Abstract
Significance: Keloids are benign fibro-proliferative raised dermal lesions that spread beyond the original borders of the wound, continue to grow, rarely regress, and are the most common in pigmented individuals after an abnormal wound healing response. The current treatment failure and respective challenges involved highlighting the underlying issue that the etiopathogenesis of keloids is still not well understood. Disease models are required to better understand the disease pathogenesis. It is not possible to establish keloids in animals because of the uniqueness of this disease to human skin. To address this challenge, along these lines, non-animal reproducible models are vital in investigating molecular mechanisms of keloid pathogenesis and therapeutics development.
Recent Advances: Various non-animal models have been developed to better understand the molecular mechanisms involved in keloid scarring and aid in identifying and evaluating the therapeutic potential of novel drug candidates. In this scenario, the current review aims at describing in vitro monocultures, co-cultures, organotypic cultures, and ex vivo whole skin keloid tissue organ culture models.
Critical Issues and Future Directions: Current treatment options for keloids are far from securing a cure or preventing disease recurrence. Identifying universally accepted effective therapy for keloids has been hampered by the absence of appropriate disease model systems. Animal models do not accurately mimic the disease, thus non-animal model systems are pivotal in keloid research. The use of these models is essential not only for a better understanding of disease biology but also for identifying and evaluating novel drug targets.
Keywords: keloid scarring and/or disease, scar models, in vitro, organotypic, ex vivo, organ culture
Ardeshir Bayat, MBBS, PhD.

Scope and Significance
Keloids are benign dermal exophytic outgrowths that can occur after cutaneous dermal injury. The lesions enlarge, do not regress, and spread beyond the confines of the original wound margins. Their prevalence rate varies from 4.5% to 16% across populations with darkly pigmented skin.1 Keloids are unique to humans,2,3 presenting a challenge to study the pathogenesis of this disease and developing novel therapeutic strategies. Therefore, developing appropriate experimental models that can faithfully represent the disease is an urgent requirement.
This review entails current and emerging research on functional testing of keloids in both in vitro and ex vivo models of keloid disease. The use of these models is not only essential to provide in-depth knowledge on disease pathogenesis but also helpful in identifying novel drug targets for its treatment and prevention of recurrence.
Translational Relevance
There are various pharmacological treatment modalities4 that are available to treat keloids; however, currently available therapies do not eradicate and cannot prevent disease recurrence. The pathophysiology of keloid scars remains ill defined, which further pose a challenge for the development of novel therapeutic strategies. Besides, keloid research is hampered by a lack of relevant animal models, as keloids do not occur in animals. Therefore, developing non-animal models for functional evaluation of keloid cells and tissue to improve our understanding of keloid pathobiology and response to putative candidate therapies is essential. The aim here is to describe current and emerging in vitro and ex vivo models employed in keloid research to date.
Clinical Relevance
Keloids can result in significant disfigurement, pruritus, inflammation, and pain. Clinically, keloids have been described as firm, raised, and exophytic lesions after any form of injury to the skin. Histological findings have described keloids with large and excessive hyalinized bundles of collagen.5 Various therapeutic modalities have been attempted for the eradication of keloid scar.6,7 However, a single effective treatment modality has yet to be established for the treatment of keloids. Unfortunately, the cure of keloids remains a therapeutic challenge,8 which further highlights current knowledge deficit in the underlying molecular mechanism of keloid development.
Hence, further research is of critical importance to identify new methods that are essential to explore the pathogenesis of keloids and to test new therapeutics.
Background Information
The term “Keloid” originated in the 17th century; it was first used by Alibert (1806),9 a French dermatologist, who initially used the term “Cheloid” derived from the Greek word chele, or crab's claw, to describe the extension of tissue beyond the original wound boundaries.10 Keloids are benign, dermal fibroproliferative tumors, characterized by raised reticular dermal lesions that extend beyond the boundaries of the original margins of the wound after any cutaneous dermal injury.
The histopathological description of keloids is represented by an expanded reticular dermis with abnormally thick hyalinized collagen fibers.11,12 Keloids are often pruritic, painful, in addition to their high recurrence rate after treatment; have a significant psychological impact; and affect a patient's quality of life.
Despite being recognized as one of the most challenging clinical problems in abnormal wound healing, the pathogenesis of this common fibroproliferative disease is still unknown and has not received enough attention or, indeed, been given adequate research funding. To date, the proposed theories for describing keloids include the following: (1) elevated expression of various growth factors and cytokines; (2) increased expression of extracellular components via cross-talks between keratinocytes and fibroblasts; (3) hypoxia; (4) abnormally stimulated angiogenic responses; (5) changes in collagen turnover; (6) reduced apoptosis of fibroblasts; (7) genetic predisposition; and (8) immune dysfunction.13–15 However, none of these theories has been proved to date or provides sufficient evidence of the proposed mechanisms involved in keloid etiopathogenesis.
Therefore, further research is critically needed to better understand the molecular mechanisms underlying the aberrant activity of keloids, which may further pave the way toward the identification of new targets for future therapeutic interventions.
To date, models that have been utilized in keloid research are mostly based on in vitro cell culture and animal models. Various animal models for wound healing and fibrosis in general (since there is no known specific animal model for keloids) have previously been extensively reviewed elsewhere.16–18 Such animal models are best suited to pharmacological treatments, because biochemical and histological changes can be observed before and after the treatment, and these results can further provide substantial information to set up clinical trials in humans.19
Animal models can also provide valuable information on keloid pathophysiology and treatment possibilities. However, the results obtained from animal models could also be misleading, because human keloid tissue after implantation is not in its in vivo environment and so offers limited viability; therefore, long-term effects of therapeutic modalities are difficult to assess. Besides, animal models do not represent a replica of the wound-healing scenario in human skin, especially in the case of keloids, which is unique to humans.20,21 Thus, keloid research is hindered by the absence of a relevant animal model.
Further, non-animal models have been employed in keloid research to identify potential drug targets, to evaluate the efficacy of various treatment modalities, as well as to identify potential hallmarks of dermal fibrosis in keloids.
This article describes a comprehensive review of currently available non-animal models such as in vitro monolayer cultures, co-cultures, and ex vivo models.
Discussion of Findings and Relevant Literature
The findings of the relevant literature will be discussed under the following headings.
In vitro models
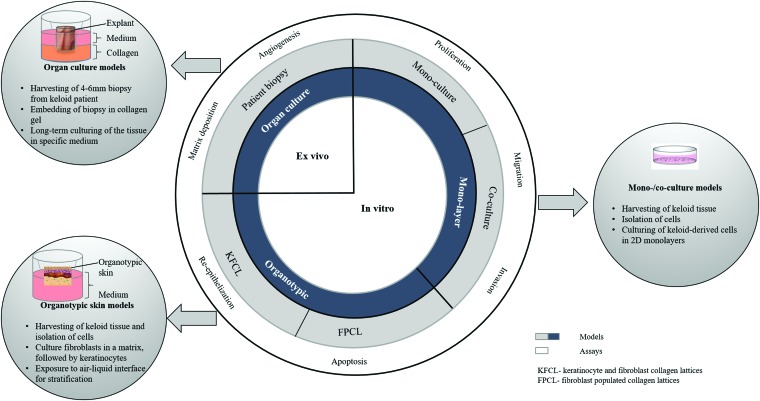
Numerous in vitro models have been used to determine the mechanisms of keloid formation and have played a pivotal role in understanding the process of wound healing and scar devlopment.22 Three categories of in vitro models include the following: Two-dimensional (2D) monolayer cultures, co-cultures (2D), and three-dimensional (3D) models have all been utilized to address different aspects of keloid pathogenesis (Fig. 1).
Figure 1.
In vitro, 2D and 3D skin models as well as ex vivo (whole skin organ culture) models of keloid. The 2D models comprise mono-/co-cultures models, whereas the 3D organotypic models include FPCL and full-thickness KFCL. The last model is the whole skin organ culture model derived from patient biopsy (3–6 mm punch). In vitro and ex vivo assays for keloid; proliferation, migration, invasion, apoptosis, angiogenesis, re-epithelization, matrix deposition, and remodeling (collagen contraction) are also described. 2D, two-dimensional; 3D, three-dimensional; FPCL, fibroblast-populated collagen lattices; KFCL, keratinocyte-fibroblast collagen lattices. To see this illustration in color, the reader is referred to the web version of this article. Color images are available online.
Two-dimensional monolayer culture models
Single-cell models provide a framework that not only permits the explicit incorporation of distinct properties of individual cells but will also enable all cells to work together as a collective unit.23 The design of monolayer culture cell models starts with harvesting of keloid-derived cells such as fibroblasts and keratinocyte cells and growing them two-dimensionally in a culture dish or flask. Conventional investigation of keloids has been based on the research of keloid-derived fibroblasts in 2D culture conditions.24,25 Since the 1970s, fibroblast monolayers have been utilized to elucidate keloid biology.
Diegelman et al. demonstrated the self-governing capacity of keloid fibroblasts (KFs) shown to synthesize collagen at significantly high levels in vitro.26 Later, Abergel et al. employed in vitro fibroblast monolayer cultures to propose two potential mechanisms for collagen accumulation in keloid lesions.11 Their first finding was that there was a markedly high production of extracellular matrix (ECM) with collagen I as the main component, in the keloid lesions. They corroborated previous findings, as they showed that their results were in line with previous observations of dysregulation of collagen production at the transcription level.
The data also suggested that decreased degradation of newly synthesized procollagen polypeptides might play a role in excessive collagen deposition. The ratio of collagen type I to collagen type III was also significantly higher in keloid lesions.
