Abstract
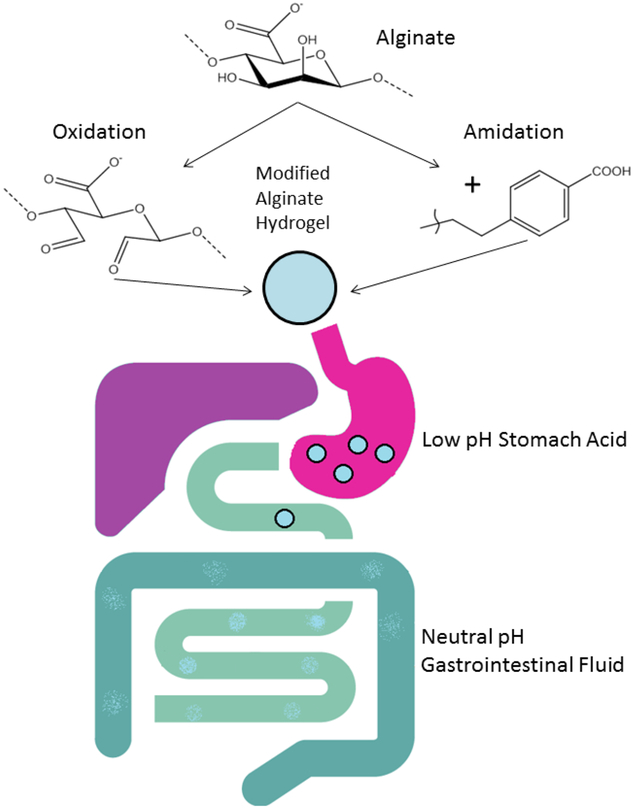
Here we report two methods that chemically-modify alginate to achieve neutral-basic pH-sensitivity of the resultant hydrogel. The first method involves direct amide bond formation between alginate and 4-(2-aminoethyl)benzoic acid. The second method that arose out of the desire to achieve better control of the degradation rate of the alginate hydrogel, involves reductive amination of oxidized alginate. The products of both methods result in a hydrogel vehicle for targeted delivery of encapsulated payload under physiological conditions in the gastrointestinal tract. 2D Diffusion-Ordered Spectroscopy as well as internal and coaxial external NMR standards were utilized to establish chemical bonding and percent incorporation of the modifying groups into the alginate polymer. The hydrogel made with the alginate modified by each method was found to be completely stable under acidic pH conditions while disintegrating within minutes to hours in neutral-basic pH conditions. We found that, while alginate oxidation did not affect the M/G ratio of the alginate, the rate of disintegration of the hydrogel made with the oxidized alginate was dependent on the degree of oxidation.
Keywords: alginate, reductive amination, oxidation, 4-(2-aminoethyl)benzoic acid, hydrogel, pH-dependence, release, bioavailability
Graphical Abstract
INTRODUCTION:
Hydrogels have been a key focus for drug delivery studies due to their natural swelling/water retention properties as well as their consistent and predictable release rate of therapeutic agents1–2. They are also used as a means of protecting the therapeutics from harmful conditions such as the acidic stomach environment during oral drug delivery. Many drug delivery hydrogels are already on the market for a wide variety of therapeutic applications including: hypertension, pain management, infections, and burn dressings1, 3. With controlled drug release being the main objective, extensive research has gone into tuning the properties of the hydrogels to respond consistently to different stimuli with temperature2, 4–5 and pH1–2, 6–13 being the most popular. Drug release based on pH sensitivity is useful for oral drug delivery due to the large difference between the acidic stomach pH (< 3.0) and the more neutral intestinal pH. Some polymers that have been examined for pH sensitivity include: chitosan-poly (ethylene oxide) (PEO)14, poly(acrylic acid):PEO11, Gelatin-PEO10, Poly(acrylamide-co-maleic acid)15, hyaluronic acid16, and alginate6–9.
There is significant interest in the use of alginate, a block copolymer consisting of (1,4)-linked β-D-mannuronate (M) and α-L-guluronate (G) monomers, in biomedical applications owing to its proven biocompatibility and ability to form hydrogels under mild physiologic conditions17–18. In addition, its controllable degradation, ease of chemical modification and self-healing properties make alginate particularly attractive in drug delivery8. Although other chemical modifications of alginate for drug delivery have been described, our objective in this study was to develop a modification method that specifically preserves alginates inherent biocompatibility and non-toxic rapid crosslinking properties when added to a divalent cation such as calcium8.
Alginate based scaffolds have been commonly used for bone and tissue regeneration19–21, absorbent dressing for wound treatment3, 22–23, and drug delivery platforms6–7, 23–28. Although alginate hydrogel microbeads are prone to swelling under physiologic conditions, they usually remain stable and slow to degradation29, which is not ideal for oral drug delivery with regards to controlled therapeutic drug release in the gastrointestinal tract (GIT). We hypothesized that alginate microbeads made with chemical modifications having basic functional groups would function inversely to those having acidic functional groups when exposed to acidic or basic solutions. Therefore, chemical modification of the alginate polymer would be an option for controlling the release rate by enhancing the swelling capacity and/or water solubility of alginate at a desired pH. A number of studies on hydrogel formation and properties based on chemical and physical crosslinking polymers23, 30 are largely based on the network structure and permeability of the material31.
Alginate derivatives via chemical modifications include reactions targeting the carboxyl groups via esterification32–33, Ugi reaction34–35, and amidation36–37 whereas modification via the vicinal hydroxyl groups on the polymer backbone include oxidation of alcohols to dialdehyde36, 38 followed by reductive-amination of the oxidized alginates38, sulfination26, and copolymerization39. Our work involves the chemical modification of alginate as a way to improve and better control the release rate of potential therapeutic agents in the GIT. Although a previous study had modified alginate using three different bioactive peptide sequences (GRGDYP, GRGDSP and KHIFSDDSSE) coupled to 8% periodate oxidized alginate32, the present study is the first application of the approach of modifying alginate by oxidizing the vicinal dialcohol backbone of alginate as well as direct amide bond formation between alginate and 4-(2-aminoethyl)benzoic acid to generate a pH-tunable alginate for drug delivery.
