Abstract
Objective:
HIV-associated neurocognitive disorders persist despite early antiretroviral therapy (ART) and optimal viral suppression. We examined the relationship between immunopathogenesis driven by various pathways of immune activation and discrete neurocognitive performance domains in youth with HIV (YWH).
Design:
Observational cross-sectional study
Methods:
YWH, ages 20 to 28, enrolled in Adolescent Medicine Trials Network 071/101 were assessed for biomarkers of macrophage, lymphocyte activation and vascular inflammation using ELISA/multiplex assays. Standardized neurocognitive tests were performed, and demographically-adjusted z-scores were combined to form indices of attention, motor, executive function, verbal and visuospatial memory. Cross-sectional analysis of the relationship between 18 plasma inflammatory biomarkers and each neurocognitive domain was performed. Linear regression models were fit for each combination of log-transformed biomarker value and neurocognitive domain score, and were adjusted for demographics, socioeconomic status, substance use, depression, CD4 T cell count, HIV viral load, and ART status.
Results:
Study included 128 YWH [mean age 23.8 (SD 1.7) years, 86% men, 68% African American]. Verbal and visuospatial memory domains were most significantly impaired in the cohort (z=−1.59 and −1.0, respectively). Higher sCD14 was associated with impaired visuospatial memory, which remained robust after adjusting for other biomarkers, demographics, and HIV-associated covariates. Among biomarkers of vascular inflammation, sICAM-1 was negatively associated with verbal memory and attention, while sVCAM-1 was positively associated with executive function and visuospatial memory. Specific neurocognitive domains were not associated with sCD163, LPS, or CCL2 levels.
Conclusions:
Impaired visuospatial memory in YWH is associated with immune activation, as reflected by higher sCD14.
Keywords: HIV, Youth, soluble CD14, inflammation, HIV-associated neurocognitive disorder, visuospatial memory
Introduction
Combination antiretroviral therapy (cART) greatly reduces morbidity and mortality in individuals living with HIV, including severe neurocognitive impairment such as HIV-associated dementia [1]. However, more subtle forms of HIV-associated neurocognitive disorders (HAND) [2] persist, even among those receiving effective cART [1, 3]. Asymptomatic neurocognitive impairment (ANI, ≥1 SD in ≥2 neurocognitive domains) increases risk for early development of symptomatic HAND by 2–6 fold compared to those with normal cognition in early disease stages [4]. Among older chronically HIV-infected adults with HAND, neurological dysfunction preferentially occurs within the frontostriato-thalamo-cortical systems, associated with deficits in executive functions, memory, and psychomotor speed, with relative sparing of basic language and visuoconstruction skills [5]. However, similar studies are limited in youth with HIV (YWH), particularly those who became infected through sexual transmission under the age of 24, representing 21% of new infections in the United States [6]. We performed the first analysis of pre-cART neurocognitive function involving YWH and showed that nearly two thirds of participants met criteria for a diagnosis of HAND, with 96% of those having ANI. These results were surprising because over half of the participants (61%) had baseline CD4 T cells counts > 350 cells/μl and were diagnosed with HIV for less than one year on average [7]. Furthermore, over 3 years of longitudinal follow-up, there were no specific treatment effect of early cART initiation on neurocognitive performance.[8].
Pathogenesis of HAND is multi-faceted. Virus and virus-infected leukocytes cross the blood-brain-interface and directly activate central nervous system (CNS)-resident microglia and T cells to cause neuroinflammation, neuronal death, and functional loss [9, 10]. Persistence of HIV in CNS despite longterm ART has shown to associate with poor neurocognitive performance [11]. On the other hand, immune activation has also shown to cause neuroinflammation and affect neurocognitive function. HIV-associated microbial translocation contributes to neuroinflammation via binding of LPS to TLR4/CD14, activating peripheral monocytes, which then infiltrate the CNS [12-16]. Soluble CD163 (sCD163), a marker of macrophage activation via hemoglobin-haptoglobin complex scavenger receptor pathway, has also been associated with poor neurocognitive function [17-19]. Monocytes/macrophages interact with endothelial cells contributing to vascular inflammation, atherosclerosis, and increased risk of cardiovascular events in HIV [20-24]. Elevated plasma levels of soluble intercellular adhesion molecule (sICAM) and soluble vascular cell adhesion molecule (sVCAM) reflect chronic injury to the vascular endothelium [22, 25]. In the CNS, ICAM-1 and VCAM-1 are both inducible on the surface of endothelial cells and astrocytes by pro-inflammatory cytokines released by activated microglia [26]. We have previously shown that YWH display persistent high levels of biomarkers of macrophage activation even after prolonged use of effective cART. Plasma levels of soluble CD14 (sCD14), soluble CD27 (sCD27), and sCD163 remained persistently elevated when compared to a cohort of healthy uninfected youth balanced for age, gender, and ethnicity [27, 28] Patterns of immune activation unique to YWH could mediate distinct neurocognitive domain performances. Furthermore, CNS inflammation may have long term implication for YWH as brain development continues through late adolescence into early adulthood [29].The evidence of association between inflammatory biomarkers and specific domains of neurocognitive function is limited and variable in YWH. Furthermore, few studies evaluating such associations all include perinatally infected youth with longer average duration of illness with limited neurocognitive assessments and biomarker measurements [30-33]. In this vulnerable population of ongoing cognitive development impacted by HIV, it is imperative to gather comprehensive yet specific evidence to establish immune-brain interactions with functional implications. Thus, we examined the relationship between eighteen inflammatory biomarkers and neurocognitive function in domains of attention, motor function, executive function, and memory in YWH after 3 years ART. We hypothesized that HIV-associated inflammatory biomarkers may be associated with impairment in specific neurocognitive domains.
Methods
Study Participants.
YWH were enrolled in the Adolescent Medicine Trials Network (ATN) for HIV/AIDS Interventions protocol 071/101, Assessment of Inflammatory Markers Associated with Neurocognitive Impairment in HIV-infected Adolescents, a sub-study of ATN 071, Neurocognitive Assessment in Youth Initiating ART, a 3-year longitudinal study (monitored every 24–48 weeks) enrolled at 22 urban sites throughout the United States and Puerto Rico (ClinicalTrials.gov Identifier ) [8]. Enrollment included YWH ages 18 to 24 years with behaviorally acquired HIV-infection and no previous ART. Details of the inclusion and exclusion criteria were previously described [7, 27]. A total of 182 participants were enrolled in ATN071, stratified at entry based on CD4 T cell counts into three study arms; 1) 56 participants who initiated ART with CD4 T cell counts >350 cells/μl, 2) 66 participants with CD4 T cell counts >350 cells/μl who delayed treatment until CD4 counts declined to <350 cells/μl, and 3) 59 participants with CD4 T cell counts <350 cells/μl who initiated ART at entry [7]. At week 144, following consent, 129 participants enrolled in ATN101 for a single blood draw to assess inflammatory biomarkers. Plasma RNA viral load levels, CD4 T cell counts, information on neurocognitive and other medical diagnoses, socioeconomic demographics, and medications were obtained from participant interviews and clinic records as previously described [7].
Depression and Substance Use.
Depressive symptomatology was assessed using the Beck Depression Inventory, 2nd Edition (BDI-II) [34]. Substance use frequency over the 3 months prior to the visit was obtained by self-report as previously described [35, 36]. The primary substances reported as used by the cohort included alcohol, marijuana, and tobacco. Use of inhalants, sedatives, cocaine, amphetamine, hallucinogens, and opioids was reported in <1% of the participants and was not included in the analysis.
ELISA and multiplex assays for plasma biomarkers.
