Abstract
BACKGROUND & AIMS:
The most recent information published on resistance of Helicobacter pylori to antibiotics in a large population in the United States is more than 10 years old. We assessed the susceptibility of H pylori to antibiotics among patients in a large metropolitan hospital, as well as demographic, clinical, and lifestyle factors associated with antimicrobial resistance.
METHODS:
We performed a cross-sectional study of a random sample of 656 patients (90.2% men) from a cohort of 1559 undergoing esophagogastroduodenoscopy with collection of gastric biopsies from 2009 through 2013 at the Houston Veterans Affairs Medical Center. We performed culture analyses of gastric tissues to detect H pylori. The minimum inhibitory concentrations of amoxicillin, clarithromycin, metronidazole, levofloxacin, and tetracycline were determined by the Epsilometer test. Logistic regression analysis was performed to estimate the association between risk factors and antimicrobial resistance.
RESULTS:
Biopsies from 135 subjects (20.6%) tested positive for H pylori; 128 of these were from men (94.8%). Only 65 strains were susceptible to all 5 antibiotics. The prevalence of resistance to levofloxacin was 31.3% (95% confidence interval [CI], 23.1%–39.4%), to metronidazole it was 20.3% (95% CI, 13.2%–27.4%), to clarithromycin it was 16.4% (95% CI, 9.9%–22.9%), and to tetracycline it was 0.8% (95% CI, 0.0%–2.3%). No isolate was resistant to amoxicillin. Clarithromycin resistance increased from 9.1% in 2009–2010 to 24.2% in 2011–2013. In multivariate analysis, prior treatment of H pylori infection and use of fluoroquinolones were significantly associated with clarithromycin and levofloxacin resistance, respectively.
CONCLUSIONS:
H pylori resistance to clarithromycin increased between 2009 and 2013; resistance to metronidazole remains high in infected men in the United States. The high frequency of resistance to levofloxacin is a new and concerning finding.
Keywords: Gastritis, Bacterial Resistance, Drug, Eradication Therapy
Eradication of Helicobacter pylori infection results in healing of gastritis, improved healing of peptic ulcers, and prevention of ulcer recurrence and reduces the risk of developing gastric cancer.1–4 Until recently, the most widely recommended anti-H pylori antimicrobial regimens worldwide have been 3 drug regimens that use a proton pump inhibitor (PPI) and 2 antimicrobial agents, such as amoxicillin, clarithromycin, or metronidazole,4–6 or a 4-drug combination of a PPI, a bismuth compound, tetracycline, and metronidazole, known as bismuth quadruple therapy.7 As with other infectious diseases, treatment success is primarily predicated on the presence of susceptible organisms and patient adherence (ie, taking the medications as prescribed). The widespread use of clarithromycin and other macrolides worldwide for upper respiratory infections has resulted in widespread development of clarithromycin resistance in H pylori as a bystander effect, such that by 2000 the success rate with clarithromycin-containing triple therapy had generally fallen to 80% or less.4,8,9
The Surveillance of H pylori Antimicrobial Resistance Partnership study compiled data that included 3624 strains from the clinical studies done in the United States during 1993 to 1999.10–12 It reported that resistance to clarithromycin was 10.1% (95% confidence interval [CI], 9.1%–11.1%) and 36.9% (95% CI, 35.1%–38.7%) to metronidazole. The subsequent H pylori Antimicrobial Resistance Monitoring Project (HARP) study included 347 strains isolated from 11 hospitals in 4 regions during 1998 to 200213 and reported resistance rates for clarithromycin and metronidazole of 13% and 25%, respectively. The last multicenter study was from a clinical trial in the United States reported in 2003,14 showing resistance rates of 10.2% to clarithromycin and 29.1% to metronidazole in 796 strains. Unfortunately, information as to antibiotic resistance of H pylori in the United States has been lacking since 2003.15
Obtaining contemporary data about H pylori antibiotic resistance is important, because the prevalence of H pylori infection continues to be high among the elderly, recent immigrants from endemic areas, and lower socioeconomic groups. Pretreatment antimicrobial susceptibility testing is generally unavailable; and H pylori eradication regimens are typically chosen empirically, thus foregoing the opportunity to maximize the likelihood of a successful result. The rate of successful H pylori eradication has declined in the United States. For example, the successful eradication rate with 10-day clarithromycin-containing triple therapy (clarithromycin, amoxicillin, and omeprazole) for patients with non-ulcer dyspepsia in 2004 was 72% in an intent-to-treat analysis16 vs 90% success reported in 1996.17
Updated information regarding antimicrobial susceptibility patterns is important to determine the best primary treatment regimens. In general, the antibiotic resistance rate parallels the antibiotic consumption rate in the population.18–20 This was recently confirmed by a recent European Helicobacter Study Group study showing a significant relationship between the use of long-acting macrolides and clarithromycin resistance.21 In the United States the prescription rates of macrolides, especially azithromycin, have increased during the 1990s and 2000s,22,23 which implies increasing clarithromycin resistance of H pylori in the United States.
Here we determined antibiotic susceptibility to commonly used drugs for which resistance has been shown to affect outcome (ie, amoxicillin, clarithromycin, metronidazole, levofloxacin, and tetracycline) among H pylori strains isolated in a major hospital in the United States. We also examined the demographic, clinical, and lifestyle factors that were associated with antibiotic-resistant H pylori strains.
