Abstract
Introduction:
Identification and retention of HIV-positive children in HIV services is essential to ensure optimal health outcomes. This systematic review and meta-analysis examines the magnitude of attrition (loss to follow-up [LTFU] and death) of HIV-positive children from HIV services in low- and middle-income countries (LMICs).
Methods:
We performed a comprehensive multi-database search of original studies reporting retention/attrition data for HIV-positive children in LMICs through April 2016. Outcomes included LTFU, death, and overall attrition (LTFU + death) at intervals up to 60 months of follow-up. At least two authors determined study eligibility, performed data extraction, and made quality assessments. We used random effects meta-analytic methods to aggregate effect sizes and perform meta-regression analyses.
Results:
We identified 3,040 unique studies; 91 met eligibility criteria and were included in the analysis. This represents 147,129 HIV-positive children; most were from Africa (83%). LTFU definitions varied widely, with significant variability in outcomes across studies. Most attrition occurred in the first six months of follow-up, increasing to 23% by 36 months. HIV-positive children receiving antiretroviral therapy (ART) had significantly better retention in care than those not on ART. Studies that performed case-finding/tracing for those LTFU had better retention in care up to 24 months of follow-up.
Conclusions:
These findings underscore the high attrition of children from HIV services in LMICs. Early ART initiation and decentralized patient support services (e.g., tracing for those LTFU) have potential to improve retention in care. Implementation research and resources are urgently needed to improve retention among this vulnerable population.
Registration of protocol: PROSPERO # CRD42016034180
Keywords: HIV, child, low- and middle-income countries, attrition, retention, loss to follow-up
Introduction
Despite progress in HIV prevention, care, and treatment, HIV in children remains a major public health burden. In 2017, there were an estimated 1.8 million children (less than 15 years old) with HIV, 180,000 children newly infected with HIV, and 110,000 children who died of HIV/AIDS.[1] The vast majority of these children were from low- and middle-income countries (LMICs), where resources to combat the HIV epidemic are limited.[1]
UNAIDS proposed the 90-90-90 goals, which state that by 2020, 90% of all people living with HIV will know their HIV status, 90% of all HIV-positive people will receive sustained antiretroviral therapy (ART), and 90% of all people receiving ART will be virally suppressed.[2] Correspondingly, success of pediatric HIV programs relies on the timely identification of HIV-positive children, prompt initiation of ART, and sustained retention in HIV care and treatment services to ensure viral suppression and optimal health outcomes.[3]
Unfortunately, many HIV-positive children in LMICs are lost to follow-up (LTFU) or die without accessing and benefiting from recommended HIV care and treatment services. Previous systematic reviews of attrition were limited to children on ART and only included studies published up to 2015 for 12-month data and 2013 for 36-month data.[4, 5] While HIV-positive children can be LTFU or die prior to ART initiation, estimates of pre-ART attrition from a prior systematic review vary widely and are difficult to interpret.[6]
In this systematic review and meta-analysis, we seek to provide comprehensive estimates of the magnitude of attrition (LTFU and death) from pediatric HIV care and treatment services in LMICs, while accounting for important patient-level and programmatic features, including ART status and whether case-finding/tracing was performed for those not retained in care. Given the impending target deadline for the UNAIDS 90-90-90 goals,[2] the extent of this attrition must be quantified.
Methods
Search strategy
This study adhered to PRISMA reporting guidelines.[7] The dataset used for this meta-analysis, a complete list of included and excluded references, and a PRISMA checklist can be found at: https://rocket.app.vumc.org/index.php?doc_id=23022.
We comprehensively searched the PubMed/MEDLINE, Embase, Cochrane Library, and Web of Science databases through April 2016 ( Table S1; Supplementary Material). We conducted cross-referencing by reviewing bibliographies of included citations. We did not restrict by publication year, language, or specific intervention.
Selection/exclusion criteria
Studies were included if they met the following criteria: 1) focused on pediatric subjects aged 0–15 years; 2) reported data on retention or attrition (i.e., death, LTFU); 3) ascertained HIV infection in subjects; and, 4) were conducted in LMIC settings as defined by the World Bank.[8] We primarily included observational/non-intervention studies that met these criteria. We also included clinical trials/intervention studies if the intervention was offered at program- rather than individual-level; control groups receiving standard of care in intervention studies were also considered if their retention/attrition data could be disaggregated. Studies reporting data including but not limited to children up to 15 years old were included only if the data could be disaggregated to the population of interest. Studies were excluded if they reported attrition outcomes but did not specify time points for attrition.
Screening and data extraction
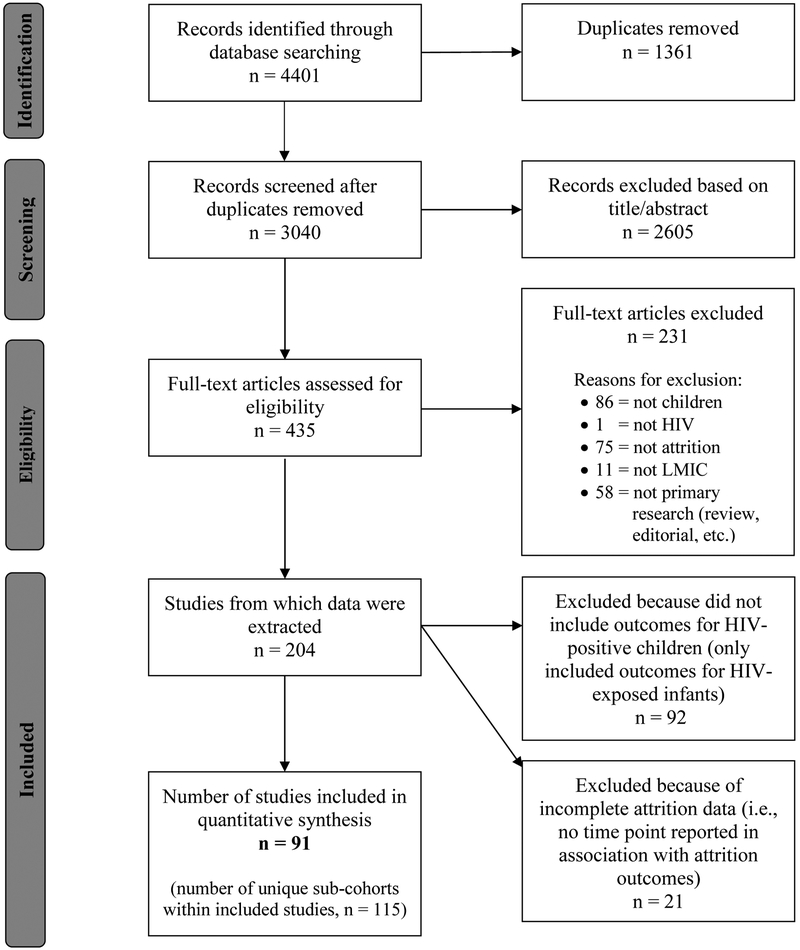
After removing duplicate records, unique study abstracts were appraised independently for eligibility by at least two reviewers. For eligible studies, full reports were obtained and independently reviewed by at least two reviewers. Any disagreement regarding study inclusion/exclusion, during either screening or full-text review, was arbitrated by a pediatrician/epidemiologist (JGC or SHV) and resolved by consensus (Figure 1).[9]
Figure 1: Flow diagram for study selection.
A complete list of references included and excluded from the meta-analysis can be found at: https://rocket.app.vumc.org/index.php?doc_id=23022
Data for each study were extracted independently by at least two reviewers and cross-checked by both reviewers, with any discrepancies arbitrated and resolved by consensus. Extracted data were managed using REDCap[10] and included: reference details (author, publication year, journal); study details (design, inclusion/exclusion criteria, intervention if applicable, sample size, study duration, location/region of study, clinical care setting, LTFU definition); outcome details (proportions of LTFU, death, and overall attrition at specified follow-up intervals, and whether case-finding/tracing was performed to clarify outcomes for those not in care); and, if applicable, notes on comparison populations or subcohorts within the study (Table S2; Supplementary Material).
