Abstract
Background:
The Hypertrophic Cardiomyopathy Registry (HCMR) is a NHLBI-funded, prospective registry of 2755 patients with HCM recruited from 44 sites in 6 countries.
Objectives:
To improve risk prediction in HCM by incorporating cardiovascular magnetic resonance (CMR), genetic, and biomarker data.
Methods:
Demographic and echocardiographic data were collected. Patients underwent CMR including cine imaging, late gadolinium enhancement imaging (LGE, replacement fibrosis) and T1 mapping for measurement of extracellular volume (ECV) as a measure of interstitial fibrosis. Blood was drawn for biomarker (N-terminal pro brain natriuretic peptide [NTproBNP] and high sensitivity troponin T [cTnT]) and genetic analysis.
Results:
2,755 patients were studied. Mean age was 49±11 years, 71% were male, and 17% non-white. Mean ESC risk score was 2.48±0.56. Eighteen % had a resting left ventricular outflow tract (LVOT) gradient ≥30mmHg. Thirty six percent had a sarcomere mutation identified and 50 percent had any LGE. Sarcomere mutation (+) patients were more likely to have reverse septal curvature morphology, LGE, and no significant resting LVOT obstruction. Those that were sarcomere mutation (−), had isolated basal septal hypertrophy, less LGE, and more LVOT obstruction. Interstitial fibrosis was present in segments both with and without LGE. Serum NTproBNP and cTnT levels correlated with increasing LGE and ECV in a graded fashion.
Conclusions:
The HCMR population has characteristics of low risk HCM. 93% had no or only mild functional limitation. Baseline data separated patients broadly into 2 categories. One group was sarcomere mutation (+), more likely had reverse septal curvature morphology, more fibrosis, but less resting obstruction whereas the other was sarcomere mutation (−), more likely had isolated basal septal hypertrophy with obstruction but less fibrosis. Further follow-up will allow better understanding of these subgroups and development of an improved risk prediction model incorporating all these markers.
Keywords: Hypertrophic cardiomyopathy, Cardiovascular magnetic resonance, Biomarkers, Fibrosis, Late gadolinium enhancement
Condensed Abstract
HCMR is a prospective registry of 2755 hypertrophic cardiomyopathy patients from 44 sites in 6 countries. Patients underwent cardiovascular magnetic resonance including cine imaging, late gadolinium enhancement imaging (LGE), and T1 mapping for measurement of extracellular volume and had blood drawn for biomarkers and genetics. Patients with sarcomere mutations more commonly had LGE and reverse septal curvature morphology but less resting outflow obstruction. Serum biomarkers correlated with the extent of LGE and ECV. Further follow-up will allow development of a useful risk prediction model incorporating these markers.
Introduction
The Hypertrophic Cardiomyopathy Registry (HCMR) is a prospective NHLBI-funded registry of 2755 HCM patients recruited across Europe and North America(1). The primary goal of the study is to improve risk prediction for important adverse clinical outcomes in HCM by integrating cardiovascular magnetic resonance (CMR) imaging, biomarker, and genetic data with standard clinical and echocardiographic findings. Insights gained by HCMR will directly impact patient care by providing a systematic evidence base to inform and advance management guidelines (2) and develop predictive models.(3) In current practice, risk stratification for sudden cardiac death (SCD) remains poorly resolved, particularly for patients at low and intermediate risk, limiting optimal utilization of implantable-cardioverter defibrillators (ICD’s).(4,5) In addition, models have not yet been developed to predict other key adverse outcomes such as incident heart failure or atrial fibrillation.
Previous large cohorts of HCM patients were gathered retrospectively and/or from one or a handful of specialist centers (5–7) and, in general, CMR has not been systematically included.(5,7) An ongoing registry in 69 centers from 18 European countries is collecting patients with HCM (n=1739), but also includes other non-ischemic cardiomyopathies, and is only collecting variables acquired at the discretion of the clinical sites.(8) For example, only 34% of patients in the latter registry underwent CMR, 46% had genetic testing, and biomarkers were not routinely collected.(8) HCMR is the first large prospective registry to include rigorous CMR imaging, genetic testing and prospective collection of blood for biomarker analysis.
Myocardial fibrosis measured by CMR has gained attention as a potential determinant of risk in patients with HCM. The presence of substantial late gadolinium enhancement (LGE), a marker of replacement fibrosis, has been associated with a 2-fold increase in SCD risk (6) and 3-fold increase in composite events(9) if present in >15% of LV mass. A meta-analysis of nearly 3000 patients from several studies demonstrated that the presence of LGE was associated with a 3.4 fold increased risk of SCD/ICD discharge and 1.8 fold increase in all-cause mortality(10). The extent of LGE was also associated with an increased risk of SCD/ICD discharge (1.36/10% LGE, p=0.005) in a continuous fashion. A recent study suggests that adding LGE to American College of Cardiology (ACCF)/American Heart Association (AHA) risk stratification, along with apical aneurysm morphology and multiple runs of NSVT improved identification of indications for ICD placement.(11) Interstitial rather than replacement fibrosis may be an additional risk marker in HCM(12). HCMR is the first large multicenter study to use T1 mapping to assess extracellular volume as a surrogate for interstitial fibrosis in HCM. Integrating these markers of fibrosis and other CMR findings with clinical information, echocardiography, genotyping and biomarker analysis may further inform risk prediction in HCM. Baseline characteristics of 2755 patients with HCM are presented in the present manuscript.
Methods
The study design for HCMR has been previously published(1); the relevant methods are summarized here. HCMR is a prospective observational study. After written informed consent, all patients underwent standard clinical evaluation, CMR, and had blood drawn for genetic and biomarker analysis. Longitudinal follow-up is being conducted to determine the incidence of cardiovascular events, adjudicated by a clinical events committee.
Inclusion/Exclusion Criteria
Patients included were ages 18–65 with an established diagnosis of HCM defined as unexplained LVH (wall thickness >15mm) without cavity dilatation or known predisposing cause (uncontrolled hypertension, aortic stenosis, etc.)(2). Patients known to have other causes of infiltrative/hypertrophic cardiomyopathies such as amyloidosis, sarcoidosis, Fabry disease, Danon disease, or Noonan’s syndrome, or discovered to have these diagnoses through HCMR genotyping, were excluded. Patients older than 65 were excluded as they have high competing mortality risks, in particular from coronary artery disease and cancer.
Additional exclusion criteria were 1) prior septal myectomy or alcohol septal ablation, 2) prior myocardial infarction or known CAD 3) incessant ventricular arrhythmias, 4) inability to lie flat, 5) contraindication to contrast-enhanced CMR including pacemakers, defibrillators, intraocular metal, certain types of intracranial aneurysm clips, severe claustrophobia, and Stage IV/V chronic kidney disease, 6) diabetes mellitus with end organ damage, 7) ongoing pregnancy, or 8) inability to provide informed consent.
Patient Enrollment
Patients were enrolled from 44 sites in the U.S. (18), Canada (4), United Kingdom (13), Italy (4), Germany (3), and the Netherlands (2) between April, 2014 and April, 2017 (Supplemental Table 1). Participating sites are experienced centers with focused care of HCM patients as well as state-of-the-art CMR capabilities. Emphasis was placed on recruiting HCM patients across the risk spectrum including higher-risk patients referred for subsequent ICD implantation. Data regarding baseline demographics and clinical variables were recorded from clinical records including data from clinically-performed echocardiographic, Holter and exercise testing studies closest to the time of enrollment. ESC risk score was calculated using baseline clinical and echocardiographic data(3).