Methods
Study Population
Gastric H pylori strains were obtained from a cross-sectional study at the Michael E. DeBakey Veterans Affairs Medical Center in Houston, Texas from June 1, 2009 to August 31, 2013, as previously described.24,25 This medical center serves as the primary healthcare provider for almost 130,000 veterans in southeast Texas. We invited all consecutive eligible patients who were scheduled for an elective esophagogastroduodenoscopy (EGD) to participate. We also invited a group of randomly selected eligible patients who were scheduled for a primary care clinic visit at the same hospital to undergo an EGD at the same time as their screening colonoscopy. Informed consent was obtained from all participants, and this study was approved by the Institutional Review Board at Baylor College of Medicine and by Research and Development at the Michael E. DeBakey Veterans Affairs Medical Center, both in Houston, Texas.
Inclusion and Exclusion Criteria
The electronic medical records of all veterans with a scheduled EGD were screened to determine study eligibility on the basis of the previously described criteria,24 including age between 40 and 80 years and complete EGD examination with gastric mapping biopsies. Subjects were excluded if they had any of the following exclusion criteria: (1) previous surgical resection of the esophagus or stomach; (2) previous cancer of the esophagus, lung, liver, colon, breast, or stomach; (3) current use of anticoagulants; (4) significant liver disease, indicated by platelet count below 70,000, ascites, or known gastroesophageal varices; and (5) history of major stroke or mental condition that would limit ability to answer questions.
Endoscopy and Biopsy
Endoscopic findings including gastric or duodenal ulcers were systematically recorded. Gastric mapping was performed by taking 7 mucosal biopsy samples from the antrum (from the greater curvature and from the lesser curvature), the corpus (from the distal greater curvature, distal lesser curvature, proximal greater curvature, proximal lesser curvature), and the cardia, as previously described.24,25 Features of gastritis, gastric atrophy, and intestinal metaplasia were identified and graded according to the standardized Updated Sydney System.26 Chronic gastritis was defined by inflammation in which mononuclear leukocytes, including lymphocytes, plasma cells, and macrophages (monocytes), predominate. Chronic active gastritis was defined by the presence of neutrophil polymorphs in a background of chronic inflammation.26 In addition, 1 gastric biopsy was collected from the lesser curvature of the antrum from all study subjects and placed in sterile vials containing cysteine medium with 20% glycerol and stored at −80°C until cultured for H pylori.
Helicobacter pylori Culture
For H pylori culture, frozen specimens were thawed, and the tissues were homogenized and inoculated onto 2 types of selective media, brain-heart infusion and H pylori Special Peptone Agar plates with 7% horse blood. The plates were incubated for up to 14 days at 37°C under microaerophilic conditions (5% O2, 10% CO2, and 85% N2) in an Anoxomat (Mart Microbiology B.V., JP Drachten, Netherlands) jar. Positive growth was transferred to a fresh, nonselective brain-heart infusion blood agar plate and incubated for 48–72 hours. H pylori was identified definitively when the oxidase, catalase, and urease reactions were positive with a compatible Gram stain. We selected and subcultured several small round colonies from each patient’s plate 1 or 2 times to obtain a pure culture. The isolated strains were stored at −80°C in cysteine storage medium containing 20% glycerol.
Antibiotic Susceptibility Testing
We used the Epsilometer test (E-test) (AB Biodisk, Solna, Sweden) to determine the minimum inhibitory concentrations (MICs) of amoxicillin, clarithromycin, metronidazole, levofloxacin, and tetracycline. Mueller–Hinton II Agar medium (Becton Dickinson, Franklin Lakes, NJ) supplemented with 5% defibrinated sheep blood was used as culture medium. The culture suspension with turbidity adjusted to be equivalent to a McFarland opacity standard of 2.0 was used to inoculate onto the plates. The E-test strip of the corresponding antibiotic was placed on the plate and incubated for 72 hours at 37°C under microaerophilic conditions. MIC was defined as the point of intersection of the elliptical inhibition zone with the E-test strip. Strains were considered resistant when the MIC was >0.12 μg/mL for amoxicillin, >0.5 μg/mL for clarithromycin, >1 μg/mL for levofloxacin, >8 μg/mL for metronidazole, and >1 μg/mL for tetracycline, according to the EUCAST H pylori breakpoints.21
Survey Questionnaire
All study participants completed a computer-assisted survey before the EGD that included questions on demographic features (age, gender, race, birthplace, education, and household income); clinical features (heartburn, acid regurgitation, stomach pain), previous treatment for H pylori; and lifestyle factors, including tobacco-smoking status and alcohol drinking, as previously described.24 Prior use of macrolides (azithromycin, clarithromycin, erythromycin, and telithromycin) and fluoroquinolones (ciprofloxacin, gatifloxacin, gemi-floxacin, levofloxacin, moxifloxacin, norfloxacin, and ofloxacin) was also examined in the Veterans Affairs pharmacy electric medical record.
Statistical Analysis
Our hypothesis was that antimicrobial resistance to clarithromycin would have increased from 6% in our previous study11 to approximately 20% in the present study. We estimated the prevalence of H pylori infection would be 20% in the present study on the basis of recent studies of H pylori prevalence in the United States.27,28 We estimated that 90 H pylori–positive subjects were required to have 80% power for a 2-sided test with a significance level of .05 to detect a difference between 2 groups. Therefore, a minimum of 450 samples needed to be cultured.
