Abstract
Breast cancer is the second leading cause of cancer-related mortality in women. In the current study, we evaluated the anti-cancer effects of an anti-protozoal drug–atovaquone against several breast cancer cell lines. Our results showed that atovaquone treatment induced apoptosis and inhibited the growth of all the breast cancer cell lines tested including several patient-derived cells. In addition, atovaquone treatment significantly reduced the expression of HER2, β-catenin and its downstream molecules such as pGSK-3β, TCF-4, cyclin D1 and c-Myc in vitro. Efficacy of atovaquone was further evaluated in an in-vivo tumor model by orthotropic implantation of two highly aggressive 4T1 and CI66 breast cancer cells in the mammary fat pad of female mice. Our results demonstrated that oral administration of atovaquone suppressed the growth of CI66 and 4T1 tumors by 70% and 60% respectively. Paclitaxel is the first line chemotherapeutic agent for metastatic breast cancer. We demonstrate that atovaquone administration suppressed the growth of 4T1 paclitaxel-resistant tumors by 40%. Tumors from atovaquone treated mice exhibited reduced HER2, β-catenin and c-Myc levels alongside an increase in apoptosis in all the three tumor models when analyzed by western blotting, IHC and TUNEL assay. Taken together, our results indicate that atovaquone effectively reduces the growth of primary and paclitaxel-resistant breast tumors. Atovaquone is already in the clinics with high safety and tolerability profile. Therefore, the findings from our studies will potentially prompt further clinical investigation into repurposing atovaquone for the treatment of patients with advanced breast cancer.
Keywords: atovaquone, HER2, β-catenin, breast cancer, c-Myc, in vivo
Introduction
Human epidermal growth receptor 2 (HER2) is a membrane tyrosine kinase that belongs to the family of epidermal growth factor receptors (EGFRs) (1). It plays a crucial role in the complex signaling network that controls normal cell growth and differentiation. However, when activated, it is responsible for rapid proliferation of cells (2,3). HER2 is known to be overexpressed in several neoplasms such as breast, ovarian, gastric, colorectal and pancreatic cancers (4–6). About 30% of breast cancer patients show overexpression of HER2 which contributes to tumor development and progression (7,8). Few studies including a recent study from our lab found that HER2 overexpression is responsible for chemotherapeutic resistance (9,10). Another study suggested that under certain conditions, cleavage of HER2 leads to the activation of intrinsic apoptotic pathway (11). Therefore, targeting HER2 signaling proves to be an effective therapeutic strategy in the clinic. Trastuzumab (herceptin) is the first humanized antibody that targets HER2. However, acquired resistance to trastuzumab occurs after initial treatment phase–providing a therapeutic roadblock in improving patient’s overall survival (12–14). Hence, there is an unmet medical need of novel therapeutic agents that can target HER2 and suppress resistant and metastatic breast cancer.
Wnt/β-catenin signaling regulate crucial aspects of cell fate such as homeostasis, cell migration and immune control (15). Wnt signaling is a complex pathway in which β-catenin is typically viewed as a central mediator. β-catenin signaling has an important role in controlling cellular proliferation and death, and has been shown to be associated with maintenance of cancer stem cells and metastasis (16,17). Perturbation of β-catenin or downstream signaling molecules lead to the development of several human cancers (18) including breast cancer (19); making it a potential therapeutic target (20,21).
Paclitaxel and other taxanes are approved for the treatment of metastatic breast cancer (22). The optimal dosage for paclitaxel as a single agent is 175 mg/m2 as 3 hour infusion (23). However, it is well established that paclitaxel administration is associated with hematological and neural adverse effects that constitute: neutropenia, peripheral neuropathy and bone marrow suppression (24). Moreover, resistance towards paclitaxel through several mechanisms has hindered its therapeutic use. To overcome the adverse effect and resistance to paclitaxel, it is important to explore novel therapeutic approaches that could be effective in treating paclitaxel-resistant breast tumors. In a recent study, we observed that overexpression of HER2/β-catenin signaling is one of the mechanisms that imparts resistance to paclitaxel (10).
Few studies have indicated the use of anti-protozoal and anti-malarial drugs for the treatment of a variety of cancers such as hepatocellular carcinoma, acute myeloid leukemia, pancreatic and colorectal cancer (25–28). Atovaquone is an anti-protozoal and an anti-malarial drug which was approved by the Food and Drug Administration (FDA) in 1999 for Pneumocystis Carinii pneumonia and was eventually approved for the treatment of malaria in combination with proguanil. Previous studies have shown the anti-cancer potency of atovaquone in various cancer models (29–31). Interestingly, clinical studies on atovaquone for lung cancer and acute myeloid leukemia are currently underway (, ). Therefore, these facts strengthen the necessity for more pre-clinical studies to establish the mechanism of the anti-cancer effects of atovaquone. This study provides an account of the pre-clinical investigation into the mechanism of atovaquone action and establishes the anticancer property of atovaquone against breast cancer. We demonstrated that oral administration of atovaquone not only suppressed the primary breast tumor growth but also reduced paclitaxel-resistant breast tumor growth by inhibiting HER2/β-catenin signaling.
Materials and Methods
Cell culture:
Human breast carcinoma cell lines MCF-7, HCC1806, T47D and murine breast cancer cells 4T1 were obtained from ATCC and were maintained in DMEM supplemented with 10% FBS, 1% PSN (Penicillin, Streptomycin and Neomycin). The MCF-7 overexpressing HER2 cells (MCF-7HH) were kindly provided by Dr. Huang Fei (Bristol-Myers Squibb) and CI66 cells were provided by Dr. Rakesh K. Singh (UNMC, Omaha, NE, USA). Both of these cell lines were cultured in DMEM supplemented with 10% FBS and 1% PSN. SKBR3 cells were kindly provided by Dr. Marc Antonyak (Cornell University, Ithaca, NY, USA) and were maintained in RPMI supplemented with 10% FBS and 1% PSN. 4T1 paclitaxel-resistant cells (4T1PR) were developed in our lab by gradually exposing the 4T1 cells to paclitaxel for several months. Patient derived cells (TX-BR-237 and TX-BR-290) were obtained from Children’s Oncology Group (Texas Tech University Health Sciences Center, Lubbock, TX). These cells were maintained in IMDM supplemented with 20% FBS, 1% PSN and 1X ITS (5μg/ml insulin, 5μg/ml transferrin, 5μg/ml selenous acid). All the cell lines were periodically authenticated by short tandem repeats (STR) analysis.
Reagents and chemicals:
HER2 shRNA was obtained from Genecopoeia (Rockville, MD). The chemicals, atovaquone, sulforhodamine B, and antibody against actin were obtained from Sigma-Aldrich (St. Louis, MO). Atovaquone (Mepron) was purchased from PRASCO laboratories (Mason, OH) and was used for in vivo study. Xfect transfection reagent kit was obtained from Clonetech (Mountain View, CA). All the antibodies and siHER2 were purchased from Cell Signaling Technology (Denvers, MA).TUNEL assay kit was purchased from Calbiochem (San Diego, CA).
Cytotoxicity Studies:
Breast cancer cells were plated at the density of 4000–5000 cells/well in 96 well plates and allowed to attach overnight. Next day, cells were treated with different concentrations of atovaquone for 24, 48 and 72 hours. At desired time points, cells were fixed with ice cold 10% trichloroacetic acid, washed and stained with sulforhodamine B dye and the optical density was measured in 10mM Tris base solution using plate reader, after washing the dye with 1% acetic acid solution as described by us previously (32).
Colony formation Assay:
Approximately 400–600 4T1 and MCF-7 cells/well were seeded in a 6-well plate. Next day, cells were treated with 10, 15 and 20 μM of atovaquone. After 72 hours of atovaquone treatment, media was re-placed with fresh medium and 4T1 and MCF-7 cells were cultured for another 5 and 10 days, respectively. At day 9 or 14, cells were fixed and stained with 0.5% crystal violet solution after washing with PBS. Finally, the colonies with > 50 cells were counted under an image J-software.