CMR methods
CMR was performed at 1.5 or 3 Tesla (T) on MR systems from the 3 primary vendors (General Electric, Philips Medical Systems, Siemens Healthineers) using a standardized protocol and multi-channel channel phased-array chest coils and electrocardiographic (ECG) gating. After rapid localization of the heart, short axis cine steady state free precession imaging (SSFP) was performed covering the whole heart in 8mm thick slices (no gap). Typical cine SSFP parameters included TR/TE 3.1/1.2 msec, in-plane resolution of 2–2.5 mm, temporal resolution of 40–50 msec. Baseline T1 mapping was performed in 3 short axis slices centered in the mid LV, representing 16 of the 17 AHA segments in the nearly 80% of the sites that had appropriate software. The Shortened Modified Look-Locker Inversion recovery technique (ShMOLLI), using a 5(1)1(1)1 Look-Locker scheme with conditional image processing (13), was used as the recommended standard on Philips and Siemens systems, both for native and post-contrast T1 mapping acquisitions. Gadolinium contrast was administered intravenously as a bolus dose of 0.15 mmol/kg. Long-axis function by SSFP cine imaging was then obtained. Post-contrast T1 mapping acquisitions were performed in the same 3 short axis slices as pre-contrast, starting at 5, 14, and 29 minutes post-contrast. LGE imaging was acquired in the same long axis and short-axis stack locations beginning at minute 17 post-contrast with a 2D breath-hold, segmented inversion-recovery sequence (inversion time (TI) optimized by the Look-Locker sequence (TI scout) to null normal myocardium). Total imaging time was approximately 60 minutes.
CMR Image Analysis
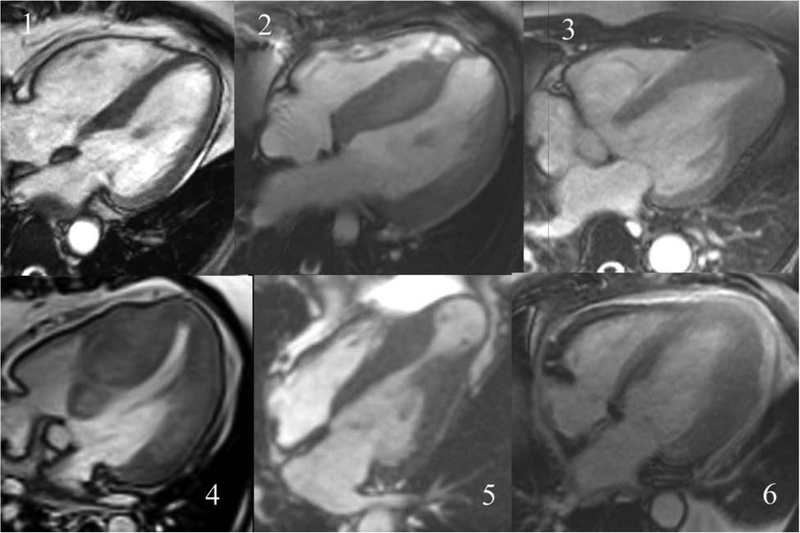
Commercially available software (MedisSuite 3.0 and QMassMR, Medis Inc., Leiden, NL) was used for analysis of all CMR images (cine, T1 maps, and LGE) in a core laboratory. LV mass, volumes, wall thickness and thickening was measured according to SCMR standards.(14) Cine images in short-axis contiguous cuts were evaluated for LV and RV volumes and myocardial mass by manually tracing endocardial and epicardial borders. Papillary muscles were included in LV volumes and excluded from LV mass. Cine images were also evaluated for morphology(15) and defined as 1) localized basal septal hypertrophy 2) reverse curvature septal hypertrophy 3) apical HCM 4) concentric HCM 5) mid-cavity obstruction with apical aneurysm or 6) other, i.e. did not fit into the preceding 5 categories (Figure 1).(16) Cine images in 2- and 4-chamber long-axis cuts were evaluated for left atrial volumes using the bi-plane area-length method, at end-ventricular systole, before atrial contraction, and end-ventricular diastole.(17)
Figure 1. Steady state free precession 4-chamber long axis cine images from individual patients with the 6 different morphologic subtypes of HCM.
The six subtypes shown are: 1) localized basal septal hypertrophy 2) reverse curvature septal hypertrophy 3) apical HCM 4) concentric HCM 5) mid-cavity obstruction with apical aneurysm or 6) other
Quantification of LGE was performed according to SCMR standards(14) using both the 6 S.D. quantitative threshold as well as visually.(18) LGE was categorized as none, >0–5%, >5–10%, >10–15% and >15% of LV mass. T1 quantification was performed on a segmental basis by non-linear least-squares fitting of the segmental inversion recovery curves, resulting in multiple T1 measurements (one pre- and 3 post-contrast) calculated. Gadolinium partition coefficient λ was calculated segmentally and globally by linear regression of pre and post-contrast R1 (=1/T1) relaxation rates in myocardium, against the corresponding R1’s in the blood pool of the same short axis slice. The linear regression slope was converted to extracellular volume (ECV) using the patient’s fractional blood volume of distribution (1-hematocrit).(19) ECV index was calculated as ECV (%) times LV mass.
Genetics
Amplicon-based sequencing for 36 cardiomyopathy-associated genes was undertaken using the Illumina MiSeq platform. Bioinformatic analysis was performed using the Genome Analysis Toolkit version 4 best practice guidelines. Variants were visually confirmed through inspection of BAM files. Variant annotation was performed using SNPEff (http://snpeff.sourceforge.net) and Ensembl’s Variant Effect Predictor (VEP version 95). Data from publicly available resources (ClinVar (version 20190211) and gnomAD r2.1) and the Oxford Regional Genetics Laboratory in-house mutation database was used to inform variant classification. Following quality control, 2,636 individuals (99.1%) were deemed suitable for subsequent genetic analyses.
Serum Biomarkers
Blood samples were transported on ice, processed within 60 minutes of phlebotomy to obtain serum and EDTA-anticoagulated plasma, aliquoted, and stored at −70°C until they were batched tested at the end of the study period in the Biomarker Research and Clinical Trials Laboratory at Brigham and Womeńs Hospital. Cardiac troponin T (cTnT) was tested using the Roche (Roche Diagnostics Corporation, Indianapolis, IN) TnT STAT Gen 5 assay to assess for myocardial injury (19,20). The analytical measurement range (AMR) for the assay is 6–10000 ng/L and coefficients of variation were 4.1% at 15.6 ng/L, 4.0% at 27.6 ng/L and 2.5% at 1893 ng/L. The N-terminal fragment of the propeptide of B-type natriuretic peptide (NT-proBNP) was measured using the Roche proBNP II assay to assess hemodynamic or myocardial wall stress. AMR of the assay is 5–35,000 pg/mL and total imprecision of the assay was 2.5% at both 138 pg/mL and 4578 pg/mL.
Data Management and Statistical Analysis
Clinical data were entered in an on-line data management system. Upon entry, data underwent a series of range and quality checks. Baseline arrhythmias were defined as a history of nonsustained ventricular tachycardia and/or atrial fibrillation. Summary statistics for continuous variables include mean ± standard deviation, and/or median and interquartile range. Most data were non-normally distributed, so the nonparametric Kruskal-Wallis rank test was used to compare independent groups. The exceptions were age and BMI where t-tests or analysis of variance were used. Categorical variables are summarized by number and percent of valid (non-missing) values and analyzed by contingency table analysis (chi-square). Odds ratios were calculated for 2 × 2 tables. Where multiple comparisons were made within tables, Bonferroni corrections were used to control Type I error rate.(20) The association of morphology categories with demographic and clinical variables was assessed by contingency table analysis (chi-square) for categorical variables and one-way analysis of variance for continuous variables. Savage Scores test was used to compare LGE distribution by morphology categories in a singly ordered (LGE) contingency table analysis.(21) Statistical testing was performed with Stata, v15 (Stata Corp., College Station, TX) and StatXact 7 (Cytel, Inc., Cambridge, MA).
Results
Baseline Demographics
Number of patients enrolled at each site is shown in Supplemental Table 1. Of 2762 patients initially enrolled, 1362 were enrolled in North America and 1400 in Europe. Seven patients were subsequently excluded as they were demonstrated to be phenocopies genetically and not have HCM, leaving 2755 for analysis. Baseline demographic and clinical information are shown in Table 1.
Table 1.