Calderon et al. designed a study to examine the in vitro mitogenic response of keloid-derived fibroblasts to wounds. They developed an in vitro wound model to compare the proliferative ability of wounded KFs versus wounded normal fibroblasts. In vitro wounds were created in confluent keloid and normal fibroblasts, followed by labeling with 3H-thymidine at various time intervals post-wounding to measure DNA synthesis. The wounded KFs demonstrated a statistically significant greater labeling index as quantified by autoradiography than wounded normal fibroblasts. Their work supported the hypothesis that keloid-derived fibroblasts have a significantly higher proliferation rate in response to wounding in vitro than normal fibroblasts.27
The intensity of ECM deposition by hyperactive uncontrolled proliferative keloid fibroblasts is the hallmark of keloid tissue formation, and the immune system has been found to also play a role in this process. A significant role of the immune system in keloid pathogenesis has been demonstrated in various studies.28–30 An association of fibroblasts with various immune cells has been observed to be enhanced in the abnormal scar.31 A type of cell interaction known as “cell talk” exists between fibroblasts and various immune cells31 (lymphocytes, macrophages, and mast cells). This cell interaction has been considered to be responsible for excessive fibrotic deposition in an abnormal scar or other fibrotic lesions.32,33
The presence of enhanced immune-regulatory cytokines production may also explain net unrestrained collagen deposition by keloid fibroblasts, thus proportional to the severity of the keloid disease lesions.34 There is evidence that T lymphocytes may play an important regulatory role in wound healing and scar formation in humans.30 T helper type 1 (Th-1) immune responses are enhanced in normal wound healing. However, Th-2 is widely linked with immune regulation and suppression, shown to be upregulated in keloid formation.35,36 Interferon (IFN)-γ and interleukin (IL)-2, which are secreted by Th-1 lymphocytes, have been shown to inhibit fibroblasts proliferation; whereas IL-4, IL-5, IL-10, and IL-13, which are secreted by Th-2 lymphocytes, have strongly been linked to fibrogenesis.35,36 In addition to lymphocytes, Mast cells have been shown to enhance scar formation and may mediate the transition from scarless to fibrotic healing during fetal development.37 One other overexpressed immune molecule in keloid scar tissue is syndecan-1 (CD138), identified as one of the potential biomarkers for keloid disease diagnosis.38
Several in vitro studies have explored the stimulation of certain cytokines on KFs in monolayer cultures.39,40 For instance, connective tissue growth factor (CTGF), was demonstrated to be involved in intracellular signaling pathways in KFs.41 It was found that CTGF could stimulate proliferation, migration, and accumulation of ECM produced by KFs. Further, it was also revealed that adiponectin suppresses' CTGF-induced KFs proliferation, migration, and ECM production with resultant attenuation of CTGF-induced phosphorylation of adipoR1 (adiponectin receptor 1), adenosine monophosphate-activated protein kinase (AMPK) mitogen activated protein kinase (p38), and extracellular-regulated kinase (ERK) signaling pathways.41
Many studies have performed experiments utilizing monolayer culture models to evaluate the potential of various chemotherapeutic drugs (5-fluorouracil, mitomycin C, bleomycin, corticosteroids) for keloid treatment with the hypothesis that these drugs work by inhibiting cell mitosis and, consequently, may induce suppression of keloid fibroblasts.42 In addition to chemotherapeutic drugs, numerous pharmacological agents such as tamoxifen citrate (synthetic anti-estrogen),43 camptothecin44 (topoisomerase I inhibitor), and dehydroxymethylepoxyquinomicin (DHMEQ; NF-kappaB inhibitor)45 are also currently being investigated in KFs for the management of keloid formation.
Aberrant wound healing could be partly because of the aberrant activity of transforming growth factor (TGF-β1) beta, which is a key cytokine in the process of tissue repair.46 Mikulec et al. evaluated the effects of tamoxifen on TGF-β production on both fetal and KFs in serum-free media. There was a progressive consistent decrease in TGF-β production with the increasing dose of tamoxifen, helping to explain the clinical benefits of tamoxifen for keloid treatment.43
A considerable higher deposition of collagen has also been considered as a hallmark of keloid formation. Therefore, Zhang et al. conducted a study to evaluate the inhibitory effect of topoisomerase I inhibitor (camptothecin) on collagen synthesis of activated dermal KFs.44 Cellular toxicity was determined with the MTT assay, and expression of collagen I and collagen II was studied at transcriptional and post-transcriptional level. A dose-dependent decrease in the production of collagen was observed with camptothecin treatment in KFs. Their data provided a piece of evidence about the inhibitory action of camptothecin on collagen synthesis of KFs, suggesting it as an effective treatment for keloid patients. However, to investigate specific molecular mechanisms, further studies are required.44
Activation of nuclear factor-kappa B has also been implicated in keloid pathogenesis.47 Proinflammatory cytokines such as Interleukin 1 and tumor necrosis factor-alpha are stimulated by the NF-kappa B pathway; therefore, blockage of the NF-Kappa B pathway is also considered an attractive option for the treatment of keloids. Therefore, Makino et al. set up a study to investigate the potential of NF-kappa B inhibitor (DHMEQ) on the activity of KFs. DHMEQ could significantly reduce type I collagen accumulation in KFs. Their results support the hypothesis that inhibitors of NG-kappa B pathways could be useful as therapeutic agents for keloid treatment.45
Recently, tremendous research efforts have also been taken to elucidate the underlying mechanism of few antifibrotic agents such as metformin,48,49 simvastin,50 and sorafenib,51 along with some natural compounds such as ginsenoside Rg352 and aspidin PB53 while employing monolayer culture models, wherein these compounds were evaluated and have shown promising results in the effective inhibition of proliferation, migration, cellular invasion, and ECM accumulation of KFs with blockade of TGF-β/Smad51,52 (SMAD; mothers against decapentaplegic homolog); PI-3K/Akt/FoxO152 (phosphoinositide-3-kinase/protein kinase B/forkhead box O1); and MAPK/ERK signaling pathways51 (p38/ERK).
Yi and Weiyuan investigated the effect of metformin on proliferation, collagen synthesis, and phosphorylation of the Akt/FoxO1 signal transduction pathway in primary KFs.48 KFs were divided into two groups: control group (media treatment) and experimental group (treated with metformin). Viability assay was performed to study the effect of metformin on the growth of KFs; whereas Akt/FoxO1 phosphorylation was detected with western blot and Hydroxyproline reagent kit was used to study collagen synthesis. It was concluded in their study that metformin can effectively suppress proliferation and collagen synthesis of KFs in a dose-dependent manner, which could also be associated with inhibition of the Akt/FoxO1 pathway.
Further, Sato et al. elucidated that metformin, an MAPK activator, inhibits TGF-β signaling through NADPH oxidase 4 (NOX4) suppression.49 In another study, the inhibitory potential of simvastatin on TGF-β1-induced production of alpha-smooth muscle actin (α-SMA), type I collagen, and CTGF in human keloid fibroblasts was investigated.50 Cell viability assay, western blot assay, and Rho activation assay were performed on primary KFs. It was demonstrated in their study that simvastatin treatment inhibited TGF-β1-induced production of collagen, CTGF, and α-SMA in a dose-dependent manner.50
A substantial inhibition of proliferation, migration, invasion, and ECM deposition was observed with sorafenib treatment on KFs in an in vitro monoculture model.51 Sorafenib could effectively inhibit the expression of inflammatory cytokines and the suppression of angiogenesis, suggesting that sorafenib could be an attractive therapeutic modality for the treatment of keloids in clinical settings.51
In a search for active natural resources that may play a role in the development of better preventive and treatment approaches, Song et al. explored the regulatory effect of aspidin PB (phloroglucinol derivative Dryopteris fragrans (L) Schott) on TGF-β-induced expression of type I collagen, CTGF, and α-SMA in KFs.53 Cell viability assay and flow cytometric analysis were performed to investigate the effect of aspidin on cell growth and apoptosis, respectively. Western blotting was used to detect the expression of type I collagen, CTGF, and α-SMA in TGF-β1-induced KFs. It was revealed in their study that aspidin PB inhibited TGF-β1 induced expression of type I collagen, CTGF, and α-SMA via PI-3K/Akt and Smad signaling pathways.
The result of their study provides substantial evidence supporting the potential therapeutic use of aspidin PB in the prevention of keloid formation.53
To identify more improved therapeutic approaches, Tang et al. aimed at investigating the effects of ginosenoside Rg3 on human KFs.52 Ginosenoside has been acknowledged as a biologically active component Panax ginseng.54 In vitro, cultured KFs were treated with various concentrations of ginsenoside and their viability was determined by using cell counting kit-8 assay. Further, the flowcytometric analysis was performed to measure the rate of apoptosis.52 The effect of ginsenoside on migration and invasion abilities of KFs was evaluated by using scratch wound assay and transwell invasion assay, respectively. To detect the expression levels of types I and III collagen, fibronectin, α-SMA, CTGF, IFN-γ, and TGF-β3 in Rg3-treated KFs, qPCR, western blot analysis, immunofluorescence, and immunohistochemical analysis were performed.52
This study has concluded that ginsenoside could inhibit proliferation, angiogenesis, and collagen synthesis of KFs. Also, results of scratch wound assay and transwell invasion assay have demonstrated that migratory and invasive capabilities of KFs were decreased with treatment of ginsenoside. These results have provided substantial evidence to suggest that Rg3 may be considered a potential therapeutic agent used to suppress keloid formation.52
The studies mentioned earlier have indicated that metformin,48,49 simvastatin50 and sorafenib,51 along with some natural compounds such as ginsenoside Rg352 and aspidin PB,53 are attractive candidates for the treatment of keloids. However, it is imperative to conduct further in vivo studies and clinical trials to confirm safe, effective, and long-lasting response in clinical use.
