Abstract
Objective:
To evaluate racial and ethnic differences in women’s postpartum pain scores, inpatient opioid administration, and discharge opioid prescriptions.
Methods:
We conducted a retrospective cohort study of all deliveries at a single, high-volume tertiary care center from December 1, 2015, through November 30, 2016. Women were included if they self-identified as non-Hispanic white, non-Hispanic black, or Hispanic, were at least 18 years of age, and did not have documented allergies to non-steroidal anti-inflammatory drugs or morphine. Medical records were queried for three outcomes: 1) patient-reported postpartum pain score (on a scale of 0 to 10) at discharge (dichotomized <5 or ≥5); 2) inpatient opioid dosing during postpartum hospitalization (reported as morphine milligram equivalents per postpartum day); and 3) receipt of an opioid prescription at discharge. The associations between each of these outcomes and maternal race–ethnicity were assessed using multivariable logistic regression models with random effects to account for clustering by discharge physician. A sensitivity analysis was conducted in which women of different race and ethnicity were matched using propensity scores.
Results:
A total of 9,900 postpartum women were eligible for analysis. Compared to non-Hispanic white women, Hispanic and non-Hispanic black women had significantly greater odds of reporting a pain score of ≥ 5 (aOR 1.61; 95% 1.26 to 2.06 and aOR 2.18; 95% 1.63 to 2.91, respectively), but received significantly fewer inpatient morphine milligram equivalents/day (aβ −5.03; 95% CI −6.91 to −3.15 and aβ −3.54; 95% CI −5.88 to −1.20, respectively). Additionally, Hispanic and non-Hispanic black women were significantly less likely to receive an opioid prescription at discharge (aOR 0.80; 95% CI 0.67 to-0.96 and aOR 0.78; 95% CI 0.62 to 0.98) compared to non-Hispanic white women. Results of the propensity score analysis largely corroborated those of primary analysis, with the exception that the difference in inpatient morphine milligram equivalents/day between non-Hispanic white and non-Hispanic black women did not reach statistical significance.
Conclusion:
Hispanic and non-Hispanic black women experience disparities in pain management in the postpartum setting that cannot be explained by less perceived pain.
PRÉCIS
Hispanic and non-Hispanic black women experience postpartum disparities in pain management that cannot be explained by less perceived pain.
INTRODUCTION
The United States (U.S.) government has declared opioid misuse a national emergency.1 Multiple studies have reported on the relatively higher impact of this epidemic among non-Hispanic white communities.2–5 In fact, a recent population-based study using data from California’s prescription drug monitoring program reported a nearly 300% difference in opioid prescription prevalence among individuals of different race–ethnicity. 2 Adults from communities with a greater proportion of white individuals were significantly more likely to receive at least 1 opioid prescription than those from lower proportion-white communities.2 Furthermore, these prescription patterns mirrored the observed rates of opioid overdoses.2
The reasons underlying these disparities in opioid prescribing have not been fully explained. However, several studies demonstrate health care disparities in treatment of pain for individuals of minority race and ethnicity. In particular, minority individuals have been shown to receive less opioid treatment than non-Hispanic white individuals for similar levels of pain and similar conditions.6,7 For example, one study examining 36.5 million emergency department visits found that non-Hispanic black individuals were significantly less likely to receive an opioid prescription at discharge for “subjective” pain complaints (e.g. back pain, abdominal pain), but not for “objective” pain complaints (e.g. kidney stones, fractures).8 Such differential prescribing may underlie some of the demonstrated disparities in the present opioid epidemic.
Whether such racial and ethnic disparities in pain management exist in the postpartum setting remains unknown, although many other racial and ethnic disparities in obstetric health services have been demonstrated.9 We hypothesize that similar disparities in pain management may exist with regard to postpartum inpatient and outpatient pain management. Thus, our aim was to evaluate racial and ethnic differences in women’s postpartum pain scores, inpatient opioid administration, and discharge opioid prescriptions in a large and diverse cohort.