Baseline characteristics of patients enrolled in HCMR.
| Variable | Summary statistic | Valid n (%) |
|---|---|---|
| Age (years) | 49 ± 11 | 2738 (99.4) |
| Male gender | 1953 (71.3) | 2740 (99.5) |
| Race / Ethnicity | 2737 (99.3) | |
| White | 2311 (84.4) | |
| Black | 204 (7.4) | |
| Asian | 205 (7.5) | |
| Other | 18 (0.7) | |
| Hispanic | 60 (2.2) | 2739 (99.4) |
| BMI (kg/m2) | 29.3 ± 5.7 | 2726 (98.9) |
| Family history of HCM | 2722 (98.9) | |
| 1st degree | 600 (22.0) | |
| 2nd degree | 84 (3.1) | |
| Both 1st and 2nd | 228 (8.4) | |
| Comorbidities | ||
| Hypertension | 997 (36.6) | 2726 (99.0) |
| Type II diabetes mellitus | 213 (7.8) | 2726 (99.0) |
| Current smoker | 387 (14.2) | 2724 (98.9) |
| NYHA | 2692 (97.7) | |
| Class I | 1792 (66.6) | |
| Class II | 706 (26.2) | |
| Class III/IV | 194 (7.2) | |
| Other clinical history | ||
| Syncope | 361 (13.2) | 2726 (98.9) |
| Heart failure | 142 (5.2) | 2733 (98.9) |
| Stroke | 76 (2.8) | 2733 (98.9) |
| Nonsustained ventricular tachycardia | 196 (12.0) | 1633 (59.3) |
| Number of runs | 3.2 ± 12.1 | |
| Atrial fibrillation | 2723 (98.8) | |
| Persistent | 77 (2.8) | |
| Paroxysmal | 244 (9.0) | |
| Symptoms at enrollment | ||
| Chest pain | 893 (32.8) | 2726 (98.9) |
| Dyspnea | 1184 (43.4) | 2726 (98.9) |
| Medications at enrollment | ||
| Beta blocker | 1547 (57.0) | 2714 (98.5) |
| Calcium channel blocker | 508 (18.7) | 2714 (98.5) |
| ACE/ARB | 644 (23.7) | 2714 (98.5) |
| Disopyramide | 84 (3.1) | 2714 (98.5) |
| Statins | 741 (27.3) | 2714 (98.5) |
| Diuretic | 314 (11.6) | 2714 (98.5) |
| Oral anticoagulant | 251 (9.2) | 2714 (98.5) |
| Oral antiplatelet agent | 431 (15.8) | 2714 (98.5) |
Summary statistics are mean ± 1 standard deviation for continuous variables; n (%) for categorical variables.
Summary statistics based on non-missing values (Valid n) of total analyzed: 2755
Echocardiography
Mean maximal wall thickness was 18.6±4.8 mm. Eighteen % of participants had a peak gradient>30 mm Hg and these patients’ average gradient was 69±31. Fifty-nine % had mitral regurgitation and 12% were graded as moderate or severe. Mean pulmonary artery pressure was 28±11 mm Hg. Maximum left atrial dimension was 4.2±0.8 cm.
Holter Monitoring and Exercise Testing
Among 1672 patients who had undergone clinically-performed 24 hour Holter monitoring, AF was seen in 4% and nonsustained ventricular tachycardia in 12%. The 1520 participants who underwent clinically-performed exercise treadmill testing achieved 9.7 METS on average and 12% had a hypotensive response to exercise or failed to increase systolic blood pressure by 20mm Hg.
CMR Cine Data
A total of 2651 patients completed the CMR as 38 (1.4%) had studies aborted due to claustrophobia and 52 (2%) for other reasons. The contrast dose used was 0.15 mM/kg (mean 20±9 ml). The rhythm at the time of the CMR was normal sinus in 93%, atrial fibrillation in 2 % and other, e.g. PVC’s, bigeminy, etc., in 5%. LV and RV structure and function results derived from SSFP cine CMR images are shown in Table 2 and examples are shown in Figure 1.
Table 2.
LV and RV volumetric results.
| Variable | Mean ± SD |
|---|---|
| LV mass (g) | |
| Males | 185±61 |
| Females | 142±50 |
| LV mass index (g/m2) | |
| Males | 89±27 |
| Females | 77±25 |
| Maximal wall thickness (mm) | 20.6±4.8 |
| LV end diastolic volume (ml) | 171±41 |
| LV end diastolic volume index | 85±17 |
| LV end systolic volume (ml) | 63±26 |
| LV end systolic volume index (ml/m2) | 31±12 |
| LV stroke volume (ml) | 108±24 |
| LV stroke volume index (ml/m2) | 54±10 |
| LV ejection fraction (%) | 64±8 |
| LV mass/EDV ratio | 1.0±0.3 |
| Cardiac output (L/min) | 6.7±1.6 |
| Cardiac index (L/min/m2) | 3.3±0.7 |
| RV mass (g) | 36±12 |
| RV mass index (g/m2) | 18±5 |
| RV end diastolic volume (ml) | 152±39 |
| RV end diastolic volume index (ml/m2) | 75±16 |
| RV end systolic volume (ml) | 50±24 |
| RV end systolic volume index (ml/m2) | 24±11 |
| RV stroke volume (ml) | 102±24 |
| RV stroke volume index (ml/m2) | 51±11 |
| RV ejection fraction (%) | 68±10 |
Data are mean ± 1 Standard Deviation.
There were 2628 studies available for morphologic evaluation with the remaining 36 incomplete for morphologic assessment. 1197 (46%) had isolated basal septal hypertrophy, 1059 (38%) reverse septal curvature, 224 (8%) apical HCM, 36 (1%) concentric HCM, 79 (3%) mid-cavity obstruction with apical aneurysm, and 33 (1%) were classified as other. Demographic and clinical characteristics associated with specific morphologies are presented in Table 3. Patients with reverse septal curvature morphology were, in general, younger, had lower BMI, more likely minority, had thicker walls, more arrhythmias, less hypertension, and less LVOT obstruction as compared to those with isolated basal septal curvature.
Table 3.
Demographic differences amongst HCM morphologies.
| Isolated basal septal | Reverse curvature | Apical | Concentric | Apical aneurysm | Other | Overall p value | |
|---|---|---|---|---|---|---|---|
| Variable | N=1199 | N=1063 | N=224 | N=36 | N=79 | N=33 | |
| Age (years) | 51.6±10.3*§ | 47.1±12.0† | 51.3±9.7§ | 50.0±11.3 | 49.7±11.4 | 45.8±13.7 | < 0.001 |
| BMI (kg/m2) | 29.8±5.5* | 28.6±5.6 | 28.9±4.8 | 33.5±8.1‡*†^ | 28.9±5.4 | 28.6±6.2 | < 0.001 |
| <Male | 839 (70.1) | 758 (71.5) | 175 (78.1) | 30 (83.3) | 47 (59.5) | 22 (66.7) | 0.014 |
| Minority | 121 (10.1)*† | 179 (16.9)† | 75 (33.5) | 11 (30.6)‡ | 22 (27.9)‡ | 4 (12.1) | < 0.001 |
| Maximal wall thickness (mm) | 17.4±3.6 * | 20.0±5.2†§ | 17.1±4.5 | 22.2±6.3‡†§ | 19.6±6.0‡† | 16.7±3.5 | < 0.001 |
| LVOT gradient ≥ 30mm Hg | 279 (30.4)* | 164 (20.8)† | 7 (5.1)‡ | 8 (33.3)† | 13 (23.6)† | 2 (7.4) | < 0.001 |
| Arrythmias | 161 (13.5)*† | 224 (21.3) | 51 (22.9) | 6 (17.1) | 28 (35.4)‡* | 6 (18.2) | < 0.001 |
| LVEF < 55% | 145 (12.4) | 168 (16.3) | 20 (9.1) | 11 (30.6)‡† | 13 (16.7) | 4 (12.1) | 0.002 |
| HTN | 504 (42.2)* | 309 (29.3) | 81 (36.3) | 16 (44.4) | 31 (39.2) | 14 (42.4) | < 0.001 |
Data are mean ± 1 standard deviation for continuous variables; n (%) for categorical variables.