We calculated the proportions and accompanying 95% CIs of patients with antibiotic-resistant and antibiotic-sensitive H pylori strains; we performed these calculations for each of the tested 4 antibiotics. Under conditions with rare events, the 95% CI was calculated by using the Clopper–Pearson exact method. Potential predictors of resistance (demographic, clinical, and lifestyle factors, history of prior treatment of H pylori and use of antibiotics) were examined in univariate analyses by using the Fisher exact test and χ2 test for categorical variables. We also conducted an adjusted analysis by using multivariable logistic regression to examine the possible predictors of antibiotic resistance. Two patients with race category “others” were excluded in the model because the number was too small to obtain a reliable estimate. Four important biological and social factors, age group, race, isolated year, and prior H pylori treatment, were included in the model, regardless of the statistical significance. For other variables, those who passed the univariate screening with P value <.25 were considered as candidates for selection. A backward variable selection procedure was used to identify the primary main-effect models. For each variable, the odds ratio (OR) and 95% CI were calculated. A 2-tailed P value <.05 was considered statistically significant. All statistical analyses were performed by using SPSS version 19 (SPSS Inc, Chicago, IL), STATA software version 13.1 (Stata Corp, College Station, TX), and SAS software version 9.3 (SAS Institute, Cary, NC).
Results
During the study period, 4996 patients were scheduled for EGD. Among them, 2478 fulfilled the study inclusion criteria, and 1595 agreed to participate in the study. A total of 1559 patients underwent study EGD with biopsy samples; 656 patients were selected by computer-based random code to have their gastric biopsies cultured, and 135 were H pylori positive (20.6%). The 135 H pylori strains were isolated from 135 patients (128 men and 7 women; age range, 40–79 years; mean age, 60.0 ± 8 years) and were examined for antibiotic resistance. One hundred seventeen strains (86.7%) were isolated from previously untreated patients; 7 strains were isolated from previously treated patients. In 11 subjects prior treatment status was unknown. The racial/ethnic distribution of patients was 39 white (28.9%), 70 black (51.9%), 24 white Hispanic (17.8%), and 2 (1.5%) of other ethnic backgrounds or unknown. A total of 31 strains were isolated in 2009, 37 strains were isolated in 2010, 25 strains were isolated in 2011, 24 strains were isolated in 2012, and 18 strains were isolated in 2013. The gastric diagnoses among the H pylori–positive subjects were chronic active gastritis in 124 (91.9%), chronic gastritis only in 5 (3.7%), and gastric ulcer in 4 (3.0%). In addition, 10 subjects also had erosive esophagitis; and 2 patients had esophageal adenocarcinoma. Information as to location of birth was obtained from 122 of the 135 subjects; 119 were born in North America, and 3 were born in Central/South America or in the Caribbean. Of the 1559 subjects, 26.7% (416) were H pylori positive by pathologic examination by using the hematoxylin-eosin stain.
Helicobacter pylori Resistance Patterns
Overall, antibiotic resistance was highest for levofloxacin (31.9%; 95% CI, 24.0%–39.7%), followed by metronidazole 21.5% (95% CI, 14.6%–28.4%) and clarithromycin 17.8% (95% CI, 11.3%–24.2%), and least for tetracycline (n = 1) 0.7% (95% CI, 0.0%–4.1%). No isolate was resistant to amoxicillin.
Among the 128 strains isolated from men, 65 (50.8%) were susceptible to all 5 antibiotics tested. The pattern of resistance was the same as for the entire group (ie, highest prevalence with levofloxacin (31.3%; 95% CI, 23.1%–39.4%), followed by metronidazole 20.3% (95% CI, 13.2%–27.4%), clarithromycin 16.4% (95% CI, 9.9%–22.9%), and finally, tetracycline (n = 1) 0.8% (95% CI, 0.0%–4.3%). Among the 110 previously untreated patients, 54.5% were susceptible to all tested antibiotics; 29.1% (95% CI, 20.5%–37.7%) were resistant to levofloxacin, 17.3% (95% CI, 10.1%–24.4%) were resistant to metronidazole, 14.5% (95% CI, 7.9%–21.2%) were resistant to clarithromycin, and 0.9% (95% CI, 0.0%–5.0%) were resistant to tetracycline. The 7 strains isolated from subjects with prior H pylori treatment were more likely to be resistant to clarithromycin and levofloxacin than strains from untreated patients (71.4% vs 14.5%, P = .002; and 71.4% vs 29.1%, P = .03, respectively). However, no strains isolated from previously treated patients were susceptible to all 5 antibiotics. Regarding the type of patient, there were no differences in antibiotic resistance rates between the 69 patients scheduled for an elective EGD compared with the 41 scheduled as a result of a primary care clinic visit for screening colonoscopy (15.9% vs 12.2% for clarithromycin, 17.4% vs 17.1% for metronidazole, and 27.5% vs 31.7% for levofloxacin, respectively; all P > .05).
The pattern of drug resistance in male patients is shown in Table 1. In the 110 patients previously untreated, 34 strains (30.9%) were resistant to only 1 antibiotic. Resistance to more than 2 antibiotics was present in 14.5% (16 of 110). Triple resistance to clarithromycin, metronidazole, and levofloxacin was observed in only 2 strains (1.8%).
Table 1.
Resistance Pattern in H pylori Strains Isolated in Male Patients During 2009 to 2013
| Total | All N = 128 | Untreated patients N = 110 |
|---|---|---|
| All susceptible | 65 (50.8%) | 60 (54.5%) |
| Mono resistance | 41 (32.0%) | 34 (30.9%) |
| Clarithromycin resistance only | 6 (4.7%) | 6 (5.5%) |
| Metronidazole resistance only | 13 (10.2%) | 9 (8.2%) |
| Levofloxacin resistance only | 21 (16.4%) | 18 (16.4%) |
| Tetracycline resistance only | 1 (0.8%) | 1 (0.9%) |
| Multiple resistance | 22 (17.2%) | 16 (14.5%) |
| Clarithromycin + metronidazole | 3 (2.3%) | 2 (1.8%) |
| Clarithromycin + levofloxacin | 9 (7.0%) | 6 (5.5%) |
| Metronidazole + levofloxacin | 7 (5.5%) | 6 (5.5%) |
| Clarithromycin + metronidazole + levofloxacin | 3 (2.3%) | 2 (1.8%) |
Among 7 strains isolated from women, resistance rates to clarithromycin, metronidazole, and levofloxacin were 42.9%, 42.9%, and 42.9%, respectively. None was resistant to amoxicillin or tetracycline.
