Abstract
Background
HbA1c, the most commonly used indicator of chronic glucose metabolism, is closely associated with cardiovascular disease. However, the relationship between HbA1c and the mortality of acute coronary syndrome (ACS) patients has not been elucidated yet. Here, we aim to conduct a systematic review assessing the effect of HbA1c on in-hospital and short-term mortality in ACS patients.
Methods
Relevant studies reported before July 2019 were retrieved from databases including PubMed, Embase, and Central. Pooled relative risks (RRs) and the corresponding 95% confidence interval (CI) were calculated to evaluate the predictive value of HbA1c for the in-hospital mortality and short-term mortality.
Results
Data from 25 studies involving 304,253 ACS patients was included in systematic review. The pooled RR of in-hospital mortality was 1.246 (95% CI 1.113–1.396, p: 0.000, I2 = 48.6%, n = 14) after sensitivity analysis in studies reporting HbA1c as categorial valuable. The pooled RR was 1.042 (95% CI 0.904–1.202, p: 0.57, I2 = 82.7%, n = 4) in random-effects model for studies reporting it as continuous valuable. Subgroup analysis by diabetic status showed that elevated HbA1c is associated increased short-term mortality in ACS patients without diabetes mellitus (DM) history and without DM (RR: 2.31, 95% CI (1.81–2.94), p = 0.000, I2 = 0.0%, n = 5; RR: 2.56, 95% CI 1.38–4.74, p = 0.003, I2 = 0.0%, n = 2, respectively), which was not the case for patients with DM and patients from studies incorporating DM and non-DM individuals (RR: 1.16, 95% CI 0.79–1.69, p = 0.451, I2 = 31.9%, n = 3; RR: 1.10, 95% CI 0.51–2.38), p = 0.809, I2 = 47.4%, n = 4, respectively).
Conclusions
Higher HbA1c is a potential indicator for in-hospital death in ACS patients as well as a predictor for short-term mortality in ACS patients without known DM and without DM.
Keywords: Glycated hemoglobin A, Acute coronary syndrome, Mortality, Predictor
Introduction
Acute coronary syndrome (ACS) is the leading cause of death worldwide. Although the prognosis of ACS patients has been improved in the past 18 years, there is a lot of room for further improvement, as the ACS patients clearly have a higher risk for a recurrent cardiovascular events compared with the corresponding general population [1]. Biomarkers are good for prognosis evaluation and risk stratification of ACS patients. Cardiac troponins, CRP and NT- BNP are the main predictors for ACS patients. However, they still have some drawbacks as BNP levels are also found to be significantly influenced by age, fluid loading and physical exercise [2], and CRP is influenced by inflammation. Cardiac troponins are affected by reperfusion modalities [3]. There is a need for more reliable biomarkers for the prognosis of ACS patients.
Hyperglycemia and newly-diagnosed diabetes mellitus (DM) are found in a large number of ACS patients and a strong predictor for the poor prognosis of these patients [4–9]. HbA1c, which reflects average blood glucose concentrations over the previous 8–12 weeks, was shown to be a better predictor of prognosis following ACS than fasting and admission glucose [10]. Recent studies proved that HbA1C is an independent predictor of mortality [11], while other studies got opposite results [12]. Furthermore, intensive glycemic control using insulin injection did not improve both short-term and long-term mortality in patients with ACS and DM [13, 14]. In spite of current uncertainty, no previous systematic review has summarized the prognostic role of HbA1c playing in ACS patients. We therefore conducted the meta-analysis of prospective and retrospective cohort studies to evaluate if high levels of HbA1c are associated with increased in-hospital and short-term mortality in ACS patients.
Methods
Search strategy
To find potentially relevant studies, we searched articles in English referenced in Medline, Central and Embase from database inception until July, 2019. The following free texts or MeSH terms were used in search: Acute coronary syndrome, unstable angina, ST-elevation, ST-segment, STEMI, NSTEMI or myocardial infarction combined with hemoglobin a1c, HbA1c, glycated hemoglobin, glycosylated hemoglobin, haemoglobin a1c, glycated haemoglobin, glycosylated haemoglobin, glycohemoglobin a or glycohaemoglobin a. The reference lists of retrieved articles were reviewed for additional eligible studies. Eligibility and study selection
Two authors (Wenjun, Haining) independently reviewed the titles and abstracts of all articles retrieved, and selected those meeting the following criteria: (1) Based on prospective and retrospective cohort studies. (2) Conducted in adults aged 18 years or older. (3) Reporting HbA1c levels as exposure. (4) Reporting the in-hospital and short-term (less than 1 year) mortality. (5) Reporting the effect of the exposure in the form of a relative risk [risk ratio (RR), odds ratio (OR) or hazard ratio (HR)] and their 95% CI or the dead number and total number of each group. Cross-sectional and case–control studies were excluded.
Data extraction and quality assessment
Two investigators (Wenjun and Baotao) independently extracted the following information from eligible studies: first authors’ name, publication year, study name, country, age range and/or mean age (years), number of participants, exposure levels, RRs and their 95% CI as well as the alive and dead numbers for each exposure category. We selected the RRs from the model with the most comprehensive covariate adjustment, and used the Newcastle–Ottawa Scale to assess the quality of the studies included [15]. Any discrepancies were resolved through discussion under supervision of a senior author (Minzhou).
Statistical analysis
We calculated the average RRs for the top compared with the bottom quantile of HbA1c levels using a fixed-effects model. We calculated the unadjusted RRs using original data published in the studies if RRs and their 95% CI were not available in the papers. The I2 statistic and Cochran’s Q test were used to evaluate heterogeneity. If there is significant heterogeneity (I2 > 50%, p < 0.1) among studies, we conducted sensitivity analysis or subgroup analysis to explore the sources of heterogeneity. Publication bias were assessed using funnel plots’ asymmetry and tested with Egger’s asymmetry test and Begg’s test (p < 0.05) when there were at least 10 studies. All analysis was conducted with Stata 13.0.
Results
Search results
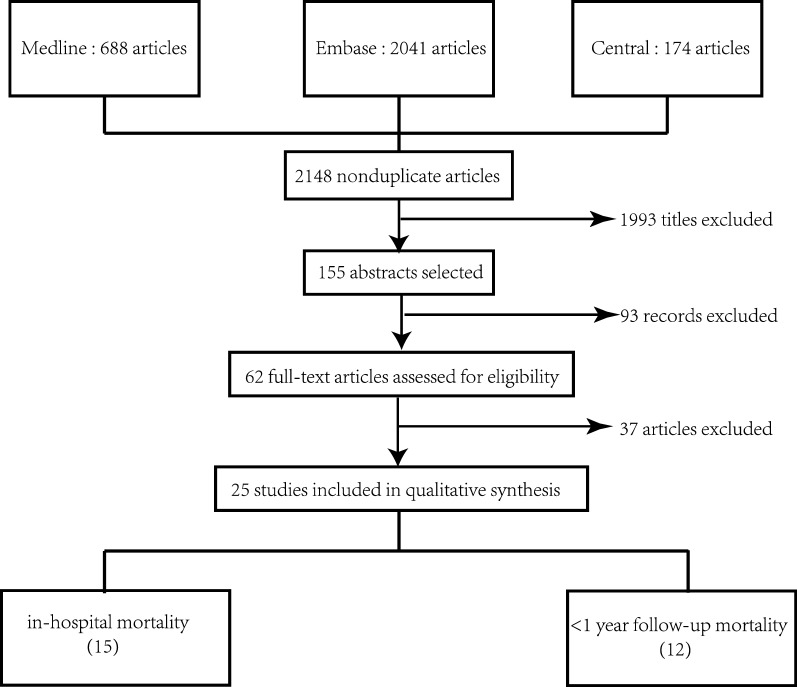
The search strategy is presented in Fig. 1. The literature search initially identified 2148 publications. After screening the titles, the number was reduced to 155 after eliminating randomized controlled trials regarding therapy, reviews and animal experiments, or irrelevant to the current analysis. After screening the remaining abstracts, there were 62 papers for full-text reading, which yielded 25 studies involving 26 papers for final analysis. There are 18 studies exploring the role of HbA1c in in-hospital mortality, and 13 studies regarding less than one-year follow-up mortality.