Percentages based on non-missing values.
For each variable, correction for Type I error was set at < 0.0055 (0.05/9). If the overall p value was <0.0055, pairwise comparisons between morphology categories were made at p < 0.0055.
p<0.0055 vs. reverse curvature
p<0.0055 vs. apical
p<0.0055 vs. apical aneurysm
p<0.0055 vs. other
p<0.0055 vs. isolated basal septal
Maximal LV wall thickness of any segment was 20.6±4.8 mm. Results comparing maximal LV wall thickness by baseline variables are presented in Supplemental Table 2. Significant relationships with wall thickness were found for age, BMI, male gender, LVOT gradient≥30 mm Hg, and sarcomere mutation (+). Left atrial width from the 3-chamber long axis view was 4.8±0.8 cm. Left atrial area from the 4-chamber long axis view was 28.9±7.6 cm2.
Myocardial fibrosis
Of 2755 patients, 254 (92%) had valid LGE values to allow assessment of replacement fibrosis. LGE was present in 50% of patients based on visual criteria, Central Illustration, and in 60% based on >6 standard deviation signal criteria. In the 50% of patients who had LGE by visual analysis, mean LGE mass was 3.7±5.2% of LV mass. In patients with LGE present, ESC risk score was higher than those without LGE (2.61±0.59 vs. 2.33±0.49, p<0.001). Only 2% of patients (n=46) had LGE>15% of LV mass. Morphologic correlates of LGE are shown in Table 4. A high percentage of patients with reverse septal curvature hypertrophy and apical aneurysm patterns had LGE whereas isolated basal septal hypertrophy demonstrated LGE less frequently than other morphologies. The reverse septal curvature pattern was associated with the majority (79%) of cases with >10% LGE.
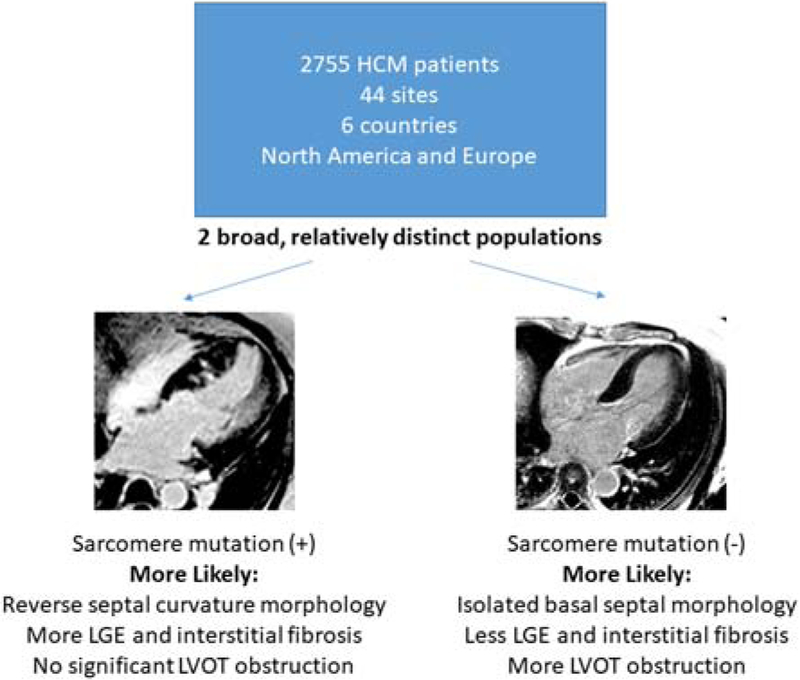
Central Illustration. Hypertrophic Cardiomyopathy Registry: Overall design and Findings.
2,755 patients from 44 sites in 6 countries were recruited. Two relatively distinct populations were identified as depicted in the slide. Left: 4-chamber inversion recovery gradient echo LGE image in a patient with reverse curvature asymmetric septal hypertrophy. Patchy LGE is noted in mid-septum. Right: similar orientation and image type in a patient with reverse curvature asymmetric septal hypertrophy. No LGE is noted. One group was sarcomere mutation positive, and more likely had reverse septal curvature morphology, more fibrosis, and less obstruction whereas the other was sarcomere mutation negative, and more likely had isolated basal septal hypertrophy with obstruction and less fibrosis.
Table 4.
LGE amount by HCM morphology.
| No LGE N=1265 |
<5% N=990 |
5–10% N=182 |
10–15% N=54 |
>15% N=46 |
|
|---|---|---|---|---|---|
| Morphology | |||||
| Isolated basal septal | 767 (66.5%) | 353 (30.6%) | 25 (2.2%) | 8 (0.7%) | 0 (0.0%) |
| Reverse curvature septal | 322 (31.4%) | 498 (48.5%) | 127 (12.4%) | 36 (3.5%) | 43 (4.2%) |
| Apical | 116 (54.2%) | 81 (37.8%) | 15 (7.0%) | 1 (0.5%) | 1 (0.5%) |
| Concentric | 19 (57.6%) | 11 (33.3%) | 0 (0.0%) | 3 (9.1%) | 0 (0.0%) |
| Apical aneurysm | 25 (32.1%) | 35 (44.9%) | 13 (16.7%) | 5 (6.4%) | 0 (0.0%) |
| Other | 16 (48.5%) | 12 (36.4%) | 2 (6.1%) | 1 (3.0%) | 2 (6.1%) |
Data are n (%).
Comparison of the presence of LGE with baseline variables is shown in Table 5. BMI, family history of HCM, maximal wall thickness, reduced LVEF, baseline arrhythmias, hypertension, and sarcomere mutation (+) were all significantly associated with LGE presence. Patients with a family history of HCM were 1.2 times more likely to have LGE present than those without. Patients with LVEF < 55% were nearly 1.3 times more likely to have LGE present. Patients with baseline arrhythmias were 1.4 times more likely to have LGE present and those with a sarcomere mutation were 1.8 times more likely to have LGE present than those without.
Table 5.
Distribution of presence of LGE for selected baseline variables.
| Variables | LGE present | Unadjusted p value | Bonferroni adjusted p value |
|---|---|---|---|
| Age (years) | 0.029 | 0.319 | |
| ≤ 40 (n = 528) | 286 (54.2) | ||
| 41–64 (n = 1944) | 958 (49.3) | ||
| ≥ 65 (n = 75) | 32 (42.7) | ||
| BMI (kg/m2) | 0.001 | 0.013 | |
| ≤ 25 (n = 606) | 338 (55.8) | ||
| 26–30 (n = 974) | 489 (50.2) | ||
| ≥ 31 (n = 969) | 451 (46.5) | ||
| Gender | 0.032 | 0.352 | |
| Male (n = 1820) | 937 (51.5) | ||
| Female (n = 729) | 341 (46.8) | ||
| Race | 0.068 | 0.748 | |
| Minority (n = 398) | 183 (46.0) | ||
| Non-minority (n = 2149) | 1095 (51.0) | ||
| Family history of HCM | < 0.001 | < 0.001 | |
| Yes (n = 870) | 489 (56.2) | ||
| No (n =1669) | 781 (46.8) | ||
| LVOT gradient ≥ 30 Hg mm | 0.036 | 0.396 | |
| Yes (n = 455) | 207 (45.5) | ||
| No (n = 1433) | 733 (51.2) | ||
| Maximal wall thickness (mm) | < 0.001 | < 0.001 | |
| ≤ 20(n=1692) | 727 (43.0) | ||
| 21–29 (n = 540) | 381 (70.6) | ||
| ≥ 30 (n = 64) | 53 (82.8) | ||
| Arrhythmias | < 0.001 | < 0.001 | |
| Yes (n = 460) | 295 (64.1) | ||
| No (n = 2080) | 975 (46.9) | ||
| Hypertension | < 0.001 | < 0.001 | |
| Yes (n = 929) | 409 (44.0) | ||
| No(n= 1613) | 863 (53.5) | ||
| LVEF < 55% | < 0.001 | < 0.001 | |
| Yes (n = 354) | 216 (61.0) | ||
| No (n = 2195) | 1062 (48.4) | ||
| Sarcomere mutation | < 0.001 | < 0.001 | |
| + (n = 878) | 614 (69.9) | ||
| − (n= 1575) | 626 (39.8) |
Data are n (%).
p values are from chi-square distribution or Savage score test for singly ordered categories, and unadjusted for multiple testing.