AnnexinV-FITC apoptosis assay:
Apoptosis assay was performed using a kit (BD Biosciences, CA) according to manufacturer’s instructions and as described by us previously (33). Approximately, 0.2×106 cells were plated in 6-well plate and left overnight for attachment and further treated with 10, 20 and 30 μM atovaquone. After 72 h of treatment, cells were harvested by trypsinization, suspended in PBS to have cell density of 1×106/ml. Further, cells were suspended in 100µl of binding buffer and 5µl of Annexin V-FITC and 5µl of propidium iodide were added to the cell suspension and incubated for additional 20 min at room temperature in dark. Additional binding buffer was further added to make the final sample volume up to 500µl. Samples were kept on ice in dark and analyzed by flow cytometer (Accuri C6, MI, USA).
Western Blot Analysis:
Various breast cancer cells were treated with varying concentrations of atovaquone (10, 20 and 30µM) for 72h. Whole cell lysates were prepared using 4% (w/v) CHAPS buffer while tumor lysates were prepared by homogenizing the tumors in PBS and lysed using RIPA buffer. About 40–60 μg of protein was subjected to SDS-PAGE and the segregated protein was transferred on PVDF membrane. The membrane was developed as described by us previously (34,35).
HER2 knockdown:
Approximately 0.2X106 SKBR3 and MCF-7HH cells were plated in 6 well plate and left overnight for attachment. Next day, cells were transfected with HER2 siRNA (Cell Signaling) as per manufacturer’s instructions using siPORT transfection reagent. After 8 hours of transfection, cells were treated with 20µM atovaquone for 72 h and the cell lysate was used for western blotting. In another experiment, HER2 shRNA and scrambled shRNA (Genecopoeia, Rockville, MD) were transfected in SKBR3 cells using Xfect Transfection reagent (Clonetech) as per manufacturer’s instructions. After 8 hours of transfection, cells were treated with 20µM atovaquone. After 72h of atovaquone treatment, cell lysate was analyzed by western blotting.
Immunoprecipitation Assay:
Immunoprecipitation assay was performed to examine the effect of atovaquone on the interaction of HER2 with β-catenin. Briefly, MCF-7 and MCF-7HH cells were treated with 20 µM atovaquone for 72 hours. The cells from control and atovaquone treated group were collected, lysed using RIPA buffer and immunoprecipitated with β-catenin antibody using Dynabeads magnetic beads as per manufacturer’s instructions. The samples were then resolved by SDS-polyacrylamide gel and transferred to PVDF membrane as described by us earlier (36). The membrane was immunoblotted using HER2 antibody. Input is the cell lysate from control and atovaquone (20 µM) treated MCF-7HH cells. Negative control is the sample with the beads not immunoprecipitated with β-catenin.
Tumor therapy model:
Female Balb/c mice (4–6 weeks old) were obtained from Envigo. Exponentially growing 4T1 and CI66 cells were harvested, washed twice with PBS, and resuspended in 1:1 PBS/matrigel at a density of 0.7×106 and 1×106 cells per ml respectively. A suspension of 0.1mL containing 0.07×106 4T1 cells or 0.1×106 CI66 cells were injected orthotropically in the right and left mammary fat pad of female Balb/c mice respectively. Tumor volumes were measured three times a week and animal weights were taken once a week as described by us previously (37). When the tumors reached a size of about 70mm3, mice were randomly segregated into two groups with 7 mice in each group. Treated group of mice received 30mg/kg atovaquone by oral gavage every day till day 25 whereas control mice received the vehicle. Mice were sacrificed on day 25 by CO2 overdose and death was confirmed by cervical dislocation in accordance with IACUC guidelines. The tumors were removed aseptically from each mouse and were snap frozen in liquid-nitrogen for western blot analysis. A part of tumor from control and treated group were kept in formalin for immunofluorescence analysis. All the mice experiments were conducted in accordance with the ethical standards and according to approved protocol by Institutional Animal Care and Use Committee (IACUC).
Resistance to paclitaxel was developed in 4T1 cells by gradually exposing these cells to paclitaxel for several months. In another in vivo study, approximately 0.07×106 4T1PR were injected orthotropically in the left and right 3rd mammary fat pad of mice. Once mice had stable tumor (approx. size: 70mm3), mice were divided into two groups (n=10) and the treatment group was administered with 25 mg/kg atovaquone everyday by oral gavage. Mice were sacrificed at day 19 and tumors were collected for further analysis as described earlier.
Immunostaining of tumor sections:
The immunohistochemistry (IHC) was performed as previously described by us (38). Briefly, paraffin-embedded tissues were sectioned into 5–7µm thick sections using microtome (Leica Microsystems Inc., Buffalo Grove, IL). After deparaffinization and rehydration, antigens were retrieved by boiling the sections in 10 mM sodium citrate buffer (pH 6.0). The slides were washed with distilled water and incubated in 3% hydrogen peroxide methanol solution. The sections were then washed, blocked in 200 μl of blocking solution (5% goat serum diluted) and incubated with anti–HER2 (1:100) (Cell Signaling, Danvers, MA) or anti-β-catenin (1:100) (Cell Signaling, Danvers, MA) overnight at 4°C. Next day, primary antibody was removed and the sections were washed with wash buffer followed by 30 minute incubation with Ultravision ONE HRP polymer (Thermofisher Scientific, Rockland, IL) as per the manufacturer’s instructions. Subsequently, sections were washed with wash buffer and incubated with DAB Plus chromogen for 15–20 minutes. The sections were counterstained with hematoxylin and dehydrated. The slides were mounted using Permount (Thermofisher Scientific, Rockland, IL) and analyzed under a bright field Olympus microscope (Olympus America Inc). TUNEL assay was carried out according to manufacturer’s protocol (Calbiochem, San Diego, CA).
Statistical Analysis:
Statistical calculations and analysis were performed using Prism 7.0 (GraphPad software Inc., San Diego, CA). Results represent means ± SD or S.E.M. of at least three independent experiments. Data was analyzed by Student’s t-test or Fischer (F) test. Differences were considered statistically significant at p<0.05.
Results
Atovaquone inhibits the proliferation of breast cancer cells
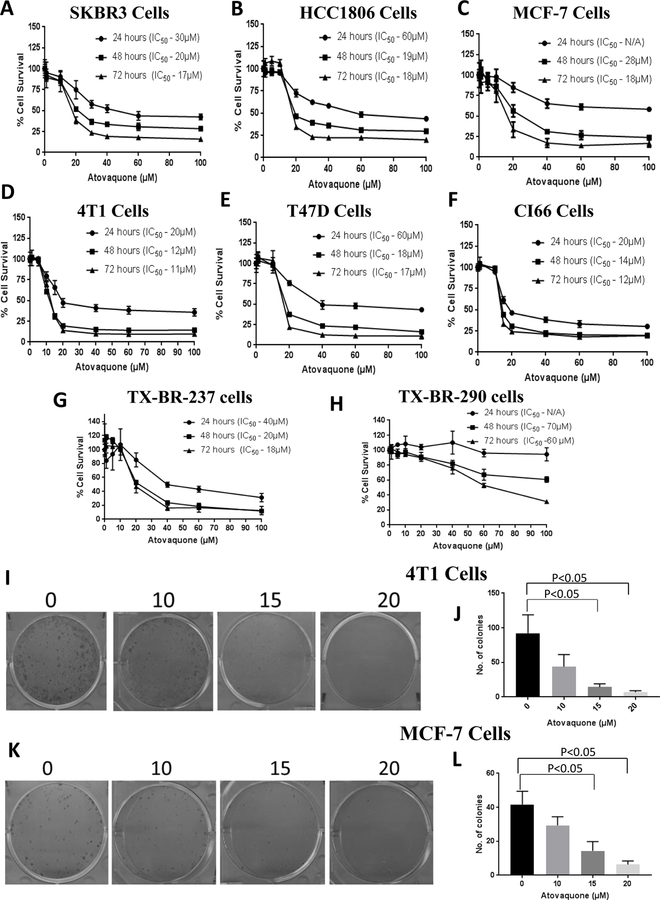
Initially, we determined the optimum concentration at which atovaquone could inhibit the proliferation of breast cancer cells. We performed the cytotoxicity assay in a panel of various breast cancer cell lines (MCF-7, SKBR3, HCC1806, 4T1, CI66 and T47D) with atovaquone. Our results showed that increasing concentrations of atovaquone significantly suppressed the growth of all the breast cancer cells in a concentration and time-dependent manner. The IC50 of atovaquone after 72 hours treatment ranged from 11–18 μM in all the cell lines tested (Fig. 1A–F). We further evaluated the growth suppressive effects of atovaquone in two adult patient-derived cells, TX-BR-237 and TX-BR-290. The IC50 of atovaquone was found to be in the range of 18–60 μM in patient-derived cells after 72 hours treatment (Fig. 1G–H). These results suggest the potential cytotoxic effects of atovaquone in all the breast cancer cells in a concentration and time-dependent manner. We also performed colony formation assay to investigate whether atovaquone could inhibit the proliferation of breast cancer cells. Results from this assay clearly showed that atovaquone treatment significantly decreased the size and number of colonies in a concentration-dependent manner (Fig 1I–L). We did not observed much toxic effects of atovaquone in HEK293 cells, which are normal human embryonic kidney cells (Supplementary Fig. S1).