Biomarkers associated with macrophage activation, vascular inflammation as well as lymphocyte activation were measured. Ten blood-based biomarkers associated with macrophage activation (sCD163, sCD14, TNFα, GM-CSF, CCL2 (monocyte chemoattractant protein-1: MCP-1)), IL-1β, IL-6, IL-8, LPS, and MMP-2) [13, 17, 37-40], four with vascular inflammation (MPO, sICAM-1, sVCAM-1, and CRP) [22, 41, 42], and four with lymphocyte activation (sCD27, IFNγ, sIL-2Rα (sCD25), and IL-10) [39, 43, 44] were measured as previously described [27]. ELISA assays were used to measure sCD14, sCD163, matrix metalloprotease-2 (MMP-2), (R&D Systems, Minneapolis, MN) and sCD27 (eBioscience, San Diego, CA). Plasma LPS was measured using the limulus amebocyte assay (LAL) Chromogenic Endpoint Assay. The remaining biomarkers were measured using the magnetic bead-based Luminex platform (Millipore, Darmstadt, Germany). Supplemental figure 1 lists lower limits of detection, inter-assay coefficients of variance, and the distributions of raw biomarker vaues for each assay.
Neurocognitive Testing.
Assessment of five neurocognitive performance domains hypothesized to be vulnerable to HIV infection was performed at week 144 as summarized in supplemental table 1. Standard scores based on normative databases were computed and converted to z-scores to allow combining of tests using a common metric, as previously described [7, 8]. Corrections provided by the z-scores differed by test and always included age, and in some cases, gender, education and/or race/ethnicity as well (supplemental table 1). Higher scores indicate better performance and lower scores indicate poorer performance. Impaired neurocognitive functioning was defined as ≥1 or ≥2 standard deviations (SDs) from published means in the direction indicating poorer performance.
Statistical analysis.
Summary statistics for neurocognitive performance, biomarkers, and potential confounders for the overall sample were calculated. For biomarkers with values below the limit of detection (LOD) that had no measured value provided by the assay, we summarized the continuous values above the LOD and provided n (%) of values that were below the LOD. For LPS, where we had estimated concentrations even if they were below the LOD, we treated these values as fully observed and not below the LOD.
The primary analysis assessed the relationships between each neurocognitive domain and primary biomarker: sCD14, sCD163, sICAM-1, and sVCAM-1. We used linear regression to fit univariable (unadjusted) and multivariable adjusted models for each neurocognitive domain and primary biomarker. The first multivariable analysis adjusted for demographic characteristics including age, gender, level of education, education risk, current employment status, annual income, race, as well as self-reported use of tobacco, cannabis, and alcohol in the past three months, BDI-II total depression score, and presence of concurrent active infections. The second multivariable adjustment included covariates from the first set, as well as HIV clinical characteristics; viral load, CD4 T-cell count, and ART status at week 144. As the antiviral drug efavirenz has been associated with neuropsychiatric side effects as well as mild/moderate neurocognitive impairment [45, 46], the ART status variable at week 144 had the following levels: efavirenz-based ART regimen, non-efavirenz-based ART regimen, and no ART regimen. Viral load was dichotomized into above or below limit of detection (< 50 copies/mL) due to the high prevalence of values below the LOD (61.7%). Regression estimates and 95% confidence intervals were used to estimate the expected change in cognitive performance index per one-unit change in the base 2 logarithm of the given biomarker.
The relationships between each neurocognitive domain and groups of related biomarkers associated with macrophage activation, vascular inflammation, and lymphocyte activation were assessed as secondary analyses. All biomarkers within a given group were included as covariates in the unadjusted model for each biomarker and the cognitive domain of interest. Several biomarkers in the secondary analysis had high proportion of values (>20%) below the LOD. Removing these biomarkers could weaken the utility of our analysis, while removing values below the LOD would deplete the sample size and increase uncertainty in our regression estimates. Therefore, we used a likelihood-based method to account for values below the LOD [47]. In brief, we first generated likely values for biomarkers below the LOD, based off the observed values. Then we found regression estimates that best fit both observed and generated values.
All analyses were conducted in SAS 9.4 (Cary, NC) and R (R Core Team 2018).
Results
Demographic characteristics.
At week 144 visit, 128 of the 129 consented participants had biomarker measurements and neurocognitive test results available for analysis (Tables 1-3). On average, participants were aged 23.8 ± 1.7 years, predominantly male (86%) and African American (68%). At study entry, mean time since HIV diagnosis was 10.8 months based on medical history [27]. At week 144, over half of participants (54%) had at least some college education, but 38% were identified as having histories of education risk (repeating a grade, receiving special education). Nearly two-thirds (64%) were currently employed; however, 63% reported earning less than $12,000 annually. Most participants were on ART (83%), and 61% had undetectable plasma viral load (<50 copies/mL). Of those with detectable virus, median viral load was 7,856 (IQR 27,029) copies/mL. Mean CD4 T cell count was 658 ± 266 cells/μl (33.06 ± 9.85%), with most participants (84%) having CD4 T cell counts of greater than 350 cells/μL. Approximately half (51%) had at least one concurrent active infection that could affect biomarkers of inflammation (Table 1). Depressive mood was assessed using BDI-II (mean 10.20 ± 11.53), with most reporting minimal (≤13; 75.8%) to mild (14–19; 10.2%) symptomatology. The most frequently reported weekly to daily substance used in the 3 months prior to week 144 visit was tobacco (43%), followed by cannabis (39%) and alcohol (26%).
Table 1: Demographic variables, HIV clinical characteristics, concurrent active infections, and psychiatric characteristics in YWH (n=128).
Values are mean (standard deviation) unless otherwise noted.
| Demographics | |
| Age, years | 23.80 (1.73) |
| Gender, Malea | 110 (85.9%) |
| African American Racea | 87 (68.0%) |
| Level of education: Some college or morea | 69 (53.9%) |
| Education riska: Repeated a grade/ Received special education | 48 (37.5%) |
| Currently employeda | 82 (64.1%) |
| Past year’s annual income < $12,000a | 81 (63.3%) |
| HIV Clinical Characteristics | |
| On ART at Week 144a | 104 (81.3%) |
| NNRTI-containing regimen | 28 (26.9%) |
| PI-containing regimen | 74 (71.2%) |
| Regimen not containing NNRTI or PI | 2 (1.9%) |
| Viral load (copies/mL)b | 7856 (27029) |
| Below Limit of Detectiona | 79 (61.7%) |
| CD4 T Cell Count (cells/μL) | 658 (266) |
| CD4 Percentage | 33.06 (9.85) |
| Concurrent Active Infectionsa | |
| Herpes | 9 (7.0%) |
| Human Papillomavirus (HPV) / Molluscum Contagiosum Virus (MCV)c | 39 (30.5%) |
| Syphilis | 7 (5.5%) |
| Other Sexually Transmitted Infection (STI)d | 5 (3.9%) |
| Fungal Infectionse | 13 (10.2%) |
| Bacterial Infectionsf | 19 (14.8%) |
| Viral Hepatitis | 1 (0.8%) |
| Participants with at least one of the above diagnoses | 65 (50.8%) |
| Psychiatric Characteristics | |
| Beck Depression Inventory – II (BDI–II) Total Score | 10.20 (11.53) |
| Tobacco useag | 55 (43.0%) |
| Alcohol useag | 33 (25.8%) |
| Cannabis useag | 50 (39.1%) |
Frequency (column percent),
Median (IQR)
Genital HPV and cutaneous MCV,
Chlamydia, Gonorrhea and Trichomonas,
Mucocutaneous candidiasis
UTI, abscesses, pneumonia, sinusitis, pharyngitis, otitis media, bacterial vaginosis or TB
Self-reported weekly or daily use over the past 3 months
NNRTI: Non-nucleoside reverse-transcriptase inhibitor
PI: Protease Inhibitor
Table 3: Standardized neurocognitive testing scores in YWH (n=128).
