Abstract
Objectives:
Adequate muscle perfusion supports the transport of nutrients, oxygen and hormones into muscle fibers. Aging is associated with a substantial decrease in skeletal muscle capillarization, fiber size and oxidative capacity, which may be improved with regular physical activity. The aim of this study was to investigate the relationship between muscle capillarization and indices of muscle hypertrophy (i.e. lean mass; fiber cross sectional area (CSA)) in older adults before and after 12 weeks of progressive resistance exercise training (RET).
Design:
Interventional study
Setting and Participants:
19 subjects (10 male and 9 female; 71.1±4.3 years; 27.6±3.2 BMI) were enrolled in the study and performed a whole body RET program for 12 weeks. Subjects where then retrospectively divided into a LOW or HIGH group, based on their pre-RET capillary-to-fiber perimeter exchange index (CFPE). Physical activity level, indices of capillarization (capillaries-to-fiber ratio, C:Fi; CFPE index and capillary-to-fiber interface, LC-PF index), muscle hypertrophy, muscle protein turnover and mitochondrial function were assessed before and after RET.
Results:
Basal capillarization (C:Fi; CFPE and LP-CF index) correlates with daily physical activity level (C:Fi, r=0.57, p=0.019; CFPE index, r=0.55, p=0.024; LC-PF index, r=0.56, p=0.022) and CFPE and LC-PF indices were also positively associated with oxidative capacity (respectively r=0.45, p=0.06; r=0.67, p=0.004). Following RET, subjects in the HIGH group underwent hypertrophy with significant improvements in muscle protein synthesis and muscle fiber CSA (p<0.05). However, RET did not promote muscle hypertrophy in the LOW group, but RET significantly increased muscle capillary density (p<0.05).
Conclusion/Implications:
Muscle fiber capillarization before starting an exercise training program may be predictive of the muscle hypertrophic response to RET in older adults. Increases in muscle fiber size following RET appear to be blunted when muscle capillarization is low, suggesting that an adequate initial capillarization is critical to achieve a meaningful degree of muscle adaptation to RET.
Keywords: capillary, muscle protein synthesis, muscle hypertrophy, aging, fiber cross-sectional area
Brief summary
Skeletal muscle capillarization is a key factor governing muscle plasticity and targeted strategies to address the age-related decline in muscle capillaries are crucial to promote an optimal hypertrophic response to exercise.
Introduction
The progressive loss of muscle mass and function that occurs with aging is associated with morphological and structural alterations within skeletal muscle. For example, aged muscles have smaller fibers (Arentson-Lantz and others 2016; Miljkovic and others 2015; Schiaffino and Reggiani 2011), a phenomenon that is particularly evident in type II fibers, along with a decrease in capillary content (Gueugneau and others 2016). The decline in skeletal muscle mass (sarcopenia) advances slowly with healthy aging but can be greatly accelerated by concurrent diseases resulting in mobility limitation, disability, frailty and loss of independence (Beaudart and others 2015; Cosquéric and others 2006). Appropriate nutritional interventions and habitual physical activity are often employed to counteract the onset of sarcopenia (Dickinson and others 2013; Fiatarone and others 1994). Resistance exercise training (RET) is the most effective strategy to increase skeletal muscle mass and strength (Bechshøft and others 2017; Raymond and others 2013). However, the muscle protein anabolic response to exercise in older adults is blunted when compared to younger adults (Fry and Rasmussen 2011; Kumar and others 2012; Kumar and others 2009; Mayhew and others 2009). The underlying mechanism(s) contributing to a blunted age-related anabolic response is not fully understood, but emerging data credit a crucial role for muscle perfusion (Charifi and others 2004; Snijders and others 2017a; Verdijk and others 2016).
Capillaries have the important function of delivering oxygen, nutrients and hormones to tissues. Adequate skeletal muscle capillarization can enhance muscle protein synthesis by ensuring the transport of amino acids and growth factors to muscle fibers (Christov and others 2007; Snijders and others 2017a). We have previously demonstrated that a moderate acute increase in physical activity can improve nutritive blood flow and stimulate the anabolic response to nutrient intake (Timmerman and others 2012). These findings, in conjunctions with others (Fujita and others 2009; Snijders and others 2017a; Timmerman and others 2010; Timmerman and Volpi 2013), led to the hypothesis that muscle capillarization above a threshold may be necessary to promote increases in protein turnover and anabolic signaling following exercise training.
It is well establish that aerobic training has an angiogenetic effect (Gavin and others 2004; Ryan and others 2006; Saltin and others 1968), promoting skeletal muscle oxidative adaptation and increasing the expression of vascular endothelial growth factor (VEGF) and angiopoietin 2 (Ang2), which are the major growth factors involved in the proliferation and stabilization of endothelial cells (Papetti and Herman 2002; Tang and others 2004; Wagner and others 2006). Increased VEGF mRNA and protein expression are also stimulated by resistance exercise training, and the acute effect of resistance exercise training on angiogenic growth factors seems to be comparable to that of endurance exercise (Gavin and others 2007a). These findings reflect a similar increase in the number of capillaries surrounding skeletal muscle fibers observed both after aerobic (Adair and others 1990; Andersen and Henriksson 1977; Kondo and others 2015) and resistant training (Gavin and others 2007a; Green and others 1999). However, due to the increase in fiber cross-sectional area that normally occurs with resistance training, increases in capillary density may be masked while increases in capillary density are generally observed after aerobic training (Green and others 1999; Snijders and others 2017a).
Recently, Snijders at al. (Snijders and others 2017a) observed, in a group of older men, that the increase in fiber size and satellite cell content in response to 24 weeks of progressive resistance exercise training was mainly driven by subjects’ preexisting muscle capillarization. The authors concluded that muscle perfusion might be a critical factor in the hypertrophic response to exercise. However, muscle capillarization didn’t change after training in that particular study (Snijders and others 2017a) but was found to increase in response to 12 weeks of RET in another study (Verdijk and others 2016). The observed discrepancy between the two studies may partially be explained by the training volume used (whole-body vs a lower limb targeted exercise protocol), which may have affected the angiogenic response to exercise. However, is worth noting that the two studies utilized subjects with similar characteristics (age, BMI) but different baseline myocellular features (fiber size, capillary-to-fiber ratio) which also may explain the different capillarization response to training.
Regular exercise can prevent a decline in muscle quality with advancing age, partially through mitigating the declines in muscle mass and insulin sensitivity (Cartee and others 2016). As such, physical activity status is crucial in determining myocellular characteristics. The most recent report from the World Health Organization shows that 32.4% of Americans and 24.5% of Europeans are physically inactive (Organization 2016). Based on the above observations, we hypothesized that reduced habitual physical activity levels in older adults would be associated with smaller muscle fiber CSA and a lower degree of muscle capillarization. The mechanisms contributing to sarcopenia are not clearly understood, and it is important to continue elucidating the cellular underpinnings of exercise adaptation in older muscle, as this will lead to more targeted strategies to improve muscle function and quality of life in older adults. Therefore, the aim of the current study was to determine whether RET alters muscle capillarization in low active (<8000 steps/day) healthy older adults and if basal muscle capillarization is predictive of the hypertrophic response to progressive resistance training.
Methods
Subjects
We performed a secondary data analysis on a study recently published from our group (Moro and others 2018) which aimed to study the acute muscle protein anabolic response to essential amino acid in older adults (registered at www.clinicaltrials.gov as ). The intent of the current secondary analysis is to explore the association between skeletal muscle capillarization and the muscle response to RET. Nineteen healthy older adults (Male, N=10, Female, N=9; age: 71.1±4.3 years; BMI: 27.6±3.2 kg•m’−2) underwent 12 weeks of RET (details of the exercise program are presented in Appendix 1). Briefly, a whole-body exercise program was performed 3 times per week for 12 weeks. Training intensity gradually increased from 60% (3 set × 15 reps) to 75% of 1RM (3 set × 10 reps), based on 1RM tests which were performed every 4 weeks (week 0, 4 and 8). A resting period of 60-75 seconds was allowed between sets and a maximum of 2 minutes rest between exercises. All participant read and signed a written informed consent form approved by local IRB before enrollment. Participants were asked to come to our facility after an overnight fast; body composition was assessed by DXA scan (dual-energy X-ray absorptiometry; Lunar iDXA, GE Healthcare, Madison, WI) and their daily activity was assessed through a step activity monitor (StepWatch activity monitors, Orthocare Innovations LLC) that subjects were wearing for 7 consecutive days the week before the infusion study day. All tests were repeated at the end of 12 weeks of RET as previously described (Moro and others 2018). Body composition was measured as total body lean and fat mass, in addition, leg lean mass, appendicular skeletal muscle (ASM) and skeletal muscle index (SMI=ASM/height2) were calculated in order to better describe the effect of exercise on limb hypertrophy and sarcopenic index.