Despite the plethora of advocated treatment options described to date, a single effective treatment is still not available to cure keloid disease patients and prevent recurrence of keloid lesion. Huang et al. elucidated the molecular-based evidence for the potential synergy implicated in combinational therapy of triamcinolone and 5-fluorouracil.55 A significantly greater inhibition in cell proliferation and collagen1 synthesis induced cell cycle arrest and downregulated expression of vascular endothelial growth factor (VEGF) in KFs with this combined therapy.
A combinatorial approach was also utilized to evaluate the use of glucocorticoids: dexamethasone, triamcinolone, and methylprednisolone in KFs by using in vitro monolayer culture models.56 This strategy was successful in inducing a significant inhibition of proliferation, migration, and invasion properties along with the differential reduction of anti-fibrotic markers in KFs.
In vitro monolayer cultures, being simple and cost-effective, provided a platform for high-throughput preclinical screening of novel therapeutics, and therefore they are a widely accepted choice in pharmaceutical industries and academic laboratories for first-round determination of lead compounds.57 These models can also be employed to establish a dose range for a drug to achieve therapeutic efficacy among humans.19
There are certain limitations of utilizing fibroblast monolayer cultures to decipher disease biology because of the heterogeneous population of fibroblasts, and such heterogeneity remains conserved over multiple divisions.58,59 Heterogeneity in keloid disease has been demonstrated in literature,60,61 and extensive differences in behavior and phenotype were observed between cells derived from different regions of keloids. Therefore, the characteristics and behavior of fibroblasts in culture will depend on the selection of the respective population at the time when the culture was initiated, which further leads to irreproducible results.
In addition to this, 2D monolayer culture models do not completely mimic cellular and substrate interactions as well as cell signaling mechanisms. Cell signaling mechanisms here refer to the molecular events activated within cells to mediate growth, proliferation, differentiation, and survival. Cell signaling is communication and response of cells to the environment. When cells are removed from the tissue and grown in 2D culture conditions, both their morphology and mode of division changed. This change in cell morphology can alter their function and cell–cell, cell–environment interactions. In addition, there is only one cell type present in monolayer models; therefore, they cannot truly represent the underlying cell interactions and mechanisms in the microenvironment of the skin, resulting in their restricted application in cell-specific responses.
Therefore, various strategies based on monolayer culture models with one cell type may have had challenges in achieving long-term success, because other cells such as keratinocytes, in addition to fibroblasts, are also driving keloid pathology.62 The secretory role of keratinocytes is pivotal regarding its influence on keloid fibroblast activity plus growth kinetics, and which is further affected by the presence of keratinocytes in the epidermis.24,63
Two-dimensional co-culture models
Recently, studies have deciphered the critical role of epithelial–mesenchymal transition (EMT). EMT is a biological process characterized by loss of epithelial functions such as cell polarity and cell–cell adhesion and gain of mesenchymal features that are critically required for fibrosis development and progression of cancer with invasion and metastasis.64 It has been demonstrated by Hahn et al. that EMT plays a significant role in keloid pathology.62
To understand the molecular scarring of keloids, genomic profiles of KFs and keratinocytes were defined by Hahn et al..62 In both cell types, keloid-derived cells exhibited significant differential expression of genes corresponding to a diverse set of functional categories. Keloid keratinocytes had higher cellular motility, reduced adhesion, and differentiation. Conversely, EMT genes in keloid-derived keratinocytes were upregulated, indicating the involvement of this process in keloid pathology. These results further revealed that keratinocytes have a profound direct role in keloid scarring. Therefore, future treatment strategies should target both keratinocytes and fibroblast population to achieve a higher efficacy.
Hence, co-culture models are employed to provide in-depth information to further elucidate pathophysiological mechanisms involved in keloid disease.
In this section, we report the main studies using co-culture models for a better understanding of the reciprocal interaction and paracrine influence of dermal fibroblasts on keratinocytes and vice versa.
In vitro co-culture models can be utilized in two ways: In the first method, cells are grown in conditioned medium (with specific growth factors known to be secreted by the effector cells), whereas the second method involves the two-chamber co-culturing technique as described by Lim et al.65 In the latter method, a permeable membrane using a transwell system separates two chambers: The upper chamber comprises a fully differentiated epithelium (effector cells), whereas the lower chamber consists of fibroblasts (cells of interest).
Lim et al. performed a study to investigate the role of keloid-derived epithelial keratinocytes on normal fibroblasts in in vitro serum-free culture-free media. Keloid-derived keratinocytes and fibroblasts were seeded together in a co-culture system separated with a permeable membrane.65 A significantly higher proliferation rate was observed among normal fibroblasts co-cultured with keloid-derived keratinocytes as compared with normal fibroblasts grown with normal keratinocytes.
Their study supports the hypothesis that keratinocytes may play a profound role in the pathogenesis of keloids by substantially affecting the proliferation of fibroblasts through paracrine signaling. In a similar co-culture method, Lim et al. demonstrated that KFs co-cultured with normal fibroblasts produced a higher amount of collagen as compared with co-culturing with normal keratinocytes.65 These data support the hypothesis that epidermal fibroblasts play a pivotal role in the formation of keloids; hence, targeting keratinocytes along with dermal fibroblasts may be a more effective treatment strategy against keloids.
Funayama et al. designed a study with the hypothesis that abnormalities in epidermal–mesenchymal interactions play a profound role in keloidogenesis by creating imbalance between fibroblast proliferation and apoptosis.66 Therefore, they investigated the influence of normal dermal keratinocytes and keloid-derived keratinocytes on normal dermal fibroblasts and keloid-derived fibroblasts by using a serum-free indirect co-culture system. Six well inserts were utilized with keratinocytes in the upper chamber and fibroblasts in the lower chamber with serum-free media. Keloid-derived fibroblasts and co-cultured keloid keratinocytes experienced enhanced proliferation with the least apoptosis than those cultured with normal keratinocytes.
Their results indicated that the abnormal wound-healing process in keloid formation could be because of prolonged proliferation and apoptosis-resistant phenotypes of KFs. Keratinocytes have a regulatory effect on the activity of fibroblasts.66
Various studies have employed both methods to study the pathophysiological mechanisms implicated in KFs.67,68
An example is a study that employed the first method utilizing conditioned medium to evaluate the paracrine effects of site-specific KFs on the cellular and molecular behavior of normal skin fibroblasts and normal scar fibroblasts.69 In their study, Ashcroft et al. stated that conditioned media were collected from cultured perilesional and intralesional KFs during the proliferation phase, and they were used to treat normal scar fibroblasts and normal fibroblasts.69 An elevated enhanced cell proliferation, viability, and migration with increased expression of fibrosis-associated molecular markers was observed in normal scar and normal fibroblasts, after treatment with conditioned media.69
A more comprehensive description of autocrine and paracrine interactions of KFs may provide a better understanding of regulatory mechanisms underlying the altered activity of KFs and will further identify targets for therapeutic interventions.
To date, several studies have used the co-culture systems to investigate the paracrine effects of stem cells on KFs. Recently, using the method of co-culturing (conditioned medium), a study evaluated the effect of conditioned media obtained from amnion-derived mesenchymal stem cells on activation of keloid fibroblasts.70 It was reported that conditioned media decreased α-smooth muscle actin and collagen I expression and cell proliferation in KFs.
Liu et al. also investigated the paracrine influence of adipose tissue-derived mesenchymal stem cells (ASCs) on proliferation and migration of KFs when exposed to ASC-derived conditioned media (ASC-CM).71 Their results have shown that ASC-CM can not only inhibit the proliferation and migration of KFs but also stop ECM synthesis. These findings were in line with another study that also demonstrated the influence of bone marrow-derived mesenchymal stem cells (BMSC)-conditioned media on paracrine signaling of BMSCs in modulating the biological behavior of KFs.72 They observed that BMSC-conditioned media could inhibit the proliferation and migration of KFs. Further, it was also postulated that treatment with BMSC-conditioned media could significantly reduce the expression levels of CTGF, plasminogen activator inhibitor-1 (PAI-1), and TGF-β3, consequently reducing ECM synthesis.
These findings were also in line with a previous report of Fong et al., in which Wharton's jelly stem cells could enhance apoptosis, as well as inhibit the proliferation and migration of KFs.73
However, contrary findings were also reported by Arno et al. in their attempt to interpret paracrine signaling of human umbilical cord Wharton's jelly-derived mesenchymal stem cells (WJ-MSCs) on KFs. They utilized both methods of the culture systems previously described (conditioned medium co-culture and transwell).74 It was shown in their study that paracrine signaling of human WJMSCs upregulated the expression of various inflammatory and profibrotic markers (IL-6, IL-8, and PAI-1) and intensified the profibrotic phenotype of KFs, whereas antifibrotic TGF-β3 was downregulated when indirectly culturing both cell types via conditioned medium or co-culturing them through a microporous membrane.
