Abstract
Significance: Wound healing is a complex and dynamic series of events influenced by a variety of intrinsic and extrinsic factors. Problematic wounds, particularly chronic wounds and pathologic scars, remain clinically significant burdens. Modeling physiologic and aberrant wound repair processes using in vitro or in vivo models have contributed to Advances in Wound Care (AWC); however, the fidelity of each model used, particularly with respect to its species-specific limitations, must be taken into account for extrapolation to human patients. Twenty-five years of wound healing models published in Wound Repair and Regeneration (1993–2017) and AWC (2012–2017) were collected and analyzed to determine trends in species utilization and models used.
Recent Advances: In 25 years, 1,521 original research articles utilizing one or more wound models were published (total of 1,665 models). Although 20 different species were used over the course of 25 years, 5 species were most commonly utilized: human, mouse, rat, pig, and rabbit. In vivo modeling was used most frequently, followed by in vitro, ex vivo, and in silico modeling of wound healing processes.
Critical Issues: A comparison of articles from 1993 to 1997 and 2013 to 2017 periods showed notable differences in model and species usage. Experiments utilizing mouse and human models increased, while the usage of pig models remained constant, rabbit and rat models declined in the more recent time period examined compared to the time period two decades before.
Future Directions: This analysis shows notable changes in types of models and species used over time which may be attributed to new knowledge, techniques, technology, and/or reagents. Explorations into mechanisms of limb regeneration and wound healing of noncutaneous tissues have also contributed to a shift in modeling over time. Changes within the journals (i.e., page expansion and increased rejection rates), research funding, and model expense may also influence the observed shifts.
Keywords: wound healing, model, animal, review, repair, regeneration
Laura K.S. Parnell, MSc, CWS.

Susan W. Volk, VMD, PhD, Dipl, ACVS.

Scope and Significance
Twenty-five years of wound healing models published in Wound Repair and Regeneration (WRR; 1993–2017) and Advances in Wound Care (AWC; 2012–2017) were collected and reviewed. These two journals are official peer-reviewed journals of several international wound healing societies that are specifically focused on advancing wound healing outcomes and represent leading wound research. Information about the species and models utilized in studies was analyzed throughout the entire 25-year time period and by comparison of the first and last 5- and 10-year periods to highlight changes in use between the two and a half decades. A review of published wound healing models subdivided by model type has not been previously undertaken.
Translational Relevance
During the 25-year review period (WRR 1993–2017 and AWC 2012–2017), 1,665 wound models were used to investigate various aspects of wound healing or regeneration. The most common species used to model physiologic and pathologic cutaneous wound healing and tissue repair processes were human, mouse, rat, pig, and rabbit. Despite the fact that pig models have been shown to have higher correlation to human healing,1 their usage has remained relatively constant over time in cutaneous wound healing studies. In vivo models were the most common model type used followed by in vitro models (ex vivo and in silico models comprised <3% of total models). Notably, research publications using more than one type of modeling or multiple species has increased in recent years. There were far fewer articles focused on regenerative wound healing (i.e., digits, limbs) compared to cutaneous wound healing (i.e., skin, tissues), but this area showed the biggest shift in species model usage over time.
Clinical Relevance
This analysis showed notable changes in wound modeling refinement, specifically the types of models and species utilized, over time. A marked increase in the number of in vitro and in vivo studies involving human tissue or human subjects from early to late time points underscores this trend, including articles which have investigated vulnerary efficacy of clinical drugs or devices previously tested in animal models. This important change may help validate experimental findings in nonhuman species and translational efficiency in the future.
Background
Greek and Shanks2 proposed nine categories by which animals are used in scientific research. In the field of wound healing, eight of the categories are used on a regular basis, but the predictive modeling of wound healing is the most common (Table 1). Unfortunately, animal models of cutaneous healing are fair at best in predicting response in humans. It is disappointing that the results from studies utilizing commonly used wound healing models, in vitro and small animal models, only show 57% and 53% concordance, respectively, with human study outcomes, while the less commonly used pig model was noted to exhibit the highest level (78%) in the study.1 Given the fact that the translational usefulness of models exhibiting a predictive value of less than 80% has been questioned,3 it is key that researchers must (1) determine which model is the most appropriate to use in a given study, (2) interpret results within the context of the limitations of the model used, and (3) refine existing and develop new models to optimize our understanding of physiologic and pathologic processes as well as translational efficiency.
Table 1.