A Bonferroni correction would require a p value of < 0.0045 to declare statistical significance at a nominal Type I error rate of 0.05 (0.05/11).
There were 2082 patients (76%) with analyzable native T1 and 2013 (73%) valid ECV measures. Mean native T1 of the entire LV myocardium was 972±74 at 1.5T and 1170±84 at 3T. Native T1 in segments without LGE was 969±74 at 1.5T and 1157±86 at 3T compared to 976±74 at 1.5T and 1179±81 at 3T (p<0.001 for both) in segments with LGE. There were no statistically significant differences in native T1 between the MR vendors. Pooled across field strengths, native T1 was 2% higher in females than males (p<0.001) and showed modest statistically significant correlations with LGE and wall thickness, but not with age.
ECV was greater in regions with LGE (0.30±0.05) than those without (0.28±0.04, p<0.001). Mean ECV was greater in females (0.31±0.04) than in males (0.28±0.04, p<0.001). For comparison purposes, ECV in normal volunteers ranges between 0.25–0.28 and tends to rise with age and be higher in females (22,23). Patients with higher ECV had smaller BMI, less likely to have a family history of HCM, had greater wall thickness, more baseline arrhythmias, and were more likely to have a sarcomere mutation (Table 6). When evaluated by morphology, ECV was lowest in isolated basal septal hypertrophy compared to reverse septal curvature, apical and mid-cavity obstruction subtypes (Supplemental Table 3). ECV index, a measure of mass of interstitium, was 49.2±20.2g.
Table 6.
Comparison of ECV by selected baseline variables.
| Variables | ECV Mean ± SD | Unadjusted p value | Bonferroni adjusted p value |
|---|---|---|---|
| Age (years) | 0.391 | 1.000 | |
| ≤ 40(n = 417) | 0.29 ± 0.04 | ||
| 41–64 (n = 1537) | 0.29 ± 0.05 | ||
| ≥ 65 (n = 58) | 0.30 ± 0.06 | ||
| BMI (kg/m2) | < 0.001 | 0.001 | |
| ≤ 25 (n = 485) | 0.30 ± 0.05 | ||
| 26–30 (n = 772) | 0.29 ± 0.04 | ||
| ≥ 31 (n = 752) | 0.29 ± 0.05 | ||
| Gender | < 0.001 | 0.001 | |
| Male (n= 1417) | 0.28 ± 0.04 | ||
| Female (n = 596) | 0.31 ± 0.04 | ||
| Race | 0.546 | 1.000 | |
| Minority (n = 323) | 0.29 ± 0.05 | ||
| Non-minority (n = 1689) | 0.29 ± 0.05 | ||
| Family history of HCM | < 0.001 | 0.001 | |
| Yes (n = 683) | 0.29 ± 0.05 | ||
| No (n = 1322) | 0.30 ± 0.05 | ||
| LVOT gradient | 0.178 | 1.000 | |
| ≥ 30 mm Hg (n = 356) | 0.29 ± 0.05 | ||
| < 30 mm Hg (n = 1103) | 0.29 ± 0.05 | ||
| Maximal wall thickness (mm) | < 0.001 | 0.002 | |
| ≤ 20 (n = 1337) | 0.29 ± 0.04 | ||
| 21–29 (n = 411) | 0.30 ± 0.05 | ||
| ≥ 30 (n = 51) | 0.31 ± 0.05 | ||
| Arrhythmias | < 0.001 | 0.002 | |
| Yes (n = 368) | 0.30 ± 0.05 | ||
| No (n = 1639) | 0.29 ± 0.04 | ||
| Hypertension | 0.123 | 1.000 | |
| Yes (n = 718) | 0.29 ± 0.04 | ||
| No (n = 1289) | 0.29 ± 0.05 | ||
| LVEF < 55% | 0.005 | 0.055 | |
| Yes (n = 264) | 0.30 ± 0.05 | ||
| No (n = 1712) | 0.29 ± 0.04 | ||
| Genetics | < 0.001 | 0.001 | |
| Sarcomere mutation + (n = 706) | 0.30 ± 0.05 | ||
| Sarcomere mutation − (n = 1261) | 0.29 ± 0.05 |
ECV data are mean ± 1 Standard Deviation.
p values are from Kruskal-Wallis test and unadjusted for multiple testing. A Bonferroni correction would require a p value of < 0.0045 to declare statistical significance at a nominal Type I error rate of 0.05 (0.05/11).
HCM Risk Factors
The mean ESC risk score (3) was 2.48±0.56, suggesting that the study group is low risk. Of the enhanced ACCF/AHA risk factors(11), 12% had a family history of SCD, 13% had a history of syncope, 9% had sustained or nonsustained VT, 4% had wall thickness >30mm, 2% had >15% LGE, and 3% had an apical aneurysm.
Genetics
DNA samples were obtained from 2,661 individuals. Genetic analyses for genes that can be reliably interpreted in HCM comprise: the core sarcomeric genes (MYH7, MYBPC3, TNNT2, TNNI3, MYL2, MYL3, ACTC1 and TPM1) and the well-established ‘phenocopy’ genes (GLA, PRKAG2, LAMP2 and TTR). Overall, 29.5% (n=774) of individuals were found to have a variant classified as “pathogenic” or “likely pathogenic” in a sarcomere gene, with variants in the MYBPC3 (18.5%) and MYH7 (8.0%) genes accounting for the majority. Only 3 individuals (0.11%) demonstrated a combination of two likely pathogenic or pathogenic variants in confirmed sarcomere genes. Seven individuals were found to harbor pathogenic variants within either GLA (n=4) or TTR (n=3), indicating a diagnosis of Fabry’s disease or hereditary amyloidosis respectively; these individuals were removed all subsequent phenotypic analyses. In 12.3% (n=325) of individuals, “variants of uncertain significance (VUS)” were detected in the sarcomere genes. See Supplemental Material for the approach to VUS. Using this approach, based on gene-specific interpretations, we dichotomized the HCMR cohort into individuals carrying a sarcomere variant, i.e. ‘sarcomere mutation positive’ (n=943; 35.8%) and those who did not, i.e. ‘sarcomere mutation negative’ (n=1693; 64.2%). Using this dichotomous criterion, just under 1% of probands carried 2 sarcomere variants (and none >2).
Those who were sarcomere mutation (+) were younger, had a lower BMI, more often female and white, had a family history of HCM, and had less hypertension, Table 7, consistent with prior findings.(24) However, they also were less likely to have a significant LVOT gradient which may, in part, reflect differences in morphology as more of the sarcomere mutation (+) demonstrated reverse curvature asymmetric septal hypertrophy (58.1%) relative to isolated basal septal hypertrophy (33.8%), ratios that were reversed in the sarcomere mutation (−) group (30.7% and 51.8%, respectively), p<0.0001. In addition, fewer sarcomere mutation (+) individuals demonstrated apical hypertrophy (4.5% vs. 10.7%), concentric hypertrophy (0.2% vs. 2.0%), and “other” forms of hypertrophy (0.8% vs, 1.6%). Incidence of mid-cavity obstruction was similar (2.6% vs. 3.2%). LVEF was similar between groups. Sarcomere mutation (+) patients were much more likely to have any LGE as well as more extensive LGE (Table 8). Native T1 was higher at 1.5T in sarcomere mutation (+) individuals (978±76 vs. 968±74, p<0.02), but similar at 3T (1175±89 and 1167±81, respectively, p=0.21), likely due to lower n at 3T and thus lower power.
Table 7.