These findings demonstrated that in contrast to the potential anti-cancer applications of WJMSCs, these cells appear to promote the keloid phenotype.74
Further, it is also noteworthy that besides the abundance of fibroblasts and keratinocytes in keloids, melanocytes have also been implicated to play an essential role in keloid disease.75 It has been postulated that fibroblasts co-cultured with melanocytes have higher expression of collagen and TGF-β whereas studies have also confirmed that α-melanocyte stimulating hormone could stimulate the proliferation and growth of fibroblasts in keloids.75
In addition to keratinocytes, inflammatory cells have also been demonstrated to play a pivotal role in keloid disease, mainly T cells and macrophages.30,31,76 A comprehensive immunophenotypic study was performed by Bagabir et al. and it demonstrated that T cells, B cells, M2, degranulated mast cells, and mature mast cells were increased in intralesional and perilesional keloid sites as compared with their normal counterparts.28
Co-culture methods have also been utilized to understand the role of mast cells in keloid pathobiology. The key role of mast cells has been implicated in neurogenic inflammation responses; therefore, they may also contribute to aberrant fibroblast activity in keloid formation.77,78
Mast cells contribute in extracellular formation in co-culture models of normal and KFs.79 KFs and mast cells were co-cultured in two ways: direct cell–cell contact and transwell co-culture system. In a system of direct cell–cell contact, human mast cells were co-cultured with a fixed number of fibroblasts on six well plates with variable cell densities ratios. Mast cells were seeded in increasing order of their cell densities, whereas fibroblasts were seeded at fixed density. The transwell system was designed as mentioned earlier with fibroblasts seeded on the bottom and mast cells laid on the transwell inserts. Both these co-cultures were incubated in hypoxia–normoxia conditions.
Their results described that co-culture of KFs and mast cells under the conditions of direct cell–cell contact exhibited a significantly elevated level of hypoxia inducible factor 1 subunit alpha (HIF-1α) and VEGF, supporting a critical role played by mast cells in keloid pathobiology.79
Further, keloids are not only composed of the cells mentioned earlier (fibroblasts, keratinocytes, melanocytes, T cells) but also importantly possess a well-defined 3D architecture along with various components of ECM. The main limitation associated with monolayer and 2D cultures is that cells are grown on rigid materials such as polystyrene and glass. These conventional unrealistic culture conditions cannot exactly mimic the complex cell-to-cell and cell-to-matrix interactions present in the in vivo environment of the skin. Because of this, there is a continued effort to overcome the limitations of single-cell models.
Three-dimensional culture models
The utilization of 3D models will provide a more physiologically relevant environment that will have widespread applications to identify drug targets for preclinical evaluation of various scar therapies. With this viewpoint, we now provide a concise overview of various 3D models of keloids.
For the first time, Ehrmann and Gey utilized an in vivo like environment to evaluate the behavior of cells; the loaded cells were placed on a natural collagen substrate instead of a glass surface.80 Since collagen's introduction as a substrate, many studies have reported the evaluation of cultured fibroblasts in a more in vivo like environment by embedding them on a collagen lattice.81 It was reported that fibroblasts maintained in monolayer culture had remarkably different physiology and morphology when compared with fibroblasts suspended in a 3D collagen matrix.
In 1979, Bell et al. designed fibroblast-populated collagen lattices (FPCLs) as a skin equivalent.82 However, organotypic culture models with cells suspended in collagen I matrix have always been of significant interest due to their physiological relevance and their ability to mimic the in vivo microenvironment of human skin. Indeed, many studies have explored the potential use of these models to study various aspects of skin biology, including epithelial–mesenchymal interactions, growth, and differentiation of keratinocytes, dynamics of wound healing, and fascinating properties of dermal fibroblasts.,83,84 Next, we explore the various keloid tissue-derived 3D models ranging from stratified organotypic to whole skin organ culture models (Fig. 1).
Organotypic skin culture models
Organotypic keloid models are designed such that keloid-derived fibroblasts are embedded in collagen lattices to form what is termed as an FPCL, or a cellularized collagen lattice (CCL). Further, several studies have also included more than just fibroblasts within such lattices, such as cultured keratinocytes, thereby yielding what could be identified as keratinocyte-fibroblast co-cultured collagen lattices (KFCLs). Since their inception, these models have been utilized to further elucidate hallmarks of keloid formation such as fibroblast hyperproliferation, migration, and invasion, as well as excess ECM deposition.85,86
Fibroblast-populated collagen lattices
The 3D in vitro keloid models derived from FPCL involve the culturing of keloid-derived fibroblasts in bovine/rat type I collagen matrices, devoid of epidermal cells.86,87 These models have proven pivotal in the study of fibrosis by researchers interested in keloids. For instance, a study by Suarez et al.88 developed a collagen-cell (fibroblasts) lattice that mimics an in vivo tension phenomenon to decipher tension-induced keloid fibroblast phenotypic and genotypic characteristics. Their study indicated that proliferation was enhanced by mechanical tension, as well as expression of well-known tension-related markers: Heat shock protein 27 (Hsp27), PAI-2 (plasminogen activator inhibitor-2), and α2β1 integrin (alpha-2 beta-1integrin).
Considering keloid heterogeneity, Suttho et al.89 isolated site-specific (peripheral, center, and non-lesional) keloid-derived fibroblasts to develop what they termed a reconstructed keloid model (RKM). This model is a result of sequential addition of site-specific fibroblasts, wherein a small volume of fibroblasts from the center of the lesion was placed in the center of a tissue culture dish. This was followed by the addition of collagen populated with cells from the center of the lesion, as a ring around the center fibroblasts. Lastly, another ring of non-lesional fibroblasts was added, yielding an RKM. The authors propose that this model's desirability lies in the fact that such heterogeneous cells can be added into the same system, wherein, microscopically, morphology, cell–cell and cell–matrix interactions could be discriminated.
Complex keratinocyte-FPCL
Three-dimensional in vitro keloid models derived from KFCL involve the culturing of keloid-derived fibroblast collagen lattices, followed by culturing of epidermal keratinocytes onto such FPCL, thereby yielding KFCL. Such models are more superior to FPCL, since they form full-thickness skin culture models of keloids, and can, therefore, be utilized in enhancing our understanding of keloid disease biology, such as a more intricate cellular process including cell–matrix (ECM remodeling), as well as cell–cell interaction in 3D.85,87
With the aim of further understanding the role of fibroblast activation (myofibroblasts) in keloids, Butler et al.85 isolated keloid-derived fibroblasts as well as normal cells (both fibroblasts and keratinocytes). Either normal or KFs were embedded in collagen gels, followed by the addition of normal keratinocytes onto both keloid and normal fibroblast-populated CCL. The cultures were exposed to an air–liquid interface (ALI) for the stratification of the epidermal layer, thus yielding full-thickness skin equivalents. Through this design, the authors report the activated state (presence of myofibroblasts) within the keloid-derived organotypic skin model. This was accompanied by the significant increase in dermal contraction, thus indicating the differences in cell–matrix as well as cell–cell interaction between the keloid-derived and normal skin models.
In another study, Supp et al. isolated keloid-derived fibroblasts and keratinocytes from various dermal depths (deep and superficial dermis), alongside both cell types from normal skin.87 Various combinations of either/both keloids and normal cells were used to construct full-thickness stratified KFCL. Here, it was shown that keloid-derived KFCL was thicker than the other constructs. Differential collagen I expression was also eminent among the various combinations of deep and superficial fibroblast-populated lattices. The authors concluded that phenotypic features of keloid-derived organotypic models are analogous to those seen in keloid scars in vivo.
Both FPCL and KFCL models are pivotal in enhancing our understanding of keloid disease biology, such as more intricate cellular processes including cell–matrix (ECM remodeling), as well as cell–cell interaction in 3D space.
Ex vivo models
In the previous section, several in vitro models (2D and 3D) were described, which are utilized to understand the pathogenesis of keloids and to investigate the potential of various therapeutic agents in keloid management to date. Although these models have provided very useful information about the pathogenesis and treatment possibilities for keloid scars, still they cannot truly replicate the actual native state of scarred skin that comprises ECM, blood vessels, and inflammatory cells along with the associated and necessary intrinsic and extrinsic biochemical interactions. The lack of these essential components of skin imposes limitations when evaluating functional testing of potential therapeutics candidates using in vitro models.
Therefore, over the past few years, ex vivo organ culture models have been generated that faithfully preserve the in vivo microenvironment and can be a valuable tool for studying keloids.90 To develop a serum-free ex vivo organ culture model of keloid diseased tissue, 3–6-mm punch biopsies were embedded in a collagen matrix, culture submerged, at ALI supplemented with two different types of serum-free media, compared, supplemented with DMEM, and supplemented with William's E medium.
The results of this study revealed that 4-mm punch biopsies cultured at ALI demonstrated the least cytotoxicity and most proliferation results after 4 and 6 weeks.90 This model exhibits persistent keloid phenotype as evident by maintained expression of collagen I and III, immune cell fraction, mesenchymal cells, and endothelial cells.
Ex vivo organ cultures (Fig. 1) are simple, convenient, and a clinically relevant model system, which further enables the relevant qualitative and quantitative keloid parameters to be evaluated. The first functional evidence of this model was described by Syed et al. for the functional testing of two candidate antifibrotic compounds in keloid disease compared with a steroid.91 Their study proves that a novel assay system90 for the long-term, serum-free ex vivo organ culture of human keloids is physiologically relevant for mechanistic keloid research. Further, this assay has the potential to test anti-keloid drugs at the pre-clinical level.
These models certainly have promising potential, although lack of a regular supply of excised keloid tissue samples and the absence of circulating immune cells pose a limitation to their widespread use.