MATERIALS AND METHODS:
Materials:
Ultra-pure low viscosity (20–200 mPa·s) sodium alginate with high guluronic acid (UPLVG) contents was purchased from Nova-Matrix (Sandvika, Norway). UPLVG alginate was reported by the manufacturer to have molecular weights 75–200kDa and G/M ratios of 1.5, and the G/M ratios were not altered by our modifications as shown in Table 2. 4-(2-aminoethyl)benzoic acid was purchased from Combi-Blocks (San Diego, CA); sodium meta-periodate >99% purity was purchased from Santa Cruz Biotechnology (Dallas, TX) and TMSP-d4 for NMR quantification was obtained from Cambridge Isotope Labs (Tewksbury, MA). All other chemicals were purchased from Sigma-Aldrich (St. Louis MO).
Table 2.
M/G ratio for UPLVG modified alginate
| Sample | A | B | C | M/G ratio |
|---|---|---|---|---|
| UPLVG 0.5% | 1.0 | 0.92 | 0.71 | 0.63 |
| UPLVG 1% | 1.0 | 0.91 | 0.78 | 0.69 |
| UPLVG 2% | 1.0 | 0.92 | 0.69 | 0.61 |
Chemical Modification of Sodium Alginate:
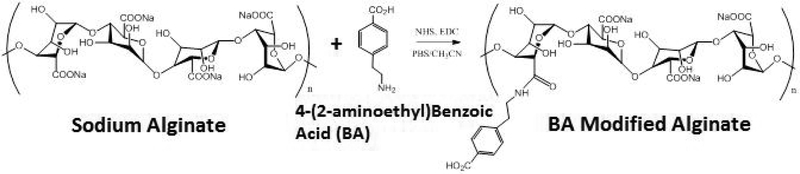
Amidation:
Amidation was done based on the general procedure described by Sakai and Kawakami with some modification40. Ultrapure alginate (200 mg, 1.14 mmol) was dissolved in 25 mL of phosphate buffer (pH 6.05) and 25 mL of acetonitrile. 240 mg (1.25 mmol) of EDC.HCl and 200 mg (1.74 mmol) of N-hydroxysuccinimide were added sequentially and the mixture was stirred in the dark under Ar atmosphere for 1 hour. 4-(2—aminoethyl)benzoic acid.HCl 300 mg (1.50 mmol) was then added and the mixture was stirred for the next 24 hours. The sample was then dialyzed with 0.1 M NaCl for 12 h followed by ultrapure water (Millipore Sigma) for 24 h and lastly lyophilized until dry obtaining 110 mg (~ 0.56 mmol) of soft cotton like material.
Reductive Amination:
Following Dalheim et al.’s protocol32, ultrapure alginate (180 mg, 1.0 mmol) was dissolved in ultrapure water (Millipore Sigma) with 10% (v/v) isopropanol to a concentration of 8 mg/mL. The solution was degassed with N2 and chilled to 2–4 °C. To generate an oxidized alginate product, a degassed solution of sodium (meta)-periodate (0.25mL of 0.25M solution, 0.0625 mmol NaIO4) was added for oxidation of about 5 mol percent of alginate units. The mixture was stirred for 48 h in the dark and then dialyzed with ultrapure water until the conductivity was below 2-μS and then dried via lyophilization to obtain 133 mg of the product. The periodate-oxidized alginate (120 mg, ~ 0.7 mmol) was dissolved in ultrapure water and methanol (12% v/v), and either used as such or used after further modification with 4-(2-aminoethyl)benzoic acid for hydrogel bead fabrication and testing.
For the modification of the oxidized alginate with 4-(2-aminoethyl)benzoic acid, after stirring for 15 minutes, 12 mg (0.06 mmol) of 4-(2-aminoethyl)benzoic acid hydrochloride and 10 mol equivalent of pic-BH3 were added. The pH of the mixture was adjusted to 6.0 with phosphate buffer and solution stirred in the dark for another 24 h. The sample was then dialyzed with 0.1 M NaCl for 12 h followed by ultrapure water for 24 h and lastly lyophilized until dry obtaining 105 mg (~ 0.58 mmol) of soft cotton like material. Batches with 1%, and 2% periodate oxidized alginates were used to obtain incorporation of approximate equivalents of small molecules via 2x and 4x scaling of the reagents for oxidation (NaIO4) followed by reductive amination with 4-(2-aminoethyl)benzoic acid hydrochloride and pic-BH3 Quantification of the small molecule incorporated was done via 1H NMR analysis detailed in the NMR analysis section below.
Quantification of alginate M/G ratios and assessment of molecular weight of alginate:
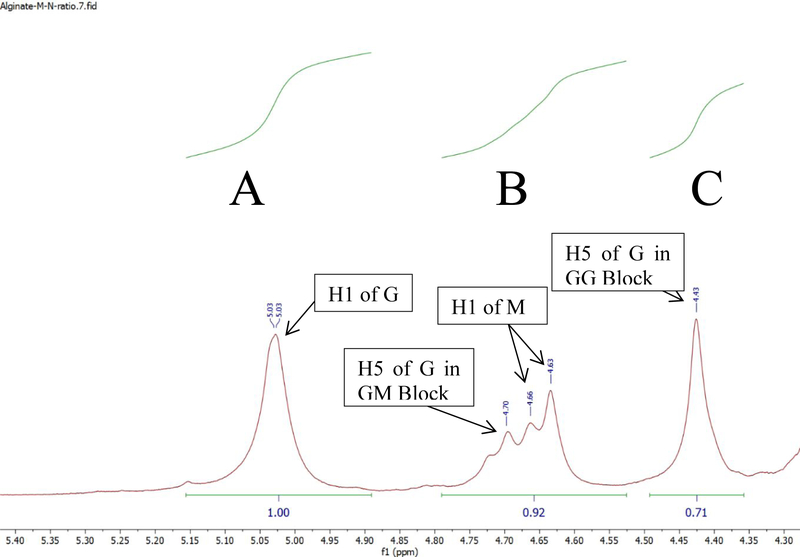
M/G ratios were determined for the modified alginates according to the published procedures41 with a few modifications; 1D 1H spectra were collected at 80° C verses 90° C in 100% D2O using a full 90 degree pulse as opposed to a 30 degree pulse. A five second recycle delay was then used to ensure full T1 relaxation when using the 90 degree pulse. Spectra were reference to the water signal (4.219 ppm) according to literature42. The equation M/G = (B + C − A)/A was used where A is the area of the G anomeric proton (H1, broad singlet, 5.03 ppm), B is the area for the multiplet centered at 4.68 ppm containing H1 of M and H5 of G in the GM blocks and C (4.43 ppm) is the area for H5 of G in the GG blocks.