METHODS
We conducted a retrospective cohort study of women who were hospitalized for delivery at a single, high volume tertiary care center over a one-year period from December 1, 2015 to November 30, 2016. Women were included in this analysis if they were 18 years of age or greater, self-identified as non-Hispanic white, non-Hispanic black or Hispanic, and did not have documented allergies to non-steroidal anti-inflammatory drugs (NSAID) or morphine. Women were excluded if they had explicit evidence of recent opioid use, defined as having been prescribed 3 or more prescriptions for an opioid in the year prior to delivery. Additionally, women were excluded if they carried a diagnosis of opioid abuse or if they had a prescription for buprenorphine, methadone, or fentanyl within a year of hospitalization. Women in other racial or ethnic groups or who had unknown self-reported race–ethnicity were excluded. This group was excluded, as the additional racial and ethnic subgroups were individually of small sample sizes, and analyzing them together was not conceptually logical. Finally, we excluded women if they received general anesthesia, were admitted to the intensive care unit, underwent a hysterectomy, or had a postpartum admission exceeding 10 days, as these conditions represent rare events10 and may reasonably be expected to result in analgesic requirements.
Electronic medical records were queried for demographic, clinical, and pharmacy data. Billing records, including International Classification of Diseases (ICD) 9 and ICD 10 codes were used to corroborate documented diagnoses of postpartum complications, substance abuse and psychiatric comorbidities. Pharmacy records were used to determine the amount of oral opioids administered during the inpatient postpartum hospitalization. This amount was standardized for differing postpartum lengths of stay by dividing the total amount of opioid used by days of hospital admission calculated to the closest hour. The details of all oral opioids were abstracted, including the type of opioid as well as the strength and number of tablets. All opioids were converted to oral morphine milligram equivalents (MME) to allow for comparison across types of opioids.11 Parenteral opioids were not included in order to decrease the likelihood of including acute immediate post-delivery analgesia needs, as it is exceedingly rare at our institution to use parenteral opioids outside of the immediate perioperative period. Whether a discharge opioid prescription was provided at discharge was also abstracted from medial and pharmacy records.
At this academic, tertiary medical center, more than 200 prescribers care for patients. Health care providers include resident and fellow physicians training in obstetrics and gynecology, emergency medicine, and midwives, advanced nurse practitioners, and both privately- and university-employed attending physicians. The patient’s obstetrical team is primarily responsible for managing postpartum pain. At the time of the study, there were no standard order sets for pain medicine prescriptions at the time of hospital discharge and no existing hospital guidelines about postpartum opioid prescriptions for either inpatient or outpatient use.
Three outcomes were evaluated for this analysis: 1) patient-reported postpartum pain score at discharge (dichotomized <5 or ≥5 on a 0 to 10 scale); 2) inpatient oral opioid dosing during postpartum hospitalization (reported as mean MME per postpartum day); and 3) receipt of an opioid prescription at discharge.
Pain scores are assessed and recorded by nurses at scheduled intervals of 8 hours, before pain medications are received, and upon discharge; women are asked to report their current perception of pain with 0 equating no pain and 10 equating the worst pain imaginable. In order to assess perceived pain by race–ethnicity, we compared women who reported a pain score of less than 5 out of 10 to those who reported a pain score of equal to or greater than 5 out of 10 (representing moderate to severe pain12) at the time of discharge. Pain scores were dichotomized at this point because a score of 5 or greater generally represents pain requiring analgesia for treatment.
Patient demographic and clinical characteristics were compared across racial and ethnic groups using chi-squared and ANOVA tests were used as appropriate. The associations of patient-reported postpartum pain score at discharge, inpatient opioid dosing during postpartum hospitalization, and receipt of opioid prescription at discharge with maternal race–ethnicity were assessed using multivariable regression models with random effects to account for clustering by discharge provider. Covariates in these models were those characteristics that were found to be significantly different (p<0.05) by race–ethnicity on bivariable analyses. Multivariable models compared non-Hispanic black and Hispanic women to non-Hispanic white women. In order to evaluate whether any observed disparity differed according to route of delivery, interaction terms between each race–ethnicity category and vaginal delivery were entered into the multivariable regression, and retained if they were significant, with the plan to perform additional stratified analyses by route of delivery for those outcomes. Finally, a sensitivity analysis was conducted in which women of different race and ethnicity were matched using propensity scores. All analyses were conducted using Stata 15 (StataCorp, College Station, TX). The Northwestern University Institutional Review Board approved this study.