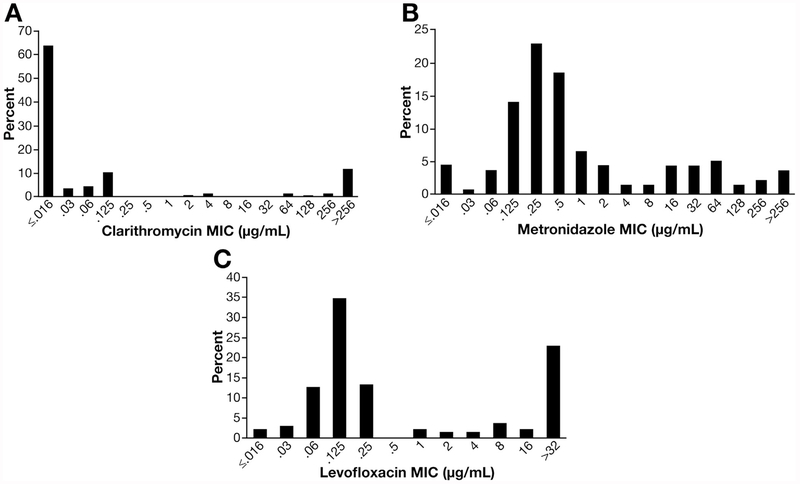
The distribution of MIC values for each antibiotic is shown in Figure 1. Most strains susceptible to clarithromycin had MIC <0.016. On the other hand, 16 of 24 resistant strains (66.7%) had high-level resistance (>256 μg/mL). The MIC values to metronidazole ranged widely. Among the 39 levofloxacin-resistant strains, 31 (72%) had high-level resistance (≥32 μg/mL). The single-strain resistance to tetracycline had MIC of 24 μg/mL.
Figure 1.
Distribution of MIC for each antibiotic: (A) clarithromycin, (B) metronidazole, and (C) levofloxacin.
Factors Associated With Antibiotic Resistance
Results of univariate analysis of factors associated with antibiotic resistance are summarized in Table 2. Strains isolated in 2011–2013 showed a significantly higher prevalence of clarithromycin resistance than those isolated in 2009–2010. Prior treatment of H pylori and prior use of macrolides were both significantly associated with clarithromycin resistance. Metronidazole resistance was significantly more common among patients who stated they never drank alcohol compared with former drinkers and current drinkers. Smoking was also significantly associated with metronidazole resistance. Levofloxacin resistance rate was significantly higher in the strains isolated from the patients with higher household incomes. Prior treatment of H pylori and prior use of fluoroquinolones were significantly associated with levofloxacin resistance. Factors significantly associated with resistance to any antibiotic included prior treatment of H pylori, education (some-college), higher household income, nonsmoking, the presence of heartburn or acid regurgitation, and prior use of fluoroquinolones.
Table 2.
Factors Associated With H pylori Resistance to Clarithromycin, Metronidazole, and Levofloxacin in Male Patients
| N | Clarithromycin resistance | P value | Metronidazole resistance | P value | Levofloxacin resistance | P value | Any resistance | P value | |
|---|---|---|---|---|---|---|---|---|---|
| Age (y) | |||||||||
| <60 | 51 | 6 (11.8%) | .25 | 11 (21.6%) | .77 | 12 (23.5%) | .13 | 26 (51.0%) | .75 |
| ≥60 | 77 | 15 (19.5%) | 15 (19.5%) | 28 (36.4%) | 37 (48.1%) | ||||
| Race/ethnic | |||||||||
| White | 39 | 7 (17.9%) | .43 | 8 (20.5%) | 1.00 | 11 (28.2%) | .49 | 18 (46.2%) | .65 |
| Black | 64 | 8 (12.5%) | 13 (20.3%) | 19 (29.7%) | 33 (51.6%) | ||||
| Hispanic | 23 | 6 (26.1%) | 5 (21.7%) | 10 (43.5%) | 12 (52.2%) | ||||
| Other | 2 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | ||||
| Isolated year | |||||||||
| 2009–2010 | 66 | 6 (9.1%) | .02 | 14 (21.2%) | .79 | 17 (25.8%) | .17 | 27 (40.9%) | .05 |
| 2011–2013 | 62 | 15 (24.2%) | 12 (19.4%) | 23 (37.1%) | 36 (58.1%) | ||||
| Prior treatment of H pylori | |||||||||
| Yes | 7 | 5 (71.4%) | .002 | 3 (42.9%) | .12 | 5 (71.4%) | .03 | 7 (100.0%) | .01 |
| No | 110 | 16 (14.5%) | 19 (17.3%) | 32 (29.1%) | 50 (45.5%) | ||||
| Missing | 11 | 0 (0.0%) | 4 (36.4%) | 3 (27.3%) | 6 (54.5%) | ||||
| Education | |||||||||
| No college | 67 | 9 (13.4%) | .11 | 10 (14.9%) | .11 | 18 (26.9%) | .23 | 28 (41.8%) | .049 |
| Some college | 48 | 12 (25.0%) | 13 (27.1%) | 18 (37.5%) | 29 (60.4%) | ||||
| Missing | 13 | 0 (0.0%) | 3 (23.1%) | 4 (30.8%) | 6 (46.2%) | ||||
| Household income ($) | |||||||||
| ≤25,000 | 61 | 9 (14.8%) | .30 | 9 (14.8%) | .20 | 13 (21.3%) | .01 | 24 (39.3%) | .02 |
| >25,000 | 54 | 12 (22.2%) | 13 (24.1%) | 24 (44.4%) | 33 (61.1%) | ||||
| Missing | 13 | 0 (0.0%) | 4 (30.8%) | 3 (23.1%) | 6 (46.2%) | ||||
| Smoking status | |||||||||
| Nonsmoker | 32 | 9 (28.1%) | .19 | 11 (34.4%) | .02 | 10 (31.3%) | .33 | 22 (68.8%) | .03 |
| Former smoker | 51 | 7 (13.7%) | 5 (9.8%) | 19 (37.3%) | 20 (39.2%) | ||||
| Current smoker | 36 | 5 (13.9%) | 7 (19.4%) | 8 (22.2%) | 16 (44.4%) | ||||
| Missing | 9 | 0 (0.0%) | 3 (33.3%) | 3 (33.3%) | 5 (55.6%) | ||||
| Drinking status | |||||||||
| Never drank | 11 | 2 (18.2%) | .81 | 6 (54.5%) | .01 | 1 (9.1%) | .24 | 8 (72.7%) | .24 |