Fig. 1.
Flowchart of study search and identification
Study characteristics
Table 1 shows the characteristics of these 25 studies from 1987 to 2019 involving 304,253 ACS patients. The mean age of patients in the included studies ranged from 50.9 to 70.1 years old. The male percentage of each studies ranged from 53.6 to 81.7%. Participants had diverse metabolic status including DM, without known DM, without DM and no specified glucose metabolic status. Most of included studies were with high quality with the average quality score as 8.27.
Table 1.
Characteristics of included studies
| First author | Year | Number screened | Age at screening | Men (%) | Follow-up | Glucose metabolic state | Country/continent | Quality score |
|---|---|---|---|---|---|---|---|---|
| Oswald/Yudkin [16, 33] | 1987/1988 | 397 | 64.6 | 76 | 3 months/in hospital | AMI without known DM | UK/Europe | 8 |
| Cao [17] | 2005 | 349 | NA | NA | In hospital | AMI with DM | US/America | 6 |
| Rasoul [34] | 2007 | 504 | 63 | 71.8 | 1 month | STEMI without known DM | Netherlands/Europe | 9 |
| Cakmak [35] | 2008 | 100 | 59.6 | 68 | 28 days | AMI | Turkey/Europe | 8 |
| Chan [19] | 2010 | 317 | 70 | 53.6 | 6 months/in hospital | ACS with DM | China/Asia | 8 |
| Timmer [36] | 2011 | 4176 | 62.2 | 74 | 30 days | STEMI without known DM | Netherlands/Europe | 9 |
| Britton [18] | 2011 | 16,004 | 64.7 | 61.2 | In hospital | AMI with known DM | US/America | 9 |
| Cicek [20] | 2011 | 374 | 55.9 | 85 | In hospital | STEMI | US/America | 9 |
| Lazzeri [21] | 2011 | 195 | 70.1 | 67.2 | In hospital | STEMI and DM | Italy/Europe | 9 |
| Lazzeri [22] | 2012 | 518 | NA | NA | In hospital | STEMI without DM | Italy/Europe | 9 |
| Liu [23] | 2012 | 4793 | 62.6 | 71.5 | 30 days/7 days | STEMI | China/Asia | 9 |
| Ahmad [37] | 2012 | 754 | 52.0 | 67.9 | 1 month | STEMI | Pakistan/Asia | 7 |
| Tian [38] | 2013 | 608 | 61.4 | 79.1 | 30 days | AMI | China/Asia | 9 |
| Pusuroglu [25] | 2014 | 443 | 54.7 | 81.7 | 1 month/in hospital | STEMI | Turkey/Europe | 7 |
| Blasco [24] | 2014 | 601 | 62 | 78 | In hospital | AMI without known DM | Spain/Europe | 9 |
| Fujino [31] | 2014 | 696 | 67.7 | 72 | In hospital | AMI | Japan/Asia | 7 |
| Rousan [26] | 2014 | 243,861 | 64 | 65.1 | In hospital | AMI | US/America | 9 |
| El-sherbiny [39] | 2015 | 60 | 57.8 | 80 | 6 months | STEMI without known DM | Egypt/Africa | 8 |
| AbuShady [27] | 2015 | 151 | 50.9 | 70.5 | In hospital | ACS without known DM | Egypt/Africa | 8 |
| Aggarwal [28] | 2016 | 1686 | 60.4 | 67.4 | In hospital | STEMI | US/America | 9 |
| Donghun[30] | 2016 | 2470 | 61.9 | 77.5 | In hospital | STEMI without DM | Korea/Asia | 9 |
| Samir [29] | 2016 | 208 | 55.9 | 54.8 | 6 months/in hospital | STEMI without DM | Egypt/Africa | 9 |
| Heller [40] | 2017 | 5380 | 61 | 67.9 | 1 month | ACS with T2DM | UK/Europe | 9 |
| Kim [32] | 2017 | 12,625 | 64 | 73.9 | In hospital | AMI | Korea/Asia | 7 |
| Hermanides [40] | 2019 | 6586 | 65.0 | 71.2 | 30 days | AMI without known DM | Netherlands/Europe | 7 |
AMI acute myocardial infarction, DM diabetes mellitus, STEMI ST segment elevated myocardial infarction, ACS acute coronary syndrome, NA not available
The baseline characteristics and therapy after admission of the highest and lowest HbA1c groups were summarized in Tables 2 and 3. There were more patients with hypertension in the highest HbA1c group, which might account for the increased number of patients taking ACEI (angiotensin-converting enzyme inhibitors) or ARB (angiotensin receptor blocker) before and after admission. However, patients with the lowest HbA1c levels had a higher hyperlipidemia incidence, resulting in the increased number of patients taking lipid lowering drugs at baseline. The adherence to antiplatelet in either group showed no difference, but patients in the highest HbA1c group got more PCI (percutaneous coronary intervention) and CABG (coronary artery bypass grafting) therapies according to their medication history. Based on the Killip grade at admission, more ACS patients had cardiac dysfunction (Killip ≥ 2) in the highest HbA1c group compared to the lowest ones, but the number of patients with severe cardiac dysfunction (Killip ≥ 3) showed no difference, indicating patients in the highest HbA1c group had worse cardiac function at admission. Patients had a higher revascularization rate in the lowest HbA1c group. Data from 4 trials showed higher percentage of ACS patients taking antiplatelet agents in highest HbA1c group while in the other three studies it showed the opposite results.
Table 2.