Demographic and clinical differences by genetic category.
| Variable | Sarcomere mutation (+) % (n) | Sarcomere mutation (−) % (n) | Unadjusted p value | Bonferroni adjusted p value |
|---|---|---|---|---|
| Age (years) | 46.2 ± 12.0 | 51.3 ± 10.4 | < 0.001 | < 0.001 |
| BMI (kg/m2) | 28.2 ± 5.4 | 29.8 ± 5.6 | < 0.001 | < 0.001 |
| Male | 65.1 (611) | 75.3 (1260) | < 0.001 | < 0.001 |
| Minority | 28.4 (116) | 37.4 (823) | 0.001 | 0.009 |
| Family history of HCM | 54.7 (511) | 22.3 (371) | < 0.001 | < 0.001 |
| LVOT gradient ≥30mm Hg | 19.0 (130) | 26.8 (335) | < 0.001 | < 0.001 |
| Arrhythmias | 38.1 (185) | 35.4 (749) | 0.255 | 1.000 |
| Hypertension | 21.3 (199) | 45.1 (752) | < 0.001 | < 0.001 |
| LVEF < 55% | 14.2 (126) | 14.2 (227) | 0.983 | 1.000 |
Data are mean ± 1 Standard Deviation for continuous variables and n (%) for dichotomous variables. A Bonferroni correction for multiplicity was made for 9 variables for nominal Type I error rate of 0.05: p < 0.0055 (0.05/9).
Table 8.
LGE categories by sarcomere mutation status.
| Category (n) | Sarcomere mutation (+) N (%) | Sarcomere mutation (–) |
|---|---|---|
| No LGE (1213) | 264 (30.1) | 949 (60.3) |
| >0–5% (965) | 458 (52.2) | 507 (32.2) |
| >5–10% (177) | 101 (11.5) | 76 (4.8) |
| > 10–15% (53) | 33 (3.8) | 20 (1.3) |
| >15% (45) | 22 (2.5) | 23 (1.5) |
Data are n (%).
LGE categories were not equally distributed for sarcomere + and − groups (p < 0.001).
Biomarkers
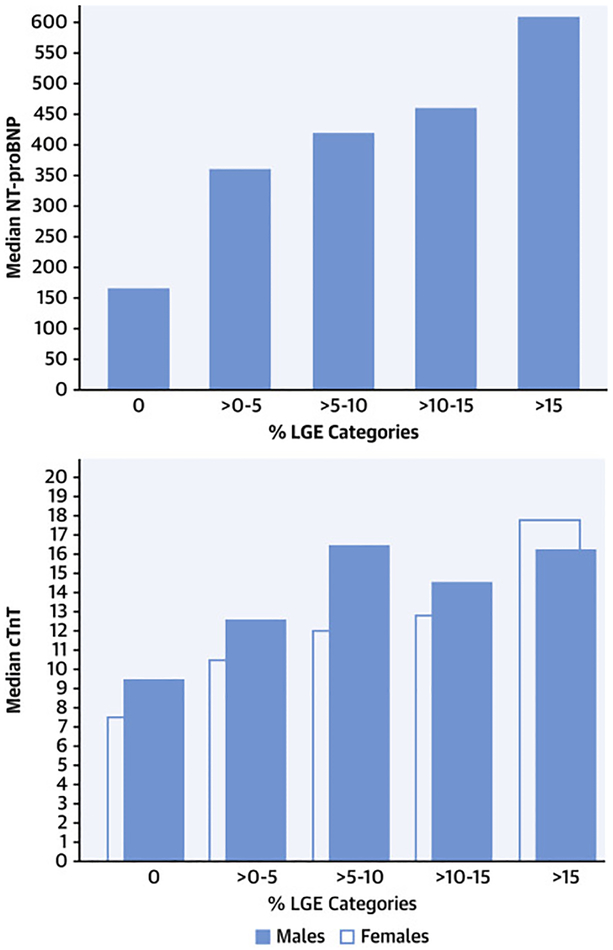
NTproBNP and cTnT were obtained in 2,665 (97%) of the 2,755 patients in the HCMR analysis database. Supplemental Table 4 presents comparisons of demographic and clinical variables and NTproBNP. Because of the extreme skewness of the NTproBNP distribution, medians and IQR are presented as well as means and standard deviations. Increasing age was associated with increasing NTproBNP. Females had higher values than males as expected, obese patients had lower levels, and patients with baseline arrhythmias had higher levels. Patients with a resting LVOT gradient ≥30mm Hg and those with a reduced LVEF had higher values. NTproBNP levels increased as maximal wall thickness increased. The relationship between NTproBNP and categories of LGE is presented in Figure 2 (Left panel). A similar relationship was seen with increasing ECV (by quartile). (Supplemental Figure 1) NTproBNP was significantly higher in sarcomere mutation (+) than (−) individuals (594±842 vs. 520±1073, p < 0.001).
Figure 2. Relationship of biomarkers to increasing amounts of late gadolinium enhancement (LGE).
N-terminal pro-brain natriuretic peptide (NTproBNP) and high sensitivity troponin T (cTnT) were measured in each patient as was the extent of LGE as a % of left ventricular mass on cardiac magnetic resonance imaging. Left – Median NTproBNP showing increasing levels relative to patients with increasing amounts of LGE (P<0.001 for trend). Right – similar trend shown for cTnT (separated by gender due to different normal values) with p<0.001 for trend for both.
Normal values for cTnT for males were ≤ 22 ng/L and ≤14 ng/L for females (per Roche Diagnostics Corporation). Of the 2665 patients with valid values, 282 males (15%) and 186 females (24%) had elevated cTnT. Supplemental Table 5 presents comparisons of demographic and imaging data and cTnT, abnormal vs. normal. Females were over 1.6 times more likely to have abnormal cTnT levels than males. Those with a history of hypertension were 1.3 times more likely to have abnormal cTnT levels and those with LVEF<55% were over 2.2 times more likely to have abnormal values. Minorities were 1.6 times more likely to have abnormal values and patients with baseline arrhythmias 1.7 times more likely. As maximal wall thickness increased, so did abnormal cTnT. The relationship between cTnT and categories of LGE is presented in Figure 2 (Right panel). In both genders, there was a stepwise increase in cTnT with categories of increasing LGE. A similar relationship was seen with increasing ECV (by quartile), although only in males. (Supplemental Figure 2) The incidence of elevated cTnT was similar in sarcomere mutation (+) and (−) groups (18% and 17%, respectively). NTproBNP was higher in sarcomere (+) than (−) groups (594±842 vs. 520±1073, respectively, p<0.001).
Discussion
HCMR is the largest systematic, prospective natural history study in HCM to date which includes comprehensive CMR data in addition to other clinical metrics, genotyping, and biomarker analysis. Prior and ongoing registries are retrospective in nature and/or do not include systematic acquisition of these data.(5–7) The 2755 patients participating in HCMR reflect a broad sampling of North American and European sites and 17% minority enrollment. A third had a family history of HCM and a third had hypertension. Eighteen % of patients had a LVOT gradient ≥30mm Hg. Only 12% of patients had moderate or more mitral regurgitation. A sarcomere variant carrier yield of 35.8% is comparable to that seen for HCM in usual routine diagnostic service laboratories, confirming that the case population on which HCMR is based is representative of ‘real world’ HCM practice. Based on the ESC risk scores, the patient group is of low risk.
The major contribution of the present study lies in the CMR, genetic, and biomarker findings and their inter-relationships in this population. Two relatively distinct populations were identified in HCMR. (Central Illustration) One was sarcomere mutation (+), and more likely to demonstrate reverse septal curvature morphology, have more extensive LGE, but less resting LVOT obstruction. The second group was sarcomere mutation (−), and more likely to demonstrate isolated basal septal hypertrophy, with less LGE, but more LVOT obstruction. The first of these groups represents the Mendelian form of familial HCM whereas the second group presumably has multifactorial disease(25), as evidenced by the higher burden of causes of secondary LVH (hypertension, high BMI, male sex, and older age, etc.). The finding that significant resting outflow obstruction indicates a lower likelihood of the familial form of HCM was not suspected. It is also notable that apical HCM is also less likely to reflect sarcomeric HCM.