NMR acquisition and analysis:
Modified alginate was dissolved in D2O (8 mg/mL) along with 3-(trimethylsilyl)-2,2,3,3-tetradeuteratepropionic acid sodium salt (TMSP-d4) as an internal standard (1 mM equivalent). The moles of substituent incorporated per moles of alginate was determined via 1H NMR analysis based on the internal standards used. A known amount of maleic acid dissolved in D2O was also used as an external standard for secondary standard for quantification. Although FTIR is often used for structural/molecular characterization of particles, the data for particle size and zeta potential were inconsistent and appeared to vary with concentration and duration of sonication. Therefore we chose not to include them in the results. We believe that NMR is much more definitive than FTIR for structural/molecular characterization. We have done SEM imaging with other alginate structures not related to this project and have found it rarely useful for this type of characterization as the particle size is too small to assess.
Validation of covalent bonding of the small molecule:
Diffusion-Ordered Spectroscopy (DOSY) is commonly used for analysis of complex mixtures based on their relative hydrodynamic radius and their derived diffusion coefficients43–44. The technique is especially useful when the 1D spectra is crowded and hard to resolve unique resonances45. Comparison of diffusion coefficients between the oxidized alginates, small molecule 4-(2-aminoethyl)benzoic acid and the covalently bonded product of the two can be used as an indirect confirmation of an association between the polymer and small molecule.
In Vitro testing of modified alginate degradation:
Modified alginate was dissolved in Hanks Balanced Salt Solution (HBSS) at a concentration of 1.5% (w/v) overnight. Hydrogel beads were formed by manually extruding the alginate through an 18-guage blunt needle into a 100 mM CaCl2 crosslinking solution. The beads were allowed to crosslink for 10–15 minutes before being incubated in either simulated gastric fluid (SGF) for up to 6 hours or simulated intestinal fluid (SIF) for 3 hours while being shaken at 60 RPM. At corresponding time points, the beads were counted to determine the degradation rate in each respective medium.
Imaging
All images were taken with a Zeiss Axiovert 200M inverted microscope.
Statistical Analysis:
Data in Figures 4, 5, & 6 are expressed as mean ± standard error and a multiple t-test was used to statistically evaluate the differences between the two groups. Differences were considered significant if p < 0.05.
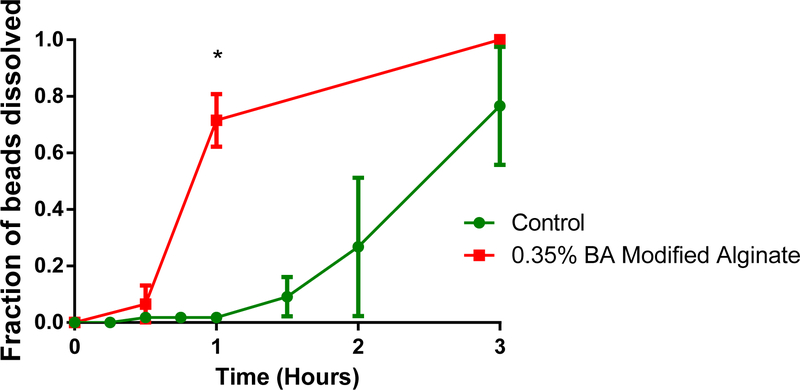
Figure 4:
Comparison of benzoic acid amidation chemistry and unmodified alginate (control) in SIF. The modified alginate falls apart much faster than the control group (*) p < 0.05, n = 4.
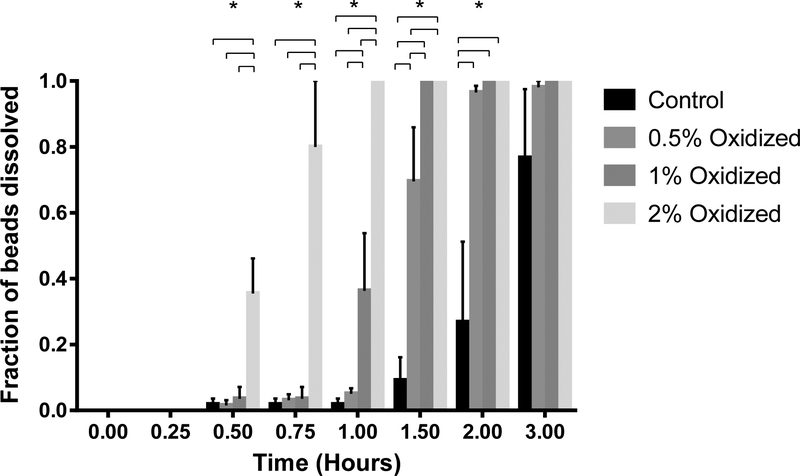
Figure 5.
Degradation rate of oxidized alginate in SGF (left) and SIF (right). Values expressed as fraction of dissolved beads. (*) p<0.05, n = 4.
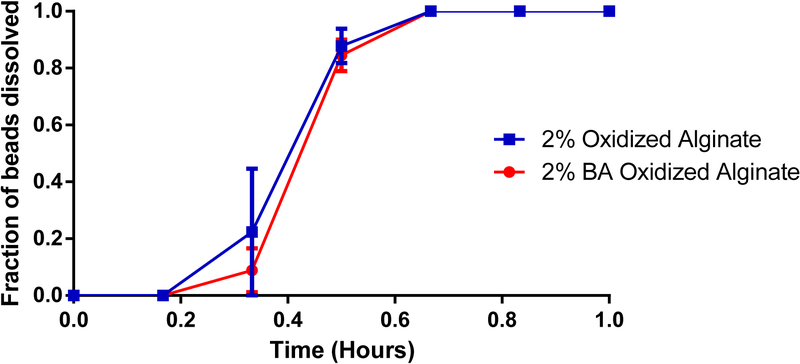
Figure 6.