Baseline characteristics of the lowest and highest HbA1c group
| Baseline characterization | Lowest | Highest | Total number | |
|---|---|---|---|---|
| Lowest | Highest | |||
| Male (%), studies = 19 | 71.69% | 73.45% | 45,354 | 10,909 |
| Age (year), studies = 19 | 61.25602 | 62.03319 | 45,354 | 10,909 |
| Smoker (%), studies = 15 | 42.25% | 39.54% | 43,277 | 9681 |
| BMI (kg/m2), studies = 11 | 25.87935 | 26.00163 | 283 | 301 |
| BMI > 30 kg/m2 (%), studies = 2 | 21.15% | 22.22% | 52 | 63 |
| Previous MI (%), studies = 11 | 16.73% | 15.57% | 39,307 | 8737 |
| Hypertension (%), studies = 15 | 38.52% | 43.27% | 9157 | 4708 |
| Heart failure (%), studies = 3 | 5.24% | 5.79% | 36,565 | 6925 |
| Cerebrovascular accident (%), studies = 6 | 4.96% | 5.58% | 37,262 | 7281 |
| Hyperlipidemia (%), studies = 10 | 47.72% | 41.92% | 41,808 | 7855 |
| Renal insufficiency (%), studies = 3 | 26.47% | 25.35% | 774 | 447 |
| Antiplatelet (%), studies = 2 | 17.47% | 20.54% | 596 | 294 |
| Aspirin (%), studies = 2 | 34.50% | 33.60% | 35,325 | 5952 |
| Clopidogrel (%), studies = 2 | 7.69% | 8.35% | 35,325 | 5952 |
| PCI (%), studies = 6 | 8.42% | 10.78% | 3031 | 2283 |
| CABG (%), studies = 4 | 2.43% | 4.08% | 1745 | 1323 |
| ACEI/ARB (%), studies = 4 | 27.97% | 34.02% | 36,013 | 6276 |
| Lipid lowering drugs (%), studies = 4 | 27.76% | 25.24% | 36,013 | 6276 |
| Beta-blocker (%), studies = 3 | 26.39% | 27.11% | 35,901 | 6194 |
| Creatinine (mg/dl), studies = 4 | 1.259,418 | 1.105723 | 1388 | 1839 |
| eGFR (mg/dl/1.73 m2), studies = 5 | 87.00478 | 68.16183 | 5507 | 1654 |
| Admission plasma glucose, studies = 11 | 7.84536 | 9.461979 | 4831 | 4171 |
| Killip ≥ 3 (%), studies = 3 | 11.75% | 14.33% | 1081 | 1584 |
| Killip ≥ 2 (%), studies = 8 | 9.88% | 16.68% | 5782 | 1915 |
BMI body mass index, MI myocardial infarction, PCI percutaneous coronary intervention, CABG coronary artery bypass grafting, ACEI angiotensin-converting enzyme inhibitors, ARB angiotensin receptor blocker, eGFR estimated glomerular filtration rate
Table 3.
Therapy after admission of the lowest and highest HbA1c groups
| Therapy after admission | Lowest (%) | Highest (%) | Total number | |
|---|---|---|---|---|
| Lowest | Highest | |||
| Thrombosis (%), studies = 3 | 7.40 | 11.86 | 36,328 | 6808 |
| PCI (%), studies = 13 | 94.86 | 88.92 | 42,985 | 9124 |
| PCI (successful) (%), studies = 6 | 92.99 | 90.05 | 5477 | 3114 |
| CABG (%), studies = 3 | 19.90 | 16.44 | 39,291 | 6326 |
| Antiplatelet agent (%), studies = 4 | 78.44 | 84.25 | 4058 | 584 |
| Aspirin (%), studies = 3 | 98.46 | 97.88 | 36,565 | 6925 |
| Clopidogrel or Tigrilo (%), studies = 3 | 72.32 | 69.16 | 36,565 | 6925 |
| Lipid lowering drugs (%), studies = 7 | 92.74 | 89.93 | 40,623 | 7509 |
| Beta blockers (%), studies = 7 | 94.79 | 90.98 | 40,623 | 7509 |
| ACEI/ARB (%), studies = 7 | 73.92 | 77.34 | 40,623 | 7509 |
PCI percutaneous coronary intervention, CABG coronary artery bypass grafting, ACEI angiotensin-converting enzyme inhibitors, ARB angiotensin receptor blocker
In-hospital mortality
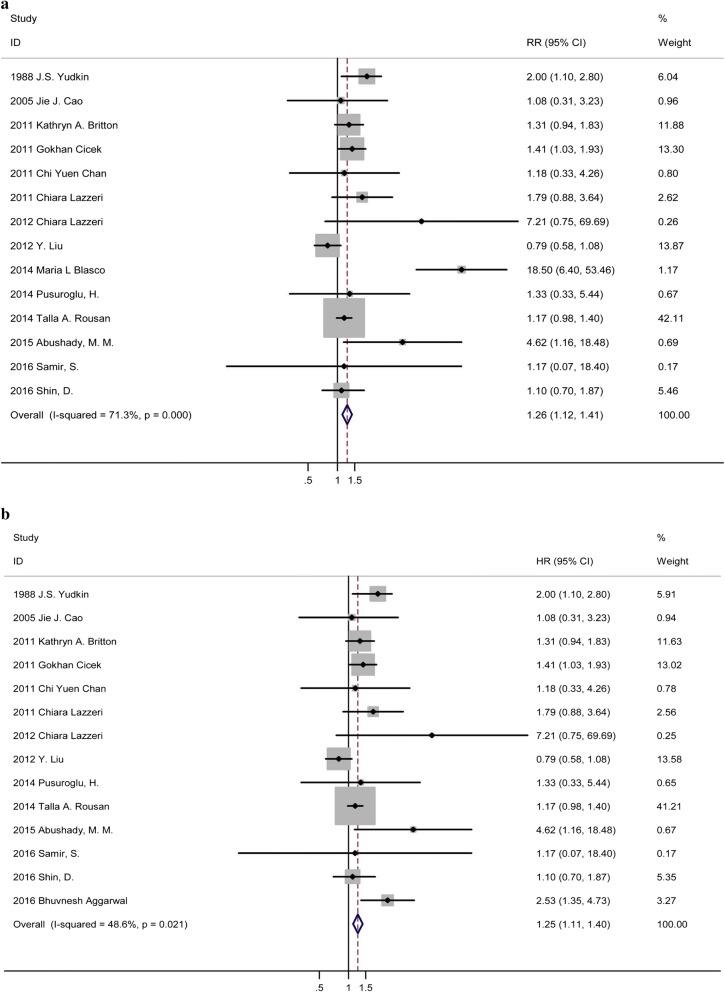
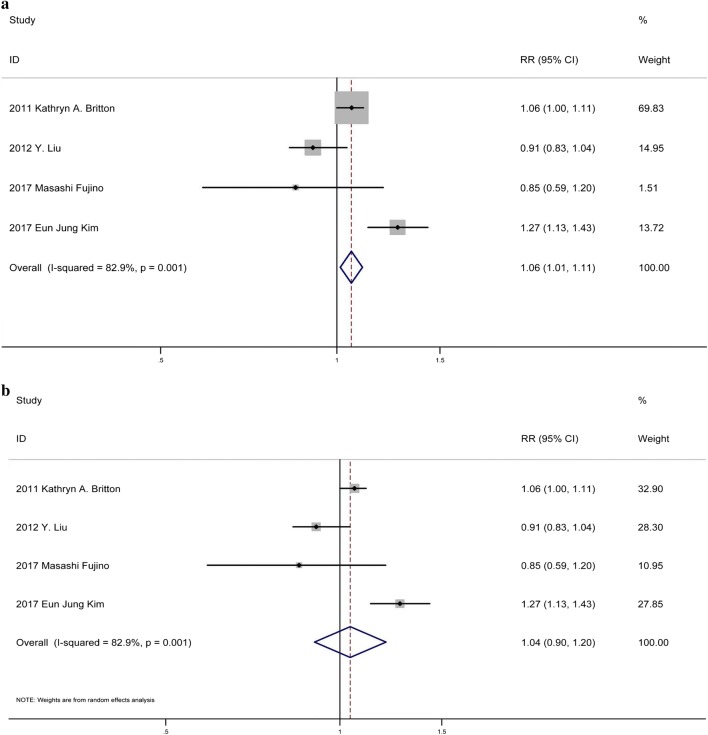
Fifteen studies referred HbA1c as a categorial valuable [16–30], and showed that when compared to lower HbA1c groups, higher HbA1c was significantly associated with increased in-hospital mortality (RR: 1.285, 95% CI 1.148–1.439, p: 0.000, I2 = 71.9%, n = 15) (Fig. 2a). Due to the significant heterogeneity, a sensitive analysis was applied. Result from the other 14 studies indicated that a significant positive relationship persisted after excluding the study causing the remarkable heterogeneity [24] (RR: 1.246, 95% CI 1.113–1.396, p: 0.000, I2 = 48.6%, n = 14) (Fig. 2b). No publication bias was found using Begg’s and Egger’s test (p > 0.05). Four studies referred HbA1c as continuous variable [18, 23, 31, 32], and they all reported RR in multivariable analysis. The pooled RR was 1.058 (95% CI 1.012–1.105, p: 0.012, I2 = 82.7%, n = 4) in fixed-effects model. The sensitivity analysis suggested that the heterogeneity did not originate single studies. Using random-effects model, there is no significant relationship between HbA1c levels and in-hospital mortality (RR: 1.042, 95% CI 0.904–1.202, p: 0.57, I2 = 82.7%, n = 4).