In vitro assays for keloid scar testing
The importance of in vitro models for exploring the pathogenesis of keloid scar formation, identification of new drug targets as well as biomarkers, and discovery of novel therapeutic candidates cannot be overemphasized.22,92,93 In vitro assays provide the opportunity for evaluation of an effect on specific cell type in a mono- or co-culture with other tissue/matrix components.16,22,94 In contrast, ex vivo testing would enable immediate and short-term examination of effects not only at the cellular level on cells but also on the surrounding tissue components. Of course, in vivo animal testing could be considered as ideal, as it would include normal physiological parameters, but it presents with several shortcomings including the lack of a relevant animal model as keloids only occur in human skin.16,19,21
However, employing in vitro and ex vivo testing without an appropriate selection of the most applicable assay(s) would not be ideal. Among the various relevant cellular assays reported, the most commonly explored include proliferation, migration, invasion, and apoptosis (Fig. 1), as well as other vital assays such as angiogenesis, senescence, fibrosis, and re-epithelialization.16,95 Most of the in vitro testing reported has explored basic questions related to cell signaling in response to cell stress or injury.22
Real-time cell analysis has been employed to study cell proliferation, migration, invasion, and compound cytotoxicity of cells by measuring electrical impedance, which is directly proportional to cell number and size.96,97 Other proliferation assays include measurement of expression markers such as Ki67 and bromodeoxyuridine incorporation in stained cells.90,98
Migration assays have also been used on dermal fibroblasts and keratinocytes, and for testing various agents for the enhancement of cell migration or re-epithelialisation.94,99 The in vitro scratch assay is a simple, versatile, cost-effective, and well-studied cell migration assay. Briefly, cells are grown in vitro; after this, a simple linear thin scratch or wound (creating a gap) is made in confluent cell culture, and subsequently, at regular intervals, images of the cells filling the gap are captured.100,101
Future models
Despite having extensive research, the development of a potential therapeutic agent for the treatment of keloids is still an important unmet clinical need. The rapid in vitro screening of the lead compounds will accelerate the developmental process of effective anti-keloid treatment. However, in vitro characterization of effective drugs has been constrained by various biological and technical problems, because fibroblasts in monolayer cultures cannot yield a considerable amount of collagen in the respective time window for screening. Therefore, the effectiveness of such therapies has been hampered by a lack of relevant fibroplasia models.
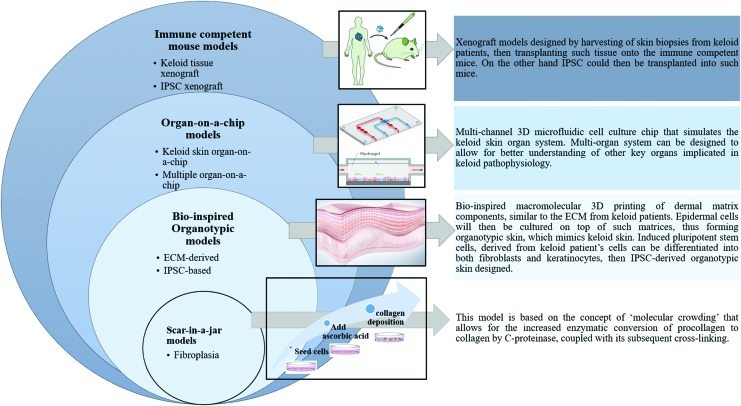
Considering this, in 2009, Chen et al. developed a pathophysiologically relevant model (scar-in-a-jar) that exhibits significant ECM (collagen) components' deposition.102 They developed a comprehensive technology to hasten collagen deposition in vitro by introducing macromolecules in the culture medium. In this model, collagen deposition and cross-linking are extensively increased by “crowding” the standard culture medium with negatively charged macromolecules such as dextran sulfate (the rapid deposition mode) and a mixture of neutral 70 and 400 kDa Ficoll polysaccharide (the accelerated mode). The rapid deposition mode yields granular collagen aggregates within 48 h, whereas the accelerated mode could produce a reticular deposition pattern of collagen 1 in 6 days.
Therefore, amount, velocity, and morphology of collagen deposition could be manipulated by using “crowders” (macromolecules) in rapid and accelerated mode. This model is based on the concept of “molecular crowding” that allows for the increased enzymatic conversion of procollagen to collagen by C-proteinase, coupled with its subsequent cross-linking. Molecular crowding induces excluded volume effect, which enhances enzymatic activity and accelerates ECM formation in vitro. This model is superior to conventional monolayer cultures, as it allows for rapid and specific quantification of collagen deposition in a single-well format that is pivotal for the screening of antifibrotic compounds.
The scar in a jar enables the analysis in a single-well format; thus, this results in a reduction in the number of processing steps, material loss, and sample variation. So far, this model has been evaluated on lung fibroblasts, but it has the flexibility and potential for relevant screening and evaluation of antifibrotic lead compounds in other cell types as well.
The various in vitro and ex vivo models described in this review provide valuable insight into the pathogenesis and potential of various drug regimens in treating keloids. However, the applicability of these models depends on the level of their similarity to the actual in vivo microenvironment of the keloid tissue. Therefore, there is an urgent unmet need for the development of more biologically complex and clinically relevant keloid models. Future prospective models could be generated with the inclusion of various innovative strategies ranging from stem cells, nanotechnology, vascularization strategies, and microfluidics to 3D bioprinting strategies.
Bioprinting is a computerized controlled deposition of cells in precise 3D patterns. Lee et al. have described the potential of 3D bioprinting for tissue engineering and organs by using human skin as a prototypical example.103 A multi-layered cell and matrix structure was obtained by growing human keratinocytes at air–liquid interphase in collagen matrix embedded with human fibroblasts. This printed skin model with effective control over the number of layers, cell density, and their precise location could represent morphological and biological features of in vivo human skin.103
By replicating the skin and its microenvironment in 3D bioprinting constructs, it is possible to investigate cell proliferation, migration, invasion, and drug testing which better mimics in vivo skin responses as compared with 2D cultures. This model has set the platform for further development and engineering of human skin with more complex structures and functions in the future.
Besides, 3D cellular models of keloid have been reported with a promising prospect of gaining new insight into keloid disease pathogenesis, to evaluate potential keloid therapeutic agents.104,105 Recent progress in the field of “organ-on a chip” may mitigate the various limitations associated with current models in keloid research.
Ataç et al. demonstrated a dynamically perfused chip-based bioreactor platform to prolong the static maintenance period of commercially available skin equivalents, ex vivo organ cultures, and biopsies of single follicular units.106 This bioreactor platform referred to as a multi-organ-chip has a built-in micropump with various tissue culture compartments having the size of one cavity of 96-well plates. This system can operate two microfluidic circuits simultaneously and can provide two important physiological features: mechanical coupling of tissues and their cross-talk with each other.
The organ on a chip or multiple organs on a chip utilized engineered tissues, mimicked in vivo equivalents, and were composed of a variety of cells interacting with each other in a better controlled cellular microenvironment provided in a microfluidic chip.106 It can comprehensively maintain various 3D tissues of human origin (cell lines, primary cells, and biopsies) over a long period and these co-cultures can further be used for pharmaceutical testing of various drugs. The controlled conditions provided with these models make it feasible to represent the environment of skin in terms of humidity, pH, oxygen levels, temperature, the elasticity of the skin, and existing cellular interactions.107
Park et al. established and propagated the first patient-derived xenograft model in immunocompetent animals.108 Immediately after surgical excision, human keloid tissue was implanted into animals and was maintained for 4 months. Primary cell culture and gene expression analysis demonstrated the structural and functional integrity of explanted keloid scars. This model could be utilized to understand keloid disease pathology, leading to the identification of predictive markers for the development of novel therapeutics.
Next-generation models (Fig. 2) such as scar-in-a-jar,102 bio-inspired macromolecular printing of dermal matrix-assisted organotypic cultures,103,109 immune-competent humanized mouse models,108 and “multiple organs on a chip”106 models may make a further contribution to our understanding of keloid pathogenesis. In addition, novel in vivo temporal keloid patient biopsy models of wound healing will, no doubt, enhance our knowledge of keloid disease formation and progression.
Figure 2.
Future in vitro, alongside ex vivo models for keloid disease. These include and scar-in-a-jar, where cells in a monolayer can produce excess collagen (fibroplasia). Bio-inspired organotypic (ECM-derived and IPSC-based) models, wherein dermal matrices can be bio-printed, followed by addition of cells. Organ-on-a-chip models (keloid skin or multiple organ-on-a-chip) comprising whole skin or multiple organs in a perfused; constant flow of nutrients. Immune competent mouse (keloid tissue and IPSC xenograft) models, where keloid biopsy of IPSC can be grafted onto non-immune compromised mouse. ECM, extracellular matrix; IPSC, induced pluripotent stem cells. To see this illustration in color, the reader is referred to the web version of this article. Color images are available online.
Summary
There is an urgent need to understand the underlying mechanism and pathobiology of keloid disease by developing functionally representative relevant models. This approach can execute the preclinical testing and validation of new treatment modalities, leading to the development of future targeted therapeutics.
Take-Home Messages.
Keloids create a significant psychological, aesthetic, and/or functional burden on the patients. The etiology of keloids remains largely unknown. Therefore, a universally accepted therapeutic regimen is still unavailable.
The major limitation in therapeutics development has been the lack of animal models to investigate keloid pathology.
Available non-animal in vitro and ex vivo models are addressed with biological and technical limitations.
Therefore, there is still an unmet need to develop a functional and physiologically relevant non-animal model to further investigate the molecular mechanism of keloid pathogenesis and therapeutic development.
Acknowledgments and Funding Sources
N.P.K. and Hair and Skin Research Lab are funded by the National Research Foundation and the South African Medical Research Council.