All participants were admitted to the UTMB Institute for Translational Sciences Clinical Research Center the evening before the study. The morning of the study a background blood sample was collected and a primed-continuous infusion of L-[ring-13C6] phenylalanine (priming dose: 2 μmol/kg; infusion rate: 0.05 μmol-kg·−1min−1) was started in order to measure muscle protein synthesis rates as previously described (Brightwell and others 2019; Moro and others 2018; Reidy and others 2013).
Two and a half hours following the initiation of the tracer infusion, after a steady state of enrichment was achieved, we collected two biopsies (3 hour apart) from the vastus lateralis muscle using a Bergström needle, aseptic technique, and local anesthesia. Approximately 50 mg of muscle was oriented and embedded in Tissue Tek optimal cutting temperature (OCT, Thermo Fisher Scientific, Rockford, IL) on a cork and frozen in liquid nitrogen-cooled isopentane for immunohistochemical analysis. The remaining sample was rinsed with ice-cold saline, blotted, frozen in liquid nitrogen, and stored at −80 °C for muscle protein synthesis and western blot analysis. Post-RET muscle tissue collection was performed 72 hours after the final exercise bout, in order to avoid any acute effect of exercise on sample analysis.
To assess the impact of muscle capillarization on the hypertrophic response to training, participants were retrospectively divided into two groups based on their capillary-to-fiber perimeter exchange index (CFPE) at baseline; the partition was made based on the median value of all subjects as previously reported by other groups (Snijders and others 2017a; Snijders and others 2017b). Quantification of muscle capillarization was performed in both a mixed and fiber type-specific manner. After assessing no difference in the subdivision using the two different medians (Supplemental Figure 2), we decided to report data specific to type II fibers to allow a better comparison with other studies using a similar method. Based on type II muscle fiber CFPE values, this subdivision allowed us to have two groups with a relative HIGH (N=10; 6 Male, 4 Female, CFPE: 5.62±1.78 capillaries · 1000 μm−1) and a relative LOW (N=9; 4 Male, 5 Female, CFPE: 3.23±1.08 capillaries · 1000 μm−1) level of capillarization (Supplemental Figure 3).
Immunohistochemistry analysis
7 μm-thick sections were cut in a cryostat, and sections were allowed to air dry for >1 hour.
Samples were fixed in ice cold acetone for 10 min and then blocked for 1 hour in 2.5% normal horse serum (no. S-2012; Vector Laboratories). Slides were then incubated overnight with specific antibodies against anti-myosin heavy chain I IgG2b BA.D5 (DHSB Iowa), anti-laminin (no. L9393; Sigma Aldrich, St. Louis, MO) and rhodamine-labeled Ulex Europaeus agglutinin I (no. RL- 1062; Vector Laboratories), a human endothelial cell marker (Holthöfer and others 1982). For immunofluorescent detection, slides were incubated in secondary antibody: goat anti-rabbit AF647 (no. A21245; Invitrogen, laminin), goat anti- mouse IgG2b, AF647 (no. A21242; Invitrogen, type 1) for 1 hour, before being mounted with fluorescent mounting media.
Images were captured at 20x magnification with a Zeiss upright microscope (AxioImager M1;Zeiss,Oberkochen,Germany) and analysis completed using the AxioVision Rel software (v4.9). For each time point at least 200 muscle fibers (pre-RET, 352±43 fiber per subject; post-RET, 376±48 fiber per subject) were included in the analysis for fiber size and characterization; a subset of at least 50 fibers per subject per time-point were used to quantify the CFPE index (Snijders and others 2017a; Snijders and others 2017b).
Relative frequencies of MyHC type I were determined by quantifying all positively stained fibers (MyHC type I+), with the remaining unstained fibers counted as MyHC type I− and referred to as MyHC type II.
Muscle capillarization indices
Quantification of capillary density and CFPE index was determined as previously described (Hepple and others 1997; Snijders and others 2017a). Capillary density was determined as the number of capillaries counted per mm2, and the capillary-to-fiber ratio (C:Fi) assessed as the total number of capillaries normalized by the capillary sharing factor and divided by the number of fibers counted in each biopsy section. The CFPE index was used as an expression of the capillary supply relative to the perimeter of each fiber and was calculated as the ratio between the number of capillaries of each fiber corrected for capillary sharing factor and muscle fiber perimeter. In addition, an index of the diffusion capacity was estimated using the capillary-to-fiber interface (LC-PF index) (Gueugneau and others 2016); the LC-PF index was calculated as the percentage of the muscle fiber perimeter in contact with the capillary wall and gives information on the diffusion capacity between the capillary network and the muscle fiber. The C:Fi, CFPE index and LC-PF index were all calculated using 50 total fibers for each time point as described by Hepple et al. 1997 and others (Charifi and others 2004; Gavin and others 2007b; Hepple and others 1997; Snijders and others 2017a; Verdijk and others 2016). A schematic of each capillary index calculation is presented in supplemental Figure 4.
Muscle protein synthesis and E3 ubiquitin ligase protein expression
Details on the specific procedure to analyze fractional synthetic rate (FSR) of mixed-muscle proteins have been previously described (Moro and others 2018). Briefly, basal muscle protein synthesis was calculated by measuring incorporation of L-[ring-13C6] phenylalanine into bound protein using the precursor-product method, where muscle intracellular free amino acids serve as the precursor:
with ΔEp is the difference in protein- bound enrichment and t is the time between two consecutive biopsies. EM(1) + EM(2) are the phenylalanine tracer enrichments in the free intracellular pool. FSR was calculated as percent per hour (%/h).
Expression of proteins involved in the ubiquitin- proteasome pathway were used to indirectly investigate muscle protein breakdown. Immunoblotting analysis was performed as previously described (Brightwell and others 2019; Moro and others 2018). Muscle tissue samples were used to measure expression of Muscle RING- finger protein- 1 (MuRF1; MP3401; ECM Biosciences, Versailles, KY) and Atrogin- 1 (AP2041; ECM Biosciences). Proteins were run in duplicate and normalized to β- tubulin (2146; Cell Signaling). Donkey anti-rabbit horseradish peroxidase-conjugated IgG (1:2000; GE Healthcare ECL #NA934V) was used as secondary antibody and protein bands were visualized via chemiluminescent detection with Super Signal Dura West (Thermo Scientific, #80196). Data are expressed as arbitrary unit (AU).
Citrate Synthase (CS) activity
CS activity was analyzed as previously described (Porter and others 2015). Briefly, muscle lysates were diluted 1:270 in a 100-mM Tris-HCl buffer (pH, 8.1) containing 30 mM acetyl-CoA and 10 mM of DTNB (5,5’ -dithiobis-2-nitrobenzoic acid). Oxaloacetate (final concentration of 50 mM) was then added to each well to initiate the CS reaction. Light absorbance at 415 nM was then recorded every 30 s for 5 min at 37°C (BioRad, Hercules, CA).
CS activity (umol/g/min) was calculated from the linear change in absorbance over time as the change in light absorbance is proportional to the reaction of DTNB with free thiol groups (coenzyme A production after the condensation of oxaloacetate and acetyl-CoA). CS activity was normalized to the protein content of the muscle lysate determined using Bradford assay.
Statistical Analysis
Statistical analysis was conducted with the statistical software GraphPad Prism version 7.0 for Mac OS X (GraphPad Prism version 8.0.0 for Mac, GraphPad Software, San Diego, California USA). Our present study was insufficiently powered to identify sex differences, however subject gender was equally distributed in the two groups. For correlation analysis the Spearman correlation coefficient was used. After assessing the normal distribution (Shapiro-Wilk W test) of CFPE measurement, a two-way ANOVA model with repeated measurements was used to the test the differences among LOW and HIGH group (condition) and the pre and post-training (time), and their interaction. To assess main effects and interactions between particular time points we used pairwise comparisons. When the ANOVA model produced significant main or interaction effects, Tukey’s post hoc paired t test was used to identify specific intragroup differences. Level of significance was set at p<0.05. All values are reported as mean ± standard error (SE).