The quality of included studies was assessed using a standardized form, based on the Newcastle-Ottawa domains for assessment of cohort studies.[11] The form included nine items/domains, each scored as 0 or 1 point, such that the total “quality score” for each study ranged from 0 to 9 (Table S3; Supplementary Material). Study quality was assessed independently by at least two reviewers, and disagreements regarding quality scores were arbitrated and resolved by consensus.
Data analysis
The primary outcome measure was the proportion of HIV-positive children not retained in care at various intervals of follow-up after enrollment in HIV care and treatment services (0 to ≤6 months, >6 to ≤12 months, >12 to ≤24 months, >24 to ≤36 months, >36 to ≤48 months, and >48 to ≤60 months follow-up). Included studies could contribute outcomes to as many of these follow-up intervals as their reported data allowed, and contributing an outcome to a particular time point was not contingent on reporting data for earlier or later time points (e.g., if only 12 month and 36 month attrition outcomes were reported, then the study contributed data to the >6 to ≤12 months and >24 to ≤36 months intervals, but not to the 0 to ≤6 months, >12 to ≤24 months, >36 to ≤48 months, or >48 to ≤60 months intervals). If a study contributed multiple outcomes within a single time period, we used the latter time point outcome only (e.g., if 10% and 15% attrition were reported at 3 and 5 months, respectively, we used 15% attrition for the 0 to ≤6 months interval). At each interval, we attempted to capture the proportion of LTFU, death, and overall attrition (LTFU+death). When studies did not specifically indicate the source of attrition (LTFU and/or death), available data were classified as “overall attrition.” Retention in care was defined as a patient continuing appointments at an HIV care and treatment facility. When studies reported data pertaining to patients who were known to have transferred to other facilities, we considered these known transfers as retained in care. Unknown/silent transfers could not be accounted for directly; however, case-finding/tracing can result in reclassification of silent transfers as retained in care, and tracing was accounted for in our quality assessment and with sub-group analyses stratified by whether or not tracing was performed, as described below. We accepted the variety of definitions for LTFU as used across studies.
To address potential across-study heterogeneity, we applied DerSimonian and Laird random effects analysis to pool proportions for each of the aforementioned follow-up intervals and by sources of attrition (LTFU, death, and overall).[12, 13] We used the Chi-square test for trend to assess if the proportion varied across intervals. We used the I2 statistic to assess between-study heterogeneity, with I2 ≤25% defined as low heterogeneity, near 50% as moderate, and 75% as high.[14]
Subgroup analyses were performed to determine if attrition differed by important study characteristics, including: study type (non-intervention vs. intervention), quality score (≥5 vs. <5), cohort type (ART vs. pre-ART), and whether case-finding was performed/documented (tracing vs. no tracing). Analyses stratified by tracing were restricted to non-intervention studies, as tracing efforts employed by intervention studies were likely more intensive than tracing performed in the context of routine programmatic activities.[15]
Univariate and multivariable meta-regression analyses were conducted to explore the potential source and magnitude of heterogeneity from a priori study- and population-level characteristics, including: study type (intervention vs. non-intervention), quality score (≥5 vs. <5), sample size (≥100 vs. <100), setting (hospital vs. non-hospital), rurality (urban vs. rural), region (Asia, Americas vs. Africa), and study initiation year (≤2003 vs. ≥2004). The 2004 cutoff corresponds to the period of expanded testing and ART availability in LMICs through the President’s Emergency Plan for AIDS Relief (PEPFAR) and the Global Fund to Fight AIDS, Tuberculosis and Malaria (Global Fund). Factors that were significant (p<0.05) in univariate meta-regression analyses were entered into the multivariable meta-regression model.
We used funnel plots to graphically evaluate the presence of publication biases and Begg’s and Egger’s linear regression test to assess funnel plot asymmetry,[16] stratified by time of measurement and type of attrition. Stata 15.0 (StataCorp, College Station, Texas) was used for all statistical analyses.
Results
We identified 3,040 unique studies, with 91 deemed eligible for inclusion (Figure 1 and Table S2), representing data from 147,129 HIV-positive children. The median sample size was 286 participants (range: 7–17,712), and 77% of included studies had sample sizes ≥100 participants. The median study start year was 2004 (range: 1990–2014), and 63% started during or after 2004. Most studies were based in African countries (83%), with fewer from LMICs in Asia (15%) and the Americas (2%), and were conducted in urban settings (88%). The median quality score was 7 (range: 2–9).
Of the 91 included studies, 25% did not provide a LTFU definition. Among the 75% of included studies that provided a LTFU definition, 66% defined LTFU relative to the last documented encounter while 34% defined LTFU relative to a missed scheduled encounter. Intervals from last/missed encounters qualifying a person as LTFU included 60 days (9%), 90 days (37%), 180 days (42%), or other time periods (12%) (Table S2; Supplementary Material).
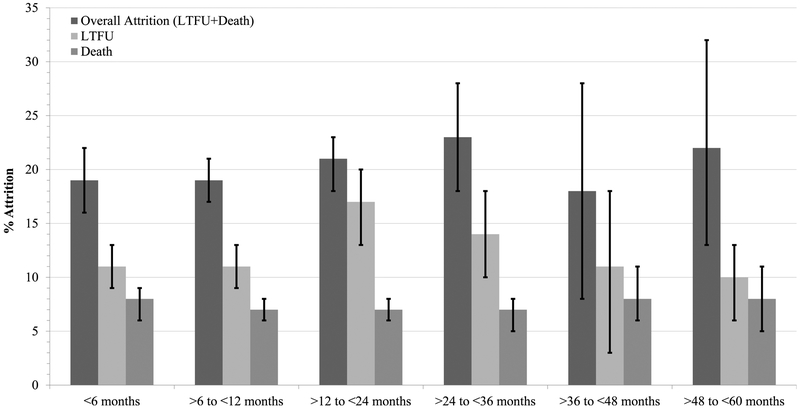
Our meta-analysis showed high prevalence of attrition from pediatric HIV care and treatment services (Figure 2 and Table S4; Forest plots shown in Figures S1–S18, Supplementary Material). Overall attrition (LTFU+death) of HIV-positive children was 19% (95%CI: 16–22%) by 6 months follow-up and increased to 23% (95%CI: 18–28%) by 36 months. Among studies contributing data up to 60 months of follow-up, overall attrition remained stable at 22% (95%CI: 13–32%). LTFU accounted for the preponderance of overall attrition, peaking at 17% (95%CI: 13–20%) by 24 months. Death accounted for 7–8% of attrition across all follow-up intervals. The proportions of pediatric patients who were either LTFU or died do not sum to the proportion of overall attrition at any of the follow-up intervals, as some studies contributing to overall attrition statistics did not specifically indicate attrition source. There was also very high heterogeneity across follow-up intervals and attrition types (I2 = 77.0–99.6%) (Figure 2, Table S4, and Figures S1–S18).
Figure 2. Meta-analysis results of attrition (loss to follow up [LTFU] or death) of HIV-positive children from HIV care and treatment services in low- and middle-income countries.
Refer to Table S4 for details of effect sizes, pooled sample sizes, number of individual cohorts, I2 statistics, and p-values for these pooled estimates.
Original forest plots and meta-analysis results are can be found in the Supplementary Material, Figures S1–S12.
At most follow-up intervals, there was little evidence for publication bias based on Egger’s test of funnel plot asymmetry. However, there was evidence of small-study effects for studies pooled for LTFU within the first 6 months of follow-up and for 6–12 months follow-up, as well as for overall attrition at 6–12 months and 36–48 months of follow-up (Table S5; Supplementary Material).
Meta-regression analyses
Meta-regression analyses were performed to investigate sources of heterogeneity (Table 1). For the 0 to 6 months interval, multivariable meta-regression analysis showed that HIV-positive children receiving ART (vs. pre-ART) were 17% less likely to have overall attrition (95%CI: 4–30%) and 27% less likely to have attrition due to LTFU alone (95%CI: 15–38%). Those receiving ART also trended toward having less mortality, but this was not statistically significant. Multivariable meta-regression analysis also revealed studies with higher quality scores (≥5) were 23% less likely to have overall attrition (95%CI: 3–44%) and 18% less likely to have attrition due to death alone (95%CI: 8–28%) during the first 6 months of follow-up (Table 1).