Nine categories of why animals are use in science and research as determined by Greek and Shanks,2 with wound healing examples
| Category | Wound Healing Example |
|---|---|
| Used as predictive models of humans for research into diseases | In vivo animal model to show wound healing. |
| Used as predictive models of humans for testing drugs or other chemicals | In vivo animal model to show affect of growth factor in wound healing. |
| Tissues used as medical treatment | Harvesting porcine small intestine submucosa for wound device. |
| Used as bioreactors or factories | Production of monoclonal antibodies. |
| Animals and animal tissues are used to study basic physiological principles | In vivo and in vitro model to show timeline of cellular influx. |
| Used as a modality for ideas or as a heuristic device, which is a component of basic science research | Development of a specific animal model. |
| Used in research to gain knowledge for knowledge sake | Discovery of specific gene and protein sequences associated with healing. |
| Used in research designed to benefit other animals of the same species or breed | Differences in cutaneous skin closure or propensity for scar formation between species or breed. |
| Used to educate and train students and to teach basic principles of anatomy | Teaching differences in cutaneous anatomy such as dermis and panniculus carnosus. |
Pragmatically, despite the drive to use models with the greatest fidelity, funding agencies often favor reductionist/mechanistic approaches and thus drive the use of in vitro and genetically modified small animal models.4 Also, the availability of knock out/knock in rodents provide diverse genetic variants to interrogate research hypotheses, ease of handling, access, and lower expense compared to large animal models make rodent models popular. However, rodents heal to a greater extent by contraction compared to tight-skinned mammals (i.e., human, pig) or high tension areas (i.e., human extremities and sternum and the distal extremities of animals). In addition, significant discrepancies have been noted in the immune system and inflammatory response to injury between rodent models and humans.5–8 Given that the initial inflammatory response postwounding directs subsequent wound healing responses, the genetic differences in mounting and maintaining a response must be considered.6,9–11 The potential confounding factors of both genetic and evolutionary differences between species used in modeling and humans have been considered a limit of their predictive value.3 As such, the incorporation of human tissue validation at early stages of research, complementary to experimental models, has been advised to limit late stage translational failures.12 Developing models such as humanized mice eliminate the original immune system and replace it with engrafted functional human cells and tissues, thereby minimizing the immunological differences between the species.13–17 Engraftment of individuals' personal tissue into these humanized small animal models have the potential not only to direct a personalized medicine approach to healing but also to explore wound healing in a new way. Although these sophisticated humanized models have other limitations that should be taken into account, the attempt to address the immunological differences between species better refines these potential wound models.
Unlike rodent models, pig wound healing models have several major unique advantages, including shared anatomical, physiological, and functional similarities of pig skin with human skin.1 In fact, porcine skin xenografts have been used temporarily when autografts were unavailable in human burn survivors.18 Yet, surprisingly, only a small percentage of studies utilize pig models in wound healing studies. Expense, difficulty of handling due to size, need for special housing, and operation and anesthesia requirements may limit the accessibility of pig models for many researchers. Despite the limited use of this model compared to rodent models in wound healing research, the contribution of the pig model to advances in human wound healing knowledge and clinical applications should not be underestimated.1,18,19
Physiologic (acute) wound healing is a dynamic process with a multitude of intrinsic and extrinsic factors documented to impact rate and quality (i.e., functionality and cosmesis) of repair. By comparison, the pathologic responses that lead to chronic wounds and pathologic scarring are even more complex. This complexity is difficult to fully capture and replicate in animal models, in part, due to the lack of superimposed comorbidities or the influence of unique genetic makeup between species. Determining the best model to utilize depends largely not only on the hypothesis to be tested but also on the validity, fidelity, and extrapolation. The best models balance the isolated phenomenon, single out variables, and yet possess the required fidelity to address the complexity of healing. Despite limitations of animal models, incorporating knowledge of these limitations into thoughtful interpretation of data promotes advancing knowledge of the wound repair process, in part or in whole. The purpose of this review is to assess how wound healing models have changed over time, not to determine the best wound model. Reviewing the published literature may provide more insight into the directions of wound healing research and the models used therein.
Discussion
From 1945 to 2017, 128,343 “wound healing” articles were identified in PubMed, review articles were excluded. More specifically using additional qualifiers, 46,479 studies were identified using the search terms “wound healing animal,” 43,832 with “wound animal model,” and 10,972 with “wound healing animal model.” The journals WRR and AWC were selected to assess wound models because both peer-reviewed journals are official journals of several international societies focused on advancing wound healing outcomes through original peer-reviewed research. Data were taken from the published abstracts and from the associated materials and methods section of the article when required for further clarification. In 25 years, 1,521 original research articles were published collectively; reviews were excluded. Over the course of the 25-year period in these 2 journals, 12 mammalian and 8 nonmammalian models were used (Table 2). Several studies used multiple models for a total of 1,665 wound models utilized. In addition to species information that was collected, the type of model used (in vivo, in vitro, ex vivo, and in silico) was also analyzed for all 1,665 models by year. Of the 1,665 models, 74% were in vivo, 23% were in vitro, <2% were ex vivo, and 0.66% were in silico.
Table 2.
Categories of species used and published as models in Wound Repair and Regeneration and Advances in Wound Care
| Category | Species | WRR | AWC |
|---|---|---|---|
| Bacterial | Bacteria | 4 | |
| Regenerative | Starfish | 1 | |
| Newt | 4 | ||
| Holothurians | 1 | ||
| Frog | 4 | ||
| Zebrafish | 7 | ||
| Salamander | 6 | ||
| Fowl | Chicken | 5 | |
| Rodent | Hamster | 1 | |
| Guinea pig | 14 | ||
| Rat | 273 | 7 | |
| Mouse | 327 | 26 | |
| Ruminants | Goat | 1 | |
| Cow | 4 | ||
| Sheep | 8 | ||
| Other mammals | Dog | 7 | |
| Horse | 9 | ||
| Rabbit | 73 | ||
| Pig | 116 | 6 | |
| Human | 729 | 32 | |
| Total | 20 | 1,594 | 71 |
All species were published in WRR.