Abbreviations and Acronyms
- 2D
two-dimensional
- 3D
three-dimensional
- α-SMA
alpha smooth muscle actin
- ALI
air–liquid interface
- AMPK
adenosine monophosphate-activated protein kinase
- ASCs
adipose tissue-derived mesenchymal stem cells
- BMSC
bone marrow-derived mesenchymal stem cells
- CCL
cellularized collagen lattice
- CTGF
connective tissue growth factor
- DHMEQ
dehydroxymethylepoxyquinomicin
- ECM
extracellular matrix
- EMT
epithelial–mesenchymal transition
- ERK
extracellular regulated kinase
- FOXO1
forkhead box O1
- FPCL
fibroblast-populated collagen lattices
- HSP27
heat shock protein 27
- HIF-1α
hypoxia inducible factor 1 alpha
- IFN
interferon
- IPSC
induced pluripotent stem cells
- KFCL
keratinocyte-fibroblast co-cultured collagen lattices
- KFs
keloid fibroblasts
- MAPK
mitogen-activated protein kinases
- p38
mitogen activated protein kinase
- RKM
reconstructed keloid models
- SMAD
mothers against decapentaplegic homolog
- TGF-β3
transforming growth factor beta 3
- Th-1
T helper type 1 lymphocyte
- Th-2
T helper type 2 lymphocyte
- VEGF
vascular endothelial growth factor
- WJ-MSCs
Wharton's jelly-derived mesenchymal stem cells
Author Disclosure and Ghostwriting
No competing financial interests exist. The content of the article was expressly written by the author(s) listed. No ghostwriters were used to write this article.
About the Authors
Jyoti R. Sharma, PhD, is a Research Officer at Hair and Skin Research Lab, involved in the research aimed at developing novel therapies for the treatment of keloids. Maribanyana Lebeko, PhD, is a postdoctoral fellow, interested in the development of in vitro 3D models of keloids. Elvis Kidzeru, BMLS, PhD, is a postdoctoral research fellow involved in elucidating the role of the immune system in keloids. Nonhlanhla P. Khumalo, MBChB, PhD, FCDerm, is the Head of the Division of Dermatology at The University of Cape Town. Her research interests include alopecias, skin fibrosis, cosmetic ingredients safety, and the use of hair as a testing substrate in Medicine. Ardeshir Bayat, MBBS, PhD, is a professor in the Division of Dermatology at University of Cape Town. His extensive research experience includes wound healing, tissue engineering, and keloid biology.
References
- 1. Cosman B, Crikelair GF, Ju DMC, Gaulin JC, Lattes RM. The surgical treatment of keloids. Plast Reconstr Surg 1961;27:335–358 [Google Scholar]
- 2. Urioste SS, Arndt KA, Dover JS. Keloids and hypertrophic scars: review and treatment strategies. Semin Cutan Med Surg 1999;18:159–171 [DOI] [PubMed] [Google Scholar]
- 3. Bloom D. Heredity of keloids; review of the literature and report of a family with multiple keloids in five generations. N Y State J Med 1956;56:511–519 [PubMed] [Google Scholar]
- 4. Ud-Din S, Bayat A. Strategic management of keloid disease in ethnic skin: a structured approach supported by the emerging literature. Br J Dermatol 2013;169(SUPPL. 3):71–81 [DOI] [PubMed] [Google Scholar]
- 5. Ehrlich HP, Desmoulière A, Diegelmann RF, et al. Morphological and immunochemical differences between keloid and hypertrophic scar. Am J Pathol 1994;145:105–113 [PMC free article] [PubMed] [Google Scholar]
- 6. McCoy BJ, Diegelmann RF, Cohen IK. In vitro inhibition of cell growth, collagen synthesis, and prolyl hydroxylase activity by triamcinolone acetonide. Exp Biol Med 1980;163:216–222 [DOI] [PubMed] [Google Scholar]
- 7. Mercer NS. Silicone gel in the treatment of keloid scars. Br J Plast Surg 1989;42:83–87 [DOI] [PubMed] [Google Scholar]
- 8. Bock O, Schmid-Ott G, Malewski P, Mrowietz U. Quality of life of patients with keloid and hypertrophic scarring. Arch Dermatol Res 2006;297:433–438 [DOI] [PubMed] [Google Scholar]
- 9. Berman B, Bieley HC. Keloids. J Am Acad Dermatol 1995;33:117–123 [DOI] [PubMed] [Google Scholar]
- 10. Rockwell WB, Cohen IK, Ehrlich HP. Keloids and hypertrophic scars: a comprehensive review. Plast Reconstr Surg 1989;84:827–837 [DOI] [PubMed] [Google Scholar]
- 11. Abergel RP, Pizzurro D, Meeker CA, et al. Biochemical composition of the connective tissue in keloids and analysis of collagen metabolism in keloid fibroblast cultures. J Invest Dermatol 1985;84:384–390 [DOI] [PubMed] [Google Scholar]
- 12. Alaish SM, Yager DR, Diegelmann RF, Cohen IK. Hyaluronic acid metabolism in keloid fibroblasts. J Pediatr Surg 1995;30:949–952 [DOI] [PubMed] [Google Scholar]
- 13. Marneros AG, Norris JEC, Olsen BR, Reichenberger E. Clinical genetics of familial keloids. Arch Dermatol 2001;137:1429–1434 [DOI] [PubMed] [Google Scholar]
- 14. Shih B, Garside E, McGrouther DA, Bayat A. Molecular dissection of abnormal wound healing processes resulting in keloid disease. Wound Repair Regen 2010;18:139–153 [DOI] [PubMed] [Google Scholar]
- 15. Luo S, Benathan M, Raffoul W, Panizzon RG, Egloff DV. Abnormal balance between proliferation and apoptotic cell death in fibroblasts derived from keloid lesions. Plast Reconstr Surg 2001;107:87–96 [DOI] [PubMed] [Google Scholar]
- 16. Ud-Din S, Bayat A. Non-animal models of wound healing in cutaneous repair: in silico, in vitro, ex vivo, and in vivo models of wounds and scars in human skin. Wound Repair Regen 2017;25:164–176 [DOI] [PubMed] [Google Scholar]
- 17. Marttala J, Andrews JP, Rosenbloom J, Uitto J. Keloids: animal models and pathologic equivalents to study tissue fibrosis. Matrix Biol 2016;51:47–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lebeko M, Khumalo NP, Bayat A. Multi-dimensional models for functional testing of keloid scars: in silico, in vitro, organoid, organotypic, ex vivo organ culture, and in vivo models. Wound Repair Regen 2019;27:298–308 [DOI] [PubMed] [Google Scholar]
- 19. Hillmer MP, MacLeod SM. Experimental keloid scar models: a review of methodological issues. J Cutan Med Surg 2002;6:354–359 [DOI] [PubMed] [Google Scholar]
- 20. Sidgwick GP, McGeorge D, Bayat A. Functional testing of topical skin formulations using an optimised ex vivo skin organ culture model. Arch Dermatol Res 2016;308:297–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Seok J, Warren HS, Cuenca AG, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A 2013;110:3507–3512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van den Broek LJ, Limandjaja GC, Niessen FB, Gibbs S. Human hypertrophic and keloid scar models: principles, limitations and future challenges from a tissue engineering perspective. Exp Dermatol 2014;23:382–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Anderson ARA, Chaplain MAJ, Rejniak KA. Single-Cell-Based Models in Biology and Medicine. Switzerland: Birkhäuser Verlag Basel, 2007 [Google Scholar]
- 24. Lim IJ, Phan T-T, Bay B-H, et al. Fibroblasts cocultured with keloid keratinocytes: normal fibroblasts secrete collagen in a keloidlike manner. Am J Physiol Physiol 2002;283:C212–C222 [DOI] [PubMed] [Google Scholar]
- 25. Ranzato E, Martinotti S, Volante A, Mazzucco L, Burlando B. Platelet lysate modulates MMP-2 and MMP-9 expression, matrix deposition and cell-to-matrix adhesion in keratinocytes and fibroblasts. Exp Dermatol 2011;20:308–313 [DOI] [PubMed] [Google Scholar]
- 26. Diegelmann RF, Cohen IK, McCoy BJ. Growth kinetics and collagen synthesis of normal skin, normal scar and keloid fibroblasts in vitro. J Cell Physiol 1979;98:341–346 [DOI] [PubMed] [Google Scholar]
- 27. Calderon M, Lawrence WT, Banes AJ. Increased proliferation in keloid fibroblasts wounded in vitro. J Surg Res 1996;61:343–347 [DOI] [PubMed] [Google Scholar]
- 28. Bagabir R, Byers RJ, Chaudhry IH, Muller W, Paus R, Bayat A. Site-specific immunophenotyping of keloid disease demonstrates immune upregulation and the presence of lymphoid aggregates. Br J Dermatol 2012;167:1053–1066 [DOI] [PubMed] [Google Scholar]
- 29. Chanpimol S, Seamon B, Hernandez H, Harris-love M, Blackman MR. Keloids: the paradigm of skin fibrosis—pathomechanisms and treatment. Matrix Biol 2017;51:37–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Martin CW, Muir IFK. The role of lymphocytes in wound healing. Br J Plast Surg 1990;43:655–662 [DOI] [PubMed] [Google Scholar]
- 31. Shaker SA, Ayuob NN, Hajrah NH. Cell talk: a phenomenon observed in the keloid scar by immunohistochemical study. Appl Immunohistochem Mol Morphol 2011;19:153–159 [DOI] [PubMed] [Google Scholar]