Table 1.
Univariate and multivariable meta-regression analyses of attrition from pediatric HIV care and treatment services.
|
Follow-up
interval Study characteristics |
Attrition type | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall (LTFU + Death) | LTFU only | Death only | ||||||||||
| Coefficient | 95% CI | P-value | Variance explained (%)† | Coefficient | 95% CI | P-value | Variance explained (%)† | Coefficient | 95% CI | P-value | Variance explained (%)† | |
| Univariate meta-regression (≤6 months) | ||||||||||||
| Intervention study (vs. non-intervention) | 0.12 | (−0.14,0.39) | 0.35 | −0.34 | 0.13 | (−0.12,0.37) | 0.29 | 0.84 | −0.04 | (−0.18,0.10) | 0.53 | −3.85 |
| ART (vs. pre-ART) | −0.27 | (−0.39,−0.16) | <0.001 | 33.71 | −0.29 | (−0.40,−0.19) | <0.001 | 66.74 | −0.06 | (−0.14,0.01) | 0.11 | −2.33 |
| Quality score ≥5 (vs. <5) | −0.34 | (−0.49,−0.20) | <0.001 | 37.90 | −0.18 | (−0.37,0.01) | 0.053 | 14.08 | −0.17 | (−0.25,−0.09) | <0.001 | 42.48 |
| Study year <2004 (vs. ≥2004) | 0.04 | (−0.11,0.18) | 0.61 | −2.03 | −0.05 | (−0.21,0.12) | 0.55 | −3.73 | 0.04 | (−0.03,0.11) | 0.24 | −2.24 |
| Sample size ≥100 (vs. <100) | −0.22 | (−0.36,−0.09) | 0.002 | 21.16 | −0.20 | (−0.36,−0.04) | 0.016 | 22.55 | −0.08 | (−0.15,−0.01) | 0.036 | 9.02 |
| Non-hospital (vs. hospital) | −0.07 | (−0.21,0.07) | 0.33 | −0.02 | 0.02 | (−0.17,0.21) | 0.81 | −5.46 | −0.03 | (−0.10,0.04) | 0.45 | −5.36 |
| Rural (vs. urban) | −0.14 | (−0.31,0.04) | 0.12 | 3.85 | −0.15 | (−0.35,0.05) | 0.13 | 7.12 | −0.04 | (−0.12,0.05) | 0.41 | −3.32 |
| Region (vs. Africa) | 3.66 | −2.65 | 5.30 | |||||||||
| Asia | −0.15 | (−0.33,0.02) | 0.088 | −0.09 | (−0.33,0.15) | 0.44 | −0.07 | (−0.16,0.02) | 0.10 | |||
| America | −0.17 | (−0.59,0.26) | 0.43 | - | - | - | −0.04 | (−0.22,0.15) | 0.68 | |||
| Multivariable meta-regression (≤6 months) | ||||||||||||
| ART (vs. pre-ART) | −0.17 | (−0.30,−0.04) | 0.012 | −0.27 | (−0.38,−0.15) | <0.001 | ||||||
| Quality score ≥5 (vs. <5) | −0.23 | (−0.44,−0.03) | 0.026 | −0.18 | (−0.28,−0.08) | 0.001 | ||||||
| Sample size ≥100 (vs. <100) | 0.01 | (−0.17,0.15) | 0.92 | −0.06 | (−0.18,0.06) | 0.31 | 0.01 | (−0.07,0.08) | 0.82 | |||
| Univariate meta-regression (>6 and ≤12 months) | ||||||||||||
| Intervention study (vs. non-intervention) | 0.09 | (−0.13,0.30) | 0.42 | −0.76 | 0.16 | (0.03,0.28) | 0.017 | 13.49 | 0.03 | (−0.07,0.13) | 0.54 | −3.78 |
| ART (vs. pre-ART) | −0.16 | (−0.25,−0.07) | 0.001 | 16.71 | −0.06 | (−0.11,−0.01) | 0.044 | 6.92 | −0.01 | (−0.04,0.04) | 0.91 | −3.45 |
| Quality score ≥5 (vs. <5) | 0.09 | (−0.16,0.34) | 0.47 | −0.92 | 0.07 | (−0.04,0.18) | 0.19 | 2.13 | 0.02 | (−0.07,0.10) | 0.68 | −2.95 |
| Study year <2004 (vs. ≥2004) | −0.09 | (−0.19,0.01) | 0.079 | 3.50 | −0.03 | (−0.09,0.03) | 0.27 | 1.24 | −0.02 | (−0.05,0.02) | 0.44 | −1.93 |
| Sample size ≥100 (vs. <100) | −0.23 | (−0.36,−0.10) | 0.001 | 16.40 | −0.11 | (−0.20,−0.02) | 0.015 | 9.97 | −0.07 | (−0.13,−0.01) | 0.031 | 5.48 |
| Non−hospital (vs. hospital) | −0.09 | (−0.19,−0.01) | 0.048 | 4.82 | −0.04 | (−0.10,0.01) | 0.10 | 5.01 | −0.02 | (−0.06,0.01) | 0.23 | 0.03 |
| Rural (vs. urban) | −0.02 | (−0.15,0.10) | 0.71 | −1.43 | −0.01 | (−0.07,0.07) | 0.95 | −3.04 | −0.01 | (−0.06,0.04) | 0.64 | −2.95 |
| Region (vs. Africa) | −1.68 | −2.16 | 10.15 | |||||||||
| Asia | −0.02 | (−0.14,0.10) | 0.77 | −0.02 | (−0.10,0.06) | 0.61 | −0.05 | (−0.10,−0.01) | 0.030 | |||
| America | - | - | - | - | - | - | - | - | - | |||
| Multivariable meta-regression (> 6 and ≤12 months) | ||||||||||||
| Intervention study (vs. non-intervention) | 0.12 | (−0.01,0.25) | 0.059 | |||||||||
| ART (vs. pre-ART) | −0.10 | (−0.19,−0.02) | 0.023 | −0.04 | (−0.09,0.01) | 0.079 | ||||||
| Sample size ≥100 (vs. <100) | −0.21 | (−0.34,−0.09) | 0.001 | −0.08 | (−0.17,0.01) | 0.090 | −0.06 | (−0.12,−0.00) | 0.050 | |||
| Non-hospital (vs. hospital) | −0.08 | (−0.17,0.01) | 0.053 | |||||||||
| Region (vs. Africa) | ||||||||||||
| Asia | −0.05 | (−0.09,−0.01) | 0.044 | |||||||||
| Univariate meta-regression (>12 and ≤24 months) | ||||||||||||
| Intervention study (vs. non-intervention) | 0.08 | (−0.06,0.22) | 0.26 | −2.42 | 0.11 | (−0.08,0.30) | 0.24 | −0.82 | −0.02 | (−0.10,0.05) | 0.53 | −0.30 |
| ART (vs. pre-ART) | −0.03 | (−0.13,0.07) | 0.58 | −2.61 | −0.12 | (−0.25,0.00) | 0.050 | 5.37 | −0.01 | (−0.05,0.04) | 0.89 | −4.43 |
| Quality score ≥5 (vs. <5) | 0.07 | (−0.08,0.23) | 0.35 | −0.31 | 0.07 | (−0.11,0.25) | 0.45 | −0.57 | −0.12 | (−0.20,−0.04) | 0.003 | 13.83 |
| Study year <2004 (vs. ≥2004) | −0.02 | (−0.11,0.06) | 0.63 | −2.06 | −0.05 | (−0.16,0.06) | 0.39 | −0.80 | −0.02 | (−0.06,0.01) | 0.18 | 4.49 |
| Sample size ≥100 (vs. <100) | −0.04 | (−0.16,0.08) | 0.54 | −2.71 | −0.15 | (−0.29,−0.01) | 0.036 | 6.43 | −0.04 | (−0.11,0.02) | 0.18 | −5.04 |
| Non-hospital (vs. hospital) | 0.03 | (−0.06,0.11) | 0.56 | −1.11 | 0.01 | (−0.11,0.13) | 0.86 | −2.38 | 0.02 | (−0.02,0.06) | 0.27 | 2.08 |
| Rural (vs. urban) | 0.01 | (−0.11,0.12) | 0.98 | −2.40 | −0.07 | (−0.22,0.09) | 0.40 | −0.97 | 0.03 | (−0.01,0.08) | 0.17 | 8.38 |