AWC, Advances in Wound Care; WRR, Wound Repair and Regeneration.
Articles from WRR covered a 25-year period of 1993–2017. There were 1,950 published articles, of which 1,460 (75% of total) original research articles used 1,594 models in 20 species. Of the 1,594 models, 1,518 were in 5 species; mouse, pig, rabbit, rat, or human (>95% of models). Articles from AWC covered a 6-year period of 2012–2017, but only one original research article with an animal model was published in 2012. The one original article published in 2012 was included in the 2008–2012 period and was excluded in the 2013–2017 period. There were a total 220 published articles, of which there were 159 review articles and 61 (28% of total) original research articles that described 71 wound models. The 61 research articles published in AWC during this 6-year period used only 4 species; mouse, pig, rat, and human.
Model overview
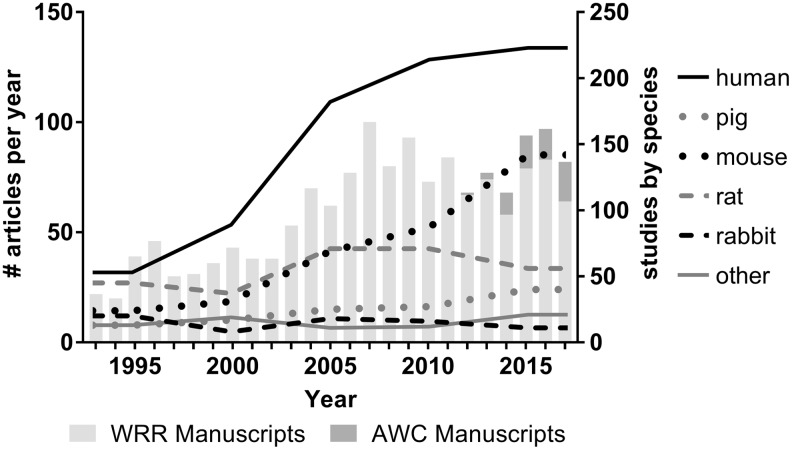
Compared to the first decade of the time period analyzed (1993–2002) when 343 original articles were published in WRR, 760 articles were published in the next decade of analysis (WRR 2003–2012 and 1 article in AWC 2012; Fig. 1). This increase in publication of original research articles, relative to review articles, coincided with a new agreement between WRR and the publisher and allowed for more articles to be accepted (pers. comm.). As noted above, similarly, the number of original research articles published post-2012 has also increased for both WRR and AWC. Considering the use of individual model species in both journals over the 25-year period, publication of articles utilizing the human and mouse models have also increased over time. In contrast, use of rabbit models have fluctuated over time but has declined overall from the beginning to the end of the period examined (during 1993–1997, 20 rabbit models were published compared to 11 during 2013–2017; a 45% decrease). The pig model showed the same percentage (8%) between time periods, but a continuous increase in pig models was also noted (increase from 13 in 1993–1997 to 34 in 2013–2017). Unlike other models, rat model use initially increased and then steadily declined over the last 10 years resulting in similar absolute numbers for early and later years even though the number of WRR articles had increased.
Figure 1.
Twenty-five years of 1,521 article publications and shifts in 1,665 animal models in WRR and AWC. The 5-year model average is located at each median year of the average. A graph line runs from 1993 to 2017 and connects each average for each species. AWC, Advances in Wound Care; WRR, Wound Repair and Regeneration.
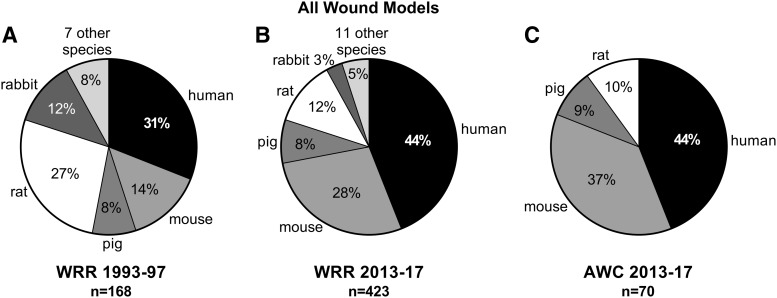
A comparison of species-specific differences in animal models used during the first and last 5-year periods of the WRR publications highlights these changes and revealed an increase in the use of human and mouse models from 31% to 45% and from 14% to 28%, respectively, of total models (Fig. 2). While the proportion of pig models remained consistent over time, the use of the rat and rabbit models, as well as the use of alternate “other” species declined between these two periods. Comparing species usage in published articles in WRR and AWC during the last 5-year period revealed nearly identical distribution of usage of human, pig, and rat models. The proportion of mouse models was higher in AWC compared to WRR, but no rabbit or “other species” models were published. Although the percentage of studies using nontraditional (i.e., not human, rodent, rabbit or pig, models) declined between the two periods examined, the diversity of these species increased over time (Fig. 2). More studies that incorporated in vivo and in vitro models and/or multiple species in tandem were noted in the later time period. In total, over the 25-year period in WRR, there were 120 articles published that utilized at least 2 models of the wound healing process. While there were only 11 articles out of 157 (7%) that utilized multiple models per publication in the early years (WRR 1993–1997), 65 of 358 publications (18%) used a multiple modeling approach in the later period (WRR 2013–2017).