RESULTS
A total of 9,900 women were eligible for analysis. The majority (68.4%) identified as non-Hispanic white, 21.0% as Hispanic, and 10.6% as non-Hispanic black (Figure 1). The population was approximately half (48.3%) nulliparous, and the majority delivered vaginally (73.4%). Non-Hispanic white women were significantly older and of lower body mass index, and were significantly more likely to be married, nulliparous, have private insurance, to smoke tobacco, and to have a history of depression or anxiety compared to Hispanic and non-Hispanic black patients. Non-Hispanic white women were less likely to have a history of non-opioid substance abuse (Table 1). Non-Hispanic white women were also less likely to have had a prior cesarean delivery (p<0.001).
Figure 1:
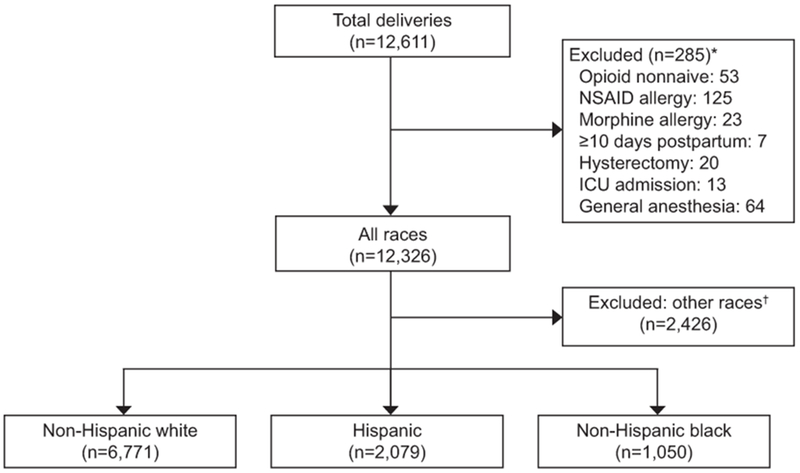
Cohort flowchart. *Items not mutually exclusive. †Includes race reported as Asian, other, unknown, or missing. NSAID, nonsteroidal anti-inflammatory drug; ICU, intensive care unit.
Table 1:
Cohort characteristics by race/ethnicity
| Characteristics | Non-Hispanic white | Hispanic | Non-Hispanic black | p-value |
|---|---|---|---|---|
| (n=6771) | (n=2079) | (n=1050) | ||
| Age, years | 33.4 ± 3.9 | 29.6 ± 5.7 | 29.9 ± 6.2 | <0.001 |
| Married * | 5,892 (88.3) | 1,074 (52.2) | 391 (38.0) | <0.001 |
| Body mass index, k/m2 | 29.5 ± 4.9 | 32.4 ± 6.2 | 33.5 ± 7.1 | <0.001 |
| Nulliparous | 3,513 (55.9) | 802 (38.6) | 464 (44.2) | <0.001 |
| Public insurance † | 2,565 (38.4) | 1,422 (69.5) | 680 (65.8) | <0.001 |
| History of tobacco use ‡ | 743 (11.1) | 173 (8.5) | 98 (9.5) | 0.002 |
| History of substance use | 41 (0.6) | 10 (0.5) | 13 (1.2) | 0.034 |
| History of depression | 935 (13.8) | 197 (9.5) | 80 (7.6) | <0.001 |
| History of anxiety | 1,019 (15.1) | 188 (9.0) | 71 (6.8) | <0.001 |
| Gestational age at delivery, weeks | 39.2 (38.5-40.1) | 39.2 (38.4-40.1) | 39.1 (38.0-40.0) | <0.001 |
| Vaginal delivery | 4,968 (73.4) | 1,566 (75.3) | 729 (69.4) | 0.002 |
| Prior cesarean delivery | 830 (12.3) | 295 (14.2) | 186 (17.7) | <0.001 |
| Health care provider training | <0.001 | |||
| Attending Physician | 4,970 (73.7) | 448 (42.9) | 1,193 (57.5) | |
| Advanced Practitioner | 1,632 (24.2) | 196 (18.8) | 625 (30.1) | |
| Trainee Physician | 144 (2.1) | 400 (38.3) | 254 (12.3) |
Data provided as mean (± standard deviation), N (%) or median (interquartile range).
Total 9760
Total 9754
Total 9733
On bivariable analysis, non-Hispanic white women were significantly less likely to report a pain score at discharge of ≥5 (4.2%) than both Hispanic (7.7%) and non-Hispanic black (11.8%) women (p<0.001). Yet, non-Hispanic white women received significantly greater MME/day as inpatients (median 24.8; IQR 12.2-39.6) than Hispanic (median 19.4; IQR 8.7-32.7) and non-Hispanic black (median 23.5; IQR 11.0-36.1) women (p<0.001). Non-Hispanic white women were also more likely to receive an opioid prescription at discharge (46.8%) than Hispanic (38.9%) and non-Hispanic black (45.3%) women (p<0.001) (Table 2).