Figure 1. Atovaquone exhibits potent anti-tumor activity in breast cancer cell lines.
A) HCC1806, B) SKBR3, C) MCF-7, D) 4T1, E) T47D, F) CI66 cells were treated with increasing concentrations of atovaquone for 24, 48 and 72h. Cell survival was measured with sulforhodamine B (SRB) assay to estimate the IC50 values. Patient derived cell lines G) TX-BR-237 and H) TX-BR-290 were also evaluated for cytotoxic effect of atovaquone. The experiments were repeated at least three times with 8 replicates in each experiment. Colony formation assay was performed by seeding 400–600 cells/well. 4T1 cells were fixed and stained using crystal violet (0.5%) after 9 days and MCF-7 cells after 14 days. Number of colonies formed in control and atovaquone treated wells were quantitated using Image J software. Representative Images of colonies and their quantification in (I-J) 4T1 cells and (K-L) MCF-7 cells. Data shown as mean ± SD; (n=3). Student’s t test for unpaired samples was used to perform statistical comparisons.
Atovaquone causes induction of apoptosis
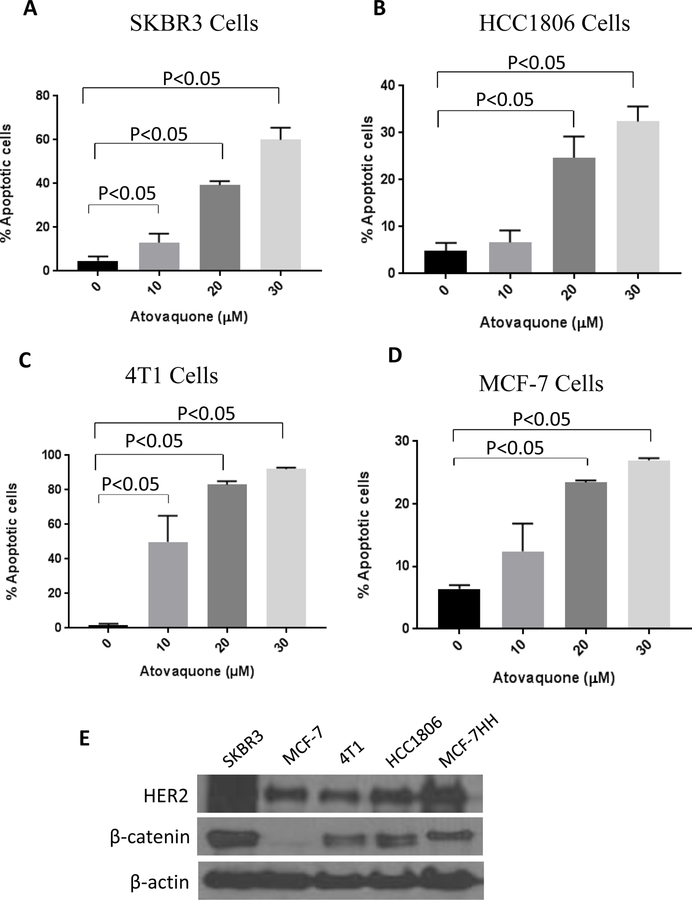
To gain further insight into the mechanism of the cell growth inhibitory effects of atovaquone, SKBR3, HCC1806, 4T1 and MCF-7 cells were treated with various concentrations of atovaquone for 72h and analyzed for apoptosis using Annexin V/PI assay. Our results showed that 20–30 µM atovaquone caused significant apoptosis in all the breast cancer cell lines after 72 hours of treatment. A concentration dependent increase in apoptosis with atovaquone treatment was observed. For instance, treatment of HCC1806 and MCF-7 cells with 20 µM and 30 µM atovaquone resulted in approximately 25–35% and 20–30% apoptosis respectively, compared to controls (Fig 2B, D). On the other hand, SKBR3 and 4T1 cells were more sensitive to atovaquone-induced apoptosis. Percentage of apoptotic cells in SKBR3 and 4T1 at 10 µM, 20 µM and 30 µM atovaquone concentrations were 15–70% and 40–95% respectively (Fig 2A, C). Approximately 30–90% apoptosis was observed in all the cell lines after 72 hours treatment with 30 μM atovaquone as shown in Fig 2A–D.
Figure 2. Atovaquone induces apoptosis in breast cancer cells.
A) SKBR3, B) HCC1806, C) 4T1, D) MCF-7 cells was treated with or without atovaquone (10, 20 and 30 µM) for 72h. Cells were then collected and labeled with Annexin V FITC. Cells that were positive for Annexin or PI or both were measured using flowcytometry. Each experiment was repeated more than three times independently. E) Western blots analyses representing the basal level of HER2 and β-catenin in breast cancer cells. *Statistically different when compared with control (p<0.05).
Atovaquone treatment depletes HER2 levels and inhibits β-catenin signaling
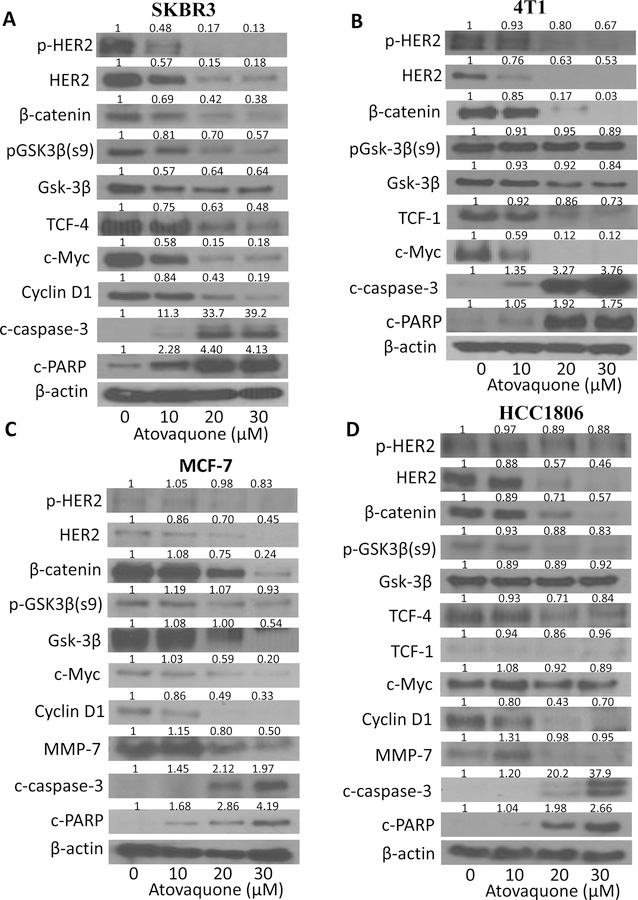
To further elucidate the mechanism of cell death induced by atovaquone, we performed western blot analysis using lysates from SKBR3, 4T1, HCC1806, MCF-7, CI66 and T47D cells treated with 10, 20, 30 μM atovaquone for 72 hours. Firstly, we compared the basal level of HER2 and β-catenin in all the cells (Fig. 2E). Consistent with previous observations, MCF-7, 4T1 and HCC1806 cells showed very low constitutive levels of HER2 (32,39–43). Our results showed that atovaquone treatment significantly reduced HER2 expression in a concentration-dependent manner in all the cell lines tested (Fig. 3 and Supplementary Fig. S2). Interestingly, complete abrogation of HER2 expression was observed in 4T1, MCF-7 and CI66 breast cancer cells after treatment with 30 μM of atovaquone (Fig. 3). Moreover, atovaquone treatment also suppressed the phosphorylation of HER2. β-catenin was also found to be reduced significantly by atovaquone treatment in all the breast cancer cells (Fig. 3 and Supplementary Fig. S2). β-catenin signaling plays a critical role in the regulation of cell proliferative pathways and is known to be activated in breast tumors. c-Myc–a proto-oncogene regulated by β-catenin was also significantly downregulated by atovaquone. In addition, atovaquone treatment substantially reduced the expression of other downstream markers of β-catenin signaling such as p-GSK3β, TCF-4, TCF-1, cyclinD1 and MMP-7. Accumulating evidences have established GSK3β as a viable therapeutic target in multiple tumor types including breast cancer (44–46). Our results showed reduction in GSK3β expression with atovaquone treatment in SKBR3 and MCF-7 cells whereas its expression remains unchanged in 4T1 and HCC1806 cells (Fig. 3A–D). As shown in Fig. 3, we also observed significant increase in the cleaved fragments of caspase-3 and PARP after treatment with 20 and 30 µM atovaquone; indicating apoptosis. These results suggest that atovaquone induces apoptosis in breast cancer cells by inhibiting HER2/β-catenin signaling (Fig. 3).