| Region (vs. Africa) | 4.06 | −0.74 | −5.78 | |||||||||
| Asia | −0.09 | (−0.19,0.01) | 0.066 | −0.08 | (−0.23,0.06) | 0.26 | −0.02 | (−0.07,0.03) | 0.39 | |||
| America | −0.07 | (−0.28,0.14) | 0.51 | −0.11 | (−0.38,0.16) | 0.42 | −0.01 | (−0.09,0.09) | 0.99 | |||
| Multivariable meta-regression (> 12 and ≤24 months) | ||||||||||||
| ART (vs. pre-ART) | −0.08 | (−0.22,0.05) | 0.21 | |||||||||
| Sample size ≥100 (vs. <100) | −0.11 | (−0.26,0.04) | 0.15 | |||||||||
| Univariate meta-regression (>24 and ≤36 months) | ||||||||||||
| Intervention study (vs. non-intervention) | −0.15 | (−0.47,0.16) | 0.31 | 0.70 | −0.09 | (−0.35,0.17) | 0.47 | −3.36 | −0.06 | (−0.14,0.03) | 0.17 | 9.83 |
| ART (vs. pre-ART) | −0.04 | (−0.25,0.18) | 0.70 | −7.86 | 0.03 | (−0.17,0.22) | 0.79 | −7.58 | 0.03 | (−0.04,0.09) | 0.39 | −2.34 |
| Quality score ≥5 (vs. <5) | - | - | - | - | - | - | - | - | - | - | - | - |
| Study year <2004 (vs. ≥2004) | −0.01 | (−0.17,0.16) | 0.94 | −7.76 | −0.04 | (−0.18,0.10) | 0.55 | −5.19 | −0.01 | (−0.06,0.04) | 0.79 | −9.53 |
| Sample size ≥100 (vs. <100) | −0.35 | (−0.70,0.01) | 0.055 | 14.74 | - | - | - | - | - | - | - | - |
| Non-hospital (vs. hospital) | −0.04 | (−0.21,0.12) | 0.60 | −3.71 | −0.10 | (−0.23,0.03) | 0.13 | 11.45 | 0.02 | (−0.03,0.07) | 0.37 | −1.29 |
| Rural (vs. urban) | 0.03 | (−0.31,0.37) | 0.84 | −7.15 | - | - | - | - | - | - | - | - |
| Region (vs. Africa) | −4.22 | −7.11 | −8.46 | |||||||||
| Asia | −0.08 | (−0.32,0.16) | 0.48 | −0.04 | (−0.23,0.16) | 0.70 | −0.01 | (−0.08,0.05) | 0.69 | |||
| America | - | - | - | - | - | - | - | - | - | |||
| Multivariable meta-regression (>24 and ≤36 months) | ||||||||||||
| N/A | ||||||||||||
| Univariate meta-regression (>36 and ≤48 months) | ||||||||||||
| Intervention study (vs. non-intervention) | 0.02 | (−0.36,0.40) | 0.89 | −23.68 | −0.03 | (−0.35,0.30) | 0.83 | −26.02 | 0.04 | (−.07,0.14) | 0.42 | 7.43 |
| ART (vs. pre−ART) | −0.12 | (−0.37,0.14) | 0.29 | 19.06 | −0.11 | (−0.32,0.11) | 0.24 | 23.40 | 0.01 | (−0.10,0.10) | 0.98 | −40.13 |
| Quality score ≥5 (vs. <5) | - | - | - | - | - | - | - | - | - | - | - | - |
| Study year <2004 (vs. ≥2004) | −0.03 | (−0.32,0.27) | 0.82 | −24.39 | 0.04 | (−0.22,0.29) | 0.70 | −19.99 | −0.03 | (−0.14,0.07) | 0.46 | −15.19 |
| Sample size ≥100 (vs. <100) | −0.24 | (−0.62,0.13) | 0.15 | 19.98 | −0.11 | (−0.46,0.25) | 0.46 | −5.29 | −0.12 | (−0.34,0.10) | 0.21 | 6.82 |
| Non−hospital (vs. hospital) | 0.02 | (−0.26,0.29) | 0.89 | −24.99 | 0.04 | (−0.22,0.30) | 0.69 | −22.58 | −0.05 | (−0.09,−0.01) | 0.041 | 87.98 |
| Rural (vs. urban) | - | - | - | - | - | - | - | - | - | - | - | - |
| Region (vs. Africa) | - | - | - | |||||||||
| Asia | - | - | - | - | - | - | - | - | - | |||
| America | - | - | - | - | - | - | - | - | - | |||
| Multivariable meta-regression (>36 and ≤48 months) | ||||||||||||
| N/A | ||||||||||||
| Univariate meta-regression (>48 and ≤60 months) | ||||||||||||
| Intervention study (vs. non-intervention) | ||||||||||||
| ART (vs. pre-ART) | −0.02 | (−0.25,0.20) | 0.81 | −13.53 | −0.02 | (−0.13,0.08) | 0.57 | −26.45 | 0.03 | (−0.05,0.12) | 0.39 | −1.46 |
| Quality score ≥5 (vs. <5) | 0.07 | (−0.23,0.38) | 0.61 | −8.14 | 0.06 | (−0.11,0.22) | 0.39 | −3.76 | −0.05 | (−0.20,0.10) | 0.45 | −5.00 |
| Study year <2004 (vs. ≥2004) | −0.02 | (−0.24,0.20) | 0.86 | −11.65 | −0.06 | (−0.12,−0.01) | 0.040 | 95.55 | 0.02 | (−0.07,0.11) | 0.56 | −6.64 |
| Sample size ≥100 (vs. <100) | −0.12 | (−0.38,0.13) | 0.30 | −1.34 | 0.06 | (−0.11,0.22) | 0.39 | −3.76 | −0.05 | (−0.20,0.10) | 0.45 | −5.00 |
| Non−hospital (vs. hospital) | 0.17 | (−0.05,0.39) | 0.12 | 11.36 | - | - | - | - | −0.03 | (−0.16,0.11) | 0.68 | −20.83 |
| Rural (vs. urban) | −0.05 | (−0.29,0.20) | 0.69 | −12.13 | 0.07 | (0.02,0.11) | 0.012 | 100.00 | −0.04 | (−0.13,0.04) | 0.29 | 2.51 |
| Region (vs. Africa) | −14.89 | 29.19 | 9.69 | |||||||||
| Asia | −0.11 | (−0.34,0.12) | 0.31 | −0.06 | (−0.17,0.05) | 0.22 | −0.01 | (−0.10,0.10) | 0.99 | |||
| America | −0.08 | (−0.50,0.34) | 0.68 | N/A | 0.09 | (−0.07,0.25) | 0.23 | |||||
| Multivariable meta-regression ( 48 and ≤60 months) | ||||||||||||
| Study year <2004 (vs. ≥2004) | 0.02 | (−0.12,0.16) | 0.66 | |||||||||
| Rural (vs. urban) | 0.09 | (−0.05,0.23) | 0.14 | |||||||||
Note: CI, confidence interval; LTFU, lost to follow-up; N/A, multivariable meta-regression not performed due to less than two significant (P<0.05) factors in the univariate analysis; “-” univariate meta-regression not performed due to no observations.
Between-study variance explained reflected as adjusted R2 statistics.
For the >6 and ≤12 months follow-up interval, multivariable meta-regression revealed that ART (vs. pre-ART) and larger sample size (≥100) were associated with 10% (95%CI: 2–19%) and 21% (95%CI: 9–34%) decreases in overall attrition, respectively. Studies in Asia (vs. Africa) were 5% (95%CI: 1–9%) less likely to have attrition due to death during this follow-up interval (Table 1). For intervals >12 months, there were no significant associations in multivariable analysis (Table 1).
