Abstract
Introduction:
Priapism is the persistent and painful erection of the penis and is a common sickle cell disease (SCD) complication.
Aim:
The goal of this study was to characterize clinical and genetic factors associated with priapism within a large multi-center SCD cohort in Brazil.
Methods:
Cases with priapism were compared to SCD type-matched controls within defined age strata to identify clinical outcomes associated with priapism. Whole blood single nucleotide polymorphism genotyping was performed using a customized array, and a genome-wide association study (GWAS) was conducted to identify single nucleotide polymorphisms associated with priapism.
Main Outcome Measure:
Of the 1,314 male patients in the cohort, 188 experienced priapism (14.3%).
Results:
Priapism was more common among older patients (P = .006) and more severe SCD genotypes such as homozygous SS (P < .0001). In the genotype- and age-matched analyses, associations with priapism were found for pulmonary hypertension (P = .05) and avascular necrosis (P = .01). The GWAS suggested replication of a previously reported candidate gene association of priapism for the gene transforming growth factor beta receptor 3 (TGFBR3) (P = 2 × 10−4).
Clinical Implications:
Older patients with more severe genotypes are at higher risk of priapism, and there is a lack of consensus on standard treatment strategies for priapism in SCD.
Strengths & Limitations:
This study characterizes SCD patients with any history of priapism from a large multi-center cohort. Replication of the GWAS in an independent cohort is required to validate the results.
Conclusion:
These findings extend the understanding of risk factors associated with priapism in SCD and identify genetic markers to be investigated in future studies to further elucidate priapism pathophysiology.
Keywords: Sickle Cell Disease, Priapism, Genome-Wide Association Study, Single Nucleotide Polymorphism
INTRODUCTION
Sickle cell disease (SCD) is a monogenic disease caused by a point mutation in the β-globin gene (HBB). This mutation leads to the generation of abnormal hemoglobin S (HbS), which polymerizes when deoxygenated and distorts red blood cells.1 Homozygous patients (HbSS) generally have higher levels of HbS and a more severe phenotype than compound heterozygous, such as HbSC or other variants. However, the course of disease, severity, and organs affected vary greatly among patients, even within HbSS patients, and HbS mutation alone is not able to completely explain the heterogeneity.
Priapism is defined as an unwanted, prolonged, and painful penile erection that may cause complications such as erectile dysfunction and impotence. Priapism is a common SCD complication, with a reported prevalence of 30–45% among homozygous HbSS male patients.2–5 In SCD patients, priapism most frequently occurs as the ischemic type,6 with decreased or absent cavernous blood flow, corporal rigidity, and pain.7 Ischemic priapism can be major, lasting 6 hours or longer, or stuttering, with repeated episodes with intervening periods of detumescence.7
Priapism in SCD patients was first linked to vascular occlusion and ischemia, as a consequence of decreased efflux of blood from corporal tissue.8,9 The decreased efflux was postulated to be a consequence of the interaction of sickled erythrocytes with endothelial cells, leukocytes, platelets, and other plasma components leading to vascular obstruction. However, later studies linked priapism in SCD patients with disruptions of the nitric oxide (NO) signal transduction pathway. NO is a key signaling molecule in pathways mediating erection.10 Hemolysis would contribute to the reduction of NO bioavailability, as plasma free hemoglobin is released and can scavenge NO.11,12 Recently, priapism has also been associated with an excess of adenosine, RhoA/Rho-kinase (ROCK), and opiorphin upregulation.13–22
Previous studies have investigated the association of single nucleotide polymorphisms (SNPs) in candidate genes with the risk of priapism in SCD patients. Associations were found for polymorphisms in the Klotho gene, involved in vascular functions and NO biology23; transforming growth factor-β receptor type III (TGFBR3), related to inflammatory pathways; aquaporin, associated with hydration; integrin α-V, which encodes a receptor related to red cell adhesion; and the A1 subunit of coagulation factor XIII, which has an important role in the coagulation system.24 However, no study has confirmed these associations and no genome-wide association studies of priapism have been conducted.
The goal of this study was to characterize clinical and genetic factors associated with priapism within a large multi-center SCD cohort in Brazil. This evaluation will help us to better understand the impact of priapism in SCD patients and the associated risk factors.
METHODS
Patient Recruiting
The REDS-III Brazil SCD cohort is part of the Recipient Epidemiology and Donor Evaluation Study-III (REDS-III) program of the National Institutes of Health’s National Heart, Lung, and Blood Institute.25 REDS-III is a collaboration among investigators in the United States, Brazil, China, and South Africa to study the safety and adequacy of the blood supply and impact of blood transfusion. The REDS-III Brazil SCD cohort study aims to characterize health and transfusion outcomes in patients with SCD.26 The Brazilian National Ethical Committee for Research, local ethical committees at each participating center, and the Institutional Review Boards at the University of California, San Francisco, and the REDS-III data coordinating center, RTI International, all reviewed and approved the study. Written informed consent was obtained from participants 18 years or older or from guardians of younger patients. Age-appropriate assent was also obtained from minors.
The REDS-III Brazil SCD cohort enrolled almost 2,800 Brazilian patients with sickle cell disease at 6 sites in Brazil: Fundação Hemominas, in the cities of Belo Horizonte, Juiz de Fora, and Montes Claros in the state of Minas Gerais; Fundação Hemorio, in Rio de Janeiro in the state of Rio de Janeiro; Fundação Hemope, in Recife in the state of Pernambuco; and Instituto da Criança, Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo in São Paulo in the state of São Paulo. The study methods and baseline results have been previously published.26 Briefly, participants were randomly selected from participating centers and recruited at routine visits. Sociodemographic information was obtained through an interview with participants (or legal guardian for participants < 18 years of age). SCD outcomes were abstracted from medical records using standardized definitions of the phenotypic manifestations of SCD as defined by Ballas et al.27 This included an abstraction of any history of priapism in a patient’s lifetime and the year of the first episode from the medical records at the enrollment visit. Two follow-up visits were conducted during the cohort study. At these visits, more detailed information regarding all priapism episodes during the follow-up time were abstracted, including duration of episodes and treatments used.