All values are mean z-score (standard deviation).
| Neurocognitive Test Scores | (n=128) |
|---|---|
| Motor Domain | −0.27 (1.18) |
| Digit Symbol | 0.03 (1.02) |
| Grooved Pegboard Dominant Hand Time | −0.19 (1.27) |
| Grooved Pegboard Non-Dominant Hand Time | −0.57 (1.36) |
| Timed Gait Average Time | −0.28 (2.50) |
| Attention Domain | −0.18 (0.85) |
| Digit Span | −0.17 (0.93) |
| Letter/Number Sequencing | −0.18 (0.92) |
| Executive Functioning Domain | 0.04 (0.85) |
| Trail-Making Test Part B Time | −0.15 (1.83) |
| Stroop Interference | 0.33 (0.84) |
| FAS Total Correct | 0.15 (1.08) |
| FAS Animals Correct | −0.18 (1.14) |
| Verbal Memory | −1.59 (1.10) |
| Hopkins Verbal Learning Test-R: Delayed Recall | −1.67 (1.25) |
| Hopkins Verbal Learning Test-R: Total Recall | −1.52 (1.10) |
| Visuospatial Memory | −1.00 (1.14) |
| Brief Visuospatial Memory Test-R: Delayed Recall | −0.95 (1.21) |
| Brief Visuospatial Memory Test-R: Total Recall | −1.05 (1.17) |
Domain scores are in bold
Biomarker measures.
Table 2 shows mean (± SD) biomarker levels of macrophage activation, vascular inflammation, and lymphocyte activation at the week 144. A notable proportion of the values were below the LOD (20.3 – 41.4%) for IFNɣ, IL-10, GM-CSF, IL-1β, and IL-6. In addition, there was considerable variability among several biomarkers (sCD163, CCL2, MMP-2, IL-1β, IL-8, sICAM-1, sVCAM-1, and MPO), with ranges >3 log2.
Table 2: Summary statistics for biomarkers levels in YWH (n=128).
Values are mean (standard deviation) unless otherwise noted.
| Biomarker | (n=128) |
|---|---|
| Macrophage Activation | |
| sCD14 (ng/mL) | 1468.04 (420.66) |
| sCD163 (ng/mL) | 548.40 (304.97) |
| CCL2 (pg/mL) | 209.54 (304.35) |
| GM-CSF (pg/mL) | 1.55 (2.42) |
| Below Limit of Detection (LOD 0.01 pg/mL)a | 53 (41.4%) |
| IL-1β(pg/mL) | 38.15 (116.63) |
| Below Limit of Detection (LOD 0.01 pg/mL)a | 33 (25.8%) |
| IL-6 (pg/mL) | 17.43 (74.66) |
| Below Limit of Detection (LOD 0.1 pg/mL)a | 26 (20.3%) |
| IL-8 (pg/mL) | 64.69 (291.50) |
| LPS (EU/mL) | 0.21 (0.08) |
| MMP-2 (ng/mL) | 195.64 (39.29) |
| TNFα (pg/mL) | 11.35 (25.71) |
| Vascular Inflammation | |
| sICAM-1 (ng/mL) | 215.07 (303.69) |
| sVCAM-1 (ng/mL) | 595.20 (238.83) |
| CRP (ng/mL) | 1424.01 (5517.78) |
| MPO (ng/mL) | 279.70 (270.26) |
| Lymphocyte Activation | |
| sCD27 (U/mL) | 57.39 (36.91) |
| IFNγ (pg/mL) | 20.23 (44.42) |
| Below Limit of Detection (LOD 0.1 pg/mL)a | 49 (38.3%) |
| IL-10 (pg/mL) | 8.25 (14.82) |
| Below Limit of Detection (LOD 0.1 pg/mL)a | 35 (27.3%) |
| sIL-2rα (pg/mL) | 67.91 (80.67) |
| Below Limit of Detection (LOD 0.1 pg/mL)a | 1 (0.8%) |
Frequency (percent)
Neurocognitive measures.
Table 3 shows results of neurocognitive index scores (z-scores) for each domain (motor, attention, executive function, verbal memory, and visuospatial memory), as well as individual test scores within each domain. Within the study cohort, verbal [z = −1.59 (1.10), with 69% of participants >1SD, 40% >2SD below mean] and visuospatial [z = −1.00 (1.14), with 44% >1SD, 22% >2SD below mean] memory scores were most impaired relative to age- and demographically-matched norms. In contrast, motor [z = - 0.27 (1.18), with 15.4% >1SD below mean], executive function [z = 0.04 (0.85), with 8.6% >1SD below mean], and attention [z= −0.18 (0.85), with 11.7% >1SD below mean] scores were close to published normed means.
Associations between key biomarkers and neurocognitive measures.
Linear regression models were fit in a sequential manner to account for potential confounders to evaluate associations between biomarkers of macrophage activation, vascular inflammation, and lymphocyte activation with five neurocognitive domains (figures 1 and 2). Supplemental tables 2 and 3 list numerical values of regression estimates and 95% confidence intervals.
Figure 1.
Associations between neurocognitive domain scores and primary biomarkers sCD14, sCD163, sICAM-1 and sVCAM-1. All biomarkers were log2 transformed. The parameter estimates (dots) and 95% confidence intervals (lines) represent the mean change in the neurocognitive score indices per 1-unit change in the log biomarker value. Lines not crossing 0 are significant. Mustard colored dots and lines represent univariate model with the sole biomarker. First adjustment (teal color) controls for age, gender, race, highest level of education retained, repeated grade, current employment status, past year’s income, self-reported tobacco, cannabis, and alcohol use in the past 3 months, BDI-II total score, concurrent active infections. Second adjustment (black color) controls for all covariates included in the first adjusted model as well as viral load (positive or negative), CD4 T cell count, cART status (Efavirenz-containing ART, non-Efavirenz containing ART, not on any ART) at week 144.
Figure 2.
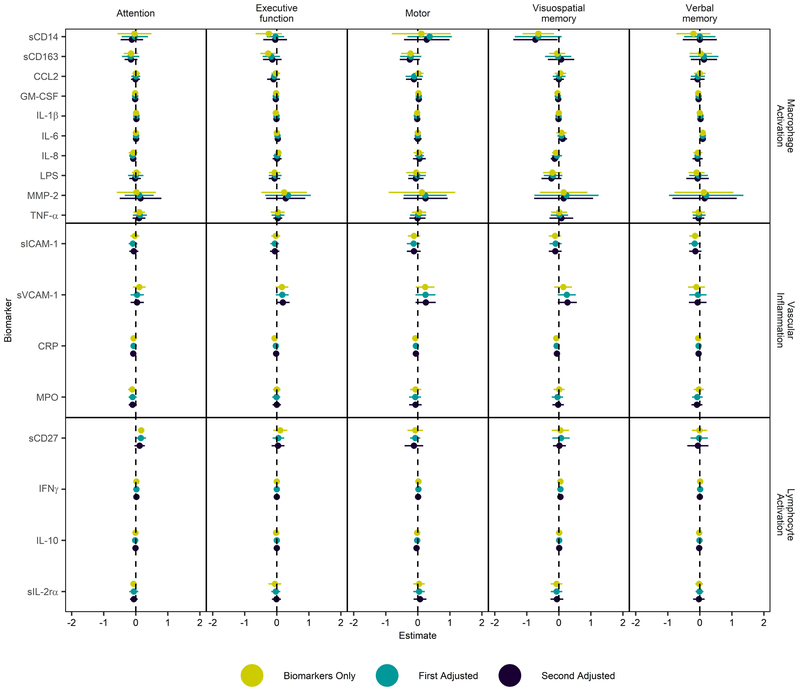
Associations between neurocognitive domain scores and 18 biomarkers of macrophage activation, vascular inflammation, and lymphocyte activation. All biomarkers were natural log-transformed. The parameter estimates (dots) and 95% confidence intervals (lines) represent the mean change in the neurocognitive score indices per 1-unit change in the log biomarker value. Lines not crossing 0 are significant. Mustard colored dots and lines represent unadjusted model that includes all biomarkers in the given group as covariates in addition to the biomarker of interest. First adjustment (teal color) controls for age, gender, race, highest level of education retained, repeated grade, current employment status, past year’s income, self-reported tobacco, cannabis, and alcohol use in the past 3 months, BDI-II total score, concurrent active infections. Second adjustment (black color) controls for all covariates included in the first adjusted model as well as viral load (positive or negative), CD4 T cell count, ART status (Efavirenz-containing ART, non-Efavirenz containing ART, not on any ART) at week 144.