Table 2:
Postpartum pain, inpatient opioid per day and Opioid prescription at discharge opioid prescription by race/ethnicity
| Characteristics | Non-Hispanic white | Hispanic | Non-Hispanic black | p-value |
|---|---|---|---|---|
| (n=6771) | (n=2079) | (n=1050) | ||
| Pain score ≥ 5 § | 282 (4.2) | 160 (7.7) | 124 (11.8) | <0.001 |
| Inpatient MME/day ‖ | 24.8 (12.2-39.6) | 19.4 (8.7-32.7) | 23.5 (11.0-36.1) | <0.001 |
| Opioid prescription at discharge | 3,171 (46.8) | 809 (38.9) | 476 (45.3) | <0.001 |
Data provided as N (%) or median (interquartile range).
Self-reported pain at the time of discharge on a scale of 0 (none) to 10 (most severe)
N for inpatient MME/day: NHW = 3,141, Hispanic = 945, NWB = 540
On multivariable analyses, the majority of significant associations from bivariable analysis remained significant (Table 3). Specifically, after we adjusted for age, marital status, nulliparity, gestational age, body mass index, public insurance, tobacco use, anxiety, depression, substance use, mode of delivery, and prior cesarean delivery, Hispanic women (compared to non-Hispanic white women) had significantly greater odds of reporting a pain score of ≥5 (aOR 1.61; 95% 1.26-2.06), but received significantly fewer adjusted inpatient MME/day (aβ −5.03; 95% CI −6.91 to −3.15) and had significantly lower odds of receiving an opioid prescription at discharge (aOR 0.80; 95% CI 0.67-0.96). Similarly, non-Hispanic black women had significantly greater odds of reporting a pain score ≥5 (aOR 2.18; 95% 1.63-2.91), but received significantly fewer adjusted inpatient MME/day (aβ −3.54; 95% CI −5.88 to −1.20) and had significantly lower odds of receiving an opioid prescription at discharge (aOR 0.78; 95% CI 0.62-0.98).No interaction terms between race–ethnicity and route of delivery were significant, indicating that the observed disparities did not differ according to route of delivery.
Table 3:
Multivariable and propensity matched analysis of postpartum pain, inpatient opioid per day and discharge opioid prescription by race/ethnicity
| Hispanic | Non-Hispanic black | |||||
|---|---|---|---|---|---|---|
| OR, 95% CI | aOR1, 95% CI | Propensity matched2 OR, 95% CI | OR, 95% CI | aOR1, 95% CI | Propensity matched3 OR, 95% CI | |
| Pain score ≥ 5‖ | 1.88, 1.54 to 2.29 | 1.61, 1.26 to 2.06 | 1.60, 1.23 to 2.09 | 3.12, 2.52 to 3.88 | 2.18, 1.63 to 2.91 | 2.94, 2.06 to 4.09 |
| Opioid prescription at discharge | 0.74, 0.67 to 0.82 | 0.80, 0.67 to 0.96 | 0.72, 0.62 to 0.84 | 0.99, 0.87 to 1.12 | 0.78, 0.62 to 0.98 | 0.81, 0.66 to 0.99 |
| β, 95% CI | Aβ1, 95% CI | Propensity matched2 β, 95% CI | β, 95% CI | Aβ1, 95% CI | Propensity matched3 β, 95% CI | |
| Inpatient MME/day | −4.82,−6.58 to −3.07 | −5.03, −6.91 to −3.15 | −4.74, −6.58 to −2.91 | −1.22, −3.40 to 0.95 | −3.54, −5.88 to −1.20 | −1.22, −3.29 to 0.85 |
MME, morphine milligram equivalents; OR, odds ratio; CI, confidence interval; aOR, adjusted odds ratio; β, beta coefficient; aβ, adjusted beta coefficient
OR and β in referent to non-Hispanic white race/ethnicity.
Adjusted: Multivariable regression accounting for age, marital status, nulliparity, gestational age, body mass index, public insurance, tobacco use, anxiety, depression, substance use, mode of delivery, prior cesarean delivery, health care provider type (attending physician, trainee physician, advanced practitioner and clustering of patients at the health care provider level.