Subgroup analyses
Subgroup meta-analyses were stratified by studies of HIV-positive children receiving ART vs. pre-ART children, with the latter having consistently higher levels of LTFU and overall attrition over time. However, differences between the groups were more extreme at earlier follow-up intervals (e.g., overall attrition up to 6 months follow-up was 38% and 11% for pre-ART and ART subgroups, respectively) and were less marked at latter follow-up intervals (e.g., overall attrition at >48 to ≤60 months was 22% and 21% for pre-ART and ART subgroups, respectively). While mortality was lower for those on ART during the first 6 months of follow-up (7% vs. 12%), mortality was similar between ART and pre-ART subgroups at latter time points (Table 2).
Table 2.
Subgroup/subgroup meta-analyses of attrition from pediatric HIV care and treatment services.
|
Attrition
type Subgroups |
Follow-up interval (months [m]) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤6 m | >6 to ≤12 m | >12 to ≤24 m | >24 to ≤36 m | >36 to ≤48 m | >48 to ≤60m | |||||||||||||
| K | EZ (95% CI) | I2,%† | K | EZ (95% CI) | I2,%† | K | EZ (95% CI) | I2,%† | K | EZ (95% CI) | I2,%† | K | EZ (95% CI) | I2,%† | K | EZ (95% CI) | I2,%† | |
| Overall (LTFU + Death) | ||||||||||||||||||
| Study type | ||||||||||||||||||
| Non-intervention | 36 | 0.18(0.15,0.21) | 98.6 | 59 | 0.19(0.16,0.21) | 99.2 | 46 | 0.20(0.17,0.23) | 99.1 | 15 | 0.24(0.18,0.29) | 98.8 | 6 | 0.18(0.07,0.28) | 99.6 | 12 | 0.22(0.13,0.32) | 98.9 |
| Intervention | 3 | 0.30(0.16,0.45) | 82.0 | 3 | 0.28(0.01,0.56) | 97.5 | 6 | 0.27(0.16,0.38) | 96.0 | 1 | 0.09(0.06,0.12) | - | 1 | 0.20(0.16,0.24) | - | 0 | - | - |
| Quality score | ||||||||||||||||||
| <5 | 7 | 0.48(0.18,0.79) | 98.5 | 2 | 0.11(0.09,0.13) | 0.0 | 4 | 0.14(0.01,0.27) | 95.6 | 0 | - | - | 0 | - | - | 2 | 0.15(−0.02,0.33) | 66.9 |
| ≥5 | 32 | 0.13(0.11,0.15) | 97.2 | 60 | 0.19(0.47,0.22) | 99.2 | 48 | 0.21(0.18,0.24) | 99.1 | 16 | 0.23(0.18,0.28) | 98.7 | 7 | 0.18(0.08,0.28) | 99.6 | 10 | 0.23(0.13,0.34) | 99.1 |
| Cohort type | ||||||||||||||||||
| ART | 26 | 0.11(0.09,0.13) | 96.7 | 42 | 0.14(0.12,0.17) | 99.2 | 39 | 0.20(0.17,0.23) | 99.2 | 13 | 0.22(0.17,0.28) | 99.0 | 5 | 0.14(0.05,0.23) | 99.4 | 7 | 0.21(0.08,0.34) | 99.4 |
| Pre-ART | 13 | 0.38(0.21,0.39) | 99.2 | 20 | 0.31(0.22,0.40) | 99.1 | 13 | 0.23(0.15,0.31) | 98.0 | 3 | 0.25(0.11,0.39) | 89.7 | 2 | 0.26(0.14,0.38) | 96.7 | 5 | 0.22(0.13,0.31) | 88.5 |
| Case-finding‡ | ||||||||||||||||||
| Tracing | 5 | 0.11(0.06,0.16) | 98.6 | 10 | 0.08(0.06,0.11) | 95.1 | 13 | 0.16(0.12,0.20) | 97.3 | 1 | 0.56(0.37,0.75) | - | 2 | 0.28(0.09,0.47) | 79.3 | 6 | 0.24(0.13,0.34) | 97.1 |
| No tracing | 34 | 0.19(0.16,0.22) | 90.4 | 52 | 0.21(0.18,0.23) | 99.1 | 39 | 0.22(0.19,0.25) | 99.1 | 15 | 0.21(0.16,0.27) | 98.8 | 5 | 0.15(0.04,0.26) | 99.7 | 6 | 0.19(0.11,0.26) | 96.0 |
| LTFU only | ||||||||||||||||||
| Study type | ||||||||||||||||||
| Non-intervention | 19 | 0.10(0.08,0.12) | 97.1 | 37 | 0.11(0.09,0.13) | 99.1 | 39 | 0.16(0.13,0.19) | 99.5 | 13 | 0.14(0.10,0.18) | 98.9 | 5 | 0.11(0.02,0.20) | 99.3 | 6 | 0.10(0.06,0.13) | 77.0 |
| Intervention | 3 | 0.27(0.12,0.41) | 83.0 | 2 | 0.27(0.20,0.34) | 0.0 | 5 | 0.28(0.10,0.46) | 96.3 | 1 | 0.05(0.03,0.07) | - | 1 | 0.09(0.01,0.11) | - | 0 | - | - |
| Quality score | ||||||||||||||||||
| <5 | 5 | 0.31(0.12,0.51) | 94.9 | 2 | 0.05(−0.01,0.11) | 96.3 | 5 | 0.08(0.02,0.14) | 86.4 | 0 | - | - | 0 | - | - | 1 | 0.04(−0.04,0.13) | - |
| ≥5 | 17 | 0.09(0.70,0.11) | 97.1 | 37 | 0.12(0.10,0.14) | 99.1 | 39 | 0.17(0.14,0.21) | 99.5 | 14 | 0.14(0.10,0.18) | 98.8 | 6 | 0.11(0.03,0.18) | 99.2 | 5 | 0.10(0.07,0.14) | 80.6 |
| Cohort type | ||||||||||||||||||
| ART | 14 | 0.05(0.04,0.07) | 95.3 | 26 | 0.10(0.08,0.12) | 99.4 | 32 | 0.14(0.11,0.17) | 99.4 | 12 | 0.14(0.10,0.18) | 99.0 | 4 | 0.06(0.01,0.10) | 96.3 | 3 | 0.08(0.05,0.12) | 59.8 |
| Pre-ART | 8 | 0.37(0.21,0.53) | 96.6 | 13 | 0.15(0.11,0.19) | 91.7 | 12 | 0.27(0.12,0.42) | 99.5 | 2 | 0.11(0.06,0.16) | 51.2 | 2 | 0.17(0.01,0.34) | 99.0 | 3 | 0.11(0.04,0.17) | 87.4 |
| Case-finding‡ | ||||||||||||||||||
| Tracing | 3 | 0.12(0.02,0.23) | 95.6 | 5 | 0.07(0.03,0.10) | 99.1 | 11 | 0.11(0.06,0.15) | 98.5 | 0 | - | - | 2 | 0.12(0.02,0.22) | 57.6 | 3 | 0.08(0.01,0.15) | 85.6 |
| No tracing | 19 | 0.11(0.09,0.13) | 97.1 | 34 | 0.12(0.10,0.14) | 95.5 | 33 | 0.19(0.15,0.23) | 99.5 | 13 | 0.14(0.10,0.18) | 98.9 | 4 | 0.10(0.00,0.19) | 99.5 | 3 | 0.10(0.06,0.14) | 73.7 |
| Death only | ||||||||||||||||||
| Study type | ||||||||||||||||||
| Non-intervention | 30 | 0.08(0.06,0.09) | 95.1 | 43 | 0.07(0.06,0.08) | 96.8 | 38 | 0.07(0.06,0.08) | 94.6 | 13 | 0.07(0.05,0.88) | 94.0 | 5 | 0.08(0.05,0.11) | 93.0 | 9 | 0.08(0.05,0.11) | 90.4 |
| Intervention | 2 | 0.05(−0.04,0.13) | 82.9 | 2 | 0.14(−0.10,0.38) | 93.6 | 4 | 0.04(0.02,0.05) | 0.0 | 1 | 0.02(0.01,0.03) | - | 1 | 0.11(0.08,0.14) | - | 0 | - | - |
| Quality score | ||||||||||||||||||
| <5 | 5 | 0.27(0.12,0.42) | 92.7 | 2 | 0.06(0.01,0.11) | 86.8 | 3 | 0.25(0.10,0.40) | 92.2 | 0 | - | - | 0 | - | - | 2 | 0.13(−0.01,0.26) | 48.4 |
| ≥5 | 27 | 0.06(0.05,0.08) | 95.1 | 43 | 0.07(0.06,0.08) | 96.8 | 39 | 0.07(0.06,0.08) | 94.1 | 14 | 0.07(0.05,0.08) | 94.7 | 6 | 0.08(0.06,0.11) | 92.1 | 7 | 0.07(0.04,0.10) | 92.4 |
| Cohort type | ||||||||||||||||||
| ART | 23 | 0.07(0.05,0.08) | 94.4 | 32 | 0.07(0.06,0.09) | 97.4 | 32 | 0.07(0.06,0.08) | 94.4 | 12 | 0.07(0.05,0.09) | 95.5 | 4 | 0.09(0.05,0.13) | 93.5 | 5 | 0.09(0.06,0.12) | 75.9 |
| Pre-ART | 9 | 0.12(0.08,0.17) | 94.6 | 13 | 0.07(0.05,0.09) | 89.1 | 10 | 0.07(0.04,0.09) | 88.3 | 2 | 0.04(0.02,0.06) | 0.0 | 2 | 0.09(0.03,0.14) | 90.9 | 4 | 0.06(0.01,0.11) | 88.7 |
| Case-finding‡ | ||||||||||||||||||
| Tracing | 5 | 0.03(0.01,0.05) | 83.5 | 9 | 0.04(0.02,0.05) | 96.9 | 12 | 0.05(0.04,0.06) | 77.3 | 0 | - | - | 2 | 0.13(0.06,0.19) | 26.6 | 4 | 0.10(0.00,0.20) | 90.5 |
| No tracing | 27 | 0.07(0.06,0.09) | 94.4 | 35 | 0.08(0.07,0.09) | 85.3 | 30 | 0.08(0.07,0.09) | 94.4 | 13 | 0.07(0.05,0.88) | 94.0 | 4 | 0.07(0.04,0.10) | 94.5 | 5 | 0.07(0.05,0.10) | 83.3 |
Note: CI, confidence interval; EZ, pooled effect size; K, number of individual effect sizes included in the meta-analysis; LTFU, lost to follow-up; m, months; “-” Subgroup/subgroup meta-analysis not performed due to ≤1 study contributing data.
When I2 was ≤25%, effect size was aggregated using fixed-effect models.
Restricted to non-intervention studies.
There were few studies with quality scores <5 from which to draw conclusions (K≤7 for each analysis). Meta-analyses of the subgroups of studies with quality scores <5 vs. ≥5 showed no clear trends in attrition (Table 2).