For an analysis of demographic characteristics, all male patients with no history of priapism were compared to patients with a history of priapism. For an analysis of clinical outcomes associated with priapism in the cohort, frequency matching was used to identify controls without priapism matched on SCD genotype and age strata to priapism cases, considering an interval of ± 2 years for participants younger than 18 years of age and an interval of ± 6 years for participants older than 18 years of age in a 1:2 ratio. Two HbSβ0 patients did not have a match among controls and were not included in the clinical analysis. Participants with a history of priapism were compared to participants without priapism using chi-squared tests for categorical variables and Wilcoxon rank-sum tests for continuous variables.
The Kaplan-Meier method was used to estimate time to event of priapism and a genome-wide association study (GWAS) was performed to identify SNPs associated with priapism. Both the Kaplan-Meier analysis and GWAS were restricted to the genotypes HbSS, HbSβ0, and HbSβ+, as these genotypes had similar prevalences of priapism in this study. HbSC patients were excluded from these analyses, as the prevalence of priapism in our cohort was significantly lower in HbSC patients. For the GWAS, cases were defined as HbSS, HbSβ0, or Hbβ+ male patients with at least 1 episode of priapism. Control subjects were HbSS, HbSβ0, or Hbβ+ male patients 12 years old or older who had never experienced priapism, to control for patients who might have priapism but had not yet experienced it because of their young age.
Genotyping
Genotyping was performed using a customized Affymetrix (Santa Clara, CA) transfusion medicine array.28 The array includes 749,000 SNPs and is enriched for blood- and transfusion-related polymorphisms. The array also includes 48 genes specifically related to SCD, including possible SCD bio-markers, such as for fetal hemoglobin (HbF) levels, hemolysis, hyperbilirubinemia, and proteinuria. Genotype calls were generated using Axiom Analysis Suite software (Affymetrix), human leukocyte antigen alleles were called with Axiom HLA Analysis software (Affymetrix), and copy number polymorphisms were called with Axiom CNV Summary Tools, with further analysis being performed with PennCNV (http://penncnv.openbioinformatics.org/en/latest/).
Samples with dish quality control values less than 0.85 or call rates < 97% were excluded. Plates with plate quality control metrics < 95% or average call rates < 98.5% were also excluded. For SNP quality control, SNPolisher was used, and SNPs in the recommended categories (PolyHighRes, MonoHighRes, NoMinorHom, and Hemizygous) were retained. Samples with inconsistent gender definitions found by genotyping and the database were excluded. PLINK29 was used to calculate identity-by-descent, and the sample with a lower call rate from pairs of samples with PI_HAT > 0, 4 or IBS > 0, 9 was excluded. SNPs with call rates < 97% or significant deviation from Hardy-Weinberg equilibrium (P ≤ 10−4) were excluded. Phasing was then conducted with SHAPEIT30 and imputation with IMPUTE2,31 with haplotypes derived from the 1000 Genomes Project Phase 332 as reference data. After quality control and imputation procedures, a final set of 831,797 SNPs was used in the GWAS.
Genome-Wide Association Study
Population substructure was determined by generating principal components using EIGENSTRAT33 software on linkage disequilibrium (LD)-pruned autosomal SNPs with unrelated participants only. The top 10 principal components were included in the association analyses to adjust for population substructure. Genome-wide association analyses were conducted using GEMMA34 software with a logistic mixed model, adjusted for age as covariate, as age was associated with priapism occurrence, as well as cryptic kinship relatedness. The online tool HaploReg v4.135 was used to explore the genes nearest to the index SNPs. LocusZoom36 was used to generate LocusZoom plots, with 1000 Genomes Project Phase 3 LD estimation. A genome-wide P value threshold of 5 × 10−8 was used to define statistical significance in the GWA analysis. Only variants with minor frequency alleles > 0.05 were considered. Odds ratios for each SNP were transformed from the effect sizes from linear mixed modelwith LMOR37 in order to obtain a comparative scale for the genetic effects.
RESULTS
Overall Characteristics of Patients
Among the REDS-III Brazil SCD cohort study participants, there were 1,314 male patients 0 to 77 years of age, of which 188 had priapism (14.3%) (Figure 1). This included 51 of 745 males younger than 18 years (6.9%) and 137 of 568 males 18 years old or older (24.1%) (Table 1). Older age was significantly associated with priapism (P < .0001). The mean age of the first priapism episode was 16 years (median, 15 years; range, 2–41 years), with 25% of the cases occurring before 10 years of age and 60% of the cases occurring before 20 years of age (Figure 2). The Kaplan-Meier analysis included 993 HbSS, HbSβ0, and HbSβ+ patients, 158 with a history of priapism and known age of first episode and 835 with no history of priapism. This demonstrated that 80% of the patients included in the analysis remained priapism free by 20 years of age and that the number of new cases stabilizes near the age of 40 (Figure 3).
Figure 1.
Flow diagram showing the number of participants included in the REDS-III Brazil SCD Cohort and subset of participants included in the clinical and genetic priapism analyses.
Table 1.