Figure 3. Atovaquone down regulates HER2/β-catenin signaling in breast cancer cells.
A) SKBR3, B) 4T1, C) MCF-7 and D) HCC1806, cells were treated with varying concentrations of atovaquone for 72h. Representative blots showing the concentration dependent effect of atovaquone on p-HER2, HER2, β-catenin, p-GSK3β, GSK3β c-Myc, TCF-4, TCF-1 cyclin D1, MMP-7, cleaved caspase 3, cleaved PARP. Actin was used as loading control. Each experiment was repeated three times independently.
Effect of Atovaquone treatment in cells stably overexpressing HER2
Next we sought to determine whether HER2 overexpression could affect atovaquone induced apoptosis. For this, we determined the effect of atovaquone on MCF-7HH cells. First, we compared the cytotoxic effects of atovaquone in MCF-7HH with MCF-7 cells. We observed that atovaquone significantly suppressed the growth of MCF-7HH cells similar to MCF-7 cells (Fig.1C) and the IC50 of atovaquone was comparable in both the cell lines (Fig. 4A). To determine the effect of atovaquone on HER2 and other oncogenic proteins, MCF-7 and MCF-7HH cells were treated with varying concentrations of atovaquone for 72h and evaluated by western blotting. Although, the expression of HER2 was drastically reduced by atovaquone treatment in both the cells lines, the extent of HER2 downregulation was diminished in MCF-7HH cells when compared to MCF-7 cells. As shown in Fig 4B, MCF-7HH cells showed diminished downregulation of β-catenin, c-Myc and other downstream markers as compared to the MCF-7 cell line in response to atovaquone treatment. Furthermore, slightly reduced apoptosis was seen by atovaquone treatment as indicated by less cleavage of PARP and caspase-3 in MCF-7HH cells when compared to MCF-7 cells. Interestingly, we observed that not only HER2 expression was greater in MCF-7HH cells but also the expression of β-catenin and its downstream markers such as p-GSK3β, c-Myc, TCF-4, MMP-7 was constitutively higher. Taken together, these results clearly indicate that atovaquone was almost equally effective in suppressing the growth of MCF-7HH cells by inhibiting HER2/β-catenin signaling.
Figure 4. Change in HER2 expression modulates the effect of atovaquone.
A) Percentage cell survival of MCF-7HH cells when treated with atovaquone at indicated concentrations and time points. B) MCF-7 and MCF-7HH cells were treated with 10, 20 and 30 μM atovaquone for 72h and whole lysate was analyzed by western blotting for HER2, β-catenin, p-GSK3β, c-Myc, TCF-4, cyclin D1, MMP-7, cleavage of caspase 3 and PARP. C) SKBR3 cells transfected with either scrambled or HER2 siRNA and treated with or without 20µM atovaquone for 72h. Whole cell lysate was evaluated for HER2, β-catenin, c-Myc, cyclin D1, cleavage of caspase 3 & PARP. MCF-7HH were treated with atovaquone (20 μM) for 72 hours after transfecting the cells with D) HER2 siRNA or scrambled siRNA and E) HER2 shRNA or scrambled shRNA. Levels of HER2, β-catenin, c-Myc, cyclin D1, cleaved caspase 3, cleaved PARP were evaluated by Western blotting. Blots were quantitated and normalized with actin. Each experiment was repeated three times independently. (F) MCF-7HH cells treated with 10μM MG-132 and 20μM atovaquone alone and in combination for 72 hours. β-catenin was immunoprecipitated from control and 20 μM atovaquone-treated cells and probed for HER2 in (G) MCF-7 and (H) MCF-7HH cells. IgG was used as loading control.
HER2 silencing enhances atovaquone-induced apoptosis
Further, to confirm the role of HER2 in atovaquone induced apoptosis in breast cancer cells, HER2 was knocked down using HER2 siRNA in SKBR3 cells, which has high constitutive level of HER2 expression. It is important to note that knocking down HER2 resulted in the reduction of β-catenin and c-Myc levels (Fig. 4C). Atovaquone treatment further reduced the expression of these proteins and enhanced the cleavage of caspase-3 and PARP in HER2 knocked down SKBR3 cells as compared to SKBR3 cells with constitutively high HER2 expression (Fig. 4C). These results were also confirmed in MCF-7 cells stably overexpressing HER2 (MCF-7HH). siRNA as well as shRNA was used to knock down HER2 in MCF-7HH cells which were further treated with 20 μM atovaquone. As shown in Fig. 4D–E, knocking down HER2 resulted in significant cleavage of caspase 3 and PARP in MCF-7HH cells. Interestingly, we observed that silencing HER2 also lead to reduced expression of β-catenin, c-Myc and cyclin D1, which was further reduced by atovaquone treatment. These results not only suggest the regulation of β-catenin and c-Myc by HER2 but also the role of HER2/β-catenin in atovaquone induced apoptosis (Fig. 4C–E).
Degradation of HER2 and β-catenin by atovaquone is proteasome dependent
We performed experiments using proteasome inhibitor MG-132 to understand the mechanism of HER2 and β-catenin degradation in MCF-7HH cells by atovaquone. MCF-7 cells were treated with either MG-132 or 20 μM atovaquone or a combination of both. We observed a humongous increase in the expression of HER2 and β-catenin in the cells treated with MG-132 and then with atovaquone, indicating that these proteins are degraded by proteasome pathway (Fig. 4F). We also observed that the effect of atovaquone was blocked when used in combination with MG-132. These results further corroborate our hypothesis that atovaquone promotes degradation of HER2 and β-catenin in a proteasome-dependent manner as evidenced by the rescue of proteins by MG-132 (Fig. 4F). Next, we used β-catenin inhibitor IWP-2 in combination with atovaquone in MCF-7HH cells. With IWP-2 treatment alone, we did not observed any change in HER2 expression indicating HER2 was not regulated by β-catenin (Supplementary Fig. S3). Nonetheless, atovaquone treatment in combination with IWP-2 further reduced the expression of β-catenin and its downstream molecules with enhanced cleavage of caspase-3 and PARP as compared to atovaquone treatment alone; indicating enhanced effects of atovaquone with IWP-2 (Supplementary Fig. S3).
Atovaquone treatment disrupts HER2/β-catenin interaction
To further analyze the cross-talk of HER2 with β-catenin and to evaluate the effect of atovaquone on the interaction of HER2 and β-catenin, immunoprecipitation studies were performed. MCF-7 cells were treated with or without 20 μM atovaquone for 72 hours, β-catenin was immunoprecipitated with β-catenin antibody and immunoblotted with HER2. We observed a clear immunoblot of HER2 in cells immunoprecipitated with β-catenin suggesting a direct interaction of β-catenin with HER2. Our results also showed that the cells treated with atovaquone and immunoprecipitated with β-catenin antibody exhibited low expression of HER2; indicating reduced association of HER2 with β-catenin after atovaquone treatment (Fig. 4G). To confirm these results, we also performed a similar experiment in HER2 overexpressing MCF-7HH cells. We observed similar results suggesting interaction between HER2 and β-catenin was completely blocked after atovaquone treatment (Fig. 4H). Taken together, these results demonstrate that HER2/β-catenin is a possible target of atovaquone in breast cancer cells.