There were also few intervention studies from which to draw conclusions (K≤6 for each analysis). However, meta-analyses of the subgroups of intervention and non-intervention studies suggested that overall attrition and attrition due to LTFU may have been higher for intervention studies compared to non-intervention studies through the first 24 months of follow-up (Table 2).
Subgroup meta-analyses suggested overall attrition was lower in non-intervention studies that performed tracing compared to those that did not at ≤6 months (11% vs. 19%), >6 to ≤12 months (8% vs. 21%), and >12 to ≤24 months (16% vs. 22%) of follow-up. Beyond 24 months follow-up, there were fewer tracing studies from which to draw conclusions (K≤6 for each analysis), but by >48 to ≤60 months of follow-up, overall attrition was higher for studies that performed tracing compared to those that did not (24% vs. 19%). Similar findings were observed when examining LTFU and death individually; generally, there were lower levels of LTFU and death among tracing studies up to 24 months of follow-up, but at later intervals, LTFU and death were similar or higher for studies with tracing compared to those without. A high degree of heterogeneity was observed across studies, even when examined in subgroup analyses (Table 2).
Discussion
To date, this work is the most comprehensive assessment of attrition from pediatric HIV care and treatment services in LMICs. Quantifying the magnitude of this attrition provides a vital benchmark for gauging programmatic successes and weaknesses. We found that most attrition occurred in the first six months of follow-up. We also found that HIV-positive children receiving ART had significantly better retention in care than those pre-ART, and that studies performing case-finding/tracing for those LTFU had better retention in care. These findings underscore the importance of early ART initiation and decentralized patient support services (e.g., tracing).
Estimates of attrition from our primary meta-analysis (Figure 2 and Table S4) were similar to those from prior systematic reviews of attrition from pediatric[4, 5] and adult[17, 18] HIV services in LMICs. However, as our primary meta-analysis was inclusive of pre-ART and ART cohorts, and we know pre-ART attrition varies widely and has the potential to bias estimates,[6, 19] we performed subgroup meta-analysis restricted to those on ART (Table 2). We found that our estimates of attrition among children on ART were slightly lower than those previously reported, with 14% of HIV-positive children on ART no longer in care at 12 months follow-up compared to previous estimates of 15%[4] and 5–29% attrition.[5] By 36 months follow-up, we found 22% of HIV-positive children on ART were no longer in care, while Fox et al. reported 23–34% attrition.[4] This might suggest slight improvement in pediatric retention as programs and guidelines have evolved over time. However, in our regression analyses, later study year (study starting during or after 2004) was not predictive of lower attrition. Abuogi et al. actually found that risk of attrition in the first 12 months after ART initiation increased in later years compared to earlier years.[5] We also found higher attrition in later years in our recent meta-analysis of HIV-exposed infant attrition.[15] Perhaps in earlier years, only successful programs were reporting outcomes (i.e., publication bias). Also, earlier publications presumably overrepresented well-established urban programs, while later publications represent expansion of services into newer programs and/or rural areas.[20] Alternatively, it is possible that prevention of mother-to-child transmission programs have succeeded in reducing incident pediatric HIV infections, and increased ART availability has “resulted in a changing demographic of HIV-positive children from families that are either more vulnerable or less motivated for treatment.”[5] Whatever the case, improvements in pediatric attrition are only modest, at best, so additional research and resources are clearly needed to ensure sustained retention in pediatric HIV services.
As expected, attrition from pediatric HIV care and treatment services increased with duration of follow-up. However, most attrition occurred within the first six months, suggesting this is an especially vulnerable period, and interventions to mitigate attrition or promote retention (improved integration/linkage of maternal and child HIV services,[21–24] phone/SMS-based messaging/reminders,[22] systems engineering and continuous quality improvement approaches,[20, 25] improved information systems for identifying patients who have missed clinical visits,[26, 27] and enhanced community outreach and patient tracing[28–30]) might have greater impact if implemented soon after HIV diagnosis.[31] While mortality was captured when documented, it must be acknowledged that the reasons for early attrition are not known and might actually be attributable in large part to undocumented mortality.
Ample data support the idea that early ART initiation improves health outcomes for children with HIV;[3, 32–35] consistent with these health benefits, our findings suggest that retention in care is also improved for those on ART. This provides support for the policy/guideline shifts to the now-standard “Test and Start” treatment paradigm.[36] We also observed more extreme differences between ART and pre-ART attrition at earlier follow-up intervals; however, this is confounded by the fact that many pre-ART patients eventually transition to ART.
Studies/programs that invested resources in tracing LTFU patients had lower levels of attrition than those not performing/reporting tracing. However, this effect was more prominent at earlier follow-up intervals. The benefits of tracing may diminish over time as patients become more treatment-experienced. By implementing intensive case-finding for those LTFU, programs can more effectively promote retention or reengagement in care, ensure that unknown/silent transfers previously characterized as LTFU can be properly characterized as retained in care, more accurately estimate mortality in their cohorts, and gain insights into barriers and facilitators to sustained retention.[37–43] Our findings provide further support to the addition of patient tracing as a standard/required component of the services provided by HIV programs in LMICs. However, additional research is needed to better understand optimal approaches to tracing HIV-affected infants and children.