Degradation rates of beads made after alginate oxidation ± benzoic acid attachment. p > 0.05, n = 3.
RESULTS:
Chemical modification and validation of covalent bonding of the small molecule:
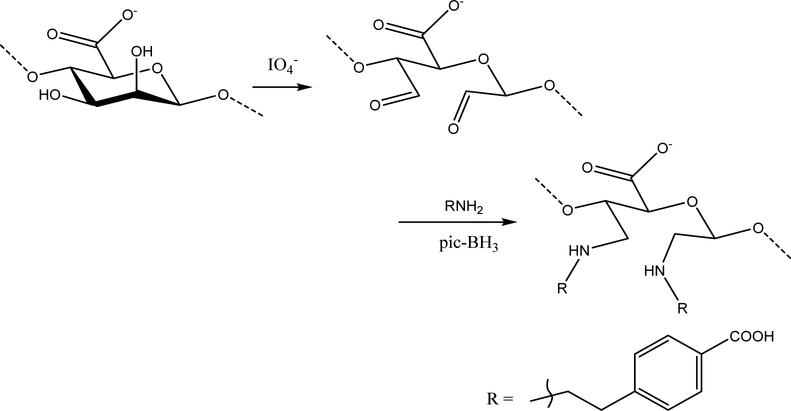
Alginate modification following the two protocols were verified for the degree of functionalization and chemical bonding. The desired structures for amidation and reductive amination are shown in Scheme 1 and Scheme 2 respectively
Scheme 1.
Chemical modification scheme for amidation represented as a reaction on an alginate monomer unit
Scheme 2.
Chemical modification scheme for reductive amination represented as a reaction on an alginate monomer unit
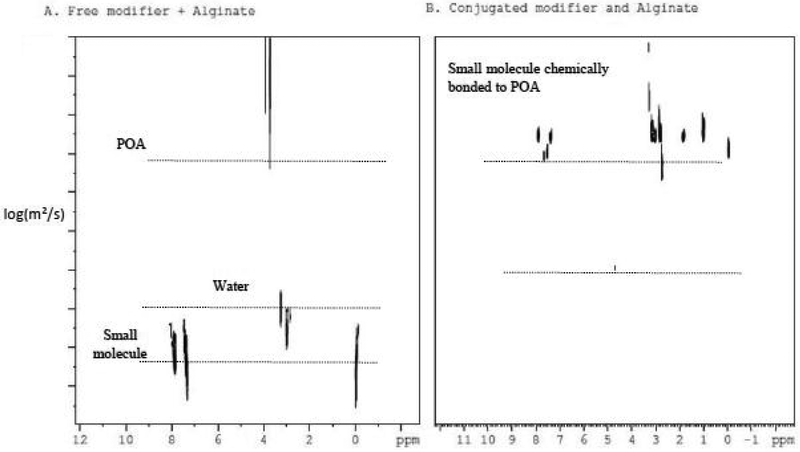
The amount of small molecule incorporated into the polymer backbone was calculated based on 1H NMR data using an internal standard (3-(trimethylsilyl) propane sulfonic acid sodium salt) and further verified with a coaxial external standard (maleic acid). Covalent bonding between the polymer backbone and the small molecule was determined using DOSY to measure the difference of diffusion coefficients between the covalently coupled product and non-coupled mixture of oxidized alginate and small molecule (Figure 1).
Figure 1.
2D DOSY (linear gradient) spectra of alginate and 4-(2-ethylamino)-benzoic acid (modifier)
Here we show the comparison of the DOSY spectra of a mixture of the oxidized polymer with free small molecule and the chemically coupled product of the two. In Figure 1A, the oxidized polymer and the small molecule both have distinctively different diffusion coefficients confirming no coupling between the two whereas in Figure 1B, the DOSY spectra after chemical modification using POA (periodate oxidized alginate) and the small molecule display one cohesive diffusion coefficient indicating the bonding of the small molecule into the alginate polymer. 2D 1H DOSY spectra were collected using the standard Bruker sequence stebpgpin1s1d. A diffusion gradient length of 100 ms and a diffusion time delay of ~3200 μs giving 95% truncation of the POA alginate 4-(2-aminoethyl)benzoic acid were used. DOSY spectra were collected with a linear gradient of 16 points and 32 scans. Processing was carried out with TopSpin3.6pl6. DOSY spectra clearly showed a difference in the diffusion coefficients between the aromatic peaks (7–8 ppm) in free 4-(2-aminoethyl)benzoic acid (Figure 1A) and the chemically bonded 4-(2-aminoethyl)-benzoic acid (Figure 1B).
NMR Acquisition and Analysis:
1D 1H NMR spectra were collected under full T1 relaxation conditions to help ensure accuracy of the integral areas. The concentration and mol% modification of POA alginates (Table 1) were determined by comparing the area of the aromatic resonances of 4-(2-aminoethyl)benzoic acid to the area generated from using a co-axial 1mm insert containing a known concentration of malic acid in D2O. Table 1 lists the concentration (and molar percent) of small molecule (4-(2-ethylamino)benzoic acid) chemically coupled to approximately 0.5, 1, and 2 percent oxidized ultrapure modified alginates (UPLVG).
Table 1.
Percent incorporation of 4-(2-aminoethyl)benzoic acid in periodate oxidized alginate (POA)
| 0.5% POA | 1% POA | 2% POA | ||||
|---|---|---|---|---|---|---|
| Concentration (mM) | Mol % | Concentration (mM) | Mol % | Concentration (mM) | Mol % | |
| UPLVG | 0.0501 | 0.6875 | 0.1242 | 1.2808 | 0.2525 | 2.4592 |
Quantification of alginate M/G ratios and assessment of molecular weight:
In order to determine if the modification had an unintended change on the structure of the alginate, the M/G ratios were measured using NMR and determined by the equation M/G = (B+C−A)/A, where A is the area under the first curve, B is the area under the centered multiplet, and C is the area under the broad singlet (Figure 2). UPLVG alginate M/G ratios (Table 2) remained within the range specified by the company’s description of the unmodified UPLVG alginate (>60% G units). The modified alginate had an M/G ratio of 0.63, 0.69, and 0.61 for 0.5%, 1%, and 2% modified alginate respectively (Table 2). This indicates G units making up about 62–64% of the alginate polymer. Therefore, the chemical modifications had no impact on the block subunit structure of the alginate.