Tumor therapy model
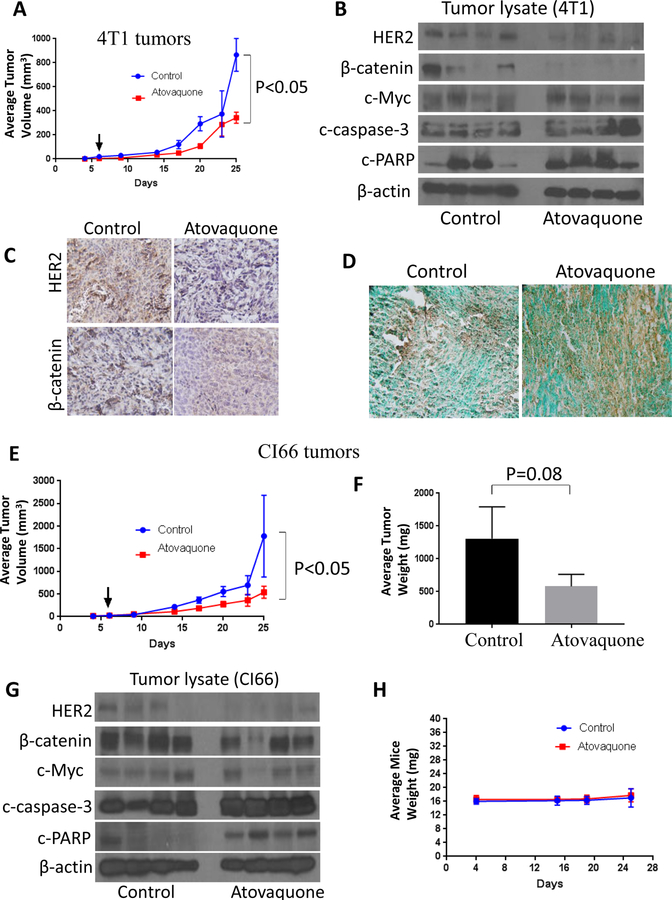
Furthermore, to evaluate the efficacy of atovaquone treatment in vivo and to establish the mechanism of tumor growth inhibition in breast cancer, highly aggressive 4T1 and CI66 cell lines were used. About 0.07X106 4T1 and 0.1X106 CI66 were orthotropically implanted in the mammary fat pad of female Balb/c mice. After each mouse had a tumor growth of about 70 mm3, mice received 30mg/kg atovaquone by oral gavage every day. Due to the high tumor burden in control mice, all animals were sacrificed at day 25. Our results showed that atovaquone treatment significantly suppressed the growth of breast tumors (Fig. 5A, 5E). At day 25, tumor volume of 4T1 tumors in atovaquone treated group was reduced by 60% as compared to control group (865.53 mm3 versus 342.99 mm3) (Fig. 5A). Similarly, atovaquone treatment significantly suppressed the growth of CI66 tumors by 70% (1778.55 mm3 versus 538.57 mm3) (Fig. 5E). In addition, average weight of CI66 tumors dissected from atovaquone treated mice was approximately 55% less than the weight of the tumors from control mice (Fig. 5F). The tumors of control and atovaquone treated mice were further analyzed by western blotting and immunohistochemistry. Consistent with our in vitro results, western blots from 4T1 tumor lysate showed that atovaquone treatment substantially down-regulated HER2 and β-catenin expression (Fig. 5B). We also observed a decrease in c-Myc expression in the tumors of atovaquone treated mice. It is noteworthy that substantial cleavage of caspase 3 and PARP was observed in the tumors from atovaquone treated group as compared to control; indicating apoptosis (Fig. 5B). Similar results were observed in the tumor lysates from CI66 tumors (Fig. 5G). These observations were confirmed by immunohistochemistry in the tumor sections from control and atovaquone treated mice. Fig. 5C shows reduced staining for HER2 and β-catenin in 4T1 tumors from atovaquone treated mice. TUNEL staining showed increased staining in the tumor sections from atovaquone treated mice as compared to control mice indicating apoptosis (Fig. 5D). No change in the body weight of atovaquone treated mice was seen as compared to control mice (Fig. 5H).
Figure 5. Atovaquone suppresses the growth of 4T1 and CI66 breast tumors and inhibits HER2/β-catenin signaling in vivo.
A) About 0.07 × 106 4T1 and 0.1X106 CI66 breast cancer cells were injected orthotropically in the right and left mammary fat pads of 4 to 6 weeks old Balb/c female mice. Mice were given 30 mg/kg of atovaquone by oral gavage every day till day 25. A) Tumor growth curve in 4T1 cells. Values were plotted as mean ± SEM. B) Orthotropically implanted 4T1 tumors were removed aseptically after terminating the experiments. Tumors were homogenized, lysed, and analyzed for HER2, β-catenin, c-Myc, c-caspase-3 and c-PARP. Blots were stripped and reprobed with actin antibody to verify equal protein loading; each lane of blot represents tumor from individual mouse. C) Tumors were sectioned and immunostained for HER2, β-catenin and c-caspase-3. D) Representative images from control and atovaquone treated 4T1 tumors by TUNEL assay. E) Tumor growth curve in CI66 cells. Values were plotted as mean ± SEM. F) Effect of atovaquone on CI66 tumor weight. G) CI66 tumors were minced, lysed and analyzed for HER2, β-catenin, c-Myc, and c-caspase-3 and c-PARP. Each band represents tumor from individual mouse. H) Weight of the mice during the study.
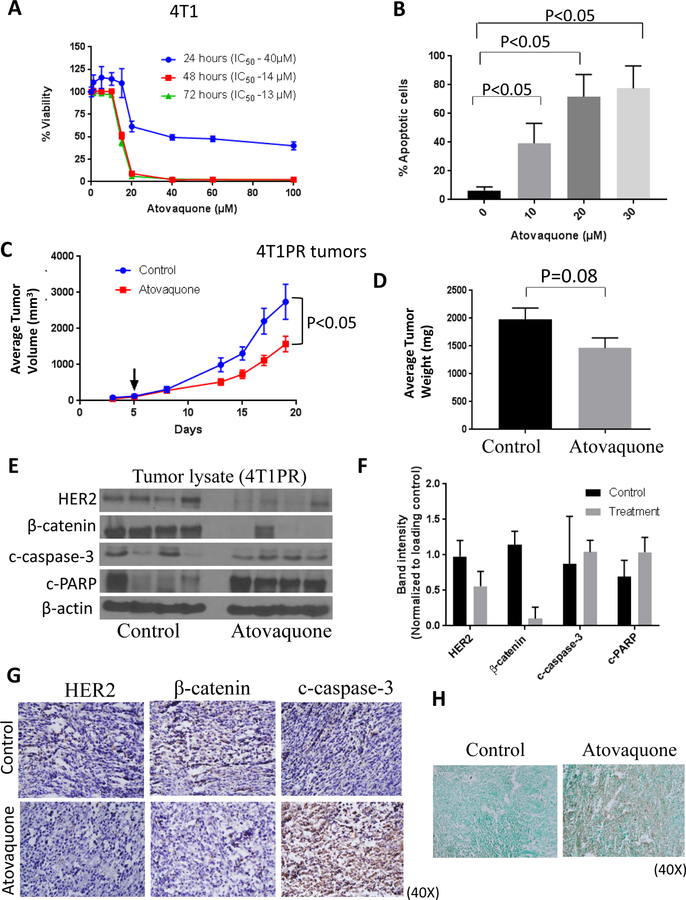
Atovaquone suppresses the growth of paclitaxel-resistant tumors
Paclitaxel is a chemotherapeutic agent used for breast cancer treatment. However, frequent development of resistance to paclitaxel dampens the clinical response. We developed >100 fold paclitaxel-resistant 4T1 breast cancer cell line (4T1PR). Interestingly, we observed enhanced expression of HER2, β-catenin and other downstream markers in 4T1PR cells when compared with 4T1 cells (10). Further, we wanted to determine whether atovaquone would be effective against paclitaxel-resistant breast cancer cells. For this purpose, we first evaluated the cytotoxicity of atovaquone in 4T1PR cells. We observed that the IC50 of atovaquone was in the range of 13–40 μM at 24–72 hours of treatment (Fig. 6A). Next, we determined the percentage apoptosis with atovaquone treatment using AnnexinV-FITC apoptosis assay in 4T1PR cells. We observed a concentration-dependent increase in apoptosis with atovaquone treatment (Fig. 6B). Further, we implanted paclitaxel-resistant 4T1 (4T1PR) cells in the left and right mammary fat pad of female mice. Once each mouse had palpable tumors, mice were treated with 25 mg/kg atovaquone every day by oral gavage for 19 days. Our results showed that atovaquone significantly suppressed the growth of 4T1PR tumors by 42% (Fig. 6C). In addition, we noted a decrease in the weight of tumors isolated from atovaquone treated mice when compared with control mice (Fig. 6D). At the end of the experiment, tumors were collected and analyzed by western blotting. Our results showed that atovaquone treatment also suppressed the expression of HER2 and β-catenin in 4T1PR tumor lysates while cleavage of caspase-3 and PARP increased (Fig. 6E). Furthermore, similar to western blot results, immunohistochemical staining showed reduction in the expression of HER2 and β-catenin in the tumor sections of atovaquone treated mice as compared to controls. In addition, increased expression of c-caspase-3 was observed in the tumor sections from atovaquone treated mice (Fig. 6G). This suggests atovaquone inhibited 4T1PR tumor growth by inhibiting HER2/β-catenin signaling. We also observed increased apoptosis in tumor samples obtained from atovaquone treated mice as analyzed by TUNEL assay (Fig. 6H). Taken together; our results indicate that paclitaxel-resistant tumor growth suppression by atovaquone treatment was associated with downregulation of HER2/β-catenin signaling and increased apoptosis.