Results
Muscle fiber capillarization
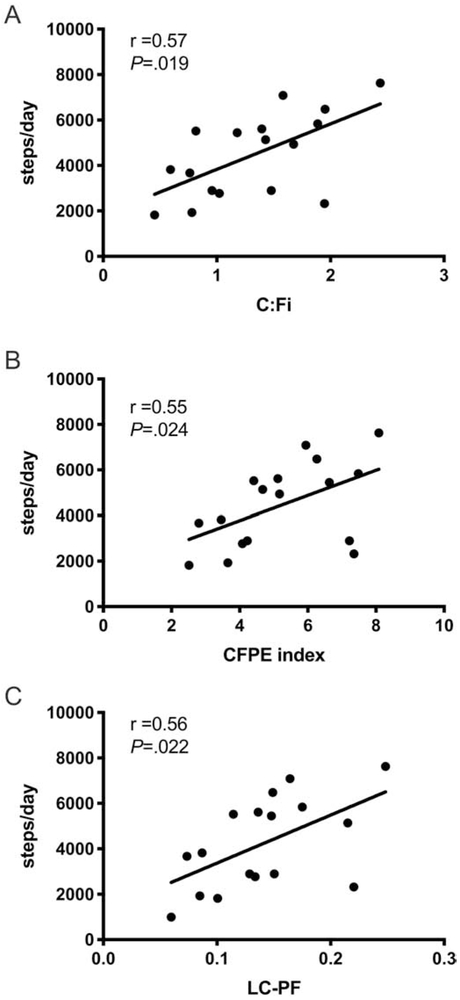
Basal capillary-to-fiber ratio (C:Fi), the perimeter exchange index (CFPE index) and the diffusion capacity (LC-PF index) between capillaries and muscle fibers were all positively correlated with the number of steps per day (Figure 1, C:Fi, Spearman r = 0.57; p=0.019; CFPE index, Spearman r = 0.55; p=0.024; LC-PF index, Spearman r = 0.56; p=0.022).
Figure 1.
Relationship between the level of physical activity (steps/day) and C:Fi, CFPE index and LC-PF index. C:Fi, capillary-to fiber ratio; CFPE, capillary-to-fiber perimeter exchange; LC-PF capillary-to-fiber interface.
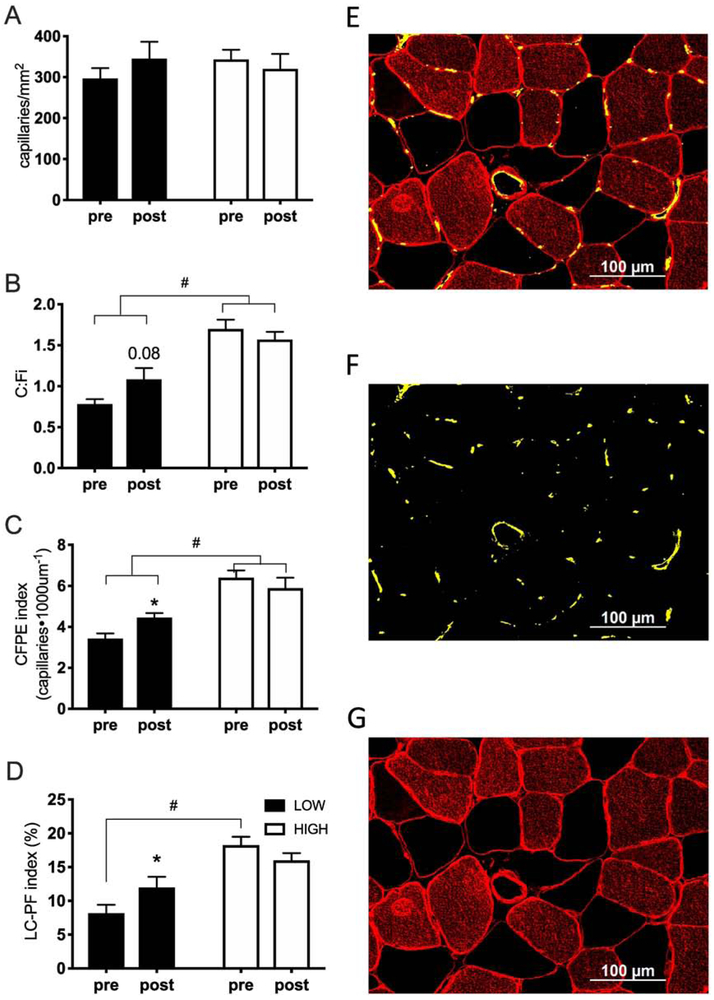
Overall, no significant change was observed pre-post RET for C:Fi, capillaries/mm2, capillary contacts (CC), CFPE or LC-PF indices. When participants were retrospectively partitioned into two groups based on their baseline CFPE index, we observed a significant interaction (p=0.038) for C:Fi, that showed a differential response in the capillary-to-fiber ratio following training in the LOW group compared to HIGH group. Only the LOW group showed an increase in C:Fi following RET (44%, p=0.087) with C:Fi in the HIGH group showing no change after RET (Figure 2B). Similar results were found with the CFPE index, which showed a significant interaction (p=0.049), and an effect for increased capillary fiber exchange capacity only in the LOW group (Figure 2C). We also observed a time by condition interaction (p=0.003) in the LC-PF index, with the percentage of the fiber perimeter directly in contact with a capillary wall significantly different between LOW and HIGH groups, and only the LOW group showing an increase in the LC-PF index following RET (Figure 2D, p=0.016).
Figure 2.
Muscle fiber capillarization indices before and after 12 weeks of RET. Muscle fiber capillarization indices before and after 12 weeks of RET (A-D) and representative immunohistochemical image demonstrating merged imaged (E), capillaries only (F) and Laminin and MyHC type I only (G). Data on fiber-type specific capillarization can be found in Table 2. (HIGH, relative high baseline CFPE index; N=9; LOW, relative low baseline CFPE index; N=10). RET, resistance exercise training; CFPE, capillary-to-fiber perimeter exchange; LC-PF capillary-to-fiber interface. Data represent mean±SE.
* significantly different from pre-RET (p<0.05).
+ main effect of condition (HIGH/LOW group) (p<0.05).
Fiber type distribution was not different following 12 weeks of RET: both groups experienced a moderate increase in type II fiber frequency with training (LOW: + 25%; HIGH: +33%), however the shift from type I to type II was not statistically significant pre-post RET (p>0.05) nor was any statistical difference found between the LOW and HIGH groups (p>0.05). Overall type I fibers had greater indices of capillarization compared to type II fibers (Table 2). Following RET, type I fiber-specific changes in muscle capillarization were similar to changes observed for type II fibers. In the LOW group, we observed an increase in CFPE (type I: 23%, p<0.001; type II: 36%), C:Fi (type I: 28%, p=0.04; type II: 52%) and LC-PF (type I, 24%, p=0.09; type II, 66%), with no significant changes occurring in the HIGH group (p>0.05) (table 2). Also in the LOW group, we observed a fiber type-specific main effect in the LC-PF index (p=0.001), with a greater increase in the LC-PF ratio in type II fibers compared to type I fibers , with no significant differences observed in the HIGH group. However, no differences were detected when comparing capillary indices expressed in a fiber type-specific or mixed fiber type manner (p>0.05).
Table 2.
Muscle fiber capillarization and muscle hypertrophy indices Pre- and Post-Intervention, grouped by basal CFPE index.
| LOW | HIGH | |||
|---|---|---|---|---|
| Pre-RET | Post-RET | Pre-RET | Post-RET | |
| Capillaries/mm2 | 296.93±75.31 | 345.24±124.25 | 334.37±72.77 | 318.00±103.36 |
| Capillaries per fiber (C:Fi) | 0.78±0.18 | 1.08±0.41* | 1.70±0.37# | 1.57±0.30 |
| Fiber type I | 0.94±0.08 | 1.20±0.15* | 1.87±0.11# | 1.69±0.16 |
| Fiber type II | 0.68±0.05‡ | 0.98±0.10‡* | 1.35±0.12‡# | 1.42±0.10 |
| CFPE index (capillaries/1000um) | 3.50±0.73 | 4.34±0.69* | 6.39±1.15# | 5.88±1.62 |
| Fiber type I | 3.87±0.15 | 4.76±0.22* | 6.80±0.32# | 6.55±0.84 |
| Fiber type II | 3.24±0.28‡ | 4.10±0.24* | 5.61±0.35‡# | 5.33±0.38 |
| LC-PF index (%) | 9.67±0.83 | 12.74±0.89* | 18.25±1.25# | 15.99±1.08 |
| Fiber type I | 11.94±0.87 | 14.25±0.99 | 20.27±1.53# | 16.93±0.84 |
| Fiber type II | 8.18±0.96‡ | 11.74±0.80* | 15.74±1.18‡# | 15.76±1.34 |
| Capillary contact (CC) | 2.25±0.53 | 2.99±0.85 | 4.53±0.82# | 4.10±0.54 |
| Fiber type I | 2.70±0.20 | 3.33±0.29* | 4.93±0.23# | 4.28±0.24 |
| Fiber type II | 1.95±0.15‡ | 2.71±0.22*‡ | 3.71±0.32*‡# | 3.89±0.17 |
| Fiber area (um2) | 3686.14±385.83 | 3965.16±604.04 | 3751.52±515.02 | 4697.06±562.41 |
| Fiber type I | 4455.80±514.89 | 4288.11±813.58 | 4244.50±596.18 | 5156.71±620.22 |
| Fiber type II | 3135.73±407.36‡ | 3428.26±526.17 | 3081.22±536.43‡ | 4131.36±557.05‡* |
Note: Values are means ± SE. CFPE, capillary-to-fiber perimeter exchange; HIGH, relative high baseline CFPE index; ASM, appendicular skeletal muscle; SMI, skeletal muscle index (ASM/height2); CSA, cross sectional area; FSR, fractional synthetic rate. N=9; LOW, relative low baseline CFPE index; N=10.
significantly different from pre-RET (p<0.05).
significantly different compared to LOW group (p<0.05).
significantly different compared to type I fibers (p<0.05).