Based on previous observations of YWH and studies of neurocognitive function, four key biomarkers of interest were included in the primary analysis (figure 1) [20, 27]. Biomarkers were differentially associated with separate neurocognitive domains. sCD14 was most negatively associated with visuospatial memory (−0.66; 95%CI: −1.1, −0.21). The association between sCD14 and visuospatial memory remained robust after adjusting for demographic variables, socioeconomic status, education, depressive symptoms (BDI-II score), and concurrent active infections [first adjustment; −0.66 (−1.14, −0.19)], as well as additional adjustment for HIV clinical status by viral load, CD4 T cell count, and ART status (Efavirenz-containing ART, non-Efavirenz-containing ART, and no ART) [second adjustment; −0.86 (−1.36, −0.37)]. sCD14 was also negatively associated with executive function [−0.39 (−0.73, −0.05)], but this association became inconclusive after first [−0.15 (−0.49, 0.2)] and second [−0.18 (−0.54, 0.19)] adjustments for the aforementioned factors. sCD163 was also negatively associated with executive function [−0.3 (−0.5, −0.1)], but similar to sCD14, this association was attenuated after first [−0.16 (−0.37, 0.06)] and second [−0.16 (−0.4, 0.08)] adjustments.
sVCAM-1 was weakly associated with visuospatial memory and executive function in the opposite direction relative to other key biomarkers. sVCAM-1 was positively associated with visuospatial memory after first [0.19 (−0.06, 0.44)] and second [0.23 (−0.03, 0.49)] adjustments. The positive association between sVCAM-1 and executive function [0.14 (−0.04, 0.32)] remained after the first adjustment [0.14 (−0.03, 0.32)] and strengthened with the second adjustment [0.18 (0, 0.36)]. In contrast, sICAM-1 most negatively associated with verbal memory, and to a lesser extent, attention, although estimates were small. Its association with verbal memory [−0.22 (−0.38, −0.06)] remained robust following both first [−0.22 (−0.38, −0.05)] and second [−0.2 (−0.37, −0.03)] adjustments. Notably, sICAM-1 association with attention [−0.11 (−0.24, 0.02)] strengthened after first [−0.16 (−0.29, −0.04)] and second [−0.14 (−0.27, −0.01)] adjustments, whereas associations with visuospatial memory [−0.17 (−0.34, 0)] and motor function [−0.17 (−0.35, 0.01)] attenuated after first or second adjustments [motor: first adjustment; −0.18 (−0.53, 0.01), second adjustment; −0.16 (−0.36, 0.01), visuospatial memory: first adjustment; −0.15 (−0.32, 0.03), second adjustment; −0.14 (−0.32, 0.04)].
Associations between individual groups of biomarkers with neurocognitive measures.
Results of the four key biomarkers (sCD14, sCD163, sICAM-1 and sVCAM-1) in the secondary analysis were similar to the primary analysis (figure 2). The negative association between sCD14 and visuospatial memory noted in the primary analysis remained robust after adjusting for all other biomarkers within the macrophage activation group [−0.64 (−1.13, −0.15), first adjustment; −0.64 (−1.37, 0.09), second adjustment; −0.73 (−1.42, −0.04)]. sCD163 was negatively associated with motor function after controlling for other biomarkers of macrophage activation in the secondary analysis [−0.23 (−0.52, 0.06], first adjustment; [−0.22 (−0.55, 0.11)], second adjustment [−0.25 (−0.56, 0.06)]. This association between sCD163 and motor function was not evident in the primary analysis. The association between sVCAM-1 and visuospatial memory in the primary analysis became inconclusive after controlling for other biomarkers of vascular inflammation [0.14 (−0.14, 0.41)], although it strengthened with first [0.25 (−0.03, 0.53)], and second [0.27 (−0.03, 0.56)] adjustments. sICAM-1 remained negatively associated with verbal memory in the secondary analysis, although to a lesser extent than in the primary analysis [−0.15 (−0.33, 0.03); first adjustment: −0.16 (−0.34, 0.03); second adjustment: −0.14 (−0.33, 0.05)]. Its negative association with attention seen in primary analysis was not observed in the secondary analysis.
A lymphocyte activation marker sCD27 was positively associated with the attention domain after controlling for other biomarkers in the lymphocyte activation group [0.17 (0.07, 0.27)]. This association was attenuated with first [0.16 (0, 0.32)], and second [0.12 (−0.04, 0.28)] adjustments. IL-6, a biomarker shown to independently predict disease progression and mortality in HIV-infected individuals [37, 48, 49], associated with verbal memory with very small estimates [0.09 (0.03, 0.15), first adjustment; 0.09 (0.01, 0.17), second adjustment; 0.09 (−0.01, 0.19)], and clinical significance of this association is questionable.
None of the neurocognitive domains were significantly associated with MMP-2, CCL2, or LPS known to associate with neurocognitive injury in HIV [14, 38, 50]. Inflammatory biomarkers that predict cardiovascular risk in HIV infected individuals, such as CRP and MPO [41], also did not exhibit significant associations with any neurocognitive domain. Finally, inclusion of whether the participant was taking Efavirenz, an antiretroviral drug known to be associated with neuropsychiatric effects and neurocognitive deficits, did not meaningfully affect any of our estimates (supplemental tables 4 and 5).
Discussion:
The study population of ATN 071/101 reflects the HIV epidemic of new infections among youth in the United States, predominantly African American men between the ages of 18 and 24 [51]. This is the first study to comprehensively evaluate the association between HIV-associated inflammatory biomarkers and specific domains of neurocognitive function in YWH recently infected through sexual transmission. A 3 year follow-up (144 weeks) was determined to be adequate time to achieve disease control on ART and to detect lasting neurocognitive and inflammatory changes. In order to be able to accurately compare our results with other studied across the life span, we studied the time point similar to previously published studies evaluating longitudinal outcomes in perinatally infected children and older adults [52-57]. After three years of initial therapy, overall viral suppression within the study cohort was 61%, considerably better than in a previous large study of YWH initiating cART, where only 24% of YWH achieved undetectable viral levels after a similar timeframe [58]. The majority of our cohort had normal CD4 T cell counts and few HIV associated infections, although there was a high prevalence of STIs [59]. Overall, participants did not exhibit significant depressive symptomatology, as BDI-II scores were mostly within minimal to mild ranges. However, substance use was common, particularly tobacco, cannabis, and alcohol [36]. Given numerous potential covariates, the comprehensive scope by which confounding factors were included in our analyses is a strength of the study.
Chronic HIV-associated macrophage activation, mediated by LPS binding to TLR4, persists despite optimal control of viral replication by cART [13, 18, 28, 39]. We previously compared biomarker profiles of uninfected youth to virally suppressed YWH in the ATN071/101 cohort and found that elevated pro-inflammatory biomarkers sCD27, sCD14, sCD163, TNFα, and LPS distinguished YWH from healthy uninfected youth [27]. In the same cohort, verbal and visuospatial memory were impaired with mean z-scores of −1.59 for verbal memory and −1.00 for visuospatial memory. Most significantly, impaired visuospatial memory was associated with higher plasma levels of sCD14. A recent study showed that plasma sCD14 was positively associated with microstructural white matter (WM) damage [32]. TLR4 is expressed on microglia and astrocytes in the CNS and plays an important role in the pathogenesis of neurodegenerative diseases such as Alzheimer’s disease [60, 61]. Furthermore, activation of TLR4 by LPS reduces hippocampal pyramidal neuron dendrite length and impairs hippocampal-dependent spatial reference memory in an inflammation-dependent manner [50, 62]. Recently, a positron emission tomography neuroimaging study of cognitively healthy HIV-infected adults showed that increased translocator protein binding, a proxy measure of brain microglial activation, in the hippocampus, amygdala, and thalamus, was associated with poorer verbal and visual memory performance [63], highlighting the possible role of macrophage-derived neuroinflammation in HIV-associated neurocognitive impairment. Together, evidence supports our finding of the association between sCD14, likely reflecting monocyte activation, and impaired visuospatial memory.
