P., Barst R.J., Badesch D.B., Frost A.E., McGoon M.D. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL Registry. Chest. 2012;142(2):448–456. doi: 10.1378/chest.11-1460. [DOI] [PubMed] [Google Scholar]
- 3.DiSalvo T.G., Mathier M., Semigram M.J., Dec G.W. Preserved right ventricular ejection fraction predicts exercise capacity and survival in advanced heart failure. J Am Coll Cardiol. 1995;25(5):1143–1153. doi: 10.1016/0735-1097(94)00511-n. [DOI] [PubMed] [Google Scholar]
- 4.Farber H.W., Miller D.P., Poms A.D. Five-year outcomes of patients enrolled in the REVEAL Registry. Chest. 2015;148(4):1043–1054. doi: 10.1378/chest.15-0300. [DOI] [PubMed] [Google Scholar]
- 5.Benza R.L., Miller D.P., Gomberg-Maitland M. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL) Circulation. 2010;122(2):164–172. doi: 10.1161/CIRCULATIONAHA.109.898122. [DOI] [PubMed] [Google Scholar]
- 6.Benza R.L., Gomberg-Maitland M., Miller D.P. The REVEAL Registry risk score calculator in patients newly diagnosed with pulmonary arterial hypertension. Chest. 2012;141(2):354–362. doi: 10.1378/chest.11-0676. [DOI] [PubMed] [Google Scholar]
- 7.Kylhammar D., Kjellstrom B., Hjalmarsson C. A comprehensive risk stratification at early follow-up determines prognosis in pulmonary arterial hypertension. Eur Heart J. 2018;39(47):4175–4181. doi: 10.1093/eurheartj/ehx257. [DOI] [PubMed] [Google Scholar]
- 8.Boucly A., Weatherald J., Savale L. Risk assessment, prognosis and guideline implementation in pulmonary arterial hypertension. Eur Respir J. 2017;50(2) doi: 10.1183/13993003.00889-2017. pii: 1700889. [DOI] [PubMed] [Google Scholar]
- 9.Hoeper M.M., Kramer T., Pan Z. Mortality in pulmonary arterial hypertension: prediction by the 2015 European pulmonary hypertension guidelines risk stratification model. Eur Respir J. 2017;50(2) doi: 10.1183/13993003.00740-2017. pii: 1700740. [DOI] [PubMed] [Google Scholar]
- 10.Abraham W.T., Stevenson L.W., Bourge R.C., Lindenfeld J.A., Bauman J.G., Adamson P.B. Sustained efficacy of pulmonary artery pressure to guide adjustment of chronic heart failure therapy: complete follow-up results from the CHAMPION randomised trial. Lancet. 2016;387(10017):453–461. doi: 10.1016/S0140-6736(15)00723-0. [DOI] [PubMed] [Google Scholar]
- 11.Martinson M., Bharmi R., Dalal N., Abraham W.T., Adamson P.B. Pulmonary artery pressure-guided heart failure management: US cost-effectiveness analyses using the results of the CHAMPION clinical trial. Eur J Heart Fail. 2017;19(5):652–660. doi: 10.1002/ejhf.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desai A.S., Bhimaraj A., Bharmi R. Ambulatory hemodynamic monitoring reduces heart failure hospitalizations in “real-world” clinical practice. J Am Coll Cardiol. 2017;69(19):2357–2365. doi: 10.1016/j.jacc.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Benza R.L., Raina A., Abraham W.T. Pulmonary hypertension related to left heart disease: insight from a wireless implantable hemodynamic monitor. J Heart Lung Transplant. 2015;34(4):329–337. doi: 10.1016/j.healun.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 14.Abraham W.T., Adamson P.B., Bourge R.C. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377(9766):658–666. doi: 10.1016/S0140-6736(11)60101-3. [DOI] [PubMed] [Google Scholar]
- 15.Tree M., White J., Midha P., Kiblinger S., Yoganathan A. Validation of cardiac output as reported by a permanently implanted wireless sensor. J Med Devices. 2015;10(1) [Google Scholar]
- 16.Karamanoglu M., Bennett T., Stahlberg M. Estimation of cardiac output in patients with congestive heart failure by analysis of right ventricular pressure waveforms. Biomed Eng Online. 2011;10:36. doi: 10.1186/1475-925X-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frantz R.P., Farber H.W., Badesch D.B. Baseline and serial brain natriuretic peptide level predicts 5-year overall survival in patients with pulmonary arterial hypertension: data from the REVEAL Registry. Chest. 2018;154(1):126–135. doi: 10.1016/j.chest.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melao F., Lopes R., Rodrigues R.A. Massive hemoptysis as an unusual complication of right heart catheterization: successful treatment with percutaneous stent. Rev Port Cardiol. 2016;35(4):235.e1–235.e4. doi: 10.1016/j.repc.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 19.Sekkal S., Cornu E., Christides C. Swan-Ganz catheter induced pulmonary artery perforation during cardiac surgery concerning two cases. J Cardiovasc Surg. 1996;37(3):313–317. [PubMed] [Google Scholar]
- 20.Fortin M., Turcotte R., Gleeton O., Bussieres J.S. Catheter-induced pulmonary artery rupture: using occlusion balloon to avoid lung isolation. J Cardiothorac Vasc Anesth. 2006;20(3):376–378. doi: 10.1053/j.jvca.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 21.Mullerworth M.H., Angelopoulos P., Couyant M.A. Recognition and management of catheter-induced pulmonary artery rupture. Ann Thorac Surg. 1998;66(4):1242–1245. doi: 10.1016/s0003-4975(98)00593-1. [DOI] [PubMed] [Google Scholar]
- 22.FDA summary of safety and effectiveness. CardioMEMS HF pressure measurement system. 2014. https://www.accessdata.fda.gov/cdrh_docs/pdf10/P100045b.pdf. Accessed July 30, 2019.
- 23.Vaduganathan M., DeFilippis E.M., Fonarow G.C., Butler J., Mehra M.R. Postmarketing adverse events related to the CardioMEMS HF System. JAMA Cardiol. 2017;2(11):1277–1279. doi: 10.1001/jamacardio.2017.3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abraham W.T., Perl L. Implantable hemodynamic monitoring for heart failure patients. J Am Coll Cardiol. 2017;70(3):389–398. doi: 10.1016/j.jacc.2017.05.052. [DOI] [PubMed] [Google Scholar]
- 25.Adamson P.B. Pathophysiology of the transition from chronic compensated and acute decompensated heart failure: new insights from continuous monitoring devices. Curr Heart Fail Rep. 2009;6(4):287–292. doi: 10.1007/s11897-009-0039-z. [DOI] [PubMed] [Google Scholar]
- 26.Weatherald J., Boucly A., Chemla D. Prognostic value of follow-up hemodynamic variables after initial management in pulmonary arterial hypertension. Circulation. 2018;137(7):693–704. doi: 10.1161/CIRCULATIONAHA.117.029254. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.