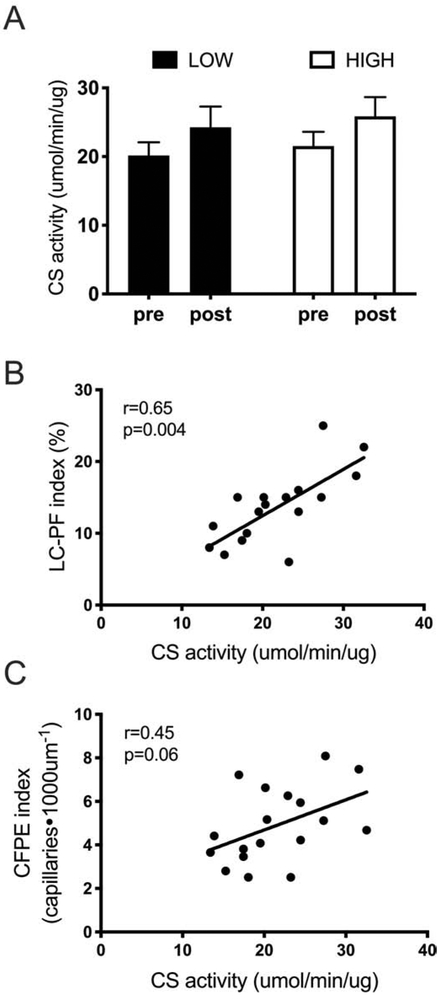
Muscle fiber citrate synthase capacity
Skeletal muscle mitochondrial protein abundance, measured by CS activity, is presented in Figure 3. The response of CS activity to RET was not different between the LOW (+20±10%) and HIGH groups (+30±18%). We observed a positive correlation between CS activity and CFPE index (r=0.45, p=0.06) and LC-PF index (r=0.65, p=0.004) pre-RET.
Figure 3.
Relationship between microvascular supply and oxidative capacity. A) Citrate synthase activity after 12 weeks of training in relative LOW (N=9) and relative HIGH (N=10) group. B) Correlation between LC-PF index and citrate synthase activity. C) Correlation between CFPE index and citrate synthase activity. CS, citrate synthase; LC-PF capillary-to-fiber interfaced; RET, resistance exercise training; HIGH, relative high baseline CFPE index. Data represent mean±SE.
Muscle hypertrophy
After 12 weeks of RET appendicular skeletal muscle and leg lean mass both significantly increased (Table 3, p=0.001). When divided by CFPE index, we observed a main effect for time in appendicular skeletal muscle (time effect p=0.004) and leg lean mass (time effect p=0.011), but post-hoc analyses reveal that only the HIGH group significantly improved in both indices of muscle hypertrophy (p=0.008). Similar results were found for type II fiber CSA (main effect of time, p=0.04), where only the HIGH group showed significant improvement in fiber CSA (LOW: +15%, HIGH +54%; p=0.049). Supportive of our data showing preferential improvements in muscle mass/fiber hypertrophy within the HIGH group, we show that the rate of basal mixed-muscle protein synthesis increased significantly following RET only in the HIGH group (Table 3, p=0.048). Muscle protein breakdown was not directly assessed, however we investigated pre- and post-RET MuRF-1 and Atrogin-1 protein expression as markers of protein degradation (Table 3). LOW and HIGH group participants had similar responses to exercise: basal levels of MuRF-1 were unchanged post-RET (LOW: 2%; HIGH −4%) while atrogin-1 expression decreased non-significantly to a similar extent in both groups post-RET (LOW: −10%; HIGH: −11%). Representative images of western blot are presented in supplemental Figure 5.
Table 3.
Muscle hypertrophy indices Pre- and Post-Intervention, grouped by basal CFPE index.
| LOW (N=9, 4 male, 5 female) |
HIGH (N=10, 6 male, 4 female) |
|||
|---|---|---|---|---|
| Hypertrophy indices | Pre-RET | Post-RET | Pre-RET | Post-RET |
| ASM (kg) | 20.2±2.3 | 20.6±2.4 | 22.7±2.7 | 23.3±2.3* |
| SMI (kg/m2) | 7.2±0.7 | 7.3±0.7 | 7.6±0.9 | 7.8±0.7* |
| Leg Lean Mass (kg) | 15.4±1.7 | 15.5±1.7 | 16.9±2.0 | 17.4±1.6* |
| Fiber type II CSA (um2) | 3135.7±407.4 | 3496.5±519.5 | 3081.2±509.8 | 4131.4±557.1* |
| FSR (%/h) | 0.06±0.01 | 0.07±0.01 | 0.06±0.01 | 0.09±0.01* |
| Protein breakdown indices | ||||
| MuRF-1 (AU) | 0.28±0.13 | 0.29±0.08 | 0.38±0.10 | 0.19±0.04 |
| Atrogin-1 (AU) | 1.38±0.40 | 1.10±0.27 | 0.91±0.34 | 0.68±0.22 |
Note: Values are means ± SE. CFPE, capillary-to-fiber perimeter exchange; HIGH, relative high baseline CFPE index; ASM, appendicular skeletal muscle; SMI, skeletal muscle index (ASM/height2); CSA, cross sectional area; FSR, fractional synthetic rate; MuRF-1, Muscle RING- finger protein-1; AU, arbitrary unit. N=9; LOW, relative low baseline CFPE index; N=10.
significantly different from pre-RET (p<0.05).
significantly different compared to LOW group (p<0.05).
Discussion
Our data provide evidence that muscle fiber capillarization may predict the hypertrophic skeletal muscle response to RET in older adults. RET is the most effective strategy to improve skeletal muscle mass and strength in both young (Kumar and others 2009; Reidy and others 2017) and older adults (Kumar and others 2012; Moro and others 2018). However, the response to training is not always homogenous, and older adults in particular show variability in their response to training (Mayhew and others 2009; Moro and others 2017). For example, in a recent study from our lab, older adults showed significant increases in leg muscle mass (+2%) and type II fiber CSA (+23%); however, there was significant heterogeneity among participants (i.e., differences in leg lean mass ranged from −4% to +9%; differences in type II fiber CSA ranged from −42% to +86%) (Moro and others 2018). The concept of muscle protein anabolic resistance is often used to describe the blunted hypertrophic response shown in older adults, yet the underlying mechanisms remain poorly understood. Previous studies have demonstrated a relationship between muscle capillarization and fiber hypertrophy (Snijders and others 2017a; Verdijk and others 2016). This is supportive of our data, where the increase in fiber size after RET was greater in individuals with higher pre-exercise muscle capillarization.