Figure 2.
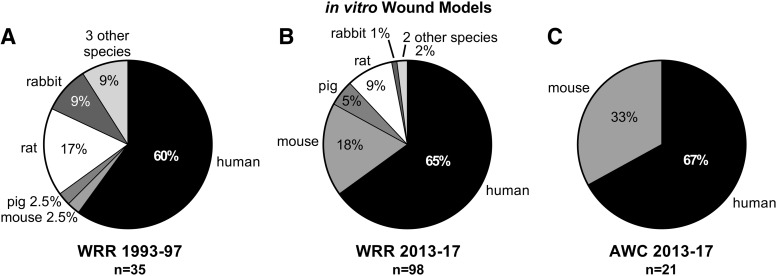
Comparison of all wound models published in WRR 1993–1997 (A), WRR 2013–2017 (B), and in AWC 2013–2017 (C).
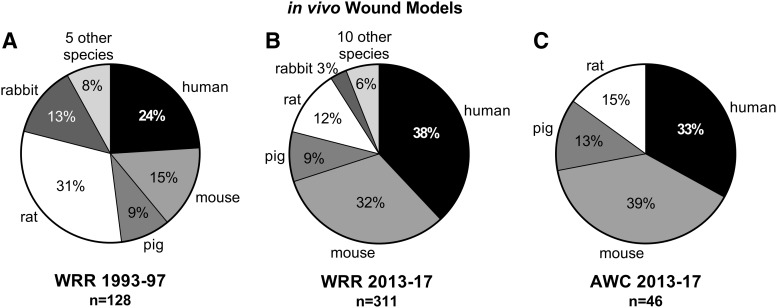
In vivo
Consistent with trends for species use in all studies published in WRR between the first 5-year period and the last 5-year period, more recent articles have increasingly incorporated mouse and human in vivo models into studies (Fig. 3). In fact, the percent of studies utilizing the mouse for in vivo wound healing studies has more than doubled (15% of 128 models vs. 32% of 311 models) between these two time periods. In contrast, performing studies in vivo using either the rat or rabbit decreased between early and later periods. The percent rat model usage dropped by more than half (31% of 128 models vs. 13% of 311 models), while use of the rabbit model fell to a proportionately greater extent (13% of 128 models vs. 3% of 311 models). The percentage of pig models remained consistent at 9% over time. Comparison of articles published in WRR and AWC during 2013–2017 showed human, mouse, pig, and rat in vivo models were used with similar percentages despite differences in numbers of original research articles published by each journal, 358 and 60, respectively.
Figure 3.
Comparison of in vivo wound models published in WRR 1993–1997 (A), WRR 2013–2017 (B), and in AWC 2013–2017 (C).
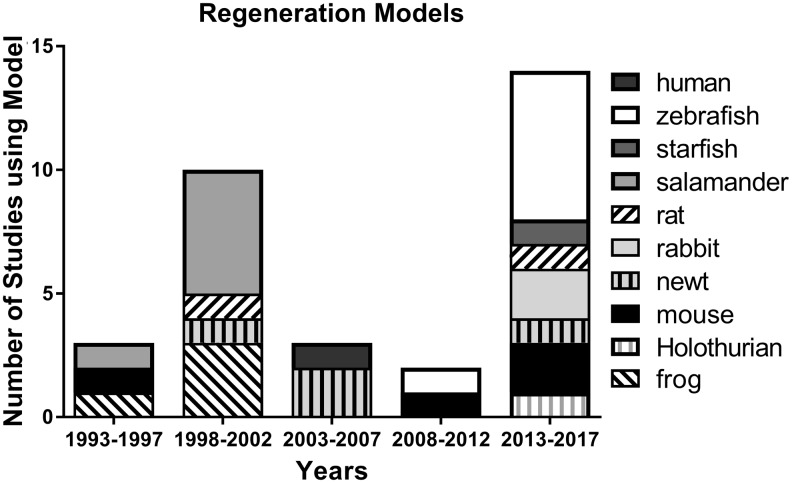
Regeneration models showed a clear shift in models over time (Fig. 4). The number of articles focusing on the process of regenerative wound healing (n = 32) published in WRR were less than 2.5% of those by volume compared to articles focusing on reparative processes (n = 1,428). While salamanders and frogs were utilized in 10 of the 13 studies modeling tissue regeneration in early years (1993–2000), these species were not used in any of the 19 studies examining this process from 2001 to 2017 published in WRR. Zebrafish were the most common species utilized in the last 5-year period (6 of 14 models). Over the 25-year period, the diversity of the 10 species used to explore regeneration models included Holothurians, newt and starfish in later years. Notably, the vast majority of regeneration models were performed in vivo (84%) with only five in vitro models (one frog, two salamander, one rabbit, and one human tissues) published.
Figure 4.