Fig. 2.
a Forest plot of categorial valuable HbA1c and relative risk of in-hospital mortality among ACS patients. b Forest plot of categorial valuable HbA1c and relative risk of in-hospital mortality among ACS patients after sensitivity analysis
Less than 1-year follow-up mortality
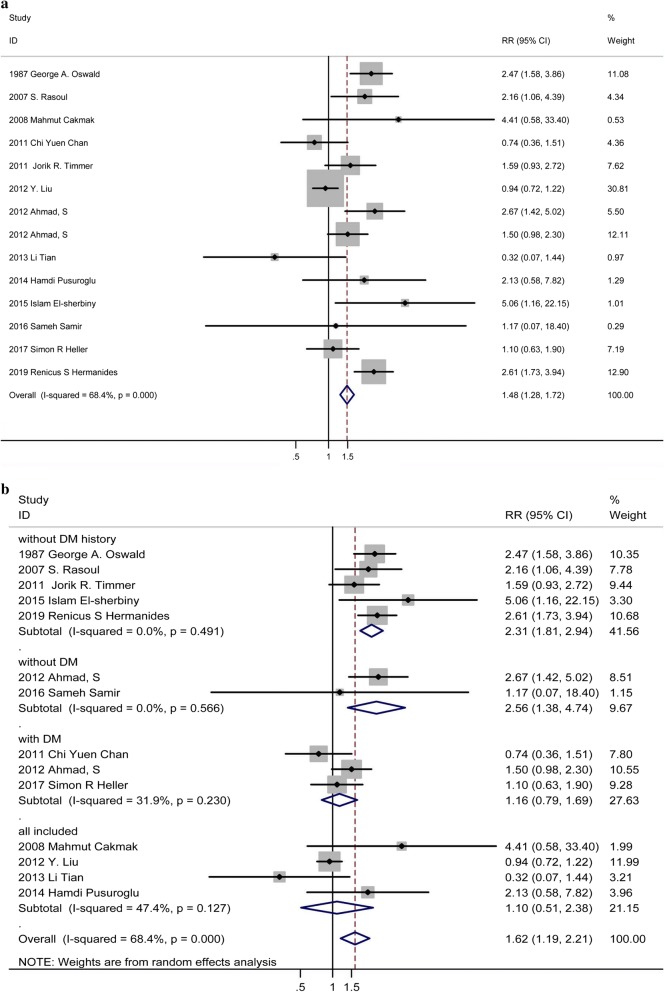
Thirteen studies [19, 23, 25, 29, 33–41] showed that incremental HbA1c levels were associated with an obviously increased risk of mortality (RR: 1.48, 95% CI 1.28–1.72, p: 0.002, I2 = 68.4%, n = 13) (Fig. 3a). Because heterogeneity test showed that there were significant differences between individual studies, we conducted subgroup analysis according to different diabetic status. Subgroup analysis, dividing the trials into four subgroups including ACS patients without DM history, without DM, with DM, and incorporated with and without DM (Fig. 4b). Patients without DM history in subgroup one is patients without previous diagnosis of DM or treatment with a diet, oral glucose-lowering medication and/or insulin. Patients with DM included patients with previous diagnosis of DM or on the anti-diabetic treatment as well as newly diagnosis of DM at admission based on the criteria of WHO (World Health Organization) or ADA (American Diabetes Association). Patients without DM excluded patients with previous or newly diagnosis of DM based on the criteria above. All included means original studies incorporated patients with and without DM. As shown in Fig. 4b, the RR of all-cause mortality in patients without DM history at admission in subgroup one was 2.31 [95% CI (1.81–2.94), p = 0.000], with little heterogeneity as I2 = 0.0%. The RR of all-cause mortality in patients without DM at admission in the subgroup two was 2.56 [95% CI 1.38–4.74, p = 0.003], with little heterogeneity I2 = 0.0%. For patients with DM at admission, the RR of all-cause mortality was 1.16 [95%CI (0.79,1.69), p = 0.451], with small heterogeneity I2 = 31.9%. For ACS patients with incorporated diabetic status, the RR of all-cause mortality was 1.10 [95% CI (0.51, 2.38), p = 0.809], with acceptable heterogeneity I2 = 47.4%. The analysis suggested that higher HbA1c in ACS patients without DM history or without DM at admission was significantly related to increased short-term all-cause mortality, but not for ACS patients with DM or not specific metabolic status. No publication bias was found among the studies (p > 0.05) (Additional file 1: Figure S1b).
Fig. 3.
a Forest plot of continuous valuable HbA1c and relative risk of in-hospital mortality among ACS patients using fixed-effects model. b Forest plot of continuous valuable HbA1c and relative risk of in-hospital mortality among ACS patients in random-effects model
Fig. 4.
a Forest plot of HbA1c and relative risk of short-term mortality among ACS patients. b Forest plot of HbA1c and relative risk of short-term mortality among ACS patients by subgroup analysis
Discussion
25 clinical trials were included in this study involving 304,253 subjects. We found: (1) higher HbA1c levels contributed to increased in-hospital mortality in ACS patients; (2) in general, HbA1c was significantly associated with increased short-term mortality in ACS patients; (3) for ACS patients without DM history and without DM, higher HbA1c had an positive relationship with increased short-term mortality; (4) for ACS patients with DM and no specified metabolic status, HbA1c was failed to show positive prediction for short-term mortality.