Exercise-induced angiogenesis normally occurs to increase oxygen diffusion and to enhance the removal of metabolites during muscle contraction (Hoier and Hellsten 2014). Three physiological factors promote skeletal muscle angiogenesis: increased blood flow, associated increase in endothelial shear stress, and enhanced oxidative metabolism. Due to its anaerobic nature, resistance exercise training primarily promotes muscle angiogenesis by stimulating blood flow and inducing mechanical shear stress. These two mechanisms stimulate the activation of specific signaling pathways (PECAM-1, VEGFR, etc.) and promote endothelial cell proliferation which leads to capillary growth (Gavin and others 2007a; Hudlicka and others 2006). Resistance exercise increases the capillary-to fiber ratio; however the concomitant accrual of fiber cross-sectional area may mask the improvement in capillary density, which often remains unchanged (Green and others 1999; Snijders and others 2017a). Moreover, resistance exercise protocols can differ in terms of intensity, volume, contraction type and duration (Paoli 2012; Paoli and Bianco 2012). Exercise programs with comparable intensity and volume have similar effects on muscle capillarization (Green and others 1999; McCall and others 1999; Snijders and others 2017a). However, levels of shear stress are mediated by specific mechano-sensors (Hoier and Hellsten 2014), and thus different contraction modalities may modify the tension applied from the sarcomere to the endothelial cell, resulting in different shear stress intensities. Some early studies suggest that muscle hypertrophy in trained individuals may occur without a parallel increase in muscle capillarization (Tesch and others 1989; Tesch and others 1984); however capillary density and capillary-to-fiber ratios are lower in untrained/sedentary individuals when compared to trained individuals (Brodal and others 1977; Zoladz and others 2005). Additionally, endurance athletes present higher capillarization when compared to power or strength athletes (Zoladz and others 2005).
We stratified our participants based on their muscle fiber capillarization (CFPE index) at the time of recruitment. A similar approach has been utilized by others (Snijders and others 2017a; Verdijk and others 2016), but, to our knowledge, this is the first time that several indices of capillarization have been studied together to comprehensively describe the role of capillaries on the muscle protein anabolic response to RET. Specifically, we assessed capillaries per mm2 in addition to C:Fi, as indices of capillary density; the CFPE index, as an estimation of capillary exchange capacity between the muscle cell and capillary bed (Hepple and others 1997; Lithell and others 1981; Tesch and Wright 1983); and the LC-PF index as an additional measure of direct fiber-to-capillary interaction (Charifi and others 2004; Gueugneau and others 2016; Sullivan and Pittman 1987). We found that subjects with a lower pre-RET CFPE index underwent an increase in C:Fi, CFPE and LC-PF indices following 12 weeks of RET, without a significant increase in muscle fiber CSA or leg/whole body lean mass. Intriguingly, the HIGH group did not show any improvement in various muscle capillarization indices yet demonstrated a significant improvement in leg muscle and fiber size. Supportive of these results is the increase in the basal rate of muscle protein synthesis following training in the HIGH group (Table 3, LOW: +18%; HIGH: +36%). As protein breakdown was not directly assessed, we utilized the expression of two markers of protein degradation as a proxy for breakdown: muscle specific ring finger protein 1 (MuRF1) and atrogin-1. These two proteins mediate the ubiquitin-proteasome pathway which is actively involved in the breakdown of myofibrillar proteins (Fry and others 2013; Masiero and others 2009). Human exercise studies have reported an acute increase in MuRF1 expression but relatively little change in the expression of atrogin-1 or other E3 ubiquitin ligases (Borgenvik and others 2012; Nedergaard and others 2007). However, longitudinal RET studies have shown a reduction in both MuRF1 and atrogin-1 expression demonstrating that protein breakdown, and thus protein degradation, is reduced with exercise training (Stefanetti and others 2014; Stefanetti and others 2015). Our results support these findings, with a subtle decrease in atrogin-1 expression observed following RET. Importantly though, we didn’t find any difference in E3 ubiquitin ligase expression between the LOW and HIGH groups. Several studies have shown that chronic RET modulates muscle-protein turnover (Phillips and others 1999; Tang and others 2008; Wilkinson and others 2008) and elevates the rate of basal post-absorptive MPS compared to pre-training levels (Damas and others 2016; Reidy and others 2017). Recently Reidy et al. demonstrated that the changes in basal post-absorptive MPS correlate positively with indices of muscle hypertrophy following RET (Reidy and others 2017). Further, vastus lateralis thickness and integrated MPS are positively correlated in the early training adaptation period in young men (Brook and others 2015), and integrated MPS over several weeks of RET is strongly associated with indices of muscle hypertrophy (Damas and others 2016). These studies suggest that the adaptation of muscle protein turnover to RET may contribute to muscle hypertrophy. Thus, the greater increase in the basal rate of muscle protein synthesis observed in the HIGH group and the similar expression of markers of protein degradation in both groups, provides support for our hypothesis that pre-existing capillarization could be a significant predictor of positive muscle protein adaptation following RET.
Previous studies have reported similar response to RET on muscle size between male and female participants (Abe and others 2000; Markofski and others 2015). Our present study was insufficiently powered to identify sex differences, but male and female participants were equally distributed in the two groups. As expected, male and female participants in our study had different lean body mass and fiber CSA pre-RET, similar to what others have shown (Bea and others 2011; Haizlip and others 2015; Staron and others 2000). Our exploratory analysis of sex-based differences in basal CFPE failed to detect a statistically significant difference between the sexes (data not presented). To our knowledge capillary distribution seems to be similar between males and females, but the influence of sex on the correlation between muscle capillarization and the hypertrophic response to RET needs to be addressed with future research.
While Snijders and colleagues report that muscle fiber growth was not accompanied by a concomitant increase in capillary density, we show that individuals with LOW muscle capillarization do undergo angiogenesis (increased C:Fi) and improve their capacity of exchange and diffusion between capillaries and myofibers following 12 weeks of training. These results were particularly evident in type II fibers, but were also confirmed in type I fibers as well. The discrepancy between our results and those reported in Snijders et al. could be explained by the difference in the baseline characteristics of our two LOW groups (Snijders and others 2017a), as our subjects show a much lower basal capillarization level compared to those published by Snijders et al. (Supplemental Figure 2; CFPE index: 3.2±0.3 in our LOW group vs 4.4±0.2 in (Snijders and others 2017a)). We also observed a strong correlation between physical activity and capillarization indices at baseline (Figure 1), which may help to address the differences between our subjects and those observed by Snijders et al. (Snijders and others 2017a). Their study was conducted in northern Europe, and the northern European population is on average more active than the typical American adult (Hallal and others 2012; Van Deveire and others 2012). Also, leg blood flow has been shown to be better preserved in trained subjects compared to sedentary (Anton and others 2006; Tanimoto and others 2009), and even a moderate acute increase in physical activity can enhance leg blood microperfusion and, thus, increase the response to anabolic stimuli in older adults (Fujita and others 2009; Timmerman and others 2012; Timmerman and others 2010). These observations, together with the results of the present study, suggest that habitual physical activity level can alter the muscle phenotype and help promote RET adaptation. Moreover, as the CFPE index was not significantly different between LOW and HIGH groups after RET, this could suggest that a longer period of training could generate a similar hypertrophic response in both groups, as the LOW group will have acquired the requisite capillarization level to support optimal muscle growth.
Many studies investigating the effect of training on muscle capillarization have utilized aerobic training (Charifi and others 2004; Gavin and others 2015; Prior and others 2014; Prior and others 2016), as aerobic training is known to promote increases in maximum oxygen uptake and microvasculature improvements. However, growing evidence suggests that progressive resistance exercise training can improve muscle oxidative capacity by increasing both qualitative and quantitative mitochondrial adaptations (i.e., increased VO2 peak, VO2max, CS activity and mitochondrial function) (Ades and others 1996; Jubrias and others 2001; Porter and others 2015; Sparks and others 2013). Endurance performance and metabolic health are strongly impacted by skeletal muscle mitochondrial content (Goodpaster 2013; MacInnis and others 2017). While increased activity of mitochondrial enzymes is an important adaptation to improve muscle performance and oxygen uptake, another important factor supporting VO2max adaptations is an delivery of O2 to the muscle (Bassett and Howley 2000). As suggested by Charifi et al. (Charifi and others 2004), older adults may respond to training by increasing their pre-existing capillary length rather than promoting angiogenesis. Their group shows a strong relationship between the LC-PF index and citrate synthase (CS) activity, demonstrating that the fiber-capillary exchange surface area responds strongly to the increased oxygen demand of muscle to exercise, as the increment in the LC-PF index post-exercise was more pronounced than the increase in CS activity (Charifi and others 2004). In our study, this relationship was partially confirmed: the LC-PF index increased only in the LOW group (LOW +34%, HIGH −4%), while CS activity increased similarly in both groups (Figure 3, LOW: +20%, HIGH +30%). Our results suggest that the early adaptive cellular response is to increase the available exchange surface area between capillaries and muscle fibers. Only when muscle fibers have an adequate capacity to exchange and diffuse nutrients/oxygen (increase in CFPE and LC-PF index) an induction in the rate of protein synthesis can occur to induce muscle growth (increase in muscle FSR and fiber CSA).