Figure 2.
NMR spectra of 0.5% modified UPLVG alginate. In the equation M/G = (B+C−A)/A, A is the area under the first curve (1.00), B is the area under the centered multiplet (0.92), and C is the area under the broad singlet (0.71).
In Vitro testing of modified alginate microbead degradation:
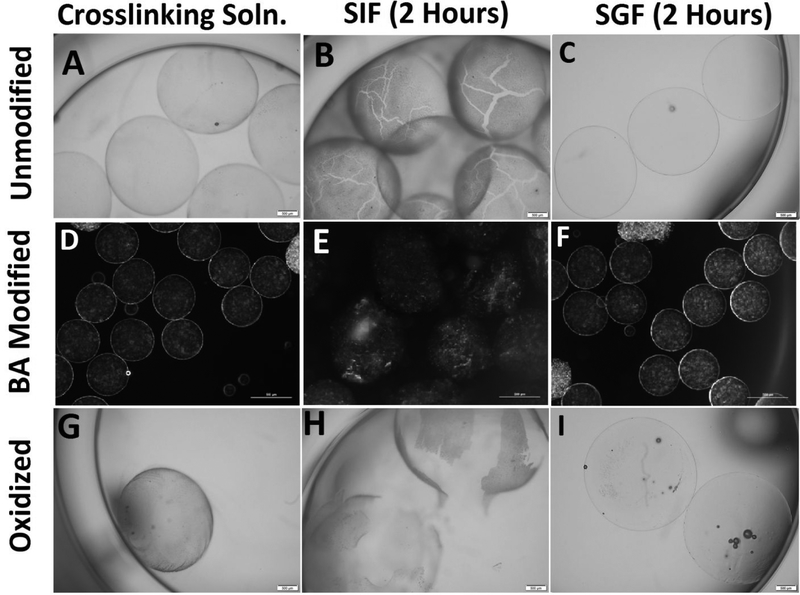
We had hypothesized that 4-(2-aminoethyl)benzoic acid modification onto the alginate backbone would lead to a structural weakness at a neutral pH. We were able to control the modification to as low as 0.5 mol percent of the total alginate units in the polymer (Table 1). Oxidation of the polymer at greater than 10% resulted in degradation of gelling capacity of the material as expected due to the disruption of the backbone structure43. Modified alginate by direct incorporation of 4-(2-aminoethyl)benzoic acid at 0.35 mol percent as well as 2% oxidized alginate was synthesized and the degradation of the resultant hydrogel microbeads in simulated acidic gastric fluid (SGF) and simulated neutral intestinal fluid (SIF) was studied. As shown in Figure 3, after 6 hours of placement of hydrogel microbeads made with either unmodified (control) or modified alginate in the SGF medium the beads remained structurally intact. However, when incubated in the neutral SIF medium while majority of the microbeads made with the unmodified alginate remained structurally intact despite an apparent swelling, all of the beads made with the modified alginate completely disintegrated within 3 hours of incubation. Also, as shown in Figure 3 when we tested alginate microbead hydrogels made with alginate that had been oxidized without covalent bonding to 4-(2-aminoethyl)benzoic acid, we found that the hydrogel was also sensitive to the simulated intestinal fluid while resistant to the gastric fluid.
Figure 3.
Visual representation of modified and unmodified alginate in different solutions after 2 hours. (A) Unmodified alginate in crosslinking solution with no signs of degradation. (B) Unmodified alginate in SIF showing signs of swelling leading to cracks in the hydrogel. (C) Unmodified alginate in SGF which shows no signs of degradation. (D) Benzoic acid modified alginate in crosslinking solution with no signs of degradation. (E) BA modified alginate with a significant increase in size due to swelling. (F) BA modified alginate in SGF showing no signs of degradation. (G) Oxidized alginate without BA in crosslinking solution with no signs of degradation. (H) Oxidized alginate without BA in SIF with an increase in size and large ruptures due to swelling. (I) Oxidized alginate without BA in SGF showing no signs of degradation. Scale bar on bright field images 500 μm. Scale bar on dark field images 250 μm.
The rate of degradation of the benzoic acid modified alginate over time can be seen in Figure 4. Within 1 hour of incubation in SIF, 71.5 ± 9.3% of the modified alginate fell apart compared to only 1.8 ± 1.8% of the unmodified alginate beads.
The effect of the degree of oxidation of the alginate backbone on the rate of degradation of the resultant microbead hydrogel in the SIF, is shown in Figure 5. As the degree of oxidation of alginate increased from 0.5% - 2%, the rate of bead disintegration was enhanced. Within 45 minutes, ~ 80% of the beads made with 2% oxidized alginate had disintegrated and this was significantly higher than was observed with beads made using lower percent oxidized alginate. Within 1.5 hours, none of the beads made with either 1% or 2% oxidized alginate remained intact and by 2 hours, less than 10% of the beads made with 0.5% oxidized alginate stayed intact. Benzoic acid was bonded to the oxidized alginate in order to determine if there was a compounding effect on the alginate bead degradation.
DISCUSSION:
Alginate is a complex polysaccharide composed of randomly oriented blocks of monomers of (1–4)-linked β-D-mannuronic acid (M) and α-L-guluronic acid (G)46. The transport of molecules within an alginate hydrogel is important for their transport in such a delivery system. Transport within this hydrogel system is largely driven by diffusion, and diffusion varies as a function of alginate composition and concentration and the rate of diffusion is greatest for hydrogels prepared with sodium alginate of low G content in contrast to those prepared with sodium alginate of high G fractions47. This is attributed to the flexibility of the polymer backbone, which suggests that higher G fractions result in higher crosslinking, less swelling and hence a greater barrier to diffusion. Measurements of simple physical parameters, such as volume fraction and size, can be used to predict solute transport in alginate hydrogel47. These parameters can be controlled based on the alginate concentration and composition for sustained release of small amounts of substances encapsulated in alginate hydrogel. However, in situations where the release of readily effective therapeutic levels is desired, it would be beneficial to modify the alginate delivery vehicle to release the encapsulated products based on prompt degradation of the alginate hydrogel. One way to achieve such immediate release and enhance the bioavailability of therapeutic molecules encapsulated in alginate hydrogel is to modify the alginate polymer to degrade based on sensitivity to the basic pH of the small intestine where absorption into the systemic circulation also takes place.