Consistent to the review by Capes et al. [42], we found positive relationship between admission hyperglycemia and in-hospital mortality in ACS patients for studies considering HbA1c as a categorial valuable. Although there was significant heterogeneity among studies which came from the one with the highest RR value, the association between HbA1c levels and in-hospital mortality remained significant after excluding it. As compared to other studies, the in-hospital mortality (14%) was higher in the top quantile of HbA1c, which might cause the great heterogeneity. For studies considering HbA1c as a continuous valuable, in fixed-effects model we found a positive relationship between HbA1c and in-hospital mortality but failed to prove it in the random-effects model. Besides the limited number of studies, the diverse valuables for adjusted in the multivariable analysis should also be taken into consideration. As reported by several independent groups, hyperglycemia is associated with impaired microvascular function and decrease coronary flow velocity even induce microvascular obstruction in ACS patients [43–45]. No reflow after reperfusion therapy due to chronic hyperglycemia could give rise to in-hospital death in ACS patients. We did pooled analysis of all the studies of short-term mortality and found that HbA1c contributes to increased short-term mortality in ACS patients. However, there was significant heterogeneity among these studies, which was resolved through subgroup analysis according to metabolic status. We found that for ACS patients without known DM, HbA1c significantly increases short-term mortality. For these included studies, besides patients with normal glucose metabolic status, there was high incidence of patients with newly diagnosed DM which have not been recognized and treated [46]. Therefore, this subgroup reflects the effect of HbA1c on short-term mortality independent of previous anti-diabetic drugs. However, consistent with Østergaard et al. [47] and She et al. [48], we found for patients with DM, it showed no significant effect of HbA1c on short-term mortality in ACS patients. Several causes should be taken into consideration. Firstly, there might be several confounding factors interfering the results, such as anti-diabetic drugs, modified lifestyle and better compliance to the doctors’ advice before and after heart attack. Secondly, as reported by Li et al. [49], higher HbA1c were associated with less risk of myocardial injury following PCI in diabetic patients because of better energy supply. As a large number of ACS patients included in our meta-analysis received PCI therapy, this might account for the confounding factor in short-term prognosis. Last but not least, a limited number of patients with a relatively short follow-up should be taken into consideration, as HbA1c has been commonly recognized as a long-term predictor of mortality in ACS patients with DM [50, 51]. Consistent with randomized controlled trials [13, 14], these patients did not benefit from intensive glycemia control after ACS. The intriguing thing we found in this systematic review is that for ACS patients with definitely no DM, HbA1c is also showed to be a predictor for short-term mortality, which is consistent with the finding by Geng et al. [52]. Overall, HbA1c is a predictor for in-hospital and short-term death in ACS patients. For DM and not specified patients, due to complicate confounding factors which might interfere the results, we found no positive relationship between HbA1c levels and short-term mortality. More studies regarding HbA1c as a continuous variable are needed to prove its effect on in-hospital mortality.
A number of biological mechanisms have been proposed to explain a potential causal association between HbA1c and increased mortality in ACS patients. Firstly, basic experimental study indicates that hyperglycemia inhibits cardiac stem cells from cardiac repair and angiogenesis in streptozotocin-induced diabetic mice undergoing AMI surgery [53], which might account for the worse prognosis for ACS patients with elevated HbA1c levels. Secondly, higher HbA1c levels reflect over-glycosylation of intracellular protein in cardiac myocytes like CaMKII [54]. This may give rise to sudden cardiac death due to fatal arrhythmia. Furthermore, as reviewed by Shah et al. [55], chronic hyperglycemia causes decreased mitochondrial biogenesis, increased reactive oxygen species (ROS) production and post-translational protein modifications, which eventually leads to malignant cardiac remodeling. Last but not least, recent experiment found that baseline plasma fibrinogen is associated HbA1c levels in ACS patients [56]. Fibrinogen is an important part of coronary thrombosis, which is consistent with that higher HbA1c levels were significantly related to increased refraction in ACS patients [25, 27, 41].
The present meta-analysis has some inevitable limitations. On one hand, for the categorial variables, we only took the highest and the lowest quantiles into consideration, which might exaggerate the effect of HbA1c. On the other hand, while we extracted the adjusted RRs if they are available, as we cannot get individual participant data, the confounding factors such as baseline characterization and therapy after admission might still interfere the results. Thus, the result should be interpreted with caution in clinical practice.
While acknowledging the inherent limitations of original data, we insist there are merits and clinical significance of our meta-analysis. To our knowledge, this is the first systematic review regarding the predictive value of HbA1c for short-term mortality in ACS patients. Although there is a similar review regarding STEMI patients [57], it did not explain the heterogeneity explicitly. As unstable angina and NSTEMI share the same pathological process as STEMI, we took all the ACS patients into account in this systematic review. Due to the increased in-hospital mortality in patients with elevated HbA1c, people at high risk of ACS should take plasma glucose and HbA1C under control. According to recent reports, there are substantial ACS patients with unknown DM at admission [34, 41], which accounts for the high risk of short-term mortality in ACS patients without known DM. It is intriguing to note that elevated HbA1c is a predictor of short-term mortality in ACS patients without DM, which instructs ACS patients especially pre-DM patients to control the plasma glucose and HbA1c levels strictly [13, 14]. While there are randomized controlled trials indicating that ACS patients with DM did not benefit from the intensive glycemia control, we recommend people at high risk of ACS without (known) DM to monitor and control HbA1c levels who might benefit from it according to our meta-analysis.
Conclusion
Elevated HbA1c is a predictor of in-hospital mortality in ACS patients and short-term mortality in ACS patients without known DM and without DM. HbA1c is a stable indicator of long-term glucose control and insulin resistance, which should be prevalently monitored by people at high risk of ACS.
Supplementary information
Additional file 1: Figure S1. a Funnel plot of categorial valuable HbA1c and relative risk of in-hospital mortality among ACS patients. b Funnel plot of HbA1c and relative risk of short-term mortality among ACS patients
Acknowledgements
We would like to thank DME center of Guangdong hospital of Chinese Medicine for providing the support of data analysis.
Abbreviations
- ACS
acute coronary syndrome
- RR
relative risk
- OR
odds ratio
- HR
hazard ratio
- CI
confidence interval
- DM
diabetes mellitus
- CRP
C reactive protein
- BNP
B-type natriuretic peptide
- ROS
reactive oxygen species
- AMI
acute myocardial infarction
- STEMI
ST-segment elevated myocardial infarction
- NSTEMI
non ST-segment elevated myocardial infarction
- ACEI
angiotensin-converting enzyme inhibitors
- ARB
angiotensin receptor blocker
- BMI
body mass index
- PCI
percutaneous coronary intervention
- CABG
coronary artery bypass grafting
- eGFR
estimated glomerular filtration rate
- WHO
World Health Organization
- ADA
American Diabetes Association
Authors’ contributions
WP and HL did the document retrieval and selection. WP and BL did the data extraction and quality score of the included studies. LG and MZ gave some advice on the study design and instructions on the discrepancies. PL gave some instructions on writing. All authors read and approved the final manuscript.