Propensity matched model for Hispanic adjusted for gestational age, nulliparity, insurance at delivery, tobacco use, anxiety, depression, mode of delivery, and prior cesarean delivery.
Propensity matched model for non-Hispanic black adjusted for insurance at delivery, anxiety, depression and mode of delivery.
Self-reported pain at the time of discharge on a scale of 0 (none) to 10 (most severe)
In the propensity score-matched cohort analysis, there were no significant differences in matched patient characteristics that persisted after nearest neighbor matching (Table 4). In the propensity score analysis, findings were overall similar to the primary analysis. Hispanic women were significantly more likely than non-Hispanic white women to report pain scores ≥5 (OR 1.60; 95% CI 1.23 to 2.09), received significantly fewer adjusted inpatient MME/day (β −4.74; 95% CI −6.58 to −2.91) and were significantly less likely to receive an opioid prescription at discharge (OR. 0.72; 95% CI 0.62 to 0.84). Non-Hispanic black women were significantly more likely than non-Hispanic white women to report pain scores ≥5 (OR 2.94; 95% CI 2.06 to 4.09) and were significantly less likely to receive an opioid prescription at discharge (OR. 0.81; 95% CI −0.66 to 0.99). The difference in inpatient MME/day between non-Hispanic white and non-Hispanic black women no longer reached statistical significance.
Table 4:
Propensity matched cohort characteristics by race/ethnicity
| Non-Hispanic white (n=1,994) | Hispanic (n=1,994) | p-value | |
|---|---|---|---|
| Nulliparous | 793 (39.8)) | 777 (39.0) | 0.603 |
| Public insurance | 1,381 (69.3) | 1,386 (69.5) | 0.864 |
| History of tobacco use | 163 (8.2) | 171 (8.6) | 0.647 |
| History of depression | 163 (8.2) | 190 (9.5) | 0.132 |
| History of anxiety | 158 (7.9) | 179 (9.0) | 0.232 |
| Vaginal delivery | 1,508 (75.6) | 1,504 (75.4) | 0.883 |
| Prior cesarean delivery | 259 (13.0) | 281 (14.1) | 0.307 |
| Gestational age at delivery, weeks | 38.8 ± 2.3 | 38.8 ± 2.4 | 0.956 |
| Outcomes | |||
| Pain score ≥ 5 § | 85 (4.3) | 151 (7.6) | <0.001 |
| Opioid prescription at discharge | 876 (43.9) | 767 (38.5) | <0.001 |
| Inpatient MME/day * | 12.5 ± 20.5 | 10.7 ± 17.9 | 0.003 |
| Non-Hispanic white (n=1,033) | Non-Hispanic black (n=1,033) | p-value | |
| Public insurance | 680 (65.8) | 680 (65.8) | 1 |
| History of depression | 78 (7.6) | 78 (7.6) | 1 |
| History of anxiety | 69 (6.7) | 69 (6.7) | 1 |
| Vaginal delivery | 721 (69.8) | 721 (69.8) | 1 |
| Outcomes | |||
| Pain score ≥ 5 § | 42 (4.1) | 122 (11.8) | <0.001 |
| Opioid prescription at discharge | 511 (49.5) | 462 (44.7) | 0.031 |
| Inpatient MME/day ‖ | 27.8 ± 20.5 | 27.4 ± 22.9 | 0.807 |
Data provided as N (%) or mean ± standard deviation
Self-reported pain at the time of discharge on a scale of 0 (none) to 10 (most severe)
N for inpatient MME/day: NHW = 861, Hispanic = 902
N for inpatient MME/day: NHW = 501, NHB = 528
DISCUSSION
In this study, we have demonstrated that Hispanic and non-Hispanic black women experience disparities in pain management in the postpartum setting that cannot be explained by less perceived pain. Even after we adjusted for potentially confounding covariates, both non-Hispanic black and Hispanic women were significantly more likely than non-Hispanic white women to report a pain score of at least 5. Despite this, Hispanic women used significantly fewer inpatient MME/day. Hispanic and non-Hispanic black women also had lower odds of receiving an opioid prescription upon discharge compared to non-Hispanic white women. Although the significant odds ratios found lie within the zone of potential bias13, these findings remain notable. The absolute differences in inpatient MME/day and rates of opioid prescriptions at discharge between non-Hispanic white and non-Hispanic black or Hispanic patients is small, this remains clinically significant in context; one may reasonably expect that groups reporting greater amount of pain would not only receive equal amount of pain management in the form of equal inpatient MME/day and rates of opioids prescriptions at discharge, but would receive an even greater amount of pain management, thus making these results especially poignant.