Figure 6. Atovaquone inhibits the growth of paclitaxel-resistant cells in vitro and in vivo by inhibiting HER2/β-catenin signaling.
A) Percentage cell survival of 4T1PR cells when treated with atovaquone at indicated concentrations and time points. B) Dose and time-dependent increase in apoptotic cell population after treatment with atovaquone in 4T1PR cells as analyzed by AnnexinV/PI co-staining. Reduction in (C) 4T1PR breast tumor volume and (D) tumor weight after treatment with 25 mg/Kg atovaquone. (E) Expression of HER2, β-catenin, c-PARP and c-caspase-3 in tumor lysates, each blot indicates tumor lysate from individual mouse. (F) Bar graph representation of quantified blots. G) Tumors from control and atovaquone treated mice were dissected out and kept in 4% formalin solution. Tumors were then sliced about 5–10 μm thick and placed on glass slides. Treated and untreated tumors were stained with HER2, β-catenin and c-caspase-3 antibodies. H) Representative images from control and atovaquone treated 4T1PR tumors by TUNEL assay.
Discussion
HER2 and β-catenin signaling pathways are upregulated in breast cancer and lead to tumor development and progression. Moreover, these pathways are also accountable to impart chemotherapeutic resistance (47,48). In order to develop effective therapies for breast cancer, one salient approach is to target HER2 and β-catenin signaling. In the present study, we demonstrate that the FDA-approved drug atovaquone is an attractive candidate for breast cancer treatment. Atovaquone is an anti-protozoal agent which belongs to the class of naphthoquinones. It is used for the prevention and treatment of malaria, toxoplasmosis and pneumocystis pneumonia (PCP) (49,50). Atovaquone primarily works by inhibiting the mitochondrial electron transport chain of malarial parasite and thus causes cell death (51). Herein, we report the novel anti-cancer mechanism of atovaquone by inhibiting HER2/β-catenin signaling in breast cancer.
HER2, an epidermal growth factor receptor (EGFR) related oncogene is found to be amplified in breast cancer and its overexpression is associated with an aggressive progression of the disease with poor prognosis (52). Wnt/β-catenin signaling has recently gained attention as an important target for therapy in various cancer types (53). Recently, a phase 2 clinical trial () has been undertaken for metastatic colorectal cancer using PRI-724–a β-catenin antagonist. The outcome of this study indicated that PRI-724 blocks the expression of many proteins by inhibiting β-catenin and thereby suppresses cancer growth. Similarly, several other clinical trials also have been initiated by the National Cancer Institute (, , and ) to evaluate the efficacy of β-catenin antagonists or inhibitors in various cancer types such as pancreatic adenocarcinoma, acute myeloid leukemia and advanced solid tumors. Hence, for the rational design of new therapies, it is crucial to establish the mechanism of interaction of HER2 with β-catenin; paving a way to better understand the underlying biology for drug targeting. Herein, our results showed significant suppression of HER2/β-catenin signaling by atovaquone treatment. Interestingly, we observed that silencing HER2 also reduced the expression of β-catenin and c-Myc in SKBR3 and MCF-HH cells; indicating a cross-talk between HER2 and β-catenin. In a previous study, we evaluated the interaction of HER2 with β-catenin and found that HER2 regulates β-catenin (10). In agreement, results from current study also show an association of HER2 and β-catenin which is further reduced by atovaquone treatment as evaluated by immunoprecipitation studies.
Our results demonstrate that atovaquone treatment significantly suppresses the viability of several breast cancer cells including MCF-7, SKBR3, HCC1806, 4T1, CI66 and T47D with IC50 ranging from 11–18 μM. In addition, atovaquone also reduces the proliferation of patient-derived breast cancer cells–TX-BR-247 and TX-BR-290. It is noteworthy that the average steady state plasma concentration of atovaquone in humans is 57 μM (54,55), concentration that is approximately 3–4 fold higher than the IC50 concentration in our cell model. This suggests that the effective therapeutic concentration of atovaquone is clinically achievable. The reduced viability of breast cancer cells by atovaquone was associated with HER2/β-catenin down-regulation. In addition, atovaquone treatment reduced the expression of downstream markers of β-catenin signaling such as p-GSK3β, TCF-4, TCF-1 as well as its transcriptionally regulated oncogenes such as c-Myc, cyclin D1, MMP-7 in most of the breast cancer cell lines tested. Knocking down HER2 using siHER2 or shRNA enhanced the cytotoxic potential of atovaquone in these cells as demonstrated by an increase in the cleavage of caspase3 and PARP. On the other hand, stable overexpression of HER2 in MCF-7 cells made the cells less sensitive towards atovaquone cytotoxicity. To determine the efficacy of atovaquone in vivo, we tested atovaquone in three different breast tumor models in mice. We used a highly aggressive cell lines, CI66 and 4T1 representing stage IV breast cancer, which is considered to be untreatable. Atovaquone suppressed the growth of both 4T1 and CI66 breast tumors. Tumor growth suppression by atovaquone was associated with reduced HER2 and β-catenin signaling markers as demonstrated by western blotting, IHC and TUNEL staining of the excised tumors. In addition, oral administration of atovaquone suppressed the growth of paclitaxel-resistant breast tumors. Paclitaxel is a clinically employed first line treatment option for the patients with metastatic breast cancer. However, inherited or acquired resistance to paclitaxel is a major limiting factor for successful therapy. Our recent studies demonstrate the significance of overexpression of HER2, β-catenin and associated proteins in paclitaxel-resistant breast cancer cells (10). As atovaquone downregulates HER2/β-catenin signaling markers, it can be successfully used as a potential agent to treat paclitaxel-resistant breast cancer as monotherapy or could also be used as combination therapy with paclitaxel; consequently reducing the toxic side effects associated with paclitaxel.