Funding
This research was funded by Project of Administration of Traditional Chinese Medicine of Guangdong Province of China (No. 20181126) and The Specific Research Fund for TCM Science and Technology of Guangdong Provincial Hospital of Chinese Medicine (No. YN2018QL06), National Natural Scientific Foundation (Nos. 81703848, 81703877), Natural Scientific Foundation of Guangzhou Province (2017A030310123), The specific Research Fund for TCM Science and Technology of Guangzhou Provincial Hospital of Chinese Medicine (YN10101915).
Availability of data and materials
All data generated or analyzed during this study are included in this published article [and its additional information files]. Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12933-019-0970-6.
References
- 1.Piironen M, Ukkola O, Huikuri H, Havulinna AS, Koukkunen H, Mustonen J, Ketonen M, Lehto S, Airaksinen J, Antero Kesaniemi Y, et al. Trends in long-term prognosis after acute coronary syndrome. Eur J Prev Cardiol. 2017;24(3):274–280. doi: 10.1177/2047487316679522. [DOI] [PubMed] [Google Scholar]
- 2.Battistoni A, Rubattu S, Volpe M. Circulating biomarkers with preventive, diagnostic and prognostic implications in cardiovascular diseases. Int J Cardiol. 2012;157(2):160–168. doi: 10.1016/j.ijcard.2011.06.066. [DOI] [PubMed] [Google Scholar]
- 3.Eggers KM, Lindahl B. Prognostic biomarkers in acute coronary syndromes: risk stratification beyond cardiac troponins. Curr Cardiol Rep. 2017;19(4):29. doi: 10.1007/s11886-017-0840-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avanzini F, Mafrici A, Riva E, Franzosi MG, Milani V, Giudici V, Marelli G, Mariani G, Piatti PM, Roncaglioni MC, et al. A multicenter observational study on the management of hyperglycemia in patients with acute coronary syndrome. Nutr Metab Cardiovasc Dis. 2015;25(10):916–923. doi: 10.1016/j.numecd.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Ambady R, Snehalatha C, Sathyamurthy I, Ravi MS, Nalini V, Vijay V, Tuomilehto J. High incidence of glucose intolerance in Asian-Indian subjects with acute coronary syndrome. Diabetes Care. 2005;28(10):2492–2496. doi: 10.2337/diacare.28.10.2492. [DOI] [PubMed] [Google Scholar]
- 6.de la Hera JM, Delgado E, Hernandez E, Garcia-Ruiz JM, Vegas JM, Avanzas P, Lozano I, Barriales-Villa R, Hevia S, Martin JS, et al. Prevalence and outcome of newly detected diabetes in patients who undergo percutaneous coronary intervention. Eur Heart J. 2009;30(21):2614–2621. doi: 10.1093/eurheartj/ehp278. [DOI] [PubMed] [Google Scholar]
- 7.Lee TF, Burt MG, Heilbronn LK, Mangoni AA, Wong VW, McLean M, Cheung NW. Relative hyperglycemia is associated with complications following an acute myocardial infarction: a post hoc analysis of HI-5 data. Cardiovasc Diabetol. 2017;16(1):157. doi: 10.1186/s12933-017-0642-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gu J, Pan JA, Fan YQ, Zhang HL, Zhang JF, Wang CQ. Prognostic impact of HbA1c variability on long-term outcomes in patients with heart failure and type 2 diabetes mellitus. Cardiovasc Diabetol. 2018;17(1):96. doi: 10.1186/s12933-018-0739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng J, Cheng J, Wang T, Zhang Q, Xiao X. Does HbA1c level have clinical implications in diabetic patients undergoing coronary artery bypass grafting? A systematic review and meta-analysis. Int J Endocrinol. 2017 doi: 10.1155/2017/1537213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kmet M, Rajer B, Pernat A. Hemoglobin A1c is a better predictor of prognosis following the non-ST elevation acute coronary syndrome than fasting and admission glucose. Wien Klin Wochenschr. 2014;126(5–6):156–162. doi: 10.1007/s00508-013-0468-2. [DOI] [PubMed] [Google Scholar]
- 11.Nichols GA, Joshua-Gotlib S, Parasuraman S. Glycemic control and risk of cardiovascular disease hospitalization and all-cause mortality. J Am Coll Cardiol. 2013;62(2):121–127. doi: 10.1016/j.jacc.2013.04.031. [DOI] [PubMed] [Google Scholar]
- 12.Pararajasingam G, Hofsten DE, Logstrup BB, Egstrup M, Henriksen FL, Hangaard J, Egstrup K. Newly detected abnormal glucose regulation and long-term prognosis after acute myocardial infarction: comparison of an oral glucose tolerance test and glycosylated haemoglobin A1c. Int J Cardiol. 2016;214:310–315. doi: 10.1016/j.ijcard.2016.03.199. [DOI] [PubMed] [Google Scholar]
- 13.Malmberg K, Rydén L, Wedel H, Birkeland K, Bootsma A, Dickstein K, Efendic S, Fisher M, Hamsten A, Herlitz J, et al. Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity. Eur Heart J. 2005;26(7):650–661. doi: 10.1093/eurheartj/ehi199. [DOI] [PubMed] [Google Scholar]
- 14.Bejan-Angoulvant T, Cornu C, Archambault P, Tudrej B, Audier P, Brabant Y, Gueyffier F, Boussageon R. Is HbA1c a valid surrogate for macrovascular and microvascular complications in type 2 diabetes? Diabetes Metab. 2015;41(3):195–201. doi: 10.1016/j.diabet.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 16.Yudkin JS, Oswald GA, McKeigue PM, Forrest RD, Jackson CA. The relationship of hospital admission and fatality from myocardial infarction to glycohaemoglobin levels. Diabetologia. 1988;31(4):201–205. doi: 10.1007/BF00290585. [DOI] [PubMed] [Google Scholar]
- 17.Cao JJ, Hudson M, Jankowski M, Whitehouse F, Weaver WD. Relation of chronic and acute glycemic control on mortality in acute myocardial infarction with diabetes mellitus. Am J Cardiol. 2005;96(2):183–186. doi: 10.1016/j.amjcard.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 18.Britton KA, Aggarwal V, Chen AY, Alexander KP, Amsterdam E, Fraulo E, Muntner P, Thomas L, McGuire DK, Wiviott SD, et al. No association between hemoglobin A1c and in-hospital mortality in patients with diabetes and acute myocardial infarction. Am Heart J. 2011;161(4):657–663.e651. doi: 10.1016/j.ahj.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan CY, Li R, Chan JY, Zhang Q, Chan CP, Dong M, Yan BP, Lam YY, Yu CM. The value of admission HbA(1c) level in diabetic patients with acute coronary syndrome. Clin Cardiol. 2011;34(8):507–512. doi: 10.1002/clc.20915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cicek G, Uyarel H, Ergelen M, Ayhan E, Abanonu GB, Eren M, Gibson CM. Hemoglobin A1c as a prognostic marker in patients undergoing primary angioplasty for acute myocardial infarction. Coron Artery Dis. 2011;22(3):131–137. doi: 10.1097/MCA.0b013e328342c760. [DOI] [PubMed] [Google Scholar]