We show in this report two methods of chemical modification of the alginate polymeric material that result in the potential use of the alginate hydrogel as a drug delivery vehicle for pH dependent release in the GIT. In this proof of concept study, 4-(2-aminoethyl)benzoic acid was used as a modifier on the alginate backbone by direct covalent bondage to generate a novel modified alginate material whose hydrogel is sensitive to neutral-basic pH prevalent in the intestine, but resistant to the simulated acid conditions of the gastric fluid. We also show that alginate can be modified by periodate-oxidation to produce an oxidized alginate material whose hydrogel has similar pH-sensitivity characteristics as the 4-(2-aminoethyl)benzoic acid-modified alginate. Successful chemical coupling of desired quantities of 4-(2-aminoethyl)benzoic acid with oxidized alginates was achieved following a facile procedure reported by Dalheim et al32. Periodate oxidized alginates were reacted with 4-(2-ethylamino)benzoic acid followed by reduction of the imine to amine using pic-BH3. However, hydrogels made with the oxidized alginates without or with the 4-(2-aminoethyl)benzoic acid were equally responsive to the neutral-basic pH effect.
Previous studies by Bharti and Roy had shown that oxidation of the alginate polymer at greater than 10% resulted in degradation of the gelling capacity of the material as expected due to the disruption of the backbone structure, but our findings constitute the first report of alginate oxidation resulting in pH-sensitive degradation of the hydrogel made with alginate oxidized by as little as 0.5%43. We hypothesize that this is caused by deprotonation of the alginate molecules at neutral pH conditions, which leads to increased water retention with subsequent swelling and eventual degradation of the hydrogel. This hypothesis would be consistent with a previous suggestion that a limited degree (typically 1–20%) of periodate oxidation of polysaccharides may give rise to derivatives with entirely altered chemical and physical properties. Specifically, it had been proposed that the ring opening caused by periodate leads to the formation of highly flexible ‘hinges’ in otherwise rather semiflexible or rigid structures48. Also, we observed that our chemical modifications had no impact on the block subunit structure of the alginate as determined by the M:G ratios in the modified alginates, which were similar to those specified by the suppliers (Novamatrix, Sandvika, Norway) of the UPLVG alginate.
We have shown through in vitro tests that this modified hydrogel is capable of staying structurally intact in the harsh acidic conditions as occurs in the stomach, but degrades readily in the more neutral pH conditions that prevail in the intestines. The rate of degradation is directly related to the percent modification of the alginate which has enormous potential in controlled release of therapeutic substances. Thus, in a therapeutic situation where multiple agents need to be delivered sequentially in a timed-release fashion, different degrees of the modification can be used to achieve such a goal. Being able to more accurately control when the modified alginate degrades and releases its therapeutic payload allows for targeted delivery throughout the GIT. In particular, a therapeutic agent would be most effective with delayed release in the distal small intestine where absorption takes place; thus encapsulating it with a lower degree of modified alginate such as 0.5% or even 0.25% oxidation would greatly increase the bioavailability of that therapeutic agent. On the other hand for a compound that needs to be delivered immediately upon entering the proximal small intestine perhaps to induce pancreatic enzyme secretion, a higher degree such as 2% oxidized alginate could be used to burst release the compound once it enters a more neutral pH in the duodenum.
We note that oral drug intake starts in the mouth where saliva might potentially influence the bioavailability of ingested substances. In this report we did not examine the stability of the hydrogel in saliva because of the extremely short transit time of swallowed substances in this fluid. Although the average pH of saliva for individuals with clinically healthy gingiva is ~ 7.0649, the instantaneous entry of a minute volume of saliva into the larger volume of very acidic gastric fluid (pH < 2), would quickly result in an acidic pH of the medium around swallowed substances during the extended transit time in the stomach, thus making the determination of the stability of the hydrogel in acidic medium more relevant, as we have done in our study consistent with the approach adopted by others1, 26, 50.
In conclusion, we have developed a novel, 4-(2-aminoethyl)benzoic acid-modified alginate material whose hydrogel is suitable as a vehicle for controlled release of therapeutic agents. The percent incorporation of small molecules in the modified alginates were reliably quantified and chemical coupling was confirmed via NMR spectroscopy. This study validates a relatively inexpensive, environmentally friendly, non-toxic and efficient method of alginate polymer modification for pH-controlled delivery of therapeutic molecules.
ACKNOWLEDGEMENT
The authors would like to thank Haley Swaim, Aubrey Peeden, and Victor Agwu for their technical help.
FUNDING SOURCES
Office of Research & Sponsored Programs of Wake Forest University the award of a Collaborative Pilot Grant and NIH Pre-doctoral Training Program: Studies in Translational Regenerative Medicine (T32 NIBIB Grant #1T32EB014836-01A1, A. Atala, PI) for Kevin Enck.