Racial and ethnic disparities in health care delivery and outcomes have been extensively documented in the literature.9 These disparities have extended to pain management, with racial and ethnic minority non-pregnant individuals having increased risk of less aggressive analgesia treatment. One retrospective study of data from more than 6,700 emergency department visits showed that non-Hispanic black patients were 22-40% less likely to receive analgesia for moderate to severe pain than non-Hispanic white patients.6 In another recent study, which examined disparities in the management of pain among over 7,000 individuals in the outpatient setting, showed that non-Hispanic black and Hispanic individuals were significantly less likely to be prescribed an opioid for management of abdominal pain (6.0% and 6.3% less likely, respectively) and back pain (7.1% and 14.8% less likely, respectively).7 Additionally, studies have shown that minority patients who present with migraines and long bone fractures receive less pain medication than non-Hispanic white patients. Although such disparities in pain control have been understudied in the obstetric setting, out data suggest that postpartum women experience similar care disparities.
Other racial and ethnic disparities in obstetrical care have been well documented. For example, a study of more than 100,000 peripartum women across 25 hospitals in the U.S demonstrated disparities in obstetrical care. Specifically, non-Hispanic white women were more likely to undergo induction of labor and initiate immediate pushing during the second stage of labor and less likely to receive general anesthesia at the time of cesarean delivery.9 However, data are sparse with regard to whether such disparities in peripartum analgesia receipt in general, and opioid receipt more specifically, exist. One particular disparity that has been noted is with regard to neuraxial analgesia in labor; multiple studies have demonstrated that non-Hispanic white women receive this type of analgesia significantly more frequently that women of minority race–ethnicity.14–17 Based on our findings in this large cohort, this disparity in analgesia persists into the postpartum period. Despite non-Hispanic black and Hispanic women reporting significantly higher pain scores, they used significantly less opioids as inpatients and were significantly less likely to be prescribed an opioid as an outpatient when compared to non-Hispanic white women. It remains unclear, however, whether such findings are due to differential prescribing by obstetricians, differential management of pain by bedside nurses, or differential patient requests for or acceptance of opioid analgesia. Additionally, our data are unable to explore adequacy of pain management and therefore, for example, we cannot draw conclusions regarding whether minority women’s pain was under-treated or non-Hispanic white women’s’ pain was over-treated.
Our study has several strengths. Although the postpartum period is increasingly recognized as a potential source of first exposure to opioids, little is understood about equity in pain management in the postpartum period. Further, the analysis is based on information directly from a medical record with highly granular data that allowed for collection of detailed opioid use, pain score, and patient characteristics. However, results must be interpreted in light of some limitations. The study was limited to a single institution, and may not be generalizable to care models in other settings. Additionally, this study is retrospective and thus susceptible to incomplete data and misclassification. Further, we are unable to explore potential etiologies for differential inpatient MME use or differential prescribing of opioids on discharge. Further work on patterns of analgesia request, comparisons to baseline pain scores, and other health services factors such as cultural differences and language barriers may address these potential inequities. Moreover, future work on whether standardized electronic medical order sets and opioid prescribing protocols work to overcome these disparities is essential.
In summary, we evaluated racial and ethnic differences in women’s postpartum pain scores, inpatient opioid administration, and discharge opioid prescriptions. Hispanic and non-Hispanic black women experienced disparities in pain management in the postpartum setting that could not be explained by less perceived pain. These data suggest that more standardized approaches to postpartum pain management may encourage racial and ethnic equity in analgesia management.