- 21.Lazzeri C, Valente S, Chiostri M, Picariello C, Attanà P, Gensini GF. The prognostic impact of glycated hemoglobin in diabetic ST-elevation myocardial infarction. Int J Cardiol. 2011;151(2):250–252. doi: 10.1016/j.ijcard.2011.06.077. [DOI] [PubMed] [Google Scholar]
- 22.Lazzeri C, Valente S, Chiostri M, Picariello C, Attana P, Gensini GF. Glycated hemoglobin in ST-elevation myocardial infarction without previously known diabetes: its short and long term prognostic role. Diabetes Res Clin Pract. 2012;95(1):e14–e16. doi: 10.1016/j.diabres.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Yang YM, Zhu J, Tan HQ, Liang Y, Li JD. Haemoglobin A(1c), acute hyperglycaemia and short-term prognosis in patients without diabetes following acute ST-segment elevation myocardial infarction. Diabet Med. 2012;29(12):1493–1500. doi: 10.1111/j.1464-5491.2012.03641.x. [DOI] [PubMed] [Google Scholar]
- 24.Blasco ML, Sanjuan R, Palacios L, Huerta R, Carratala A, Nunez J, Sanchis J. Prognostic value of admission glycated haemoglobin in unknown diabetic patients with acute myocardial infarction. Eur Heart J Acute Cardiovasc Care. 2014;3(4):347–353. doi: 10.1177/2048872614530574. [DOI] [PubMed] [Google Scholar]
- 25.Pusuroglu H, Akgul O, Cakmak HA, Erturk M, Surgit O, Celik O, Ozturk D, Uzun F, Akkaya E, Yildirim A. Long-term prognostic value of admission haemoglobin A1c(HbA1c) levels in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Postepy w Kardiologii Interwencyjnej. 2014;10(3):166–174. doi: 10.5114/pwki.2014.45143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rousan TA, Pappy RM, Chen AY, Roe MT, Saucedo JF. Impact of diabetes mellitus on clinical characteristics, management, and in-hospital outcomes in patients with acute myocardial infarction (from the NCDR) Am J Cardiol. 2014;114(8):1136–1144. doi: 10.1016/j.amjcard.2014.07.031. [DOI] [PubMed] [Google Scholar]
- 27.Abushady MM, Mohamady Y, Enany B, Nammas W. Prevalence of prediabetes in patients with acute coronary syndrome: impact on in-hospital outcomes. Intern Med J. 2015;45(2):183–188. doi: 10.1111/imj.12651. [DOI] [PubMed] [Google Scholar]
- 28.Aggarwal B, Shah GK, Randhawa M, Ellis SG, Lincoff AM, Menon V. Utility of glycated hemoglobin for assessment of glucose metabolism in patients with ST-segment elevation myocardial infarction. Am J Cardiol. 2016;117(5):749–753. doi: 10.1016/j.amjcard.2015.11.060. [DOI] [PubMed] [Google Scholar]
- 29.Samir S, Naseem M. Effect of admission glycometabolic state on clinical outcome in non diabetic subjects with acute st segment elevation myocardial infarction. Egypt J Crit Care Med. 2016;4(2):73–78. doi: 10.1016/j.ejccm.2016.03.003. [DOI] [Google Scholar]
- 30.Shin D, Ahn J, Cha KS, Park JS, Oh JH, Lee HW, Hong JY, Kim BW, Hong TJ. Impact of initial glycosylated hemoglobin level on cardiovascular outcomes in prediabetic patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Coron Artery Dis. 2016;27(1):40–46. doi: 10.1097/MCA.0000000000000305. [DOI] [PubMed] [Google Scholar]
- 31.Fujino M, Ishihara M, Honda S, Kawakami S, Yamane T, Nagai T, Nakao K, Kanaya T, Kumasaka L, Asaumi Y, et al. Impact of acute and chronic hyperglycemia on in-hospital outcomes of patients with acute myocardial infarction. Am J Cardiol. 2014;114(12):1789–1793. doi: 10.1016/j.amjcard.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 32.Kim EJ, Jeong MH, Kim JH, Ahn TH, Seung KB, Oh DJ, Kim HS, Gwon HC, Seong IW, Hwang KK, et al. Clinical impact of admission hyperglycemia on in-hospital mortality in acute myocardial infarction patients. Int J Cardiol. 2017;236:9–15. doi: 10.1016/j.ijcard.2017.01.095. [DOI] [PubMed] [Google Scholar]
- 33.Oswald GA, Yudkinb JS. Hyperglycaemia following acute myocardial infarction The contribution of undiagnosed diabetes. Diabet Med. 1987;4:68–70. doi: 10.1111/j.1464-5491.1987.tb00833.x. [DOI] [PubMed] [Google Scholar]
- 34.Rasoul S, Ottervanger JP, Bilo HJ, Timmer JR, van ‘t Hof AW, Dambrink JH, Dikkeschei LD, Hoorntje JC, de Boer MJ, Zijlstra F. Glucose dysregulation in nondiabetic patients with ST-elevation myocardial infarction acute and chronic glucose dysregulation in STEMI. Neth J Med. 2007;65(3):95–100. [PubMed] [Google Scholar]
- 35.Cakmak M, Cakmak N, Cetemen S, Tanriverdi H, Enc Y, Teskin O, Kilic ID. The value of admission glycosylated hemoglobin level in patients with acute myocardial infarction. Can J Cardiol. 2008;24(5):375–378. doi: 10.1016/S0828-282X(08)70600-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Timmer JR, Hoekstra M, Nijsten MW, van der Horst IC, Ottervanger JP, Slingerland RJ, Dambrink JH, Bilo HJ, Zijlstra F, van ‘t Hof AW. Prognostic value of admission glycosylated hemoglobin and glucose in nondiabetic patients with ST-segment-elevation myocardial infarction treated with percutaneous coronary intervention. Circulation. 2011;124(6):704–711. doi: 10.1161/CIRCULATIONAHA.110.985911. [DOI] [PubMed] [Google Scholar]
- 37.Ahmad S, Ahmad S, Kashif M. Increasing HbA1C level; A prognostic indicator of increased thirty days mortality in patients of ST-Segment elevation in myocardial infarction. Pak J Med Health Sci. 2012;6(3):724–726. [Google Scholar]
- 38.Tian L, Zhu J, Liu L, Liang Y, Li J, Yang Y. Hemoglobin A1c and short-term outcomes in patients with acute myocardial infarction undergoing primary angioplasty: an observational multicenter study. Coron Artery Dis. 2013;24(1):16–22. doi: 10.1097/MCA.0b013e32835b3971. [DOI] [PubMed] [Google Scholar]