Sociodemographic characteristics of patients with and without history of priapism
| Demographic | No priapism (N = 1,126) n (%) | Priapism (N = 188) n (%) | Total | P value |
|---|---|---|---|---|
| Clinical site, Brazilian city | ||||
| Hemominas, Belo Horizonte | 276 (80) | 67 (20) | 343 | .0001 |
| Hemominas, Juiz de Fora | 113 (90) | 13 (10) | 126 | |
| Hemominas, Montes Claros | 170 (95) | 9 (5) | 179 | |
| Hemorio, Rio de Janeiro | 289 (84) | 54 (16) | 343 | |
| Hemope, Recife | 220 (84) | 41 (16) | 261 | |
| Institute of Childhood Cancer, Sao Paulo | 56 (93) | 4 (7) | 60 | |
| Ethnicity | ||||
| Caucasian | 129 (87) | 20 (13) | 148 | .18 |
| Black | 285 (82) | 62 (18) | 347 | |
| Mixed | 674 (87) | 101 (13) | 773 | |
| Other | 37 (88) | 5 (12) | 42 | |
| Income (reais*), ≥18 y old | ||||
| <700 | 181 (90) | 21 (10) | 201 | .19 |
| 701–1400 | 642 (86) | 107 (14) | 747 | |
| 1401–3000 | 220 (86) | 37 (14) | 257 | |
| ≥3000 | 49 (79) | 13 (21) | 62 | |
| Age (y) | ||||
| 0–9 | 360 (94) | 22 (6) | 382 | <.0001 |
| 10–17 | 334 (92) | 29 (8) | 363 | |
| 18–29 | 252 (76) | 79 (24) | 331 | |
| 30–41 | 113 (73) | 42 (27) | 155 | |
| 42+ | 66 (80) | 16 (20) | 82 | |
| Sickle cell disease genotype | ||||
| SS | 763 (82) | 168 (18) | 931 | <.0001 |
| SC | 286 (97) | 9 (3) | 295 | |
| Sβ0 | 36 (86) | 6 (14) | 42 | |
| Sβ+ | 37 (88) | 5 (12) | 42 | |
| Other† | 3 (100%) | 0 (0) | 3 |
Exchange rate in 2014: 1 BRL = 0.43 USD.
Includes 1 SD, 1 S/HPFH, and 1 S/K-Woolwich.
Figure 2.
Distribution of the age at first priapism occurrence among participants with priapism in the REDS III Brazil SCD cohort study.
Figure 3.
Time estimates for first priapism episode with Kaplan-Meier analysis method.
Clinical Variables Associated with the Occurrence of Priapism
Patients with a history of priapism were mainly HbSS patients (89.4% of the total number of cases and 18% of the total of HbSS males); a smaller number were HbSβ+ (3% of the total number of cases and 12% of the total HbSβ+ males) and HbSβ0 (3% of the total number of cases and 14% of the total HbSβ0 males). Patients with HbSC were proportionally rarer (5% of the total number of cases and 3% of the total of 295 HbSC males). There was no occurrence of priapism in the small number of patients with other SCD variants, such as SD, S/Hereditary Persistence of Fetal Hemoglobin, S/K-Woolwich, or HbS/Quebec-Chori genotypes. No association was found for biomarkers and priapism (Table 2). Pulmonary hypertension and avascular necrosis were significantly more frequent in the patients with priapism (15% and 19%, respectively) than in patients who never had priapism (9% and 11%, respectively) (Table 3).
Table 2.
Clinical characteristics of patients with priapism and patients matched on age and HBB genotype without history of priapism
| No priapism (N = 372) | Priapism (N = 186) | ||||
|---|---|---|---|---|---|
| Variable | Mean/median | Range | Mean/median | Range | P value |
| Reticulocyte (%) | 10.15/9.5 | 1–25.8 | 10.36/9.77 | 0.8–26.2 | .66 |
| Total bilirubin (mg/dL) | 1.76/1.35 | 0.12–15.21 | 1.75/1.37 | 0.13–12.37 | .86 |
| HbF (%) | 10.9/6.95 | 0.1–45 | 11.3/8.9 | 0.1–42 | .36 |
| Indirect bilirubin (mg/dL) | 1.2/0.87 | 0–12.11 | 1.26/0.87 | 0.02–11.29 | .73 |
| Leukocyte (/mm3) | 11,218/10,810 | 2,370–28,800 | 10,664.06/10,200 | 3,560–23,700 | .11 |
| Hemoglobin (g/dL) | 8.82/8.6 | 3.52–15.9 | 9.04/8.98 | 4.86–15.7 | .11 |
| Platelet count (103/mm3) | 397/387 | 82–999 | 396/373 | 89.8–930 | .94 |
Table 3.
Clinical complications of patients with priapism and patients matched on age and HBB genotype without history of priapism
| Complication | No priapism (N = 372) n (%) | Priapism (N = 186) n (%) | Total | P value |
|---|---|---|---|---|
| Pulmonary hypertension | 33 (9) | 28 (15) | 61 | .05 |
| Leg ulcers | 66 (18) | 31 (17) | 97 | .83 |
| Vaso-occlusive pain episodes | 348 (94) | 176 (95) | 524 | .84 |
| Acute chest syndrome | 262 (72) | 146 (80) | 408 | .07 |
| Avascular necrosis | 42 (11) | 36 (19) | 78 | .01 |
| Ischemic stroke | 39 (10) | 17 (9) | 56 | .74 |
Priapism Episodes Reported During the Follow-Up Time
During the follow-up period, 37 participants had priapism episodes; 13 of the 37 had priapism for the first time, and 24 of the 37 were patients with a history of priapism at enrollment had recurrent episodes in the follow-up period. Most of the patients had 1 episode (21 of 37, 57%) although 16 of the 37 participants had repeated episodes, with a range of 2 to 6 episodes. Episodes lasted from 1 to 6 hours, with the majority of the episodes lasting for 1 hour (16%) or 3 hours (17.5%), and a minority were of cases of stuttering priapism (13%). There were 18 episodes in which patients did not receive treatment (28.5%), although 81% of the episodes without treatment lasted only 1 or 2 hours and may have resolved prior to presentation for care. Among the treated episodes, the most common treatment was red blood cell transfusion (28.5%). The remainder were treated with various treatments and combinations of treatments (Table 4).