ICAM and VCAM on brain microvascular endothelial cells play important roles in transmigration of HIV-infected monocytes through the blood brain barrier, potentially contributing to neuroinflammation [64, 65]. We hypothesized that sICAM-1 and sVCAM-1 may be associated with impaired neurocognitive function. While estimates were small, sICAM-1 was most significantly associated with impaired verbal memory and attention. Elevated sICAM-1 and sVCAM-1 levels are found in the serum and CSF of HIV infected adults, children, as well as SIV-infected non-human primates, and vascular inflammation have been associated with HIV encephalopathy and poor neurocognitive function [32, 66, 67]. To our surprise, sVCAM-1 was positively associated with executive function and visuospatial memory. The contrasting findings between sICAM-1 and sVCAM-1 were not expected and are difficult to explain. Limited studies assessing inflammatory markers and cognition in YWH further lead to a challenge in the interpretation of these findings, although elevated sVCAM-1 levels coupled with impaired visuomotor integration and higher cerebral blood flow has been reported in HIV-infected children [68]. Kapetanovic et al. observed higher sVCAM-1 and sICAM-1 levels in perinatally-infected youth compared to healthy controls, but did not find any association between these vascular inflammatory markers and neurocognitive function [31]. Unexpected associations between higher expression of sVCAM-1 and higher cognitive function could indicate effect modification, but small sample size prevented us from exploring further. The exploratory findings from the ATN 071/101 cohort indicate that more sophisticated studies of CSF and imaging of cerebral blood flow may be needed to clarify the inflammatory mechanisms.
In contrast to verbal and visuospatial memory, domains of attention, executive function, and motor function were relatively intact in our cohort. Prior studies of older HIV-infected adults and young children infected through maternal transmission have shown that multiple cognitive domains are impaired in advanced disease, including attention, motor, executive functions, as well as learning and memory [1, 5, 19, 69, 70]. Differences across affected cognitive domains suggest HIV infection may impair selective components of brain in early disease states and expand as disease progresses.
Studies of chronically HIV-infected adults have shown associations between impaired learning and memory and higher sCD14 and sCD163 levels [14, 19, 70]. In contrast to our cohort, these older adults had lower CD4 T cell counts, more co-morbidities, and longer infection durations. A surprising finding in our YWH cohort was the lack of association between sCD163 levels and neurocognitive domains. In chronically infected adults, higher levels of sCD163 were associated with poorer overall cognitive performance, specifically in domains of verbal learning, verbal memory, executive functions, psychomotor speed, and fine motor skills [19]. Normalization of sCD163 occurs on cART along with reversal of neurocognitive impairment to an extent, but this observation is limited when treatment is initiated past Fiebig stage II [71]. In YWH treated with cART prior to CD4 T cell decline, sCD163 levels decline but sCD14 levels remain persistently elevated [28], indicating that different pathways of macrophage activation may distinctively affect HIV-associated neuropathogenesis [72].
Associations between other biomarkers of macrophage activation such as LPS, a trigger of CD14/TLR4 activation, and neurocognitive function were not observed. In other studies, LPS, along with CCL2, were associated with HIV associated dementia [14]. However, unlike sCD14, these biomarkers were only associated with significant neurocognitive impairment in advanced HIV disease [14, 70]. The lack of association between LPS and neurocognitive function may have been due to poor ability of the limulus amoebocyte lysate (LAL) assay to accurately reflect the amount of endotoxin in the plasma [73]. None of the lymphocyte activation markers had strong associations with neurocognitive outcomes. While sCD27 distinguishes YWH from uninfected youth, the clinical significance of a positive association between attention and sCD27 is unclear given the lack of significant impairment in this domain.
While it is beyond the scope of our investigation to identify clear mechanisms on how macrophage activation and vascular inflammation directly impact neurocognitive function, our results highlight that these biomarkers are clearly important in HIV inflammatory pathogenesis in YWH. Together with newer imaging techniques targeting the hippocampus and other brain regions involved in learning and memory, assessment of neurocognitive function can be further refined.
Our study was limited to examining plasma biomarkers and levels in CSF were not examined, although CSF biomarkers do not necessarily better correlate with neurocognitive function [18]. There were no healthy controls in this study, and the normative samples for the neurocognitive tests differ from the demographic characteristics of youth with HIV in systematic ways. We addressed this through both normative corrections and our statistical models with adjustment for demographic characteristics, but this remains a limitation of interpreting the z-scores. Viral load was dichotomized due to large number of values below the LOD, as inclusion as a continuous variable would have considerably increased the level of uncertainty in the estimate. Dichotomizing the viral load could have left some residual confounding, but doing so likely provided significant reductions in uncertainty in the parameter estimate. We did not impute the 18 values for LPS that were below the LOD of 0.1 EU/mL, as we reasoned that the observed concentrations likely contained less measurement error than values imputed from the LOD regression procedure. However, these 18 values do have more measurement error than values for LPS above the LOD, so the results for LPS should be interpreted with additional caution. In general, the estimated associations between the biomarkers and the neurocognitive domains were small. Some of the biomarker associations had large confidence intervals, possibly due to large percentages of values below the LOD. However, because we explicitly modeled the possible distribution of values below the LOD and accounted for the uncertainty due to not observing them, our confidence intervals honestly represent our uncertainty in those parameter estimates, given the large proportion of values below the LOD. Considering the number of models fit, the evidence for robust, clinically significant associations for many of these biomarkers is limited. Nonetheless, the assessment of inflammatory biomarkers can be reliably applied to clinical trials for interventions to improve neurocognitive function.
Plasma sCD14 is an independent predictor of mortality in HIV and may serve as the best intermediate measure for clinical interventions designed to lower the inflammatory consequences of HIV infection [27, 70, 74]. Results of ATN 071/101 provide evidence that immune activation in the CNS selectively impairs visuospatial memory, although studies with larger cohorts are needed. As new interventions are developed to inhibit microbial translocation, resultant attenuation of CD14/TLR4 activation and its association with neurocognitive performance will become an important clinical endpoint.
Supplementary Material
Supplemental Figure 1. Distribution of biomarker and viral load density measurements at week 144. Biomarkers were measured by Millipore Multiplex Luminex platform, ELISA, or Limulus Amebocyte Lysate Assay. Thirteen biomarkers were measured by Millipore Multiplex assay, with the lower limit of detection and inter-assay coefficient of variation listed in parenthesis: GM-CSF (0.01 pg/mL; <15%), TNFα (0.20 pg/mL; <20%), IL-6 (0.1 pg/mL; <15%), IL-8 (0.30 pg/mL; <15%), IL-1β (0.01 pg/mL; <10%), IFNγ (0.1 pg/mL; <10%), IL-10 (0.1 pg/mL; <10%), sICAM-1 (19 pg/mL; <20%), sVCAM-1 (24 pg/mL; <20%), MPO (36 pg/mL; <20%), CRP (1 pg/mL; <20%), sIL-2Rα (0.1 pg/mL; <10%), MCP-1 (1.2 pg/mL; <15%). Four biomarkers were assayed by ELISA, with the lower limit of detection and inter-assay coefficient of variation listed in parenthesis: sCD14 (125 pg/mL; <10%), sCD163 (613 pg/mL; <10%), MMP-2 (289 pg/mL; <10%), and sCD27 (0.2 U/mL; <10%). Plasma LPS was measured using the limulus Amebocyte Lysate (LAL) Chromogenic Endpoint Assay, with lower limit of detection of 0.1 EU/mL and inter-assay coefficient of variation of < 10%.