- 39.El-Sherbiny I, Nabil B, Saber T, Abdelgawad FE. Impact of admission glycosylated hemoglobin A1c on angiographic characteristics and short term clinical outcomes of nondiabetic patients with acute ST-segment elevation myocardial infarction. Cardiol Res Pract. 2015 doi: 10.1155/2015/274892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heller SR, Bergenstal RM, White WB, Kupfer S, Bakris GL, Cushman WC, Mehta CR, Nissen SE, Wilson CA, Zannad F, et al. Relationship of glycated haemoglobin and reported hypoglycaemia to cardiovascular outcomes in patients with type 2 diabetes and recent acute coronary syndrome events: the EXAMINE trial. Diabetes Obes Metab. 2017;19(5):664–671. doi: 10.1111/dom.12871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hermanides RS, Kennedy MW, Kedhi E, van Dijk PR, Timmer JR, Ottervanger JP, Dambrink JH, Gosselink AM, Roolvink V, Miedema K, et al. Impact of elevated HbA1c on long-term mortality in patients presenting with acute myocardial infarction in daily clinical practice: insights from a ‘real world’ prospective registry of the Zwolle Myocardial Infarction Study Group. Eur Heart J Acute Cardiovasc Care. 2019 doi: 10.1177/2048872619849921. [DOI] [PubMed] [Google Scholar]
- 42.Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet. 2000;355(9206):773–778. doi: 10.1016/S0140-6736(99)08415-9. [DOI] [PubMed] [Google Scholar]
- 43.Wang Z-B, Qiu C-G, Wang S-J, Han Z-Y, Huang Z-W, Sun G-J. Effect of hemoglobinA1C on the coronary flow velocity after percutaneous coronary intervention. Biomed Res. 2015;26(4):677–681. [Google Scholar]
- 44.Ota S, Tanimoto T, Orii M, Hirata K, Shiono Y, Shimamura K, Matsuo Y, Yamano T, Ino Y, Kitabata H, et al. Association between hyperglycemia at admission and microvascular obstruction in patients with ST-segment elevation myocardial infarction. J Cardiol. 2015;65(4):272–277. doi: 10.1016/j.jjcc.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 45.Iwakura K, Ito H, Ikushima M, Kawano S, Okamura A, Asano K, Kuroda T, Tanaka K, Masuyama T, Hori M, et al. Association between hyperglycemia and the no-reflow phenomenon in patients with acute myocardial infarction. J Am Coll Cardiol. 2003;41(1):1–7. doi: 10.1016/S0735-1097(02)02626-8. [DOI] [PubMed] [Google Scholar]
- 46.Norhammar A, Tenerz A, Nilsson G, Hamsten A, Efendic S, Ryden L, Malmberg K. Glucose metabolism in patients with acute myocardial infarction and no previous diagnosis of diabetes mellitus: a prospective study. Lancet. 2002;359(9324):2140–2144. doi: 10.1016/S0140-6736(02)09089-X. [DOI] [PubMed] [Google Scholar]
- 47.Østergaard HB, Mandrup-Poulsen T, Berkelmans GFN, van der Graaf Y, Visseren FLJ, Westerink J. Limited benefit of haemoglobin glycation index as risk factor for cardiovascular disease in type 2 diabetes patients. Diabetes Metab. 2019;45(3):254–260. doi: 10.1016/j.diabet.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 48.She J, Deng Y, Wu Y, Xia Y, Li H, Liang X, Shi R, Yuan Z. Hemoglobin A1c is associated with severity of coronary artery stenosis but not with long term clinical outcomes in diabetic and nondiabetic patients with acute myocardial infarction undergoing primary angioplasty. Cardiovasc Diabetol. 2017;16(1):97. doi: 10.1186/s12933-017-0578-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li XL, Li JJ, Guo YL, Zhu CG, Xu RX, Li S, Qing P, Wu NQ, Jiang LX, Xu B, et al. Relationship of glycated hemoglobin levels with myocardial injury following elective percutaneous coronary intervention in patients with type 2 diabetes mellitus. PLoS ONE. 2014;9(7):e101719. doi: 10.1371/journal.pone.0101719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kowalczyk J, Mazurek M, Zielinska T, Lenarczyk R, Sedkowska A, Swiatkowski A, Sredniawa B, Mencel G, Francuz P, Kalarus Z. Prognostic significance of HbA1c in patients with AMI treated invasively and newly detected glucose abnormalities. Eur J Prev Cardiol. 2015;22(6):798–806. doi: 10.1177/2047487314527850. [DOI] [PubMed] [Google Scholar]
- 51.Savonitto S, Morici N, Nozza A, Cosentino F, Perrone Filardi P, Murena E, Morocutti G, Ferri M, Cavallini C, Eijkemans MJ, et al. Predictors of mortality in hospital survivors with type 2 diabetes mellitus and acute coronary syndromes. Diabetes Vasc Dis Res. 2018;15(1):14–23. doi: 10.1177/1479164117735493. [DOI] [PubMed] [Google Scholar]
- 52.Geng J, Zhang Y, Wang B, Xie J, Xu B, Li J. Glycosylated hemoglobin levels and clinical outcomes in nondiabetic patients with coronary artery disease. Medicine. 2017;96(17):e6784. doi: 10.1097/MD.0000000000006784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Molgat AS, Tilokee EL, Rafatian G, Vulesevic B, Ruel M, Milne R, Suuronen EJ, Davis DR. Hyperglycemia inhibits cardiac stem cell-mediated cardiac repair and angiogenic capacity. Circulation. 2014;130(11 Suppl 1):S70–S76. doi: 10.1161/CIRCULATIONAHA.113.007908. [DOI] [PubMed] [Google Scholar]
- 54.Erickson JR, Pereira L, Wang L, Han G, Ferguson A, Dao K, Copeland RJ, Despa F, Hart GW, Ripplinger CM, et al. Diabetic hyperglycaemia activates CaMKII and arrhythmias by O-linked glycosylation. Nature. 2013;502(7471):372–376. doi: 10.1038/nature12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shah MS, Brownlee M. Molecular and cellular mechanisms of cardiovascular disorders in diabetes. Circ Res. 2016;118(11):1808–1829. doi: 10.1161/CIRCRESAHA.116.306923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang L, Xu C, Liu J, Bai X, Li R, Wang L, Zhou J, Wu Y, Yuan Z. Baseline plasma fibrinogen is associated with haemoglobin A1c and 2-year major adverse cardiovascular events following percutaneous coronary intervention in patients with acute coronary syndrome: a single-centre, prospective cohort study. Cardiovasc Diabetol. 2019;18(1):52. doi: 10.1186/s12933-019-0858-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li G, Hou X, Li Y, Zhang P, Zhao Q, Li J, Shi J. Prognostic value of glycated hemoglobin among patients with ST-segment elevation myocardial infarction: a systematic review and meta-analysis. Clin Chem Lab Med. 2017;55(8):1090–1099. doi: 10.1515/cclm-2016-0792. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. a Funnel plot of categorial valuable HbA1c and relative risk of in-hospital mortality among ACS patients. b Funnel plot of HbA1c and relative risk of short-term mortality among ACS patients
Data Availability Statement
All data generated or analyzed during this study are included in this published article [and its additional information files]. Not applicable.