Atovaquone is an anti-protozoal agent with a dose of 750 mg orally twice a day for the treatment of PCP. As of now, the lethal dose of atovaquone is not known. The maximum tolerated oral dose tested in mice is 1825 mg/kg/day, which did not show any signs of adverse effects (https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/020500s010lbl.pdf). In a clinical study performed in AIDS patients (n=109) for the treatment of PCP, 750 mg of atovaquone was given to patients three times a day for 21 days. The results showed only minor side effects such as rash and fever in 4% patients suggesting high tolerability of atovaquone in humans (56,57). Moreover, it is important to note that the intended dose of atovaquone to induce anticancer effects is six-fold less, as established in our study, than its upper anti-malarial dose used clinically. Dose of atovaquone used in the current study is 30mg/kg, which, when converted to human equivalent dose is 2.5 mg/kg. Therefore, human equivalent dose of atovaquone would be approximately 150 mg for females considering an average body weight of 60 kg. Prior studies have shown the anti-cancer effects of atovaquone by targeting mitochondrial respiration and inhibiting signal transducer and activator of transcription 3 (STAT3) (31,58,59). In line with these studies, our work provides observable evidences to establish a strong antitumor effect of atovaquone in primary and paclitaxel-resistant breast cancer. The mechanism of atovaquone is depicted in Supplementary Fig. S4. Such observations draw us to conclude that atovaquone could serve as a highly safe, non-toxic and efficacious anti-cancer agent in humans for breast cancer treatment and should be pursued for further clinical investigations. To the best of our knowledge, ours is the first study to demonstrate the use of atovaquone for breast cancer by inhibiting HER2/β-catenin signaling.
Supplementary Material
Acknowledgements
We gratefully acknowledge Dr. Huang Fei and Dr. Karen Reeves (Bristol-Myers Squibb) for providing us MCF7 overexpressing HER2 cells. We are thankful to Dr. Marc Antonyak (Cornell University, Ithaca, NY, USA) for providing SKBR3 cells. CI66 cells were kindly provided by Dr. Rakesh K. Singh (UNMC, Omaha, NE, USA).
Grant support: Financial support for this work was partially covered by R01 grant CA129038 (to S. K. Srivastava) awarded by the National Cancer Institute (NIH)
Footnotes
Disclosure of Potential Conflict of Interest
Authors declare that there are no competing interests.
REFERENCES
- 1.Gutierrez C, Schiff R. HER2: biology, detection, and clinical implications. Archives of pathology & laboratory medicine 2011;135(1):55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rubin I, Yarden Y. The basic biology of HER2. Annals of oncology 2001;12(suppl_1):S3–S8. [DOI] [PubMed] [Google Scholar]
- 3.Gupta P, Gupta N, Fofaria NM, Ranjan A, Srivastava SK. HER2-mediated GLI2 stabilization promotes anoikis resistance and metastasis of breast cancer cells. Cancer Lett 2019;442:68–81 doi 10.1016/j.canlet.2018.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gravalos C, Jimeno A. HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Annals of oncology 2008;19(9):1523–9. [DOI] [PubMed] [Google Scholar]
- 5.Berchuck A, Kamel A, Whitaker R, Kerns B, Olt G, Kinney R, et al. Overexpression of HER-2/neu is associated with poor survival in advanced epithelial ovarian cancer. Cancer research 1990;50(13):4087–91. [PubMed] [Google Scholar]
- 6.Abrahao-Machado LF, Scapulatempo-Neto C. HER2 testing in gastric cancer: An update. World Journal of gastroenterology 2016;22(19):4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.English DP, Roque DM, Santin AD. HER2 expression beyond breast cancer: therapeutic implications for gynecologic malignancies. Molecular diagnosis & therapy 2013;17(2):85–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim CG, Lee H, Gupta N, Ramachandran S, Kaushik I, Srivastava S, et al. Role of Forkhead Box Class O proteins in cancer progression and metastasis. Semin Cancer Biol 2018;50:142–51 doi 10.1016/j.semcancer.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu S, Lee JS, Jie C, Park M-H, Iwakura Y, Patel Y, et al. HER2 overexpression triggers an IL-1α pro-inflammatory circuit to drive tumorigenesis and promote chemotherapy resistance. Cancer research 2018:canres. 2761.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta N, Gupta P, Srivastava SK. Penfluridol overcomes paclitaxel resistance in metastatic breast cancer. . Scientific Reports 2019;9(1):5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strohecker AM, Yehiely F, Chen F, Cryns VL. Caspase cleavage of HER-2 releases a Bad-like cell death effector. Journal of Biological Chemistry 2008;283(26):18269–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ye H, Chai X, Wang X, Zheng Q, Zheng D, Wu F, et al. Autophagy flux inhibition augments gastric cancer resistance to the anti-human epidermal growth factor receptor 2 antibody trastuzumab. Oncology letters 2018;15(4):4143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang L, Long Z, Feng G, Guo X, Yu M. NES1/KLK10 promotes trastuzumab resistance via activation of PI3K/AKT signaling pathway in gastric cancer. Journal of cellular biochemistry 2017. [DOI] [PubMed] [Google Scholar]
- 14.Shi J, Li F, Yao X, Mou T, Xu Z, Han Z, et al. The HER4-YAP1 axis promotes trastuzumab resistance in HER2-positive gastric cancer by inducing epithelial and mesenchymal transition. Oncogene 2018:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pai SG, Carneiro BA, Mota JM, Costa R, Leite CA, Barroso-Sousa R, et al. Wnt/beta-catenin pathway: modulating anticancer immune response. Journal of hematology & oncology 2017;10(1):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teng Y, Wang X, Wang Y, Ma D. Wnt/β-catenin signaling regulates cancer stem cells in lung cancer A549 cells. Biochemical and biophysical research communications 2010;392(3):373–9. [DOI] [PubMed] [Google Scholar]
- 17.Prasad S, Ramachandran S, Gupta N, Kaushik I, Srivastava SK. Cancer cells stemness: A doorstep to targeted therapy. Biochim Biophys Acta Mol Basis Dis 2019. doi 10.1016/j.bbadis.2019.02.019. [DOI] [PubMed] [Google Scholar]
- 18.Morin PJ. beta-catenin signaling and cancer. Bioessays 1999;21(12):1021–30 doi . [DOI] [PubMed] [Google Scholar]
- 19.R Prosperi J, H Goss K. A Wnt-ow of opportunity: targeting the Wnt/β-catenin pathway in breast cancer. Current drug targets 2010;11(9):1074–88. [DOI] [PubMed] [Google Scholar]
- 20.Thakur R, Mishra DP. Pharmacological modulation of beta‐catenin and its applications in cancer therapy. Journal of cellular and molecular medicine 2013;17(4):449–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luu HH, Zhang R, Haydon RC, Rayburn E, Kang Q, Si W, et al. Wnt/β-catenin signaling pathway as novel cancer drug targets. Current cancer drug targets 2004;4(8):653–71. [DOI] [PubMed] [Google Scholar]
- 22.Cortes J, Pérez-García J, Whiting S, Wan Y, Solem C, Tai M-H, et al. Quality-Adjusted Survival With nab-Paclitaxel Versus Standard Paclitaxel in Metastatic Breast Cancer: A Q-TWiST Analysis. Clinical breast cancer 2018. [DOI] [PubMed] [Google Scholar]
- 23.Perez EA. Paclitaxel in breast cancer. The oncologist 1998;3(6):373–89. [PubMed] [Google Scholar]
- 24.Guastalla J Iii, Dieras V. The taxanes: toxicity and quality of life considerations in advanced ovarian cancer. British journal of cancer 2003;89(S3):S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan M, Rong Y, Su Q, Chen Y. Artesunate induces apoptosis via inhibition of STAT3 in THP-1 cells. Leukemia research 2017;62:98–103. [DOI] [PubMed] [Google Scholar]
- 26.Kim JH, Cho EB, Lee J, Jung O, Ryu BJ, Kim SH, et al. Emetine inhibits migration and invasion of human non-small-cell lung cancer cells via regulation of ERK and p38 signaling pathways. Chemico-biological interactions 2015;242:25–33. [DOI] [PubMed] [Google Scholar]