Acknowledgements:
This work was supported in part by funding from the Adolescent Trials Network for HIV/AIDS Interventions (ATN) from the National Institutes of Health (U01-HD040533 and U01-HD040474) through the Eunice Kennedy Shriver National Institute of Child Health and Human Development, with supplemental funding from the National Institute on Drug Abuse and the National Institute for Mental Health. It was also supported by indpendent grant from the National Institute on Drug Abuse (R01-DA031017 and U01-DA044571). The study was co-endorsed by the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) which is supported by the National Institute of Allergy and Infectious Diseases (NIAID), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Institute of Mental Health (NIMH) (U01-A1068632). Further support was provided by the UNC coordinating center (NIH U24-HD089880) and Duke University Center for AIDS Research (CFAR), (NIH 5P30-AI064518). Dr. Kim-Chang was supported by NIH T32-AI007062.
The following ATN sites participated in this study: University of South Florida, Tampa (Emmanuel, Lujan-Zilberman, Julian), Children’s Hospital of Los Angeles (Belzer, Flores, Tucker), University of Southern California at Los Angeles (Kovacs, Homans, Lozano), Childrens National Medical Center (D’Angelo, Hagler, Trexler), Children’s Hospital of Philadelphia (Douglas, Tanney, DiBenedetto), John H. Stroger Jr. Hospital of Cook County and the Ruth M. Rothstein CORE Center (Martinez, Bojan, Jackson), University of Puerto Rico (Febo, Ayala-Flores, Fuentes-Gomez), Montefiore Medical Center (Futterman, Enriquez-Bruce, Campos), Mount Sinai Medical Center (Steever, Geiger), University of California-San Francisco (Moscicki, Auerswald, Irish), Tulane University Health Sciences Center (Abdalian, Kozina, Baker), University of Maryland (Peralta, Gorle), University of Miami School of Medicine (Friedman, Maturo, Major-Wilson), Children’s Diagnostic and Treatment Center (Puga, Leonard, Inman), St. Jude’s Children’s Research Hospital (Flynn, Dillard), and Children’s Memorial (Garofalo, Brennan, Flanagan).
The following IMPAACT sites participated in the study: Children’s Hospital of Michigan – Wayne State (Moore, Rongkavilit, Hancock), Duke University Medical Center Pediatric CRS (Cunningham, Wilson), Johns Hopkins University (Ellen, Chang, Noletto), New Jersey Medical School CTU/CRS (Dieudonne, Bettica, Monti), St. Jude/Memphis CTU/CRS (Flynn, Dillard, McKinley), University of Colorado School of Medicine/The Children’s Hospital (Reirden, Kahn, Witte) University of Southern California Medical Center (Homans, Lozano), Howard University Hospital (Rana, Deressa),
Four of the ATN and IMPAACT sites utilized their General Clinical Research Center (GCRC)/Pediatric Clinical Research Center (PCRC) for the study. The centers were supported by grants from the General Clinical Research Center Program of the National Center for Research Resources (NCRR), National Institutes of Health, Department of Health and Human Services as follows: Children’s National Medical Center, M01RR020359; Howard University Hospital, MO1-RR010284; University of California at San Francisco, UL1 RR024131; and University of Colorado School of Medicine/Children’s Hospital, UL1 RR025780. The University of Pennsylvania/Children’s Hospital of Philadelphia utilized its Institutional Clinical and Translational Science Award Research Center (CTRC), supported by grant UL1 RR024134 from NCRR. The Tulane University Health Sciences Center utilized its Clinical and Translational Research Center (CTRC) for the study which was supported in whole or in part by funds provided through the Louisiana Board of Regents RC/EEP (RC/EEP - 06).
The authors wish to thank Micah McCumber and Laura Renshaw for their assistance with manuscript preparation. We are grateful to all of the youth who agreed to participate in the study.
Sources of Funding: NIH grants (R01-DA031017, U01-DA044571, U01-HD040533, U01-HD040474, U01-A1068632, 5P30-AI064518, U24-HD089880), Duke University Center for AIDS Research (CFAR), (5P30-AI064518). Dr. Kim-Chang was supported by NIH T32-AI007062.
Justification for greater than 10 authors: Study represents the combined work of two ATN clinical trials (ATN071, ATN101) and therefore has multiple authors exceeding 10.
Footnotes
Conflicts of Interest: None declared
Data presented previously at 2019 Conference on Retroviruses and Opportunistic infections (CROI) annual meeting.
References:
- 1.Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol 2011; 17(1):3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 2007; 69(18):1789–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heaton RK, Clifford DB, Franklin DR Jr., Woods SP, Ake C, Vaida F, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 2010; 75(23):2087–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grant I, Franklin DR Jr., Deutsch R, Woods SP, Vaida F, Ellis RJ, et al. Asymptomatic HIV-associated neurocognitive impairment increases risk for symptomatic decline. Neurology 2014; 82(23):2055–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woods SP, Moore DJ, Weber E, Grant I. Cognitive neuropsychology of HIV-associated neurocognitive disorders. Neuropsychol Rev 2009; 19(2):152–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.HIV Surveillance Report, 2016; Vol. 28 Centers for Disease Control and Prevention (CDC); 2017. [Google Scholar]
- 7.Nichols SL, Bethel J, Garvie PA, Patton DE, Thornton S, Kapogiannis BG, et al. Neurocognitive functioning in antiretroviral therapy-naive youth with behaviorally acquired human immunodeficiency virus. J Adolesc Health 2013; 53(6):763–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nichols SL, Bethel J, Kapogiannis BG, Li T, Woods SP, Patton ED, et al. Antiretroviral treatment initiation does not differentially alter neurocognitive functioning over time in youth with behaviorally acquired HIV. J Neurovirol 2016; 22(2):218–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hong S, Banks WA. Role of the immune system in HIV-associated neuroinflammation and neurocognitive implications. Brain Behav Immun 2015; 45:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watkins CC, Treisman GJ. Cognitive impairment in patients with AIDS - prevalence and severity. HIV AIDS (Auckl) 2015; 7:35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spudich S, Robertson KR, Bosch RJ, Gandhi RT, Cyktor JC, Mar H, et al. Persistent HIV-infected cells in cerebrospinal fluid are associated with poorer neurocognitive performance. J Clin Invest 2019; 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wright S, Ramos R, Tobias P, Ulevitch R, Mathison J. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 1990; 249(4975):1431–1433. [DOI] [PubMed] [Google Scholar]
- 13.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 2006; 12(12):1365–1371. [DOI] [PubMed] [Google Scholar]
- 14.Ancuta P, Kamat A, Kunstman KJ, Kim EY, Autissier P, Wurcel A, et al. Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS One 2008; 3(6):e2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischer-Smith T, Croul S, Sverstiuk AE, Capini C, L’Heureux D, Regulier EG, et al. CNS invasion by CD14+/CD16+ peripheral blood-derived monocytes in HIV dementia: perivascular accumulation and reservoir of HIV infection. J Neurovirol 2001; 7(6):528–541. [DOI] [PubMed] [Google Scholar]
- 16.Persidsky Y, Stins M, Way D, Witte MH, Weinand M, Kim KS, et al. A model for monocyte migration throught the blood-Brain barrier during HIV-1 Encephalitis. The Journal of Immunology 1997; 158:3499–3510. [PubMed] [Google Scholar]