Conclusions and Implications
Our data imply that a minimal level of muscle capillarization is needed to achieve muscle hypertrophy in older adults. Our data suggest the need for more targeted strategies to address capillarization deficits in older adults that appear to be integral in the response to RET. We conclude that muscle capillarization plays a crucial role in regulating muscle adaptation. We are unable to physically define a minimal thresholds for capillarization that can predict the hypertrophic response to training in older adults; however, our data underscore the role for capillaries in regulating positive muscle adaptation. For example, in an individual with lower level of capillarization, perhaps a targeted aerobic regimen could be useful to improve the available exchange surface area between muscle and capillaries prior to the initiation of a resistance training program to better support the hypertrophic response to RET.
Supplementary Material
Supplemental Figure 1. Exercise program.
Supplemental Figure 2. Comparison between basal fiber type II CFPE index in different studies.
Supplemental Figure 3. Representative image for LOW and HIGH group muscle capillarization.
Supplemental Figure 4. Schematic for capillary idiocies measurement.
Supplemental Figure 5. Representative image of western blot.
Table 1.
Anthropometric Data of Participants, Grouped by basal CFPE index.
| LOW (N=9, 4 male, 5 female) |
HIGH (N=10, 6 male, 4 female) |
|
|---|---|---|
| Age (years) | 72.2±5.2 | 70.0±3.3 |
| Body height (m) | 1.7±0.1 | 1.7±0.1 |
| Body mass (kg) | 77.1±14.9 | 83.1±11.4 |
| BMI (kg/m2) | 27.5±3.0 | 28.0±3.1 |
| Fat mass (%) | 40.4±5.5 | 36.2±9.9 |
| ASM (kg) | 20.2±5.4 | 22.7±4.8 |
| SMI (kg/m2) | 7.2±1.3 | 7.6±1.2 |
| Leg Lean Mass (kg) | 15.4±4.1 | 16.9±3.2 |
Note: BMI = Body mass index, CFPE = capillary-to-fiber perimeter exchange index; ASM, appendicular skeletal muscle; SMI, skeletal muscle index (ASM/height2). LOW and HIGH represent the two groups obtained with the retrospective subdivision based on the median value of all subject’s CFPE index. Values are means ± SD.
Acknowledgments
Funding sources:
This work was supported by the National Institute on Aging at the National Institute of Health grants (R56 AG051267 and P30 AG024832) and the National Center for Advancing Translational Sciences at the National Institute of Health grant (UL1 TR001439).
Acknowledgments
This study was approved by the UTMB Institutional Review Board, and complied with the guidelines set out in the Declaration of Helsinki as revised in 2008. Participants gave their informed written consent prior to their inclusion in the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
All authors report no conflict of interest.
Online supplementary material
Supporting information may be found in the online version of this article.
References
- Abe T; DeHoyos DV; Pollock ML; Garzarella L Time course for strength and muscle thickness changes following upper and lower body resistance training in men and women. Eur J Appl Physiol. 81:174–180; 2000 [DOI] [PubMed] [Google Scholar]
- Adair TH; Gay WJ; Montani JP Growth regulation of the vascular system: evidence for a metabolic hypothesis. Am J Physiol. 259:R393–404; 1990 [DOI] [PubMed] [Google Scholar]
- Ades PA; Ballor DL; Ashikaga T; Utton JL; Nair KS Weight training improves walking endurance in healthy elderly persons. Ann Intern Med. 124:568–572; 1996 [DOI] [PubMed] [Google Scholar]
- Andersen P; Henriksson J Capillary supply of the quadriceps femoris muscle of man: adaptive response to exercise. J Physiol. 270:677–690; 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton MM; Cortez-Cooper MY; DeVan AE; Neidre DB; Cook JN; Tanaka H Resistance training increases basal limb blood flow and vascular conductance in aging humans. J Appl Physiol (1985). 101:1351–1355; 2006 [DOI] [PubMed] [Google Scholar]
- Arentson-Lantz EJ; Paddon-Jones D; Fry CS The intersection of disuse-induced muscle atrophy and satellite cell content: reply to Snijders, Nederveen, and Parise. J Appl Physiol (1985). 120:1491; 2016 [DOI] [PubMed] [Google Scholar]
- Bassett DR; Howley ET Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med Sci Sports Exerc. 32:70–84; 2000 [DOI] [PubMed] [Google Scholar]
- Bea JW; Zhao Q; Cauley JA; LaCroix AZ; Bassford T; Lewis CE; Jackson RD; Tylavsky FA; Chen Z Effect of hormone therapy on lean body mass, falls, and fractures: 6-year results from the Women's Health Initiative hormone trials. Menopause. 18:44–52; 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudart C; Reginster JY; Petermans J; Gillain S; Quabron A; Locquet M; Slomian J; Buckinx F; Bruyère O Quality of life and physical components linked to sarcopenia: The SarcoPhAge study. Exp Gerontol. 69:103–110; 2015 [DOI] [PubMed] [Google Scholar]
- Bechshøft RL; Malmgaard-Clausen NM; Gliese B; Beyer N; Mackey AL; Andersen JL; Kjær M; Holm L Improved skeletal muscle mass and strength after heavy strength training in very old individuals. Exp Gerontol. 92:96–105; 2017 [DOI] [PubMed] [Google Scholar]
- Borgenvik M; Apró W; Blomstrand E Intake of branched-chain amino acids influences the levels of MAFbx mRNA and MuRF-1 total protein in resting and exercising human muscle. Am J Physiol Endocrinol Metab. 302:E510–521; 2012 [DOI] [PubMed] [Google Scholar]
- Brightwell CR; Markofski MM; Moro T; Fry CS; Porter C; Volpi E; Rasmussen BB Moderate-intensity aerobic exercise improves skeletal muscle quality in older adults. Transl Sports Med. 2:109–119; 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodal P; Ingjer F; Hermansen L Capillary supply of skeletal muscle fibers in untrained and endurance-trained men. Am J Physiol. 232:H705–712; 1977 [DOI] [PubMed] [Google Scholar]
- Brook MS; Wilkinson DJ; Mitchell WK; Lund JN; Szewczyk NJ; Greenhaff PL; Smith K; Atherton PJ Skeletal muscle hypertrophy adaptations predominate in the early stages of resistance exercise training, matching deuterium oxide-derived measures of muscle protein synthesis and mechanistic target of rapamycin complex 1 signaling. FASEB J. 29:4485–4496; 2015 [DOI] [PubMed] [Google Scholar]
- Cartee GD; Hepple RT; Bamman MM; Zierath JR Exercise Promotes Healthy Aging of Skeletal Muscle. Cell Metab. 23:1034–1047; 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charifi N; Kadi F; Féasson L; Costes F; Geyssant A; Denis C Enhancement of microvessel tortuosity in the vastus lateralis muscle of old men in response to endurance training. J Physiol. 554:559–569; 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christov C; Chrétien F; Abou-Khalil R; Bassez G; Vallet G; Authier FJ; Bassaglia Y; Shinin V; Tajbakhsh S; Chazaud B; Gherardi RK Muscle satellite cells and endothelial cells: close neighbors and privileged partners. Mol Biol Cell. 18:1397–1409; 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosquéric G; Sebag A; Ducolombier C; Thomas C; Piette F; Weill-Engerer S Sarcopenia is predictive of nosocomial infection in care of the elderly. Br J Nutr. 96:895–901; 2006 [DOI] [PubMed] [Google Scholar]
- Damas F; Phillips SM; Libardi CA; Vechin FC; Lixandrão ME; Jannig PR; Costa LA; Bacurau AV; Snijders T; Parise G; Tricoli V; Roschel H; Ugrinowitsch C Resistance training-induced changes in integrated myofibrillar protein synthesis are related to hypertrophy only after attenuation of muscle damage. J Physiol. 594:5209–5222; 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson JM; Volpi E; Rasmussen BB Exercise and Nutrition to Target Protein Synthesis Impairments in Aging Skeletal Muscle. Exercise and Sport Sciences Reviews. 41:216–223 210.1097/JES.1090b1013e3182a1094e1699; 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiatarone MA; O'Neill EF; Ryan ND; Clements KM; Solares GR; Nelson ME; Roberts SB; Kehayias JJ; Lipsitz LA; Evans WJ Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med. 330:1769–1775; 1994 [DOI] [PubMed] [Google Scholar]