- 27.Huang C-H, Lee Y-C, Chen Y-J, Wang L-J, Shi Y-J, Chang L-S. quinacrine induces the apoptosis of human leukemia U937 cells through Foxp3/mir-183/β-trcp/sp1 axis-mediated Bax upregulation. Toxicology and applied pharmacology 2017;334:35–46. [DOI] [PubMed] [Google Scholar]
- 28.Verma S, Das P, Kumar VL. Chemoprevention by artesunate in a preclinical model of colorectal cancer involves down regulation of β-catenin, suppression of angiogenesis, cellular proliferation and induction of apoptosis. Chemico-biological interactions 2017;278:84–91. [DOI] [PubMed] [Google Scholar]
- 29.Ashton TM, McKenna WG, Kunz-Schughart LA, Higgins GS. Oxidative phosphorylation as an emerging target in cancer therapy. Clinical Cancer Research 2018:clincanres. 3070.2017. [DOI] [PubMed] [Google Scholar]
- 30.Tian S, Chen H, Tan W. Targeting mitochondrial respiration as a therapeutic strategy for cervical cancer. Biochemical and biophysical research communications 2018. [DOI] [PubMed] [Google Scholar]
- 31.Xiang M, Kim H, Ho VT, Walker SR, Bar-Natan M, Liu S, et al. Gene expression–based discovery of atovaquone as a STAT3 inhibitor and anticancer agent. Blood 2016;128(14):1845–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gupta P, Srivastava SK. Antitumor activity of phenethyl isothiocyanate in HER2-positive breast cancer models. BMC medicine 2012;10(1):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verma K, Gupta N, Zang T, Wangtrakuldee P, Srivastava SK, Penning TM, et al. AKR1C3 Inhibitor KV-37 Exhibits Antineoplastic Effects and Potentiates Enzalutamide in Combination Therapy in Prostate Adenocarcinoma Cells. Molecular cancer therapeutics 2018:molcanther. 1023.2017. [DOI] [PubMed] [Google Scholar]
- 34.Pramanik KC, Fofaria NM, Gupta P, Srivastava SK. CBP-mediated FOXO-1 acetylation inhibits pancreatic tumor growth by targeting SirT. Molecular cancer therapeutics 2014;13(3):687–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verma K, Zang T, Gupta N, Penning TM, Trippier PC. Selective AKR1C3 Inhibitors Potentiate Chemotherapeutic Activity in Multiple Acute Myeloid Leukemia (AML) Cell Lines. ACS Med Chem Lett 2016;7(8):774–9 doi 10.1021/acsmedchemlett.6b00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pramanik KC, Srivastava SK. Apoptosis signal-regulating kinase 1–thioredoxin complex dissociation by capsaicin causes pancreatic tumor growth suppression by inducing apoptosis. Antioxidants & redox signaling 2012;17(10):1417–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ranjan A, Gupta P, Srivastava SK. Penfluridol: an antipsychotic agent suppresses metastatic tumor growth in triple-negative breast cancer by inhibiting integrin signaling axis. Cancer research 2016;76(4):877–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sahu RP, Srivastava SK. The role of STAT-3 in the induction of apoptosis in pancreatic cancer cells by benzyl isothiocyanate. Journal of National Cancer Institute 2009;101(3):176–93 doi djn470 [pii] 10.1093/jnci/djn470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siddiqa A, Long LM, Li L, Marciniak RA, Kazhdan I. Expression of HER-2 in MCF-7 breast cancer cells modulates anti-apoptotic proteins Survivin and Bcl-2 via the extracellular signal-related kinase (ERK) and phosphoinositide-3 kinase (PI3K) signalling pathways. BMC cancer 2008;8(1):129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Asada S, Choi Y, Yamada M, Wang S-C, Hung M-C, Qin J, et al. External control of Her2 expression and cancer cell growth by targeting a Ras-linked coactivator. Proceedings of the National Academy of Sciences 2002;99(20):12747–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gupta P, Srivastava SK. HER2 mediated de novo production of TGFβ leads to SNAIL driven epithelial‐to‐mesenchymal transition and metastasis of breast cancer. Molecular oncology 2014;8(8):1532–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu J, Ma R, Cao H, Wang Z, Jing C, Sun Y, et al. Intraoperative imaging of metastatic lymph nodes using a fluorophore-conjugated antibody in a HER2/neu-expressing orthotopic breast cancer mouse model. Anticancer research 2013;33(2):419–24. [PubMed] [Google Scholar]
- 43.Wang J, Li G, Wang Y, Tang S, Sun X, Feng X, et al. Suppression of tumor angiogenesis by metformin treatment via a mechanism linked to targeting of HER2/HIF-1α/VEGF secretion axis. Oncotarget 2015;6(42):44579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walz A, Ugolkov A, Chandra S, Kozikowski A, Carneiro BA, O’Halloran TV, et al. Molecular Pathways: Revisiting Glycogen Synthase Kinase-3beta as a Target for the Treatment of Cancer. Clin Cancer Res 2017;23(8):1891–7 doi 10.1158/1078-0432.ccr-15-2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ugolkov A, Gaisina I, Zhang JS, Billadeau DD, White K, Kozikowski A, et al. GSK-3 inhibition overcomes chemoresistance in human breast cancer. Cancer Lett 2016;380(2):384–92 doi 10.1016/j.canlet.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim HM, Kim CS, Lee JH, Jang SJ, Hwang JJ, Ro S, et al. CG0009, a novel glycogen synthase kinase 3 inhibitor, induces cell death through cyclin D1 depletion in breast cancer cells. PLoS One 2013;8(4):e60383 doi 10.1371/journal.pone.0060383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moasser MM. The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene 2007;26(45):6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhan T, Rindtorff N, Boutros M. Wnt signaling in cancer. Oncogene 2017;36(11):1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosenberg DM, McCarthy W, Slavinsky J, Chan CK, Montaner J, Braun J, et al. Atovaquone suspension for treatment of Pneumocystis carinii pneumonia in HIV-infected patients. Aids 2001;15(2):211–4. [DOI] [PubMed] [Google Scholar]
- 50.Lundgren J, Dragsted U. Atovaquone/proguanil. Prophylaxis and treatment of malaria. Ugeskrift for laeger 2000;162(31):4177–81. [PubMed] [Google Scholar]
- 51.Srivastava IK, Rottenberg H, Vaidya AB. Atovaquone, a broad spectrum antiparasitic drug, collapses mitochondrial membrane potential in a malarial parasite. Journal of Biological Chemistry 1997;272(7):3961–6. [DOI] [PubMed] [Google Scholar]
- 52.Perrier A, Gligorov J, Lefèvre G, Boissan M. The extracellular domain of Her2 in serum as a biomarker of breast cancer. Laboratory Investigation 2018:1. [DOI] [PubMed] [Google Scholar]
- 53.Zhang X, Hao J. Development of anticancer agents targeting the Wnt/β-catenin signaling. American journal of cancer research 2015;5(8):2344. [PMC free article] [PubMed] [Google Scholar]
- 54.Nixon GL, Moss DM, Shone AE, Lalloo DG, Fisher N, O’neill PM, et al. Antimalarial pharmacology and therapeutics of atovaquone. journal of Antimicrobial Chemotherapy 2013;68(5):977–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sabchareon A, Attanath P, Phanuaksook P, Chanthavanich P, Poonpanich Y, Mookmanee D, et al. Efficacy and pharmacokinetics of atovaquone and proguanil in children with multidrug-resistant Plasmodium falciparum malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene 1998;92(2):201–6. [DOI] [PubMed] [Google Scholar]
- 56.Dohn MN, Weinberg WG, Torres RA, Follansbee SE, Caldwell PT, Scott JD, et al. Oral atovaquone compared with intravenous pentamidine for Pneumocystis carinii pneumonia in patients with AIDS. Annals of internal medicine 1994;121(3):174–80. [DOI] [PubMed] [Google Scholar]
- 57.Baggish AL, Hill DR. Antiparasitic agent atovaquone. Antimicrobial agents and chemotherapy 2002;46(5):1163–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fiorillo M, Lamb R, Tanowitz HB, Mutti L, Krstic-Demonacos M, Cappello AR, et al. Repurposing atovaquone: targeting mitochondrial complex III and OXPHOS to eradicate cancer stem cells. Oncotarget 2016;7(23):34084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lv Z, Yan X, Lu L, Su C, He Y. Atovaquone enhances doxorubicin’s efficacy via inhibiting mitochondrial respiration and STAT3 in aggressive thyroid cancer. Journal of bioenergetics and biomembranes 2018:1–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.