ABBREVIATIONS
- GIT
gastrointestinal tract
- PEO
poly (ethylene oxide)
- UPLVG
ultra-pure low viscosity high guluronic acid
- NaIO4
sodium (meta)-periodate
- TMSP-d4
3-(trimethylsilyl)-2,2,3,3-tetradeuteratepropionic acid sodium salt
- DOSY
diffusion-ordered spectroscopy
- HBSS
hanks balanced salt solution
- SGF
simulated gastric fluid
- SIF
simulated intestinal fluid
- POA
periodate oxidized alginate
REFERENCES
- 1.Gupta P; Vermani K; Garg S, Hydrogels: from controlled release to pH-responsive drug delivery. Drug Discov Today 2002, 7 (10), 569–79. [DOI] [PubMed] [Google Scholar]
- 2.Qiu Y; Park K, Environment-sensitive hydrogels for drug delivery. Advanced Drug Delivery Reviews 2001, 53 (3), 321–339. [DOI] [PubMed] [Google Scholar]
- 3.Thomas S, Alginate dressings in surgery and wound management--Part 1. Journal of Wound Care 2000, 9 (2), 56–60. [DOI] [PubMed] [Google Scholar]
- 4.Jeong B; Bae YH; Kim SW, Drug release from biodegradable injectable thermosensitive hydrogel of PEG–PLGA–PEG triblock copolymers. Journal of controlled release 2000, 63 (1–2), 155–163. [DOI] [PubMed] [Google Scholar]
- 5.Bhattarai N; Gunn J; Zhang M, Chitosan-based hydrogels for controlled, localized drug delivery. Advanced Drug Delivery Reviews 2010, 62 (1), 83–99. [DOI] [PubMed] [Google Scholar]
- 6.Tønnesen HH; Karlsen J, Alginate in drug delivery systems. Drug development and industrial pharmacy 2002, 28 (6), 621–630. [DOI] [PubMed] [Google Scholar]
- 7.Chen S-C; Wu Y-C; Mi F-L; Lin Y-H; Yu L-C; Sung H-W, A novel pH-sensitive hydrogel composed of N, O-carboxymethyl chitosan and alginate cross-linked by genipin for protein drug delivery. Journal of Controlled Release 2004, 96 (2), 285–300. [DOI] [PubMed] [Google Scholar]
- 8.Cikrikci S; Mert B; Oztop MH, Development of pH Sensitive Alginate/Gum Tragacanth Based Hydrogels for Oral Insulin Delivery. Journal of agricultural and food chemistry 2018, 66 (44), 11784–11796. [DOI] [PubMed] [Google Scholar]
- 9.Yang J; Chen J; Pan D; Wan Y; Wang Z, pH-sensitive interpenetrating network hydrogels based on chitosan derivatives and alginate for oral drug delivery. Carbohydrate Polymers 2013, 92 (1), 719–725. [DOI] [PubMed] [Google Scholar]
- 10.Amiji M; Tailor R; Ly M-K; Goreham J, Gelatin-Poly(Ethylene Oxide) Semi-interpenetrating Polymer Network with pH-Sensitive Swelling and Enzyme-Degradable Properties for Oral Drug Delivery. Drug Development and Industrial Pharmacy 1997, 23 (6), 575–582. [Google Scholar]
- 11.Bilia A; Carelli V; Di Colo G; Nannipieri E, In vitro evaluation of a pH-sensitive hydrogel for control of GI drug delivery from silicone-based matrices. International Journal of Pharmaceutics 1996, 130 (1), 83–92. [Google Scholar]
- 12.Simoes S; Figueiras A; Veiga F, Modular Hydrogels for Drug Delivery. 2012; Vol. 3, p 185. [Google Scholar]
- 13.Yang J; Wang Y; Li M; Wu H; Zhen T; Xiong L; Sun Q, pH-Sensitive Chitosan–Sodium Phytate Core–Shell Hollow Beads and Nanocapsules for the Encapsulation of Active Ingredients. Journal of Agricultural and Food Chemistry 2019, 67 (10), 2894–2905. [DOI] [PubMed] [Google Scholar]
- 14.Patel VR; Amiji MM, Preparation and Characterization of Freeze-dried Chitosan-Poly(Ethylene Oxide) Hydrogels for Site-Specific Antibiotic Delivery in the Stomach. Pharmaceutical Research 1996, 13 (4), 588–593. [DOI] [PubMed] [Google Scholar]
- 15.Şen M; Uzun C; Güven O, Controlled release of terbinafine hydrochloride from pH sensitive poly(acrylamide/maleic acid) hydrogels. International Journal of Pharmaceutics 2000, 203 (1), 149–157. [DOI] [PubMed] [Google Scholar]
- 16.Buffa R; Odstrčilová L; Šedová P; Basarabová I; Novotný J; Velebný V, Conjugates of modified hyaluronic acid with amino compounds for biomedical applications. Carbohydrate polymers 2018, 189, 273–279. [DOI] [PubMed] [Google Scholar]
- 17.Augst AD; Kong HJ; Mooney DJ, Alginate hydrogels as biomaterials. Macromolecular bioscience 2006, 6 (8), 623–633. [DOI] [PubMed] [Google Scholar]
- 18.Smidsrød O; Skja G, Alginate as immobilization matrix for cells. Trends in biotechnology 1990, 8, 71–78. [DOI] [PubMed] [Google Scholar]
- 19.Chen C-Y; Ke C-J; Yen K-C; Hsieh H-C; Sun J-S; Lin F-H, 3D Porous Calcium-Alginate Scaffolds Cell Culture System Improved Human Osteoblast Cell Clusters for Cell Therapy. Theranostics 2015, 5 (6), 643–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim J; Park Y; Tae G; Lee KB; Hwang CM; Hwang SJ; Kim IS; Noh I; Sun K, Characterization of low-molecular-weight hyaluronic acid-based hydrogel and differential stem cell responses in the hydrogel microenvironments. Journal of Biomedical Materials Research Part A 2009, 88A (4), 967–975. [DOI] [PubMed] [Google Scholar]
- 21.Venkatesan J; Bhatnagar I; Manivasagan P; Kang K-H; Kim S-K, Alginate composites for bone tissue engineering: a review. International journal of biological macromolecules 2015, 72, 269–281. [DOI] [PubMed] [Google Scholar]
- 22.Kiene K; Porta F; Topacogullari B; Detampel P; Huwyler J, Self-assembling chitosan hydrogel: A drug-delivery device enabling the sustained release of proteins. Journal of Applied Polymer Science, n/a-n/a. [Google Scholar]