- Fry CS; Drummond MJ; Glynn EL; Dickinson JM; Gundermann DM; Timmerman KL; Walker DK; Volpi E; Rasmussen BB Skeletal muscle autophagy and protein breakdown following resistance exercise are similar in younger and older adults. J Gerontol A Biol Sci Med Sci. 68:599–607; 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry CS; Rasmussen BB Skeletal muscle protein balance and metabolism in the elderly. Curr Aging Sci. 4:260–268; 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita S; Glynn EL; Timmerman KL; Rasmussen BB; Volpi E Supraphysiological hyperinsulinaemia is necessary to stimulate skeletal muscle protein anabolism in older adults: evidence of a true age-related insulin resistance of muscle protein metabolism. Diabetologia. 52:1889–1898; 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin TP; Drew JL; Kubik CJ; Pofahl WE; Hickner RC Acute resistance exercise increases skeletal muscle angiogenic growth factor expression. Acta Physiol (Oxf). 191:139–146; 2007a [DOI] [PubMed] [Google Scholar]
- Gavin TP; Kraus RM; Carrithers JA; Garry JP; Hickner RC Aging and the Skeletal Muscle Angiogenic Response to Exercise in Women. J Gerontol A Biol Sci Med Sci. 70:1189–1197; 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin TP; Robinson CB; Yeager RC; England JA; Nifong LW; Hickner RC Angiogenic growth factor response to acute systemic exercise in human skeletal muscle. J Appl Physiol (1985). 96:19–24; 2004 [DOI] [PubMed] [Google Scholar]
- Gavin TP; Ruster RS; Carrithers JA; Zwetsloot KA; Kraus RM; Evans CA; Knapp DJ; Drew JL; McCartney JS; Garry JP; Hickner RC No difference in the skeletal muscle angiogenic response to aerobic exercise training between young and aged men. J Physiol. 585:231–239; 2007b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodpaster BH Mitochondrial deficiency is associated with insulin resistance. Diabetes. 62:1032–1035; 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green H; Goreham C; Ouyang J; Ball-Burnett M; Ranney D Regulation of fiber size, oxidative potential, and capillarization in human muscle by resistance exercise. Am J Physiol. 276:R591–596; 1999 [DOI] [PubMed] [Google Scholar]
- Gueugneau M; Coudy-Gandilhon C; Meunier B; Combaret L; Taillandier D; Polge C; Attaix D; Roche F; Féasson L; Barthélémy JC; Béchet D Lower skeletal muscle capillarization in hypertensive elderly men. Exp Gerontol. 76:80–88; 2016 [DOI] [PubMed] [Google Scholar]
- Haizlip KM; Harrison BC; Leinwand LA Sex-based differences in skeletal muscle kinetics and fiber-type composition. Physiology (Bethesda). 30:30–39; 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallal PC; Andersen LB; Bull FC; Guthold R; Haskell W; Ekelund U; Group LPASW Global physical activity levels: surveillance progress, pitfalls, and prospects. Lancet. 380:247–257; 2012 [DOI] [PubMed] [Google Scholar]
- Hepple RT; Mackinnon SL; Goodman JM; Thomas SG; Plyley MJ Resistance and aerobic training in older men: effects on VO2peak and the capillary supply to skeletal muscle. J Appl Physiol (1985). 82:1305–1310; 1997 [DOI] [PubMed] [Google Scholar]
- Hoier B; Hellsten Y Exercise-induced capillary growth in human skeletal muscle and the dynamics of VEGF. Microcirculation. 21:301–314; 2014 [DOI] [PubMed] [Google Scholar]
- Holthöfer H; Virtanen I; Kariniemi AL; Hormia M; Linder E; Miettinen A Ulex europaeus I lectin as a marker for vascular endothelium in human tissues. Lab Invest. 47:60–66; 1982 [PubMed] [Google Scholar]
- Hudlicka O; Brown MD; May S; Zakrzewicz A; Pries AR Changes in capillary shear stress in skeletal muscles exposed to long-term activity: role of nitric oxide. Microcirculation. 13:249–259; 2006 [DOI] [PubMed] [Google Scholar]
- Jubrias SA; Esselman PC; Price LB; Cress ME; Conley KE Large energetic adaptations of elderly muscle to resistance and endurance training. J Appl Physiol (1985). 90:1663–1670; 2001 [DOI] [PubMed] [Google Scholar]
- Kondo H; Fujino H; Murakami S; Tanaka M; Kanazashi M; Nagatomo F; Ishihara A; Roy RR Low-intensity running exercise enhances the capillary volume and pro-angiogenic factors in the soleus muscle of type 2 diabetic rats. Muscle Nerve. 51:391–399; 2015 [DOI] [PubMed] [Google Scholar]
- Kumar V; Atherton PJ; Selby A; Rankin D; Williams J; Smith K; Hiscock N; Rennie MJ Muscle protein synthetic responses to exercise: effects of age, volume, and intensity. J Gerontol A Biol Sci Med Sci. 67:1170–1177; 2012 [DOI] [PubMed] [Google Scholar]
- Kumar V; Selby A; Rankin D; Patel R; Atherton P; Hildebrandt W; Williams J; Smith K; Seynnes O; Hiscock N; Rennie MJ Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol. 587:211–217; 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lithell H; Lindgärde F; Nygaard E; Saltin B Capillary supply and lipoprotein-lipase activity in skeletal muscle in man. Acta Physiol Scand. 111:383–384; 1981 [DOI] [PubMed] [Google Scholar]
- MacInnis MJ; Zacharewicz E; Martin BJ; Haikalis ME; Skelly LE; Tarnopolsky MA; Murphy RM; Gibala MJ Superior mitochondrial adaptations in human skeletal muscle after interval compared to continuous single-leg cycling matched for total work. J Physiol. 595:2955–2968; 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markofski MM; Dickinson JM; Drummond MJ; Fry CS; Fujita S; Gundermann DM; Glynn EL; Jennings K; Paddon-Jones D; Reidy PT; Sheffield-Moore M; Timmerman KL; Rasmussen BB; Volpi E Effect of age on basal muscle protein synthesis and mTORC1 signaling in a large cohort of young and older men and women. Exp Gerontol. 65:1–7; 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masiero E; Agatea L; Mammucari C; Blaauw B; Loro E; Komatsu M; Metzger D; Reggiani C; Schiaffino S; Sandri M Autophagy is required to maintain muscle mass. Cell Metab. 10:507–515; 2009 [DOI] [PubMed] [Google Scholar]
- Mayhew DL; Kim JS; Cross JM; Ferrando AA; Bamman MM Translational signaling responses preceding resistance training-mediated myofiber hypertrophy in young and old humans. J Appl Physiol (1985). 107:1655–1662; 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall GE; Byrnes WC; Fleck SJ; Dickinson A; Kraemer WJ Acute and chronic hormonal responses to resistance training designed to promote muscle hypertrophy. Can J Appl Physiol. 24:96–107; 1999 [DOI] [PubMed] [Google Scholar]
- Miljkovic N; Lim JY; Miljkovic I; Frontera WR Aging of skeletal muscle fibers. Ann Rehabil Med. 39:155–162; 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro T; Brightwell CR; Deer RR; Graber TG; Galvan E; Fry CS; Volpi E; Rasmussen BB Muscle Protein Anabolic Resistance to Essential Amino Acids Does Not Occur in Healthy Older Adults Before or After Resistance Exercise Training. J Nutr. 148:900–909; 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro T; Tinsley G; Bianco A; Gottardi A; Gottardi GB; Faggian D; Plebani M; Marcolin G; Paoli A High intensity interval resistance training (HIIRT) in older adults: Effects on body composition, strength, anabolic hormones and blood lipids. Exp Gerontol. 98:91–98; 2017 [DOI] [PubMed] [Google Scholar]
- Nedergaard A; Vissing K; Overgaard K; Kjaer M; Schjerling P Expression patterns of atrogenic and ubiquitin proteasome component genes with exercise: effect of different loading patterns and repeated exercise bouts. J Appl Physiol (1985). 103:1513–1522; 2007 [DOI] [PubMed] [Google Scholar]
- Organization, W.H. Physical Inactivity: A Global Public Health Problem. 2016
- Paoli A Resistance training: the multifaceted side of exercise. Am J Physiol Endocrinol Metab. 302:E387; 2012 [DOI] [PubMed] [Google Scholar]
- Paoli A; Bianco A Not all exercises are created equal. Am J Cardiol. 109:305; 2012 [DOI] [PubMed] [Google Scholar]
- Papetti M; Herman IM Mechanisms of normal and tumor-derived angiogenesis. Am J Physiol Cell Physiol. 282:C947–970; 2002 [DOI] [PubMed] [Google Scholar]
- Phillips SM; Tipton KD; Ferrando AA; Wolfe RR Resistance training reduces the acute exercise-induced increase in muscle protein turnover. Am J Physiol. 276:E118–124; 1999 [DOI] [PubMed] [Google Scholar]