- 32. Chizzolini C. T cells, B cells, and polarized immune response in the pathogenesis of fibrosis and systemic sclerosis. Curr Opin Rheumatol 2008;20:707–712 [DOI] [PubMed] [Google Scholar]
- 33. Chujo S, Shirasaki F, Kondo-Miyazaki M, Ikawa Y, Takehara K. Role of connective tissue growth factor and its interaction with basic fibroblast growth factor and macrophage chemoattractant protein-1 in skin fibrosis. J Cell Physiol 2009;220:189–195 [DOI] [PubMed] [Google Scholar]
- 34. Kashiyama K, Mitsutake N, Matsuse M, et al. miR-196a downregulation increases the expression of type I and III collagens in keloid fibroblasts. J Invest Dermatol 2012;132:1597–1604 [DOI] [PubMed] [Google Scholar]
- 35. Sziksz E, Pap D, Lippai R, et al. Fibrosis related inflammatory mediators: role of the IL-10 cytokine family. Mediators Inflamm 2015;2015:764641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xiong L-X, Li W-L, Cai Z-Y, et al. Suppressive effect of interferon γ on IL-13-induced fibrosis in fibroblasts. Chinese J Pathophysiol 2013;03 [Google Scholar]
- 37. Wulff BC, Parent AE, Meleski MA, DiPietro LA, Schrementi ME, Wilgus TA. Mast cells contribute to scar formation during fetal wound healing. J Invest Dermatol 2012;132:458–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bagabir RA, Wu PA, Chen CA, Stern RS. Identification of a potential molecular diagnostic biomarker in keloid disease: syndecan-1 (CD138) is overexpressed in keloid scar tissue. J Invest Dermatol 2016;136:2319–2323 [DOI] [PubMed] [Google Scholar]
- 39. Colwell AS, Phan T-T, Kong W, Longaker MT, Lorenz PH. Hypertrophic scar fibroblasts have increased connective tissue growth factor expression after transforming growth factor-beta stimulation. Plast Reconstr Surg 2005;116:1387–1390; discussion 1391–1392 [DOI] [PubMed] [Google Scholar]
- 40. Smith P, Mosiello G, Deluca L, Ko F, Maggi S, Robson MC. TGF-beta2 activates proliferative scar fibroblasts. J Surg Res 1999;82:319–323 [DOI] [PubMed] [Google Scholar]
- 41. Luo L, Li J, Liu H, et al. Adiponectin is involved in connective tissue growth factor-induced proliferation, migration and overproduction of the extracellular matrix in keloid fibroblasts. Int J Mol Sci 2017;18:1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jones CD, Guiot L, Samy M, Gorman M, Tehrani H. The use of chemotherapeutics for the treatment of keloid scars. Dermatol Rep 2015;7:5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mikulec AA, Hanasono MM, Lum J, Kadleck JM, Kita M, Koch RJ. Effect of tamoxifen on transforming growth factor beta1 production by keloid and fetal fibroblasts. Arch Facial Plast Surg 2001;3:111–114 [DOI] [PubMed] [Google Scholar]
- 44. Zhang G-Y, Gao W-Y, Li X, et al. Effect of camptothecin on collagen synthesis in fibroblasts from patients with keloid. Ann Plast Surg 2009;63:94–99 [DOI] [PubMed] [Google Scholar]
- 45. Makino S, Mitsutake N, Nakashima M, et al. DHMEQ, a novel NF-kappaB inhibitor, suppresses growth and type I collagen accumulation in keloid fibroblasts. J Dermatol Sci 2008;51:171–180 [DOI] [PubMed] [Google Scholar]
- 46. Yamauchi T. Effects of TGF-beta and TGF-beta-neutralizing antibody on normal skin fibroblasts and scar-derived fibroblasts. Kurume Med J 2002;49:171–176 [DOI] [PubMed] [Google Scholar]
- 47. Messadi D, Doung H, Zhang Q, et al. Activation of NFkappB signal pathways in keloid fibroblasts. Arch Dermatol Res 2004;296:125–133 [DOI] [PubMed] [Google Scholar]
- 48. Yi S, Weiyuan M. The effect of Metformin on the proliferation and collagen synthesis of human keloids fibroblasts. Zhonghua Zheng Xing Wai Ke Za Zhi 2015;31:291–295 [PubMed] [Google Scholar]
- 49. Sato N, Takasaka N, Yoshida M, et al. Metformin attenuates lung fibrosis development via NOX4 suppression. Respir Res 2016;17:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mun J-H, Kim Y-M, Kim B-S, Kim J-H, Kim M-B, Ko H-C. Simvastatin inhibits transforming growth factor-β1-induced expression of type I collagen, CTGF, and α-SMA in keloid fibroblasts. Wound Repair Regen 2014;22:125–133 [DOI] [PubMed] [Google Scholar]
- 51. Wang W, Qu M, Xu L, et al. Sorafenib exerts an anti-keloid activity by antagonizing TGF-β/Smad and MAPK/ERK signaling pathways. J Mol Med (Berl) 2016;94:1181–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tang M, Bian W, Cheng L, et al. Ginsenoside Rg3 inhibits keloid fibroblast proliferation, angiogenesis and collagen synthesis in vitro via the TGF-β/Smad and ERK signaling pathways. Int J Mol Med 2018;41:1487–1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Song R, Li G, Li S. Aspidin PB, a novel natural anti-fibrotic compound, inhibited fibrogenesis in TGF-β1-stimulated keloid fibroblasts via PI-3K/Akt and Smad signaling pathways. Chem Biol Interact 2015;238:66–73 [DOI] [PubMed] [Google Scholar]
- 54. Soldati F, Sticher O. HPLC separation and quantitative determination of ginsenosides from Panax ginseng, Panax quinquefolium and from Ginseng drug preparations. Planta Med 1980;39:348–357 [DOI] [PubMed] [Google Scholar]
- 55. Huang L, Cai YJ, Lung I, Leung BCS, Burd A. A study of the combination of triamcinolone and 5-fluorouracil in modulating keloid fibroblasts in vitro. J Plast Reconstr Aesthet Surg 2013;66:e251–e259 [DOI] [PubMed] [Google Scholar]
- 56. Syed F, Singh S, Bayat A. Superior effect of combination vs. single steroid therapy in keloid disease: a comparative in vitro analysis of glucocorticoids. Wound Repair Regen 2013;21:88–102 [DOI] [PubMed] [Google Scholar]
- 57. Chen CZ, Raghunath M. Focus on collagen: in vitro systems to study fibrogenesis and antifibrosis—state of the art. Fibrogenesis Tissue Repair 2009;2:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fleischmajer R, Perlish JS, Krieg T, Timpl R. Variability in collagen and fibronectin synthesis by scleroderma fibroblasts in primary culture. J Invest Dermatol 1981;76:400–403 [DOI] [PubMed] [Google Scholar]
- 59. Botstein GR, Sherer GK, Leroy EC. Fibroblast selection in scleroderma. An alternative model of fibrosis. Arthritis Rheum 1982;25:189–195 [DOI] [PubMed] [Google Scholar]
- 60. Lu F, Gao J, Ogawa R, Hyakusoku H, Ou C. Biological differences between fibroblasts derived from peripheral and central areas of keloid tissues. Plast Reconstr Surg 2007;120:625–630 [DOI] [PubMed] [Google Scholar]
- 61. Sayah DN, Soo C, Shaw WW, et al. Downregulation of apoptosis-related genes in keloid tissues. J Surg Res 1999;87:209–216 [DOI] [PubMed] [Google Scholar]
- 62. Hahn JM, Glaser K, McFarland KL, Aronow BJ, Boyce ST, Supp DM. Keloid-derived keratinocytes exhibit an abnormal gene expression profile consistent with a distinct causal role in keloid pathology. Wound Repair Regen 2013;21:530–544 [DOI] [PubMed] [Google Scholar]
- 63. Maas-Szabowski N, Shimotoyodome A, Fusenig NE. Keratinocyte growth regulation in fibroblast cocultures via a double paracrine mechanism. J Cell Sci 1999;112 (Pt 1):1843–1853 [DOI] [PubMed] [Google Scholar]
- 64. Thiery JP, Acloque H, Huang RYJ, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell 2009;139:871–890 [DOI] [PubMed] [Google Scholar]
- 65. Lim IJ, Phan TT, Song C, Tan WT, Longaker MT. Investigation of the influence of keloid-derived keratinocytes on fibroblast growth and proliferation in vitro. Plast Reconstr Surg 2001;107:797–808 [DOI] [PubMed] [Google Scholar]
- 66. Funayama E, Chodon T, Oyama A, Sugihara T. Keratinocytes promote proliferation and inhibit apoptosis of the underlying fibroblasts: an important role in the pathogenesis of keloid. J Invest Dermatol 2003;121:1326–1331 [DOI] [PubMed] [Google Scholar]
- 67. Lim CP, Phan TT, Lim IJ, Cao X. Cytokine profiling and Stat3 phosphorylation in epithelial–mesenchymal interactions between keloid keratinocytes and fibroblasts. J Invest Dermatol 2009;129:851–861 [DOI] [PubMed] [Google Scholar]
- 68. Kuwahara H, Tosa M, Egawa S, Murakami M, Mohammad G, Ogawa R. Examination of epithelial mesenchymal transition in keloid tissues and possibility of keloid therapy target. Plast Reconstr surgery Glob open 2016;4:e1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ashcroft KJ, Syed F, Bayat A. Site-specific keloid fibroblasts alter the behaviour of normal skin and normal scar fibroblasts through paracrine signalling. PLoS One 2013;8:e75600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sato C, Yamamoto Y, Funayama E, et al. Conditioned medium obtained from amnion-derived mesenchymal stem cell culture prevents activation of keloid fibroblasts. Plast Reconstr Surg 2018;141:390–398 [DOI] [PubMed] [Google Scholar]