Table 4.
Priapism recurrences between visits
| Priapism episodes | n (%) |
|---|---|
| Number of episodes during follow-up | |
| 1 | 21 (57) |
| 2 | 11 (30) |
| 3 | 3 (8) |
| 4 | 0 (0) |
| 5 | 1 (2.5) |
| 6 | 1 (2.5) |
| Duration (h) | |
| 1 | 10 (16) |
| 2 | 6 (9.5) |
| 3 | 11 (17.5) |
| 4 | 2 (3) |
| 6 | 6 (9.5) |
| Unknown | 28 (44.5) |
| Stuttering priapism | |
| Yes | 8 (13) |
| No | 31 (49) |
| Unknown | 24 (38) |
| Treatment* | |
| Transfusion | 18 (28.5) |
| No treatment | 18 (28.5) |
| Cavernous aspiration | 11 (17.5) |
| Finasteride | 8 (13) |
| Analgesics | 8 (13) |
| Hydroxyurea | 1 (1.5) |
Most of the patients were treated with a combination of different treatments. Additionally, 3 patients were treated with undescribed medicine, 1 with hydration, and 1 with estrogen.
Genome-Wide Association Analysis
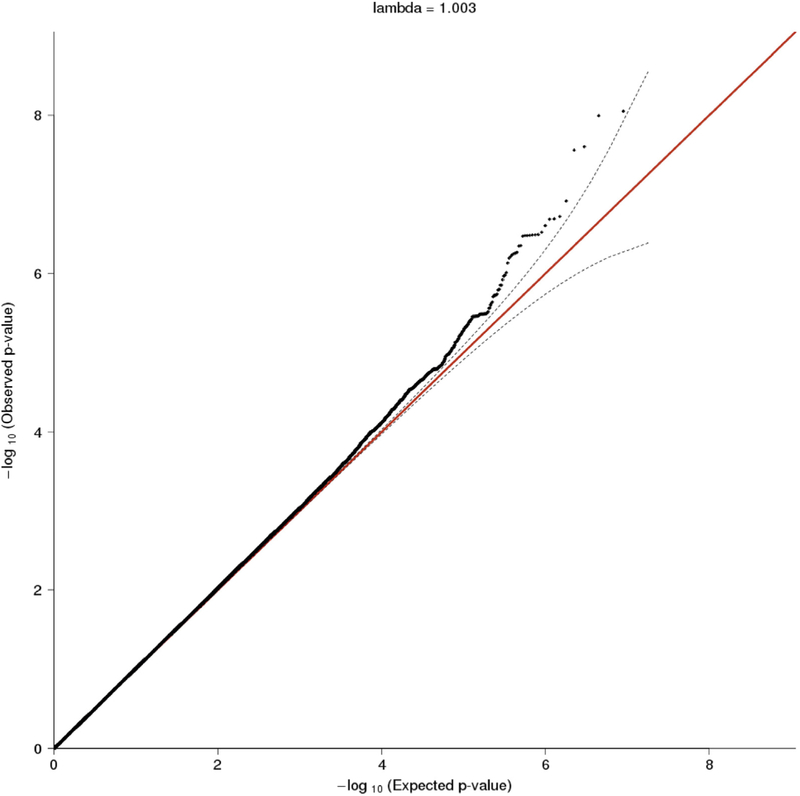
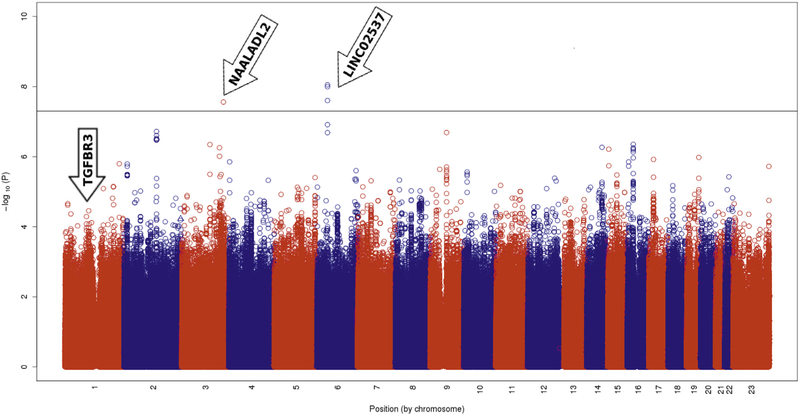
The GWAS included 169 HbSS, HbSβ0, or Hbβ+ male patients with a history of priapism as cases and 433 HbSS, HbSβ0, or Hbβ+ males older than 12 years without a history of priapism as controls (Figure 1). The analysis did not show any P value bias (λGC = 1.003), and the Q-Q plot (Figure 4) suggests that there was no genomic inflation or any additional confounding factors. Figure 5 shows the distribution of P values for the association of the SNPs and priapism, summarizing the results of the GWAS. SNP rs77635018 reached genome-wide significance (P < 5 × 10−8), and SNPs rs116116525, rs60503510, and rs190103771 reached P values approximately to the threshold of genome-wide significance (Table 5). The odds ratios show a protective effect of the minor alleles against priapism. SNP rs190103771 is located in an intronic region of chromosome 3q26.31, in the N-acetylated alpha-linked acidic dipeptidase like 2 (NAALADL2) gene (Figure 6). The 3 other SNPs are located on chromosome 6p21.1 in a nonintronic region that contains long intergenic non-protein coding RNA 2537 (LINC02537) (Table 5).
Figure 4.