- Porter C; Reidy PT; Bhattarai N; Sidossis LS; Rasmussen BB Resistance Exercise Training Alters Mitochondrial Function in Human Skeletal Muscle. Med Sci Sports Exerc. 47:1922–1931; 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior SJ; Blumenthal JB; Katzel LI; Goldberg AP; Ryan AS Increased skeletal muscle capillarization after aerobic exercise training and weight loss improves insulin sensitivity in adults with IGT. Diabetes Care. 37:1469–1475; 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior SJ; Ryan AS; Blumenthal JB; Watson JM; Katzel LI; Goldberg AP Sarcopenia Is Associated With Lower Skeletal Muscle Capillarization and Exercise Capacity in Older Adults. J Gerontol A Biol Sci Med Sci. 71:1096–1101; 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond MJ; Bramley-Tzerefos RE; Jeffs KJ; Winter A; Holland AE Systematic review of high-intensity progressive resistance strength training of the lower limb compared with other intensities of strength training in older adults. Arch Phys Med Rehabil. 94:1458–1472; 2013 [DOI] [PubMed] [Google Scholar]
- Reidy PT; Borack MS; Markofski MM; Dickinson JM; Fry CS; Deer RR; Volpi E; Rasmussen BB Post-absorptive muscle protein turnover affects resistance training hypertrophy. Eur J Appl Physiol. 117:853–866; 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidy PT; Walker DK; Dickinson JM; Gundermann DM; Drummond MJ; Timmerman KL; Fry CS; Borack MS; Cope MB; Mukherjea R; Jennings K; Volpi E; Rasmussen BB Protein blend ingestion following resistance exercise promotes human muscle protein synthesis. J Nutr. 143:410–416; 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan NA; Zwetsloot KA; Westerkamp LM; Hickner RC; Pofahl WE; Gavin TP Lower skeletal muscle capillarization and VEGF expression in aged vs. young men. J Appl Physiol (1985). 100:178–185; 2006 [DOI] [PubMed] [Google Scholar]
- Saltin B; Blomqvist G; Mitchell JH; Johnson RL; Wildenthal K; Chapman CB Response to exercise after bed rest and after training. Circulation. 38:VII1–78; 1968 [PubMed] [Google Scholar]
- Schiaffino S; Reggiani C Fiber types in mammalian skeletal muscles. Physiol Rev. 91:1447–1531; 2011 [DOI] [PubMed] [Google Scholar]
- Snijders T; Nederveen JP; Joanisse S; Leenders M; Verdijk LB; van Loon LJ; Parise G Muscle fibre capillarization is a critical factor in muscle fibre hypertrophy during resistance exercise training in older men. J Cachexia Sarcopenia Muscle. 8:267–276; 2017a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijders T; Nederveen JP; Verdijk LB; Houben AJHM; Goossens GH; Parise G; van Loon LJC Muscle fiber capillarization as determining factor on indices of insulin sensitivity in humans. Physiol Rep. 5; 2017b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks LM; Johannsen NM; Church TS; Earnest CP; Moonen-Kornips E; Moro C; Hesselink MK; Smith SR; Schrauwen P Nine months of combined training improves ex vivo skeletal muscle metabolism in individuals with type 2 diabetes. J Clin Endocrinol Metab. 98:1694–1702; 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staron RS; Hagerman FC; Hikida RS; Murray TF; Hostler DP; Crill MT; Ragg KE; Toma K Fiber type composition of the vastus lateralis muscle of young men and women. J Histochem Cytochem. 48:623–629; 2000 [DOI] [PubMed] [Google Scholar]
- Stefanetti RJ; Lamon S; Rahbek SK; Farup J; Zacharewicz E; Wallace MA; Vendelbo MH; Russell AP; Vissing K Influence of divergent exercise contraction mode and whey protein supplementation on atrogin-1, MuRF1, and FOXO1/3A in human skeletal muscle. J Appl Physiol (1985). 116:1491–1502; 2014 [DOI] [PubMed] [Google Scholar]
- Stefanetti RJ; Lamon S; Wallace M; Vendelbo MH; Russell AP; Vissing K Regulation of ubiquitin proteasome pathway molecular markers in response to endurance and resistance exercise and training. Pflugers Arch. 467:1523–1537; 2015 [DOI] [PubMed] [Google Scholar]
- Sullivan SM; Pittman RN Relationship between mitochondrial volume density and capillarity in hamster muscles. Am J Physiol. 252:H149–155; 1987 [DOI] [PubMed] [Google Scholar]
- Tang JE; Perco JG; Moore DR; Wilkinson SB; Phillips SM Resistance training alters the response of fed state mixed muscle protein synthesis in young men. Am J Physiol Regul Integr Comp Physiol. 294:R172–178; 2008 [DOI] [PubMed] [Google Scholar]
- Tang K; Breen EC; Gerber HP; Ferrara NM; Wagner PD Capillary regression in vascular endothelial growth factor-deficient skeletal muscle. Physiol Genomics. 18:63–69; 2004 [DOI] [PubMed] [Google Scholar]
- Tanimoto M; Kawano H; Gando Y; Sanada K; Yamamoto K; Ishii N; Tabata I; Miyachi M Low-intensity resistance training with slow movement and tonic force generation increases basal limb blood flow. Clin Physiol Funct Imaging. 29:128–135; 2009 [DOI] [PubMed] [Google Scholar]
- Tesch PA; Thorsson A; Essén-Gustavsson B Enzyme activities of FT and ST muscle fibers in heavy-resistance trained athletes. J Appl Physiol (1985). 67:83–87; 1989 [DOI] [PubMed] [Google Scholar]
- Tesch PA; Thorsson A; Kaiser P Muscle capillary supply and fiber type characteristics in weight and power lifters. J Appl Physiol Respir Environ Exerc Physiol. 56:35–38; 1984 [DOI] [PubMed] [Google Scholar]
- Tesch PA; Wright JE Recovery from short term intense exercise: its relation to capillary supply and blood lactate concentration. Eur J Appl Physiol Occup Physiol. 52:98–103; 1983 [DOI] [PubMed] [Google Scholar]
- Timmerman KL; Dhanani S; Glynn EL; Fry CS; Drummond MJ; Jennings K; Rasmussen BB; Volpi E A moderate acute increase in physical activity enhances nutritive flow and the muscle protein anabolic response to mixed nutrient intake in older adults. Am J Clin Nutr. 95:1403–1412; 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmerman KL; Lee JL; Dreyer HC; Dhanani S; Glynn EL; Fry CS; Drummond MJ; Sheffield-Moore M; Rasmussen BB; Volpi E Insulin stimulates human skeletal muscle protein synthesis via an indirect mechanism involving endothelial-dependent vasodilation and mammalian target of rapamycin complex 1 signaling. J Clin Endocrinol Metab. 95:3848–3857; 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmerman KL; Volpi E Endothelial function and the regulation of muscle protein anabolism in older adults. Nutr Metab Cardiovasc Dis. 23 Suppl 1:S44–50; 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Deveire KN; Scranton SK; Kostek MA; Angelopoulos TJ; Clarkson PM; Gordon PM; Moyna NM; Visich PS; Zoeller RF; Thompson PD; Devaney JM; Gordish-Dressman H; Hoffman EP; Maresh CM; Pescatello LS Variants of the ankyrin repeat domain 6 gene (ANKRD6) and muscle and physical activity phenotypes among European-derived American adults. J Strength Cond Res. 26:1740–1748; 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdijk LB; Snijders T; Holloway TM; VAN Kranenburg J; VAN Loon LJ Resistance Training Increases Skeletal Muscle Capillarization in Healthy Older Men. Med Sci Sports Exerc. 48:2157–2164; 2016 [DOI] [PubMed] [Google Scholar]
- Wagner PD; Olfert IM; Tang K; Breen EC Muscle-targeted deletion of VEGF and exercise capacity in mice. Respir Physiol Neurobiol. 151:159–166; 2006 [DOI] [PubMed] [Google Scholar]
- Wilkinson SB; Phillips SM; Atherton PJ; Patel R; Yarasheski KE; Tarnopolsky MA; Rennie MJ Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle. J Physiol. 586:3701–3717; 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoladz JA; Semik D; Zawadowska B; Majerczak J; Karasinski J; Kolodziejski L; Duda K; Kilarski WM Capillary density and capillary-to-fibre ratio in vastus lateralis muscle of untrained and trained men. Folia Histochem Cytobiol. 43:11–17; 2005 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Exercise program.
Supplemental Figure 2. Comparison between basal fiber type II CFPE index in different studies.
Supplemental Figure 3. Representative image for LOW and HIGH group muscle capillarization.
Supplemental Figure 4. Schematic for capillary idiocies measurement.
Supplemental Figure 5. Representative image of western blot.