- 71. Liu J, Ren J, Su L, et al. Human adipose tissue-derived stem cells inhibit the activity of keloid fibroblasts and fibrosis in a keloid model by paracrine signaling. Burns 2018;44:370–385 [DOI] [PubMed] [Google Scholar]
- 72. Fang F, Huang R-L, Zheng Y, Liu M, Huo R. Bone marrow derived mesenchymal stem cells inhibit the proliferative and profibrotic phenotype of hypertrophic scar fibroblasts and keloid fibroblasts through paracrine signaling. J Dermatol Sci 2016;83:95–105 [DOI] [PubMed] [Google Scholar]
- 73. Fong C-Y, Biswas A, Subramanian A, Srinivasan A, Choolani M, Bongso A. Human keloid cell characterization and inhibition of growth with human Wharton's jelly stem cell extracts. J Cell Biochem 2014;115:826–838 [DOI] [PubMed] [Google Scholar]
- 74. Arno AI, Amini-Nik S, Blit PH, et al. Effect of human Wharton's jelly mesenchymal stem cell paracrine signaling on keloid fibroblasts. Stem Cells Transl Med 2014;3:299–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Gao FL, Jin R, Zhang L, Zhang YG. The contribution of melanocytes to pathological scar formation during wound healing. Int J Clin Exp Med 2013;6:609–613 [PMC free article] [PubMed] [Google Scholar]
- 76. Boyce DE, Ciampolini J, Ruge F, Harding KG, Murison MMSC. Inflammatory cell subpopulations in keloid scars. Br J Plast Surg 2001;54:511–516 [DOI] [PubMed] [Google Scholar]
- 77. Akaishi S, Ogawa R, Hyakusoku H. Keloid and hypertrophic scar: neurogenic inflammation hypotheses. Med Hypotheses 2008;71:32–38 [DOI] [PubMed] [Google Scholar]
- 78. Arck PC, Slominski A, Theoharides TC, Peters EMJ, Paus R. Neuroimmunology of stress: skin takes center stage. J Invest Dermatol 2006;126:1697–1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zhang Q, Oh CK, Messadi DV, et al. Hypoxia-induced HIF-1 α accumulation is augmented in a co-culture of keloid fibroblasts and human mast cells: involvement of ERK1/2 and PI-3K/Akt. Exp Cell Res 2006;312:145–155 [DOI] [PubMed] [Google Scholar]
- 80. Ehrmann RL, Gey GO. The growth of cells on a transparent gel of reconstituted rat-tail collagen. J Natl Cancer Inst 1956;16:1375–1403 [PubMed] [Google Scholar]
- 81. Elsdale T, Bard J. Collagen substrata for studies on cell behavior. J Cell Biol 1972;54:626–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bell E, Ivarsson B, Merrill C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc Natl Acad Sci U S A 1979;76:1274–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Muffler S, Stark H-J, Amoros M, et al. A stable niche supports long-term maintenance of human epidermal stem cells in organotypic cultures. Stem Cells 2008;26:2506–2515 [DOI] [PubMed] [Google Scholar]
- 84. Sriram G, Bigliardi PL, Bigliardi-Qi M. Fibroblast heterogeneity and its implications for engineering organotypic skin models in vitro. Eur J Cell Biol 2015;94:483–512 [DOI] [PubMed] [Google Scholar]
- 85. Butler PD, Ly DP, Longaker MT, Yang GP. Use of organotypic coculture to study keloid biology. Am J Surg 2008;195:144–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Muthusubramaniam L, Zaitseva T, Paukshto M, Martin G, Desai T. Effect of collagen nanotopography on keloid fibroblast proliferation and matrix synthesis: implications for dermal wound healing. Tissue Eng A 2014;20:2728–2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Supp DM, Hahn JM, Glaser K, McFarland KL, Boyce ST. Deep and superficial keloid fibroblasts contribute differentially to tissue phenotype in a novel in vivo model of keloid scar. Plast Reconstr Surg 2012;129:1259–1271 [DOI] [PubMed] [Google Scholar]
- 88. Suarez E, Syed F, Rasgado TA, Walmsley A, Mandal P, Bayat A. Skin equivalent tensional force alters keloid fibroblast behavior and phenotype. Wound Repair Regen 2014;22:557–568 [DOI] [PubMed] [Google Scholar]
- 89. Suttho D, Mankhetkorn S, Binda D. 3D modeling of keloid scars in vitro by cell and tissue engineering. Arch Dermatology Res 2017;309:55–62 [DOI] [PubMed] [Google Scholar]
- 90. Bagabir R, Syed F, Paus R, Bayat A. Long-term organ culture of keloid disease tissue. Exp Dermatol 2012;21:376–381 [DOI] [PubMed] [Google Scholar]
- 91. Syed F, Bagabir RA, Paus R, Bayat A. Ex vivo evaluation of antifibrotic compounds in skin scarring: EGCG and silencing of PAI-1 independently inhibit growth and induce keloid shrinkage. Lab Invest 2013;93:946–960 [DOI] [PubMed] [Google Scholar]
- 92. Mathes SH, Ruffner H, Graf-Hausner U. The use of skin models in drug development. Adv Drug Deliv Rev 2014;69–70:81–102 [DOI] [PubMed] [Google Scholar]
- 93. Lebonvallet N, Jeanmaire C, Danoux L, Sibille P, Pauly G, Misery L. The evolution and use of skin explants: potential and limitations for dermatological research. Eur J Dermatol 2010;20:671–684 [DOI] [PubMed] [Google Scholar]
- 94. Wolf NB, Küchler S, Radowski MR, et al. Influences of opioids and nanoparticles on in vitro wound healing models. Eur J Pharm Biopharm 2009;73:34–42 [DOI] [PubMed] [Google Scholar]
- 95. Grada A, Otero-Vinas M, Prieto-Castrillo F, Obagi Z, Falanga V. Research techniques made simple: analysis of collective cell migration using the wound healing assay. J Invest Dermatol 2017;137:e11–e16 [DOI] [PubMed] [Google Scholar]
- 96. Limame R, Wouters A, Pauwels B, et al. Comparative analysis of dynamic cell viability, migration and invasion assessments by novel real-time technology and classic endpoint assays. PLoS One 2012;7:e46536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Dowling CM, Herranz Ors C, Kiely PA. Using real-time impedance-based assays to monitor the effects of fibroblast-derived media on the adhesion, proliferation, migration and invasion of colon cancer cells. Biosci Rep 2014;34:415–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Rehders M, Grosshäuser BB, Smarandache A, et al. Effects of lunar and mars dust simulants on HaCaT keratinocytes and CHO-K1 fibroblasts. Adv Space Res 2011;47:1200–1213 [Google Scholar]
- 99. Harmon AM, Kong W, Buensuceso CS, Gorman AJ, Muench TR. Effects of fibrin pad hemostat on the wound healing process in vivo and in vitro. Biomaterials 2011;32:9594–9601 [DOI] [PubMed] [Google Scholar]
- 100. Cory G. Scratch-wound assay. Methods Mol Biol 2011;769:25–30 [DOI] [PubMed] [Google Scholar]
- 101. Bindschadler M, McGrath JL. Sheet migration by wounded monolayers as an emergent property of single-cell dynamics. J Cell Sci 2007;120(Pt 5):876–884 [DOI] [PubMed] [Google Scholar]
- 102. Chen CZC, Peng YX, Wang ZB, et al. The Scar-in-a-Jar: studying potential antifibrotic compounds from the epigenetic to extracellular level in a single well. Br J Pharmacol 2009;158:1196–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Lee V, Singh G, Trasatti JP, et al. Design and fabrication of human skin by three-dimensional bioprinting. Tissue Eng C Methods 2014;20:473–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Sato M, Ishikawa O, Miyachi Y. Distinct patterns of collagen gene expression are seen in normal and keloid fibroblasts grown in three-dimensional culture. Br J Dermatol 1998;138:938–943 [DOI] [PubMed] [Google Scholar]
- 105. Lee WJ, Ahn HM, Na Y, Wadhwa R, Hong J, Yun C. Mortalin deficiency suppresses fibrosis and induces apoptosis in keloid spheroids. Sci Rep 2017;7:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ataç B, Wagner I, Horland R, et al. Skin and hair on-a-chip: in vitro skin models versus ex vivo tissue maintenance with dynamic perfusion. Lab Chip 2013;13:3555–3561 [DOI] [PubMed] [Google Scholar]
- 107. Wagner I, Materne E-M, Brincker S, et al. A dynamic multi-organ-chip for long-term cultivation and substance testing proven by 3D human liver and skin tissue co-culture. Lab Chip 2013;13:3538. [DOI] [PubMed] [Google Scholar]
- 108. Park TH, Rah DK, Chang CH, Kim SY. Establishment of patient-derived keloid xenograft model. J Craniofac Surg 2016;27:1670–1673 [DOI] [PubMed] [Google Scholar]
- 109. Tarassoli SP, Jessop ZM, Al-Sabah A, et al. Skin tissue engineering using 3D bioprinting: an evolving research field. J Plast Reconstr Aesthet Surg 2018;71:615–623 [DOI] [PubMed] [Google Scholar]