Q-Q plot of the observed (y axis) versus expected (x axis) -log(P-values), REDS III SCD cohort study.
Figure 5.
Manhattan plot showing the genome wide −log 10 P-values plotted against the position on each chromosome showing the association of SNPs with priapism in the REDS-III Brazil SCD cohort study. The black horizontal line indicates genome wide significance at 5 × 10 −8.
Table 5.
SNPs associated with priapism with genome-wide significance (P < 10−8)
| Chromosome | SNP | Locus | P value | Odds ratio |
|---|---|---|---|---|
| 6 | rs77635018 | Long intergenic non-protein coding RNA 2537 (LINC02537) | 8.9 × 10−9 | 0.22 |
| 6 | rs116116525 | Long intergenic non-protein coding RNA 2537 (LINC02537) | 1 × 10−8 | 0.22 |
| 6 | rs60503510 | Long intergenic non-protein coding RNA 2537 (LINC02537) | 2.5 × 10−8 | 0.25 |
| 3 | rs190103771 | N-acetylated-alpha-linked acidic dipeptidase-like 2 (NAALADL2) | 2.8 × 10−8 | 0.3 |
Figure 6.
Regional plot on chromosomes 6, 3 and 1. Axis y represents the negative of the logarithm of Pvalues for the association of the SNPs with priapism. SNPs are represented as dots. Axis x shows the relative positions of the SNPs in this region of the chromosome, representing the recombination rate in centimorgans (cM) per megabase (Mb). Colors coding the level of linkage disequilibrium (measured by r2) of SNPs with A) rs77635018, B) rs190103771 and C) rs3103333 represented by a purple diamond.
A targeted analysis of previously reported SNPs associated with priapism in candidate gene studies using a less stringent threshold found rs3103333 to be nominally significant (P = 2 × 10−4; odds ratio = 0.56), suggesting some evidence of an association of priapism with the transforming growth factor beta receptor 3 (TGFBR3) gene. Other previously reported SNPs did not show P values < 10−3 and were not considered significant.
DISCUSSION
Priapism is a painful SCD complication with complex pathophysiology. This study evaluated the clinical and genetic predictors of priapism in a large cohort of Brazilian SCD patients. Older age, pulmonary hypertension, and avascular necrosis were associated with priapism. A GWAS replicated the association of 1 SNP in the TGFBR3 gene and identified 2 previously unreported genetic markers with unclear functional significance associated with priapism. In the present cohort, priapism occurred at a lower prevalence (24.2% in adults) than the 30–45% prevalence reported in other studies.2–5 Considering only patients younger than 18 years, the prevalence was 6.9%, which is similar to the reported prevalence of priapism in pediatric SCD populations (12%,26 6.9%,38 and 3.6%39).
HbSS patients represented 89% of the priapism cases, consistent with other reports that identified HbSS accounted for 81–87% of priapism cases.38,40 Although HbSS patients were at the highest risk of suffering from priapism, cases were also seen in HbSβ0 (14% of HbSβ0), HbSβ+ (12% of all HbSβ+), and HbSC (3% of all HbSC) patients. Although HbSβ+ is typically considered to exhibit a milder phenotype, there is significant heterogeneity based on the type of β-thalassemia mutation and resulting amount of normal hemoglobin A produced. In this cohort, many HbSβ+ participants were known to have more severe β+mutations, which could explain the higher prevalence of priapism in our HbSβ+ participants. Cases were rarer in HbSC patients, consistent with some studies (0 out of 10 HbSC patients5 and 3 out of 91 cases40), although a prevalence of 20% (16 out of 82 HbSC patients) was reported in one study of SC patients41 and 19% (5 out of 27 HbSC patients in the study) in another.42
Bilirubin levels were not associated with the occurrence of priapism, although the association with biomarkers of hemolysis has been previously reported.12,43 Priapism is assumed to be associated with hemolysis12,43,44 as a result of the impaired bioavailability of NO, which has an important role in penile erection. During hemolysis episodes, free hemoglobin consumes NO that would be used to convert guanosine triphosphate to cyclic guanosine monophosphate to induce vasodilatation; therefore, the balance is skewed toward vasoconstriction, favoring the occurrence of priapism.11
Priapism is postulated to be associated with pulmonary hypertension, stroke, and leg ulcers, with hemolysis as a biomarker for these complications.44 Although we replicated the finding of pulmonary hypertension, leg ulcers and stroke were not associated with priapism in our analysis. In addition, avascular necrosis, which has been postulated to be related to blood-viscosity and vaso-occlusion and therefore is more common in SCD patients with higher hemoglobin/lower hemolysis,11,42,45 was associated with priapism in this cohort.
Twenty-eight percent of the priapism episodes during the follow-up period were not treated. Some of these may have resolved prior to presenting for medical care, as 81% of the untreated episodes lasted for only 1 or 2 hours. Among the patients that were treated, the majority received conservative treatments, and urologic procedures were rarely performed, consistent with other literature reporting on priapism treatment.46 Even though intravenous hydration and oxygen supplementation are commonly applied to treat priapism, these treatments were not frequently described in the medical records and are not listed here. There was heterogeneity among treatments, reflecting the lack of consensus in standard treatment strategies for priapism in SCD.
Recent studies suggest that priapism is mainly associated with disruption of the endothelium-derived NO signaling and phosphodiesterase 5 (PDE5) pathways and an excess of adenosine, as well as disruption of the RhoA/ROCK pathway and opiorphin upregulation.13–22 Alterations in NO/PDE5 pathways would result in priapism, as the pathways play important roles in the erection control mechanisms.10,47,48 Adenosine is a potent vasodilator, and adenosine signaling is part of a regulatory control of signal transduction systems interacting with the PDE5 pathway.15,49–51 ROCK is a vasoconstrictor factor involved in endothelial NO synthase regulation, and the RhoA/ROCK pathway alters erectile function.20,21,52–54 Ophiorphins are pentapeptides associated with erectile function,55,56 and enhanced activation and expression of these peptides can cause smooth muscle relaxation.57 However, the genomic regions identified by our GWAS as significantly associated with priapism in SCD patients were related to different pathways.