Changes in species used for wound healing models of regeneration from 1992 to 2017 published in WRR.
In vitro
In vitro models were the second most popular model type used over the 25-year period. In vitro experiments most commonly utilized human cells, 60% of all studies between 1993 and 1997 and up to 65% between 2013 and 2017 (Fig. 5). In the early years (WRR 1993–1997), 2.5% of in vitro studies incorporated murine cells, however, incorporation of tissues and cells from mice in experiments in the late period (WRR 2013–2017) made the mouse the second most commonly used in vitro model (18% of all in vitro studies utilized murine cells). Although in vitro models using rat cells were the second most common in vitro model in the early time period, the percent use of rat in vitro models declined by half in the later period (17% down to 9%), following trends for the overall decline in rat use for in vivo studies. Rabbit in vitro models followed the same pattern, dropping from 9% in the early years to 1% in the later years. Pig in vitro models comprised a small percentage of the total in vitro assays performed, with 2.5% in early years and 5% in later years. A comparison of the percentages of human in vitro models published in WRR and AWC during 2013 and 2017 showed that they were similar (65% vs. 67%, respectively). Although the proportion of human in vitro models between the two journals was similar, the distribution of nonhuman species in in vitro studies published was striking. While WRR publications included five nonhuman mammalian (mouse, rat, pig, rabbit, and horse) and bacterial models during 2013–2017, in vitro studies utilizing nonhuman cells in AWC publications was limited to the mouse.
Figure 5.
Comparison of in vitro wound models published in WRR 1993–1997 (A), WRR 2013–2017 (B), and in AWC 2013–2017 (C).
Ex vivo and in silico
Ex vivo models, typically cultured tissues, which attempt to recreate some of the complexity of in vivo tissues outside the body, were first published in WRR and AWC in 1995 and 2014, respectively. The ex vivo model type was used much less frequently than in vivo and in vitro models, with only 29 WRR and 2 AWC publications over 25 years. The first 13 years of WRR had 4 mouse and 3 human ex vivo models whereas the last 12 years had 1 mouse, 1 rat, 3 pig, and 17 human models. Likewise, in silico models are relatively new in wound healing articles and the first one was published in WRR in 2001 and 2017 in AWC with only 10 and 1 publications in each of the respective 2 journals.
Model comparisons in literature
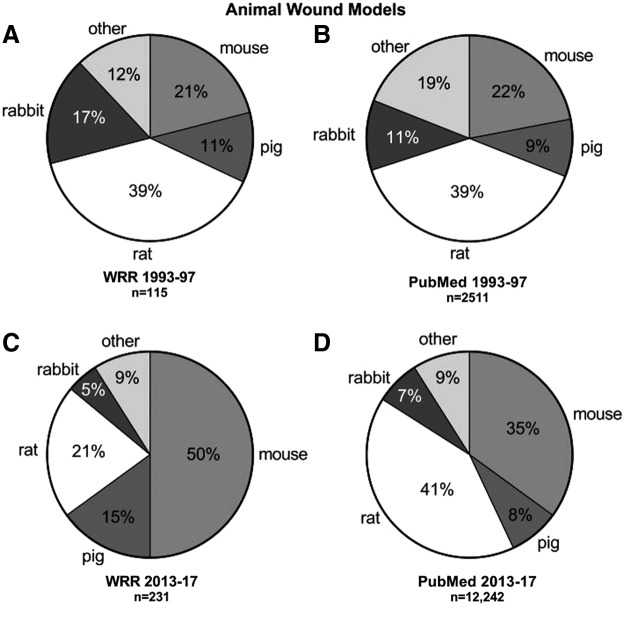
To assess the preclinical animal models published in WRR compared to other publications, the percentage of “animal model wound” excluding humans published in PubMed and in WRR were compared (Fig. 6). The early years (1993–1997) between WRR and PubMed publications (Fig. 6A, B) were similar in percent usage with the exception of rabbit models and “other species” models. WRR had 17% rabbit and 12% “other species” models, whereas PubMed publications for the same years had 11% rabbit and 19% “other” models. The percent usage in later years (2013–2017), however, was markedly different (Fig. 6C, D). PubMed publications were slightly different from the early years with modest growth in the use of rat (41%) and mouse (35%) models. During this time in WRR, 50% of studies utilized mice, while only 21% of studies used rat models. The percentage of studies utilizing pig models in later year WRR articles (15%) was higher than in PubMed (8%) in the same years and from WRR 1993–1997 years (11%). Rabbit and “other species” model usage were similar between WRR and PubMed for 2013–2017.
Figure 6.
Comparison of nonhuman wound models published in WRR 1993–1997 (A) and 2013–2017 (C), and in PubMed 1993–1997 (B) and 2013–2017 (D).