| Former drinker | 47 | 7 (14.9%) | 6 (12.8%) | 15 (31.9%) | 21 (44.7%) | ||||
| Current drinker | 61 | 12 (19.7%) | 11 (18.0%) | 21 (34.4%) | 29 (47.5%) | ||||
| Missing | 9 | 0 (0.0%) | 3 (33.3%) | 3 (33.3%) | 5 (55.6%) | ||||
| Heartburn or acid regurgitation | |||||||||
| Yes | 73 | 16 (21.9%) | .12 | 16 (21.9%) | .37 | 26 (35.6%) | .18 | 41 (56.2%) | .04 |
| No | 46 | 5 (10.9%) | 7 (15.2%) | 11 (23.9%) | 17 (37.0%) | ||||
| Missing | 9 | 0 (0.0%) | 3 (33.3%) | 3 (33.3%) | 5 (55.6%) | ||||
| Stomach pain | |||||||||
| Yes | 37 | 6 (16.2%) | .78 | 10 (27.0%) | .15 | 11 (29.7%) | .83 | 20 (54.1%) | .44 |
| No | 82 | 15 (18.3%) | 13 (15.9%) | 26 (31.7%) | 38 (46.3%) | ||||
| Missing | 9 | 0 (0.0%) | 3 (33.3%) | 3 (33.3%) | 5 (55.6%) | ||||
| Prior use of macrolides | |||||||||
| Yes | 30 | 9 (30.0%) | .02 | 8 (26.7%) | .32 | 11 (36.7%) | .46 | 18 (60.0%) | .18 |
| No | 98 | 12 (12.2%) | 18 (18.4%) | 29 (29.6%) | 45 (45.9%) | ||||
| Prior use of fluoroquinolones | |||||||||
| Yes | 39 | 9 (23.1%) | .18 | 12 (30.8%) | .05 | 17 (43.6%) | .046 | 25 (64.1%) | .03 |
| No | 89 | 12 (13.5%) | 14 (15.7%) | 23 (25.8%) | 38 (42.7%) |
Multiple logistic analysis revealed that those with prior H pylori treatment had a significantly higher likelihood of clarithromycin resistance than previously untreated patients, and this persisted after adjustment by age, race, isolated year, education, smoking status, the presence of heartburn or acid regurgitation, prior use of macrolides, and prior use of fluoroquinolones (Table 3). With regard to metronidazole, former drinkers were significantly less likely to have resistance than never drinkers. For levofloxacin, higher household income was significantly associated with levofloxacin resistance in the multivariate analysis. Prior use of fluoroquinolones was also significantly associated with levofloxacin resistance. The year of H pylori isolation (2011–2013 vs 2009–2010), higher household income, and prior use of macrolides were significantly associated with resistance to any antibiotics; prior treatment of H pylori was not included in the model because all 7 patients with prior H pylori treatment showed resistance to any antibiotics.
Table 3.
Factors Associated With H pylori Resistance to Clarithromycin, Metronidazole, and Levofloxacin in Male Patients in Multivariate Analysis
| Clarithromycin resistance |
Metronidazole resistance |
Levofloxacin resistance |
Any resistance |
|
|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Age (y) | ||||
| ≥60 (vs <60) | 2.16 (0.67–6.99) | 0.74 (0.26–2.10) | 2.34 (0.87–6.28) | 1.04 (0.44–2.48) |
| Race/ethnic | ||||
| Black (vs white) | 1.10 (0.32–3.83) | 0.89 (0.27–2.95) | 1.80 (0.62–5.27) | 2.18 (0.81–5.83) |
| Hispanic (vs white) | 1.47 (0.35–6.11) | 1.87 (0.46–7.66) | 2.48 (0.65–9.52) | 1.78 (0.52–6.07) |
| Isolated year | ||||
| 2011–2013 (vs 2009–2013) | 2.57 (0.83–8.00) | 0.69 (0.23–2.03) | 1.89 (0.72–4.93) | 2.52 (1.06–5.97) |
| Prior treatment of H pylori | ||||
| Yes (vs no) | 11.37 (1.79–72.21) | 4.64 (0.86–24.92) | 3.83 (0.60–24.36) | — |
| Household income ($) | ||||
| >25,000 (vs ≤25,000) | — | — | 3.15 (1.21–8.25) | 2.61 (1.10–6.18) |
| Drinking status | ||||
| Former drinker (vs never drank) | — | 0.12 (0.02–0.65) | — | — |
| Current drinker (vs never drank) | — | 0.19 (0.04–0.94) | — | — |
| Prior use of macrolides | ||||
| Yes (vs no) | — | — | — | 3.92 (1.39–11.07) |
| Prior use of fluoroquinolones | ||||
| Yes (vs no) | — | — | 3.49 (1.30–9.36) | — |
Discussion
Successful H pylori eradication therapy depends in part on whether antimicrobial resistance is present to 1 or more of the drugs used.29 H pylori strains isolated from veteran patients in Houston were often resistant to commonly prescribed antibiotics, including levofloxacin, clarithromycin, and metronidazole; 32.0% showed resistance to 1 antibiotic, but only 50.8% were susceptible to all 5 antibiotics tested.