Myocardial replacement fibrosis is prevalent (50%), although the frequency of extensive LGE is less than that noted in the study by Chan et al(6), in which the 4 sites included were highly specialized referral centers. Patients with LGE had thicker walls, more baseline arrhythmias, and were more likely to be sarcomere mutation (+), in keeping with the concept of a higher burden of LGE in clearly identified genetic disease. One prior study of 82 patients showed that the extent of LGE had an odds ratio of 2.1 to predict mutation-positive HCM.(26) The ESC risk score increases modestly with any LGE compared to no LGE. Whether the presence and/or extent of LGE improves risk stratification compared to the ESC risk score remains to be determined with longer follow-up. One recent study does suggest it adds to ACCF/AHA guidelines for identification of patients who subsequently require an ICD.(11)
The vast majority of patients in this study (86%) have a form of asymmetric septal hypertrophy, either isolated basal or reverse curvature. Patients with the reverse curvature form were younger, less commonly had hypertension, and were more likely sarcomere mutation (+). They represented most of the cases of >10% LGE, providing further data for a link between genetics, morphology, and fibrosis. Follow-up will test whether this morphologic subtype with its link to sarcomere mutation positivity and increased fibrosis is a risk factor for outcome events. The other morphologic subgroup with extensive LGE was the mid-cavity obstruction with apical aneurysm subtype, which has been shown to be associated with higher risk and adds to ACCF/AHA risk stratification.(11,27)
In the HCMR cohort, there was evidence of interstitial fibrosis indicated by the elevated mean ECV when compared to prior measurements using the same techniques in normal controls.(28,29) ECV was mildly elevated even in regions without LGE, suggesting that interstitial fibrosis is a characteristic of HCM. Similar to LGE, increased wall thickness, baseline arrhythmias, and sarcomere mutation positivity were associated with interstitial fibrosis. Unlike LGE, there was more interstitial fibrosis in females.
The 2 biomarkers that were measured were both elevated in subsets of patients in this cohort. NTproBNP was elevated subsets with resting LVOT gradient≥30mm Hg, reduced LVEF, more baseline arrhythmias, and sarcomere mutation (+). CTnT was higher in minorities and patients with hypertension, LVOT gradient≥30 mm Hg, increased wall thickness, and reduced LVEF. Whether elevated biomarkers are predictive of worse outcome in HCM will only be clarified with further follow-up. This is an understudied area, especially for NTproBNP.(30) One smaller study in Japan demonstrated worse outcomes with increasing levels of troponin.(31) cTnT has been shown to improve risk prediction in women, but only in the setting of coronary heart disease.(30) Both biomarkers demonstrated stepwise increases in relationship to LGE extent and ECV. Since LGE extent is a marker of SCD/ICD discharge in HCM, it may be that elevated biomarkers are a synergistic risk marker with either or both replacement and interstitial fibrosis. This points to the importance of developing a multivariable model using all of these potential risk markers to predict outcome events once follow-up is long enough to allow sufficient numbers of events to occur.
Limitations
This cohort excluded patients with HCM who had prior invasive septal therapy or ICD placement. Although minority recruitment was less than planned, the overall numbers may allow analysis of subpopulation differences and will likely be hypothesis-generating. The echocardiographic data were derived from clinical echo reports and thus protocols were not standardized. Therefore, reporting of provoked obstruction was incomplete. Stress testing and Holter monitoring at entry were also not protocol-driven and thus were not performed in every patient. While the use of ECV reduces the impact of magnetic field choice for T1 mapping, all T1 mapping techniques may be method and vendor-dependent.
Conclusions
The HCMR study population is characteristic of patients with low risk HCM by ESC risk score. 93% had no or only mild functional limitation. These patients have predominantly septal hypertrophy, 18% have resting LVOT gradient≥30mm Hg, and half have LGE. Over a third are sarcomere mutation (+). Interstitial fibrosis is prevalent even in segments without LGE. Serum biomarkers are elevated and relate to both replacement and interstitial fibrosis in a graded fashion. Two relatively distinct populations were identified. One group was sarcomere mutation (+) and more likely had reverse septal curvature morphology, more fibrosis, and less resting obstruction whereas the other was sarcomere mutation (−), and more likely had isolated basal septal hypertrophy with resting obstruction and less fibrosis. Further follow-up will allow development of a model inclusive of the demographic, clinical, echocardiographic, CMR, biomarker and genetic variables that best predict risk of major adverse cardiac events in HCM.
Supplementary Material
CLINICAL PERSPECTIVES.
Competency in Medical Knowledge:
Patients with hypertrophic cardiomyopathy and a sarcomere mutation associated with reversed septal curvature, more extensive myocardial fibrosis, and less resting ventricular outflow tract obstruction, can be distinguished from others without this sarcomere mutation, who more often have isolated basal septal hypertrophy with less fibrosis and greater resting obstruction.
Translational Outlook:
Further analysis of this registry and other sources should facilitate patient-specific risk profiling based on demographic, echocardiographic, magnetic resonance imaging, genetic, and biomarker data to identify those at risk of heart failure, arrhythmias and other adverse outcomes, including mortality.
Acknowledgements -
we would like to acknowledge the technical assistance of Michael Bowman PhD and image analysis skills of Kathleen Cheng BA, Sebastian Estrada BA, Hannah King BA, and Evan Yang
Funding: National Heart Lung Blood Institute U01HL117006-01A1 and Oxford NIHR Biomedical Research Centre
Disclosures:
Evan Appelbaum - none
Milind Desai – none
Patrice Desvigne-Nickens - none
John DiMarco - consultant to Novartis, Celgene, and Daiichi Sankyo
Sarahfaye Dolman – none
Matthias Friedrich - board member, shareholder, and consultant of Circle Cardiovascular Imaging Inc.
Nancy Geller – none
Dong-Yun Kim - none
Andrew Harper – none
Carolyn Ho – consultant for and research support from MyoKardia, Inc.
Petr Jarolim – research support from Abbott Laboratories, AstraZeneca, LP, Daiichi-Sankyo, Inc., GlaxoSmithKline, Merck & Co., Inc., Roche Diagnostics Corporation, Takeda Global Research and Development Center, and Waters Technologies Corporation, and consulting fees from Roche Diagnostics Corporation.
Michael Jerosch-Herold - none
Paul Kolm – none
Christopher M. Kramer MD – consultant for Cytokinetics, research grants from Biotelemetry, MyoKardia
Raymond Y. Kwong MD – research support from Siemens Healthineers, Bayer AG, Myokardia
Martin Maron – Consulting and research support from iRhythm and consultant for Celltrion, Cytokinetics
Stefan K Piechnik – SKP has patent authorship rights for U.S. patent 9,285,446 B2. Systems and methods for shortened look locker inversion recovery (Sh-MOLLI) cardiac gated mapping of T1.
Granted March 15, 2016. IP is managed by Oxford University Innovations; the license exclusively transferred to Siemens Healthcare.
Jeanette Schulz-Menger MD – consultant for Bayer, research grants from Bayer, Siemens Healthineers, Circle Cardiovascular Imaging
Stefan Neubauer – consultant for Pfizer and Cytokinetics. Research grants from Boehringer Ingelheim.