There was a high degree of heterogeneity in reported outcomes across follow-up intervals and attrition subgroups. While this was also a major issue for other meta-analyses, this degree of between-study variance should lead to cautious interpretation of our primary meta-analysis. That said, we performed subgroup meta-analyses and random effects meta-regression analyses to explore the potential sources and magnitude of this heterogeneity.
In particular, we acknowledge that inclusion of both non-intervention and intervention studies, which often differ in very important ways, might have biased attrition/retention outcomes resulting in enhanced heterogeneity. However, to ensure that we captured all relevant data (including from control groups receiving standard of care in intervention studies offering program-level interventions) we chose not to categorically exclude all intervention studies. Interestingly, our subgroup analysis revealed the counterintuitive finding that intervention studies had higher levels of attrition than non-intervention studies, despite the extra-programmatic research resources that are typically available to promote retention for intervention studies. That said, it is difficult to draw conclusions from this subgroup analysis for two important reasons: 1) relatively few intervention studies were included in the subgroup (K≤6), and 2) most intervention studies reported pooled attrition outcomes for intervention and control arms. Among those reporting attrition outcomes disaggregated by intervention status, those receiving the intervention typically had lower attrition. Intervention studies may not have the external validity needed to compare attrition outcomes to reports of more routine programmatic data.
A strength of our study was that we synthesized data from more than 145,000 HIV-positive children. Consistent with the global burden of HIV and PEPFAR and Global Fund investments, most of these children were from African countries. We were surprised to find little evidence of regional variation in attrition between Africa, Asia, and the Americas, though the small number of studies from the Americas limits inference. More variability is likely to be observed if we stratified by country, but too few studies were available from any given country for us to make these comparisons. There was also disproportionate representation from urban compared to rural areas. This may limit our ability to generalize our findings to non-urban areas in Africa and highlights the need for additional outcomes research in other regions and rural areas.
Another limitation was the wide variation in LTFU definition across studies, which at least partially accounts for the high degree of heterogeneity in reported outcomes.[44, 45] We accepted all provided definitions of LTFU, but because of this heterogeneity and the lack of a clear LTFU definition in 25% of studies, we were unable to directly account for LTFU definition in meta-regression or subgroup analyses. However, our quality assessments did consider duration of follow-up and whether an LTFU definition was provided. Heterogeneity in definitions and its impact on reporting outcomes have been raised by several studies.[44, 46, 47] Adoption of a universal LTFU definition would help standardize reporting and allow for more reliable comparisons between programs.
The factors/covariates used in our meta-regression and subgroup meta-analyses were selected based on our experience working with HIV programs in LMICs and on literature reviews, but other important factors influence attrition from pediatric HIV care (e.g., health literacy/numeracy).[48, 49] As the studies reviewed here overwhelmingly represented routinely collected programmatic data, there was no consistent exploration of other factors. Attrition/retention outcomes were infrequently disaggregated by age, sex, or World Health Organization (WHO) clinical stage, CD4 count, or HIV viral load, so we were unable to assess for differences in attrition by these variables. In-depth exploration of other factors influencing retention in pediatric HIV services should be a focus of future research.
Conclusions
Our findings of high attrition of HIV-positive children from HIV care and treatment services in LMICs underscore the urgent need for implementation research and resources to mitigate attrition and improve retention among this vulnerable population. The magnitude of attrition from pediatric HIV programs in LMICs remains unacceptably high, with minimal improvements shown in recent years. By quantifying this magnitude of attrition, this report may also serve as a benchmark for programmatic successes and weaknesses, potentially useful for HIV program directors in LMICs and global policymakers.
Supplementary Material
Acknowledgments
The authors would like to thank Donna Ingles for assistance with writing and manuscript preparation, and Elizabeth Frakes for assistance with constructing a comprehensive search strategy. Halle Friedman, Brenda E. Pelayo, Kimberly Robelin, and Emily K. Sheldon provided valued early assistance in data collection and management.
Funding:
The authors acknowledge support from: the National Institutes of Health (NIH) funded Tennessee Center for AIDS Research (P30AI110527; for SHV and JGC), the Yale Center for Interdisciplinary Research on AIDS (P30MH062294; for SHV), NIH grants T32HD060554 for JGC and K01MH107258 for KC, and the Amos Christie Chair Endowment Global Health Fellowship at Vanderbilt University Medical Center for YL.
List of abbreviations
- ART
antiretroviral therapy
- ARV
antiretroviral medication
- CI
confidence interval
- HIV
human immunodeficiency virus
- LMIC
low- and middle-income countries
- LTFU
loss to follow-up
- PEPFAR
President’s Emergency Plan for AIDS Relief
- UNAIDS
Joint United Nations Programme on HIV/AIDS
- WHO
World Health Organization
Footnotes
Publisher's Disclaimer: Disclaimer: The funders of this study had no role in study design, data collection, analysis, interpretation, or writing of this report. The corresponding author had full access to all of the data in the study and had final responsibility for the decision to submit for publication.
Supplementary material includes: Table S1 (search strategy), Table S2 (details of included studies), Table S3 (quality assessment tool), Table S4 (meta-analysis results), Table S5 (results of Egger’s linear regression test to assess funnel plot asymmetry), and Figures S1–S18 (Forest plots contributing to Table S4 and Figure 2).
The dataset used for this meta-analysis, a complete list of references included and excluded from the meta-analysis, and a PRISMA checklist can be found at: https://rocket.app.vumc.org/index.php?doc_id=23022.
Competing interests
The authors declare no competing interests.
References
- 1.Joint United Nations Programme on HIV/AIDS (UNAIDS). UNAIDS Data 2018. In. Geneva, Switzerland; 2018. [Google Scholar]
- 2.Joint United Nations Programme on HIV/AIDS (UNAIDS). 90-90-90 - An ambitious treatment target to help end the AIDS epidemic. In. Geneva, Switzerland; 2017. [Google Scholar]
- 3.World Health Organization (WHO). March 2014 Supplement to the 2013 Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. In. Geneva, Switzerland; 2014. [PubMed] [Google Scholar]
- 4.Fox MP, Rosen S. Systematic review of retention of pediatric patients on HIV treatment in low and middle-income countries 2008–2013. AIDS 2015; 29(4):493–502. [DOI] [PubMed] [Google Scholar]
- 5.Abuogi LL, Smith C, McFarland EJ. Retention of HIV-Infected Children in the First 12 Months of Anti-Retroviral Therapy and Predictors of Attrition in Resource Limited Settings: A Systematic Review. PloS one 2016; 11(6):e0156506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mugglin C, Wandeler G, Estill J, Egger M, Bender N, Davies MA, et al. Retention in care of HIV-infected children from HIV test to start of antiretroviral therapy: systematic review. PloS one 2013; 8(2):e56446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009; 62(10):1006–1012. [DOI] [PubMed] [Google Scholar]
- 8.The World Bank. World Bank Country and Lending Groups. In.