We identified four SNPs significantly associated with priapism in LINC02537 and NAALADL2. LINC02537 is a long intergenic non-protein coding RNA with unknown function, previously associated with vascular endothelial growth factor phenotypic variance,58,59 a growth factor associated with prostate cancer.60–64 NAALADL2 is ubiquitously expressed in the prostate, and, although its function is unknown, it has been associated with Kawasaki disease, a pediatric, autoimmune vascular disease,65 as well as venous thromboembolism66 and prostate carcinoma.67 The disposition of NAALADL2 near genes involves vascular development and maintenance,68 suggesting a possible relationship with vascular pathways. Further studies are needed to determine the functional significance of LINC02537 and NAALADL2 in the occurrence of priapism.
In accordance with previous literature, our data showed that TGFBR3 was associated with priapism. TGFBR3 encodes a membrane proteoglycan expressed in endothelial cells that acts as a co-factor in inflammatory pathways. It is important in endothelial cell migration and transformation.69 TGFBR3 is also a member of the TGF-β receptor superfamily. TGF-β pathways have been correlated with risks of stroke,70 leg ulcers,71 bacteremia,72 and priapism.24 However, TGF-β pathways are also hypothesized to be related to SCD severity and survival73; therefore, the TGFBR3 polymorphism could be associated with disease severity in general rather than priapism pathophysiology specifically.
CONCLUSION
We described the burden of priapism and identified clinical factors associated with priapism development in a large cohort of Brazilian SCD patients. These findings extend our understanding of the risk factors associated with priapism in SCD and identify genetic markers to be investigated in future studies to further elucidate priapism pathophysiology and to be considered as drug targets for priapism treatment.
Funding:
This work was partly supported by Grant # NIH/NHLBI HHSN268201100007I. This work was conducted during a visiting scholar period at Vitalant Research Institute, sponsored by the Capes Foundation within the Ministry of Education, Brazil.
Footnotes
Conflict of Interest: None.
REFERENCES
- 1.Pauling L, Itano HA, Singer S, et al. Sickle cell anemia, a molecular disease. Science 1949;110:543–548. [DOI] [PubMed] [Google Scholar]
- 2.Adeyoju AB, Olujohungbe AB, Morris J, et al. Priapism in sickle-cell disease; incidence, risk factors and complications -an international multicentre study. BJU Int 2002;90:898–902. [DOI] [PubMed] [Google Scholar]
- 3.Bruno D, Wigfall DR, Zimmerman SA, et al. Genitourinary complications of sickle cell disease. J Urol 2001;166:803–811. [PubMed] [Google Scholar]
- 4.Emond AM, Holman R, Hayes RJ, et al. Priapism and impotence in homozygous sickle cell disease. Arch Intern Med 1980;140:1434–1437. [PubMed] [Google Scholar]
- 5.Fowler JE Jr, Koshy M, Strub M, et al. Priapism associated with the sickle cell hemoglobinopathies: prevalence, natural history and sequelae. J Urol 1991;145:65–68. [DOI] [PubMed] [Google Scholar]
- 6.Broderick GA. Priapism and sickle-cell anemia: diagnosis and nonsurgical therapy. J Sex Med 2012;9:88–103. [DOI] [PubMed] [Google Scholar]
- 7.Montague DK, Jarow J, Broderick GA, et al. American Urological Association guideline on the management of priapism. J Urol 2003;170:1318–1324. [DOI] [PubMed] [Google Scholar]
- 8.Burnett AL. Priapism pathophysiology: clues to prevention. Int J Impot Res 2003;15(Suppl 5):S80–S85. [DOI] [PubMed] [Google Scholar]
- 9.Kimmelsteil P. Vascular occlusion and ischemic infarction in sickle cell disease. Am J Med Sci 1948;216:11–19. [PubMed] [Google Scholar]
- 10.Champion HC, Bivalacqua TJ, Takimoto E, et al. Phosphodiesterase-5A dysregulation in penile erectile tissue is a mechanism of priapism. Proc Natl Acad Sci U S A 2005;102:1661–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kato GT, Gladwin MT, Steinberg MH. Deconstructing sickle cell disease: reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Rev 2007;21:37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nolan VG, Wyszynski DF, Farrer LA, et al. Hemolysis-associated priapism in sickle cell disease. Blood 2005;106:3264–3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bivalacqua TJ, Musicki B, Kutlu O, et al. New insights into the pathophysiology of sickle cell disease-associated priapism. J Sex Med 2012;9:79–87. [DOI] [PubMed] [Google Scholar]
- 14.Kanika ND, Tar M, Tong Y, et al. The mechanism of opiorphin-induced experimental priapism in rats involves activation of the polyamine synthetic pathway. Am J Physiol Cell Physiol 2009;297:C916–C927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mi T, Abbasi S, Zhang H, et al. Excess adenosine in murine penile erectile tissues contributes to priapism via A2B adenosine receptor signaling. J Clin Invest 2008;118:1491–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Claudino MA, Franco-Penteado CF, Corat MA, et al. Increased cavernosal relaxations in sickle cell mice priapism are associated with alterations in the NO-cGMP signaling pathway. J Sex Med 2009;6:2187–2196. [DOI] [PubMed] [Google Scholar]
- 17.Fu S, Tar MT, Melman A, et al. Opiorphin is a master regulator of the hypoxic response in corporal smooth muscle cells. FASEB J 2014;28:3633–3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ning C, Wen J, Zhang Y, et al. Excess adenosine A2B receptor signaling contributes to priapism through HIF-1α mediated reduction of PDE5 gene expression. FASEB J 2014;28:2725–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hurt KJ, Musicki B, Palese MA, et al. Akt-dependent phosphorylation of endothelial nitric-oxide synthase mediates penile erection. Proc Natl Acad Sci U S A 2002;99:4061–4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang H, Eto M, Steers WD, et al. RhoA-mediated Ca2+ sensitization in erectile function. J Biol Chem 2002; 277:30614–30621. [DOI] [PubMed] [Google Scholar]