Summary
During the review process, models were compiled, but were not assessed for effectiveness, accuracy, or concordance of predictive wound healing. The prevalence of a model or species should not be construed as an indication of its effectiveness, but rather a look at how usage of models have evolved over the past 25 years. Scope limitations of this initial review precluded a more in depth analysis of critical features that refine models further, such as: the type of injury (i.e., incisional, excisional, burn, etc), the presence of infection (i.e., sepsis, biofilm, polymicrobial, etc), age of the animal subject, immunocompetency, or other potential cohorts. The scientist utilizing animal models is encouraged to model as many aspects of wound and patient specific factors as possible to improve model fidelity in their studies. Published models from WRR and AWC articles were not assessed categorically by acute versus chronic wound models. Individual humans and companion animals that exhibit chronic wounds have unique intrinsic genetics and are exposed to diverse environments (i.e., nutrition, smoking, obesity, and so on) that impact their health that cannot be modeled in a laboratory setting. Outside of human chronic wound studies and a limited number of veterinary publications, in vivo models typically utilize young, healthy animals in clean, uniform environments, which are incapable of developing a chronic wound or the associated comorbidities. In fact, although many animal model studies may classify themselves as chronic wound models, in reality, the vast majority of these models are more appropriately labeled as impaired. Current wound models have yet to be able to model a chronic wound and the milieu of factors contributing to its chronicity in a consistent and reproducible manner, although newly published models have been developed that incorporate many features of chronic wounds that should increase our understanding about pathology in these wounds and their response to treatments.20 The lack of a replicable chronic wound model that represents the range of pathologies that exist in patients emphasizes the need to cross-validate in vivo wound models with chronic wound patient studies to refine these or new models for better translational research outcomes.12
In 25 years of wound healing research, animal models utilizing 20 different species have been published in WRR and AWC. Models using mice, pigs, rats, and humans, continue to dominate in vivo and in vitro wound healing experiments, even after 25 years. As the wound models were refined, the types of models and species used have shifted over time. A difference in species utilized as models in WRR was seen when compared over the years and more so with the 5-year time endpoints (1993–1997 vs. 2013–1017). Exploration into limb regeneration and noncutaneous wound healing also contributed to a shift in species of animal models used, even though regeneration models make up a small proportion of WRR published wound models. Regeneration research showed the clearest shift in animal model usage during the 25-year period. Initially amphibians were the regeneration models of choice and now there have been 10 species, including rodents and mammals, investigated. This research area will likely continue to expand. Likewise, ex vivo and in silico models currently only make up <3% of wound models published in WRR and AWC, but future usage of these model types will probably increase as new techniques and knowledge are discovered.
Wound healing is a complex system and wound model selection is heavily dependent on the hypothesis being tested. The validity, fidelity, and extrapolation of each species should also be taken into consideration during the selection process. Some factors, such as lacking species-specific reagents and model expense can pressure model selection toward a model with less fidelity, but one in which the hypothesis can be fully tested. For example, pig models have been shown to have higher correlation to human healing,1 but the model expense, housing requirements and lack of comparative reagents can limit study parameters if this model is pursued. This may result in revision of the hypothesis being tested or may drive selection of species that are less expensive and “easier” to work with. The availability of genetically modified mice has provided significant contributions by defining the impact of specific gene pathways on wound healing. Likewise, the use of CRISPR technology has opened new research avenues to more easily edit the genome of larger animals to allow this technology to leverage benefits of novel models in those species.21–23 Over time, the expense of these new technologies has decreased and more researchers are able to incorporate these approaches with their areas of expertise thereby contributing to shifts in both in vitro and in vivo modeling. The mouse, however, does remain the mainstay for first-line exploratory research and to test mechanisms critical to the wound healing process. Once these have been established, researchers have the premise to translate into a large animal model with potentially higher fidelity to human wound healing. This may explain, in part, why the porcine model usage has not grown more during the 25-year period. Both rat and rabbit model use declined, despite the growth of reagents available for these species and the relative inexpensiveness compared to the pig model.
Studies incorporating in vitro models made up 21% of all models published in the early time period (WRR 1993–1997) and more recently 23% of all models (WRR 2013–2017). The percentage change in published in vitro species models between the comparison time periods belie the striking changes of percent between species with the exception of the human and pig models (Fig. 5). In vitro model usage changed from rat and rabbit models comprising 27% in the early years to rat and rabbit comprising only 10% in later years. The mouse in vitro models were almost the complete inverse of the rat and rabbit models. In the early years, only 2.5% of the models were in vitro mouse and then the usage jumped to 18% in later years. Numerous advantages of the mouse model can explain the sharp increase in usage. The introduction of humanized and genetically modified species, especially modified mouse models, has enabled wound healing discovery like never before. These altered models can provide and have provided more unique in vivo and in vitro data and knowledge about wound healing and associated pathways that were previously unattainable. The rationale to use these models in the discovery phase is sound and provides a better understanding of the cellular and molecular mechanisms involved in wound healing. These models also provide a premise for subsequent experiments using models with more fidelity to further interrogate the hypothesis. It should be noted that in vitro models lack the complexity of the in vivo wound such as the dynamic influence of an immune system, other systemic influences or local three-dimensional influences which are critical to the wound healing process and thus are limited in their modeling capacity. Utilization of in vitro models in conjunction with immunocompetent models provides a more complete modeling of the healing process. Use of multiple model types and species in such a manner should allow for more successful translational research in the future.