- 17.Burdo TH, Lentz MR, Autissier P, Krishnan A, Halpern E, Letendre S, et al. Soluble CD163 made by monocyte/macrophages is a novel marker of HIV activity in early and chronic infection prior to and after anti-retroviral therapy. J Infect Dis 2011; 204(1):154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burdo TH, Weiffenbach A, Woods SP, Letendre S, Ellis RJ, Williams KC. Elevated sCD163 in plasma but not cerebrospinal fluid is a marker of neurocognitive impairment in HIV infection. AIDS 2013; 27(9):1387–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imp BM, Rubin LH, Tien PC, Plankey MW, Golub ET, French AL, et al. Monocyte Activation Is Associated With Worse Cognitive Performance in HIV-Infected Women With Virologic Suppression. J Infect Dis 2017; 215(1):114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papasavvas E, Azzoni L, Pistilli M, Hancock A, Reynolds G, Gallo C, et al. Increased soluble vascular cell adhesion molecule-1 plasma levels and soluble intercellular adhesion molecule-1 during antiretroviral therapy interruption and retention of elevated soluble vascular cellular adhesion molecule-1 levels following resumption of antiretroviral therapy. AIDS 2008; 22(10):1153–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muller MM, Griesmacher A. Markers of endothelial dysfunction. Clin Chem Lab Med 2000; 38(2):77–85. [DOI] [PubMed] [Google Scholar]
- 22.Syed SS, Balluz RS, Kabagambe EK, Meyer WA 3rd, Lukas S, Wilson CM, et al. Assessment of biomarkers of cardiovascular risk among HIV type 1-infected adolescents: role of soluble vascular cell adhesion molecule as an early indicator of endothelial inflammation. AIDS Res Hum Retroviruses 2013; 29(3):493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol 2013; 13(10):709–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin J, Collot-Teixeira S, McGregor L, McGregor J. The Dialogue Between Endothelial Cells and Monocytes/Macrophages in Vascular Syndromes. Current Pharmaceutical Design 2007; 13(17):1751–1759. [DOI] [PubMed] [Google Scholar]
- 25.Engelhardt B Immune cell entry into the central nervous system: involvement of adhesion molecules and chemokines. J Neurol Sci 2008; 274(1–2):23–26. [DOI] [PubMed] [Google Scholar]
- 26.Seilhean D, Dzia-Lepfoundzou A, Sazdovitch V, Cannella B, Raine CS, Katlama C, et al. Astrocytic adhesion molecules are increased in HIV-1-associated cognitive/motor complex. Neuropathol Appl Neurobiol 1997; 23(2):83–92. [PubMed] [Google Scholar]
- 27.Williams JC, Zhang X, Karki M, Chi YY, Wallet SM, Rudy BJ, et al. Soluble CD14, CD163, and CD27 biomarkers distinguish ART-suppressed youth living with HIV from healthy controls. J Leukoc Biol 2018; 103(4):671–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rudy BJ, Kapogiannis BG, Worrell C, Squires K, Bethel J, Li S, et al. Immune Reconstitution but Persistent Activation After 48 Weeks of Antiretroviral Therapy in Youth With Pre-Therapy CD4 >350 in ATN 061. Journal of acquired immune deficiency syndromes (1999) 2015; 69(1):52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lebel C, Beaulieu C. Longitudinal development of human brain wiring continues from childhood into adulthood. The Journal of neuroscience : the official journal of the Society for Neuroscience 2011; 31(30):10937–10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eckard AR, Rosebush JC, O’Riordan MA, Graves CC, Alexander A, Grover AK, et al. Neurocognitive dysfunction in HIV-infected youth: investigating the relationship with immune activation. Antiviral therapy 2017; 22(8):669–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kapetanovic S, Griner R, Zeldow B, Nichols S, Leister E, Gelbard HA, et al. Biomarkers and neurodevelopment in perinatally HIV-infected or exposed youth: a structural equation model analysis. AIDS 2014; 28(3):355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blokhuis C, Peeters CFW, Cohen S, Scherpbier HJ, Kuijpers TW, Reiss P, et al. Systemic and intrathecal immune activation in association with cerebral and cognitive outcomes in paediatric HIV. Sci Rep 2019; 9(1):8004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ananworanich J, Kerr SJ, Jaimulwong T, Vibol U, Hansudewechakul R, Kosalaraksa P, et al. Soluble CD163 and monocyte populations in response to antiretroviral therapy and in relationship with neuropsychological testing among HIV-infected children. J Virus Erad 2015; 1(3):196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. In. 2nd Edition ed. San Antonio, Texas: Psychological Corporation; 1996. [Google Scholar]
- 35.WHO ASSIST Working Group. The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): development, reliability and feasibility. Addiction (Abingdon, England) 2002; 97(9):1183–1194. [DOI] [PubMed] [Google Scholar]
- 36.Nichols SL, Lowe A, Zhang X, Garvie PA, Thornton S, Goldberger BA, et al. Concordance between self-reported substance use and toxicology among HIV-infected and uninfected at risk youth. Drug Alcohol Depend 2014; 134:376–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boulware DR, Hullsiek KH, Puronen CE, Rupert A, Baker JV, French MA, et al. Higher levels of CRP, D-dimer, IL-6, and hyaluronic acid before initiation of antiretroviral therapy (ART) are associated with increased risk of AIDS or death. J Infect Dis 2011; 203(11):1637–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li S, Wu Y, Keating SM, Du H, Sammet CL, Zadikoff C, et al. Matrix metalloproteinase levels in early HIV infection and relation to in vivo brain status. J Neurovirol 2013; 19(5):452–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wallet MA, Rodriguez CA, Yin L, Saporta S, Chinratanapisit S, Hou W, et al. Microbial translocation induces persistent macrophage activation unrelated to HIV-1 levels or T-cell activation following therapy. AIDS 2010; 24(9):1281–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilson EM, Singh A, Hullsiek KH, Gibson D, Henry WK, Lichtenstein K, et al. Monocyte-activation phenotypes are associated with biomarkers of inflammation and coagulation in chronic HIV infection. J Infect Dis 2014; 210(9):1396–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Borato DC, Parabocz GC, Ribas SR, Kalva-Filho CA, Borba LM, Ito CA, et al. Changes of metabolic and inflammatory markers in HIV infection: glucose, lipids, serum Hs-CRP and myeloperoxidase. Metabolism 2012; 61(10):1353–1360. [DOI] [PubMed] [Google Scholar]
- 42.Fourie CM, Schutte AE, Smith W, Kruger A, van Rooyen JM. Endothelial activation and cardiometabolic profiles of treated and never-treated HIV infected Africans. Atherosclerosis 2015; 240(1):154–160. [DOI] [PubMed] [Google Scholar]
- 43.Milito AD, Aleman S, Marenzi R, SÖNnerborg A, D F, Zazzi M, et al. Plasma levels of soluble CD27: a simple marker to monitor immune activation during potent antiretroviral therapy in HIV-1-infected subjects. Clinical & Experimental Immunology 2002; 127(3):486–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wada NI, Jacobson LP, Margolick JB, Breen EC, Macatangay B, Penugonda S, et al. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS 2015; 29(4):463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Apostolova N, Funes HA, Blas-Garcia A, Galindo MJ, Alvarez A, Esplugues JV. Efavirenz and the CNS: what we already know and questions that need to be answered. J Antimicrob Chemother 2015; 70(10):2693–2708. [DOI] [PubMed] [Google Scholar]
- 46.Ciccarelli N, Fabbiani M, Di Giambenedetto S, Fanti I, Baldonero E, Bracciale L, et al. Efavirenz associated with cognitive disorders in otherwise asymptomatic HIV-infected patients. Neurology 2011; 76(16):1403–1409. [DOI] [PubMed] [Google Scholar]
- 47.May RC, Ibrahim JG, Chu H. Maximum likelihood estimation in generalized linear models with multiple covariates subject to detection limits. Stat Med 2011; 30(20):2551–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kalayjian RC, Machekano RN, Rizk N, Robbins GK, Gandhi RT, Rodriguez BA, et al. Pretreatment levels of soluble cellular receptors and interleukin-6 are associated with HIV disease progression in subjects treated with highly active antiretroviral therapy. J Infect Dis 2010; 201(12):1796–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hunt. HIV and inflammation: mechanisms and consequences. Current HIV/AIDS Rep 2012; 9(2):139–147. [DOI] [PubMed] [Google Scholar]