Kate Thomson – none
Hugh Watkins – consultant for Cytokinetics
William Weintraub - consultant for Janssen, AstraZeneca, SC Pharma. Research grants from Amarin
Cheng Zhang - none
Abbreviations
- HCM
hypertrophic cardiomyopathy
- CMR
cardiovascular magnetic resonance
- LGE
late gadolinium enhancement
- ECV
extracellular volume
- NTproBNP
N-terminal pro-brain natriuretic peptide
- cTnT
high sensitivity troponin T
- LVOT
left ventricular outflow tract
References
- 1.Kramer CM, Appelbaum E, Desai MY et al. Hypertrophic Cardiomyopathy Registry: The rationale and design of an international, observational study of hypertrophic cardiomyopathy. Am Heart J 2015;170:223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gersh BJ, Maron BJ, Bonow RO et al. 2011 ACCF/AHA Guideline for the Diagnosis and Treatment of Hypertrophic Cardiomyopathy: Executive Summary:. J Am Coll Cardiol 2013;58:2703–2738. [DOI] [PubMed] [Google Scholar]
- 3.O’Mahony C, Jichi F, Pavlou M et al. A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM Risk-SCD). Eur Heart J 2014;35:2010–2020. [DOI] [PubMed] [Google Scholar]
- 4.Maron BJ, Spirito P, Shen WK et al. Implantable Cardioverter-Defibrillators and Prevention of Sudden Cardiac Death in Hypertrophic Cardiomyopathy. JAMA 2007;298:405–412. [DOI] [PubMed] [Google Scholar]
- 5.O’Mahony C, Jichi F, Ommen SR et al. International External Validation Study of the 2014 European Society of Cardiology Guidelines on Sudden Cardiac Death Prevention in Hypertrophic Cardiomyopathy (EVIDENCE-HCM). Circulation 2018;137:1015–1023. [DOI] [PubMed] [Google Scholar]
- 6.Chan RH, Maron BJ, Olivotto I et al. Prognostic Value of Quantitative Contrast-Enhanced Cardiovascular Magnetic Resonance for the Evaluation of Sudden Death Risk in Patients With Hypertrophic Cardiomyopathy. Circulation 2014;130:484–495. [DOI] [PubMed] [Google Scholar]
- 7.Ho CY, Day SM, Ashley EA et al. Genotype and Lifetime Burden of Disease in Hypertrophic Cardiomyopathy: Insights from the Sarcomeric Human Cardiomyopathy Registry (SHaRe). Circulation 2018;138:1387–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charron P, Elliott PM, Gimeno JR et al. The Cardiomyopathy Registry of the EURObservational Research Programme of the European Society of Cardiology: baseline data and contemporary management of adult patients with cardiomyopathies. Eur Heart J 2018;39:1784–1793. [DOI] [PubMed] [Google Scholar]
- 9.Mentias A, Raeisi-Giglou P, Smedira NG et al. Late Gadolinium Enhancement in Patients With Hypertrophic Cardiomyopathy and Preserved Systolic Function. J Am Coll Cardiol 2018;72:857–870. [DOI] [PubMed] [Google Scholar]
- 10.Weng Z, Yao J, Chan RH et al. Prognostic Value of LGE-CMR in HCM. JACC Cardiovasc Imaging 2016;9:1392–1402. [DOI] [PubMed] [Google Scholar]
- 11.Maron MS, Rowin EJ, Wessler BS et al. Enhanced American College of Cardiology/American Heart Association Strategy for Prevention of Sudden Cardiac Death in High-Risk Patients With Hypertrophic CardiomyopathyEnhanced ACC/AHA Strategy for Prevention of Sudden Cardiac Death in Patients With Hypertrophic CardiomyopathyEnhanced ACC/AHA Strategy for Prevention of Sudden Cardiac Death in Patients With Hypertrophic Cardiomyopathy. JAMA Cardiol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ho CY, Abbasi SA, Neilan TG et al. T1 Measurements Identify Extracellular Volume Expansion in Hypertrophic Cardiomyopathy Sarcomere Mutation Carriers With and Without Left Ventricular Hypertrophy. Circ Cardiovasc Imaging 2013;6:415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piechnik S, Ferreira V, Dall’Armellina E et al. Shortened Modified Look-Locker Inversion recovery (ShMOLLI) for clinical myocardial T1-mapping at 1.5 and 3 T within a 9 heartbeat breathhold. J Cardiovasc Magn Reson 2010;12:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schulz-Menger J, Bluemke DA, Bremerich J et al. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) Board of Trustees Task Force on Standardized Post Processing. J Cardiovasc Magn Reson 2013;15:35–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maron M Clinical Utility of Cardiovascular Magnetic Resonance in Hypertrophic Cardiomyopathy. J Cardiovasc Magn Reson 2012;14:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Binder J, Ommen SR, Gersh BJ et al. Echocardiography-Guided Genetic Testing in Hypertrophic Cardiomyopathy: Septal Morphological Features Predict the Presence of Myofilament Mutations. Mayo Clin Proc 2006;81:459–467. [DOI] [PubMed] [Google Scholar]
- 17.Dodson JA, Neilan TG, Shah RV et al. Left Atrial Passive Emptying Function Determined by Cardiac Magnetic Resonance Predicts Atrial Fibrillation Recurrence after Pulmonary Vein Isolation. Circ Cardiovasc Imaging 2014;7:586–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Appelbaum E, Maron BJ, Adabag S et al. Intermediate Signal Intensity Late Gadolinium Enhancement Predicts Ventricular Tachyarrhythmias in Patients with Hypertrophic Cardiomyopathy. Circ Cardiovasc Imaging 2012;5:78–85. [DOI] [PubMed] [Google Scholar]
- 19.Flett AS, Hayward MP, Ashworth MT et al. Equilibrium Contrast Cardiovascular Magnetic Resonance for the Measurement of Diffuse Myocardial Fibrosis. Circulation 2010;122:138–144. [DOI] [PubMed] [Google Scholar]
- 20.Hochberg Y, Tamhane A. Multiple comparison procedures. New York: John Wiley & Sons, 1987. [Google Scholar]
- 21.Lehmann E Nonparametrics: Statistical Methods Based on Ranks. San Francisco: Holden-Day, 1975. [Google Scholar]
- 22.Liu C-Y, Liu Y-C, Wu C et al. Evaluation of Age-Related Interstitial Myocardial Fibrosis With Cardiac Magnetic Resonance Contrast-Enhanced T1 Mapping: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 2013;62:1280–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neilan TG, Coelho-Filho OR, Shah RV et al. Myocardial Extracellular Volume Fraction From T1 Measurements in Healthy Volunteers and Mice: Relationship to Aging and Cardiac Dimensions. JACC: Cardiovasc Imaging 2013;6:672–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ingles JBC, Bagnall RD, Lam L, et al. Nonfamilial hypertrophic cardiomyopathy: Prevalence, natural history, and clinical implications. Circ Cardiovasc Genet 2017;10. [DOI] [PubMed] [Google Scholar]
- 25.Maron BJ, Maron MS, Maron BA, Loscalzo J. Moving Beyond the Sarcomere to Explain Heterogeneity in Hypertrophic Cardiomyopathy: JACC Review Topic of the Week. J Am Coll Cardiol 2019;73:1978–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teramoto R, Fujino N, Konno T et al. Late Gadolinium Enhancement for Prediction of Mutation-Positive Hypertrophic Cardiomyopathy on the Basis of Panel-Wide Sequencing. Circ J 2018;82:1139–1148. [DOI] [PubMed] [Google Scholar]
- 27.Rowin EJ, Maron BJ, Haas TS et al. Hypertrophic Cardiomyopathy WithLeft Ventricular Apical Aneurysm: Implications for Risk Stratification and Management. J Am Coll Cardiol 2017;69:761–773. [DOI] [PubMed] [Google Scholar]
- 28.Rosmini S, Bulluck H, Treibel TA et al. Native myocardial T1 and ECV with age and gender developing normal reference ranges - a 94 healthy volunteer study. J Cardiovasc Magn Reson 2016;18:O42. [Google Scholar]
- 29.Liu JM, Liu A, Leal J et al. Measurement of myocardial native T1 in cardiovascular diseases and norm in 1291 subjects. J Cardiovasc Magn Reson 2017;19:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manson JE, Bassuk SS. Biomarkers of cardiovascular disease risk in women. Metabolism 2015;64:S33–S39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kubo T, Kitaoka H, Yamanaka S et al. Significance of High-Sensitivity Cardiac Troponin T in Hypertrophic Cardiomyopathy. J Am Coll Cardiol 2013;62:1252–1259. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.