- 23.Lee KY; Mooney DJ, Alginate: Properties and biomedical applications. Progress in Polymer Science 2012, 37 (1), 106–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sosnik A Alginate Particles as Platform for Drug Delivery by the Oral Route: State-of-the-Art. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwanaga S; Saito N; Sanae H; Nakamura M, Facile fabrication of uniform size-controlled microparticles and potentiality for tandem drug delivery system of micro/nanoparticles. Colloids and Surfaces B: Biointerfaces 2013, 109, 301–306. [DOI] [PubMed] [Google Scholar]
- 26.Alvarez-Lorenzo C; Blanco-Fernandez B; Puga AM; Concheiro A, Crosslinked ionic polysaccharides for stimuli-sensitive drug delivery. Advanced Drug Delivery Reviews 2013, 65 (9), 1148–1171. [DOI] [PubMed] [Google Scholar]
- 27.Ramdas M; Dileep K; Anitha Y; Paul W; Sharma CP, Alginate encapsulated bioadhesive chitosan microspheres for intestinal drug delivery. Journal of biomaterials applications 1999, 13 (4), 290–296. [DOI] [PubMed] [Google Scholar]
- 28.Wee n.; Gombotz n., Protein release from alginate matrices. Advanced Drug Delivery Reviews 1998, 31 (3), 267–285. [DOI] [PubMed] [Google Scholar]
- 29.Darrabie MD; Kendall WF; Opara EC, Effect of alginate composition and gelling cation on micro-bead swelling. Journal of microencapsulation 2006, 23 (1), 29–37. [DOI] [PubMed] [Google Scholar]
- 30.Lee KY; Yuk SH, Polymeric protein delivery systems. Progress in Polymer Science 2007, 32 (7), 669–697. [Google Scholar]
- 31.Patil J, Hydrogel System: An Approach for Drug Delivery Modulation. Advances in Pharmacoepidemiology & Drug Safety 2015. [Google Scholar]
- 32.Dalheim MØ; Vanacker J; Najmi MA; Aachmann FL; Strand BL; Christensen BE, Efficient functionalization of alginate biomaterials. Biomaterials 2016, 80 (Supplement C), 146–156. [DOI] [PubMed] [Google Scholar]
- 33.Yang J-S; Xie Y-J; He W, Research progress on chemical modification of alginate: A review. Carbohydrate Polymers 2011, 84 (1), 33–39. [Google Scholar]
- 34.Bu H; Kjøniksen A-L; Knudsen KD; Nyström B, Rheological and Structural Properties of Aqueous Alginate during Gelation via the Ugi Multicomponent Condensation Reaction. Biomacromolecules 2004, 5 (4), 1470–1479. [DOI] [PubMed] [Google Scholar]
- 35.Bu H; Kjøniksen A-L; Elgsaeter A; Nyström B, Interaction of unmodified and hydrophobically modified alginate with sodium dodecyl sulfate in dilute aqueous solution: Calorimetric, rheological, and turbidity studies. 2006; Vol. 278, p 166. [Google Scholar]
- 36.Gomez CG; Chambat G; Heyraud A; Villar M; Auzély-Velty R, Synthesis and characterization of a β-CD-alginate conjugate. Polymer 2006, 47 (26), 8509–8516. [Google Scholar]
- 37.Galant C; Kjøniksen A-L; Nguyen GTM; Knudsen KD; Nyström B, Altering Associations in Aqueous Solutions of a Hydrophobically Modified Alginate in the Presence of β-Cyclodextrin Monomers. The Journal of Physical Chemistry B 2006, 110 (1), 190–195. [DOI] [PubMed] [Google Scholar]
- 38.Kang H-A; Shin MS; Yang J-W, Preparation and characterization of hydrophobically modified alginate. Polymer Bulletin 2002, 47 (5), 429–435. [Google Scholar]
- 39.Zhu LM; Xin CL, Preparation of a Superabsorbent Resistant to Saline Solution by Copolymerization of Acrylic Acid with Sodium Polymannuronate. Chinese Journal of Applied Chemistry 2002, 05. [Google Scholar]
- 40.Sakai S; Kawakami K, Synthesis and characterization of both ionically and enzymatically cross-linkable alginate. Acta biomaterialia 2007, 3 (4), 495–501. [DOI] [PubMed] [Google Scholar]
- 41.Grasdalen H; Larsen B; Smidsrød O, A pmr study of the composition and sequence of uronate residues in alginates. Carbohydrate Research 1979, 68 (1), 23–31. [Google Scholar]
- 42.Gottlieb HE; Kotlyar V; Nudelman A, NMR chemical shifts of common laboratory solvents as trace impurities. The Journal of organic chemistry 1997, 62 (21), 7512–7515. [DOI] [PubMed] [Google Scholar]
- 43.Bharti SK; Roy R, Quantitative 1H NMR spectroscopy. TrAC Trends in Analytical Chemistry 2012, 35, 5–26. [Google Scholar]
- 44.Johnson CS Jr, Diffusion ordered nuclear magnetic resonance spectroscopy: principles and applications. Progress in Nuclear Magnetic Resonance Spectroscopy 1999, 34 (3–4), 203–256. [Google Scholar]
- 45.Kirschning A; Dibbert N; Dräger G, Chemical Functionalization of Polysaccharides—Towards Biocompatible Hydrogels for Biomedical Applications. Chemistry–A European Journal 2018, 24 (6), 1231–1240. [DOI] [PubMed] [Google Scholar]
- 46.Gandhi JK; Opara EC; Brey EM, Alginate-based strategies for therapeutic vascularization. Therapeutic delivery 2013, 4 (3), 327–341. [DOI] [PubMed] [Google Scholar]
- 47.Amsden B; Turner N, Diffusion characteristics of calcium alginate gels. Biotechnology and Bioengineering 1999, 65 (5), 605–610. [DOI] [PubMed] [Google Scholar]
- 48.Kristiansen KA; Potthast A; Christensen BE, Periodate oxidation of polysaccharides for modification of chemical and physical properties. Carbohydrate Research 2010, 345 (10), 1264–1271. [DOI] [PubMed] [Google Scholar]
- 49.Baliga S; Muglikar S; Kale R, Salivary pH: A diagnostic biomarker. Journal of Indian Society of Periodontology 2013, 17 (4), 461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guo B-L; Gao Q-Y, Preparation and properties of a pH/temperature-responsive carboxymethyl chitosan/poly (N-isopropylacrylamide) semi-IPN hydrogel for oral delivery of drugs. Carbohydrate research 2007, 342 (16), 2416–2422. [DOI] [PubMed] [Google Scholar]