- 50.Hauss-Wegrzyniak BVP, Wenk GL. LPS-induced neuroinflammatory effects do not recover with time. Neuroreport 2000; 11:1759–1763. [DOI] [PubMed] [Google Scholar]
- 51.Zanoni BC, Mayer KH. The adolescent and young adult HIV cascade of care in the United States: exaggerated health disparities. AIDS Patient Care STDS 2014; 28(3):128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bosch RJ, Bennett K, Collier AC, Zackin R, Benson CA. Pretreatment factors associated with 3-year (144-week) virologic and immunologic responses to potent antiretroviral therapy. Journal of acquired immune deficiency syndromes (1999) 2007; 44(3):268–277. [DOI] [PubMed] [Google Scholar]
- 53.Weinberg A, Dickover R, Britto P, Hu C, Patterson-Bartlett J, Kraimer J, et al. Continuous improvement in the immune system of HIV-infected children on prolonged antiretroviral therapy. AIDS 2008; 22(17):2267–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Olmo M, Saumoy M, Alonso-Villaverde C, Penaranda M, Gutierrez F, Romeu J, et al. Impact of antiretroviral therapy interruption on plasma biomarkers of cardiovascular risk and lipids: 144-week final data from the STOPAR study. Hiv Med 2012; 13(8):488–498. [DOI] [PubMed] [Google Scholar]
- 55.Currier JS, Kendall MA, Henry WK, Alston-Smith B, Torriani FJ, Tebas P, et al. Progression of carotid artery intima-media thickening in HIV-infected and uninfected adults. AIDS 2007; 21(9):1137–1145. [DOI] [PubMed] [Google Scholar]
- 56.Samri A, Goodall R, Burton C, Imami N, Pantaleo G, Kelleher A, et al. Three-year immune reconstitution in PI-sparing and PI-containing antiretroviral regimens in advanced HIV-1 disease. Antiviral therapy 2007; 12(4):553–558. [PubMed] [Google Scholar]
- 57.Lederman HM, Williams PL, Wu JW, Evans TG, Cohn SE, McCutchan JA, et al. Incomplete immune reconstitution after initiation of highly active antiretroviral therapy in human immunodeficiency virus-infected patients with severe CD4+ cell depletion. J Infect Dis 2003; 188(12):1794–1803. [DOI] [PubMed] [Google Scholar]
- 58.Flynn PM, Rudy BJ, Lindsey JC, Douglas SD, Lathey J, Spector SA, et al. Long-term observation of adolescents initiating HAART therapy: three-year follow-up. AIDS Res Hum Retroviruses 2007; 23(10):1208–1214. [DOI] [PubMed] [Google Scholar]
- 59.Mullins TLK, Li SX, Bethel J, Goodenow MM, Hudey S, Sleasman JW, et al. Sexually transmitted infections and immune activation among HIV-infected but virally suppressed youth on antiretroviral therapy. J Clin Virol 2018; 102:7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fiebich BL, Batista CRA, Saliba SW, Yousif NM, de Oliveira ACP. Role of Microglia TLRs in Neurodegeneration. Front Cell Neurosci 2018; 12:329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Okun E, Griffioen KJ, Mattson MP. Toll-like receptor signaling in neural plasticity and disease. Trends Neurosci 2011; 34(5):269–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maezawa I, Zaja-Milatovic S, Milatovic D, Stephen C, Sokal I, Maeda N, et al. Apolipoprotein E isoform-dependent dendritic recovery of hippocampal neurons following activation of innate immunity. J Neuroinflammation 2006; 3:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vera JH, Guo Q, Cole JH, Boasso A, Greathead L, Kelleher P, et al. Neuroinflammation in treated HIV-positive individuals: A TSPO PET study. Neurology 2016; 86(15):1425–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nottet HSLM, Persidsky Y, Sasseville VG, Nukuna AN, Bock P, Zhai QH, et al. Mechanisms for the transendothelial migration of HIV-1-infected monocytes into brain. Journal of Immunology 1996; 156(3):1284–1295. [PubMed] [Google Scholar]
- 65.Nottet HSLM. Interactions between macrophages and brain microvascular endothelial cells: role in pathogenesis of HIV-1 infection and blood-brain barrier function. Journal of Neurovirology 1999; 5(6):659–669. [DOI] [PubMed] [Google Scholar]
- 66.Heidenreich F, Arendt G, Jander S, Jablonowski H, Stoll G. Serum and cerebrospinal fluid levels of soluble intercellular adhesion molecule 1 (sICAM-1) in patients with HIV-1 associated neurological diseases. J Neuroimmunol 1994; 52(2):117–126. [DOI] [PubMed] [Google Scholar]
- 67.Sasseville VG, Newman WA, Lackner AA, Smith MO, Lausen NC, Beall D, et al. Elevated vascular cell adhesion molecule-1 in AIDS encephalitis induced by simian immunodeficiency virus. Am J Pathol 1992; 141(5):1021–1030. [PMC free article] [PubMed] [Google Scholar]
- 68.Blokhuis C, Cohen S, Scherpbier HJ, Mutsaerts HJMM, Meijers JCM, Kootstra NA, et al. Vascular Health and Cerebral Blood Flow in Perinatally HIV-Infected Children. [Abstract 825] 24th Conference on Retroviruses and Opportunistic Infections (CROI) February 2017. [Google Scholar]
- 69.Nichols SL, Brummel SS, Smith RA, Garvie PA, Hunter SJ, Malee KM, et al. Executive Functioning in Children and Adolescents With Perinatal HIV Infection. Pediatr Infect Dis J 2015; 34(9):969–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lyons JL, Uno H, Ancuta P, Kamat A, Moore DJ, Singer EJ, et al. Plasma sCD14 is a biomarker associated with impaired neurocognitive test performance in attention and learning domains in HIV infection. Journal of acquired immune deficiency syndromes (1999) 2011; 57(5):371–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.D’Antoni ML, Byron MM, Chan P, Sailasuta N, Sacdalan C, Sithinamsuwan P, et al. Normalization of Soluble CD163 Levels After Institution of Antiretroviral Therapy During Acute HIV Infection Tracks with Fewer Neurological Abnormalities. J Infect Dis 2018; 218(9):1453–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brown JN, Kohler JJ, Coberley CR, Sleasman JW, Goodenow MM. HIV-1 activates macrophages independent of Toll-like receptors. PLoS One 2008; 3(12):e3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gnauck A, Lentle RG, Kruger MC. The Limulus Amebocyte Lysate assay may be unsuitable for detecting endotoxin in blood of healthy female subjects. J Immunol Methods 2015; 416:146–156. [DOI] [PubMed] [Google Scholar]
- 74.So-Armah KA, Tate JP, Chang CH, Butt AA, Gerschenson M, Gibert CL, et al. Do Biomarkers of Inflammation, Monocyte Activation, and Altered Coagulation Explain Excess Mortality Between HIV Infected and Uninfected People? Journal of acquired immune deficiency syndromes (1999) 2016; 72(2):206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Distribution of biomarker and viral load density measurements at week 144. Biomarkers were measured by Millipore Multiplex Luminex platform, ELISA, or Limulus Amebocyte Lysate Assay. Thirteen biomarkers were measured by Millipore Multiplex assay, with the lower limit of detection and inter-assay coefficient of variation listed in parenthesis: GM-CSF (0.01 pg/mL; <15%), TNFα (0.20 pg/mL; <20%), IL-6 (0.1 pg/mL; <15%), IL-8 (0.30 pg/mL; <15%), IL-1β (0.01 pg/mL; <10%), IFNγ (0.1 pg/mL; <10%), IL-10 (0.1 pg/mL; <10%), sICAM-1 (19 pg/mL; <20%), sVCAM-1 (24 pg/mL; <20%), MPO (36 pg/mL; <20%), CRP (1 pg/mL; <20%), sIL-2Rα (0.1 pg/mL; <10%), MCP-1 (1.2 pg/mL; <15%). Four biomarkers were assayed by ELISA, with the lower limit of detection and inter-assay coefficient of variation listed in parenthesis: sCD14 (125 pg/mL; <10%), sCD163 (613 pg/mL; <10%), MMP-2 (289 pg/mL; <10%), and sCD27 (0.2 U/mL; <10%). Plasma LPS was measured using the limulus Amebocyte Lysate (LAL) Chromogenic Endpoint Assay, with lower limit of detection of 0.1 EU/mL and inter-assay coefficient of variation of < 10%.