- 21.Bivalacqua TJ, Champion HC, Usta MF, et al. RhoA/Rho-kinase suppresses endothelial nitric oxide synthase in the penis: a mechanism for diabetes-associated erectile dysfunction. Proc Natl Acad Sci U S A 2004;101:9121–9126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chitaley K, Weber D, Webb RC. RhoA/Rho-kinase, vascular changes, and hypertension. Curr Hypertens Rep 2001; 3:139–144. [DOI] [PubMed] [Google Scholar]
- 23.Nolan VG, Baldwin C, Ma Q, et al. Association of single nucleotide polymorphisms in klotho with priapism in sickle cell anaemia. Br J Haematol 2005;128:266–272. [DOI] [PubMed] [Google Scholar]
- 24.Elliott L, Ashley-Koch AE, De Castro L, et al. Genetic polymorphisms associated with priapism in sickle cell disease. Br J Haematol 2005;128:266–272. [DOI] [PubMed] [Google Scholar]
- 25.Kleinman S, Busch MP, Murphy EL, et al. The National Heart, Lung, and Blood Institute Recipient Epidemiology and Donor Evaluation Study (REDS-III): a research program striving to improve blood donor and transfusion recipient outcomes. Transfusion 2014;54:942–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carneiro-Proietti ABF, Kelly S, Miranda Teixeira C, et al. Clinical and genetic ancestry profile of a large multi-centre sickle cell disease cohort in Brazil. Br J Haematol 2018;182:895–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ballas SK, Lieff S, Benjamin LJ, et al. Definitions of the phenotypic manifestations of sickle cell disease. Am J Hematol 2010;85:6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo Y, Busch MP, Seielstad M, et al. Development and evaluation of a transfusion medicine genome wide genotyping array. Transfusion 2019;59:101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 2007;81:559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Delaneau O, Coulonges C, Zagury JF. Shape-IT: new rapid and accurate algorithm for haplotype inference. BMC Bioinformatics 2008;9:540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Howie BN, Donnelly P, Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet 2009; 5:e1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.1000 Genomes Project Consortium. Auton A, Brooks LD, et al. A global reference for human genetic variation. Nature 2015; 526:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Price AL, Patterson NJ, Plenge RM, et al. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet 2006;38:904–909. [DOI] [PubMed] [Google Scholar]
- 34.Zhou X, Stephens M. Efficient multivariate linear mixed model algorithms for genome-wide association studies. Nat Methods 2014;11:407–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ward LD, Kellis M. HaploReg: a resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic Acids Res 2012;40:D930–D934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pruim RJ, Welch RP, Sanna S, et al. LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics 2010;26:2336–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lloyd-Jones LR, Robinson MR, Yang J, et al. Transformation of summary statistics from linear mixed model association on all-or-none traits to odds ratio. Genetics 2018;208:1397–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tarry WF, Duckett JW Jr, Snyder HM 3rd. Urological complications of sickle cell disease in a pediatric population. J Urol 1987;138:592–594. [DOI] [PubMed] [Google Scholar]
- 39.Furtado PS, Costa MP, Ribeiro do Prado Valladares F, et al. The prevalence of priapism in children and adolescents with sickle cell disease in Brazil. Int J Hematol 2012;95:648–651. [DOI] [PubMed] [Google Scholar]
- 40.Sharpsteen JR Jr, Powars D, Johnson C, et al. Multisystem damage associated with tricorporal priapism in sickle cell disease. Am J Med 1993;94:289–295. [DOI] [PubMed] [Google Scholar]
- 41.Lionnet F, Hammoudi N, Stojanovic KS, et al. Hemoglobin sickle cell disease complications: a clinical study of 179 cases. Haematologica 2012;97:1136–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hamre MR, Harmon EP, Kirkpatrick DV, et al. Priapism as a complication of sickle cell disease. J Urol 1991;145:1–5. [DOI] [PubMed] [Google Scholar]
- 43.Cita KC, Brureau L, Lemonne N, et al. Men with sickle cell anemia and priapism exhibit increased hemolytic rate, decreased red blood cell deformability and increased red blood cell aggregate strength. PLoS One 2016;11:e0154866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kato GJ, McGowan V, Machado RF, et al. Lactate dehydrogenase as a biomarker of hemolysis-associated nitric oxide resistance, priapism, leg ulceration, pulmonary hypertension, and death in patients with sickle cell disease. Blood 2006; 107:2279–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ballas SK. Sickle cell anemia with few painful crises is characterized by decreased red cell deformability and increased number of dense cells. Am J Hematol 1991;36:122–130. [DOI] [PubMed] [Google Scholar]
- 46.Kalpatthi R, Lee BR, Woods GM, et al. Epidemiology, management and outcome of priapism in children and adolescents with sickle cell disease: a pediatric health information system database analysis. Blood 2013;122:1698. [Google Scholar]