The animal model role humans play in developing the wound healing knowledge base should not be overlooked.4 As shown in Fig. 2B, wound researchers publishing in WRR (2013–2017) use human subjects or use of human tissue samples in 45% of studies. During the same time, humans accounted for only 22% of PubMed wound models (data not shown), far fewer than rat or mouse models. Interestingly, distribution of animal models published in PubMed during early and later time periods are similar although there is a modest increase in mouse usage. In contrast, distribution of animal models published in WRR reveal a switch from a preference for rats (39% of models used from 1993 to 1997) over mice (21%) to a much heavier reliance on mouse models (50% of nonhuman models) compared to rats (only 21% from 2013 to 2017). Notably, in later years, pig models in the specialty journal WRR accounted for nearly twice the usage compared to articles published in all journals in PubMed (15% vs. 8%) (Fig. 6C, D). The increase in mouse and pig models in WRR and AWC compared to PubMed journals may indicate more models are being used for discovery and fidelity purposes. Journals that are not dedicated to wound research found in PubMed may not have adapted to the changes in animal models as those that are published in WRR and AWC. Wound healing research is as complex as the wounds being studied and researchers must take this into account. The predictive concordance rate of wound healing models should improve as strides are made to decrease confounding genetic and evolutionary factors and increase extrapolation. The responsibility of contributing appropriate and high quality, rigorous data to the knowledge base rests with wound researchers. This reiterates the point made at the 2012 Wound Healing Society symposium on wound models, that “As leaders in the field of wound healing, we must hold our peers accountable for producing research that is valid for the human or veterinary condition if that is the clinical endpoint.”12
Although not commonly discussed, changes in journal logistics, such as number of issues and pages can influence what models are published and disseminated. When WRR increased the journal pages, the average number of published articles more than doubled the 31.4 per year average in the first 5 years to 71.6 per year average in the last 5 years. Author tendency to publish more than one model per article also increased from an average of 33.2 per year in the first 5 years to 84.6 per year in the last 5 years. Likewise, funding sources can also influence hypothesis development and model usage.4 The push for translational research in the last decade by federal funding agencies has led to a shift from solely basic, applied and clinical research to a more multidirectional, translational style which can be interpreted as encouraging multiple model usage.24 Nonetheless, selecting accurate wound healing models that correlate well to the target population to prove the hypothesis is important. In the field of wound healing, WRR and AWC journals can continue to disseminate and influence the most appropriate animal models to use.
Take-Home Messages.
Standard and innovative wound models in 20 species were published from 1993 to 2017 for a total of 1,665 models in 2 prestigious wound healing research journals.
As wound modeling was refined over time, types of models and species shifted.
Models using mice, pigs, rats, and humans, dominate cutaneous wound healing studies over the last 25 years.
Exploration into limb regeneration and wound healing of noncutaneous tissues has contributed to the shift in species of models utilized in tissue repair research.
Compared to published animal wound model literature in PubMed, use of the pig as model is more common in cutaneous wound healing research published in WRR and AWC.
Vast differences in animal wound model usage between WRR and AWC and PubMed are seen in the percentage of human, mouse, rat, and pig models used. The increasing number of human studies in WRR and AWC present a unique opportunity in the future to reassess the concordance of particular nonhuman models with translatability in wound healing.
Changes in wound models observed can be attributed to new knowledge, techniques, and reagents. To some extent, funding sources and changes in journal logistics (i.e., number of pages, issues, and percent rejection rates) can influence which articles are ultimately published.
Selecting and executing wound healing models with high fidelity, combining multiple model systems that correlate well to target clinical population and validating research findings in human tissues remains important in translatability.
Acknowledgments and Funding Sources
The authors thank Becky K. Brisson, PhD for her assistance in preparing the article figures. No outside funding was used in the research or preparation of this article.
Abbreviations and Acronyms
- AWC
Advances in Wound Care
- CRISPR
Clustered Regularly Interspaced Short Palindromic Repeats
- WRR
Wound Repair and Regeneration
Author Disclosure and Ghostwriting Statement
Author L.K.S.P. is the current president of the Wound Healing Foundation. All views in this article are the author's and not the official position of the Wound Healing Foundation. No competing financial interests exist for either author. The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article.
About the Authors
Laura K.S. Parnell, MSc, CWS, is the current president of the Wound Healing Foundation (woundhealingfoundation.org). She serves on the Board of Directors for both the Foundation and the Society. She founded Precision Consulting in 1998 and specializes in wound healing and burn research. Laura designs research protocols, develops scientific niche products and provides scientific knowledge, medical writing and regulatory background based on client needs and budget. She has an international clientele and works extensively with industry representatives and clinicians on research investigations. Her personal goal is to see wound healing and associated problems be solved in more innovative and creative ways.
Susan W. Volk, VMD, PhD, Dipl, ACVS, is an Associate Professor of Small Animal Surgery at the University of Pennsylvania School of Veterinary Medicine. As a clinician-scientist, her clinical interest in wound healing complements her basic and translational research program focused on the role of the extracellular matrix in modulating cell activities and fate during tissue repair and regeneration. Recent work has defined cell-matrix interactions that regulate the wound healing-fibrosis-cancer triad. Dr. Volk has served on the Board of Directors of both the Wound Healing Society and the North American Veterinary Regenerative Medical Association.