Supplementary Material
STROBE Statement—checklist of items that should be included in reports of observational studies
| Item No | Recommendation | Page No | |
|---|---|---|---|
| Title and abstract | 1 | (a) Indicate the study’s design with a commonly used term in the title or the abstract | 4 |
| (b) Provide in the abstract an informative and balanced summary of what was done and what was found | 4 | ||
| Introduction | |||
| Background/rationale | 2 | Explain the scientific background and rationale for the investigation being reported | 5 |
| Objectives | 3 | State specific objectives, including any prespecified hypotheses | 5 |
| Methods | |||
| Study design | 4 | Present key elements of study design early in the paper | 6 |
| Setting | 5 | Describe the setting, locations, and relevant dates, including periods of recruitment, exposure, follow-up, and data collection | 6 |
| Participants | 6 | (a) Cohort study—Give the eligibility criteria, and the sources and methods of selection of participants. Describe methods of follow-up Case-control study—Give the eligibility criteria, and the sources and methods of case ascertainment and control selection. Give the rationale for the choice of cases and controls Cross-sectional study—Give the eligibility criteria, and the sources and methods of selection of participants |
6 |
| (b) Cohort study—For matched studies, give matching criteria and number of exposed and unexposed Case-control study—For matched studies, give matching criteria and the number of controls per case |
|||
| Variables | 7 | Clearly define all outcomes, exposures, predictors, potential confounders, and effect modifiers. Give diagnostic criteria, if applicable | 6-7 |
| Data sources/measurement | 8* | For each variable of interest, give sources of data and details of methods of assessment (measurement). Describe comparability of assessment methods if there is more than one group | 7 |
| Bias | 9 | Describe any efforts to address potential sources of bias | 7 |
| Study size | 10 | Explain how the study size was arrived at | 8 |
| Quantitative variables | 11 | Explain how quantitative variables were handled in the analyses. If applicable, describe which groupings were chosen and why | |
| Statistical methods | 12 | (a) Describe all statistical methods, including those used to control for confounding | 8 |
| (b) Describe any methods used to examine subgroups and interactions | |||
| (c) Explain how missing data were addressed | |||
| (d) Cohort study—If applicable, explain how loss to follow-up was addressed Case-control study—If applicable, explain how matching of cases and controls was addressed Cross-sectional study—If applicable, describe analytical methods taking account of sampling strategy |
|||
| (e) Describe any sensitivity analyses | 9 | ||
| Results | |||
| Participants | 13* | (a) Report numbers of individuals at each stage of study—eg numbers potentially eligible, examined for eligibility, confirmed eligible, included in the study, completing follow-up, and analysed | 8 |
| (b) Give reasons for non-participation at each stage | |||
| (c) Consider use of a flow diagram | 13 | ||
| Descriptive data | 14* | (a) Give characteristics of study participants (eg demographic, clinical, social) and information on exposures and potential confounders | 8 |
| (b) Indicate number of participants with missing data for each variable of interest | |||
| (c) Cohort study—Summarise follow-up time (eg, average and total amount) | |||
| Outcome data | 15* | Cohort study—Report numbers of outcome events or summary measures over time | 9 |
| Case-control study—Report numbers in each exposure category, or summary measures of exposure | |||
| Cross-sectional study—Report numbers of outcome events or summary measures | |||
| Main results | 16 | (a) Give unadjusted estimates and, if applicable, confounder-adjusted estimates and their precision (eg, 95% confidence interval). Make clear which confounders were adjusted for and why they were included | 9 |
| (b) Report category boundaries when continuous variables were categorized | |||
| (c) If relevant, consider translating estimates of relative risk into absolute risk for a meaningful time period | |||
| Other analyses | 17 | Report other analyses done—eg analyses of subgroups and interactions, and sensitivity analyses | |
| Discussion | |||
| Key results | 18 | Summarise key results with reference to study objectives | 9 |
| Limitations | 19 | Discuss limitations of the study, taking into account sources of potential bias or imprecision. Discuss both direction and magnitude of any potential bias | 11 |
| Interpretation | 20 | Give a cautious overall interpretation of results considering objectives, limitations, multiplicity of analyses, results from similar studies, and other relevant evidence | 11–12 |
| Generalisability | 21 | Discuss the generalisability (external validity) of the study results | 11 |
| Other information | |||
| Funding | 22 | Give the source of funding and the role of the funders for the present study and, if applicable, for the original study on which the present article is based | 1 |
Give information separately for cases and controls in case-control studies and, if applicable, for exposed and unexposed groups in cohort and cross-sectional studies.
Note: An Explanation and Elaboration article discusses each checklist item and gives methodological background and published examples of transparent reporting. The STROBE checklist is best used in conjunction with this article (freely available on the Web sites of PLoS Medicine at http://www.plosmedicine.org/, Annals of Internal Medicine at http://www.annals.org/, and Epidemiology at http://www.epidem.com/). Information on the STROBE Initiative is available at www.strobe-statement.org.