Antibiotic resistance patterns differ geographically and can change dramatically in relation to an increase in use of an antibiotic. A systematic review of articles published during 2006 to 2009 worldwide reported antibiotic resistance rates of 17.2% to clarithromycin, 26.7% to metronidazole, 16.2% to levofloxacin, 5.9% to tetracycline, and 11.2% to amoxicillin.30 A recent study from Europe showed overall resistance rates of 17.5% to clarithromycin, 34.9% to metronidazole, 14.1% to levofloxacin, 0.9% to tetracycline, and 0.7% to amoxicillin, but with marked regional differences.21 We previously examined antibiotic resistance rates between 1988 and 1997 in the Veterans Affairs hospital in which the current study was conducted.11 Among 179 strains examined, resistance to clarithromycin was present in 6.1% (95% CI, 2.6%–9.7%), and 37.4% (95% CI, 30.3%–44.6%) were resistant to metronidazole. Although a higher cutoff value MIC (>2 μg/mL) of clarithromycin was used in the previous study, when we used that cutoff for the current study, the clarithromycin resistance rate was 17% (95% CI, 10.6%–23.5%). Furthermore, clarithromycin resistance significantly increased between 2009 to 2010 and 2011 to 2013 in this study, likely related to increased use of macrolides for respiratory and otorhinolaryngology reasons.31,32 Although the metronidazole resistance rate had not increased compared with the previous study, it remains high. Dual resistance to clarithromycin and metronidazole was present in 5% in the HARP study and in 2.8% in our previous study in the Michael E. DeBakey Veteran Affairs Medical Center in Houston.11,13 Importantly, in our current study dual resistance to clarithromycin and metronidazole was present in only 1.8%, which makes concomitant therapy (PPI, clarithromycin, metronidazole, and amoxicillin) an excellent choice as an empiric therapy.29
This study reports an H pylori resistance rate of levofloxacin in the United States outside Alaska.33 Levofloxacin has been recommended as a rescue drug to eradicate H pylori in patients who fail first-line therapy.4,34,35 Locally, it would seem to be a poor choice on the basis of the high resistance rate (31.9%), which is higher than the 10% limit suggested as a cutoff for use of fluoroquinolone-containing triple therapy for H pylori.29 Tetracycline resistance was rare in our study, and no strain exhibited amoxicillin resistance, which is consistent with previous studies.10,13
MIC values for clarithromycin showed a bimodal pattern; 16 strains (66.7% of resistant strains) showed high MIC (>256 μg/mL), a finding suggesting the presence of limited target sites for antimicrobial activity such as the well-known point mutations in the 23S rRNA gene of H pylori.36 On the other hand, MIC values to metronidazole were widely distributed, which suggests several resistance mechanisms such as modulation in the enzymatic reductive activities.37 With regard to levofloxacin, 31 strains (72% of resistant strains) showed high MIC (≥32 μg/mL), consistent with other reports.33
It is clinically useful to be able to predict which populations are at risk for resistant strains. Prior treatment of H pylori was significantly associated with clarithromycin, consistent with the suggestion that clarithromycin-containing triple therapy should not be used for patients with a history of prior clarithromycin use. In the HARP study, black race was a risk factor significantly associated with infection with resistant H pylori strains13; however, this study did not confirm that observation. Although it did not reach statistical significance, we found that antibiotic resistances in 3 antibiotics tended to be higher in Hispanic patients. It was previously reported that inappropriate use of antibiotics, including inadequate knowledge about health, healthcare, and the role of antibiotics, can contribute to a higher antimicrobial resistance rate among Hispanic patients.38 Multivariate analysis showed that less alcohol drinking was associated with metronidazole resistance. Antibiotic resistance of H pylori has also been reported to be higher in patients with lower alcohol use in Italy.39 It is possible that physicians do not prescribe metronidazole to patients who drink alcohol because of a disulfiram-like reaction, which might contribute to a low metronidazole resistance rate among drinkers.40 Levofloxacin resistance was significantly associated with prior use of fluoroquinolones. The increasing use of fluoroquinolones in the United States22 is consistent with the increasing prevalence of levofloxacin resistance among those with H pylori. Last, high household income was previously associated with antibiotic resistance, especially to levofloxacin,41 a finding that may relate to increased access to healthcare. The antibiotic resistance rate of Streptococcus pneumoniae was also shown to be higher among patients with high household income in the United States.42 Further studies are necessary to elucidate these associations.