- 9.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. In. Ottawa: Ottawa Hospital Research Institute; 2011. [Google Scholar]
- 12.Brockwell SE, Gordon IR. A comparison of statistical methods for meta-analysis. Stat Med 2001; 20(6):825–840. [DOI] [PubMed] [Google Scholar]
- 13.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7(3):177–188. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003; 327(7414):557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carlucci JG, Liu Y, Friedman H, Pelayo BE, Robelin K, Sheldon EK, et al. Attrition of HIV-exposed infants from early infant diagnosis services in low- and middle-income countries: a systematic review and meta-analysis. J Int AIDS Soc 2018; 21(11):e25209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315(7109):629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fox MP, Rosen S. Retention of Adult Patients on Antiretroviral Therapy in Low- and Middle-Income Countries: Systematic Review and Meta-analysis 2008–2013. J Acquir Immune Defic Syndr 2015; 69(1):98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosen S, Fox MP, Gill CJ. Patient retention in antiretroviral therapy programs in sub-Saharan Africa: a systematic review. PLoS Med 2007; 4(10):e298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosen S, Fox MP. Retention in HIV care between testing and treatment in sub-Saharan Africa: a systematic review. PLoS Med 2011; 8(7):e1001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vermund SH, Blevins M, Moon TD, Jose E, Moiane L, Tique JA, et al. Poor clinical outcomes for HIV infected children on antiretroviral therapy in rural Mozambique: need for program quality improvement and community engagement. PloS one 2014; 9(10):e110116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ciampa PJ, Burlison JR, Blevins M, Sidat M, Moon TD, Rothman RL, et al. Improving retention in the early infant diagnosis of HIV program in rural Mozambique by better service integration. J Acquir Immune Defic Syndr 2011; 58(1):115–119. [DOI] [PubMed] [Google Scholar]
- 22.Geldsetzer P, Yapa HM, Vaikath M, Ogbuoji O, Fox MP, Essajee SM, et al. A systematic review of interventions to improve postpartum retention of women in PMTCT and ART care. J Int AIDS Soc 2016; 19(1):20679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tudor Car L, Brusamento S, Elmoniry H, van Velthoven MH, Pape UJ, Welch V, et al. The uptake of integrated perinatal prevention of mother-to-child HIV transmission programs in low- and middle-income countries: a systematic review. PloS one 2013; 8(3):e56550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aliyu MH, Blevins M, Audet CM, Kalish M, Gebi UI, Onwujekwe O, et al. Integrated prevention of mother-to-child HIV transmission services, antiretroviral therapy initiation, and maternal and infant retention in care in rural north-central Nigeria: a cluster-randomised controlled trial. Lancet HIV 2016; 3(5):e202–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rustagi AS, Gimbel S, Nduati R, Cuembelo Mde F, Wasserheit JN, Farquhar C, et al. Implementation and Operational Research: Impact of a Systems Engineering Intervention on PMTCT Service Delivery in Cote d’Ivoire, Kenya, Mozambique: A Cluster Randomized Trial. J Acquir Immune Defic Syndr 2016; 72(3):e68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clouse K, Phillips T, Myer L. Understanding data sources to measure patient retention in HIV care in sub-Saharan Africa. Int Health 2017; 9(4):203–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clouse K, Mongwenyana C, Musina M, Bokaba D, Long L, Maskew M, et al. Acceptability and feasibility of a financial incentive intervention to improve retention in HIV care among pregnant women in Johannesburg, South Africa. AIDS Care 2017:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mugavero MJ. Elements of the HIV Care Continuum: Improving Engagement and Retention in Care. Top Antivir Med 2016; 24(3):115–119. [PMC free article] [PubMed] [Google Scholar]
- 29.Clouse K, Vermund SH, Maskew M, Lurie MN, MacLeod W, Malete G, et al. Mobility and Clinic Switching Among Postpartum Women Considered Lost to HIV Care in South Africa. J Acquir Immune Defic Syndr 2017; 74(4):383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sibanda EL, Weller IV, Hakim JG, Cowan FM. The magnitude of loss to follow-up of HIV-exposed infants along the prevention of mother-to-child HIV transmission continuum of care: a systematic review and meta-analysis. AIDS 2013; 27(17):2787–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanters S, Park JJ, Chan K, Socias ME, Ford N, Forrest JI, et al. Interventions to improve adherence to antiretroviral therapy: a systematic review and network meta-analysis. Lancet HIV 2017; 4(1):e31–e40. [DOI] [PubMed] [Google Scholar]
- 32.Phelps BR, Ahmed S, Amzel A, Diallo MO, Jacobs T, Kellerman SE, et al. Linkage, initiation and retention of children in the antiretroviral therapy cascade: an overview. Aids 2013; 27 Suppl 2:S207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Essajee S, Vojnov L, Penazzato M, Jani I, Siberry GK, Fiscus SA, et al. Reducing mortality in HIV-infected infants and achieving the 90-90-90 target through innovative diagnosis approaches. J Int AIDS Soc 2015; 18:14–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cotton MF, Violari A, Otwombe K, Panchia R, Dobbels E, Rabie H, et al. Early time-limited antiretroviral therapy versus deferred therapy in South African infants infected with HIV: results from the children with HIV early antiretroviral (CHER) randomised trial. Lancet 2013; 382(9904):1555–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Violari A, Cotton MF, Gibb DM, Babiker AG, Steyn J, Madhi SA, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med 2008; 359(21):2233–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.World Health Organization (WHO). Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. In; 2015. [PubMed]
- 37.Brinkhof MWG, Pujades-Rodriguez M, Egger M. Mortality of Patients Lost to Follow-Up in Antiretroviral Treatment Programmes in Resource-Limited Settings: Systematic Review and Meta-Analysis. PloS one 2009; 4(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Egger M, Spycher BD, Sidle J, Weigel R, Geng EH, Fox MP, et al. Correcting mortality for loss to follow-up: a nomogram applied to antiretroviral treatment programmes in sub-Saharan Africa. PLoS Med 2011; 8(1):e1000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Geng EH, Bangsberg DR, Musinguzi N, Emenyonu N, Bwana MB, Yiannoutsos CT, et al. Understanding reasons for and outcomes of patients lost to follow-up in antiretroviral therapy programs in Africa through a sampling-based approach. J Acquir Immune Defic Syndr 2010; 53(3):405–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Geng EH, Glidden DV, Emenyonu N, Musinguzi N, Bwana MB, Neilands TB, et al. Tracking a sample of patients lost to follow-up has a major impact on understanding determinants of survival in HIV-infected patients on antiretroviral therapy in Africa. Trop Med Int Health 2010; 15 Suppl 1:63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lamb MR, El-Sadr WM, Geng E, Nash D. Association of adherence support and outreach services with total attrition, loss to follow-up, and death among ART patients in sub-Saharan Africa. PloS one 2012; 7(6):e38443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilkinson LS, Skordis-Worrall J, Ajose O, Ford N. Self-transfer and mortality amongst adults lost to follow-up in ART programmes in low- and middle-income countries: systematic review and meta-analysis. Trop Med Int Health 2015; 20(3):365–379. [DOI] [PubMed] [Google Scholar]
- 43.Zurcher K, Mooser A, Anderegg N, Tymejczyk O, Couvillon MJ, Nash D, et al. Outcomes of HIV-positive patients lost to follow-up in African treatment programmes. Trop Med Int Health 2017; 22(4):375–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shepherd BE, Blevins M, Vaz LM, Moon TD, Kipp AM, Jose E, et al. Impact of definitions of loss to follow-up on estimates of retention, disease progression, and mortality: application to an HIV program in Mozambique. Am J Epidemiol 2013; 178(5):819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grimsrud AT, Cornell M, Egger M, Boulle A, Myer L. Impact of definitions of loss to follow-up (LTFU) in antiretroviral therapy program evaluation: variation in the definition can have an appreciable impact on estimated proportions of LTFU. J Clin Epidemiol 2013; 66(9):1006–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chi BH, Yiannoutsos CT, Westfall AO, Newman JE, Zhou J, Cesar C, et al. Universal definition of loss to follow-up in HIV treatment programs: a statistical analysis of 111 facilities in Africa, Asia, and Latin America. PLoS Med 2011; 8(10):e1001111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rollins NC, Becquet R, Orne-Gliemann J, Phiri S, Hayashi C, Baller A, et al. Defining and analyzing retention-in-care among pregnant and breastfeeding HIV-infected women: unpacking the data to interpret and improve PMTCT outcomes. J Acquir Immune Defic Syndr 2014; 67 Suppl 2:S150–156. [DOI] [PubMed] [Google Scholar]
- 48.Howard LM, Tique JA, Gaveta S, Sidat M, Rothman RL, Vermund SH, et al. Health literacy predicts pediatric dosing accuracy for liquid zidovudine. Aids 2014; 28(7):1041–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tique JA, Howard LM, Gaveta S, Sidat M, Rothman RL, Vermund SH, et al. Measuring Health Literacy Among Adults with HIV Infection in Mozambique: Development and Validation of the HIV Literacy Test. AIDS Behav 2017; 21(3):822–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.