Acknowledgments
FUNDING:
Lynn M. Yee is supported by the NICHD K12 HD050121-11 at the time of the study.
Research reported in this publication was supported, in part, by the National Institutes of Health’s National Center for Advancing Translational Sciences, Grant Number UL1TR001422. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This study is supported by the Society for Maternal-Fetal Medicine/AMAG 2017 Health Policy Award.
Footnotes
Financial Disclosure
The authors did not report any potential conflicts of interest.
Each author has confirmed compliance with the journal’s requirements for authorship.
PRESENTATION: Presented as a poster at the 2019 Society of Maternal-Fetal Medicine 39th Annual Meeting in Las Vegas, NV (February 10-17, 2019).
REFERENCES
- 1.Azar A Renewal of Determination that a Public Health Emergency Exists. In: Services USDoHaH, ed. [Google Scholar]
- 2.Friedman J, Kim D, Schneberk T, et al. Assessment of Racial/Ethnic and Income Disparities in the Prescription of Opioids and Other Controlled Medications in California. JAMA Intern Med 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pletcher MJ, Kertesz SG, Kohn MA, Gonzales R. Trends in opioid prescribing by race/ethnicity for patients seeking care in US emergency departments. JAMA 2008;299:70–8. [DOI] [PubMed] [Google Scholar]
- 4.Jayawardhana J, Abraham AJ, Perri M. Opioid Analgesics in Georgia Medicaid: Trends in Potential Inappropriate Prescribing Practices by Demographic Characteristics, 2009-2014. J Manag Care Spec Pharm 2018;24:886–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Groenewald CB, Rabbitts JA, Hansen EE, Palermo TM. Racial differences in opioid prescribing for children in the United States. Pain 2018;159:2050–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah AA, Zogg CK, Zafar SN, et al. Analgesic Access for Acute Abdominal Pain in the Emergency Department Among Racial/Ethnic Minority Patients: A Nationwide Examination. Med Care 2015;53:1000–9. [DOI] [PubMed] [Google Scholar]
- 7.Ly DP. Racial and Ethnic Disparities in the Evaluation and Management of Pain in the Outpatient Setting, 2006–2015. Pain Med 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singhal A, Tien YY, Hsia RY. Racial-Ethnic Disparities in Opioid Prescriptions at Emergency Department Visits for Conditions Commonly Associated with Prescription Drug Abuse. PLoS One 2016;11:e0159224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grobman WA, Bailit JL, Rice MM, et al. Racial and ethnic disparities in maternal morbidity and obstetric care. Obstetrics and gynecology 2015;125:1460–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grobman WA, Bailit JL, Rice MM, et al. Frequency of and factors associated with severe maternal morbidity. Obstetrics and gynecology 2014;123:804–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain - United States, 2016. MMWR Recomm Rep 2016;65:1–49. [DOI] [PubMed] [Google Scholar]
- 12.Boonstra AM, Stewart RE, Koke AJ, et al. Cut-Off Points for Mild, Moderate, and Severe Pain on the Numeric Rating Scale for Pain in Patients with Chronic Musculoskeletal Pain: Variability and Influence of Sex and Catastrophizing. Front Psychol 2016;7:1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grimes DA, Schulz KF. False alarms and pseudo-epidemics: the limitations of observational epidemiology. Obstetrics and gynecology 2012;120:920–7. [DOI] [PubMed] [Google Scholar]
- 14.Lange EMS, Rao S, Toledo P. Racial and ethnic disparities in obstetric anesthesia. Semin Perinatol 2017;41:293–8. [DOI] [PubMed] [Google Scholar]
- 15.Toledo P, Sun J, Grobman WA, Wong CA, Feinglass J, Hasnain-Wynia R. Racial and ethnic disparities in neuraxial labor analgesia. Anesth Analg 2012;114:172–8. [DOI] [PubMed] [Google Scholar]
- 16.Rust G, Nembhard WN, Nichols M, et al. Racial and ethnic disparities in the provision of epidural analgesia to Georgia Medicaid beneficiaries during labor and delivery. Am J Obstet Gynecol 2004;191:456–62. [DOI] [PubMed] [Google Scholar]
- 17.Glance LG, Wissler R, Glantz C, Osler TM, Mukamel DB, Dick AW. Racial differences in the use of epidural analgesia for labor. Anesthesiology 2007;106:19–25; discussion 6-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