Our study has several limitations. First, our findings were based on veteran patients in 1 hospital. Most patients were men between 40 and 79 years of age. Therefore, this study population may not be adequately reflective of the general population. In addition, we cannot exclude the possibility of selection bias, in that patients in whom H pylori was previously detected and successfully treated were more likely to be referred for endoscopy for persistent symptoms. Although our data were based on a sample of 135 cultured H pylori strains randomly selected from 656 gastric biopsies from among 1559 EGDs, the proportion of H pylori isolated was similar to the proportion with histologically documented H pylori in the entire population. Although prior antibiotic use was significantly associated with antimicrobial resistance, the VA electronic medical records cover only antibiotic use since 1997. Finally, we used the E-test to examine the antibiotic resistance, which may have exaggerated the rate of metronidazole resistance, because the E-test showed significantly higher MIC values than that of agar dilution.12
In conclusion, clarithromycin, metronidazole, and levofloxacin resistances were all high among untreated patients, suggesting that they all should be avoided as components of empiric triple therapy (eg, PPI, amoxicillin, plus a third antibiotic). The 4-drug concomitant therapy and bismuth quadruple therapy, or antibiotic susceptibility-guided therapy, are likely be the best strategies locally and are recommended for previously untreated patients with H pylori infection.
Acknowledgments
The authors thank David Ramsey and Xiaoying Yu for their contributions to this project.
Funding
Supported in part by National Institutes of Health grant NCI R01 116845 and the Texas Digestive Disease Center NIH DK58338 and NIDDK K24-04-107 to H.B.E-S. Also supported in part with resources at the VA HSR&D Center for Innovations in Quality, Effectiveness and Safety (#CIN 13-413) at the Michael E. DeBakey VA Medical Center, Houston, TX. The opinions expressed reflect those of the authors and not necessarily those of the Department of Veterans Affairs, the US government, or Baylor College of Medicine
Abbreviations used in this paper:
- CI
confidence interval
- EGD
esophagogastroduodenoscopy
- E-test
Epsilometer test
- HARP study
H pylori Antimicrobial Resistance Monitoring Project study
- MIC
minimum inhibitory concentration
- OR
odds ratio
- PPI
proton pump inhibitor
Footnotes
Conflicts of interest
The authors disclose no conflicts.
References
- 1.Hosking SW, Chung SCS, Yung MY, et al. Duodenal ulcer healing by eradication of Helicobacter pylori without anti-acid treatment: randomised controlled trial. Lancet 1994; 343:508–510. [DOI] [PubMed] [Google Scholar]
- 2.Shiota S, Murakami K, Fujioka T, et al. Population-based strategies for Helicobacter pylori-associated disease management: a Japanese perspective. Expert Rev Gastroenterol Hepatol 2010;4:149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takenaka R, Okada H, Kato J, et al. Helicobacter pylori eradication reduced the incidence of gastric cancer, especially of the intestinal type. Aliment Pharmacol Ther 2007;25:805–812. [DOI] [PubMed] [Google Scholar]
- 4.Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection: the Maastricht IV/Florence Consensus Report. Gut 2012;61:646–664. [DOI] [PubMed] [Google Scholar]
- 5.Fock KM, Katelaris P, Sugano K, et al. Second Asia-Pacific consensus guidelines for Helicobacter pylori infection. J Gastroenterol Hepatol 2009;24:1587–1600. [DOI] [PubMed] [Google Scholar]
- 6.Chey WD, Wong BC. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol 2007;102:1808–1825. [DOI] [PubMed] [Google Scholar]
- 7.Liang X, Xu X, Zheng Q, et al. Efficacy of bismuth-containing quadruple therapies for clarithromycin-, metronidazole-, and fluoroquinolone-resistant Helicobacter pylori infections in a prospective study. Clin Gastroenterol Hepatol 2013;11:802–807. [DOI] [PubMed] [Google Scholar]
- 8.Graham DY, Lu H, Yamaoka Y. A report card to grade Helicobacter pylori therapy. Helicobacter 2007;12:275–278. [DOI] [PubMed] [Google Scholar]
- 9.Asaka M, Kato M, Takahashi S, et al. Guidelines for the management of Helicobacter pylori infection in Japan: 2009 revised edition. Helicobacter 2010;15:1–20. [DOI] [PubMed] [Google Scholar]
- 10.Meyer JM, Silliman NP, Wang W, et al. Risk factors for Helicobacter pylori resistance in the United States: the surveillance of H. pylori antimicrobial resistance partnership (SHARP) study, 1993-1999. Ann Intern Med 2002;136:13–24. [DOI] [PubMed] [Google Scholar]
- 11.Osato MS, Reddy R, Graham DY. Metronidazole and clarithromycin resistance amongst Helicobacter pylori isolates from a large metropolitan hospital in the United States. Int J Antimicrob Agents 1999;12:341–347. [DOI] [PubMed] [Google Scholar]
- 12.Osato MS, Reddy R, Reddy SG, et al. Comparison of the Etest and the NCCLS-approved agar dilution method to detect metronidazole and clarithromycin resistant Helicobacter pylori. Int J Antimicrob Agents 2001;17:39–44. [DOI] [PubMed] [Google Scholar]
- 13.Duck WM, Sobel J, Pruckler JM, et al. Antimicrobial resistance incidence and risk factors among Helicobacter pylori-infected persons, United States. Emerg Infect Dis 2004;10:1088–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bochenek WJ, Peters S, Fraga PD, et al. Eradication of Helicobacter pylori by 7-day triple-therapy regimens combining pantoprazole with clarithromycin, metronidazole, or amoxicillin in patients with peptic ulcer disease: results of two double-blind, randomized studies. Helicobacter 2003;8:626–642. [DOI] [PubMed] [Google Scholar]