- 47.Lin G, Xin ZC, Lue TF, et al. Up and down-regulation of phosphodiesterase-5 as related to tachyphylaxis and priapism. J Urol 2003;170:S15–S18; discussion S19. [DOI] [PubMed] [Google Scholar]
- 48.Corbin JD. Mechanisms of action of PDE5 inhibition in erectile dysfunction. Int J Impot Res 2004;16(Suppl 1):S4–S47. [DOI] [PubMed] [Google Scholar]
- 49.Christ GJ, Richards S, Winkler A. Integrative erectile biology: the role of signal transduction and cell-to-cell communication in coordinating corporal smooth muscle tone and penile erection. Int J Impot Res 1997;9:69–84. [DOI] [PubMed] [Google Scholar]
- 50.Prieto D. Physiological regulation of penile arteries and veins. Int J Impot Res 2008;20:17–29. [DOI] [PubMed] [Google Scholar]
- 51.Tostes RC, Giachini FR, Carneiro FS, et al. Determination of adenosine effects and adenosine receptors in murine corpus cavernosum. J Pharmacol Exp Ther 2007;322:678–685. [DOI] [PubMed] [Google Scholar]
- 52.Musicki B, Ross AE, Champion HC, et al. Posttranslational modification of constitutive nitric oxide synthase in the penis. J Androl 2009;30:352–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chitaley K, Wingard CJ, Clinton Webb R, et al. Antagonism of Rho-kinase stimulates rat penile erection via a nitric oxide-independent pathway. Nat Med 2001;7:119–122. [DOI] [PubMed] [Google Scholar]
- 54.Mills TM, Chitaley K, Wingard CJ, et al. Effect of Rho-kinase inhibition on vasoconstriction in the penile circulation. J Appl Physiol (1985) 2001;91:1269–1273. [DOI] [PubMed] [Google Scholar]
- 55.Davies KP. The role of opiorphins (endogenous neutral endopeptidase inhibitors) in urogenital smooth muscle biology. J Sex Med 2009;6(Suppl 3):286–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thanawala V, Kadam VJ, Ghosh R. Enkephalinase inhibitors: potential agents for the management of pain. Curr Drug Targets 2008;9:887–894. [DOI] [PubMed] [Google Scholar]
- 57.Tong Y, Tar M, Davelman F, et al. Variable coding sequence protein A1 as a marker for erectile dysfunction. BJU Int 2006; 98:396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang Y, Zhang F, Wang J, et al. lncRNA LOC100132354 promotes angiogenesis through VEGFA/VEGFR2 signaling pathway in lung adenocarcinoma. Cancer Manag Res 2018; 10:4257–4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Choi SH, Ruggiero D, Sorice R, et al. Six novel loci associated with circulating VEGF levels identified by a meta-analysis of genome-wide association studies. PLoS Genet 2016; 12:e1005874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fryczkowski M, Bułdak RJ, Hejmo T, et al. Circulating levels of omentin, leptin, VEGF, and HGF and their clinical relevance with PSA marker in prostate cancer. Dis Markers 2018; 2018:3852401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sfar S, Hassen E, Saad S, et al. Association of VEGF genetic polymorphisms with prostate carcinoma risk and clinical outcome. Cytokine 2006;35:21–28. [DOI] [PubMed] [Google Scholar]
- 62.Peyromaure M, Camparo P, Badoual C, et al. The expression of vascular endothelial growth factor is associated with the risk of cancer progression after radical prostatectomy. BJU Int 2007;99:1150–1153. [DOI] [PubMed] [Google Scholar]
- 63.Green MM, Hiley CT, Shanks JH, et al. Expression of vascular endothelial growth factor (VEGF) in locally invasive prostate cancer is prognostic for radiotherapy outcome. Int J Radiat Oncol Biol Phys 2007;67:84–90. [DOI] [PubMed] [Google Scholar]
- 64.Ye Y, Li SL, Wang SY. Construction and analysis of mRNA, miRNA, lncRNA, and TF regulatory networks reveal the key genes associated with prostate cancer. PLoS One 2018; 13:e0198055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Burgner D, Davila S, Breunis WB, et al. A genome-wide association study identifies novel and functionally related susceptibility loci for Kawasaki disease. PLoS Genet 2009; 5:e1000319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Greliche N, Germain M, Lambert JC, et al. A genome-wide search for common SNP x SNP interactions on the risk of venous thrombosis. BMC Med Genet 2013;14:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Whitaker HC, Shiong LL, Kay JD, et al. N-acetyl-L-aspartyl-L-glutamate peptidase-like 2 is overexpressed in cancer and promotes a pro-migratory and pro-metastatic phenotype. Oncogene 2014;33:5274–5287. [DOI] [PubMed] [Google Scholar]
- 68.Berndt SI, Wang Z, Yeager M, et al. Two susceptibility loci identified for prostate cancer aggressiveness. Nat Commun 2015;6:6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brown CB, Boyer AS, Runyan RB, et al. Requirement of type III TGF-β receptor for endocardial cell transformation in the heart. Science 1999;283:2080–2082. [DOI] [PubMed] [Google Scholar]
- 70.Sebastiani P, Ramoni MF, Nolan V, et al. Genetic dissection and prognostic modeling of overt stroke in sickle cell anemia. Nat Genet 2005;37:435–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nolan VG, Adewoye A, Baldwin C, et al. Sickle cell leg ulcers: associations with haemolysis and SNPs in Klotho, TEK and genes of the TGF-beta/BMP pathway. Br J Haematol 2006; 133:570–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Adewoye AH, Nolan VG, Ma Q, et al. Association of polymorphisms of IGF1R and genes in the transforming growth factor-beta/bone morphogenetic protein pathway with bacteremia in sickle cell anemia. Clin Infect Dis 2006;43:593–598. [DOI] [PubMed] [Google Scholar]
- 73.Ashley-Koch AE, De Castro LM, Lennon-Graham F, et al. Clinical and genetic profiles of the aging sickle cell patient. Blood 2005;106:26A. [Google Scholar]