References
- 1. Sullivan TP, Eaglstein WH, Davis SC, Mertz P. The pig as a model for human wound healing. Wound Repair Regen 2001;9:66–76 [DOI] [PubMed] [Google Scholar]
- 2. Greek R, Shanks N, eds. FAQs About the Use of Animals in Science: A Handbook for the Scientifically Perplexed, 1st ed. Lanham: University Press of America, 2009 [Google Scholar]
- 3. Greek R, Menache A. Systematic reviews of animal models. Int J Med Sci 2013;10:206–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pastar I, Wong LL, Egger AN, Tomic-Canic M. Descriptive versus mechanistic scientific approach to study wound healing and its inhibition: is there a value of translational research involving human subjects? Exp Dermatol 2018;27:551–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A 2013:110:3507–3512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wagar L, DiFazio RM, Davis MM. Advanced model systems and tools for basic and translational human immunology. Genome Med 2018;10:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Drake AC. Of mice and men: what rodent models don't tell us. Cell Mol Immunol 2013;10:284–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fingleton B. MMPS as therapeutic targets—still a viable option? Semin Cell Dev Biol 2008;19:61–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ariffin JK, Kapetanovic R, Schaale K, Gatica-Andrades M, Blumenthal A, Schroder K, et al. The E3 ubiquitin ligae RNF144B is LPS-inducible in human, but not mouse, macrophages and pronmotes inducible IL-1β expression. J Leukoctye Biol 2016;100:155–161 [DOI] [PubMed] [Google Scholar]
- 10. Martinez FO, Helming L, Milde R, Varin A, Melgert BN, Draijer C, et al. Genetic programs expressed in resting and IL-4 alternatively activiated mouse and human macrophages: similarities and differences. Blood 2013;121:e57–e69 [DOI] [PubMed] [Google Scholar]
- 11. Jones CN, Hoang AH, Martel JM, Dimisko L, Mikkola A, Inoue Y, et al. Technical advance: microfluidic assay for precise measurements of mouse, rat, and human neutrophil chemtaxis in whole-blood droplets. J Leukoctye Biol 2016;100:241–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gordillo GM, Bernatchez SF, Diegelmann , Di Pietro LA, Eriksson E, Hinz B, et al. Preclinical models of wound healing: is man the model? Proceedings of the Wound Healing Society symposium. Adv Wound Care (New Rochelle) 2013;2:1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schultz LD, Brehm MA, Garcia JV, Griener DL. Humanized mice for immune system investigation: progress, promise and challenges. Nat Rev Immunol 2012;12:786–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Walsh N, Kenney L, Jangalwe S, Aryee KE, Greiner DL, Brehm MA, et al. Humanized mouse models of clinical disease. Annu Rev Pathol 2017;12:187–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang HM, Choi JJ, Kim HN, Yang SJ, Park SJ, Kang C, et al. Reconstituting human cutaneous regeneration in humanized mice under endothelial cell therapy. J Invest Derm 2018 [DOI] [PubMed] [Google Scholar]
- 16. Martinez-Santamaria L, Conti CJ, Llames S, Garcia E, Retamosa L, Holquin A, et al. The regenerative potential of fibroblasts in a new diabetes-induced delayed humanised wound healing model. Exp Dermatol 2013;22:195–201 [DOI] [PubMed] [Google Scholar]
- 17. Baird A, Deng C, Eliceiri MH, Haghi F, Dang X, Coimbra R, et al. Mice engrafted with human hematopoietic stem cells support a human myeloid cell inflammatory response in vivo. Wound Repair Regen 2016;24:1004–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yamamoto T, Iwase H, King TW, Hara H, Cooper DKC. Skin xenotransplantation: historical review and clinical potential. Burns 2018;44:1738–1749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Meyer W, Scharz R, Neurand K. The skin of domestic mammals as a model for the human skin with special reference to the domestic pig. Curr Probl Dermatol 1978;7:39–52 [DOI] [PubMed] [Google Scholar]
- 20. Dhall S, Do DC, Garcia M, Kim J, Mirebrahim SH, Lyubovitsky J, et al. Generating and reversing chronic wounds in diabetic mice by manipulating wound redox parameters. J Diabetes Res 2014;2014:562625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shrock E, Güell M. CRISPR in animals and animal models. Prog Mol Biol Transl Sci 2017;152:95–114 [DOI] [PubMed] [Google Scholar]
- 22. Sun S, Xiao J, Huo J, Geng Z, Ma K, Sun X, et al. Targeting ectodysplasin promotor by CRISPR/dCas9-effector effectively induces the reprogramming of human bone marrow-derived mesenchymal stem cells into sweat gland-like cells. Stem Cell Res Ther 2018;9:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sessions JW, Armstrong DG, Hope S, Jensen BD. A review of genetic engineering biotechnologies for enhanced chronic wound healing. Exp Dermatol 2017;26:179–185 [DOI] [PubMed] [Google Scholar]
- 24. Rubio DM, Schoenbaum EE, Lee LS, Schteingart DE, Marantz PR, Anderson KE, et al. Defining translational research: implications for training. Acad Med 2010;85:470–475 [DOI] [PMC free article] [PubMed] [Google Scholar]