- 15.Moss SF. How can we rationally select Helicobacter pylori therapy without resistance data? Clin Gastroenterol Hepatol 2014;12:1578. [DOI] [PubMed] [Google Scholar]
- 16.Vakil N, Lanza F, Schwartz H, et al. Seven-day therapy for Helicobacter pylori in the United States. Aliment Pharmacol Ther 2004;20:99–107. [DOI] [PubMed] [Google Scholar]
- 17.Laine L, Estrada R, Trujillo M, et al. Randomized comparison of differing periods of twice-a-day triple therapy for the eradication of Helicobacter pylori. Aliment Pharmacol Ther 1996; 10:1029–1033. [DOI] [PubMed] [Google Scholar]
- 18.Megraud F H pylori antibiotic resistance: prevalence, importance, and advances in testing. Gut 2004;53:1374–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Megraud F. Epidemiology and mechanism of antibiotic resistance in Helicobacter pylori. Gastroenterology 1998;115:1278–1282. [DOI] [PubMed] [Google Scholar]
- 20.Perez Aldana L, Kato M, Nakagawa S, et al. The relationship between consumption of antimicrobial agents and the prevalence of primary Helicobacter pylori resistance. Helicobacter 2002;7:306–309. [DOI] [PubMed] [Google Scholar]
- 21.Megraud F, Coenen S, Versporten A, et al. Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut 2013;62:34–42. [DOI] [PubMed] [Google Scholar]
- 22.Grijalva CG, Nuorti JP, Griffin MR. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA 2009;302:758–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hicks LA, Chien YW, Taylor TH Jr, et al. Outpatient antibiotic prescribing and nonsusceptible Streptococcus pneumoniae in the United States, 1996-2003. Clin Infect Dis 2011;53:631–639. [DOI] [PubMed] [Google Scholar]
- 24.Nordenstedt H, Graham DY, Kramer JR, et al. Helicobacter pylori-negative gastritis: prevalence and risk factors. Am J Gastroenterol 2013;108:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fischbach LA, Graham DY, Kramer JR, et al. Association between Helicobacter pylori and Barrett’s esophagus: a case-control study. Am J Gastroenterol 2014;109:357–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dixon M, Genta R, Yardley J, et al. Classification and grading of gastritis: the updated Sydney System—International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol 1996;20:1161–1181. [DOI] [PubMed] [Google Scholar]
- 27.Sonnenberg A, Lash RH, Genta RM. A national study of Heli-cobactor pylori infection in gastric biopsy specimens. Gastroenterology 2010;139:1894–1901; quiz e12. [DOI] [PubMed] [Google Scholar]
- 28.Grad YH, Lipsitch M, Aiello AE. Secular trends in Helicobacter pylori seroprevalence in adults in the United States: evidence for sustained race/ethnic disparities. Am J Epidemiol 2012; 175:54–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Graham DY, Lee YC, Wu MS. Rational Helicobacter pylori therapy: evidence-based medicine rather than medicine-based evidence. Clin Gastroenterol Hepatol 2014;12:177–186; discussion e12–e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Francesco V, Giorgio F, Hassan C, et al. Worldwide H. pylori antibiotic resistance: a systematic review. J Gastrointest Liver Dis 2010;19:409–414. [PubMed] [Google Scholar]
- 31.McMahon BJ, Hennessy TW, Bensler JM, et al. The relationship among previous antimicrobial use, antimicrobial resistance, and treatment outcomes for Helicobacter pylori infections. Ann Intern Med 2003;139:463–469. [DOI] [PubMed] [Google Scholar]
- 32.Suda KJ, Hicks LA, Roberts RM, et al. Trends and seasonal variation in outpatient antibiotic prescription rates in the United States, 2006 to 2010. Antimicrob Agents Chemother 2014; 58:2763–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tveit AH, Bruce MG, Bruden DL, et al. Alaska sentinel surveillance study of Helicobacter pylori isolates from Alaska Native persons from 2000 to 2008. J Clin Microbiol 2011;49:3638–3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gisbert JP, De La Morena F. Systematic review and metaanalysis: levofloxacin-based rescue regimens after Helicobacter pylori treatment failure. Aliment Pharmacol Ther 2006;23:35–44. [DOI] [PubMed] [Google Scholar]
- 35.Gisbert JP. Second-line rescue therapy of Helicobacter pylori infection. Therap Adv Gastroenterol 2009;2:331–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Megraud F, Lehours P. Helicobacter pylori detection and antimicrobial susceptibility testing. Clin Microbiol Rev 2007; 20:280–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rimbara E, Fischbach LA, Graham DY. Optimal therapy for Helicobacter pylori infections. Nat Rev Gastroenterol Hepatol 2011;8:79–88. [DOI] [PubMed] [Google Scholar]
- 38.Cespedes A, Larson E. Knowledge, attitudes, and practices regarding antibiotic use among Latinos in the United States: review and recommendations. Am J Infect Control 2006; 34:495–502. [DOI] [PubMed] [Google Scholar]
- 39.Pilotto A, Rassu M, Leandro G, et al. Prevalence of Helicobacter pylori resistance to antibiotics in Northeast Italy: a multicentre study—GISU: Interdisciplinary Group for the Study of Ulcer. Dig Liver Dis 2000;32:763–768. [DOI] [PubMed] [Google Scholar]
- 40.Williams CS, Woodcock KR. Do ethanol and metronidazole interact to produce a disulfiram-like reaction? Ann Pharmacother 2000;34:255–257. [DOI] [PubMed] [Google Scholar]
- 41.Kirby A, Herbert A. Correlations between income inequality and antimicrobial resistance. PLoS One 2013;8:e73115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen FM, Breiman RF, Farley M, et al. Geocoding and linking data from population-based surveillance and the US census to evaluate the impact of median household income on the epidemiology of invasive Streptococcus pneumoniae infections. Am J Epidemiol 1998;148:1212–1218. [DOI] [PubMed] [Google Scholar]